Abstract
The mechanical properties of the local microenvironment may have important influence on the fate and function of adult tissue progenitor cells, altering the regenerative process. This is particularly critical following a myocardial infarction, in which the normal, compliant myocardial tissue is replaced with fibrotic, stiff scar tissue. In this study, we examined the effects of matrix stiffness on adult cardiac side population (CSP) progenitor cell behavior. Ovine and murine CSP cells were isolated and cultured on polydimethylsiloxane substrates, replicating the elastic moduli of normal and fibrotic myocardium. Proliferation capacity and cell cycling were increased in CSP cells cultured on the stiff substrate with an associated reduction in cardiomyogeneic differentiation and accelerated cell ageing. In addition, culture on stiff substrate stimulated upregulation of extracellular matrix and adhesion proteins gene expression in CSP cells. Collectively, we demonstrate that microenvironment properties, including matrix stiffness, play a critical role in regulating progenitor cell functions of endogenous resident CSP cells. Understanding the effects of the tissue microenvironment on resident cardiac progenitor cells is a critical step toward achieving functional cardiac regeneration.
Keywords: matrix stiffness, cell cycle regulation, cardiac side population cell, proliferation, differentiation, cellular ageing
the adult heart possesses limited regenerative potential (5, 19) and contains within it a population of cardiac stem/progenitor cells (4, 17, 28, 35). Following injury, such as that which occurs with myocardial infarction (MI), the heart's native regenerative potential is insufficient to replace lost myocardium and prevent progression toward heart failure. Interestingly, cardiac progenitor cell quantities are reported to be normal or even increased in injured versus healthy hearts. When isolated from diseased tissue, these progenitor cells retain their ability to differentiate into cardiomyocytes in vitro (26, 32), suggesting that endogenous factors may be preventing the differentiation of progenitor cells.
Following MI, the adult heart undergoes characteristic structural and functional remodeling with the formation of fibrosis and changes in the ECM composition. This results in an escalation in stiffness of the local microenvironment (6, 11). Emerging studies of pluripotent stem cells have suggested that the mechanical properties of the microenvironment may modulate stem cell function, including self-renewal and lineage-specific differentiation (10, 20, 48). Stem/progenitor cell function can be tuned for highly specific differentiation by altered matrix stiffness in embryonic stem cells (25), mesenchymal stem cells (36, 37, 44, 47), neural stem cells (27), and adipose-derived stem cells (3, 33). Similarly, mesenchymal stem cells (14, 29, 45, 49), CD34+ bone marrow cells (50), and induced pluripotent stem cells (16) are suggested to differentiate into cardiac cells at higher percentages when the underlying matrix stiffness approximates that of the myocardium. However, the influence of tissue stiffness in regulating the function of resident cardiac stem/progenitor cells has not been reported. Understanding the modulation of resident cardiac stem/progenitor cells is critical toward aiding functional cardiac regeneration.
Herein, we tested the hypothesis that matrix stiffness modulates resident cardiac stem/progenitor cell function. Using biomaterials to simulate microenvironment stiffness, we studied the response of cardiac side population (CSP) cells to substrates spanning the elastic moduli of normal (soft) and fibrotic (stiff) myocardium. We find that matrix stiffness is a critical regulator of CSP cell fate and function, with stiff matrix, mimicking diseased myocardium, increasing the proliferation capacity and decreasing the cardiomyogenic differentiation of CSP cells.
MATERIALS AND METHODS
Polydimethylsiloxane matrix preparation.
To mimic the matrix stiffness of normal (soft) and scarred myocardium (stiff), polydimethylsiloxane (PDMS; Sylgard 184 silicone elastomer, Dow Corning) was used to prepare volumetric base monomer to curing agent in 60:1 and 30:1 ratios, respectively. The PDMS and curing agent mixtures were then vacuum degassed. Nine hundred sixty milliliters of the PDMS mixtures were dispensed into 35-mm (P35) tissue culture polystyrene dishes or each well of a six-well culture plate, respectively, to produce 1-mm-thick PDMS matrix. PDMS polymerization was achieved by incubating the PDMS mixture in culture dishes at 65–70°C for 18 to 19 h.
Chemical surface treatment and extracellular matrix conjugation.
To generate a hydrophilic surface on PDMS matrixes for cell attachment, we employed a published methodology (1, 7) that involves alternate layer-by-layer coating with 0.2% polyethylenimine (PEI) and 0.3% polystyrene sulfate (PSS) layers to create a hydrophilic surface on the PDMS matrix. PEI (0.2%) and PSS (0.3%) were prepared in 10% phosphate-buffered saline (PBS) at pH 7.4. All reagents were purchased from Sigma-Aldrich. The PEI layer was always applied first, followed by incubation for 30 min at room temperature and rinsing with 10% PBS before application of the PSS layer. These steps were repeated to produce a total of three bilayers [(PSS/PEI)3] for the 60:1 PDMS substrates representing soft matrix and four bilayers [(PSS/PEI)4] for the 30:1 PDMS substrates representing stiff matrix.
Laminin coating and binding assay.
To further ensure homogeneous CSP cell attachment to these PDMS matrixes, 20 μg of mouse laminin (Sigma-Aldrich) was loaded onto each P35 plate or each well of a six-well plate coated with PDMS matrix. Laminin-coated plates were than incubated for 16–20 h at standard cell culture conditions (37°C, 5% CO2) before cell seeding or surface stiffness measurement. Homogenous and equal laminin binding was verified using immunofluorescent tagging of the substrate surface (data not shown).
Characterization of substrate elastic moduli.
