Abstract
Increases in airborne particulate matter (PM) are linked to increased mortality from myocardial ischemia. PM contains environmentally persistent free radicals (EPFRs) that form as halogenated hydrocarbons chemisorb to transition metal oxide-coated particles, and are capable of sustained redox cycling. We hypothesized that exposure to the EPFR DCB230 would increase cardiac vulnerability to subsequent myocardial ischemia-reperfusion (MI/R) injury. Rats were exposed to DCB230 or vehicle via nose-only inhalation (230 μg max/day) over 30 min/day for 7 days. MI/R or sham MI/R (sham) was initiated 24 h after the final exposure. Following 1 or 7 days of reperfusion, left ventricular (LV) function was assessed and infarct size measured. In vehicle-exposed rats, MI/R injury did not significantly reduce cardiac output (CO), stroke volume (SV), stroke work (SW), end-diastolic volume (EDV), or end-systolic volume (ESV) after 1 day of reperfusion, despite significant reductions in end-systolic pressure (ESP). Preload-recruitable SW (PRSW; contractility) was elevated, presumably to maintain LV function. MI/R 1-day rats exposed to DCB230 also had similarly reduced ESP. Compared with vehicle controls, CO, SV, and SW were significantly reduced in DCB230-exposed MI/R 1-day rats; moreover, PRSW did not increase. DCB230’s effects on LV function dissipated within 8 days of exposure. These data show that inhalation of EPFRs can exacerbate the deficits in LV function produced by subsequent MI/R injury. Infarct size was not different between the MI/R groups. We conclude that inhalation of EPFRs can compromise cardiac function during MI/R injury and may help to explain the link between PM and MI/R-related mortality.
Keywords: particulate matter, rat, pressure volume, cardiac toxicity
increased levels of ambient particulate matter (PM) are consistently linked with cardiopulmonary and cardiovascular morbidity and mortality (4, 5, 51, 52, 55). There is a strong correlation between increased ambient fine (PM2.5, diameter <2.5μm) or ultrafine (PM0.1, diameter <0.1μm) particulates and mortality due to acute myocardial ischemia (MI) (9, 10, 13, 45, 49–52). Additionally, increases in ambient PM levels also lead to increased emergency MI admissions and, in MI survivors, increased risk of developing heart failure or suffering a second MI (17, 60, 61).
PM consists of a mixture of suspended solid and liquid particles, the composition of which varies widely depending on emission source. This heterogeneity of PM complicates mechanistic studies, making it difficult to identify the constituents of PM responsible for the observed cardiovascular toxicity (11, 42). While the exact mechanism(s) underlying PM-induced cardiovascular effects are largely unknown, systemic inflammation and oxidative stress secondary to pulmonary inflammation and/or the translocation of PM from the lung into the circulation are believed to play important roles (5, 44).
Our colleagues, as well as others, have demonstrated that ambient PM2.5 contains environmentally persistent free radicals (EPFRs) (14, 40). EPFRs are a unique particle-pollutant system that forms during combustion processes, wherein halogenated hydrocarbons chemisorb to the surfaces of transition metal-coated particles, forming surface-stabilized quinone and semiquinone-type radicals (38). EPFRs are capable of prolonged (days to weeks) redox cycling in air and soils, resulting in the generation of large quantities of reactive oxygen species (ROS) (21, 33, 34). Although studies of the biological actions of EPFRs have only just begun, our group, and others, have demonstrated that these redox active particles produce oxidative stress in vitro (2, 19, 31), as well as oxidative stress and inflammation in the lung, heart, and blood of experimental animals (3, 39, 41, 57). Exposure to EPFRs has also been shown to alter pulmonary and cardiac function in vivo (3, 39, 41). Our group has shown that short-term, inhalational exposure to EPFRs decreases baseline cardiac output (CO), stroke volume (SV), end-diastolic pressure (EDP), and end-diastolic volume (EDV), while increasing pulmonary artery pressure in otherwise healthy rats (39, 41).
Despite the links between PM, oxidative stress, and ischemic heart disease, surprisingly few studies have examined the relationship between exposure to PM and the severity or extent of ischemic cardiac injury. Bagate and colleagues (1) observed decreased left ventricular (LV) developed pressures as well as slower recovery of contractile function following a 35-min period of global ischemia in Langendorff perfused hearts from spontaneously hypertensive rats into whom PM10 had been intratracheally (IT) instilled 4 h prior. Prior IT instillation of PM0.1 (24 h) also produced significantly greater oxidative stress and larger areas of infarction in the myocardium of mice in vivo after a 20 min ischemic period followed by 2 h of reperfusion (12). Additionally, Knuckles and colleagues (35) demonstrated that prior (24 h) IT instillation of PM10 leads to microvascular dysfunction in isolated coronary arterioles of rats. These data indicate that ambient PM, and perhaps the EPFRs contained therein, may exacerbate LV dysfunction resulting from myocardial ischemia/reperfusion (MI/R) injury; however, this has yet to be systematically studied, especially in an inhalational model. Therefore, this study was designed to test the hypothesis that prior inhalation of EPFRs may degrade LV function and/or increase myocardial infarct size either 1 or 7 days post-MI/R injury. An MI/R model of ischemic cardiac injury was chosen, as this model has a well-characterized association with increased inflammation and oxidative stress (16, 18, 54, 59). We predicted that oxidative stress produced by the exposure to EPFRs may have an additive effect on oxidative stress produced by MI/R injury, thereby increasing the severity of cardiac injury. This study provides further insight into the link between PM air pollution and cardiovascular morbidity/mortality and provides additional information regarding the health risks posed by EPFRs within ambient PM.
METHODS
EPFR synthesis.
The EPFR particle used in this study was DCB230 (41). DCB230 is a model pollutant particle system consisting of silica particles (silica gel powder grade 923, 100–200 mesh; Sigma-Aldrich, St. Louis, MO) dosed with 5% CuO by mass and chemisorbed to 1,2-dichlorobenzene at 230°C by established procedures (38). The temperature of the reaction is designed to mimic postcombustion cool-zone temperatures, where excess reactants condense to form EPFRs via surface-mediated reactions (38). DCB230 produces an electron paramagnetic resonance (EPR) spectrum indicative of an oxygen-centered semiquinone-type radical, which is consistent with the EPR spectra of airborne PM2.5 samples (14, 38).
Animals.
Experiments were performed with male Sprague-Dawley rats (297 ± 1.8 g; Harlan Laboratories, Indianapolis, IN). Rats were housed in a temperature- and humidity-controlled environment on a 12-h light/dark cycle. Standard rat chow and autoclaved tap water were available ad libitum. All procedures were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Experimental Animals and were approved by the Institutional Animal Care and Use Committee of Louisiana State University Health Sciences Center.
DCB230 exposure.
