This randomized, double-blind comparison demonstrates that biosimilar filgrastim (EP2006) and the US-licensed reference filgrastim are similar with no clinically meaningful differences regarding efficacy and safety in prevention of severe neutropenia. Biosimilar filgrastim could represent an important alternative to the reference product, potentially increasing access to filgrastim treatment.
Keywords: granulocyte colony-stimulating factor, filgrastim, neutropenia, biosimilars
Abstract
Background
Biosimilars of filgrastim are in widespread clinical use in Europe. This phase III study compares biosimilar filgrastim (EP2006), with the US-licensed reference product, Neupogen®, in breast cancer patients receiving (neo)adjuvant myelosuppressive chemotherapy (TAC).
Patients and methods
A total of 218 patients receiving 5 µg/kg/day filgrastim over six chemotherapy cycles were randomized 1:1:1:1 into four arms. Two arms received only one product (nonalternating), biosimilar or reference, and two arms (alternating) received alternating treatments during each cycle (biosimilar then reference or vice versa). The primary end point was duration of severe neutropenia (DSN) during cycle 1.
Results
The baseline characteristics were balanced between the four treatment arms. Noninferiority of biosimilar versus the reference was demonstrated: DSN (days) in cycle 1 was 1.17 ± 1.11 (biosimilar, N = 101) and 1.20 ± 1.02 (reference, N = 103), 97.5% confidence interval lower boundary for the difference was −0.26 days (above the predefined limit of −1 day). No clinically meaningful differences were observed regarding any other efficacy parameter: incidence of febrile neutropenia (FN); hospitalization due to FN; incidence of infections; depth and time of absolute neutrophil count (ANC) nadir and time to ANC recovery during cycle 1 and across all cycles. The pattern and frequency of adverse events were similar across all treatments.
Conclusion
This study demonstrates that biosimilar and the reference filgrastim are similar with no clinically meaningful differences regarding efficacy and safety in prevention of severe neutropenia. Biosimilar filgrastim could represent an important alternative to the reference product, potentially benefiting public health by increasing access to filgrastim treatment.
Study number
introduction
Biosimilars are biologic medicines that have been proven, through a regulatory process, to be highly similar to a reference product. Several biosimilars of filgrastim are now in clinical use in Europe and elsewhere. Acceptance of biosimilar filgrastim is reflected in EORTC guidelines, recommending biosimilar filgrastim as well as the reference product to prevent chemotherapy-induced neutropenia [1].
Previous studies of granulocyte colony-stimulating factor (G-CSF) use have suggested that under-utilization is frequent, with patients either not receiving treatment at all or receiving delayed and/or shorter courses than recommended [2–4]. Such suboptimal use of G-CSF is associated with poorer outcomes, with patients facing reduced protection against febrile neutropenia (FN) [5]. In a time of increasing cost constraints in healthcare, cost considerations may be a factor in restricted access to G-CSF for some patients [6]. Moreover, in countries in which patients may need to contribute to treatment costs, high drug prices may result in reduced adherence. In Europe, the availability of filgrastim biosimilars has been accompanied by increased use, suggesting physicians are more able and/or willing to use filgrastim in accordance with guidelines [7].
This randomized, double-blind, multicenter phase III study was designed to show noninferiority of a biosimilar filgrastim (EP2006) compared with US-licensed reference filgrastim (Neupogen®) in the prevention of duration of severe neutropenia (DSN) in breast cancer patients receiving myelosuppressive chemotherapy. The study also examined if alternating between the biosimilar and reference filgrastim or vice versa affected efficacy, safety or immunogenicity.
patients and methods
patients
Patients were women ≥18 years with histologically proven breast cancer scheduled to receive (neo-)adjuvant chemotherapy with docetaxel 75 mg/m2, doxorubicin 50 mg/m2 and cyclophosphamide 500 mg/m2 (TAC regimen). Other key inclusion criteria included Eastern Cooperative Oncology Group performance status ≤2 and adequate bone marrow function. Exclusion criteria included history of myelogenous leukemia, myelodysplastic syndrome or concomitant sickle cell disease, concurrent or prior radiotherapy within 4 weeks of randomization, use of prophylactic antibiotics, prior chemotherapy or anticancer treatment of breast cancer or previous G-CSF therapy.
