Abstract
Purpose
Prior research has shown a significant relationship between six minute walking distances (6MWD) and health related quality of life (HRQL) in patients with chronic obstructive pulmonary disease (COPD). However, few have examined this relationship above and below the 350 meters (m) threshold that prognosticates survival. We further investigated whether serum biomarkers could provide insight into the causes of quality of life differences above and below this threshold.
Methods
Measures of lung function, 6MWD, HRQL (SGRQ and SF-36) were compared in patients with COPD. Differences in HRQL domains and serum biomarkers were compared in patients whose 6MWD were > or < 350m.
Results
In patients walking <350m, scores in the physical domains of the SF-36 and SGRQ were significantly different than their counterparts with greater 6MWD. However, there was no association between any biomarkers and the physical domains of the SF-36 and the SGRQ. In patients walking <350m, only the Il-8 levels were associated with lower scores in SF-36 domains of emotional role, pain, vitality and mental health (average r=−0.702, p=0.01). In contrast, in patients walking >350m, surfactant protein D (SP-D) levels were associated with higher SF-36 scores in general pain, vitality and social functioning (average r = 0.42, p=0.04).
Conclusions
In COPD, there is an association between 6MWD and the physical domains of the SF-36 and SGRQ in those patients walking < 350m. The physical differences between patients walking less or more than 350m are not related to systemic inflammation. The association between IL-8 with nonphysical domains in patients with 6MWD < 350m suggests that inflammation may play a larger role in the perceptive domain than previously recognized.
Introduction and Statement of Purpose
Chronic obstructive pulmonary disease (COPD) is a leading cause of mortality and disability in the world.1 Impaired functional capacity is common in COPD and likely multifactorial, including severe airflow limitation2, muscle wasting3 and depression4, amongst other systemic manifestations of the disease. Systemic inflammation has been implicated in the development of these extrapulmonary manifestations of COPD5,6. Therefore, serum biomarkers of local and systemic inflammation have gained increasing attention as associations to meaningful clinical outcomes, such as mortality and hospitalizations, have been established.7 Studies thus far, however, have not examined the relationship between these serum biomarkers and health related quality of life domains in COPD patients with severe functional limitations.
In COPD, forced expiratory volume in 1 second (FEV1) has been historically used to predict functional outcomes and even mortality despite its weak relationship to both of these important outcomes. More recently exercise capacity has emerged as one of the most important prognostic indicators in COPD. Although VO2max obtained from cardiopulmonary exercise testing has been shown to be a predictor of mortality in COPD, such testing is not always readily available or cost effective.8 The six minute walking distance (6MWD), on the other hand, is a quick reproducible exercise field test that predicts mortality in patients with COPD.9,10 Although the average 6MWD is low in severe COPD, the distribution is wide within all severity categories of COPD.11 In studies where both have been compared, the 6MWD was a better predictor of mortality than the FEV19 and evidence suggests that a value of 350 meters (m) is the threshold below which patients experience a linear increased risk of hospitalization and mortality.12,13
Relationships between 6MWD as a continuous physiologic measure and health related quality of life (HRQL) measures have been established in COPD. 6MWD has been shown to correlate with both disease specific and generic HRQL instruments, such as the St. George’s Respiratory Questionnaire (SGRQ)14 and the Medical Outcomes SF-36 questionnaire (SF-36),15 respectively. Although the disease specific SGRQ has been validated in large therapeutic trials, such as TORCH and UPLIFT,16 and in observational studies such as ECLIPSE,2 relatively few studies have examined the relationship of the 6MWD with generic measures of quality of life such as the SF-36, which measures overall health status including effects of co-morbid diseases. Of importance, no study has evaluated the relationship between HRQL domains of the SGRQ and SF-36 and patients’ ability to walk more or less than the 350m,17 despite the considerable prognostic value of this functional threshold.
HRQL measures have been shown to be robust predictors of 6MWD2 and mortality18 in COPD. Thus, understanding the HRQL profile of COPD patients with poor functional status is essential to our clinical understanding of true burden of illness engendered by COPD in this functionally limited population. In addition, understanding possible biologic causes of the HRQL profile of those with 6MWD <350m may provide insight into how best stratify and manage these patients. Therefore, the purpose of this study was two-fold. First, this study sought to establish which domains of HRQL are related to poor functional capacity as measured by the 6MWD <350m in COPD. Subsequently, this study examined the link between inflammatory biomarkers and these domains of HRQL in patients with 6MWD above and below the 350m threshold. We hypothesized that HRQL domain scores and the relationships between HRQL domains and biomarker profiles would differ above and below the 350m threshold that confers survival prognosis and is currently proposed as a stratification tool by the COPD Foundation Biomarker Qualification Consortium.12,13,19
Subjects and Methods
Study Population
Subjects were participants in the observational study RES19044 (“A Multi-center Cohort Study to Evaluate Radiological, Physiological and Biochemical Biomarkers in Patients with Chronic Obstructive Pulmonary Disease and Age and Gender Matched Controls”) enrolled in 2004 and followed until 2008. RES19044 was approved by the Institutional Review Boards of St. Elizabeth’s Medical Center (Protocol #00195) and the University of Pennsylvania (Protocol #801096). Subjects were diagnosed based on the 2007 Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria of airflow obstruction20 and assigned to one of five groups: healthy non-smokers, current or former smokers without airflow obstruction, GOLD COPD stages II, III, or IV. Patients with respiratory disorders other than COPD were excluded.