Rats were exposed once a day to either aerosolized vehicle (7 ml 0.9% NaCl + 0.02% Tween-80) or DCB230 (20 mg suspended in 7 ml of vehicle) using a flow-past, nose-only inhalational system (InExpose; SciReq, Montreal, Canada). The dose of DCB230 was chosen based on its known ability to significantly reduce baseline CO, SV, and EDV in otherwise healthy rats (41). We did not include silica particles as a control in this study as our previous studies have shown inhalation of the silica particles used does not degrade baseline LV function (41). The 20 mg DCB230 samples were nebulized at a rate of 0.0055 m3/min and combined with 0.002 m3/min of room air. Exposures lasted ∼30 min/day (0.225 m3/day) and consisted of a gravimetrically determined maximum flow-past exposure of 230 μg per rat per session (41). This exposure creates an ambient concentration of ∼1,022 μg/m3 during the 30-min exposure period each day. By using the method detailed by de Winter-Sorkina and Cassee (13a) to standardize the deposited thoracic mass rates of inhaled particulates in rats vs. humans, this daily exposure produces the equivalent deposited mass of particulate per alveolus in our rats as a human receives in the same 30-min period if exposed to PM2.5 levels of 205 μg/m3, an ambient level relevant to many locations (24, 30, 62).
Myocardial infarction.
Twenty-four hours after the final EPFR exposure, rats were anesthetized in a Plexiglas induction chamber (Braintree Scientific, Braintree, MA) fed by a vaporizer supplying 5% isoflurane. Once immobile and unresponsive to tail pinch, rats were removed from the chamber and intubated with a 14-gauge plastic vascular cannula with an ophthalmoscope. The cannula was then connected to an anesthesia work station (Hallowell EMC, Pittsfield, MA), providing ventilation with isoflurane (2.5%) and oxygen for the duration of the surgery. Thoracotomy was performed through an incision in the fourth intercostal space to expose the base of the heart. The pericardial sac was removed with a sterile cotton tip applicator. Occlusion of the left anterior descending (LAD) coronary artery was induced by tying 6-0 prolene suture around the proximal portion of the LAD. A short segment of silicon tubing was inserted under the knot before it was cinched, padding the myocardium and facilitating subsequent suture release. Effectiveness of the occlusion was verified visually through pallor of the myocardium distal to the ligature. After the 45-min ischemic period, the suture was removed to allow reperfusion. Sham MI/R rats (sham) received pericardiotomy without coronary occlusion; however, the rats remained anesthetized and ventilated for the same 45-min period. The intercostal incision was closed with 4-0 silk suture and pleural pressure restored via manual aspiration with a 5-ml syringe attached to silicon tubing (Silastic Laboratory Tubing; Dow Corning, Midland, MI). The surgical incision was closed superficially with 5-0 silk suture, and buprenorphine (0.1 mg/kg, i.p.) was administered every 12 h for up to 6 days.
Assessment of left ventricular function.
Following a reperfusion period of either 1 or 7 days, rats were reanesthetized, intubated, and ventilated as before. A pressure-volume catheter (SPR-838; Millar instruments, Houston, TX) was introduced into the LV as outlined by Pacher et al. (48). Briefly, the right common carotid artery was accessed via an incision in the ventral neck, and the catheter was advanced within this vessel towards the LV. Mean arterial blood pressure (MABP) was recorded from the aortic arch by the pressure sensor on the Millar catheter prior to reaching the LV. Once in the LV, the catheter generates real-time pressure-volume data of cardiac function, allowing direct recording of closed-chest cardiac parameters in vivo, including end-systolic pressure (ESP), end-systolic volume (ESV), EDP, EDV, SV, CO, stroke work (SW), and ejection fraction percent (EF%). Additionally, independent indices of contractile function were assessed via caval occlusion maneuvers that reduce cardiac preload (48). The inferior vena cava was approached through an incision in the anterior abdominal wall and slowly occluded with padded forceps. During the occlusion, the preload-recruitable stroke work (PRSW) and end-diastolic pressure-volume relationship (EDPVR) were estimated from the SW-EDV and EDP-EDV regressions, respectively. Volume calibration and parallel conductance of the catheter was performed as previously described (39). LV function and infarct size were measured 24 h after MI/R to correspond with the acute oxidative burst and inflammation of reperfusion, and after 7 days when the oxidative burst had subsided and the infarct area became more defined.
Infarct size quantification.
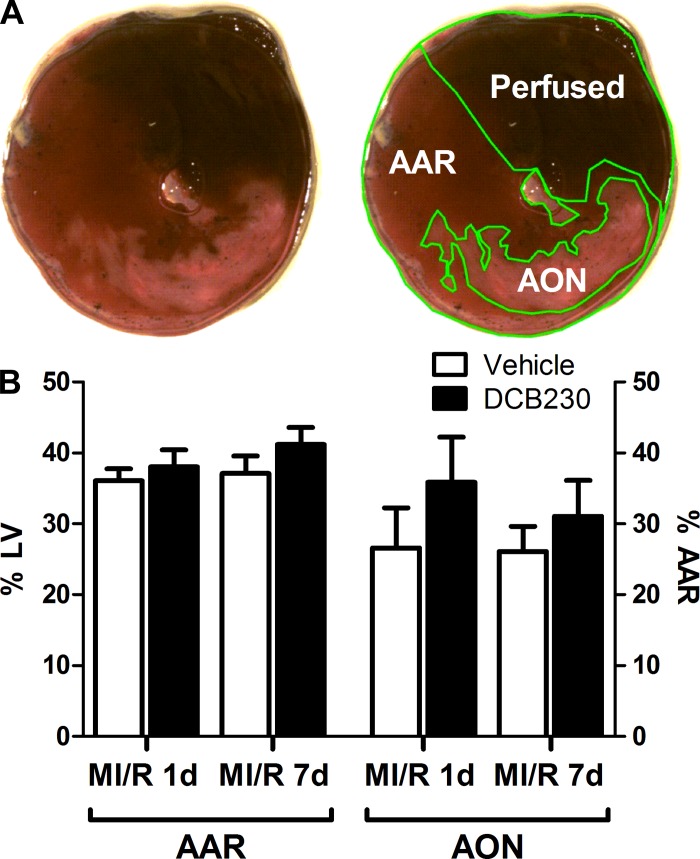
Immediately following cardiac catheterization, the thoracic cavity was breached through the diaphragm (via the previous abdominal incision) to expose the heart. The LAD was religated with 6-0 prolene suture, and 5% phthalocyanine blue dye (Quantum Ink, Louisville, KY) was infused into the systemic circulation via the right jugular vein. Dye in the patent coronary circulation demarcated the area of the LV that remained perfused throughout the ischemic period (area not at risk) from the area at risk (AAR) of ischemic damage. Whole hearts were immediately excised, wrapped in plastic wrap to prevent freeze drying, and stored overnight at −20°C. The following day, hearts were unwrapped and sliced into 2-mm transverse sections. Slices at or below the level of the coronary ligation were incubated for 10 min in 2% triphenyltetrazolium chloride (Sigma-Aldrich) solution to stain viable myocardium a deep red (the AAR), demarcating the area of myocardial infarction in pale white (the area of necrosis, AON) (Fig. 1A). The slices were photographed and analyzed for relative area of infarction with NIH image software (ImageJ). Infarct size was normalized to section weight and calculated as AON expressed as a percentage of the AAR according to established methods (63).