For determination of substrate stiffness, surface-treated and laminin-coated PDMS samples were subjected to microscale mechanical indentation (21, 30) using a TI 900 TriboIndenter (Hysitron). A 50 μm-diameter cylindrical punch tip was preloaded to 1 μN and displaced 1,000 nm into the surface of 1-mm-thick PDMS samples at a rate of 20 nm/s (i.e., a quasistatic indentation). A piezoelectric sensor measured force generation, and a portion of the loading region of the force-displacement curve (100–400 nm) was used to calculate a linear regression. The calibration of the nanoindenter was performed before each testing session to ensure the robust characterization of substrate properties using TriboScan software package (Hysitron). This procedure was implemented to reduce system drift and to keep the piezo scanner in range for the duration of the test. The sample surface was first imaged. Upon contact of the probe, scanning was initiated with the set point reduced to 1 μN and scan size set to 0 μm. Before measurements, the piezo scanner was allowed to stabilize for 10 min, and the linear range was confirmed. Small offsets in the in X or Y directions were then performed. To account for the possibility that localized PDMS stiffness may vary because of nonuniformity of base/curing agent mixing, PDMS stiffness was measured in 3–5 random regions. It is noteworthy that there are a number of parameters that may impact the stiffness reading, including but not limited to, inhomogeneous polymerization, nanoindenter system drift, and thermal noises.
The elastic modulus (E) was determined using Eq. 1.
| (1) |
where S is the slope of the linear regression, D is the punch tip diameter (50 μm), and ν is the Poisson's ratio for PDMS (0.5), which was assumed to be a perfectly incompressible material.
CSP cell isolation and culture.
CSP cells from sheep and mice were isolated and cultured using our previously reported protocol (38). Briefly, heart tissue from adult male 10–12-mo-old sheep (Parson's Farm) and 8-wk-old male C57BL/6 mice (strain no. 027; Charles River Laboratories) were excised, and the left ventricle was separated from the whole heart by manual dissection and digested. Residual red cells were removed, and the mononuclear cell suspension was stained with Hoechst 33342 dye and 7-aminoactinomycin D (7-AAD). With the use of fluorescence-activated cell sorting (FACS), CSP cells were distinguished from the main population by the ability to efflux the Hoechst dye, as we have previously reported (32, 41). FACS-sorted 7-AAD-negative CSP cells were cultured in medium (growth media) consisting of 20 vol/vol% fetal bovine serum (HyClone), 2.5 mM l-glutamine (Sigma-Aldrich), and 1.0 vol/vol% penicillin-streptomycin (Life Technologies) in α-MEM (Lonza). Cells in passages 4–6 were used for experimentation. All animal studies strictly adhered to the guidelines of the Harvard Medical School Institutional Animal Care and Use Committee, National Society for Medical Research, National Research Council, National Institutes of Health, and Institute of Laboratory Animal Resources and the protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Harvard Medical School (protocol no. 04745).
Cell attachment and proliferation measurements.
CSP cells were seeded on each substrate condition at a density of 10 cells/mm2 in the growth medium described above. Eight hours following initial seeding, adherent cells were lifted using 0.05% trypsin-EDTA solution. and cell number was determined by hemocytometer. Total initial cell number before seeding was also determined by the same counting method. The percent cell seeding was determined by the ratio of adherent to total initial cell numbers. Proliferation capacity was defined by the calculated doubling time following 6 days in culture using methods similar to ones previously reported (42). The doubling time was calculated using Eq. 2.
| (2) |
where T is the incubation time in any units; Xb and Xe are the cell numbers at the beginning and the end of the incubation time, respectively (2).
Terminal deoxynucleotidyl transferase dUTP nick end labeling assay for cell death measurement.
CSP cell death was determined using terminal deoxynucleotidyl transferase dUTP nick end labeling. Briefly, CSP cells were fixed after 3 days in culture and stained with In-situ Cell Death Detection Kit-TMR Red (Roche Applied Science). Fluorescent images of dead cells were captured using an LSM 700 Flexible Confocal Microscope (Carl Zeiss). Data were then analyzed using ImageJ (National Institute of Health). Investigators were blinded to groups while performing the experiments.
Measurements of CSP cell proliferation capacity.
CSP cell proliferation capacity was determined using a complementary set of methodologies including bromodeoxyuridine (BrdU) cell cycle flow cytometry and analysis of fluorescence ubiquitination cell cycle indicator (FUCCI). To characterize the proportion of cells actively replicating DNA, CSP cells were cultured for 4 days in growth media as described above. BrdU (10 μM) was pulsed for 30 min and CSP cells subsequently lifted, fixed, and stained using BD Pharmingen BrdU Flow Kit (BD Biosciences) according to the manufacturer's instructions. Analysis of cell cycle profile was performed using Accuri C6 Flow Cytometer (BD Biosciences), and the proportions of cells in the G0/G1, S, and G2/M phases were determined through software algorithms based on the BrdU and 7-AAD staining intensity.
Cell cycling profiles were also characterized in CSP cells expressing FUCCI (40). FUCCI was delivered to the CSP cells via lentivirual-mediated gene transfer methodologies as we have previously described (41), and FUCCI-positive CSP cells were sorted with a FACSAria II sorter (BD Biosciences). The FUCCI+ CSP cells were then seeded onto soft and stiff substrates and cultured for 4 days. Flow cytometry with a DxP12 Flow Analyzer (Cytek) was performed to obtain cell cycling profiles for CSP cells, and data were exported to FlowJo software (Tree Star) for analysis.
Analysis of mode of cell division.
The mode of cell division, i.e., symmetrical versus asymmetrical division, was determined following 4-day culture on respective PDMS substrates. Ovine CSP cells were lifted using 0.05% trypsin solution and fixed with 4% paraformaldehyde for 30 min. The cells were then permeabilized with BD Perm/Wash solution (BD Biosciences) according to the manufacturer's instructions. Blocking was performed on the cells with 1% BSA, followed by incubation with 1:200 dilution anti-numb (Cat No. ab14140; Abcam) and 1:500 dilution of anti-phospho-histone H3 (phospho S10, Cat No. ab32107; Abcam) antibodies, followed by secondary Alexa 488 and Alexa 555 antibodies (Molecular Probes), respectively. The cells were analyzed with the Accuri C6 Flow Cytometer.