The study was conducted between December 2011 and June 2013 at 25 centers (Russia, N = 10; Ukraine, N = 6; Hungary, N = 6; Latvia, Slovakia, Czech Republic, all N = 1). An independent data safety monitoring board monitored patient safety. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice; the protocol was approved by the independent ethics review board for each center. All patients provided written informed consent.
study design
Eligible patients were randomized (stratified by adjuvant or neoadjuvant chemotherapy) by an interactive web-based system to one of four treatment arms (1:1:1:1) before six cycles of chemotherapy. Allocation was to either the biosimilar (EP2006) or reference filgrastim (Neupogen®) for the duration of chemotherapy, or patients alternated between biosimilar and reference filgrastim at each cycle (supplementary Figure S1, available at Annals of Oncology online). This design assessed the efficacy, safety and immunogenicity of alternating between the biosimilar and reference filgrastim or vice versa. The required filgrastim volume was transferred from vials into identical application syringes by unblinded site staff who did not participate in study assessments. Patients, investigators and other site staff involved in study assessments were blinded. Chemotherapy was administered i.v. on day 1 of each cycle and given every 3 weeks for six cycles. Filgrastim was administered (5 µg/kg body weight/day, s.c.) from day 2 of each cycle until the absolute neutrophil count (ANC) recovered to 10 × 109/l after its nadir or for a maximum of 14 days.
study assessments
Patient baseline characteristics including laboratory assessments were reported at screening, on day 1 of each chemotherapy cycle and at the end of treatment. Complete blood counts (CBCs) were done on day 1 and then daily in cycle 1 until the ANC reached 10 × 109/l after its nadir or until day 15, whichever occurred first. For cycles 2–6, CBC was assessed on day 1, day 7 and daily thereafter. Body temperature was recorded on days 1–21 of each cycle and at treatment end. Injection-site reactions were assessed following each application. Concomitant medication and adverse events (AEs) were assessed at every visit.
Anti-recombinant human G-CSF (rhG-CSF) antibody formation was assessed by radioimmunoprecipitation at day 1 of each cycle, at end of treatment and at study end. Eight time points were collected per patient. Serum samples were immediately frozen and shipped to HEXAL AG (Oberhaching, Germany) on dry ice and stored at ≤−70°C until analysis. Samples were incubated with [125I]-labeled rhG-CSF tracer. After equilibrium was reached between anti-rhG-CSF antibodies and rhG-CSF, the formed complex was precipitated with Protein-G Sepharose. Specificity of anti-rhG-CSF antibodies was further tested in a separate assay after addition of excess amounts of unlabeled rhG-CSF. The sensitivity of the validated radioimmunoprecipitation was in the single-digit nanogram range.
statistical analysis
This noninferiority trial design utilized a difference of −1 day (noninferiority margin) in DSN in cycle 1 as the largest acceptable difference between the biosimilar compared with reference treatment. TAC chemotherapy induces a median DSN of 7 days in breast cancer patients receiving no G-CSF treatment [8], while G-CSF treatment reduces the mean DSN to 1.4 days [95% confidence interval (CI) 1.07–1.69] [9]. Assuming an expected mean difference of 0.25 days with a common standard deviation of 1.5 days, a 10% dropout rate and a noninferiority margin of −1 day, 96 patients per group (biosimilar or reference) were required to have 90% power for showing noninferiority based on a one-sided 97.5% CI.
