Abstract
Background
Children with congenital heart disease (CHD) are at risk for developmental delay (DD). This study sought to identify early risk factors for abnormal developmental trajectories in children with CHD.
Methods and Results
Children with CHD at high risk for DD, without known genetic abnormality, and with ≥3 assessments using the Bayley Scales of Infant Development-III (BSID-III) were studied. Logistic regression was used to assess the impact of patient and clinical factors on cognitive, language, and motor score trajectories; classified as: “Average or Improved” if all scores were ≥ 85 (< 1SD below mean) or increased to ≥ 85 and never decreased; or “Abnormal” if all scores were < 85, fell to < 85 and never improved, or fluctuated above and below 85. Data on 131 children with 527 BSID-III assessments were analyzed. Subject age was 5.5–37.4 months. Overall, 56% had cognitive, language, and motor development in the average range. Delays occurred in single domains in 23%. Multiple domains were delayed in 21%. More cardiac surgeries, longer hospital stay, poorer linear growth, and tube feeding were associated with worse outcomes in all domains (p<0.05). In the multivariable model, need for tube feeding was a risk factor for having an abnormal developmental trajectory (OR = 5.1–7.9). Minority race and lack of private insurance had significant relationships with individual domains.
Conclusions
Longitudinal developmental surveillance identified early factors that can help quantify risk of DD over time. Strategies to improve modifiable factors and early therapeutic intervention can be targeted to children at highest risk.
Keywords: cardiac surgery, pediatrics, congenital cardiac defect, congenital heart disease, neuropediatrics, neurodevelopment, outcomes research
Background
It is well-established that children with congenital heart disease (CHD) experience a higher prevalence of developmental delays and disabilities in early childhood than typically developing healthy children.1,2 This has been demonstrated in children with isolated lesions that have been anatomically corrected such as transposition of the great arteries,3,4 children with palliated single ventricle heart disease,5–8 as well as in heterogenous groups of children with CHD that required surgery in infancy.9–11
Severe global developmental delay is relatively uncommon; however, a characteristic pattern of mild or combined disabilities across multiple domains has emerged.1,12,13 The range of developmental outcomes for children with CHD varies widely; similar to the range of severity of lesions in CHD. To date, no single diagnostic or treatment characteristic has been found to reliably predict developmental outcomes. Despite intense study, patient and treatment related factors, such as diagnosis, birth history, and perioperative events, typically account for < 40% of the variance in developmental outcomes in children with CHD.2,6,8,9
Children with CHD experience multiple sources of developmental risk including genetic abnormalities,14 prenatal alterations in cerebral blood flow and brain maturation,15,16 the impact of early diagnosis and surgery,17,18 perioperative risk related to low cardiac output,19 and long-term issues that are associated with physiology, socioeconomic status, and parenting practices.20–22 The American Heart Association (AHA) and the American Academy of Pediatrics (AAP) have recommended systematic surveillance, evaluation and management of developmental outcomes in children with CHD throughout childhood to promote early detection of delays and to optimize long-term outcomes.1
It is not known how specific patient and clinical factors impact the patterns of development in early childhood. Individual children with CHD may display different patterns of developmental competencies over time.11,12 The aim of this study was to examine factors that contribute to developmental trajectories in cognitive, language, and motor skills over the first 3 years of life in a cohort of children with CHD who were being systematically evaluated and referred for early intervention services.
Methods
Patient Population
Children with CHD believed to be at high risk for developmental delay as defined by the AHA/AAP guideline,1 were recruited from the Herma Heart Center Developmental Follow-up Clinic (HHCDC) at Children’s Hospital of Wisconsin (CHW). Eligibility criteria and operation of the HHCDC have been previously described.12,23 Families were invited to participate in systematic developmental follow-up as part of routine clinical care. HHCDC visits were scheduled to occur approximately every 6 months during the first 3 years of life. Referrals for early intervention services (Birth to 3 in the state of Wisconsin) were made for all children who required cardiac surgery at < 1 year of age and for those who scored > 1 SD below the mean (composite score < 85) on cognitive, language, or motor assessment. To be eligible for this study, children had undergone formal developmental evaluation of cognitive, language, and motor skills using the Bayley Scales of Infant Development, Third Edition (BSID-III)24 a minimum of 3 and up to 5 times during the first 3 years of life. Parents provided informed consent to have their child’s data included in a databank approved by the institutional review board at CHW. No subjects were excluded based on race, language, or other coexisting medical condition. In order to focus specifically on structural heart disease, subjects were excluded from this analysis if they had a clinically diagnosed genetic abnormality or if their primary diagnosis was cardiomyopathy (Figure 1).
Figure 1.
