Anabolic resistance to conventional nutritional supplements is present in advanced cancer. The present study shows a high anabolic response to dietary essential amino acid intake in advanced nonsmall-cell lung cancer patients which is independent of nutritional status or disease trajectory, suggesting a role for dietary essential amino acids mixtures to prevent and treat cancer cachexia.
Keywords: nonsmall-cell lung cancer, cachexia, protein anabolism, dietary essential amino acids, stable isotopes, translational research
Abstract
Background
Conventional nutritional supplements are not or only partly successful in inducing protein accretion in advanced cancer, suggesting an attenuated anabolic response. To prevent muscle wasting and its deleterious consequences, generating an anabolic response is crucial. Dietary essential amino acids (EAA) have anabolic properties in other wasting diseases; however, data in advanced cancer are lacking.
Patients and methods
In 13 patients with advanced nonsmall-cell lung cancer (NSCLC) (stage III and IV) and 11 healthy age-matched subjects, we measured protein synthesis and breakdown of the whole body, and net protein anabolism (difference between protein synthesis and breakdown) after intake of 14 g of free EAA with high leucine levels (EAA/leucine) versus a balanced amino acid mixture containing both EAA and non-EAA as present in whey protein, according to a randomized, double-blind, crossover design.
Results
Protein synthesis and net protein anabolism were higher after intake of the EAA/leucine than the balanced amino acid mixture (P < 0.001), independent of presence of cancer. A highly significant linear relationship between net protein anabolism and the amount of EAA available in the systemic circulation (R2: 0.85, P < 0.001) was found in both groups. The presence of muscle or recent weight loss, systemic inflammatory response, or length of survival did not influence this relationship. High leucine levels in the EAA/leucine mixture was of no anabolic benefit.
Conclusions
There is no anabolic resistance or attenuated anabolic potential to intake of 14 g of EAA/leucine or balanced amino acid mixture in advanced (mainly stage III) NSCLC. The high anabolic potential of dietary EAA in cancer patients is independent of their nutritional status, systemic inflammatory response or disease trajectory, suggesting a key role of EAA in new nutritional approaches to prevent muscle loss, thereby improving outcome of patients with advanced cancer.
ClinicalTrails.gov
introduction
Nonsmall-cell lung cancer (NSCLC) is characterized by poor survival and involuntary weight loss is a significant prognostic factor for this disease [1, 2]. Skeletal muscle wasting is commonly present in NSCLC despite a normal of high body weight [3, 4], and negatively affects the response and tolerance to therapy and survival [5]. Lung cancer patients often experience a cluster of symptoms (i.e. appetite loss, altered taste, and nausea), which remain over the course of their disease and is an independent predictor of the patient's death [2]. The patient's ability to ingest main classes of protein-containing foods such as meats is compromised, justifying an approach using concentrated high-quality protein sources to supplement the diet.
Muscle wasting in cancer is related to alterations in skeletal muscle protein metabolism in a state of elevated systemic inflammation [6]. Conventional nutritional supplementations are ineffective in stimulating muscle protein synthesis in advanced cancer [7], and a lower anabolic potential was suggested in those with <3 months life expectancy and muscle loss [8]. A recent euglycemic, hyperinsulinemic clamp study in NSCLC [4] revealed a blunted protein anabolic response to low levels of amino acids but a normal response to hyperaminoacidemia. This suggests that substantial protein intake is required to induce protein anabolism in cancer which might be difficult when appetite loss is present. Recently we showed that a high protein formula containing high essential amino acid (EAA) and leucine levels was able to stimulate muscle protein synthesis in advanced cancer [7]. This suggests that the suppressed anabolic responsiveness to a conventional nutritional supplement in advanced cancer can be (at least partly) overcome by providing specially formulated nutrition.
Mixtures containing single (free) EAA are capable of stimulating muscle protein synthesis in older healthy adults [9] without suppressing appetite, while supplementing extra leucine further increases muscle protein synthesis [10]. We recently showed that a leucine-enriched EAA mixture was highly anabolic in patients with cystic fibrosis with severe nutritional failure [11].