Fig. 1.
Inhalational exposure to DCB230 does not affect infarct size. A: representative cardiac section from a vehicle-exposed myocardial ischemia/reperfusion injury 1-day (MI/R 1d) rat showing how the area at risk (AAR) for MI/R injury and area of necrosis (AON) were determined. B: the AAR was consistent between all groups receiving MI/R injury. Additionally, no significant difference in AON was measured between exposure groups at either MI/R 1d or MI/R 7-day (7d) time periods. In this and all figures, n = 10/group except the MI/R 7d vehicle group where n = 9. LV, left ventricle.
Statistical analysis.
All data are expressed as means ± SE. Parameters of LV function were tested via one-way analysis of variance (ANOVA) with Dunnett's post hoc analysis. Infarct size data were compared by an unpaired Student's t-test. In all cases, statistical significance was assumed at P < 0.05.
RESULTS
In this study, 25 rats exposed to DCB230 and 21 rats exposed to vehicle were subjected to MI/R injury. Five of the DCB230-exposed rats and two of the vehicle-exposed rats died either during the ischemic period or within 1 h of initiating reperfusion.
Following pressure-volume catheterization, the hearts were excised, sectioned, and stained to determine infarct size in each heart. Figure 1A shows typical histological sections of the LV from a vehicle-exposed MI/R animal, illustrating how the AAR and the AON were determined. There was no significant difference in AAR, expressed as a percentage of the LV, between DCB230- and vehicle-exposed rats after either 1 or 7 days of reperfusion. Additionally, no significant difference in AON, expressed as a percentage of the AAR, was observed between the two exposure groups 1 or 7 days post-MI/R injury (Fig. 1B).
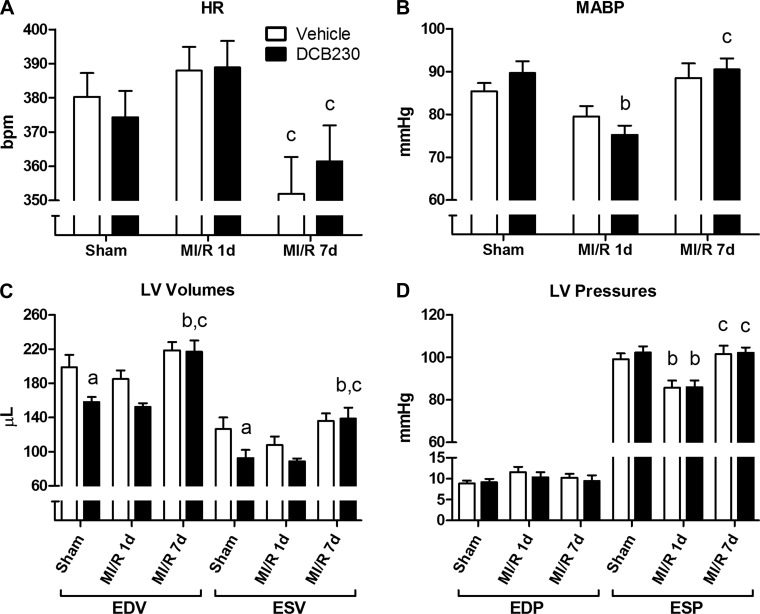
Compared with vehicle, exposure to DCB230 did not significantly affect heart rate (HR) in the sham (n = 10/group) or infarcted rats after 1 day of reperfusion (MI/R 1d; n = 10/group) (Fig. 2A). Additionally, DCB230 exposure did not significantly affect HR in a separate group of infarcted rats allowed 7 days of reperfusion (MI/R 7d; vehicle n = 9; DCB230 n = 10); however, both MI/R 7d exposure groups had significantly lower HR compared with the corresponding MI/R 1d groups (Fig. 2A). In sham rats, exposure to DCB230 did not significantly alter MABP (Fig. 2B). MABP was also not significantly different between sham and MI/R 1d rats exposed to vehicle; however, MABP was significantly lower in the MI/R 1d rats exposed to DCB230 compared with DCB230-exposed sham rats (Fig. 2B). MI/R 7d rats in both exposure groups had MABPs similar to their exposure-matched sham counterparts (Fig. 2B).
Fig. 2.
Effects of inhaled DCB230 and MI/R on heart rate (HR) (A), mean arterial blood pressure (MABP) (B), LV volumes (C), and LV pressures (D). Rats were exposed (20 min/day for 7 days) by nose-only inhalation to either DCB230 (230 μg max/day) or vehicle. Twenty-four hours after the final exposure, rats underwent sham or MI/R surgery followed by 1 or 7 days of reperfusion (MI/R 1d and MI/R 7d, respectively) before assessing ventricular function. A: exposure to DCB230 or MI/R did not markedly affect HR, except for the significant differences in HR between the DCB230- and vehicle-exposed rats in the MI/R 1d and MI/R 7d groups. B: MABP in DCB230-exposed rats at MI/R 1d was significantly lower than in DCB230-exposed sham rats, but was not different than baseline in the MI/R 7d group. C: inhalational exposure to DCB230 reduced baseline cardiac end-diastolic volume (EDV) and end-systolic volume in (ESV) sham rats, although this effect dissipated within 8 days of the final exposure (MI/R 7d). D: neither DCB230 nor MI/R injury significantly affected end-diastolic pressure (EDP); however, MI/R acutely decreased end-systolic pressure (ESP) in both exposure groups at the 1 day time point. Lower case letters indicated the following: a, P < 0.05 vs. vehicle-exposed sham; b, P < 0.05 vs. exposure-matched sham; c, P < 0.05 vs. exposure-matched MI/R 1d.
In sham rats, exposure to DCB230 significantly reduced both EDV and ESV compared with vehicle exposure (Fig. 2C). A similar downward trend in EDV and ESV was seen between vehicle- and DCB230-exposed rats in the MI/R 1d group, although these differences were not statistically significant. In vehicle-exposed rats, neither MI/R 1d nor MI/R 7d had a significant effect on LV volumes (Fig. 2C). In DCB230-exposed MI/R 7d rats, both EDV and ESV were significantly elevated compared with DCB230-exposed sham and MI/R 1d rats and nearly identical to those in the corresponding vehicle-exposed MI/R 7d rats (Fig. 2C). No differences in EDP were observed between any of the experimental groups following either exposure to DCB230 or MI/R injury (Fig. 2D). In both vehicle- and DCB230-exposed rats, a significant decrease in ESP was observed after MI/R 1d; however, ESP returned to control levels in both groups within 7 days following MI/R (Fig. 2D).