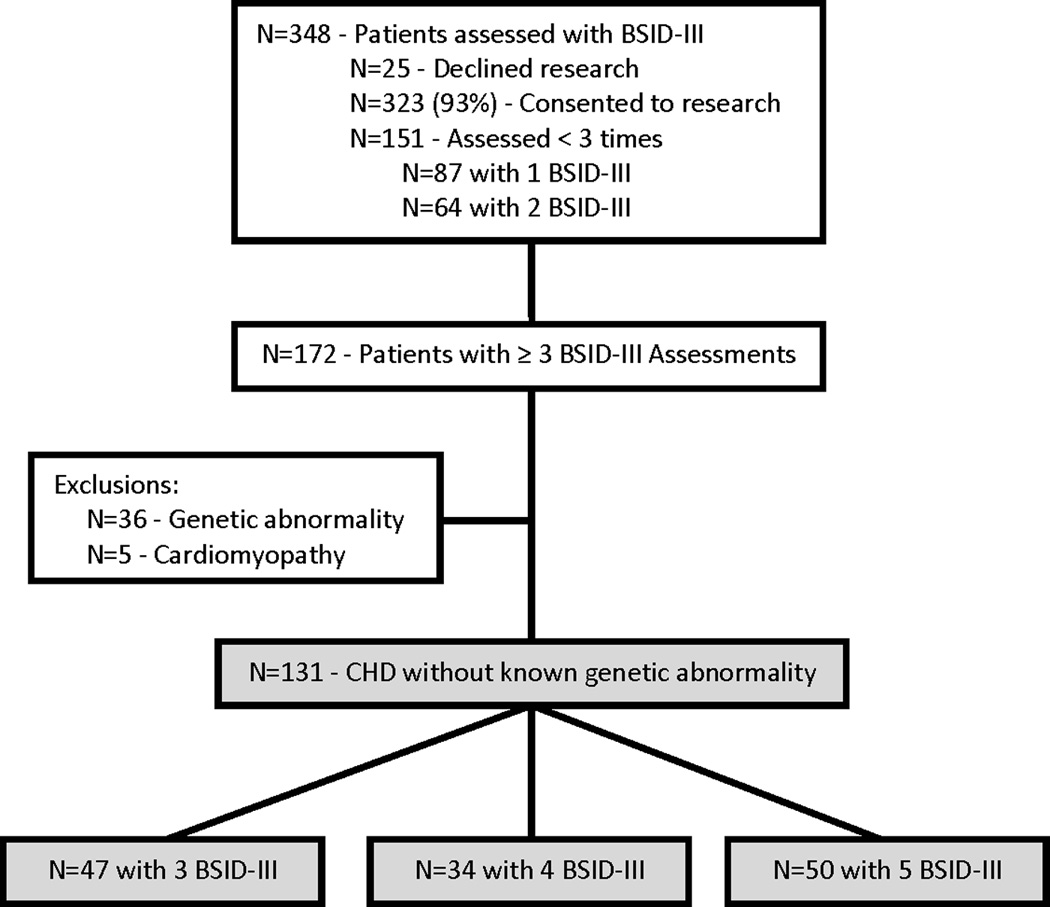
Herma Heart Center Developmental Follow-up Clinic (HHCDC) patients. Shaded boxes identify current study cohort.
Statistical Analysis
Sample characteristics and clinical variables are presented as medians with interquartile range (IQR) for continuous data and frequencies (%) for categorical data. Cognitive, language, and motor composite scores on the BSID-III were compared to the population mean of 100, standard deviation (SD) 15. Univariate and multivariable logistic regression analyses were used to assess the impact of patient and clinical factors on the cognitive, language, and motor composite score trajectories for each patient. Trajectories in each domain were classified as “Average or Improved” if all scores were ≥ 85 (< 1 SD below the population mean), or scores increased to ≥ 85 and never decreased. Trajectories were classified as “Abnormal” if all scores were < 85, if scores fell to < 85 and never improved, or if scores fluctuated above and below 85 over time. P < 0.05 was considered significant. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina) software.
Results
From January 2007 through March 2014, 131 subjects with CHD completed a total of 527 BSID-III assessments, which are included in this analysis (Figure 1). Median age at first BSID-III assessment was 7.5 months, IQR 6.6–8.6. Age at evaluation ranged from 5.5 to 37.4 months. The median time interval between BSID-III assessments was 6.0 months, IQR 6.0–6.4. The subjects represented a wide spectrum of CHD, but all were considered to be at risk of developmental delay as defined by the AHA/AAP guideline.1 Anatomy was classified according to the child’s fundamental diagnosis at birth. The sample was further classified as to whether the child’s anatomy resulted in achievement of a two ventricle repair or a single functional ventricle and whether or not aortic arch obstruction was present. These categories have been previously established as representing increasing complexity.25 Aortic arch obstruction is associated with altered fetal cerebral blood flow and may therefore have an impact on neurodevelopmental outcomes.26 Thirty-eight percent of the subjects had anatomy that required surgical palliation resulting in a single functional ventricle. Of these, 2 subjects with hypoplastic left heart syndrome underwent orthotopic heart transplantation during the study period. No subjects with two ventricle anatomy underwent transplantation. Thirty-two percent (n = 42) of the subjects had a known medical comorbidity in addition to their CHD involving the following systems: airway issues (n = 14), chronic lung disease (n = 5), neurologic or neuromuscular (n = 6), orthopedic (n = 5), gastrointestinal (n = 3), hearing loss (n = 3), and multisystem (n = 6). Characteristics of the sample are presented in Table 1a. Table 1b presents detailed anatomic diagnostic information for the subjects using the fundamental diagnosis assigned in the Society of Thoracic Surgeons (STS) Congenital Heart Surgery Database.
Table 1a.
Sample Demographics N = 131,
| Gender | Male = 57% | |
| Race/ethnicity | White = 65%, Black = 9%, Hispanic = 16%, Other = 10% | |
| Maternal education | Post-High School = 76% | |
| Insurance | Private = 56% | |
| Prenatal diagnosis | Yes = 59% | |
| Premature: GA < 37 wks | Yes = 15% | |
| Comorbidities | Other medical = 32% | |
| Anatomy | Two Ventricle = 81 (62%) Without AAO = 68 (52%) With AAO = 13 (10%) |
Single Ventricle = 50 (38%) Without AAO = 16 (12%) With AAO = 34 (26%) |
GA indicates gestational age.
AAO indicates aortic arch obstruction.
Table 1b.