A group of 59 patients aged 45–75 years old with COPD were enrolled. All subjects provided written consent.
Inclusion criteria included a diagnosis of COPD (FEV1/FVC < 0.7), stable on medical therapy, smoking history > 10 years (current and ex-smokers), and ability to complete the questionnaires. Exclusion criteria included concomitant anticoagulant (except aspirin) therapy or recent use of investigational drugs, alcohol abuse, medical conditions such as (but not limited to) prior lung surgery, ventilator dependence, asthma, New York Heart Association class 3 or 4 congestive heart failure, cirrhosis, end stage renal disease requiring dialysis, uncontrolled diabetes, alpha-1 antitrypsin disease, rheumatoid arthritis or other known significant inflammatory disorders, chronic respiratory failure (defined as PaO2 < 60), right heart failure, recent infections (within 21 days), pregnancy, hospitalization for COPD exacerbation within 3 months.
Data Collection
Each subject participated in up to 5 study sessions. The duration of each subject’s participation from screening to last follow-up telephone call was 3 years and a total of 7 visits. Data from the initial visit were used for this analysis.
HRQL was assessed with generic and disease specific quality of life questionnaires14,21. The Medical Outcomes Study short-form survey (SF-36) consists of 36 questions that cover 9 health domains including physical and social functioning, physical and emotional role limitations, mental health, pain index, vitality, general health perceptions and health transitions. Scores for each domain range from 0 to 100 with the higher scores indicating better functioning. The SGRQ consists of 76 items addressing the effect of respiratory disease on HRQL. A composite score and 3 domain scores for symptoms, activity and impact range from 0% to 100%, with higher scores indicating worse impairment.
Patients performed pulmonary function testing following ATS /ERS guidelines.22 The symptom limited non-invasive CPET was performed following ATS/ACCP standards.23 Exercise tolerance was measured using the 6 minute walk distance testing following the recommendations of the ATS24. The longer of two walk tests measured with a 30 minute rest was used for analysis.
The selection of biomarkers was based on a review of the literature and their biological plausibility. For instance, plasma c-reactive protein (CRP),25 fibrinogen,26 and IL-627 have been well studied and associated with poor clinical outcomes and mortality in COPD. The levels of IL-67, TNF –alpha4 and surfactant protein D (SDP)28 are associated with disease specific health related quality of life. Additionally, relationships between functional limitations and Il-6, IL-8 and TNF-alpha have also been described7. As proteloytic enzyme activity and lung epithelial injury have been implicated in the development and progression of COPD, we included assays for matrix metalloproteinases (MMP)29 and clara cell secretory protein 16 (CC-16)30 in our analysis. Therefore, our biomarker panel included Il-6, Il-8, CRP, SPD, TNF-alpha, MMP and CC-16.
For biomarker analysis, the blood was allowed to clot for 30 minutes and serum was obtained by centrifugation at 1500 g for 10–15 minutes. For plasma preparation, whole blood was collected into vacutainer tubes containing EDTA. Plasma was obtained by centrifugation at 2000 g for 10–15 minutes. Serum and plasma samples were stored at −80°C until analyzed. With the exception of fibrinogen, all biomarkers, including SP-D and CC-16, were measured using validated immunoassays (Aushon Biosystems, Inc., Billerica, MA USA). Fibrinogen was measured using an immunoturbidometric assay validated for use with EDTA plasma (K-ASSAY fibrinogen test, Kamiya Biomedical Co., Seattle, WA, USA). Assays were performed in duplicate to allow assessment of assay variation.
Statistical Analysis
Descriptive statistics were performed for all data. Categorical data were summarized using frequency counts and percentages while continuous data were summarized using means and standard deviations. The number of missing data points was low and missing values were not imputed. However, for biomarkers with missing values due to the concentration being below the limit of detection of the assay, values were imputed to be half the limit of detection.
Comparisons of means or frequencies between groups were performed using a t-test or a Cochran-Mantel-Haenszel test, respectively. The relationship between pairs of continuous variables were assessed using linear regression and the Pearson correlation coefficient was used to express strength and direction of the relationship. Differences between groups and tests of association were declared significant at the 5% level. There was no adjustment for multiple testing.
Results
Subject Characteristics
The baseline characteristics of the 59 COPD subjects (25 female, 34 male) are summarized in table 1. The mean age was 63 years and the mean BMI was 29 kg/m2. As compared to patients able to walk more than 350m during testing, COPD patients who walked less than 350m had more significant airflow obstruction with a mean FEV1 0.96 L (34% predicted), lower diffusing capacity for carbon monoxide (48% predicted) and lower resting and peak oxygen saturations. Total lung capacity was similar amongst all patients with COPD.
Table 1.