primary efficacy analysis
The primary efficacy end point was a comparison of mean DSN (number of consecutive days from ANC <0.5 × 109/l to ≥0.5 × 109/l) during cycle 1 of chemotherapy between biosimilar and reference treatments. The primary efficacy analysis was based on the per-protocol (PP) set including all patients who completed the first chemotherapy cycle without major protocol deviations. A one-sided 97.5% CI for the difference in mean DSN was derived from an analysis of co-variance adjusted for treatment group, adjuvant or neoadjuvant chemotherapy (stratification factor) and baseline ANC used, with noninferiority of the biosimilar confirmed if the lower limit was larger than −1 day. In addition, an analysis of variance including only the factor ‘treatment group’ was carried out for the primary efficacy end point. To evaluate the robustness of the PP results, the primary end point using the full analysis set (FAS: all patients who received ≥1 dose of study medication, analyzed according to randomization allocation) was also analyzed. Since five patients had a missing ANC at baseline, their ANC at screening visit was used. The ANC adjacent to a missing post-baseline ANC was used for DSN for a further five patients.
secondary efficacy analysis
The number of patients who reported ≥1 fever episode (oral temperature ≥38.3°C), total number of days of fever, incidence of FN (oral temperature ≥38.3°C and ANC <0.5 × 109/l on the same day) and hospitalization due to FN were analyzed descriptively for each cycle and across all cycles. Depth of ANC nadir was analyzed descriptively for each cycle (median and day on which the nadir occurred). Time to ANC recovery (time after ANC nadir to ANC increase to ≥2 × 109/l) was analyzed descriptively for cycle 1.
Frequency of infections (recorded as AE, coded to system organ class of infections and infestations) and incidence of hospitalizations due to FN were analyzed descriptively for each cycle and across all cycles. Anti-rhG-CSF antibody formation was reported as the number of patients with a positive or negative result. AEs were summarized.
During cycle 1, comparisons were made between biosimilar filgrastim (biosimilar arm plus alternating arm starting with biosimilar) and reference filgrastim (reference arm plus alternating arm starting with reference). Efficacy analyses carried out in cycle 1 were analyzed for the PP and the FAS population.
Across all cycles, comparisons were made between alternating and nonalternating treatment, as well as between nonalternating treatments. Efficacy analyses carried out across all cycles (alternating and nonalternating comparisons) were analyzed by the alternating PP population (PP-I: all patients who completed six cycles without a major protocol deviation).
Safety analyses were carried out for the safety population (SAF: all patients who received ≥1 dose of study medication and had ≥1 post-baseline safety assessment) and the alternating safety population (SAF-I: all patients who received ≥1 dose of study medication after cycle 1).
All statistical analyses were carried out using SAS (SAS Institute, Cary, NC).
results
patients
A total of 218 patients were randomized. Patient disposition is described in supplementary Figure S2, available at Annals of Oncology online. Baseline characteristics were balanced between treatment arms (Table 1). Overall, median age was 50 (range: 23−76) years and median duration since breast cancer diagnosis was one month; most patients had stage II–III cancer (93%).
Table 1.
Baseline characteristics (full analysis set/safety set)
| Cycle 1 |
All cycles |
All cycles |
||||
|---|---|---|---|---|---|---|
| Pooled biosimilar (B + B − R) N = 107 |
Pooled reference (R + R − B) N = 107 |
Pooled nonalternating (B + R) N = 105 |
Pooled alternating (B − R + R − B) N = 109 |
Biosimilar N = 53 |
Reference N = 52 |
|
| Age (years) | ||||||
| Mean (SD) | 49.5 (11.52) | 48.4 (11.02) | 49.2 (11.22) | 48.6 (11.35) | 51.5 (11.16) | 46.9 (10.91) |
| Time (months) since initial diagnosis of breast cancer | ||||||
| Median (min, max) | 1.0 (0, 171a) | 1.0 (0, 16b) | 1.0 (0, 171a) | 1.0 (0, 16b) | 1.0 (0, 171a) | 1.0 (0.7) |
| Stage at initial diagnosis of breast cancer, N (%) | ||||||
| I | 7 (6.5) | 8 (7.5) | 9 (8.6) | 6 (5.5) | 5 (9.4) | 4 (7.7) |
| II | 57 (53.3) | 53 (49.5) | 49 (46.7) | 61 (56.0) | 24 (45.3) | 25 (48.1) |
| III | 43 (40.2) | 46 (43.0) | 47 (44.7) | 42 (38.5) | 24 (45.3) | 23 (44.2) |
aOne patient was enrolled in the study with contralateral breast cancer diagnosis 1 month before enrollment, the initial diagnosis was 171 months before randomization.
bThe second longest duration since initial breast cancer diagnosis was 16 months before enrollment.