Analysis of cellular ageing.
Ovine CSP cells were lifted from the substrate following a 3-day culture period. After fixation with 4% paraformaldehyde, rinsing in 0.5% wt/vol BSA solution in PBS and permeabilization with ethanol, the cells were sprayed onto glass slides with a Shandon Cytospin III (Thermo Scientific) at 700 rpm. Two commercially available cell lines, K562 human leukemia cells (Sigma-Aldrich) and 1301 human T-cell leukemia cells (Sigma-Aldrich), were identically prepared for calibration of telomere length. Cell-sprayed slides were then stained with Dako Telomere PNA FISH Kit/Cy3 (Dako Denmark A/S). Z-stack images (stack of 25 images with interval of 0.39 μm) of peptide nucleic acid-hybridized interphase nuclei were taken with an LSM700 Confocal Microscope (Carl Zeiss). The image stacks were then analyzed with FIJI open-source image processing software to determine the fluorescence intensity as a marker of telomere length.
Cardiomyogenic differentiation capacity of CSP cells.
The effect of matrix stiffness on cardiomyogenic potential of CSP cells was determined using our well-established coculture method (38, 42). Neonatal rat ventricular myocytes (NRVMs) were isolated from neonatal Wistar rats (strain no. 003; Charles River Laboratories) as previously described (13). Forty-eight hours after the initial seeding of NRVMs, green fluorescent protein (GFP)-expressing CSP cells were seeded onto the NRVM culture to reach a total density of ∼500 cells/mm2. Six days following coculture, cells were fixed in 4% paraformaldehyde for 23 min and were permeabilized with methanol at −20°C for 23 min. Cells were stained with antibodies for sarcomeric α-actinin following 1% bovine serum albumin blocking procedure. Nuclei in all samples were identified via 4′,6-diamidino-2-phenylindole counterstain.
Fluorescent images were acquired using an LSM 700 confocal microscope (Carl Zeiss). As previously described (38, 42), differentiation of CSP cells was determined by assessing the ratio between total GFP-positive CSP cells and GFP-positive cells expressing cardiac-specific α-actinin by immunocytochemical staining and confocal microscope. All investigators were blinded to groups during the data analysis.
ECM and adhesion protein RT-PCR gene array.
mRNA extracted from murine CSP cells cultured on both the soft and stiff substrates was used to synthesize cDNA using RT2-First Strand Kits (SABiosciences). Extracellular Matrix and Adhesion Molecules PCR array (Cat No. PAMM-013Z, SABiosciences) and CFX96 Real-Time System (Bio-Rad) were used to establish the ECM and adhesion protein expression profile according to the manufacturer's guidelines. β-Actin was used as the internal control.
Statistical analysis.
Data are presented as means ± SE. Significance was determined by a paired or unpaired Student's t-test with GraphPad Prism software. P value < 0.05 was considered significant.
RESULTS
Generation of substrates mimicking normal and fibrotic myocardium.
To examine the effects of ECM stiffness on CSP cell fate and function, PDMS substrates representing normal and fibrotic myocardium were generated with 60:1 and 30:1 PDMS, curing agent ratios, respectively. Using nanoindentation, we found that the elastic moduli of soft (60:1) and stiff (30:1) PDMS were 17.5 ± 4.2 and 145.3 ± 18.0 kPa, respectively (Fig. 1A). The contact angle tests performed before and after the layer-by-layer treatment and laminin coating showed that hydrophilicity was similar between surfaces of soft and stiff substrates (Fig. 1B). Ovine CSP cell attachment at 8 h was >90% of seeding quantity and independent of substrate stiffness (Fig. 1C).
Fig. 1.

Characterization of soft and stiff polydimethylsiloxane (PDMS) substrates. A: mechanical characterization of PDMS stiffness substrate using a nanoindenter. B: contact angle measurement before and after the layer-by-layer (LBL) surface hydrophilization treatment. C: ovine cardiac side population (CSP) cell attachment onto surface-coated PDMS substrate. *P < 0.05; #P < 0.05 vs. before treatment.
Elevated substrate stiffness promotes CSP proliferation.
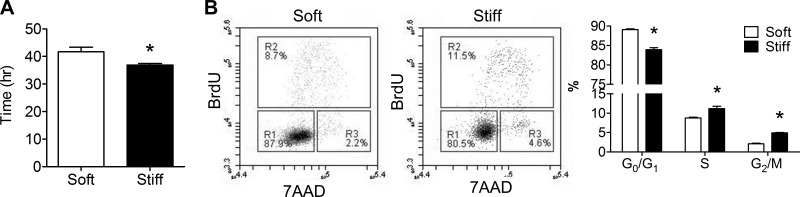
Six days following culture, ovine CSP cells proliferated with a doubling time of 29.4 ± 0.5 and 23.3 ± 0.2 h (P < 0.05) (Fig. 2A) on soft and stiff substrate conditions, respectively. Baseline levels of CSP cell death were equally low on both PDMS substrates (Fig. 2B), suggesting that the cell number difference can be predominantly attributed to the differential proliferation capacity of CSP. Like other proliferating, non-neoplastic cells (24), CSP cells slow or stop cell-cycle progression once they perceive restrictive boundary conditions. We did not observe overconfluency in our proliferation assays or find evidence that differential boundary conditions may have influenced proliferation results. Consistent with enhanced proliferation, CSP cells cultured on stiff substrate conditions were proportionally less present in G0/G1 phases (75 vs. 81%, P < 0.05) by a BrdU/7-AAD assay and more present in S and G2/M phases (15 vs. 10%, P < 0.05), as shown in the representative flow cytometric profiles (Fig. 2C). An alternative characterization of cell cycling by FUCCI (40) revealed a similar effect of ECM stiffness on ovine CSP cell cycling in favor of increasing proliferation rate on stiffer substrates as demonstrated by less CSP cells in G0/G1 phase and more in G1/S phase when cultured on the stiff substrate condition (Fig. 2D). Similar to that in the observed ovine CSP cells, the shorter doubling time of cells on stiff substrate was also observed in murine CSP cells (Fig. 3A), with consistent BrdU/7-AAD results (Fig. 3B). Collectively, these results suggest that ECM stiff alters CSP proliferation independent of species.