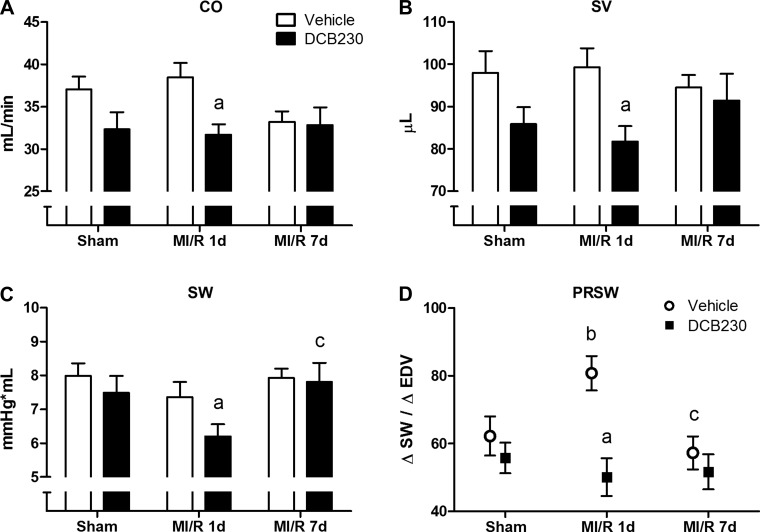
Compared to vehicle, exposure to DCB230 significantly decreased CO, SV, and SW in MI/R 1d rats (Fig. 3, A–C). Exposure to DCB230 also tended to decrease CO, SV, and SW in sham rats; however, these values were not significantly different from those in vehicle-exposed sham rats. In the MI/R 7d cohort, no differences in SV, CO, or SW were seen between the two exposure groups, and both groups were statistically similar to their respective sham counterparts (Fig. 3, A–C). No significant differences in CO, SV, or SW were observed between sham, MI/R 1d, and MI/R 7d rats receiving vehicle (Fig. 3, A–C). Similarly, in DCB230-exposed rats, MI/R 1d or 7d injury did not further reduce CO, SV, or SW compared with sham (Fig. 3, A–C), although SW was significantly higher in the MI/R 7d group compared with the MI/R 1d group (Fig. 3C).
Fig. 3.
Effects of DCB230 and MI/R injury on LV systolic function. Prior inhalational exposure to DCB230 produced significant reductions in cardiac output (CO) (A), stroke volume (SV) (B), and stroke work (SW) at the MI/R 1d time point (C); preload-recruitable stroke work (PRSW) was significantly elevated compared with sham controls at the MI/R 1d time point in vehicle-exposed, but not those exposed to DCB230 (D). Lower case letters indicated the following: a, P < 0.05 vs. vehicle-exposed sham; b, P < 0.05 vs. exposure-matched sham; c, P < 0.05 vs. exposure-matched MI/R 1d.
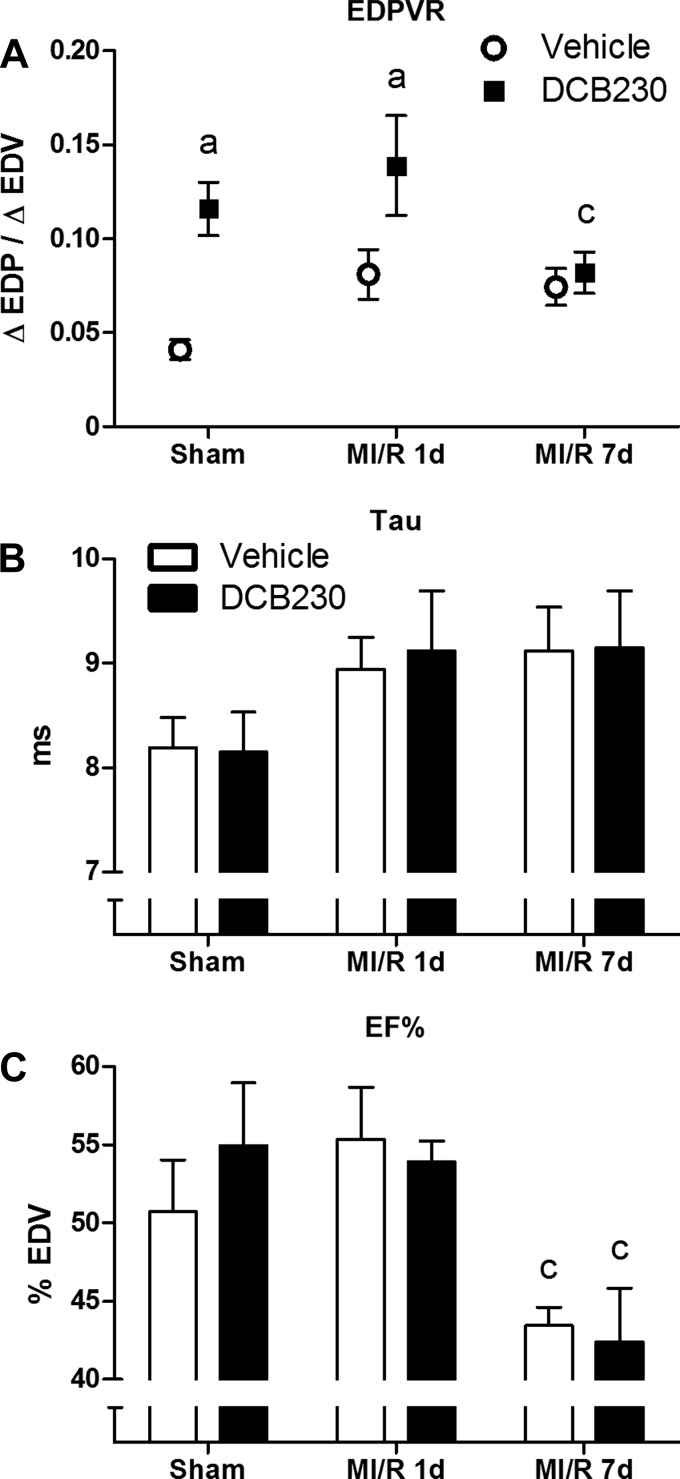
Manual occlusion of the inferior vena cava was performed to allow measurement of preload-independent indices of contraction (PRSW) and relaxation (EDPVR). Exposure to DCB230 did not affect PRSW in sham rats (Fig. 3D). After infarction and 1 day of reperfusion, PRSW in the vehicle-exposed group was significantly increased compared with that in the MI/R 1d group exposed to DCB230 (Fig. 3D). PRSW in the vehicle-exposed rats returned to control levels after 7 days of reperfusion (Fig. 3D). EDPVR was significantly elevated in sham rats exposed to DCB230, and this increase was maintained after 1 day of reperfusion, before returning to control levels after 7 days of reperfusion (Fig. 4A). No significant differences in the isovolumetric relaxation time constant, Tau, were seen between any experimental groups, despite an upward trend in all rats receiving MI/R injury (Fig. 4B).
Fig. 4.
Effects of DCB230 and MI/R injury on LV diastolic function and ejection fraction (EF%). A: prior inhalational exposure to DCB230 significantly increased the end-diastolic pressure-volume relationship (EDPVR) in sham and MI/R 1d rats; however, this effect dissipated by the MI/R 7d time point. B: no significant changes to the isovolumetric relaxation constant, Tau, were caused by either DCB230 exposure or MI/R injury. C: Prior inhalational exposure to DCB230 had no effect on LV EF%. Additionally, no significant change in EF% was elicited by MI/R 1d injury, although EF% significantly declined in both exposure groups by the MI/R 7d time point. Lower case letters indicated the following: a, P < 0.05 vs. vehicle-exposed sham; c, P < 0.05 vs. exposure-matched MI/R 1d.
