Abstract
Once released, manure-borne bacteria can enter runoff via interaction with the thin mixing layer near the soil surface. The objectives of this work were to document temporal changes in profile distributions of manure-borne Escherichia coli and enterococci in the near-surface soil layers after simulated rainfalls and to examine differences in survival of the two fecal indicator bacteria. Rainfall simulations were performed in triplicate on soil-filled boxes with grass cover and solid manure application for 1 h with rainfall depths of 30, 60, and 90 mm. Soil samples were collected weekly from depth ranges of 0 to 1, 1 to 2, 2 to 5, and 5 to 10 cm for 1 month. Rainfall intensity was found to have a significant impact on the initial concentrations of fecal indicator bacteria in the soil. While total numbers of enterococci rapidly declined over time, E. coli populations experienced initial growth with concentration increases of 4, 10, and 25 times the initial levels at rainfall treatment depths of 30, 60, and 90 mm, respectively. E. coli populations grew to the approximately the same level in all treatments. The 0- to 1-cm layer contained more indicator bacteria than the layers beneath it, and survival of indicator bacteria was better in this layer, with decimation times between 12 and 18 days after the first week of growth. The proportion of bacteria in the 0- to 1-cm layer grew with time as the total number of bacteria in the 0- to 10-cm layer declined. The results of this work indicate the need to revisit the bacterial survival patterns that are assumed in water quality models.
INTRODUCTION
Fecal contamination of soil and water presents a major worldwide health risk. When pathogenic microorganisms enter soil or water, they can infect humans via drinking or recreational activities and through consumption or handling of contaminated produce. The Centers for Disease Control and Prevention (1) estimated in 2011 that each year nearly 1 in 6 Americans (or 46 million people) get sick and roughly 3,000 die from foodborne diseases. Another study performed in the same year estimated that foodborne pathogens resulted in medical care costs in the range from $4.4 billion to $33.0 billion (2). Contamination of soil and water leading to foodborne illness can originate from numerous sources, such as direct deposition by animals, overflow or leakage of faulty septic and sewage systems, or manure application to agricultural fields and pastures (3–5). The potential presence of pathogens that cause foodborne illness can be inferred via the monitoring of fecal indicator bacteria (FIB), such as Escherichia coli and enterococci (6).
It has been found that both E. coli and enterococci can survive in the soil for several weeks after deposition, if not longer (7, 8). Some studies have found that populations of these organisms can experience initial growth in the soil that can extend their prevalence for longer than previously thought. For example, Lau and Ingham (9) reported that E. coli and enterococci survived in soil following manure incorporation for more than 19 weeks. Avery et al. (10) observed E. coli persistence in soils amended with cattle, sheep, and swine manure for up to 19 weeks, while Entry et al. (11) observed prolonged survival of fecal coliforms and enterococci (at least 42 weeks) in soils amended with dairy manure. Such extended survival in soils can result in the occurrence of indicator organisms in runoff and infiltration waters from sites that have not had prior manure applications for weeks, months, or even years (12, 13).
Suspended, dissolved, and adsorbed substances and organisms are known to be released to runoff from a thin near-surface layer of soil. Ahuja et al. (14) proposed the term “effective depth of interaction” (EDI), defined as the thickness of surface soil in which the degree of interaction is equal to that at the soil surface. The soil layer between the surface and EDI is commonly called the “mixing layer.” Sharpley (15) estimated the EDI to be 3 to 5 mm for a 5° slope in experiments with clay loam and sandy loam soils under 50 mm per h of simulated rainfall. Yang et al. (16) found EDI values in the range from 1 to 11 mm, such that the value increased with increased rainfall intensity and slope. Sanchez and Boll (17) experimented with a 5-mm-thick mixing layer enriched with phosphorus. Tong et al. (18) estimated a mixing layer thickness of 1.5 cm for unsaturated soil and noted the decrease of the EDI with increasing saturation. Mamo et al. (19) and Owens et al. (20) indicated the need to account for profile distributions of P in topsoil to correctly model the phosphorus losses from land to water. E. coli concentrations in runoff were related to E. coli contents in the top 1-cm soil layer by Muirhead and Monaghan (21).
Field data on survival of manure-borne organisms in soils have typically been collected from core samples encompassing a range of depths much deeper than the mixing layer thickness estimates. For example, in experiments with surface-applied manures, samples were taken from the surface to depths of 1 cm (13), 2.6 cm (22), 3 cm (23), 4 cm (24), 5 cm (10, 25–27), 7.5 cm (28), 10 cm (11, 29–31), 15 cm (32, 33), and 20 cm (34–36). In only one study (37) were concentrations of E. coli reported for several depth ranges: 0 to 2.5 cm, 2.5 to 5 cm, and 5 to 25 cm. That study applied dairy cattle slurry on surfaces of grassed lysimeters and found only 1% of bacteria from the applied manure in the 2.5- to 5-cm layer, while the rest of the bacteria were in the 0- to 2.5-cm layer.