The aim of the present study was to examine whether an EAA mixture induces protein anabolism in cancer patients to the same degree as in healthy control subjects and is independent of the presence of muscle or recent weight loss, systemic inflammation or length of survival. Furthermore, a comparison of the anabolic properties of the EAA mixture versus a balanced mixture of both EAA and non-EAA mixtures as present in whey proteins was carried out, as these milk proteins are often used in commercially available supplements in cancer care. Stable isotope methodology was used as a promising tool that can measure personalized anabolic potential of cancer patients in the course of their disease and can guide preventive measures. Our results could initiate novel nutritional approaches to prevent or minimize muscle wasting, thereby, improving clinical and overall outcome of advanced cancer patients.
methods
study population
The study population consisted of 13 subjects with advanced NSCLC and 11 healthy age-matched subjects (supplementary Figure S7, available at Annals of Oncology online). The subjects with NSCLC were clinically diagnosed as stage III (unresectable) or stage IV cancer at the University of Arkansas for Medical Sciences (UAMS) or the Central Arkansas Veterans Healthcare System (Table 1). Exclusion criteria were anticancer therapy (e.g. radiotherapy, chemotherapy) and surgery <4 weeks before the study, and presence of cardiovascular or unstable metabolic diseases. All healthy subjects were recruited via flyers in the local community. The study was approved by the Institutional Review Board, UAMS.
Table 1.
Characterization of the individual patients with nonsmall-cell lung cancer
| Subject | Age | Histology | Stage | Treatment | Chemotherapy drugs | Time study day until death (months) |
|---|---|---|---|---|---|---|
| 1 | 79 | Adenocarcinoma | IIIA (T1N2M0) | Thoracic radiation therapy, no chemotherapy | 4 | |
| 2 | 62 | Squamous cell carcinoma | IIIA (T2N2M0) | Concurrent chemo-radiotherapy | Cisplatin + etoposide | 29 |
| 3 | 62 | Adenocarcinoma | IIIA | Surgery + adjuvant chemotherapy + concurrent chemo-radiotherapy | Taxol + carboplatin cisplatin + etoposide |
26 |
| 4 | 66 | Adenocarcinoma | IV (pleural effusion) | Chemotherapy | Paclitaxel + carboplatin | 1 |
| 5 | 69 | Squamous cell carcinoma | III | Concurrent chemo-radiotherapy | Cisplatin + etoposide | 45 |
| 6 | 58 | Adenocarcinoma | III → IV | Surgery + chemotherapy + brain radiation therapy | Paclitaxel + carboplatin | 36 |
| 7 | 57 | Poorly differentiated adenocarcinoma | IIIA → IV | Concurrent chemo-radiotherapy | Cisplatin + etoposide | 5 |
| 8 | 61 | Adenocarcinoma | III | Concurrent chemo-radiotherapy | Cisplatin + etoposide | 26 |
| 9 | 71 | Poorly differentiated adenocarcinoma | III | Concurrent chemo-radiotherapy | Cisplatin + etoposide | 44 |
| 10 | 74 | Squamous cell carcinoma | IIIB | Concurrent chemo-radiotherapy + chemotherapy | Paclitaxel + carboplatin | 12 |
| 11 | 68 | Adenocarcinoma | IV (brain metastasis to pleura) | Chemotherapy | Pemetrexed + carboplatin | 4 |
| 12 | 76 | Squamous cell carcinoma | IIIA | Concurrent chemo-radiotherapy | Cisplatin + etoposide | 11 |
| 13 | 78 | Adenocarcinoma | IIIA | Thoracic radiation therapy + chemotherapy | Paclitaxel + carboplatin | 44 |
Time study day until death in the cancer group ranged from <6 months (n = 4), between 6 and 12 months (n = 2), and >12 months (n = 7). Median survival was 26 months between study date and date of death. Average time period between cancer diagnosis and study enrollment was 12 months, and between last treatment and study enrollment was 5 months (data not shown).
study protocol
All subjects were studied at the UAMS Clinical Center of the Translational Research Institute on two different study days. Body weight, height, fat, and fat-free mass (FFM) were measured by dual-energy X-ray absorptiometry and standardized for height [12]. Respiratory muscle function, handgrip strength, and endurance were assessed as described previously [13], and habitual dietary intake by 24-h recall. Disease and treatment history were obtained from the medical chart until 3.5 years after enrollment.