B, biosimilar; R, reference; SD, standard deviation.
Most frequent concomitant medications were corticosteroids (81.8% of patients in SAF set) including dexamethasone (71.0%), anti-emetics (73.4%) including ondansetron (67.8%), and anti-inflammatory/antirheumatics (23.8%). Concomitant medications were similar across treatment arms in all cycles.
efficacy—during cycle 1
Mean DSN for the PP set was 1.17 ± 1.11 days in the biosimilar (N = 101) and 1.20 ± 1.02 days in the reference arm (N = 103). Mean treatment difference was 0.04 days with lower limit of the 97.5% CI of −0.26 days. For the FAS set, DSN was 1.18 ± 1.12 days in the biosimilar (N = 107) and 1.20 ± 1.02 days in the reference arm (N = 107), with a mean treatment difference of 0.02 days and a lower limit of the 97.5% CI of −0.27 days. Biosimilar was noninferior to the reference, because the lower bound of the 97.5% CI was entirely above the predefined noninferiority margin of −1 day (Figure 1).
Figure 1.
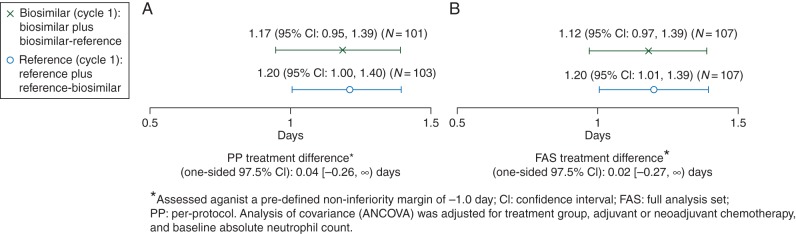
Duration of severe neutropenia with biosimilar and reference filgrastim during cycle 1 in the (A) per-protocol and (B) full analysis set populations.
Secondary efficacy parameters (FAS) are described in supplementary Table S1, available at Annals of Oncology online. Fever episodes were reported in seven (6.6%) patients in the biosimilar arm and three (2.8%) patients in the reference arm. Most resolved on the same day, with a maximum duration in both arms of 2 days. In patients with fever episodes, ≥1 episode of FN was recorded in five (4.7%) patients receiving biosimilar and two (1.9%) patients receiving reference and led to the hospitalization of one (0.9%) patient in each arm. Two (1.9%) patients in each arm experienced infections, of whom one (0.9%) patient receiving biosimilar also experienced ≥1episode of FN.
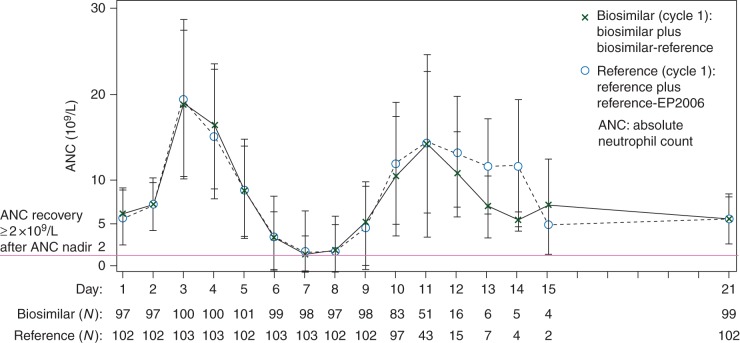
Mean ANC time courses with biosimilar and reference were superimposable (Figure 2), showing the expected increase at day 3 and subsequent decrease with nadir around days 7–8, followed by recovery and stable values from day 10 onwards. Only a small number of patients were still on treatment after day 12 so results after this time should be interpreted with caution. In all patients still on treatment after day 12, the ANC was above what would be considered critical.
Figure 2.
Time course of ANC (mean ± SD) in cycle 1 (PP set).