Given the scarcity of the information on survival of bacteria near the soil surface at depths comparable with the depth of the mixing layer estimates, the objectives of this study were (i) to document temporal changes in profile distributions of manure-borne E. coli and enterococci in the near-surface soil layers after simulated rainfalls and (ii) to examine differences in survival characteristics of the two fecal indicator bacteria.
MATERIALS AND METHODS
Experiments were performed at the Beltsville Agricultural Research Center (BARC). A variable controlled-intensity rainfall simulator (38) was used to apply rainfall for the release of manure-borne E. coli and enterococci from soil-applied dairy cattle manure to runoff and infiltration. The rainfall simulator sprinkler nozzles (Veejet 80150; Spraying Systems Co., Wheaton, IL) were positioned 3 m above the soil surface, which allowed rain drops to reach near-terminal velocity upon landing, with an energy impact of approximately 275 kJ ha−1 mm−1, which is similar to natural rainfall events greater than 25 mm h−1. The rainfall simulator was calibrated to deliver a relatively uniform rainfall distribution for a central 1-m2 area with a Christiansen coefficient of uniformity in the range from 84% to 86%.
The 100-cm by 26.5-cm by 15-cm experimental boxes, described by Isensee and Sadeghi (39) and Faucette et al. (40), were filled with a sandy loam soil. A 2-cm-thick sand layer was evenly placed over the bottom of the box to facilitate infiltration release through the three mesh-covered drains. On top of this initial sand layer, six additional layers of an air-dried sandy loam soil (a mixture of various USDA-ARS Beltsville A horizons of no single soil series) that had been screened prior to placement in the soil boxes were added. Each layer was evenly spread throughout the box, packed flat with a plywood board, and then scored at the surface prior to placement of additional layers atop it. This packing procedure was performed to create a uniform bulk density throughout the box. The final bulk density of the soil boxes was 1.34 ± 0.07 g cm−3. The soil textural composition was 63.8% sand, 24.8% silt, and 11.4% clay, and the chemical properties were pH 7.0, electrical conductivity in 1:2 paste of 0.36 mmhos cm−1, and an average total C of 2.23%.
Packed soil boxes were placed in a temperature-controlled hoop house set to operate at 18°C at the USDA-ARS BARC North Farm in Beltsville, MD. The soil in each box was watered and cross-scored at the surface before Kentucky 31 tall fescue grass seed was applied at a rate of 49 g m−2. Boxes were watered twice daily until germination and then once a day following germination. After 20 days of grass growth, the soil boxes were overseeded with additional grass seed at the same rate as previously indicated to fill in spots of uneven growth in the boxes. An additional 2 kg of topsoil was added to cover the newly added seed. Daily watering continued until the newly added seeds germinated, and then watering was reduced to once every 2 to 3 days.
Manure was prepared by mixing fresh dairy cattle excreta collected at the USDA-ARS Dairy Research Facility, with sawdust bedding to reach a 30% dry solid content. The manure application rate of each soil box was 60 tons ha−1 (2.1 kg box−1). The manure properties as determined in the Penn State Agricultural Analytical Services Laboratory were pH 8.25 ± 0.16, carbon content of 14.9% ± 1.0%, and C/N ratio of 40.9 ± 5.6. The contents of E. coli and enterococci were (5.30 ± 4.24) × 105 CFU per g dry weight (gdw−1) and (3.81 ± 1.64) × 106 CFU gdw−1, respectively, shown as the average ± standard error (R. Blaustein, personal communication).
The synthetic rainwater was prepared by adding reagent-grade chemicals to deionized (DI) water to obtain a rainfall composition typical for the Maryland, Pennsylvania, and Delaware region, with concentrations of Ca2+, Mg2+, K+, Na+, NH4+, NO3−, Cl−, and SO42− of 0.08, 0.03, 0.02, 0.12, 0.34, 1.36, 0.26, and 1.9 mg liter−1, respectively (41). Rainfall water pH was adjusted to 4.5 just prior to the experiments. Boxes were placed within the central 1-m2 area and adjusted to a 5% slope steepness. Antecedent water contents in each soil box were made uniform by applying a pre-wetting rainfall simulation event for 30 min at an intensity of 3 cm h−1 24 h prior to the actual experiment. Rainfall in the amounts of 30, 60, and 90 mm was applied for 1 h in triplicate for each rainfall intensity according to the randomized design for the sequence of the irrigations.
Each box was transported to the hoop house shortly after the simulated rainfall. Soil was sampled in triplicate from four depths and at three locations from each box weekly for 1 month. Specifically, soil samples were taken with a sterilized handheld soil core probe to a depth of 10 cm in triplicate at 30-, 50-, and 70-cm length marks along the box. The 10-cm-length cores were then subdivided into 0- to 1-cm, 1- to 2-cm, 2- to 5-cm, and 5- to 10-cm sections. This sampling was performed at 30-, 50-, and 70-cm length marks in the box to see if any effect of location existed. All subsamples were immediately placed in sterile bottles on ice until processing.