Each study day started in the early morning after an overnight fast (supplementary Figure S5, available at Annals of Oncology online). Body weight, height, and vital signs were measured and two peripheral lines were placed. One catheter was placed in an antecubital vein for stable isotope infusion, and the other in a superficial dorsal vein of the hand or lower arm of the contralateral arm for blood sampling. The hand was placed in a thermostatically controlled hot box to mimic direct arterial sampling [14].
A primed, constant, and continuous infusion protocol was carried out [11] with the stable isotopes of phenylalanine and tyrosine (supplementary Table S2, available at Annals of Oncology online). After 3 h of infusion, each subject ingested within 5 min a 250-ml non-caloric soft-drink containing L-15N-phenylalanine isotope and one of two amino acid mixtures (supplementary Table S1, available at Annals of Oncology online: 14 g of leucine-enriched (40%) EAA (EAA/leucine) mixture versus 14 g of balanced [(essential and nonessential) amino acid mixture], according to a randomized and double-blind cross-over design. Carbohydrates are a large component of daily food intake; therefore, maltodextrin (30 g) was added to the mixture. Combined intake of amino acid and carbohydrates increases the insulin response that is required for maximal protein anabolism [11]. Blood was processed and analyzed batch-wise by LC-MS/MS and routine techniques [11].
protein metabolism and amino acid kinetics
We calculated protein kinetic measures (whole-body protein synthesis, breakdown, and net protein anabolism (=protein synthesis – breakdown)) from the isotope enrichments [11, 12] in the postabsorptive and postprandial state [12]. Amino acid efficiency, reflecting the extent to which EAA from the mixtures are used for net protein anabolism, was calculated as a ratio of net protein anabolism to dietary intake of phenylalanine (as analog for dietary EAA) [12]. Plasma amino acid concentrations were also analyzed to get information about the availability of the dietary EAA and non-EAAs in the circulation for protein anabolism.
statistical analysis
Results are mean ± standard error (SE). If data failed the normality or equal variance test, data were log-transformed. Unpaired Student's t-test was used to determine differences in general characteristics and plasma metabolites between the NSCLC and control groups. Protein metabolism in the postabsorptive state was measured as the median value of the measures at time points 150, 165, and 180 min and in the prandial state as the 3 h integral (nmol/kg FFM/3 h) and per hour (nmol/kg FFM/h) to enable postabsorptive/postprandial comparisons. Two-way repeated-measures analysis of variance (two-way RM ANOVA; general linear model) was carried out with group (NSCLC versus healthy controls) and amino acid mixture (EAA/leucine versus balanced amino acid mixture) as factors. Bonferroni post hoc test was applied when significant interactions were observed. The relations between net protein anabolism versus dietary EAA intake and EAA appearance in circulation after splanchnic extraction were analyzed with two-tailed tests of significance by using Pearson's correlation coefficients and linear regression analysis. The level of significance was set at P < 0.05. Graphpad Prism (Version 6.05) and SPSS (version 21) were used for data analysis.
results
Eleven patients (Table 1) had stage III NSCLC and two of them developed stage IV during follow-up. Median survival (supplementary Figure S6, available at Annals of Oncology online) from diagnosis was 48 months. No differences were found between the groups in age, body mass index, fat mass, FFM, and muscle function(Table 2), but reduced values for leg FFM (P < 0.05) and a tendency for reduced handgrip endurance (P = 0.07) in NSCLC. Weight loss >5% in the past 3–6 months (average 8.4% in past 5 months) was observed in 38% of the NSCLC patients, although a comparable daily energy and macronutrient intake was found in both groups (Table 2).
Table 2.