Fig. 2.
Ovine CSP cell proliferation and analysis of cell cycle. A: doubling time of ovine CSP cells on soft and stiff PDMS substrates. B: terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining of ovine CSP cell culture on both PDMS substrates. C: flow cytometric analysis of bromodeoxyuridine (BrdU)-7-aminoactinomycin D (7-AAD) stained ovine CSP cells. D: flow cytometric analysis of fluorescence ubiquitination cell cycle indicator expressing ovine CSP cells. Cdt1, Cdc10-dependent transcript 1. *P < 0.05.
Fig. 3.
Murine CSP cell proliferation and analysis of cell cycle. A: doubling time of murine CSP cells on soft and stiff PDMS substrates. B: flow cytometric analysis of BrdU–7-AAD stained murine CSP cells. *P < 0.05.
Stiffer substrate accelerates cellular ageing of CSP cells.
Telomere length is one of the most commonly used indicators of cellular ageing (8). Given that cell replication was accelerated by substrate stiffness, it stood to reason that a faster cell cycling rate may lead to telomere length shortening. Accordingly, the telomere lengths of ovine CSP cells cultured on the soft and stiff substrates for 3 days were quantified using methods described above. The fluorescence intensity values of K562 and 1301 leukemia cells with known telomere lengths (9) were recorded (Fig. 4A) to obtain a calibration curve (Fig. 4A). CSP cells in interphase, which presented three-dimensional spatial telomere distribution within the nuclei (Fig. 4B), were used to determine the telomere length. Telomere lengths of ovine CSP cells on the stiff substrate were ∼200 base pair shorter than those on the soft substrate with statistically significant difference (P < 0.05, Fig. 4B), suggesting the stiffer substrate stimulates cellular ageing.
Fig. 4.
Quantitative fluorescence in situ hybridization tests of CSP cell telomere length. A: Z-stack images and Z-projection images for K562 and 1301 cell telomeres (top); calibration curve of absolute telomere length using leukemia cell lines with known telomere lengths (bottom). kbp, kilo-base pair. B: Z-stack images and Z-projection images for CSP cell telomeres (top); comparison of telomere lengths of CSP cells on both PDMS substrates (bottom). *P < 0.05.
CSP cells favor asymmetric division in a soft environment.
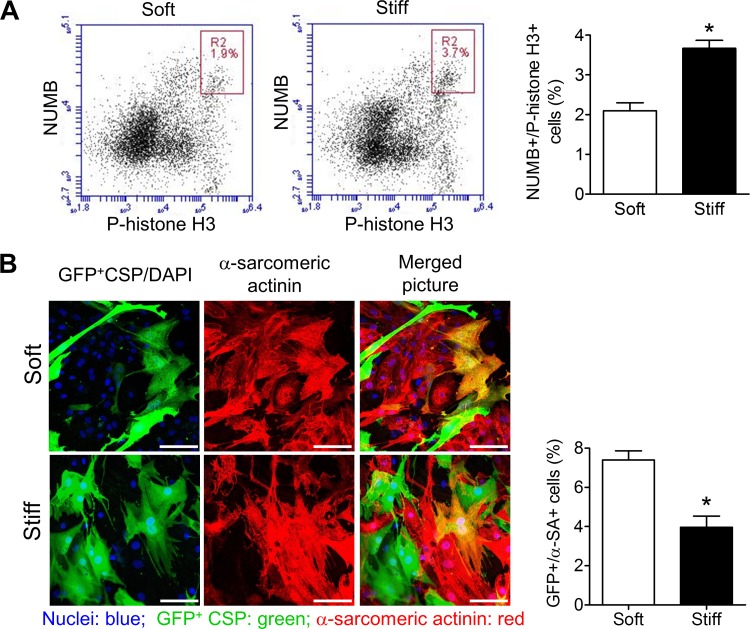
Asymmetric division is essential for stem cell fate determination, as it produces daughter cells for both self-renewal and differentiation (31). Numb was used in this study to label the cells undergoing mitosis, where unequal segregation of numb indicated asymmetric cell division (15, 41). Flow cytometric results showed there was a larger numb-positive population in CSP cells on the stiff substrate than on the soft substrate (P < 0.05) (Fig. 5A), consistent with equal numb segregation. This suggests that increased substrate stiffness promoted symmetric division.
Fig. 5.
Asymmetric cell division and cardiomyogenic differentiation of CSP cells. A: flow cytometric analysis of numb and P-histone H3 stained ovine CSP cells and comparison of numb-positive P-histone H3-positive cell percentage. B: confocal microscopic images of ovine CSP cells in coculture with neonatal rat ventricular myocytes and comparison of CSP cardiomyogenic differentiation after a 6-day coculture with neonatal rat ventricular myocytes. GFP, green fluorescent protein; DAPI, 4′,6-diamidino-2-phenylindole; α-SA, α-sarcomeric actinin. *P < 0.05.
Stiff substrates hinder the cardiomyogenic differentiation potential of CSP cells.