Characteristics of Patients with COPD Stratified by Six Minute Walking Distance (6MWD)
| Characteristics | 6MWD < 350m | 6MWD> 350m | p-value |
|---|---|---|---|
| N | 17 | 38 | |
| Age (y), mean | 64 (59 – 69) | 62 (59 – 65) | 0.42 |
| Sex (F) | 10 | 15 | 0.19 |
| BMI (kg/m2) | 30.3 (28.1 – 32.5) | 27.8 (25.8 – 29.8) | 0.12 |
| Smoking history, pack years, mean | 58 (44 – 72) | 53 (41 – 64) | 0.54 |
| Smoking status (Current smoker) (n) | 7 | 17 | 0.81 |
| CAD (n) | 0 | 3 | NS‡ |
| Congestive Heart Failure (n) | 0 | 1 | NS |
| Diabetes Mellitus (n) | 6 | 1 | <0.01 |
| FEV1 (L), mean | 0.96 (0.71 – 1.21) | 1.42 (1.22 – 1.62) | <0.01 |
| FEV1 (% predicted), mean | 34 (26 – 42) | 46 (41 – 52) | 0.01 |
| FEV1/FVC, mean | 54 (46 – 61) | 57 (51 – 63) | 0.49 |
| RV, L, mean | 3.9 (3.2 – 4.7) | 3.8 (3.4 – 4.2) | 0.8 |
| FRC, L, mean | 4.5 (3.7 – 5.2) | 4.8 (4.3 – 5.2) | 0.51 |
| TLC, L, mean | 6.5 (5.9 – 7.2) | 7.2 (6.6 – 7.8) | 0.2 |
| DLCO, uncorrected (ml/min/mmHg), mean | 10.27 (7.88 – 12.65) | 13.83 (12.33 – 15.33) | 0.01 |
| DLCO, % predicted, mean | 48 (39 – 57) | 61 (56 – 66) | <0.01 |
| VO2 max, % predicted, mean | 54 (46 – 61) | 63 (57– 69) | 0.08 |
| VE/MVV %, mean | 71 (65 – 78) | 69 (62 – 77) | 0.74 |
| HRR %, mean | 46 (34 – 58) | 69 (62 – 76) | <0.01 |
| Pre-SPO2 %, mean | 92 (89 – 95) | 95 (94 – 96) | <0.01 |
| Peak-SPO2 %, mean | 90 (87 – 93) | 94 (93 – 95) | <0.01 |
Numbers in brackets denote 95% confidence intervals. 6MWD denotes the six minute walking distance. FEV1 denotes the forced expiratory volume in 1 second, FVC the forced vital capacity, RV the residual volume, FRC the functional residual capacity, TLC the total lung capacity, DLCO the diffusing capacity of the lung for carbon monoxide.
VO2 max is the peak oxygen uptake. VE/MVV is the maximum minute ventilation during exercise relative to minimum voluntary ventilation at rest; a ratio > 80% suggests a pulmonary limitation to exercise. O2 pulse approximates stroke volume. HRR is the heart rate recovery and SpO2 denotes arterial oxygen saturation.
n, number of cases
NS, non-significant
Relationship between HRQL domains and functional status in COPD subjects walking < 350 meters
The analysis comparing the HRQL domains in COPD patients able to walk more or less than 350m is shown in figure 1. SGRQ scores for all subgroups were on average 8 points higher in COPD patients who walked less than 350m during the 6 minute walk test, compared to COPD patients able to walk greater than 350m although only the score in the activity domain was significantly different between the groups (p=0.02). The score in the SF-36 domain of physical functioning was significantly different (p < 0.01) when comparing patients based on a 6MWD above or below 350m, and there was a trend towards significance in the domain of physical role. Scores in the domains of mental health, emotional role, vitality and pain, on the other hand, were similar between groups regardless of 6MWD achieved.
Figure 1.
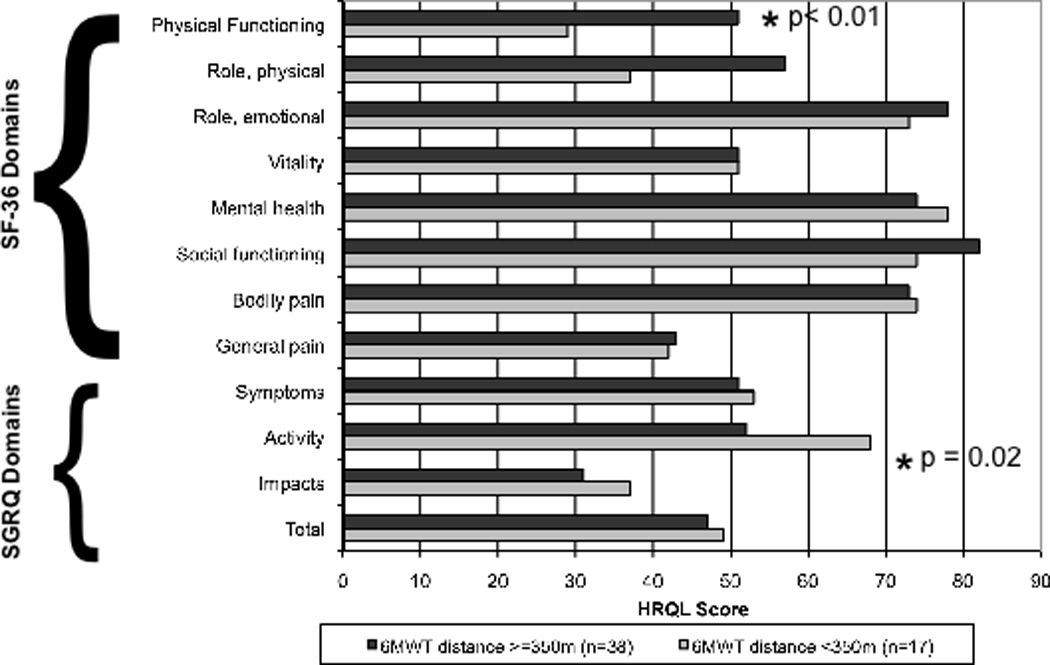
SF-36 and SGRQ scores in COPD subjects stratified by 6MWD.