Hoop house air temperature was recorded with a HOBO Pendant temperature/light data logger (Onset Computer Corporation, Bourne, MA). The grass in each box was sprinkler watered with approximately 7 mm of water once a week immediately after soil sampling.
Microbiological analyses were done with an approximately 2-g subsample of each soil sample. Subsamples were placed into sterile blenders, where they were ground with 200 ml of sterile DI water for 2 min to create an initial dilution factor of 10−2. Samples were then poured into sterile beakers and left to settle for 1 h. After settling, the supernatant was diluted, and E. coli contents were determined using a Colilert 18 and a QuantiTray 2000 (IDEXX Laboratories, Inc., Westbrook, MA). From the initial blended sample, 250 μl of supernatant was pipetted onto m-Enterococcus agar (Neogen Corporation, Lansing, MI) and then spread plated to enumerate enterococci. Plates were then incubated for 48 h at 35 ± 0.5°C. A portion of the unblended original sample was weighed and placed in a drying oven for at least 24 h to determine water content. Results of microbial analysis are presented as most probable number (MPN) per gram of dry weight and CFU per gram of dry weight for E. coli and enterococci, respectively. Total numbers of bacteria in soil were computed for columns having the 1-cm2 cross-section and height equal to the soil layer thickness.
Decimal reduction times were calculated by fitting the data in coordinates of time versus total organism number to the exponential decay equation
| (1) |
with two estimated parameters: a, the decimal reduction time (i.e., the time when the computed concentration is 10 times less than the initial concentration), and c0, the effective concentration at time zero. The values of a and c0 were estimated from concentrations observed on days 7 to 21 when equation 1 was deemed to be valid; therefore c0 did not represent the actual initial concentration, whereas a represented the inactivation rate.
The total numbers of organisms in soil, NT, were obtained as the results of summation by layers
| (2) |
where NT is the total number of organisms within the 10-cm-thick soil layer per 1-cm2 surface of soil, ci (i = 1, 2, 3, 4) is the concentration of microorganisms (in MPN and CFU per gram of dry soil for E. coli and enterococci, respectively) in layer i, hi (i = 1, 2, 3, 4) represents the thicknesses of layers 0 to 1, 1 to 2, 2 to 5, and 5 to 10 cm (i.e., 1, 1, 3, and 5 cm, respectively), and ρi (i = 1, 2, 3, 4) represents the soil bulk densities in layers.
Data analysis.
All experiments were performed in triplicate. Tray and plate counts for E. coli and enterococci, respectively, were converted to dry weight equivalents and subjected to a multifactorial analysis of variance (Sigmaplot 12.5; Systat Software, Inc., San Jose, CA). Statistical significance was evaluated at the 0.05 probability level.
RESULTS
Soil water content was not significantly different between any of the rainfall intensities immediately after rainfall application or at any point during the experiment (P = 0.934). Soil moisture significantly declined from week to week in all instances (P < 0.001). Soil water storage changes over time are shown in the top panel of Fig. 1. The bars represent the mean soil moisture of all depths.
FIG 1.

Mean soil water contents in the beginning of each week (top) and mean daily air temperatures (bottom). The plotted values are averages across three replications. Error bars show standard errors.
Average daily air temperatures are shown in the bottom panel of Fig. 1. Temperature regimes were slightly different among the treatments due to the randomized experimental design, which resulted in boxes arriving at the hoop house at slightly different times.
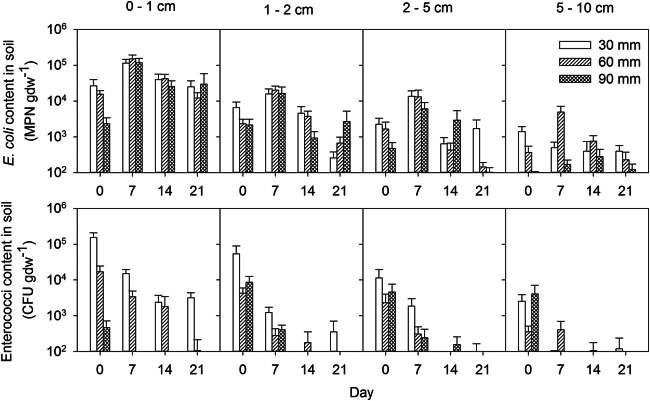
Bacterial survival by layers is shown in Fig. 2. The pattern of the initial increase of bacterial content followed by decrease was observed at all depths for E. coli. Enterococcal contents steadily decreased at all depths. The decimation times could be approximately estimated for E. coli but not for enterococci. The latter organism had survival dynamics that did not follow equation 1. Decimal reduction times for concentrations of E. coli at the four examined depths and across the three rainfall intensities are shown in Table 1. The estimated decimation times decreased with depth in the 0- to 5-cm layer but were very large in the 5- to 10-cm layer.