Figure 4C summarizes the effect of DCB230 exposure and MI/R on EF% in the rats. Exposure to DCB230 did not significantly change EF% in sham, MI/R 1d, or MI/R 7d rats compared with the relevant vehicle-exposed controls. Additionally, infarction followed by 1 day of reperfusion did not decrease EF% in either exposure group; however, under MI/R 7d conditions both exposure groups had significantly lower EF% compared with their MI/R 1d counterparts.
DISCUSSION
This study showed for the first time that, compared with vehicle exposure, the prior inhalation (30 min/day for 7 days) of EPFRs, specifically DCB230, significantly degraded LV function in rats 24 h after MI/R injury and 48 h after the last exposure to EPFRs. However, the EPFR-mediated reductions in LV function in the injured animals were transient and dissipated within 8 days of the last particle exposure, as evidenced by similarities in LV function between vehicle- and DCB230-exposed rats 7 days post-MI/R. Infarct size in the LV of DCB230- and vehicle-exposed rats was very similar at both 1 day and 7 days following MI/R.
As expected, 48 h after the last inhalational exposure to DCB230, sham MI/R rats showed significant reductions in EDV and ESV, coupled with nonsignificant reductions in CO, SV, and SW. These results are consistent with our previous studies showing that both IT and inhalational exposure to DCB230 significantly reduces baseline diastolic filling (EDV), CO, SV, and SW in otherwise healthy rats 24 h postexposure (39, 41). The decrease in ventricular filling and subsequent LV dysfunction were attributed to increases in pulmonary artery resistance, secondary to EPFR-mediated pulmonary inflammation (41). The fact that EPFR-mediated decreases in CO, SV, and SW did not reach statistical significance in the current study likely reflects the longer time interval (48 h in the current study vs. 24 h in previous studies) between final exposure to EPFRs and functional testing of the LV. As noted above, our data indicate that EPFR-mediated decreases in LV function dissipate with time after the last exposure, and thus it is reasonable to expect that less EPFR-related effect on cardiac function may be seen 48 h as opposed to 24 h after the last exposure. Nonetheless, compared with vehicle-exposed controls, CO, SV, and SW were significantly lower in the DCB230-exposed rats following MI/R with 1 day of reperfusion, indicating LV function was further compromised by exposure to DCB230. The difference in LV function after infarction between vehicle- and DCB230-treated rats was not due to differences in infarct size between the groups. The LAD occlusion procedure used in our study subjected ∼36% of the LV to risk of ischemia (AAR), and produced an approximate area of infarction in both groups that was 26% of the AAR when normalized by mass.
Our data showing EPFR-mediated reductions in LV function following MI/R are consistent with those of Bagate and colleagues (1), who instilled urban PM10 into the tracheas of rats 4 h prior to subjecting the isolated hearts to global ischemia. The hearts of rats exposed to particles showed greater contractile deficits during the 20 min of ischemia, and were slower to recover function postischemia, than vehicle-exposed controls. We have also shown that, compared with vehicle, prior instillation of DCB230 (24 h prior) results in significantly greater reductions in LV function following a brief period (90 s) of ischemia produced by occluding the LAD coronary artery (39).
Remarkably, MI/R injury in vehicle-exposed rats did not significantly reduce CO, SV, SW, or LV volumes (EDV, ESV) when measured 1 or 7 days after initiating reperfusion. ESP, however, was significantly reduced in both the vehicle- and DCB230-exposed rats 1 day after MI/R. Previous studies involving comparable infarcts and reperfusion periods have reported similar decreases in ESP, as well as increases in EDP, 1 day after infarction (28, 29). Janahmadi and colleagues (28), using echocardiography, reported significant reductions in CO, SV, and EF% 1 day after infarction; however, their study employed a chronic model of coronary ligation, without reperfusion. In vehicle-exposed MI/R 1d rats, PRSW was significantly elevated, indicating that, in spite of structural damage, the infarcted hearts were able to acutely increase contractility to maintain LV function. In contrast, the hearts of DCB230-exposed rats did not increase PRSW 1 day after infarction, possibly preventing compensation to maintain CO, SV, or SW. PRSW was selected as a measure of contractile performance, as opposed to end-systolic pressure-volume (ESPVR) or dP/dtmax/EDV relationships, as PRSW integrates the entire cardiac cycle and is reported to be a more linear and reliable marker of the intrinsic inotropic state of the heart, less effected by conditions such as ventricular filling, geometry, and HR (20, 22, 37). At this time the mechanism(s) by which EPFRs prevent the injured heart from increasing contractility are unknown, although autonomic imbalance and or oxidative stress may play important roles.
Heart rate variability (HRV) describes variations in both instantaneous heart rate and R-R intervals and is used as a noninvasive, quantitative marker of sympathetic and parasympathetic autonomic activity (32). Clinical and experimental studies have demonstrated a positive correlation between decreased HRV (increased sympathetic or diminished vagal activity) and increased risk of cardiovascular morbidity and mortality, including sudden cardiac death (55a). Experimental studies using healthy animals or models of cardiovascular disease (e.g., hypertension and heart failure) frequently report that exposure to diesel exhaust decreases HRV, producing a state of sympathetic dominance (6, 7, 25, 26, 36). Although not as common, there are also reports of diesel exhaust or other particulates increasing HRV, producing states of parasympathetic dominance (8). In terms of cardiac function, Carll and colleagues (6) showed that inhalation of diesel exhaust (4 h) by spontaneously hypertensive heart failure-prone rats produces time- and state-dependent oscillations in HRV that alternate between sympathetic and parasympathetic dominance. More importantly, the periods of sympathetic dominance were associated with decreases in cardiac contractility and impaired LV relaxation. The decrease in HRV appeared to reflect the particulate fraction of diesel exhaust since, in separate studies, exposure to the filtered gaseous component of diesel exhaust increased HRV. In otherwise healthy rats, IT instillation of a large dose of diesel exhaust particles (DEP) decreased HRV, contractility, and relaxation (27). Likewise, inhalation of DEP also reduced cardiac contractility (23). These findings suggest that particle-mediated decreases in HRV may have prevented the infarcted, DCB230-exposed hearts from increasing contractility to maintain LV function. Whether EPFRs similarly reduce HRV has not been directly tested; however, diesel exhaust does contain EPFRs (46). The specific effect of EPFRs on HRV is currently being tested in a model of cardiac MI/R injury.