Fundamental Diagnoses.
| Two Ventricle Congenital Heart Defects | N = 81 |
| Transposition of the Great Arteries with intact ventricular septum | 12 |
| Transposition of the Great Arteries with ventricular septal defect | 6 |
| Tetralogy of Fallot | 11 |
| Ventricular septal defect | 7 |
| Double outlet right ventricle | 7 |
| Pulmonary atresia + ventricular septal defect | 6 |
| Aortic arch hypoplasia or coarctation | 6 |
| Aortic valve stenosis | 5 |
| Total anomalous pulmonary venous connection | 4 |
| Atrioventricular septal defect | 3 |
| Ebstein's malformation of tricuspid valve | 3 |
| Double aortic arch | 2 |
| Interrupted aortic arch | 2 |
| Mitral valve stenosis | 2 |
| Pulmonary valve stenosis | 2 |
| Truncus arteriosus | 1 |
| Congenitally corrected transposition of the great arteries with ventricular septal defect | 1 |
| Aortico-left ventricular tunnel | 1 |
| Single Ventricle Congenital Heart Defects | N = 50 |
| Hypoplastic left heart syndrome | 24 |
| Double outlet right ventricle | 7 |
| Tricuspid atresia | 7 |
| Double inlet left ventricle | 5 |
| Pulmonary atresia + intact ventricular septum | 3 |
| Single ventricle, Heterotaxia syndrome | 3 |
| Unbalanced atrioventricular septal defect | 1 |
Subject and treatment characteristics at the time of first BSID-III assessment are presented in Table 2. For variables with the potential to change over time, i.e. cumulative length of hospitalization, the value at the time of the subject’s first BSID-III assessment was used in all statistical analyses. The majority of subjects, 115/131 (88%) had undergone at least one open heart surgery and 49/131 (37%) had undergone deep hypothermic circulatory arrest (DHCA) prior to their first BSID-III assessment. There were no differences in cognitive, language or motor outcomes for the group that did have cardiac surgery with CPB prior to their first developmental assessment compared to those who did not undergo CPB. One quarter of the subjects required some nutritional support with tube feeding ranging from complete tube feedings to supplementation of oral intake to reach a targeted daily volume. Just under half of the subjects were actively enrolled in early intervention services at the time of their first BSID-III assessment.
Table 2.
Subject and Treatment Characteristics.
| Median | 25th–75th percentile | |
|---|---|---|
| Age at first open heart, days | 14 | 7–97 |
| Total # open & closed heart operations*† | 1 | 1–2 |
| STS risk category* | 4 | 3–5 |
| Length of hospitalization, days* | 38 | 24–65 |
| CPB time, minutes* | 196 | 127–252 |
| DHCA time, minutes*‡ | 13 | 9–19 |
| Feeding at 1st discharge | All Oral = 75% | Supplemental Tube Feeds = 25% |
| Feeding at 1st BSID-III assessment | All Oral = 75% | Supplemental Tube Feeds = 25% |
| Enrolled in Birth to 3* | Yes = 47% | No = 53% |
| Birth weight percentile | 42 | 13–69 |
| Birth height percentile | 55 | 15–80 |
| Weight percentile* | 32 | 8–52 |
| Height percentile* | 28 | 8–63 |
| Head circumference percentile* | 40 | 20–66 |
| Arterial oxygen saturation* | 96 | 84–99 |
BSID-III indicates Bayley Scales of Infant and Toddler Development®; CPB, cardiopulmonary bypass; DHCA, deep hypothermic circulatory arrest; STS, Society of Thoracic Surgeons.
At 1st BSID-III assessment.
9 subjects never had open heart surgery.
49 subjects had DHCA.
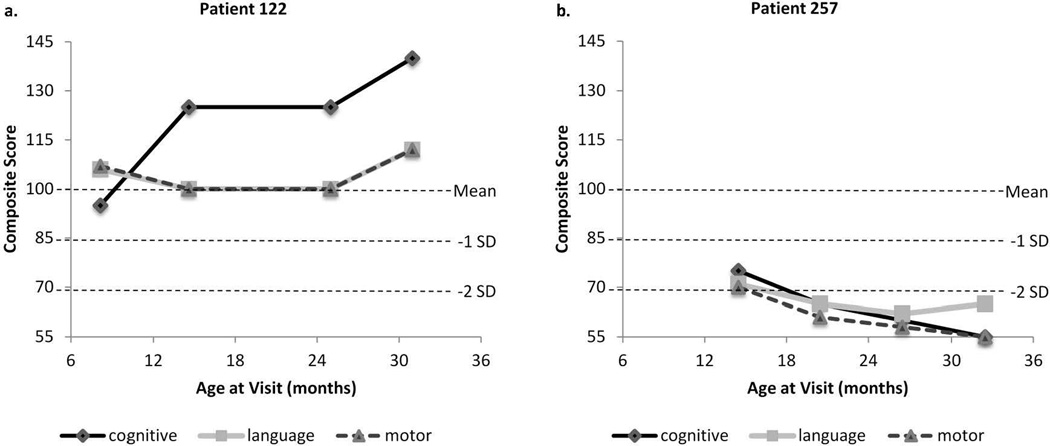
The majority of subjects had average or improving developmental trajectories: cognitive, 104/131 (79%); language, 89/131 (68%); and motor, 102/131 (78%). A smaller portion had developmental trajectories that were significantly below average or declined over time: cognitive, 16/131 (12%); language, 28/131 (21%); and motor, 15/131 (11%). Scores fluctuated above and below -1 SD below the mean in others: cognitive, 11/131 (8%); language, 14/131 (11%); and motor, 14/131 (11%). Overall, 56% of subjects had all trajectories for cognitive, language, and motor development in the average range (Figure 2). Trajectories were abnormal in single domains in 23%; cognitive, 4%, language, 13%, and motor, 6%. Twenty-one percent of the subjects had multiple domains delayed. Two example subject trajectories are shown in Figure 3. Both children were born with hypoplastic left heart syndrome, were non-Hispanic white, had undergone 2 cardiac surgeries prior to their first BSID-III assessment, and had prolonged hospitalizations. They differed on feeding status, insurance, and the occurrence of postoperative seizures. As the graphs demonstrate, their developmental trajectories were profoundly different.