FIG 2.
Changes in E. coli and enterococcus contents in soil layers over time. Error bars show standard errors of concentrations.
TABLE 1.
Average decimal reduction time for E. coli for the period from day 7 to day 21 of the experiment
| Depth (cm) | Decimation time (days) at simulated rainfall depth of: |
||
|---|---|---|---|
| 30 mm | 60 mm | 90 mm | |
| 0–1 | 18.0 | 12.7 | 15.7 |
| 1–2 | 11.7 | 9.4 | 7.1 |
| 2–5 | 6.0 | 4.8 | 15.7 |
| 5–10 | 130.8 | NDa | 150.6 |
ND, not determined.
The total initial numbers of bacteria in the soil boxes following simulated rainfall varied with the amounts of water applied (Fig. 3). Total E. coli numbers significantly differed between the 30- and 90-mm treatments (P < 0.05) and between the 60- and 90-mm treatments (P < 0.05) but did not significantly differ between the 30-mm and 60-mm. Total numbers of enterococci varied significantly between the 30- and 60-mm treatments (P < 0.05) as well as between the 30- and 90-mm treatments (P < 0.05) but were not significantly different for the 60- and 90-mm treatments. No significant trends were observed between the total number of organisms and sampling locations along the box (data not shown). E. coli and enterococcus populations exhibited very different survival dynamics (Fig. 3). The E. coli population at all three rainfall intensities exhibited initial growth during the week following the application of simulated rainfall. After the initial growth phase, E. coli numbers began to decline but remained relatively similar to initial values by the end of the 21-day-long experiment. Initial total numbers of E. coli in the soil were not significantly different from the final total numbers in the 30-, 60-, and 90-mm/h treatments (P = 0.418, P = 0.122, and P = 0.808, respectively). Concentrations of E. coli in the 90-mm treatment boxes were higher than initial concentrations at the end of the experiment. The total numbers of enterococci in the soil boxes consistently declined throughout the duration of the experiment, with concentrations in the 60- and 90-mm treatment boxes being undetectable by the end of the experiment.
FIG 3.
Total number of E. coli cells (MPN per square centimeter) and enterococci (CFU per square centimeter) in soil over time following the application of 30-, 60-, or 90-mm h−1 rainfalls. Error bars show standard errors of concentrations.
E. coli populations in soil did not significantly differ between rainfall treatments 1 week after the application of simulated rainfall (P = 0.587). Conversely, enterococcus populations were significantly different between the three rainfall intensities 1 week after the application of simulated rainfall (P < 0.001).
Figure 4 shows the relative contribution of each soil layer to the vertical distribution of the total number of organisms within the soil profile. Both indicator organisms were overwhelmingly concentrated in the surface layer of 0 to 1 cm. In almost all instances, the number of bacteria in the surface layer accounted for a greater proportion of the overall population than the next three depths combined. Additionally in most cases, the overall proportion of bacteria in the surface layer increased over time as concentrations in the lower depths declined.
FIG 4.
Distributions of indicator organisms in soil for three rainfall intensities on observation times. Error bars show standard errors.
DISCUSSION
The initial populations of fecal indicators in soil after rainfall simulation showed a dependence on the amount of simulated rainfall applied (Fig. 2). The total numbers were generally lower as the amount of rain water increased. Possible reasons for this include the increased leaching of bacteria through macropores in the soil with an increase in rainfall intensity (42), formation of a layer of the dispersed manure material on the soil surface that would prevent bacterial accumulation within the soil layer (43), and/or the slowdown of manure bacterial release with the increased amount of applied water (44) that would lead to a larger dilution of released bacteria with rainfall water within the manure layer before it reaches soil.
All treatments in this work resulted in qualitatively similar microbial population dynamics within each microorganism group. E. coli concentrations initially increased and then slowly decreased, whereas populations of enterococci began to decrease from the start and were not detectable after 4 weeks in all cases, except for treatments that received the least intense rainfall application, where they remained at a total number that was 1.3% of the initial population. The initial growth of E. coli populations was observed in the top 1-cm layer, where large manure particles were strained and provided a nutrient-rich environment. Some of the dissolved manure nutrient material may have moved to deeper layers with the infiltrating water. Soil water contents also changed with depth such that greater water contents were observed close to the surface, where the conditions were more favorable for E. coli survival. Additionally, the presence of oxygen results in aerobic respiration, which is the most productive metabolic mode for E. coli (45). Increased enterococcus survival in top layers may also be explained by this as enterococci are facultative anaerobes as well. The loss of some water from the soil occurred due to plant transpiration and evaporation from the soil surface; dissolved nutrients moved to the surface with the evapo-transpired water and accumulated there. E. coli may have responded to the increasing concentrations of nutrients near the surface via chemotaxis (46).