The mechanism responsible for the particulate-mediated decrease in HRV and LV contractility is unknown. Rhoden and colleagues (53) suggest that oxidative stress can increase sympathetic tone and decrease HRV. There is also evidence that diesel exhaust may directly inhibit parasympathetic function (7). It is generally accepted that inhalation of particulates produces systemic inflammation and oxidative stress in a number of organ systems (5, 43). Not surprisingly, EPFRs, such as DCB230, similarly produce systemic inflammation, as well as oxidative stress in the lungs and hearts of experimental animals (2, 39, 41, 56). In addition to changes in HRV, oxidative stress arising from neural, humoral, immune, or exogenous sources may directly decrease myocardial function by a diverse number of mechanisms, including protein and lipid oxidation, disruption of subcellular organelles, and calcium overload (15). Particulates may also have more direct actions on organs and tissues. There is accumulating evidence that inhaled fine and ultrafine particulates can transit from the lung to the systemic circulation (5, 47), raising the possibility that particles may directly produce localized inflammatory and oxidative responses. Because of the ability of EPFRs to generate oxygen radicals, the EPFR may produce a level of toxicity greater than that of the particle alone.
In addition to contractile deficits, inhalational exposure to EPFRs also produced diastolic dysfunction (decreased compliance) as evidenced by significant increases in the EDPVR, before returning to control levels after 7 days of reperfusion. Notably, the relaxation time constant Tau was not altered by exposure to EPFRs. While there was a tendency for Tau to increase after MI/R in vehicle- and DCB230-exposed rats, these changes were not significant. EPFR-mediated diastolic dysfunction has also been previously documented after IT instillation of EPFRs (39) and inhalation of diesel exhaust (7), or the instillation of DEP (26). The mechanism responsible for the decreased compliance is not currently known.
It is well documented that reperfusion of ischemic myocardium produces an oxidative and inflammatory burst, increasing the severity and size of LV injury beyond that caused by ischemia alone (15). Given that EPFRs and other particles produce systemic inflammation and oxidative stress, we hypothesized that prior exposure to EPFRs, coupled with subsequent reperfusion injury, would increase the level of oxidative stress leading to increased infarct size and greater LV dysfunction. Consistent with our hypothesis was the observation that infarct size doubled in mice that had received a large IT-instilled dose of environmentally-collected PM0.1 24 h prior to coronary artery ligation (12). The increase in infarct size was accompanied by a significant increase in inflammation and oxidative stress. Additionally, a study by Zhu et al. (63) showed that inhalation of tobacco smoke (another source of particulates) dose dependently increases infarct size in rats subjected to MI/R within 1 h of the final exposure. Unexpectedly, in the present study, prior exposure to DCB230 did not result in a larger necrotic infarct when measured either 1 or 7 days after the ischemic event. The reason for the discrepancy between our study and previous studies is unknown, but likely reflects differences in the concentration of particles used and the route of administration. In the mouse study, a relatively large, single dose of particles (∼3.3 mg/kg) was instilled directly into the airways. In contrast, our flow-past, nose-only inhalational protocol provided a maximum daily presentation of 230 μg/day over 7 days to each rat, of which only a small fraction is actually inhaled into the lungs. Previous studies by our group have shown that IT instillation of DCB230 (8 mg/kg) in rats produces a greater degree of LV dysfunction than inhalational exposure at a dose similar to that used in the present study (39, 41). Differences in infarct size could also reflect differences in the type of particles administered or species differences.
Results from this study corroborate our previous finding that inhalational exposure to the EPFR DCB230 reduces baseline cardiac filling and LV function in otherwise healthy rats. Vehicle-exposed rats were also found to be capable of acutely maintaining LV function post-MI/R injury by increasing contractility (PRSW). In contrast, rats exposed to EPFRs and subsequent MI/R injury were unable to increase PRSW to maintain CO, SV, or SW. The deleterious effects of DCB230 dissipated within 8 days after the final exposure. Surprisingly, in spite of the ability of EPFR exposure to decrease LV function, infarct size was similar in vehicle- and particle-treated rat hearts. The finding that prior inhalational exposure to EPFR-containing PM reduces ventricular filling and prevents the heart from increasing PRSW to maintain ventricular function following MI/R injury potentially has important clinical implications regarding prognosis for individuals exposed to high ambient PM who subsequently suffer an MI/R event. Although the EPFR-mediated effects dissipated within 8 days of the final exposure, the question of whether subsequent inhalational exposure to EPFRs can affect long-term survival following an MI/R event is currently being investigated.
GRANTS
This study was supported by National Institutes of Health Grants P42ES013648 and P30GM106392.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
B.R.B. and K.J.V. conception and design of research; B.R.B. performed experiments; B.R.B. and K.J.V. analyzed data; B.R.B. and K.J.V. interpreted results of experiments; B.R.B. prepared figures; B.R.B. drafted manuscript; B.R.B. and K.J.V. approved final version of manuscript; K.J.V. edited and revised manuscript.
REFERENCES
- 1.Bagate K, Meiring JJ, Gerlofs-Nijland ME, Cassee FR, Wiegand H, Osornio-Vargas A, Borm a PJ. Ambient particulate matter affects cardiac recovery in a Langendorff ischemia model. Inhal Toxicol 18: 633–643, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Balakrishna S, Lomnicki S, McAvey KM, Cole RB, Dellinger B, Cormier SA. Environmentally persistent free radicals amplify ultrafine particle mediated cellular oxidative stress and cytotoxicity. Part Fibre Toxicol 6: 11, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balakrishna S, Saravia J, Thevenot P, Ahlert T, Lominiki S, Dellinger B, Cormier SA. Environmentally persistent free radicals induce airway hyperresponsiveness in neonatal rat lungs. Part Fibre Toxicol 8: 11, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC, Tager I. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation 109: 2655–2671, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Brook RD, Rajagopalan S, Pope CA, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC, Whitsel L, Kaufman JD. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 121: 2331–2378, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Carll AP, Hazari MS, Perez CM, Krantz QT, King CJ, Haykal-Coates N, Cascio WE, Costa DL, Farraj AK. An autonomic link between inhaled diesel exhaust and impaired cardiac performance: insight from treadmill and dobutamine challenges in heart failure-prone rats. Toxicol Sci 135: 425–436, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carll AP, Hazari MS, Perez CM, Krantz QT, King CJ, Winsett DW, Costa DL, Farraj AK. Whole and particle-free diesel exhausts differentially affect cardiac electrophysiology, blood pressure, and autonomic balance in heart failure-prone rats. Toxicol Sci 128: 490–499, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carll AP, Lust RM, Hazari MS, Perez CM, Krantz QT, King CJ, Winsett DW, Cascio WE, Costa DL, Farraj AK. Diesel exhaust inhalation increases cardiac output, bradyarrhythmias, and parasympathetic tone in aged heart failure-prone rats. Toxicol Sci 131: 583–595, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cesaroni G, Forastiere F, Stafoggia M, Andersen ZJ, Badaloni C, Beelen R, Caracciolo B, de Faire U, Erbel R, Eriksen KT, Fratiglioni L, Galassi C, Hampel R, Heier M, Hennig F, Hilding A, Hoffmann B, Houthuijs D, Jöckel KH, Korek M, Lanki T, Leander K, Magnusson PKE, Migliore E, Ostenson CG, Overvad K, Pedersen NL, JJP, Penell J, Pershagen G, Pyko A, Raaschou-Nielsen O, Ranzi A, Ricceri F, Sacerdote C, Salomaa V, Swart W, Turunen AW, Vineis P, Weinmayr G, Wolf K, de Hoogh K, Hoek G, Brunekreef B, Peters A. Long term exposure to ambient air pollution and incidence of acute coronary events: prospective cohort study and meta-analysis in 11 European cohorts from the ESCAPE Project. BMJ 348: f7412, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang CC, Kuo CC, Liou SH, Yang CY. Fine particulate air pollution and hospital admissions for myocardial infarction in a subtropical city: Taipei, Taiwan. J Toxicol Environ Health A 76: 440–448, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Conklin D. Beware the air! Why particulate matter matters. Circ Res 108: 644–647, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cozzi E, Hazarika S, Stallings HW, Cascio WE, Devlin RB, Lust RM, Wingard CJ, Van Scott MR. Ultrafine particulate matter exposure augments ischemia-reperfusion injury in mice. Am J Physiol Heart Circ Physiol 291: H894–H903, 2006. [DOI] [PubMed] [Google Scholar]
- 13.D'Ippoliti D, Forastiere F, Ancona C, Agabiti N, Fusco D, Michelozzi P, Perucci CA. Air pollution and myocardial infarction in Rome: a case-crossover analysis. Epidemiology 14: 528–535, 2003. [DOI] [PubMed] [Google Scholar]
- 13a.de Winter-Sorkina R, Cassee FR. From concentration to dose: factors influencing airborne particulate matter deposition in humans and rats (Online). Rivm Repository. http://hdl.handle.net/10029/9272 [9 Dec. 2014].