Figure 2.
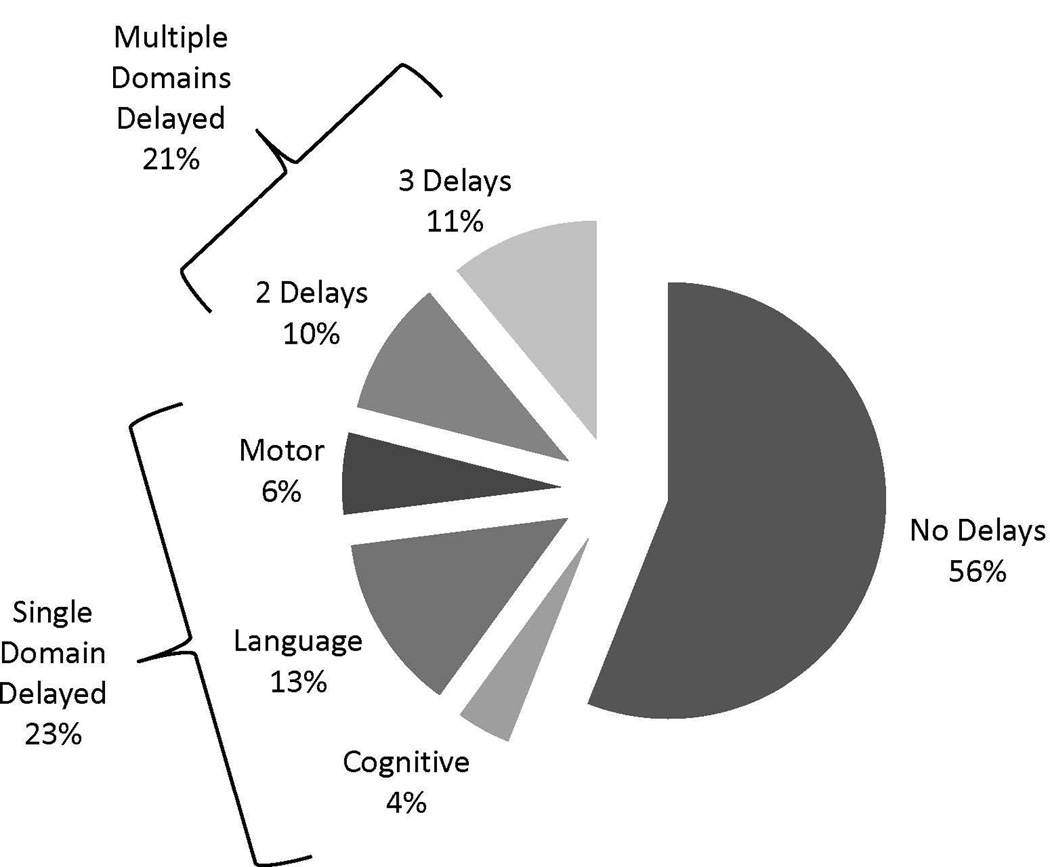
Patterns of development in cognitive, language and motor domains.
Figure 3.
Example subject trajectories. (a.) HLHS, female, non-Hispanic white, oral feeds, private insurance, total open + closed = 2, hospital length of stay = 73 days. (b.) HLHS, male, non-Hispanic white, tube feeds, public insurance, total open+closed = 2, hospital length of stay = 103 days, history of seizures.
Univariate logistic regression identified multiple patient and clinical factors associated with cognitive, language, and motor development (Table 3). An odds ratio (OR) > 1 represented an increased likelihood of having an abnormal developmental trajectory. A greater number of cardiac surgical procedures, longer hospital stay, poorer linear growth, and need for supplemental tube feeding were associated with worse outcomes in all areas of development. The presence of single ventricle anatomy was associated with higher odds of having an abnormal cognitive trajectory (OR = 2.5, 95% CI = 1.04–5.83, p < 0.05), but was not associated with language (OR = 1.4, 95% CI = 0.63–2.83, p > 0.05) or motor (OR = 1.4, 95% CI = 0.62–3.29, p > 0.05) development. The 34 patients with single ventricle anatomy and aortic arch obstruction (AAO) were compared to those without AAO and there were no differences in cognitive, language or motor outcomes. All of the patients with single ventricle anatomy and AAO had undergone either a Norwood procedure (n = 31) or a coarctation repair in the neonatal period followed by a Damus-Kaye-Stansel procedure (n = 3). There were also no differences in outcomes detected for patients with two ventricle anatomy with AAO compared to those without AAO. Of note, gender, prematurity (< 37 weeks gestational age), prenatal diagnosis, birth weight or height percentile, age at first open heart operation, highest Society of Thoracic Surgeons risk category of operation,27 weight, oxygen saturation, and minutes of DHCA at time of first BSID-III assessment were not significantly associated with any developmental domains.
Table 3.