General characteristics, body composition, muscle function, habitual dietary intake and laboratory values of the healthy control and NSCLC groups
| Healthy controls (n = 11) | NSCLC (n = 13) | Statistical values, P | |
|---|---|---|---|
| Gender (m/f) | 11/0 | 13/0 | |
| Age (years) | 65.8 ± 1.6 | 68.5 ± 2.1 | NS |
| Weight (kg) | 86.2 ± 3.1 | 79.6 ± 3.7 | NS |
| Body mass index (kg/m2) | 27.8 ± 1.1 | 26.5 ± 1.1 | NS |
| Body composition | |||
| Fat-free mass index (kg/m2) | 18.9 ± 0.6 | 18.0 ± 0.7 | NS |
| Fat mass index (kg/m2) | 8.0 ± 0.6 | 7.8 ± 0.6 | NS |
| Whole-body fat-free mass (kg) | 59.2 ± 1.9 | 54.0 ± 2.4 | NS |
| Arms fat-free mass (kg) | 6.7 ± 0.3 | 6.5 ± 0.3 | NS |
| Legs fat-free mass (kg) | 19.4 ± 0.8 | 16.8 ± 0.8 | 0.02* |
| Muscle function | |||
| Maximal inspiratory pressure (cmH2O) | 85.2 ± 10.8 | 73.3 ± 8.1 | NS |
| Maximal expiratory pressure (cmH2O) | 110.7 ± 8.3 | 98.2 ± 7.7 | NS |
| Handgrip strength | |||
| N | 260.3 ± 19.3 | 267.5 ± 9.7 | NS |
| N/FFM | 4.4 ± 0.3 | 5.1 ± 0.3 | NS |
| Handgrip endurance (%) | 82.8 ± 5.8 | 72.6 ± 3.1 | 0.07 |
| Habitual dietary intake | |||
| Energy | |||
| kcal | 2139 ± 167 | 1944 ± 215 | NS |
| kcal/kg bw | 24 ± 2 | 25 ± 3 | NS |
| kcal/kg FFM | 109 ± 8 | 110 ± 12 | NS |
| Protein | |||
| g | 85.5 ± 7.3 | 70.4 ± 8.4 | NS |
| g/kg bw | 1.0 ± 0.1 | 0.9 ± 0.1 | NS |
| g/kg FFM | 4.4 ± 0.4 | 4.0 ± 0.5 | NS |
| energy% | 16.3 ± 1.2 | 14.6 ± 0.8 | NS |
| Fat (energy%) | 35.0 ± 1.7 | 36.8 ± 1.9 | NS |
| Carbohydrates (energy%) | 48.8 ± 1.9 | 48.6 ± 1.9 | NS |
| Laboratory data plasma | |||
| C-reactive protein (mg/l) | 1.5 ± 0.4 | 9.8 ± 3.7 | 0.04* |
| Insulin (µIU/ml) | 8.1 ± 1.9 | 9.4 ± 1.1 | NS |
| Glucose (mmol/l) | 5.7 ± 0.4 | 5.6 ± 0.2 | NS |
| HOMA score (MU) | 2.0 ± 0.5 | 2.4 ± 0.3 | NS |
All values are means ± SEM. Data were analyzed by unpaired Student's t-test.
Significantly different from the control group. *P < 0.05.
NSCLC, nonsmall-cell lung cancer; bw, body weight; FFM, fat-free mass; HOMA, Homeostasis Model Assessment score (marker of insulin resistance).
metabolism
Higher plasma CRP (Table 2), but comparable insulin and glucose values were found in NSCLC. Postabsorptive protein synthesis and breakdown (supplementary Table S3, available at Annals of Oncology online) were comparable, but net protein anabolism was less negative in NSCLC (P < 0.05). Higher values were present for protein synthesis and net protein anabolism (Table 3, supplementary Figure S8, available at Annals of Oncology online) after intake of the EAA/leucine mixture (P < 0.001), and higher postprandial protein breakdown in NSCLC (P < 0.05). Postprandial protein synthesis and breakdown and net protein anabolism remained elevated at 3 h after intake (P < 0.05) (supplementary Figure S7, available at Annals of Oncology online), but amino acid efficiency was not different between groups or mixtures (Table 3).
Table 3.