To study the cardiomyogenic differentiation of CSP cells, we used an established coculture system, coculturing GFP-positive CSP cells with NRVMs (38, 42). Cardiomyogensis was assessed by the expression of cardiomyocyte-specific sarcomeric α-actinin (Fig. 5B). Immunocytochemistry revealed an increase in cardiomyogenic differentiation with decreasing stiffness, consistent with prior reports (11, 22, 43). Mean CSP cell differentiation was 6.9 ± 0.5% on soft substrate versus 3.7 ± 0.4% on stiff substrate, representing a 46% decrease on the stiff substrate (P < 0.05) (Fig. 5B).
Microenvironment stiffness alters ECM gene expression.
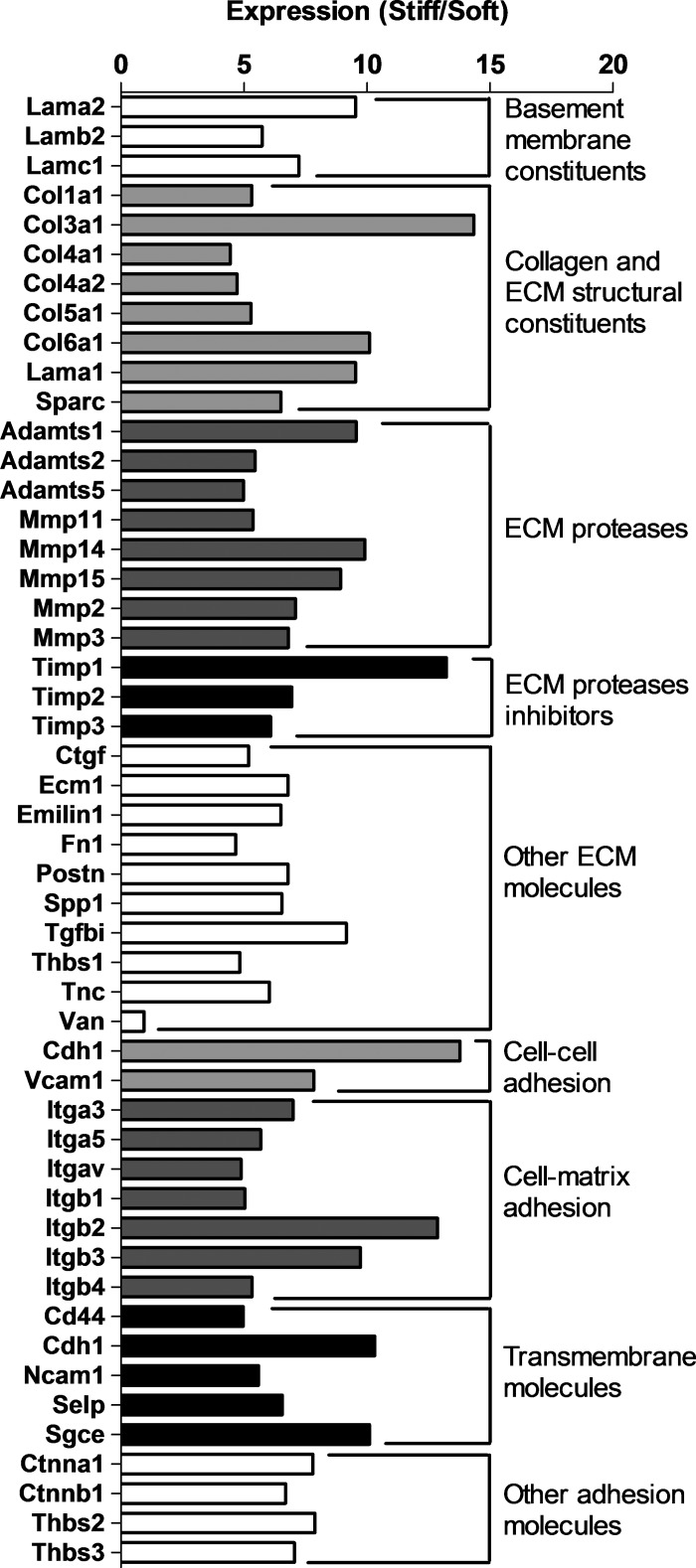
After a 3-day culture, mouse CSP cells on the two stiffness substrates were harvested and mRNA was extracted for quantitative PCR of gene expression of ECM and adhesion proteins. The expression of 50 out of 84 genes tested in the preset array was reliably measured. Forty-nine genes exhibited fourfold or higher expression in the CSP cells cultured on stiff substrate relative to soft substrate (n = 3) (Fig. 6).
Fig. 6.
Up- and downregulation of ECM and adhesion protein genes in murine CSP cells by quantitative PCR. Adamts, a disintegrin-like and metallopeptidase (reprolysin type) with thrombospondin type 1; Cd, cluster of differentiation; Cdh, cadherin; Col, collagen; Ctgf, connective tissue growth factor; Ctnn, catenin (cadherin-associated protein); Ecm1, extracellular matrix protein 1; Emilin, elastin microfibril interfacer; Fn, fibronectin; Itg, integrin; Lam, laminin; Mmpl matrix metallopeptidase; Ncam, neural cell adhesion molecule; Postn, periostin, osteoblast-specific factor; Sele, selectin, endothelial cell; Selp, selectin, platelet; Sgce, sarcoglycan, epsilon; Sparc, secreted acidic cysteine-rich glycoprotein; Spp, secreted phosphoprotein; Tgfbi, transforming growth factor, β-induced; Thbs, thrombospondin; Timp, tissue inhibitor of metalloproteinase; Tnc, tenascin; Vcam, vascular cell adhesion molecule; Vcan, versican.