Median depth of ANC nadir was 0.30 × 109/l (range: 0−8.87) for the biosimilar and 0.25 × 109/l (range: 0−8.39) for the reference. Time of ANC nadir for the majority of patients in both treatment arms was day 7 (biosimilar, N = 60, 56.1%, reference, N = 59, 55.1%) or day 8 (biosimilar, N = 33, 30.8%, reference, N = 39, 36.4%). Time to ANC recovery (median) was 2.0 days for biosimilar (range: 0−6) and reference (range: 0−4). Grade 3/4 neutropenia (<1.0 × 109/l) was reported in 82 (77%) patients in the biosimilar arm and 85 (79%) patients in the reference arm.
efficacy—alternating versus nonalternating (over all cycles)
In the PP-I set, fever episodes occurred in 12 patients (13.5%) in the alternating and 8 patients (9.3%) in the nonalternating arm (supplementary Table S1, available at Annals of Oncology online). Median duration of fever was 0 days, with a maximum of 2 days in the alternating and 9 days in the nonalternating arm. In patients with fever, ≥1 episode of FN was recorded in six (6.7%) patients in the alternating and two (2.3%) patients in the nonalternating arm. Episodes of FN led to hospitalization in one (1.1%) patient in the alternating and two (2.3%) patients in the nonalternating arm. Infections occurred in nine (10.1%) patients in the alternating and six (7.0%) patients in the nonalternating arm. One (1.1%) patient with infection in the alternating arm experienced ≥1 fever episode and ≥1 episode of FN. Three (3.5%) patients with infection in the nonalternating arm also experienced ≥1 fever episode. Depth of ANC nadir in each cycle was similar in alternating and nonalternating treatment arms and occurred on days 7 or 8 in each cycle for the majority of patients in alternating and nonalternating arms.
efficacy—nonalternating (over all cycles)
Fever episodes were reported in 6 (15.0%) patients receiving biosimilar and 2 (4.3%) patients receiving reference (PP-I set, supplementary Table S1, available at Annals of Oncology online). Median duration of fever was 0 days, with a maximum of 2 days in both arms. Among patients with fever episodes, two (5.0%) in the biosimilar and none in the reference arm had ≥1 FN episode. Both patients with FN in the biosimilar arm were hospitalized. Infections occurred in two (5.0%) patients receiving biosimilar and four (8.7%) receiving reference. One (2.5%) patient with infection in the biosimilar and two (4.3%) in the reference arm also experienced ≥1 fever episode. Depth of ANC nadir in each cycle was similar in both treatments and was recorded on days 7 or 8 of each cycle in the majority of patients for both arms.
safety
anti-rhG-CSF antibody formation
No patient developed binding or neutralizing antibodies against G-CSF in any arm at any time during the study.
adverse events
Treatment-emergent AEs (TEAEs) over all cycles are summarized in supplementary Table S2, available at Annals of Oncology online. The number of patients who experienced TEAEs with suspected relationship to filgrastim was similar between the biosimilar (N = 22, 20.6%) and the reference arm (N = 21, 19.6%) in cycle 1.
One patient in the biosimilar arm died due to a pulmonary embolism (day 6, cycle 1), where a study drug relationship was not suspected. Serious AEs included FN, anemia, leukopenia, diarrhea, pulmonary embolism and hypertensive crisis. Numerically, but not significantly, more were reported in patients receiving the biosimilar (five patients) than in patients receiving the reference (two patients). Injection-site reactions were rare and of mild or moderate intensity in all treatment arms.
discussion
This study demonstrates that biosimilar filgrastim is noninferior to the US-licensed product in reducing the DSN in breast cancer patients receiving (neo-)adjuvant myelosuppressive chemotherapy. Similar ANC profiles were observed in both treatments. The number of patients with FN, although slightly higher in the biosimilar arm (4.7% versus 1.9% with reference), was still in the expected range of variability. Previous studies have shown that G-CSF is effective in reducing FN risk following chemotherapy, with incidence of FN in the first cycle ranging from 7% to 15% [10–13], higher than observed in this study. Hospitalization due to FN, incidence of infections, depth and time of ANC nadir and time to ANC recovery in cycle 1 and across all cycles were all similar between the biosimilar and reference groups.