Postprandial protein metabolism after intake of the leucine-enriched essential amino acid mixture and the balanced total amino acid mixtures in the healthy control and NSCLC groups
| Control group (n = 11) |
NSCLC group (n = 13) |
Statistical values, P | |||
|---|---|---|---|---|---|
| Balanced total amino acid mixture | EAA/leucine mixture | Balanced total amino acid mixture | EAA/leucine mixture | ||
| Protein synthesis (µmol/kg FFM/3 h) | 178.8 ± 8.3 | 199.3 ± 9.9 | 189.5 ± 6.1 | 209.9 ± 6.0 | AA: P < 0.0001 |
| Protein breakdown (µmol/kg FFM/3 h) | 138.4 ± 4.0 | 142.7 ± 4.2 | 153.2 ± 5.3 | 155.5 ± 4.1 | G: P = 0.026 |
| Net protein anabolism (µmol/kg FFM/3 h) | 30.7 ± 3.4 | 49.8 ± 3.0 | 36.2 ± 2.0 | 54.4 ± 3.5 | AA: P < 0.0001 |
| Splanchnic extraction (%) | 36 ± 3 | 40 ± 2 | 40 ± 2 | 42 ± 2 | |
| Amino acid efficiency (%) | 47 ± 4 | 49 ± 2 | 51 ± 2 | 50 ± 3 | |
Values are means ± SEM. Amino acid efficiency: the extent to which phenylalanine (as measure of EAA) from the meals are used for net protein anabolism, two-factor repeated-measures ANOVA was used to test amino acid (AA) mixture and group (G) effects and the G × AA mixture interaction (I).
NSCLC, nonsmall-cell lung cancer; EAA/leucine, leucine-enriched essential amino acid mixture; FFM, fat-free mass.
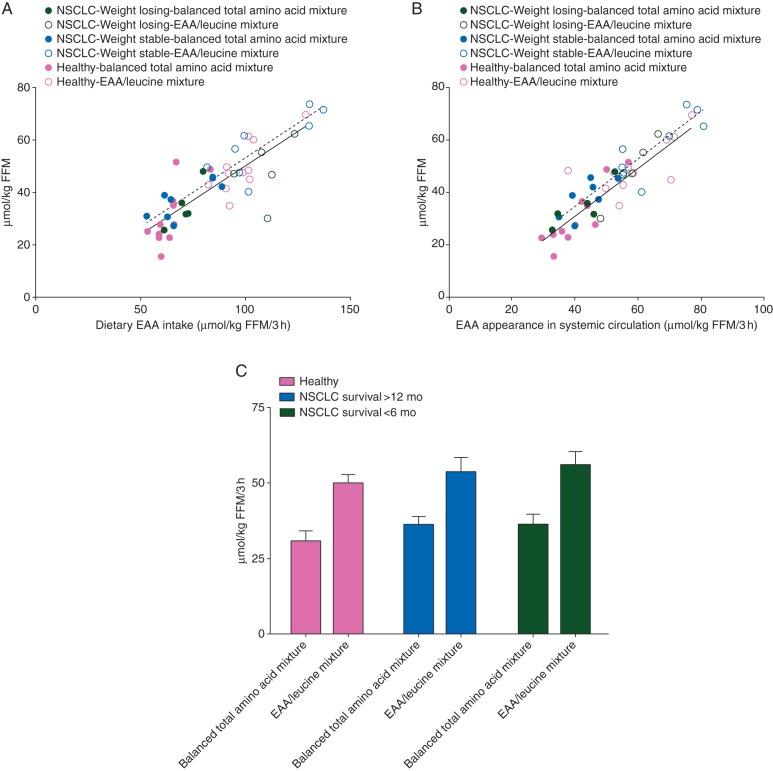
In both groups, a highly significant relationship was present between net protein anabolism and dietary EAA intake (Figure 1A) and EAA appearance in systemic circulation (Figure 1B). This relationship in the NSCLC group remained after stratification for recent weight loss (weight stable versus weight losing) or survival (<6 versus >12 months, data not shown). Stratification into survival <6 versus >12 months (Figure 1C) showed higher values for net protein anabolism after intake of the EAA/leucine mixture (P < 0.001). Stratification of the cancer group into muscle loss (FFM Index <25% percentile, n = 4) versus muscle preservation (FFM Index >25% percentile, n = 9) showed comparable data for net protein anabolism (data not shown).