Univariate Predictors of Abnormal Developmental Trajectories.
| Cognitive | Language | Motor | |
|---|---|---|---|
| Odds Ratio (95% Confidence Interval) | |||
| Non-Hispanic white vs. others | 0.23† (0.09–0.55) | 0.39‡ (0.18–0.84) | 0.49 (0.21–1.13) |
| Single ventricle anatomy | 2.57‡ (1.09–6.10) | 1.40 (0.66–2.97) | 1.24 (0.53–2.88) |
| Maternal education – ≤ HS vs. > HS | 3.06‡ (1.17–7.98) | 2.96‡ (1.23–7.12) | 0.56 (0.17–1.78) |
| Public or no Insurance vs. Private | 5.17§ (2.00–13.37) | 1.47 (0.70–3.08) | 1.53 (0.67–3.50) |
| Total # Open & Closed* operations | 1.79‡ (1.07–3.01) | 1.94‡ (1.16–3.24) | 2.04‡ (1.18–3.53) |
| Height %ile* | 0.98‡ (0.97–1.00) | 0.98‡ (0.97–1.00) | 0.98‡ (0.97–1.00) |
| Head circumference %ile* | 0.98‡ (0.97–1.00) | 0.99 (0.98–1.00) | 0.99 (0.97–1.00) |
| Other medical condition* vs. none | 2.92‡ (1.22–6.98) | 1.49 (0.69–3.23) | 1.15 (0.48–2.76) |
| Supplemental tube feeding* vs. none | 15.27§ (5.62–41.49) | 5.32§ (2.29–12.36) | 6.15§ (2.51–15.11) |
| Length of Hospital Stay (per 10 days)* | 1.32§ (1.16–1.49) | 1.21§ (1.08–1.34) | 1.18§ (1.07–1.31) |
| CPB time (per 60 minutes)* | 1.27‡ (1.06–1.52) | 1.20‡ (1.06–1.43) | 1.20 (1.00–1.35) |
BSID-III indicates Bayley Scales of Infant and Toddler Development®; CPB, cardiopulmonary bypass; HS, high school.
At 1st BSID-III assessment.
p < 0.01.
p < 0.05.
p < 0.001.
Predictors identified from the univariate analysis were used in a multivariable logistic regression model. Potential predictors (as noted at time of first BSID-III assessment) included race, anatomy (single ventricle vs. two ventricle), total number of open and closed cardiac surgeries, height percentile, head circumference percentile, presence of other medical conditions, feeding status, length of hospital stay, cardiopulmonary bypass (CPB) time, maternal education, and insurance status. A forward stepwise selection method was used and the final model included predictors that were significant or had p < 0.1. Need for supplemental tube feeding was highly correlated with length of hospital stay, CPB time and total number of surgeries (p < 0.01). As it was difficult to determine whether feeding status was an outcome or a predictor of developmental progress over time, models were created with and without feeding status at first BSID-III assessment. Results are presented in Table 4. When feeding status was included, the need for supplemental tube feeding was associated with 5.1–7.9 increased odds of having an abnormal developmental trajectory. When feeding status was excluded from the model, length of hospital stay became the dominant predictor of outcomes. Socioeconomic factors including race, insurance status, and maternal education also demonstrated significant relationships with individual domains.
Table 4.
Multivariable Predictors of Abnormal Developmental Trajectories.
| Odds Ratio (95% Confidence Interval) | |||
| Model 1: With Feeding Status at 1st BSID-III Assessment | |||
| Predictor | Cognitive | Language | Motor |
| Supplemental tube feeding* | 7.95† (2.14–29.53) | 5.23§ (2.21–12.42) | 5.09§ (2.01–12.87) |
| Public or no insurance | 5.39† (1.68–17.30) | NS | NS |
| White/non-Hispanic | NS | 0.40‡ (0.18–0.90) | NS |
| Model 2: Without Feeding Status at 1st BSID-III Assessment | |||
| Predictor | Cognitive | Language | Motor |
| Length of hospital stay (per 10 days)* | 1.31§ (1.15–1.49) | 1.20† (1.07–1.33) | 1.18§ (1.07–1.31) |
| Public or no insurance | 4.53† (1.55–13.28) | NS | NS |
| Maternal education HS or less | NS | 3.12‡ (1.22–7.98) | NS |
BSID-III indicates Bayley Scales of Infant and Toddler Development®; HS, high school; NS, not significant.
At 1st BSID-III assessment.
p < 0.01.
p < 0.05.
p < 0.001.
Discussion
Consistent with previous research on developmental outcomes for children with CHD in the modern era of pediatric cardiac surgery and neuroprotective strategies, our research has identified that developmental delays are both common and dynamic in this population.2,6,7,9–11 This study provides unique characterization of changes in developmental trajectories in multiple domains over time in a large sample treated with a consistent approach to developmental evaluation and support. This is a novel approach to longitudinal data using a person-centered method addressing patterns of development over time in individual children28 as opposed to a single time point or a change from one time point to another. Despite known risk due to the severity of their CHD, developmental outcomes for individual subjects varied widely. Over half of the sample demonstrated normal development in all domains but more than 1 in 5 children had delays in multiple domains. It was shown that children with similar clinical backgrounds can have very different developmental outcomes.
Several factors have consistently emerged as predictors of poorer developmental outcomes including longer duration of hospital stay, poorer linear growth, problems with feeding, and socioeconomic risks.1,4,6,8–12 Presence of any of these factors should alert clinicians to the need for systematic surveillance of development and the need for early intervention to minimize delays. However, despite intense scrutiny there has been no clear composite of patient and clinical factors which can be identified early in life that consistently predict development over time. This highlights the importance of incorporating evaluation and management of developmental outcomes into our pediatric cardiology and cardiovascular surgery programs. It should also guide our prenatal and postnatal counseling of families.