Results of this study substantiate findings of previous studies with this biosimilar filgrastim [14] and are consistent with results of reference filgrastim studies in patients with breast cancer [11, 12]. Similar results comparable with the reference have also been reported with filgrastim biosimilars approved in Europe other than EP2006 [13, 15, 16].
No clinically meaningful differences were observed between the well-known safety profile of the reference and the biosimilar filgrastim. Most frequently reported AEs were alopecia, nausea, asthenia, fatigue and bone pain. The observed safety pattern is consistent with findings in other studies of filgrastim [17, 18].
Alternating between the biosimilar and the reference or vice versa showed no clinically meaningful differences regarding efficacy and safety. The immunogenic response to filgrastim assessed under the conditions of repeated alternating and nonalternating of products also showed no increased risk of developing anti-rhG-CSF antibodies, which is in accordance with the low immunogenic potential of filgrastim. The lack of an immunogenic response against biosimilar filgrastim is consistent with postmarketing surveillance where there have been no reports of neutralizing antibodies [7].
The study was conducted in Eastern European countries primarily for feasibility reasons. Patients were treated with TAC according to the approved label and international treatment guidelines. Inclusion and exclusion criteria ensured a homogenous population that is representative of breast cancer patients worldwide. The dose-limiting hematological toxicity of frequent grade 3–4 neutropenia represents a well-established model for studying and comparing G-CSFs. Since the mechanism of action of filgrastim is mediated by selective binding to the G-CSF receptor and is the same across different patient populations, similarity between the biosimilar and reference in the prevention of severe neutropenia shown in breast cancer patients can be extrapolated to other patient populations.
Medical products are approved on the basis of clinical trials that involve relatively small numbers of patients. Once medical products become available to the general population and for long-term use, postmarketing surveillance is required to collect safety data. This is a continuous process of evaluation that is accompanied by steps to improve their safe use.
This study demonstrates that biosimilar and reference filgrastim are similar with no clinically meaningful differences regarding efficacy and safety (also while alternating between the treatments) in the prevention of severe neutropenia in breast cancer patients receiving (neo)-adjuvant myelosuppressive chemotherapy. Biosimilar filgrastim could represent an important alternative, benefiting public health by increasing access to treatment.
funding
This work study was supported by Sandoz GmbH, Austria. No grant number is applicable.
disclosure
KB has provided advisory or consulting services to Amgen, Hospira, Novartis and Sandoz. NH has provided advisory or consulting services to Sandoz. RN, GS, PS and AS are employees of Sandoz. All remaining authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
The following investigators also enrolled and treated patients in this study: Jan Vydra, Nova Ves pod Plesi (Czech Republic); Janos Szanto, Debrecen; Jozsef Erfan, Nyiregyhaza; Jozsef Cseh, Szekesfehervar; Judit Kocsis, Budapest; Laszlo Landherr, Budapest; Miklos Wenczl, Szombathely (Hungary); Marianna Bitina, Daugavpils (Latvia); Marian Stresko, Nitra (Slovakia); Alexey Manikhas, Saint-Petersburg; Iya Smirnova, Obninsk; Marina Matrosova, Nizhny Novgorod; Oleg Gladkov, Chelyabinsk; Vadim Popov, Voronezh; Vladimir Vladimirov, Pyatigorsk (Russia); Grigoriy Adamchu, Lviv; Igor Bondarenko, Dnipropetrovsk; Oleksandr Berzoy, Odessa; Petro Odarchenko, Vinnytsya; Tetyana Shchetinina, Lugansk; Yaroslav Schparyk, Kriviy Rig (Ukraine).