HRQL = Health Related Quality of Life.
Relationship between HRQL and biomarkers in COPD patients stratified by 6MWD
The association between HRQL domains and biomarkers in COPD patients walking more or less than 350m is shown in table 2. Higher levels of serum interleukin 8 (IL-8) were associated with lower scores in SF-36 domains of emotional role (r= −0.717, p=0.02), bodily pain (r=−0.662, p=0.02), general pain (r=−0.741, p=0.01), vitality (r=−0.748, p=0.01) and mental health (r=−0.789, p=0.01) in COPD patients with a 6MWD less than 350m. There was no correlation between IL-8 levels and any domains of the SGRQ in this group. In contrast, surfactant protein D (SP-D) levels were significantly associated with higher SF-36 scores in the domains of general pain (r=0.417, p=0.05), vitality (0.425, p=0.05) and social functioning (0.441, p=0.04) and in all SGRQ domains except “activity” in COPD patients walking greater than 350m. In the absence of stratification by walking distance, there was no association between any biomarkers and the SF-36 domains of physical functioning and physical role across all patients.
Table 2.
Relationship Between Serum Biomarkers and Health Status in COPD Patients According to Six Minute Walking Distance (6MWD)
| Biomarkers† | IL-6 *Corr (*p) |
IL-8 Corr (p) |
CRP Corr (p) |
Fibrinogen Corr (p) |
SPD Corr (p) |
TNF-α Corr (p) |
MMP-9 Corr (p) |
CC16 Corr (p) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6MWD (m) | <=350m | >350m | <=350m | >350m | <=350m | >350m | <=350m | >350m | <=350m | >350m | <=350m | >350m | <=350m | >350m | <=350m | >350m |
| **SF-36 | ||||||||||||||||
| Physical Function | 0.516 (0.13) |
−0.304 (0.16) |
−0.202 (0.58) |
−0.232 (0.29) |
0.217 (0.48) |
−0.011 (0.95) |
0.391 (0.19) |
−0.005 (0.98) |
0.012 (0.98) |
0.322 (0.14) |
0.180 (0.62) |
0.121 (0.58) |
−0.425 (0.22) |
−0.295 (0.17) |
0.135 (0.73) |
0.191 (0.39) |
| Role, physical | 0.147 (0.69) |
−0.009 (0.97) |
−0.539 (0.11) |
0.249 (0.25) |
−0.273 (0.37) |
−0.112 (0.54) |
0.090 (0.77) |
−0.052 (0.77) |
0.202 (0.60) |
0.375 (0.09) |
0.029 (0.94) |
0.198 (0.36) |
0.038 (0.92) |
0.039 (0.86) |
−0.357 (0.35) |
0.140 (0.53) |
| Role, emotional | −0.114 (0.75) |
0.196 (0.37) |
−0.717 (0.02) |
0.129 (0.56) |
0.248 (0.41) |
−0.205 (0.25) |
0.199 (0.51) |
−0.088 (0.63) |
0.302 (0.43) |
0.300 (0.18) |
−0.068 (0.85) |
0.079 (0.72) |
0.086 (0.81) |
−0.289 (0.18) |
−0.182 (0.64) |
0.229 (0.30) |
| Bodily Pain | −0.342 (0.33) |
0.362 (0.09) |
−0.662 (0.04) |
0.284 (0.19) |
0.217 (0.48) |
−0.199 (0.27) |
0.384 (0.20) |
0.274 (0.12) |
0.405 (0.28) |
0.394 (0.07) |
−0.334 (0.35) |
0.127 (0.56) |
−0.092 (0.80) |
−0.244 (0.26) |
0.427 (0.25) |
0.366 (0.09) |
| General Pain | 0.267 (0.46) |
−0.215 (0.33) |
−0.741 (0.01) |
0.052 (0.81) |
0.209 (0.49) |
−0.125 (0.49) |
0.245 (0.42) |
−0.350 (0.05) |
0.120 (0.76) |
0.417 (0.05) |
0.084 (0.82) |
0.141 (0.52) |
−0.130 (0.72) |
−0.274 (0.21) |
0.219 (0.57) |
0.075 (0.74) |
| Vitality | 0.120 (0.74) |
−0.232 (0.29) |
−0.748 (0.01) |
−0.063 (0.77) |
0.403 (0.17) |
−0.342 (0.05) |
0.528 (0.06) |
−0.509 (0.00) |
0.393 (0.29) |
0.425 (0.05) |
−0.055 (0.88) |
0.103 (0.64) |
0.173 (0.63) |
−0.024 (0.91) |
0.270 (0.48) |
0.282 (0.20) |
| Social Function | −0.605 (0.06) |
0.190 (0.39) |
−0.403 (0.25) |
0.194 (0.38) |
0.399 (0.18) |
−0.351 (0.05) |
0.491 (0.09) |
−0.094 (0.60) |
0.679 (0.04) |
0.441 (0.04) |
−0.608 (0.06) |
0.171 (0.44) |
0.238 (0.51) |
−0.043 (0.84) |
0.149 (0.70) |
0.423 (0.05) |
| Mental Health | −0.412 (0.24) |
−0.076 (0.73) |
−0.789 (0.01) |
−0.110 (0.62) |
0.318 (0.29) |
−0.136 (0.45) |
0.384 (0.19) |
−0.266 (0.13) |
0.508 (0.16) |
0.363 (0.10) |
−0.439 (0.20) |
0.195 (0.37) |
0.103 (0.78) |
−0.318 (0.14) |
0.355 (0.35) |
0.295 (0.18) |
| **SGRQ | ||||||||||||||||
| Symptoms | −0.491 (0.15) |
0.286 (0.19) |
0.447 (0.20) |