Parents should be educated that it cannot be assumed that if a child is doing well at one time point that they will continue to do well, as evidenced by the fact that 11–21% of patients in the present cohort had scores that declined over time. This supports the need for serial developmental assessments, as some children may not show any deficits until later in life and early results on the Bayley Scales of Infant Development are not highly predictive of school age performance.29 Formal evaluation of development can provide parents and clinicians with information and unique insight into each individual child’s strengths and weaknesses. The examination of patterns of development over time is a better predictor of later outcomes than single BSID scores or early risk factors alone.29
The need for supplemental tube feeding was once again found to be an important risk factor for abnormal development in cognitive, language and motor domains. The inability to achieve full oral feeding was multifactorial and was correlated with a more complex clinical course involving longer hospitalization, more CPB time, and a greater number of cardiac surgeries. Some infants did not feed due to the inability to protect their airway and risk of aspiration. Other infants did not have the stamina to consume the number of calories needed to sustain adequate growth. Further research is ongoing to improve our understanding of why feeding problems occur and what approach to feeding management over time can reduce long term feeding problems. Similar to previous work we found that height percentile at the time of BSID-III assessment was related to development but not weight percentile.8 Neither birth weight nor height percentile was significantly associated with developmental outcomes.
The effects of socioeconomic disadvantage have long been known to put child development at risk.30 In this cohort the presence of social risk factors including minority race, lack of private insurance, and lower maternal education emerged as significant risk factors for poorer outcomes in specific domains. These factors are typically not modifiable and tend to exert their influence more in the second and third year of life. Using the patterns of development over time as the primary outcome allowed detection of these risk factors. Presence of social risk factors should alert clinicians to the need for ongoing monitoring and support. Luby and colleagues30 found that more supportive parenting practices mediated the effect of poverty on child brain development. Interventions designed to provide guidance to parents and help them manage the stress of having a child with a chronic health condition may be beneficial. One study that targeted promotion of maternal coping and adjustment after the birth of an infant with CHD was able to demonstrate significantly lower maternal worry at 6 months and a statistically significant improvement in the mental development index (MDI) on the BSID-II in the infants of mothers who participated in the intervention.31
There are some important limitations to the current study. The cohort studied represents a single center experience and the findings may not be generalizable to the population of young children with CHD as a whole. Not all children who were eligible attended the developmental follow-up program and it is likely that parents of children with more obvious developmental delays were more motivated to participate. The most common reason parents cited for not participating was that they perceived their child was “doing well”. Nonattendees lived farther away from the treatment center and had less complex cardiac surgery; however, in most cases we do not know why families chose not to attend the program.
We excluded children with any clinically diagnosed genetic abnormality despite the fact that they make up approximately 20% of the CHD population. Further research is needed to understand the combined impact of CHD and genetic abnormalities on child development. We also excluded 5 children with cardiomyopathy as their clinical course was quite different than the children with structural heart disease.
We relied on parent-report of use of early intervention services, but we know very little about the quality or quantity of the services the children were receiving. This makes it challenging to measure the impact of early developmental surveillance and intervention on later outcomes. While some children demonstrated notable improvements over time (cognitive, 3%; language, 7.6%; and motor, 24%) it is impossible to know if these improvements would have occurred without regular surveillance. It has been repeatedly shown that children at known developmental risk do not necessarily receive the school-based support services mandated by the Individuals with Disabilities Education Act.32,33 Future studies should attempt to recruit age-matched subjects who did not participate in developmental follow-up to determine if differences can be detected between those who did and did not receive longitudinal assessments.
While it is too soon to speculate on the cost effectiveness and impact of developmental follow-up programs within our cardiac centers, the societal cost of early childhood developmental delay are enormous and have an impact across the lifespan. One recent study found that for every child with mental development greater than 1.5 standard deviations below the mean at less than 3 years of age, there was a cost of $34,532 due to increased preschool special education services.34 This cost will increase exponentially if needs for special education persist and if developmental delays have a negative impact on the potential for lifetime employment and earnings.
Conclusions
Developmental delays and disabilities are the most common and perhaps the most costly, long-term morbidity associated with congenital heart disease. Each year approximately 10,000 infants are born with CHD that will require surgical intervention putting them at higher risk for developmental delay as defined by the AHA/AAP guideline. Few studies have characterized factors that contribute to the patterns of development over time. In this study longitudinal developmental surveillance identified early factors that can help quantify risk of developmental delay over time. Strategies to improve modifiable factors and early therapeutic intervention can be targeted to children at highest risk. Research to understand and improve these outcomes is our ongoing obligation.
Acknowledgments
The authors would like to acknowledge the indispensable support of Mara Koffarnus, MA for scheduling, data management support, and manuscript preparation and Ann Chin, RN for her role as nurse clinician and lead for patient recruitment in the HHCDC research effort. In addition we thank all of the therapists that conducted the BSID-III assessments, as well as the students and research coordinators that supported data collection efforts. Finally, we are most grateful to the parents who have entrusted the care of their children to us and whose willingness to participate in research will help us to improve outcomes for future generations.
Funding Sources: This publication was supported in part by the National Center for Research Resources and the National Center for Advancing Translations Sciences, National Institutes of Health (NIH), through Grant Number 8UL1TR000055. Its contents are solely the responsibility of the authors and to not necessarily represent the official views of the NIH. Funded by the NIH.