Figure 1.
Correlation between net protein anabolism (expressed in µmol/kg FFM/3 h) and dietary EAA intake (A), and EAA appearance in the systemic circulation (B) in the NSCLC groups with recent weight loss (WL) (black, green circles online) and without recent weight loss (dark grey, blue circles online), and the healthy control (light grey, pink circles online) group after intake of EAA/leucine (open circles) and balanced total amino acid mixture (closed circles) mixtures. Net protein anabolism versus dietary EAA intake in NSCLC WL: R2 = 0.50, P < 0.01, NSCLC no WL: R2 = 0.83, P < 0.001, healthy controls: R2 = 0.74, P < 0.001. Net protein anabolism versus EAA appearance in the systemic circulation: NSCLC WL: R2 = 0.85, P < 0.001, NSCLC no WL: R2 = 0.84, P < 0.001, healthy controls: R2 = 0.75, P < 0.001. Mean (±SEM) net protein anabolism (C) in the NSCLC patients with survival <6 months (WL) (black, green bars online) and those with survival >12 month (dark grey, blue bars online), and the healthy control (light grey, pink bars online) group after intake of EAA/leucine and balanced total amino acid mixtures. Two-factor repeated-measures ANOVA was used to test the AA mixture (AA) and group (G) effect. Net protein anabolism: AA effect: P < 0.001. There is no Group or AA mixture X Group interaction.
Changes in postprandial plasma amino acid kinetics provide important information on the availability of dietary EAA and non-EAAs for protein anabolism. Lower postabsorptive plasma concentrations were found for sum of all EAA (P < 0.01) and sum all amino acids (P < 0.05) in the NSCLC group, and sum non-EAAs (P = 0.09) tended to be lower (supplementary Table S3, available at Annals of Oncology online). The postprandial (3 h) increase in sum EAA and decrease in sum non-EAAs was larger after intake of the EAA/leucine mixture (P < 0.001), and less negative values were found in the NSCLC group (P < 0.05) (supplementary Table S4 and Figure S9B, available at Annals of Oncology online). After intake of both mixtures, sum EAA immediately increased (peak value ∼45 min) but normalization toward baseline values was still not present at 3 h after EAA/leucine intake (supplementary Figure S9A, available at Annals of Oncology online). The absolute 3-h change in sum all amino acids was different between the NSCLC and control groups (supplementary Table S4 and Figure S9C, available at Annals of Oncology online).
discussion
In the present study, intake of 14 g of EAA resulted in a high anabolic response in patients with stage III/IV NSCLC. The magnitude of the response was comparable with that found in the healthy control group indicating a preserved anabolic potential in advanced cancer. The highly significant relationship between the dietary EAA intake and net protein anabolism indicates that the anabolic response to feeding is depending on the amount of EAA in the diet, but independent of the presence of cancer.
At enrollment, most cancer patients had stage III NSCLC, diagnosed ∼1 year before study participation, and were studied during a relatively stable period of their disease. The cancer group was characterized by preserved nutritional status and muscle function, in line with previous studies in advanced (lung) cancer [4, 7]. Wasting of skeletal muscle mass despite a normal or high body weight or fat mass was previously observed in NSCLC [3, 4]. Our subjects had a habitual protein intake of 0.9 g/kg day which is above the RDA of 0.8 g/kg for healthy older adults but substantially lower than recommended for cancer patients (1.2–2 g/kg/bw [15]). Moreover, 39% of the studied NSCLC patients had lost weight involuntarily, in line with the 30%–60% reported previously [16], despite preserved caloric intake. It remains unclear whether a change in eating habits might have played a role. We found reduced postabsorptive plasma amino acid levels (including EAA) in NSCLC, but no alterations in postabsorptive protein synthesis and breakdown although an unexplained lower net protein breakdown was found in NSCLC. The studied NSCLC patients had an increased systemic inflammatory response which is known to be associated with greater weight loss, poorer performance status, more fatigue, and poorer survival [1, 17], albeit at higher CRP levels than seen in the present study (38–40 versus 10 mg/l).