−0.033 (0.88) |
−0.427 (0.15) |
0.235 (0.19) |
−0.270 (0.37) |
0.332 (0.06) |
0.199 (0.61) |
−0.634 (0.00) |
−0.618 (0.06) |
−0.110 (0.62) |
0.104 (0.78) |
0.280 (0.20) |
−0.330 (0.39) |
−0.291 (0.19) |
| Activity | −0.723 (0.02) |
0.236 (0.29) |
0.088 (0.81) |
0.078 (0.73) |
−0.182 (0.55) |
−0.004 (0.98) |
−0.294 (0.33) |
0.256 (0.16) |
0.163 (0.68) |
−0.313 (0.17) |
−0.448 (0.19) |
−0.113 (0.62) |
0.083 (0.82) |
0.398 (0.07) |
0.035 (0.93) |
−0.032 (0.89) |
| Impacts | −0.493 (0.15) |
0.167 (0.44) |
0.407 (0.24) |
−0.013 (0.95) |
−0.253 (0.41) |
0.133 (0.46) |
−0.283 (0.35) |
0.282 (0.11) |
−0.007 (0.98) |
−0.463 (0.03) |
−0.415 (0.23) |
−0.156 (0.48) |
−0.111 (0.76) |
0.235 (0.28) |
0.103 (0.79) |
−0.209 (0.35) |
| Total | −0.608 (0.06) |
0.219 (0.33) |
0.351 (0.32) |
0.005 (0.98) |
−0.273 (0.37) |
0.144 (0.44) |
−0.307 (0.31) |
0.350 (0.05) |
0.075 (0.85) |
−0.450 (0.04) |
−0.493 (0.15) |
−0.151 (0.50) |
−0.027 (0.94) |
0.333 (0.13) |
0.035 (0.93) |
−0.176 (0.45) |
The bolded cells represent correlations that were significant to a p-value < 0.05. For the SF-36, higher scores indicate better functioning. Therefore, negative correlations between domains of the SF-36 and IL-8 reflect higher IL-8 levels found in patients with lower SF-36 scores, or poor functioning. For the SGRQ, on the other hand, higher scores indicate worse impairment. Therefore, negative correlation correlations between domains of SGRQ and SDP reflect higher SDP levels found in patients with lower SGRQ scores, or better functioning.
Corr denotes the correlation coefficient and (p), p-value.
SF-36 denotes the Medical Outcomes SF-36 questionnaire and SGRQ, the St. George’s Respiratory Questionnaire.
IL-6 denotes interleukin-6, IL-8 interleukin 8, CRP c-reactive protein, SPD surfactant associated protein D, TNF-αtumor necrosis factor alpha, MMP matrix metalloproteinase, CC16 clara cell protein.
Discussion
This prospective study of patients with COPD provides two novel findings. First, in functionally limited COPD patients who walk less than 350m during the 6MWD test, the physical domains of the SF-36 and SGRQ were significantly associated with 6MWD and constituted the only important difference in HRQL compared with patients that walked more than 350m. Second, inflammatory markers were not associated with HRQL domains of physical functioning and physical role suggesting that the physical differences between COPD patients who were able to walk less than 350m and their counterparts with better functional capacity may not be driven by systemic inflammation. However, the inflammatory marker IL-8 was associated with emotional, pain, vitality, and mental health in those with 6MWD less than 350m suggesting that inflammation may play a role in non-physical domains of well-being that impact performance on this functional field test.
The 6MWD is an excellent indicator of physical function and therapeutic response in patients with COPD31 and has gained wide acceptance given its simplicity and standardization.32 Specifically, the 6MWD been used effectively to evaluate the effect of pulmonary rehabilitation and changes in exercise capacity33. A recent systematic review from the European Respiratory and American Thoracic Society concluded that the 6MWD responsiveness was high, especially to interventions that included exercise training.34 This test has also been shown to provide prognostic information in COPD, as a more reliable predictor of mortality in patients completing rehabilitation35 than other tests such as spirometry.34 In spite of this strong evidence for the 6MWD as a robust test of functional capacity and prognosis, there are no studies evaluating the relationship between the distances walked by patients with COPD and biomarkers, using the 6MWD threshold that has been found to predict outcomes.