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Marino BS, Lipkin PH, Newburger JW, Peacock G, Gerdes M, Gaynor JW, Mussatto KA, Uzark K, Goldberg CS, Johnson WH, Jr, Li J, Smith SE, Bellinger DC, Mahle WT American Heart Association Congenital Heart Defects Committee, Council on Cardiovascular Disease in the Young, Council on Cardiovascular Nursing, and Stroke Council. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation. 2012;126:1143–1172. doi: 10.1161/CIR.0b013e318265ee8a. [DOI] [PubMed] [Google Scholar]
- 2.Snookes SH, Gunn JK, Eldridge BJ, Donath SM, Hunt RW, Galea MP, Shekerdemian L. A systematic review of motor and cognitive outcomes after early surgery for congenital heart disease. Pediatrics. 2010;125:e818–e827. doi: 10.1542/peds.2009-1959. [DOI] [PubMed] [Google Scholar]
- 3.Bellinger DC, Newburger JW, Wypij D, Kuban KCK, duPlesssis AJ, Rappaport LA. Behaviour at eight years in children with surgically corrected transposition: The Boston Circulatory Arrest Trial. Cardiol Young. 2009;19:86–97. doi: 10.1017/S1047951108003454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellinger DC, Wypij D, Rivkin MJ, DeMaso DR, Robertson RL, Jr, Dunbar-Masterson C, Rappaport LA, Wernovsky G, Jonas RA, Newburger JW. Adolescents with d-transposition of the great arteries corrected with the arterial switch procedure: neuropsychological assessment and structural brain imaging. Circulation. 2011;124:1361–1369. doi: 10.1161/CIRCULATIONAHA.111.026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahle WT, Visconti KJ, Freier MC, Kanne SM, Hamilton WG, Sharkey AM, Chinnock RE, Jenkins KJ, Isquith PK, Burns TG, Jenkins PC. Relationship of surgical approach to neurodevelopmental outcomes in hypoplastic left heart syndrome. Pediatrics. 2006;117:e90–e97. doi: 10.1542/peds.2005-0575. [DOI] [PubMed] [Google Scholar]
- 6.Newburger JW, Sleeper LA, Bellinger DC, Goldberg CS, Tabbutt S, Lu M, Mussatto KA, Williams IA, Gustafson KE, Mital S, Pike N, Sood E, Mahle WT, Cooper DS, Dunbar-Masterson C, Krawczeski CD, Lewis A, Menon SC, Pemberton VL, Ravishankar C, Atz TW, Ohye RG, Gaynor JW Pediatric Heart Network I. Early developmental outcome in children with hypoplastic left heart syndrome and related anomalies: the single ventricle reconstruction trial. Circulation. 2012;125:2081–2091. doi: 10.1161/CIRCULATIONAHA.111.064113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldberg CS, Mussatto K, Licht D, Wernovsky G. Neurodevelopment and quality of life for children with hypoplastic left heart syndrome: current knowns and unknowns. Cardiol Young. 2011;21:88–92. doi: 10.1017/S104795111100165X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ravishankar C, Zak V, Williams IA, Bellinger DC, Gaynor JW, Ghanayem NS, Krawczeski CD, Licht DJ, Mahony L, Newburger JW, Pemberton VL, Williams RV, Sananes R, Cook AL, Atz T, Khaikin S, Hsu DT Pediatric Heart Network I. Association of impaired linear growth and worse neurodevelopmental outcome in infants with single ventricle physiology: a report from the pediatric heart network infant single ventricle trial. J Pediatr. 2013;162:250.e2–256.e2. doi: 10.1016/j.jpeds.2012.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tabbutt S, Gaynor JW, Newburger JW. Neurodevelopmental outcomes after congenital heart surgery and strategies for improvement. Curr Opin Cardiol. 2012;27:82–91. doi: 10.1097/HCO.0b013e328350197b. [DOI] [PubMed] [Google Scholar]
- 10.Gaynor JW, Gerdes M, Nord AS, Bernbaum J, Zackai E, Wernovsky G, Clancy RR, Heagerty PJ, Solot CB, McDonald-McGinn D, Jarvik GP. Is cardiac diagnosis a predictor of neurodevelopmental outcome after cardiac surgery in infancy? J Thorac Cardiovasc Surg. 2010;140:1230–1237. doi: 10.1016/j.jtcvs.2010.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sananes R, Manlhiot C, Kelly E, Hornberger LK, Williams WG, MacGregor D, Buncic R, McCrindle BW. Neurodevelopmental outcomes after open heart operations before 3 months of age. Ann Thorac Surg. 2012;93:1577–1583. doi: 10.1016/j.athoracsur.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Mussatto KA, Hoffmann RG, Hoffman GM, Tweddell JS, Bear L, Cao Y, Brosig C. Risk and prevalence of developmental delay in young children with congenital heart disease. Pediatrics. 2014;133:e570–e577. doi: 10.1542/peds.2013-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wernovsky G. Current insights regarding neurological and developmental abnormalities in children and young adults with complex congenital cardiac disease. Cardiol Young. 2006;16:92–104. doi: 10.1017/S1047951105002398. [DOI] [PubMed] [Google Scholar]