- 14.Dellinger B, Pryor WA, Cueto R, Squadrito GL, Hegde V, Deutsch WA. Role of free radicals in the toxicity of airborne fine particulate matter. Chem Res Toxicol 14: 1371–1377, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. J Hypertens 18: 655–673, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Dianat M, Hamzavi GR, Badavi M, Samarbafzadeh A. Effects of losartan and vanillic Acid co-administration on ischemia-reperfusion-induced oxidative stress in isolated rat heart. Iran Red Crescent Med J 16: e16664, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dominici F, Peng R, Bell M. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA 295: 1127–1134, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elahi MM, Kong YX, Matata BM. Oxidative stress as a mediator of cardiovascular disease. Oxid Med Cell Longev 2: 259–269, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fahmy B, Ding L, You D, Lomnicki S, Dellinger B, Cormier SA. In vitro and in vivo assessment of pulmonary risk associated with exposure to combustion generated fine particles. Environ Toxicol Pharmacol 29: 173–182, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feneley MP, Skelton TN, Kisslo KB, Davis JW, Bashore TM, Rankin JS. Comparison of preload recruitable stroke work, end-systolic pressure-volume and dPdtmax-end-diastolic volume relations as indexes of left ventricular contractile performance in patients undergoing routine cardiac catheterization. J Am Coll Cardiol 19: 1522–1530, 1992. [DOI] [PubMed] [Google Scholar]
- 21.Gehling W, Khachatryan L, Dellinger B. Hydroxyl radical generation from environmentally persistent free radicals (EPFRs) in PM2.5. Environ Sci Technol 48: 4266–4272, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glower DD, Spratt JA, Snow ND, Kabas JS, Davis JW, Olsen CO, Tyson GS, Sabiston DC, Rankin JS. Linearity of the Frank-Starling relationship in the intact heart: the concept of preload recruitable stroke work. Circulation 71: 994–1009, 1985. [DOI] [PubMed] [Google Scholar]
- 23.Gordon CJ, Schladweiler MC, Krantz T, King C, Kodavanti UP. Cardiovascular and thermoregulatory responses of unrestrained rats exposed to filtered or unfiltered diesel exhaust. Inhal Toxicol 24: 296–309, 2012. [DOI] [PubMed] [Google Scholar]
- 24.Guo L, Hu Y, Hu Q, Lin J, Li C, Chen J, Li L, Fu H. Characteristics and chemical compositions of particulate matter collected at the selected metro stations of Shanghai, China. Sci Total Environ 496: 443–452, 2014. [DOI] [PubMed] [Google Scholar]
- 25.Hazari MS, Haykal-Coates N, Winsett DW, Krantz QT, King C, Costa DL, Farraj AK. TRPA1 and sympathetic activation contribute to increased risk of triggered cardiac arrhythmias in hypertensive rats exposed to diesel exhaust. Environ Health Perspect 119: 951–957, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang CH, Lin LY, Tsai MS, Hsu CY, Chen HW, Wang TD, Chang WT, Cheng TJ, Chen WJ. Acute cardiac dysfunction after short-term diesel exhaust particles exposure. Toxicol Lett 192: 349–355, 2010. [DOI] [PubMed] [Google Scholar]
- 27.Huang W, Zhu T, Pan X, Hu M, Lu SE, Lin Y, Wang T, Zhang Y, Tang X. Air pollution and autonomic and vascular dysfunction in patients with cardiovascular disease: interactions of systemic inflammation, overweight, and gender. Am J Epidemiol 176: 117–126, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janahmadi Z, Nekooeian AA, Moaref AR, Emamghoreishi M. Oleuropein offers cardioprotection in rats with acute myocardial infarction. Cardiovasc Toxicol 15: 61–68, 2015. [DOI] [PubMed] [Google Scholar]
- 29.Jin YC, Kim KJ, Kim YM, Ha YM, Kim HJ, Yun UJ, Bae KH, Kim YS, Kang SS, Seo HG, Lee JH, Chang KC. Anti-apoptotic effect of magnolol in myocardial ischemia and reperfusion injury requires extracellular signal-regulated kinase1/2 pathways in rat in vivo. Exp Biol Med (Maywood) 233: 1280–1288, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Kan H, London SJ, Chen G, Zhang Y, Song G, Zhao N, Jiang L, Chen B. Differentiating the effects of fine and coarse particles on daily mortality in Shanghai, China. Environ Int 33: 376–384, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelley MA, Hebert VY, Thibeaux TM, Orchard MA, Hasan F, Cormier SA, Thevenot PT, Lomnicki SM, Varner KJ, Dellinger B, Latimer BM, Dugas TR. Model combustion-generated particulate matter containing persistent free radicals redox cycle to produce reactive oxygen species. Chem Res Toxicol 26: 1862–1871, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kerut EK, McKinnie JJ, Giles TD. Modern evaluation of the hypertensive patient: autonomic tone in cardiovascular disease and the assessment of heart rate variability. Blood Press Monit 4, Suppl 1: S7–S14, 1999. [PubMed] [Google Scholar]
- 33.Khachatryan L, Dellinger B. Environmentally persistent free radicals (EPFRs)-2. Are free hydroxyl radicals generated in aqueous solutions? Environ Sci Technol 45: 9232–9239, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khachatryan L, Vejerano E, Lomnicki S, Dellinger B. Environmentally persistent free radicals (EPFRs). 1. Generation of reactive oxygen species in aqueous solutions. Environ Sci Technol 45: 8559–8566, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knuckles TL, Stapleton a P, Minarchick VC, Esch L, McCawley M, Hendryx M, Nurkiewicz TR. Air pollution particulate matter collected from an Appalachian mountaintop mining site induces microvascular dysfunction. Microcirculation 20: 158–169, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lamb CM, Hazari MS, Haykal-Coates N, Carll AP, Krantz QT, King C, Winsett DW, Cascio WE, Costa DL, Farraj AK. Divergent electrocardiographic responses to whole and particle-free diesel exhaust inhalation in spontaneously hypertensive rats. Toxicol Sci 125: 558–568, 2012. [DOI] [PubMed] [Google Scholar]