Two large studies and a systematic review from the ERS/ATS show that a 6MWD of 350m separates COPD patients at an increased risk for death and supports the existence of a curvilinear association between 6MWD and mortality, where the relationship is linear below that threshold.2,8,34 This asymptotic relationship may help explain the modest associations that have been observed between 6MWD as a continuous variable and both the SGRQ36,37 and SF-3615 scores. A prior study of the ECLIPSE cohort revealed significantly higher total SGRQ scores between COPD patients with a 6MWD less than 350m compared to their more functional counterparts regardless of GOLD stage.2 However, differences in the individual domains of the disease specific SGRQ and more generic HRQL measures have never been examined. When we examined the HRQL measures in this severely functionally limited group of COPD patients at increased risk of mortality, we found that both the SF-36 and SGRQ questionnaires accurately reflect the decrement in functional status. Indeed, the SGRQ activity domain and the SF-36 domain of physical functioning were significantly different between COPD patients who walked less than or greater than 350m suggesting that this difference in functional capacity is reflected similarly within both instruments.
In this study, we also explored the relationship between levels of biomarkers and HRQL measures in these two COPD patient populations (6MWD > and < 350m) in an effort to elucidate possible biological mechanisms behind the difference in HRQL outcomes. In prior studies of COPD patients, biomarkers such as serum IL-6, Il-16 and TNF alpha have been independently associated with 6MWD as a continuous variable and total SGRQ scores.7 IL-627, fibrinogen26 and CRP27,38 have also been associated with disease severity and poor outcomes. However, no studies to our knowledge have examined the relationship between HRQL measures and serum biomarker levels in these functionally distinct groups.
In our study, there were no associations between any biomarkers and the SF-36 domains of physical functioning and physical role in either group suggesting that a systemic inflammatory process may not be contributing directly to physical and functional limitations. However, in COPD patients who walked less than 350m, the IL-8 level was inversely associated with SF-36 domains of emotional role, bodily pain, general pain, vitality and mental health. Prior literature does support the association of inflammation with psychological changes such as depression in COPD, although few have examined IL-839,40,41,4. Given the absence of elevation in other inflammatory markers in this population, IL-8 may play a more important role in psychological limitations and perceptions of pain than previously recognized. The association between higher levels of SP-D and HRQL in patients who walked more than 350m reflects this marker’s association with fewer COPD exacerbations as observed in the large ECLIPSE cohort of COPD patients.42
This study has several limitations including a limited sample size. Therefore, some findings lacking statistical significance may have been due to inadequate statistical power. Replication with a larger cohort would help solidify these findings. Second, the results are correlative in nature, limiting our ability to define causality. Interventional strategies in randomized trials evaluating the multiple domains here analyzed will yield more mechanistic insights. Third, we recognize that, as is commonly the case in health status research, we measured multiple functional domains and thus we used multiple comparisons in our statistical approach. As such, some of our significant findings may be due to chance alone. However, the results are not only biologically plausible, but also clinically meaningful and can serve to generate further research in this field. We certainly encourage further efforts to define predictors of various patient-centered outcomes and their relationship to biological processes.
In summary, this study shows that in functionally limited COPD patients who walk less than 350m during the 6MWD test, the physical domains of the SF-36 and SGRQ are important measures of HRQL that reflect the relationship between functional status and HRLQ. Also, the lack of association between inflammatory markers and the HRQL domains related to physical functioning and physical role suggest that the functional difference between COPD patients who were able to walk less than 350m and their counterparts with better functional capacity may not driven by systemic inflammation. However, IL-8 is inversely correlated to scores in non-physical SF-36 domains of emotional, pain, vitality and mental health in those that walk less than 350m suggesting IL-8 may play a more important role in psychological limitations and perceptions of pain than previously recognized. There is a need for more studies relating patient centered outcomes with potential biological mechanisms.
Acknowledgements
Funding/Support: Support for this research was provided by an unrestricted grant from GalaxoSmithKline.
Atul Malhotra, MD has clinical and/or research income from NIH, AHA, Philips, Pfizer, SGS, Apnex, Apnicure but has relinquished all outside personal income since May 2012.
Aili Lazaar, MD is an employee and shareholder of GlaxoSmithKline.
Aiden Flynn, PhD is a shareholder of GlaxoSmithKline.
Ruth Tal-Singer, PhD is an employee and shareholder of GlaxoSmithKline.
Reynold Panettieri, MD is a consultant for AstraZeneca and Johnson & Johnson. He has research grants from the National Institutes of Health, Merck and Roche.
Bartolome Celli, MD. Dr. Celli has participated in advisory boards for GlaxoSmithKline, Boehringer Ingleheim, Forrest, Almirall, Astra Zeneca, Sunovion, and Rox therapeutics. He has received grants from NIH, Astra Zeneca and GlaxoSmithKline.