DISCUSSION
In the post-MI heart, the activation of endogenous cardiac stem/progenitor cells has been observed with increased mitotic activity, proliferation, and ultimately cell number (26, 38, 46). Activation alone, however, does not imply augmentation of stem cell-mediated regeneration of lost cardiomyocytes. An increasing number of reports suggest that changes in mechanical properties within the local cardiac microenvironment may alter stem/progenitor cell behavior. In this study, we describe the influence of stiffness on CSP cell fate and function.
We used PDMS to mimic the mechanical properties of normal myocardium and scar tissue by tuning the mixing ratio of PDMS base to curing agent. Because of biological heterogeneity and differences in experimental settings, the reported elastic moduli of myocardium range from 18 to 60 kPa in normal myocardium and 55 to 295 kPa in fibrotic myocardium (6, 18).
The elastic moduli of our experimental substrates determined by microscale, mechanical indentation were within this range. For reference, polystyrene, which is used to make culture dishes, is reported by the manufacturer (BD Biosciences) to have an elastic modulus range of 3,200-3,241 MPa. Importantly, cell adhesion and initial growth conditions were unaltered on the PDMS substrate, providing an opportunity to evaluate the effects of microenvironment stiffness on CSP cells.
Cell proliferation capacity has been previously found to increase in response to microenvironment stiffening for other non-cardiac stem and progenitor cell populations (12, 39). Thus we postulated that a stiffer substrate, mimicking the scar tissue generated following cardiac injury, would also promote proliferation in CSP cells. Our observation of an increase in CSP cell numbers when cultured on the stiffer substrate is consistent with our previous in vivo finding (32) as well as other studies (26, 46) in which resident cardiac stem/progenitor cell numbers in response to scar formation exit quiescence and become proliferative. When we examined the proliferation processes in detail, cell-cycle profiles suggested that cells on the stiff substrate are more likely to proceed to the S phase and increase DNA synthesis. Provided that the difference between cell-doubling times of ovine CSP cells grown on soft and stiff substrates is 6.1 h over a 3-day culture period, cells on the stiff substrate would complete an additional 0.64 cell cycles versus those on the normal substrate. Although we cannot rigorously predict the telomere shortening for ovine CSP cells using a linear model, we estimate shortening of 128 to 256 base pair and observed results within these bounds. With the accelerated proliferation of CSP cells on the stiff substrate, telomere shortening is thus also accelerated, decreasing the time to stem/progenitor cell exhaustion. Similarly, prior reports have suggested increased exhaustion of endothelial progenitor cells in patients with post-MI heart failure (23), which may be explained by critically short telomeres (34). Given the effects of altered substrate stiffness on CSP proliferation, scar formation may therefore also contribute to cardiac stem/progenitor cell exhaustion post-MI.
Our results are in line with reports suggesting that matrix stiffness regulates behavior of stem/progenitors cells (14, 16, 29, 45, 49, 50). However, we fully recognize the intrinsic limitations of extrapolating the data obtained from an in vitro static environment to the in vivo state in which the heart is actively contracting, particularly as the heart is highly organized with anisotropic structure. In addition, its mechanical properties are likely to be different in various physical regions. Nonetheless, our findings have implications for cardiac regeneration in that endogenous stem/progenitor cells and those delivered to the diseased myocardium may be subject to microenvironment changes that favor proliferation over differentiation. Increases in microenvironment stiffness also led to greater ECM and adhesion protein expression in CSP cells. Of the 50 ECM genes examined in mouse CSP cells, 49 of those genes showed fourfold higher expression with culture on stiff substrate versus soft substrate. ECM synthesis may translate to increased fibrosis and tissue stiffness, potentially suggesting a positive feedback mechanism in mechanotransducing pathways in CSP cells.
In conclusion, stiffening of the microenvironment of cardiac stem cells leads to greater cell proliferation than differentiation, which impedes cardiac stem/progenitor cell regeneration of lost myocardium. To address this potential challenge, it may be possible to target the cellular pathways responsible for transducing mechanical signals into cell fate decisions in situ through genetic or pharmacologic therapy. Alternatively, stem/progenitor cells may be implanted in a vehicle or matrix engineered specifically to promote cardiomyogenic differentiation despite the stiffened myocardium. Eventually, post-MI cardiac regeneration may be realized through investigation of cell-matrix interactions and application of this knowledge to therapy design.
GRANTS
This work was support in part by the National Institutes of Health Grant HL-099073 (to R. Liao) M. Bauer was a recipient of the American Heart Association Founder Affiliate Postdoctoral Fellowship. M. V. Gomez was a summer student at the Research Science Institute. A. F. Bayomy was a recipient of the Sarnoff Cardiovascular Research Fellowship for medical students. P. Du and X. Zhang would like to acknowledge support from National Science Foundation Grant CMMI-0826191.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Y.Q., A.F.B., and R.L. conception and design of research; Y.Q., A.F.B., M.V.G., M.B., P.D., Y.Y., and X.Z. performed experiments; Y.Q., A.F.B., M.V.G., M.B., P.D., Y.Y., and X.Z. analyzed data; Y.Q., A.F.B., and R.L. interpreted results of experiments; Y.Q. prepared figures; Y.Q. and A.F.B. drafted manuscript; M.V.G., M.B., A.F.B., and R.L. edited and revised manuscript; R.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank G. Losyev at Brigham and Women's Hospital Flow Cytometry Core for assisting in cell sorting and analysis. Sheep cardiac tissues were kindly provided by F. Y. Chen in the Department of Surgery at Brigham and Women's Hospital.