E. coli concentrations in soil were not significantly different among the treatments 1 week after simulated rainfall, whereas the initial concentrations of indicator organisms in the soil decreased significantly with increasing amounts of water applied. One possible explanation is that E. coli growth was limited by competition or predation such that E. coli populations above a certain level did not depend on the differences in initial concentrations within the observed range.
Decimal reduction times for E. coli demonstrated a trend of decreasing with increased depth. This observation held true in most cases, but E. coli survival data for the deepest depth could not be adequately fitted with equation 1; hence, the 5- to 10-cm values are many times higher than values for shallower depths. These values, however, cannot be considered reliable since they are outside the duration of the experiment.
Numerous factors could be responsible for the difference in survival dynamics of E. coli and enterococci. E. coli populations have been shown to experience initial growth phases in soil following rainfall events and rises in water tables (47, 48). Sinton et al. (49) found that after flooding events, enterococci did not show growth in deposited cow feces and that the organism was quickly inactivated in all seasons. These conditions are comparable to the conditions in the soil environment following the simulated rainfall events performed in this study. Wang et al. (50) observed the initial growth phases of both organisms in cow manure but found a survival-enhancing moisture effect only for fecal streptococci, which may indicate that enterococci are sensitive to low-moisture conditions. Another study that looked at survival in cowpats found that when water content falls between 70 and 75%, fecal streptococcus exhibits slower decay than E. coli; however, under dry conditions, E. coli exhibited slower decay (49). This finding is consistent with the decrease of soil water content in the soil boxes throughout the experiment and with each organism's unique survival. Solo-Gabriele et al. (51) found that E. coli was able to multiply as soil was drying, with the concentration increasing several orders of magnitude. The authors hypothesized that this was due to the capability of E. coli to survive under dry conditions with limited competitor/predator involvement. Howell et al. (52) observed that fecal coliforms experienced greater growth under warm conditions than fecal streptococcus. This study found that fecal coliforms often experienced regrowth, while fecal streptococci did not, which is similar to what was observed for E. coli versus enterococci in the present study. Kibbey et al. (53) posited that increased soil temperatures resulted in increased microflora activity within soils. This increased activity may have had a greater negative effect on Enterococcus faecalis than on E. coli. Byappanahalli (54) also found that enterococcal growth is hampered by competition from native soil biota. Yet another possible explanation for different survival dynamics is the availability of nutrients within the soil. Both E. coli and enterococci are facultative anaerobic bacteria and can thus survive in both oxygen-rich and oxygen-poor environments. However, E. coli is versatile in its ability to obtain energy and only requires simple carbon and nitrogen sources (55), whereas enterococci have more complex nutrient requirements (56). The soil boxes did not receive any nutrient addition after the initial application of manure, and this lack of nutrient inputs may have had detrimental effects on both organisms, of which enterococcus was more susceptible because of its complex nutrient requirements. Both organisms were less persistent at lower depths, and it has been has speculated that this may be partially due to low nitrogen availability (57).
We realize that the weekly refreshing irrigation, albeit in small amounts, may have influenced the results. Lau and Ingham (9) found no significant difference in population decline between E. coli and enterococci when biweekly watering was implemented, but when watered once a week, E. faecalis declined significantly faster than E. coli. We also note that the observed differences in survival patterns may be specific for the sandy loam texture of the soil used in this study. Cools et al. (58) found that Enterococcus spp. outsurvived E. coli in fine-textured soils, whereas in sandy soil E. coli thrived and Enterococcus spp. showed relatively poor survival.
Substantial temperature oscillations were encountered by bacteria in experiments of this work. Temperature oscillations have been shown to impact bacterial survival in manure. Semenov et al. (59) demonstrated the dependence of E. coli O157:H7 survival in cow manure on the amplitude of temperature oscillations. Freeze-thaw cycles were detrimental for survival of Yersinia enterocolitica in the work of Asadishad et al. (60). The effect of temperature oscillations on survival of manure-borne bacteria in soil has not been studied and can present an interesting avenue for future work.
The initial growth of E. coli and enterococcus populations in soils after their release from manure can undoubtedly complicate the development of regulatory guidance. The extended persistence of indicator bacteria can lead to false indications of recent fecal contamination where there has been none. On the other hand, the common assumption that once deposited into extraenteric environments E. coli experiences immediate exponential decay (61) is obviously not valid in the present study. More information on the survival patterns of manure-borne indicator microorganisms after their release in soil needs to be collected to make best management practices more efficient.