Role of Sponsors: This study was funded with an unrestricted grant provided by GlaxoSmithKline.
Footnotes
Author contributions: Dr. Celli had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr. Kohli: contributed to the data interpretation and analysis, and writing and revision of the manuscript.
Dr. Pinto-Plata: contributed to the study concept and design, data collection, data analysis and revision of the manuscript.
Dr. Divo: contributed to data collection, data analysis and revision of the manuscript.
Dr. Malhotra: contributed to the data interpretation and writing and revision of the manuscript.
Dr. Harris: contributed to the data interpretation and writing and revision of the manuscript.
Dr. Lazaar: contributed to the study concept and design, data analysis and revision of the manuscript.
Dr. Flynn: contributed to the data analysis and writing and revision of the manuscript.
Dr. Tal-Singer: contributed to the study concept and design, data analysis and revision of the manuscript.
Dr. Panetierri: contributed to the study concept and design, data collection and revision of the manuscript.
Dr. Celli: contributed to the study concept and design, data collection and analysis, and writing and revision of the manuscript.
Other Contributions:
Jane Gilbert contributed to the data analysis of the manuscript.
Financial/nonfinancial disclosures:
Puja Kohli, MD has no conflicts to disclose.
Victor Pinto-Plata, MD has no conflicts to disclose.
Miguel Divo, MD has no conflicts to disclose.
Scott Harris, MD has no conflicts to disclose.
References
- 1.Pauwels RA, Buist AS, Calverley PM, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 2.Spruit MA, Watkins ML, Edwards LD, et al. Determinants of poor 6-min walking distance in patients with COPD: the ECLIPSE cohort. Respir Med. 2010;104:849–857. doi: 10.1016/j.rmed.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Eisner MD, Blanc PD, Yelin EH, et al. COPD as a systemic disease: impact on physical functional limitations. Am J Med. 2008;121:789–796. doi: 10.1016/j.amjmed.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-shair K, Kolsum U, Dockry R, et al. Biomarkers of systemic inflammation and depression and fatigue in moderate clinically stable COPD. Respir Res. 2011;12:3. doi: 10.1186/1465-9921-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fabbri LM, Luppi F, Beghe B, et al. Complex chronic comorbidities of COPD. Eur Respir J. 2008;31:204–212. doi: 10.1183/09031936.00114307. [DOI] [PubMed] [Google Scholar]
- 6.Gan WQ, Man SF, Senthilselvan A, et al. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59:574–580. doi: 10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinto-Plata V, Casanova C, Mullerova H, et al. Inflammatory and repair serum biomarker pattern: association to clinical outcomes in COPD. Respir Res. 2012;13:71. doi: 10.1186/1465-9921-13-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cote CG, Pinto-Plata V, Kasprzyk K, et al. The 6-min walk distance, peak oxygen uptake, and mortality in COPD. Chest. 2007;132:1778–1785. doi: 10.1378/chest.07-2050. [DOI] [PubMed] [Google Scholar]
- 9.Pinto-Plata VM, Cote C, Cabral H, et al. The 6-min walk distance: change over time and value as a predictor of survival in severe COPD. Eur Respir J. 2004;23:28–33. doi: 10.1183/09031936.03.00034603. [DOI] [PubMed] [Google Scholar]
- 10.Casanova C, Cote C, Marin JM, et al. Distance and oxygen desaturation during the 6-min walk test as predictors of long-term mortality in patients with COPD. Chest. 2008;134:746–752. doi: 10.1378/chest.08-0520. [DOI] [PubMed] [Google Scholar]
- 11.Agusti A, Calverley PM, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11:122. doi: 10.1186/1465-9921-11-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spruit MA, Polkey MI, Celli B, et al. Predicting outcomes from 6-minute walk distance in chronic obstructive pulmonary disease. J Am Med Dir Assoc. 2012;13:291–297. doi: 10.1016/j.jamda.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Cote CG, Casanova C, Marin JM, et al. Validation and comparison of reference equations for the 6-min walk distance test. Eur Respir J. 2008;31:571–578. doi: 10.1183/09031936.00104507. [DOI] [PubMed] [Google Scholar]
- 14.Jones PW, Quirk FH, Baveystock CM, et al. A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. Am Rev Respir Dis. 1992;145:1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 15.Boueri FM, Bucher-Bartelson BL, Glenn KA, et al. Quality of life measured with a generic instrument (Short Form-36) improves following pulmonary rehabilitation in patients with COPD. Chest. 2001;119:77–84. doi: 10.1378/chest.119.1.77. [DOI] [PubMed] [Google Scholar]
- 16.Tashkin DP, Celli BR, Decramer M, et al. Efficacy of tiotropium in COPD patients with FEV1 >/= 60% participating in the UPLIFT(R) trial. COPD. 2012;9:289–296. doi: 10.3109/15412555.2012.656211. [DOI] [PubMed] [Google Scholar]