references
- 1.Aapro MS, Bohlius J, Cameron DA et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer 2011; 47: 8–32. [DOI] [PubMed] [Google Scholar]
- 2.Almenar D, Mayans J, Juan O et al. Pegfilgrastim and daily granulocyte colony-stimulating factor: patterns of use and neutropenia-related outcomes in cancer patients in Spain—results of the LEARN Study. Eur J Cancer Care (Engl) 2009; 18: 280–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falandry C, Campone M, Cartron G et al. Trends in G-CSF use in 990 patients after EORTC and ASCO guidelines. Eur J Cancer 2010; 46: 2389–2398. [DOI] [PubMed] [Google Scholar]
- 4.Barni S, Lorusso V, Giordano M et al. A prospective observational study to evaluate G-CSF usage in patients with solid tumors receiving myelosuppressive chemotherapy in Italian clinical oncology practice. Med Oncol 2014; 31: 797. [DOI] [PubMed] [Google Scholar]
- 5.Weycker D, Barron R, Edelsberg J et al. Incidence of reduced chemotherapy relative dose intensity among women with early stage breast cancer in US clinical practice. Breast Cancer Res Treat 2012; 133: 301–310. [DOI] [PubMed] [Google Scholar]
- 6.Potosky AL, Malin JL, Kim B et al. Use of colony-stimulating factors with chemotherapy: opportunities for cost savings and improved outcomes. J Natl Cancer Inst 2011; 103: 979–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gascón P, Tesch H, Verpoort K et al. Clinical experience with Zarzio® in Europe: what have we learned? Support Care Cancer 2013; 21: 2925–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nabholtz JM, Mackey JR, Smylie M et al. Phase II study of docetaxel, doxorubicin, and cyclophosphamide as first-line chemotherapy for metastatic breast cancer. J Clin Oncol 2001; 19: 314–321. [DOI] [PubMed] [Google Scholar]
- 9.Kaufman PA, Paroly W, Rinaldi D et al. Randomized, double-blind, phase 2 study evaluating the safety of same vs next day administration of pegfilgrastim with docetaxel, doxorubicin and cyclophosphamide (TAC) in women with breast cancer. Breast Cancer Res Treat 2004; 88(S59): abstr 1054. [Google Scholar]
- 10.Cooper KL, Madan J, Whyte S et al. Granulocyte colony-stimulating factors for febrile neutropenia prophylaxis following chemotherapy: systematic review and meta-analysis. BMC Cancer 2011; 11: 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holmes FA, O'Shaughnessy JA, Vukelja S et al. Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage II or stage III/IV breast cancer. J Clin Oncol 2002; 20: 727–731. [DOI] [PubMed] [Google Scholar]
- 12.Green MD, Koelbl H, Baselga J et al. A randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol 2003; 14: 29–35. [DOI] [PubMed] [Google Scholar]
- 13.del Giglio A, Eniu A, Ganea-Motan D et al. XM02 is superior to placebo and equivalent to Neupogen in reducing the duration of severe neutropenia and the incidence of febrile neutropenia in Cycle 1 in breast cancer patients receiving docetaxel/doxorubicin chemotherapy. BMC Cancer 2008; 8: 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gascón P, Fuhr U, Sörgel F et al. Development of a new G-CSF product based on biosimilarity assessment. Ann Oncol 2010; 21: 1419–1429. [DOI] [PubMed] [Google Scholar]
- 15.Gascón P. Presently available biosimilars in hematology-oncology: G-CSF. Targ Oncol 2012; 7(suppl 1): S29–S34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waller CF, Semiglazov VF, Tjulandin S et al. A phase III randomized equivalence study of biosimilar filgrastim versus Amgen filgrastim in patients receiving myelosuppressive chemotherapy for breast cancer. Onkologie 2010; 33: 504–511. [DOI] [PubMed] [Google Scholar]
- 17.Abraham I, Tharmarajah S, MacDonald K. Clinical safety of biosimilar recombinant human granulocyte colony-stimulating factors. Expert Opin Drug Saf 2013; 12: 235–246. [DOI] [PubMed] [Google Scholar]
- 18.Renner P, Milazzo S, Liu JP et al. Primary prophylactic colony-stimulating factors for the prevention of chemotherapy-induced febrile neutropenia in breast cancer patients. Cochrane Database Syst Rev 2012; 10: CD007913. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.