Results on retention, growth, and survival of indicator microorganisms in the thin top layer of soil present substantial interest for microbial water quality modeling. Popular watershed models, such as SWAT and KINEROS/STWIR, account for bacterial contributions from the top 1 cm of soil, where mass exchange between the soil and runoff is assumed to occur. In the present study, 40 to 100% of all detected bacteria were found within the top 1 cm. Results from this work show that much higher concentrations of bacteria are available to be released from soil to runoff when concentrations of bacteria in the mass exchange layer are computed from sampling of surface soil layers several centimeters thick. To be used in modeling with the mass exchange layer, or mixing zone, such data on bacterial concentrations have to be adjusted to reflect realistic profile distributions. Another consequence of the observations in this work is the limited validity of the model assumption of the immediate exponential decay of microorganisms excreted by livestock or wildlife. In this study, it was found that E. coli released from manure with other components of the manure matrix can experience initial growth in soils. Therefore, the assumption of immediate exponential decay for this indicator organism may also introduce errors in modeling that need to be evaluated to decide on the need for model modifications.
Conclusions.
Rainfall intensity had a significant impact on initial concentrations of indicator bacteria deposited from manure to soil. Increasing rainfall intensity resulted in reduced concentrations of deposited bacteria. Concentrations of indicator bacteria decreased with increasing depth. E. coli and enterococci were both found to persist in soil layers up to 10 cm deep. Most of the released bacteria stayed in the top 1-cm layer of soil, which is the mixing zone for resuspension of manure particles and bacteria during subsequent rainfall-runoff events. The best survival conditions were also in the top 1-cm layer. Total numbers of E. coli cells increased at all rainfall intensities between simulated rainfall and 1 week afterwards, whereas enterococci declined in each treatment and at each depth. Different survival dynamics were observed for each rainfall intensity, which demonstrated rainfall intensity as a factor in survival, although this relationship varied by organism and by depth. The findings of this work indicate the need to investigate whether current microbial water quality models need to be amended to account for the initial bacterial growth phase.
ACKNOWLEDGMENTS
We thank Ryan Blaustein and Billie Griffith for superb technical assistance.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2011. CDC estimates of foodborne illness in the United States. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/foodborneburden/PDFs/FACTSHEET_A_FINDINGS_updated4-13.pdf Accessed 24 August 2014. [Google Scholar]
- 2.Hoffmann S, Batz MB, Morris JG Jr. 2012. Annual cost of illness and quality-adjusted life year losses in the United States due to 14 foodborne pathogens. J Food Prot 75:1292–1302. doi: 10.4315/0362-028X.JFP-11-417. [DOI] [PubMed] [Google Scholar]
- 3.Van Donsel DJ, Geldreich EE, Clarke NA. 1967. Seasonal variations in survival of indicator bacteria in soil and their contribution to storm-water pollution. Appl Microbiol 15:1362–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang T, Wang H, Wu L, Lou J, Wu J, Brookes PC, Xu J. 2013. Survival of Escherichia coli O157:H7 in soils from Jiangsu Province, China. PLoS One 8:e81178. doi: 10.1371/journal.pone.0081178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sjogren RE. 1993. Prolonged survival of an environmental Escherichia coli in laboratory soil microcosms. Water Air Soil Pollut 75:389–403. [Google Scholar]
- 6.US Environmental Protection Agency. 1986. Bacteriological water quality criteria for marine and fresh recreational waters. EPA-440/5-84-002. US Environmental Protection Agency, Office of Water Regulations and Standards, Cincinnati, OH. [Google Scholar]
- 7.Rogers SW, Donnelly M, Peed L, Kelty CA, Mondal S, Zhong Z, Shanks OC. 2011. Decay of bacterial pathogens, fecal indicators, and real-time quantitative PCR genetic markers in manure-amended soils. Appl Environ Microbiol 77:4839–4848. doi: 10.1128/AEM.02427-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharples KE, Stratton GW, Madani SA, Gordon RJ, Patterson G. 2004. The survival of E. coli in agricultural soil treated with dairy manure, paper 042209. 2004 ASAE Annu Meet American Society of Agricultural and Biological Engineers, St. Joseph, MI. doi: 10.13031/2013.16415. [DOI] [Google Scholar]