- 17.Polkey MI, Spruit MA, Edwards LD, et al. Six-minute-walk test in chronic obstructive pulmonary disease: minimal clinically important difference for death or hospitalization. Am J Respir Crit Care Med. 2013;187:382–386. doi: 10.1164/rccm.201209-1596OC. [DOI] [PubMed] [Google Scholar]
- 18.Domingo-Salvany A, Lamarca R, Ferrer M, et al. Health-related quality of life and mortality in male patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:680–685. doi: 10.1164/rccm.2112043. [DOI] [PubMed] [Google Scholar]
- 19.Casaburi R, Celli B, Crapo J, et al. The COPD Biomarker Qualification Consortium (CBQC) COPD. 2013;10:367–377. doi: 10.3109/15412555.2012.752807. [DOI] [PubMed] [Google Scholar]
- 20.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 21.Reardon JZ, Lareau SC, ZuWallack R. Functional status and quality of life in chronic obstructive pulmonary disease. Am J Med. 2006;119:32–37. doi: 10.1016/j.amjmed.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 23.Ross RM. ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:1451. doi: 10.1164/ajrccm.167.10.950. author reply 1451. [DOI] [PubMed] [Google Scholar]
- 24.ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 25.Broekhuizen R, Wouters EF, Creutzberg EC, et al. Raised CRP levels mark metabolic and functional impairment in advanced COPD. Thorax. 2006;61:17–22. doi: 10.1136/thx.2005.041996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mannino DM, Valvi D, Mullerova H, et al. Fibrinogen, COPD and mortality in a nationally representative U.S. cohort. COPD. 2012;9:359–366. doi: 10.3109/15412555.2012.668249. [DOI] [PubMed] [Google Scholar]
- 27.Ferrari R, Tanni SE, Caram LM, et al. Three-year follow-up of Interleukin 6 and C-reactive protein in chronic obstructive pulmonary disease. Respir Res. 2013;14:24. doi: 10.1186/1465-9921-14-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sin DD, Leung R, Gan WQ, et al. Circulating surfactant protein D as a potential lung-specific biomarker of health outcomes in COPD: a pilot study. BMC Pulm Med. 2007;7:13. doi: 10.1186/1471-2466-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Churg A, Zhou S, Wright JL. Series "matrix metalloproteinases in lung health and disease": Matrix metalloproteinases in COPD. Eur Respir J. 2012;39:197–209. doi: 10.1183/09031936.00121611. [DOI] [PubMed] [Google Scholar]
- 30.Lomas DA, Silverman EK, Edwards LD, et al. Evaluation of serum CC-16 as a biomarker for COPD in the ECLIPSE cohort. Thorax. 2008;63:1058–1063. doi: 10.1136/thx.2008.102574. [DOI] [PubMed] [Google Scholar]
- 31.Enright PL. The six-minute walk test. Respir Care. 2003;48:783–785. [PubMed] [Google Scholar]
- 32.Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44:1428–1446. doi: 10.1183/09031936.00150314. [DOI] [PubMed] [Google Scholar]
- 33.Puhan M, Scharplatz M, Troosters T, et al. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2009:CD005305. doi: 10.1002/14651858.CD005305.pub2. [DOI] [PubMed] [Google Scholar]
- 34.Singh SJ, Puhan MA, Andrianopoulos V, et al. An official systematic review of the European Respiratory Society/American Thoracic Society: measurement properties of field walking tests in chronic respiratory disease. Eur Respir J. 2014;44:1447–1478. doi: 10.1183/09031936.00150414. [DOI] [PubMed] [Google Scholar]
- 35.Gerardi DA, Lovett L, Benoit-Connors ML, et al. Variables related to increased mortality following out-patient pulmonary rehabilitation. Eur Respir J. 1996;9:431–435. doi: 10.1183/09031936.96.09030431. [DOI] [PubMed] [Google Scholar]
- 36.Brown CD, Benditt JO, Sciurba FC, et al. Exercise testing in severe emphysema: association with quality of life and lung function. COPD. 2008;5:117–124. doi: 10.1080/15412550801941265. [DOI] [PubMed] [Google Scholar]
- 37.Lacasse Y, Goldstein R, Lasserson TJ, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006:CD003793. doi: 10.1002/14651858.CD003793.pub2. [DOI] [PubMed] [Google Scholar]
- 38.Celli BR, Locantore N, Yates J, et al. Inflammatory biomarkers improve clinical prediction of mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185:1065–1072. doi: 10.1164/rccm.201110-1792OC. [DOI] [PubMed] [Google Scholar]
- 39.Sethi S, Mahler DA, Marcus P, et al. Inflammation in COPD: implications for management. Am J Med. 2012;125:1162–1170. doi: 10.1016/j.amjmed.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 40.Eagan TM, Ueland T, Wagner PD, et al. Systemic inflammatory markers in COPD: results from the Bergen COPD Cohort Study. Eur Respir J. 2010;35:540–548. doi: 10.1183/09031936.00088209. [DOI] [PubMed] [Google Scholar]
- 41.Lu Y, Feng L, Nyunt MS, et al. Systemic inflammation, depression and obstructive pulmonary function: a population-based study. Respir Res. 2013;14:53. doi: 10.1186/1465-9921-14-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lomas DA, Silverman EK, Edwards LD, et al. Serum surfactant protein D is steroid sensitive and associated with exacerbations of COPD. Eur Respir J. 2009;34:95–102. doi: 10.1183/09031936.00156508. [DOI] [PubMed] [Google Scholar]