Cancer patients might lose their anabolic potential in the 3 month time window before death [8]. This so-called refractory period is characterized by severe muscle wasting, ongoing catabolism, low performance status, and metastatic disease refractory to antineoplastic therapy. Still 65% of the cancer patients were able to maintain or increase their skeletal muscle mass in this period [8], suggesting exploitable anabolic potential. When evaluating the disease trajectory of the studied NSCLC group, 31% passed away within 6 months after study participation whereas the remaining 69% were still alive after 12 months. The anabolic response remained the same in both groups, suggesting a preserved anabolic potential to the amino acid mixtures in the last 6 months of life. Whether this is also the case for the 3-month refractory period remains unclear. The high anabolic potential of 14 g of free EAA in patients with NSCLC was independent of their body weight, muscle mass, and the presence of recent weight loss. Furthermore, the anabolic response to amino acids was not affected by previous chemotherapy which is remarkable as the prescribed platinum-based chemotherapy is known to be associated with weight loss [18]. Others [6] have suggested that cancer-induced inflammation reduces the sensitivity of skeletal muscle protein synthesis to amino acid supplementation, but our data on whole-body level do not confirm this. Leucine supplementation is used to improve this sensitivity, but our study shows no additional anabolic benefit in line with our previous data in cystic fibrosis and COPD [11, 12]. Our data also revealed that intake of dietary EAA did not reach a plateau in anabolism, suggesting that higher quantities of amino acids might be useful to advanced cancer patients.
Particularly in patients with anorexia, intake of dietary EAA is preferable above a mixture containing both EAA and non-EAAs as less supplement is needed to obtain the same anabolic response. Measuring postprandial plasma kinetics of amino acids provides important information on the availability of the dietary amino acids for systemic protein build up (anabolism). The plasma amino acids kinetics are dependent on the extraction of these amino acids by the splanchnic area and in this way might affect the anabolic response. No difference was observed in splanchnic extraction between the cancer and the healthy subjects. Plasma EAA levels and net protein anabolism remained elevated above baseline values 3 h after EAA intake, indicating a sustained anabolic effect.
Limitation of the study is the small sample size, although sufficient to answer our research aim. A potential selection bias toward the more fit and motivated patients cannot be excluded as patients were enrolled who were physically able to spend 2 study days at our research unit. Furthermore, all studied patients appeared to be men (mostly veterans), and a large proportion had stage IIIA NSCLC. Although CRP levels were comparable with those previously reported in advanced NSCLC [4], inflammation as potential driver of anabolic resistance was ‘only’ mild to moderate. Still, the patients with CRP >20 mg/l had a comparable anabolic response as those with lower CRP levels, in line with our previous study in cancer patients with average CRP levels of 25 mg/l [7]. Further research is needed to confirm this finding in cancer patients with more progressive disease and high CRP levels, as well as, during anticancer treatment associated with active muscle loss.
To prevent and treat involuntary muscle loss in cancer, it is of crucial importance to use nutritional supplements that are able to generate a very high anabolic response. The present study shows that dietary free EAA are very efficient in inducing anabolism in advanced cancer. The linear and highly significant relationship between anabolism and EAA available from the diet in cancer was independent of disease trajectory (<versus> 6 months before death), mild-to-moderate systemic inflammation, presence of muscle or recent weight loss, and comparable with that observed in the healthy group. Therefore, our data provide a path to a novel nutritional approach to prevent and treat cancer cachexia and improve their outcome.
funding
This work was supported by a grant from the American Institute for Cancer Research (grant number #09A051 to MPKJE), National Institutes of Health (S10RR027047 to NEPD, and Clinical and Translational Science Award UL1RR029884 to NEPD).