- 9.Lau MM, Ingham SC. 2001. Survival of faecal indicator bacteria in bovine manure incorporated into soil. Lett Appl Microbiol 33:131–136. doi: 10.1046/j.1472-765x.2001.00962.x. [DOI] [PubMed] [Google Scholar]
- 10.Avery SM, Moore A, Hutchison ML. 2004. Fate of Escherichia coli originating from livestock faeces deposited directly onto pasture. Lett Appl Microbiol 38:355–359. doi: 10.1111/j.1472-765X.2004.01501.x. [DOI] [PubMed] [Google Scholar]
- 11.Entry JA, Leytem AB, Verwey S. 2005. Influence of solid dairy manure and compost with and without alum on survival of indicator bacteria in soil and on potato. Environ Pollut 138: 212–218. doi: 10.1016/j.envpol.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Brennan FP, O'Flaherty V, Kramers G, Grant J, Richards KG. 2010. Long-term persistence and leaching of Escherichia coli in temperate maritime soils. Appl Environ Microbiol 76:1449–1455. doi: 10.1128/AEM.02335-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muirhead RW. 2009. Soil and faecal material reservoirs of Escherichia coli in a grazed pasture. N Z J Agric Res 52:1–8. doi: 10.1080/00288230909510483. [DOI] [Google Scholar]
- 14.Ahuja LR, Sharpley AN, Yamamoto M, Menzel RG. 1981. The depth of rainfall-runoff-soil interaction as determined by 32P. Water Resour Res 17:969–974. doi: 10.1029/WR017i004p00969. [DOI] [Google Scholar]
- 15.Sharpley AN. 1985. Depth of surface soil-runoff interaction as affected by rainfall, soil slope, and management. Soil Sci Soc Am J 49:1010–1015 doi: 10.2136/sssaj1985.03615995004900040044x. [DOI] [Google Scholar]
- 16.Yang T, Wang Q, Xu D, Lv J. 2015. A method for estimating the interaction depth of surface soil with simulated rainfall. CATENA 124:109–118. doi: 10.1016/j.catena.2014.09.009. [DOI] [Google Scholar]
- 17.Sanchez M, Boll J. 2005. The effect of flow path and mixing layer on phosphorus release: physical mechanisms and temperature effects. J Environ Qual 34:1600–1609. doi: 10.2134/jeq2004.0306. [DOI] [PubMed] [Google Scholar]
- 18.Tong JX, Yang JZ, Hu BX, Bao RC. 2010. Experimental study and mathematical modeling of soluble chemical transfer from unsaturated/saturated soil to surface runoff. Hydrol Processes 24:3065–3073. doi: 10.1002/hyp.7722. [DOI] [Google Scholar]
- 19.Mamo M, Ginting D, Zanner CW, McCallister DL, Renken RR, Shapiro CA. 2005. Phosphorus stratification and potential for runoff loss following long term manure application. J Soil Water Conserv 60:243–250. [Google Scholar]
- 20.Owens PN, Deeks LK, Wood GA, Betson MJ, Lord EI, Davison PS. 2008. Variations in the depth distribution of phosphorus in soil profiles and implications for model-based catchment-scale predications of phosphorus delivery to surface waters. J Hyrdrol 350:317–328. doi: 10.1016/j.jhydrol.2007.10.043. [DOI] [Google Scholar]
- 21.Muirhead RW, Monaghan RM. 2012. A two reservoir model to predict Escherichia coli losses to water from pastures grazed by dairy cows. Environ Int 40:8–14. doi: 10.1016/j.envint.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Islam M, Doyle MP, Phatak SC, Millner P, Jiang X. 2004. Persistence of enterohemorrhagic Escherichia coli O157:H7 in soil and on leaf lettuce and parsley grown in fields treated with contaminated manure composts or irrigation water. J Food Prot 67:1365–1370. [DOI] [PubMed] [Google Scholar]
- 23.Chandler DS, Craven JA. 1980. Relationship of soil moisture to survival of Escherichia coli and Salmonella typhimurium in soils. Aust J Agric Res 31:547–555. doi: 10.1071/AR9800547. [DOI] [Google Scholar]
- 24.Wood JD, Bezanson GS, Gordon RJ, Jamieson R. 2010. Population dynamics of Escherichia coli inoculated by irrigation into the phyllosphere of spinach grown under commercial production conditions. Int J Food Microbiol 143:198–204. doi: 10.1016/j.ijfoodmicro.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 25.Nyberg KA, Vinneras B, Ottoson JR, Aronsson P, Albihn A. 2010. Inactivation of Escherichia coli O157:H7 and Salmonella Typhimurium in manure-amended soils studied in outdoor lysimeters. Appl Soil Ecol 46:398–404. doi: 10.1016/j.apsoil.2010.10.004. [DOI] [Google Scholar]
- 26.Entry JA, Hubbard RK, Thies JE, Fuhrmann JJ. 2000. The influence of vegetation in riparian filterstrips on coliform bacteria. II Survival in soils. J Environ Qual 29:1215–1224. [Google Scholar]
- 27.Crane SR, Westerman PW, Overcash MR. 1980. Die-off of fecal indicator organisms following land application of poultry manure. J Environ Qual 9:531–537. doi: 10.2134/jeq1980.00472425000900030042x. [DOI] [Google Scholar]