- 14.Fahed AC, Gelb BD, Seidman JG, Seidman CE. Genetics of congenital heart disease: the glass half empty. Circ Res. 2013;112:707–720. doi: 10.1161/CIRCRESAHA.112.300853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Limperopoulos C, Tworetzky W, McElhinney DB, Newburger JW, Brown DW, Robertson RL, Jr, Guizard N, McGrath E, Geva J, Annese D, Dunbar-Masterson C, Trainor B, Laussen PC, du Plessis AJ. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation. 2010;121:26–33. doi: 10.1161/CIRCULATIONAHA.109.865568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Licht DJ, Shera DM, Clancy RR, Wernovsky G, Montenegro LM, Nicolson SC, Zimmerman RA, Spray TL, Gaynor JW, Vossough A. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg. 2009;137:529–536. doi: 10.1016/j.jtcvs.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuller S, Rajagopalan R, Jarvik GP, Gerdes M, Bernbaum J, Wernovsky G, Clancy RR, Solot C, Nicolson SC, Spray TL, Gaynor JW. J. Maxwell Chamberlain Memorial Paper for congenital heart surgery. Deep hypothermic circulatory arrest does not impair neurodevelopmental outcome in school-age children after infant cardiac surgery. Ann Thorac Surg. 2010;90:1985–1994. doi: 10.1016/j.athoracsur.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahle WT, Clancy RR, McGaurn SP, Goin JE, Clark BJ. Impact of prenatal diagnosis on survival and early neurologic morbidity in neonates with the hypoplastic left heart syndrome. Pediatrics. 2001;107:1277–1282. doi: 10.1542/peds.107.6.1277. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman GM, Brosig CL, Mussatto KA, Tweddell JS, Ghanayem NS. Perioperative cerebral oxygen saturation in neonates with hypoplastic left heart syndrome and childhood neurodevelopmental outcome. J Thorac Cardiovasc Surg. 2013;146:1153–1164. doi: 10.1016/j.jtcvs.2012.12.060. [DOI] [PubMed] [Google Scholar]
- 20.Bellinger DC, Newberger JW. Neuropsychological, psychosocial, and quality-of-life outcomes in children and adolescents with congenital heart disease. Prog Pediatr Cardiol. 2010;29:87–92. [Google Scholar]
- 21.Cassedy A, Drotar D, Ittenbach R, Hottinger S, Wray J, Wernovsky G, Newburger JW, Mahony L, Mussatto K, Cohen MI, Marino BS. The impact of socio-economic status on health related quality of life for children and adolescents with heart disease. Health Qual Life Outcomes. 2013;11:99. doi: 10.1186/1477-7525-11-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rempel GR, Harrison MJ, Williamson DL. Is "treat your child normally" helpful advice for parents of survivors of treatment of hypoplastic left heart syndrome? Cardiol Young. 2009;19:135–144. doi: 10.1017/S1047951109003485. [DOI] [PubMed] [Google Scholar]
- 23.Soto CB, Olude O, Hoffmann RG, Bear L, Chin A, Dasgupta M, Mussatto K. Implementation of a routine developmental follow-up program for children with congenital heart disease: early results. Congenit Heart Dis. 2011;6:451–460. doi: 10.1111/j.1747-0803.2011.00546.x. [DOI] [PubMed] [Google Scholar]
- 24.Bayley N. The Bayley Scales of Infant Development - III. San Antonio, TX: The Psychological Corporation; 2006. [Google Scholar]
- 25.Clancy RR, McGaurn SA, Wernovsky G, Spray TL, Norwood WI, Jacobs ML, Murphy JD, Gaynor JW, Goin JE. Preoperative risk-of-death prediction model in heart surgery with deep hypothermic circulatory arrest in the neonate. J Thorac Cardiovasc Surg. 2000;119:347–357. doi: 10.1016/S0022-5223(00)70191-7. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto Y, Khoo NS, Brooks PA, Savard W, Hirose A, Hornberger LK. Severe left heart obstruction with retrograde arch flow influences fetal cerebral and placental blood flow. Ultrasound Obstet Gynecol. 2013;42:294–299. doi: 10.1002/uog.12448. [DOI] [PubMed] [Google Scholar]
- 27.O'Brien SM, Clarke DR, Jacobs JP, Jacobs ML, Lacour-Gayet FG, Pizarro C, Welke KF, Maruszewski B, Tobota Z, Miller WJ, Hamilton L, Peterson ED, Mavroudis C, Edwards FH. An empirically based tool for analyzing mortality associated with congenital heart surgery. J Thorac Cardiovasc Surg. 2009;138:1139–1153. doi: 10.1016/j.jtcvs.2009.03.071. [DOI] [PubMed] [Google Scholar]
- 28.Fay TB, Yeates KO, Wade SL, Drotar D, Stancin T, Taylor HG. Predicting longitudinal patterns of functional deficits in children with traumatic brain injury. Neuropsychology. 2009;23:271–282. doi: 10.1037/a0014936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benson JB, Haith MM, editors. Language, Memory, and Congition in Infancy and Early Childhood. 1st ed. Oxford, United Kingdom: Academic Press; 2010. [Google Scholar]; Lennon EM, Gardner JM, Karmel BZ, Flory MJ, editors. Bayley Scales of Infant Development. [Google Scholar]
- 30.Luby J, Belden A, Botteron K, Marrus N, Harms MP, Babb C, Nishino T, Barch D. The effects of poverty on childhood brain development: the mediating effect of caregiving and stressful life events. JAMA Pediatrics. 2013;167:1135–1142. doi: 10.1001/jamapediatrics.2013.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCusker CG, Doherty NN, Molloy B, Rooney N, Mulholland C, Sands A, Craig B, Stewart M, Casey F. A controlled trial of early interventions to promote maternal adjustment and development in infants born with severe congenital heart disease. Child Care Health Dev. 2010;36:110–117. doi: 10.1111/j.1365-2214.2009.01026.x. [DOI] [PubMed] [Google Scholar]
- 32.Litt JS, Glymour M, Hauser-Cram P, Hehir T, McCormick MC. The Effect of the Infant Health and Development Program on Special Education Use at School Age. J Pediatr. 2015;166:457.e1–462.e1. doi: 10.1016/j.jpeds.2014.09.066. [DOI] [PubMed] [Google Scholar]
- 33.Individuals with Disabilities Education Act Reauthorization 2004. Pub L No. 108–446, 118 Stat 2647. [Google Scholar]
- 34.Weiland K, Neidell M, Rauh V, Perera F. Cost of developmental delay from prenatal exposure to airborne polycyclic aromatic hydrocarbons. J Health Care Poor Underserved. 2011;22:320–329. doi: 10.1353/hpu.2011.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]