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
We thank the cancer patients and the healthy control subjects who have participated in this research and who have made this work possible.
references
- 1.Scott HR, McMillan DC, Forrest LM et al. The systemic inflammatory response, weight loss, performance status and survival in patients with inoperable non-small-cell lung cancer. Br J Cancer 2002; 87: 264–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gift AG, Stommel M, Jablonski A, Given W. A cluster of symptoms over time in patients with lung cancer. Nurs Res 2003; 52: 393–400. [DOI] [PubMed] [Google Scholar]
- 3.Baracos VE, Reiman T, Mourtzakis M et al. Body composition in patients with non-small cell lung cancer: a contemporary view of cancer cachexia with the use of computed tomography image analysis. Am J Clin Nutr 2010; 91: 1133S–1137S. [DOI] [PubMed] [Google Scholar]
- 4.Winter A, MacAdams J, Chevalier S. Normal protein anabolic response to hyperaminoacidemia in insulin-resistant patients with lung cancer cachexia. Clin Nutr 2012; 31: 765–773. [DOI] [PubMed] [Google Scholar]
- 5.Prado CM, Baracos VE, Xiao J et al. The association between body composition and toxicities from the combination of Doxil and trabectedin in patients with advanced relapsed ovarian cancer. Appl Physiol Nutr Metab 2014; 39: 693–698. [DOI] [PubMed] [Google Scholar]
- 6.Dillon EL, Volpi E, Wolfe RR et al. Amino acid metabolism and inflammatory burden in ovarian cancer patients undergoing intense oncological therapy. Clin Nutr 2007; 26: 736–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deutz NE, Safar A, Schutzler S et al. Muscle protein synthesis in cancer patients can be stimulated with a specially formulated medical food. Clin Nutr 2011; 30: 759–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prado CM, Sawyer MB, Ghosh S et al. Central tenet of cancer cachexia therapy: do patients with advanced cancer have exploitable anabolic potential? Am J Clin Nutr 2013; 98: 1012–1019. [DOI] [PubMed] [Google Scholar]
- 9.Volpi E, Kobayashi H, Sheffield-Moore M et al. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr 2003; 78: 250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katsanos CS, Kobayashi H, Sheffield-Moore M et al. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab 2006; 291: E381–E387. [DOI] [PubMed] [Google Scholar]
- 11.Engelen MP, Com G, Wolfe RR, Deutz NE. Dietary essential amino acids are highly anabolic in pediatric patients with cystic fibrosis. J Cyst Fibros 2013; 12: 445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jonker R, Deutz NE, Erbland ML et al. Hydrolyzed casein and whey protein meals comparably stimulate net whole-body protein synthesis in COPD patients with nutritional depletion without an additional effect of leucine co-ingestion. Clin Nutr 2014; 33: 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engelen MP, Schols AM, Does JD, Wouters EF. Skeletal muscle weakness is associated with wasting of extremity fat-free mass but not with airflow obstruction in patients with chronic obstructive pulmonary disease. Am J Clin Nutr 2000; 71: 733–738. [DOI] [PubMed] [Google Scholar]
- 14.Abumrad NN, Rabin D, Diamond MP, Lacy WW. Use of a heated superficial hand vein as an alternative site for the measurement of amino acid concentrations and for the study of glucose and alanine kinetics in man. Metabolism 1981; 30: 936–940. [DOI] [PubMed] [Google Scholar]
- 15.Deutz NE, Bauer JM, Barazzoni R et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr 2014; 33: 929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan BH, Fearon KC. Cachexia: prevalence and impact in medicine. Curr Opin Clin Nutr Metab Care 2008; 11: 400–407. [DOI] [PubMed] [Google Scholar]
- 17.McMillan DC, Elahi MM, Sattar N et al. Measurement of the systemic inflammatory response predicts cancer-specific and non-cancer survival in patients with cancer. Nutr Cancer 2001; 41: 64–69. [DOI] [PubMed] [Google Scholar]
- 18.Sánchez-Lara K, Turcott JG, Juárez-Hernández E et al. Effects of an oral nutritional supplement containing eicosapentaenoic acid on nutritional and clinical outcomes in patients with advanced non-small cell lung cancer: randomised trial. Clin Nutr 2014; 33: 1017–1023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.