REFERENCES
- 1.Ai H, Lvov YM, Mills DK, Jennings M, Alexander JS, Jones SA. Coating and selective deposition of nanofilm on silicone rubber for cell adhesion and growth. Cell Biochem Biophys 38: 103–114, 2003. [DOI] [PubMed] [Google Scholar]
- 2.American Type Culture Collection. ATCC Animal Cell Culture Guide: Tips and Techniques for Continuous Cell Lines. Manassas, VA: ATCC, 2014. http://www.atcc.org/∼/media/PDFs/Culture%20Guides/AnimCellCulture_Guide.ashx. [Google Scholar]
- 3.Banks JM, Mozdzen LC, Harley BAC, Bailey RC. The combined effects of matrix stiffness and growth factor immobilization on the bioactivity and differentiation capabilities of adipose-derived stem cells. Biomaterials 35: 8951–8959, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114: 763–776, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science 324: 98–102, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berry MF, Engler AJ, Woo YJ, Pirolli TJ, Bish LT, Jayasankar V, Morine KJ, Gardner TJ, Discher DE, Sweeney HL. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circ Physiol 290: H2196–H2203, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Brown XQ, Ookawa K, Wong JY. Evaluation of polydimethylsiloxane scaffolds with physiologically-relevant elastic moduli: interplay of substrate mechanics and surface chemistry effects on vascular smooth muscle cell response. Biomaterials 26: 3123–3129, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Campisi J, Kim Sh Lim CS, Rubio M. Cellular senescence, cancer and aging: the telomere connection. Exp Gerontol 36: 1619–1637, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Derradji H, Bekaert S, van Oostveldt P, Baatout S. Comparison of different protocols for telomere length estimation by combination of quantitative fluorescence in situ hybridization (Q-FISH) and flow cytometry in human cancer cell lines. Anticancer Res 25: 1039–1050, 2005. [PubMed] [Google Scholar]
- 10.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science 324: 1673–1677, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engler AJ, Carag-Krieger C, Johnson CP, Raab M, Tang HY, Speicher DW, Sanger JW, Sanger JM, Discher DE. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J Cell Sci 121: 3794–3802, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans ND, Minelli C, Gentleman E, LaPointe V, Patankar SN, Kallivretaki M, Chen X, Roberts CJ, Stevens MM. Substrate stiffness affects early differentiation events in embryonic stem cells. Eur Cell Mater 18: 1–13, 2009. [DOI] [PubMed] [Google Scholar]
- 13.Fisch S, Gray S, Heymans S, Haldar SM, Wang B, Pfister O, Cui L, Kumar A, Lin Z, Sen-Banerjee S, Das H, Petersen CA, Mende U, Burleigh BA, Zhu Y, Pinto Y, Liao R, Jain MK. Kruppel-like factor 15 is a regulator of cardiomyocyte hypertrophy. Proc Natl Acad Sci USA 104: 7074–7079, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gershlak JR, Resnikoff JI, Sullivan KE, Williams C, Wang RM, Black LD. Mesenchymal stem cells ability to generate traction stress in response to substrate stiffness is modulated by the changing extracellular matrix composition of the heart during development. Biochem Biophys Res Commun 439: 161–166, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gulino A, Di Marcotullio L, Screpanti I. The multiple functions of Numb. Exp Cell Res 316: 900–906, 2010. [DOI] [PubMed] [Google Scholar]
- 16.Heras-Bautista CO, Katsen-Globa A, Schloerer NE, Dieluweit S, Abd El Aziz OM, Peinkofer G, Attia WA, Khalil M, Brockmeier K, Hescheler J, Pfannkuche K. The influence of physiological matrix conditions on permanent culture of induced pluripotent stem cell-derived cardiomyocytes. Biomaterials 35: 7374–7385, 2014. [DOI] [PubMed] [Google Scholar]
- 17.Hierlihy AM, Seale P, Lobe CG, Rudnicki MA, Megeney LA. The post-natal heart contains a myocardial stem cell population. FEBS Lett 530: 239–243, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Hiesinger W, Brukman MJ, McCormick RC, Fitzpatrick JR 3rd Frederick JR, Yang EC, Muenzer JR, Marotta NA, Berry MF, Atluri P, Woo YJ. Myocardial tissue elastic properties determined by atomic force microscopy after stromal cell-derived factor 1alpha angiogenic therapy for acute myocardial infarction in a murine model. J Thorac Cardiovasc Surg 143: 962–966, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD, Robbins J, Lee RT. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med 13: 970–974, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang S, Ingber DE. The structural and mechanical complexity of cell-growth control. Nat Cell Biol 1: E131–E138, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Jacot JG, Dianis S, Schnall J, Wong JY. A simple microindentation technique for mapping the microscale compliance of soft hydrated materials and tissues. J Biomed Mater Res A 79: 485–494, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Jacot JG, McCulloch AD, Omens JH. Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes. Biophys J 95: 3479–3487, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kissel CK, Lehmann R, Assmus B, Aicher A, Honold J, Fischer-Rasokat U, Heeschen C, Spyridopoulos I, Dimmeler S, Zeiher AM. Selective functional exhaustion of hematopoietic progenitor cells in the bone marrow of patients with postinfarction heart failure. J Am Coll Cardiol 49: 2341–2349, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Klug WS, Cummings MR, Spencer CA, Palladino MA (editors). Concepts of Genetics. San Francisco: Pearson, 2011. [Google Scholar]
- 25.Kothapalli CR, Kamm RD. 3D matrix microenvironment for targeted differentiation of embryonic stem cells into neural and glial lineages. Biomaterials 34: 5995–6007, 2013. [DOI] [PubMed] [Google Scholar]