- 37.Little WC, Cheng CP, Mumma M, Igarashi Y, Vinten-Johansen J, Johnston WE. Comparison of measures of left ventricular contractile performance derived from pressure-volume loops in conscious dogs. Circulation 80: 1378–1387, 1989. [DOI] [PubMed] [Google Scholar]
- 38.Lomnicki S, Truong H, Vejerano E, Dellinger B. Copper Oxide-Based Model of Persistent Free Radical Formation on Combustion-Derived Particulate Matter. Environ Sci Technol 42: 4982–4988, 2008. [DOI] [PubMed] [Google Scholar]
- 39.Lord K, Moll D, Lindsey JK, Mahne S, Raman G, Dugas T, Cormier S, Troxlair D, Lomnicki S, Dellinger B, Varner K. Environmentally persistent free radicals decrease cardiac function before and after ischemia/reperfusion injury in vivo. J Recept Signal Transduct Res 31: 157–167, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lyons MJ, Spence JB. Environmental free radicals. Br J Cancer 14: 703–708, 1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahne S, Chuang GC, Pankey E, Kiruri L, Kadowitz PJ, Dellinger B, Varner KJ. Environmentally persistent free radicals decrease cardiac function and increase pulmonary artery pressure. Am J Physiol Heart Circ Physiol 303: H1135–H1142, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martinelli N, Olivieri O, Girelli D. Air particulate matter and cardiovascular disease: a narrative review. Eur J Intern Med 24: 295–302, 2013. [DOI] [PubMed] [Google Scholar]
- 43.Miller MR, Shaw CA, Langrish JP. From particles to patients: oxidative stress and the cardiovascular effects of air pollution. Future Cardiol 8: 577–602, 2012. [DOI] [PubMed] [Google Scholar]
- 44.Miller MR. The role of oxidative stress in the cardiovascular actions of particulate air pollution. Biochem Soc Trans 42: 1006–1011, 2014. [DOI] [PubMed] [Google Scholar]
- 45.Mustafić H, Jabre P, Caussin C. Main air pollutants and myocardial infarction: a systematic review and meta-analysis. JAMA 307: 713–721, 2012. [DOI] [PubMed] [Google Scholar]
- 46.Nel AE, Diaz-Sanchez D, Li N. The role of particulate pollutants in pulmonary inflammation and asthma: evidence for the involvement of organic chemicals and oxidative stress. Curr Opin Pulm Med 7: 20–26, 2001. [DOI] [PubMed] [Google Scholar]
- 47.Nemmar A, Vanbilloen H, Hoylaerts MF, Hoet PH, Verbruggen A, Nemery B. Passage of intratracheally instilled ultrafine particles from the lung into the systemic circulation in hamster. Am J Respir Crit Care Med 164: 1665–1668, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Pacher P, Nagayama T, Mukhopadhyay P, Bátkai S, Kass DA. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc 3: 1422–1434, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peters A, Dockery DW, Muller JE, Mittleman MA. Increased particulate air pollution and the triggering of myocardial infarction. Circulation 103: 2810–2815, 2001. [DOI] [PubMed] [Google Scholar]
- 50.Peters A. Particulate matter and heart disease: evidence from epidemiological studies. Toxicol Appl Pharmacol 207: 477–482, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Pope CA, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation 109: 71–77, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Pope CA, Muhlestein JB, May HT, Renlund DG, Anderson JL, Horne BD. Ischemic heart disease events triggered by short-term exposure to fine particulate air pollution. Circulation 114: 2443–2448, 2006. [DOI] [PubMed] [Google Scholar]
- 53.Rhoden CR, Wellenius GA, Ghelfi E, Lawrence J, González-Flecha B. PM-induced cardiac oxidative stress and dysfunction are mediated by autonomic stimulation. Biochim Biophys Acta 1725: 305–313, 2005. [DOI] [PubMed] [Google Scholar]
- 54.Rodrigo R, Libuy M, Feliú F, Hasson D. Oxidative stress-related biomarkers in essential hypertension and ischemia-reperfusion myocardial damage. Dis Markers 35: 773–790, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Silva RA, West JJ, Zhang Y, Anenberg SC, Lamarque JF, Shindell DT, Collins WJ, Dalsoren S, Faluvegi G, Folberth G, Horowitz LW, Nagashima T, Naik V, Rumbold S, Skeie R, Sudo K, Takemura T, Bergmann D, Cameron-Smith P, Cionni I, Doherty RM, Eyring V, Josse B, MacKenzie IA, Plummer D, Righi M, Stevenson DS, Strode S, Szopa S, Zeng G. Global premature mortality due to anthropogenic outdoor air pollution and the contribution of past climate change. Environ Res Lett 8: 034005, 2013. [Google Scholar]
- 55a.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation 93: 1043–1065, 1996. [PubMed] [Google Scholar]
- 56.Wang P, Thevenot P, Saravia J, Ahlert T, Cormier SA. Radical-containing particles activate dendritic cells and enhance Th17 inflammation in a mouse model of asthma. Am J Respir Cell Mol Biol 45: 977–983, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang P, You D, Saravia J, Shen H, Cormier SA. Maternal exposure to combustion generated PM inhibits pulmonary Th1 maturation and concomitantly enhances postnatal asthma development in offspring. Part Fibre Toxicol 10: 29, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang M, Chen J, Zhao J, Meng M. Etanercept attenuates myocardial ischemia/reperfusion injury by decreasing inflammation and oxidative stress. PLoS One 9: e108024, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zanobetti A, Schwartz J. Air pollution and emergency admissions in Boston, MA. J Epidemiol Community Health 60: 890–895, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zanobetti A, Schwartz J. Particulate air pollution, progression, and survival after myocardial infarction. Environ Health Perspect 115: 769–775, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang F, Li L, Krafft T, Lv J, Wang W, Pei D. Study on the association between ambient air pollution and daily cardiovascular and respiratory mortality in an urban district of Beijing. Int J Environ Res Public Health 8: 2109–2123, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu BQ, Sun YP, Sievers RE, Glantz SA, Parmley WW, Wolfe CL. Exposure to environmental tobacco smoke increases myocardial infarct size in rats. Circulation 89: 1282–1290, 1994. [DOI] [PubMed] [Google Scholar]