- 26.Kubo H, Jaleel N, Kumarapeli A, Berretta RM, Bratinov G, Shan X, Wang H, Houser SR, Margulies KB. Increased cardiac myocyte progenitors in failing human hearts. Circulation 118: 649–657, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee IC, Wu YC, Cheng EM, Yang WT. Biomimetic niche for neural stem cell differentiation using poly-L-lysine/hyaluronic acid multilayer films. J Biomater Appl 2014. December 12 pii: 0885328214563341. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 28.Leri A, Kajstura J, Anversa P. Cardiac stem cells and mechanisms of myocardial regeneration. Physiol Rev 85: 1373–1416, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Li Z, Guo X, Palmer AF, Das H, Guan J. High-efficiency matrix modulus-induced cardiac differentiation of human mesenchymal stem cells inside a thermosensitive hydrogel. Acta Biomater 8: 3586–3595, 2012. [DOI] [PubMed] [Google Scholar]
- 30.Lin IK, Ou KS, Liao YM, Liu Y, Chen KS, Zhang X. Viscoelastic characterization and modeling of polymer transducers for biological applications. J Microelectromech Syst 18: 1087–1099, 2009. [Google Scholar]
- 31.Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature 441: 1068–1074, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Mouquet F, Pfister O, Jain M, Oikonomopoulos A, Ngoy S, Summer R, Fine A, Liao R. Restoration of cardiac progenitor cells after myocardial infarction by self-proliferation and selective homing of bone marrow-derived stem cells. Circ Res 97: 1090–1092, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Nii M, Lai JH, Keeney M, Han LH, Behn A, Imanbayev G, Yang F. The effects of interactive mechanical and biochemical niche signaling on osteogenic differentiation of adipose-derived stem cells using combinatorial hydrogels. Acta Biomater 9: 5475–5483, 2013. [DOI] [PubMed] [Google Scholar]
- 34.Oeseburg H, Westenbrink BD, de Boer RA, van Gilst WH, van Veldhuisen DJ, van der Harst P. Can critically short telomeres cause functional exhaustion of progenitor cells in postinfarction heart failure? J Am Coll Cardiol 50: 1911–1912, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, Entman ML, Schneider MD. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci USA 100: 12313–12318, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park JS, Chu JS, Tsou AD, Diop R, Tang Z, Wang A, Li S. The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-β. Biomaterials 32: 3921–3930, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pek YS, Wan ACA, Ying JY. The effect of matrix stiffness on mesenchymal stem cell differentiation in a 3D thixotropic gel. Biomaterials 31: 385–391, 2010. [DOI] [PubMed] [Google Scholar]
- 38.Pfister O, Mouquet F, Jain M, Summer R, Helmes M, Fine A, Colucci WS, Liao R. CD31- but Not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res 97: 52–61, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Rowlands AS, George PA, Cooper-White JJ. Directing osteogenic and myogenic differentiation of MSCs: interplay of stiffness and adhesive ligand presentation. Am J Physiol Cell Physiol 295: C1037–C1044, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Sakaue-Sawano A, Kurokawa H, Morimura T, Hanyu A, Hama H, Osawa H, Kashiwagi S, Fukami K, Miyata T, Miyoshi H, Imamura T, Ogawa M, Masai H, Miyawaki A. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell 132: 487–498, 2008. [DOI] [PubMed] [Google Scholar]
- 41.Sereti KI, Oikonomopoulos A, Unno K, Cao X, Qiu Y, Liao R. ATP-binding cassette G-subfamily transporter 2 regulates cell cycle progression and asymmetric division in mouse cardiac side population progenitor cells. Circ Res 112: 27–34, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sereti KI, Oikonomopoulos A, Unno K, Liao R. Methods to study the proliferation and differentiation of cardiac side population (CSP) cells. Methods Mol Biol 1036: 95–106, 2013. [DOI] [PubMed] [Google Scholar]
- 43.Shapira-Schweitzer K, Seliktar D. Matrix stiffness affects spontaneous contraction of cardiomyocytes cultured within a PEGylated fibrinogen biomaterial. Acta Biomater 3: 33–41, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Shih YR, Tseng KF, Lai HY, Lin CH, Lee OK. Matrix stiffness regulation of integrin-mediated mechanotransduction during osteogenic differentiation of human mesenchymal stem cells. J Bone Miner Res 26: 730–738, 2011. [DOI] [PubMed] [Google Scholar]
- 45.Sullivan KE, Quinn KP, Tang KM, Georgakoudi I, Black LD. Extracellular matrix remodeling following myocardial infarction influences the therapeutic potential of mesenchymal stem cells. Stem Cell Res Ther 5: 14–14, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Urbanek K, Torella D, Sheikh F, De Angelis A, Nurzynska D, Silvestri F, Beltrami CA, Bussani R, Beltrami AP, Quaini F, Bolli R, Leri A, Kajstura J, Anversa P. Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proc Natl Acad Sci USA 102: 8692–8697, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang LS, Boulaire J, Chan PPY, Chung JE, Kurisawa M. The role of stiffness of gelatin-hydroxyphenylpropionic acid hydrogels formed by enzyme-mediated crosslinking on the differentiation of human mesenchymal stem cell. Biomaterials 31: 8608–8616, 2010. [DOI] [PubMed] [Google Scholar]
- 48.Wozniak MA, Chen CS. Mechanotransduction in development: a growing role for contractility. Nat Rev Mol Cell Biol 10: 34–43, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu H, Tay CY, Pal M, Leong WS, Li H, Wen F, Leong DT, Tan LP. A bio-inspired platform to modulate myogenic differentiation of human mesenchymal stem cells through focal adhesion regulation. Adv Healthc Mater 2: 442–449, 2013. [DOI] [PubMed] [Google Scholar]
- 50.Zhang S, Ma X, Yao K, Zhu H, Huang Z, Shen L, Qian J, Zou Y, Sun A, Ge J. Combination of CD34-positive cell subsets with infarcted myocardium-like matrix stiffness: a potential solution to cell-based cardiac repair. J Cell Mol Med 18: 1236–1238, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]