- 28.Nicholson FA, Groves SJ, Chambers BJ. 2004. Pathogen survival during livestock manure storage and following land application. Bioresour Technol 96:135–143. doi: 10.1016/j.biortech.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 29.Saunders O, Harrison J, Fortuna A-M, Whitefield E, Bary A. 2011. Effect of anaerobic digestion and application method on the presence and survivability of E. coli and fecal coliforms in dairy waste applied to soil. Water Air Soil Pollut 223:1055–1063. doi: 10.1007/s11270-011-0923-5. [DOI] [Google Scholar]
- 30.Ohtomo R, Minato K, Saito M. 2004. Survival of Escherichia coli in a field amended with cow feces slurry. Soil Sci Plant Nutr 50:575–581. doi: 10.1080/00380768.2004.10408514. [DOI] [Google Scholar]
- 31.Entry JA, Bjornberg DL, Vervey S. 2010. Influence of tillage and daily manure application on the survival of bacterial pathogens indicators in soil and on radish. Appl Environ Soil Sci 2010:973925. doi: 10.1155/2010/973925. [DOI] [Google Scholar]
- 32.Stoddard CS, Coyne MS, Grove JH. 1998. Fecal bacteria survival and infiltration through a shallow agricultural soil: timing and tillage effects. J Environ Qual 27:1516–1523. doi: 10.2134/jeq1998.00472425002700060031x. [DOI] [Google Scholar]
- 33.Hutchison ML, Walters LD, Moore T, Thomas DJ, Avery SM. 2005. Fate of pathogens present in livestock wastes spread onto fescue plots. Appl Environ Microbiol 71:691–696. doi: 10.1128/AEM.71.2.691-696.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trevisan D, Vansteelant JY, Dorioz JM. 2002. Survival and leaching of fecal bacteria after slurry spreading on mountain hay meadows: consequences for the management of water contamination risk. Water Res 36:275–283. doi: 10.1016/S0043-1354(01)00184-1. [DOI] [PubMed] [Google Scholar]
- 35.Scott A, Conn KL, Lazarovits G, Topp E. 2006. Dynamics of Escherichia coli in agricultural soils receiving swine manure slurry or liquid municipal biosolids. Can J Soil Sci 86:841–849. doi: 10.4141/S06-011. [DOI] [Google Scholar]
- 36.Cote C, Quessy S. 2005. Persistence of Escherichia coli and Salmonella in surface soil following application of liquid hog manure for production of pickling cucumbers. J Food Prot 68:900–905. [DOI] [PubMed] [Google Scholar]
- 37.Fenlon DR, Ogden ID, Vinten A, Svoboda I. 2000. The fate of Escherichia coli and E. coli O157 in cattle slurry after application to land. J Appl Microbiol Symp Suppl 88:149S–156S. doi: 10.1111/j.1365-2672.2000.tb05343.x. [DOI] [PubMed] [Google Scholar]
- 38.Meyer LD, Harmon WC. 1979. Multiple intensity rainfall simulator for erosion research on row side slopes. Trans ASAE 24:1152–1157. [Google Scholar]
- 39.Isensee AR, Sadeghi AM. 1999. Quantification of runoff in laboratory-scale chambers. Chemosphere 38:1733–1744. doi: 10.1016/S0045-6535(98)00390-7. [DOI] [PubMed] [Google Scholar]
- 40.Faucette LB, Cardoso-Gendreau FA, Codling E, Sadeghi AM, Pachepsky YA, Shelton DR. 2009. Storm water pollutant removal performance of compost filter stocks. J Environ Qual 38:1233–1239. doi: 10.2134/jeq2008.0306. [DOI] [PubMed] [Google Scholar]
- 41.Green VS, Dao TH, Stone G, Cavigelli MA, Baumhardt RL, Devine TE. 2007. Bioactive phosphorus loss in simulated runoff from a phosphorus-enriched soil under two forage management systems. Soil Sci 172:721–732. doi: 10.1097/SS.0b013e31809eda32. [DOI] [Google Scholar]
- 42.Guber AK, Shelton DR, Pachepsky YA. 2005. Transport and retention of manure-borne coliforms in soil. Vadose Zone J 4:828–837. doi: 10.2136/vzj2004.0097. [DOI] [Google Scholar]
- 43.Burkhardt M, Stamm C, Waul C, Singer H, Müller S. 2005. Surface runoff and transport of sulfonamide antibiotics and tracers on manured grassland. J Environ Qual 34:1363–1371. doi: 10.2134/jeq2004.0261. [DOI] [PubMed] [Google Scholar]
- 44.Dao TH, Guber AK, Sadeghi AM, Karns JS, Van Kessel JS, Shelton DR, Pachepsky YA, Mccarty GW. 2008. Loss of bioactive phosphorus and enteric bacteria in runoff from dairy manure applied to sod. Soil Sci 173:511–521. doi: 10.1097/SS.0b013e31817d9d02. [DOI] [Google Scholar]
- 45.Partridge JD, Scott C, Tang Y. 2006. Escherichia coli transcriptome dynamics during the transition from anaerobic to aerobic conditions. J Biol Chem 281:27806–27815. doi: 10.1074/jbc.M603450200. [DOI] [PubMed] [Google Scholar]
- 46.Duffy KJ, Ford RM, Cummings PT. 1997. Residence time calculation for chemotactic bacteria within porous media. Biophys J 73:2930–2936. doi: 10.1016/S0006-3495(97)78321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tate RL. 1978. Cultural and environmental factors affecting the longevity of Escherichia coli in histosols. Appl Environ Microbiol 35:925–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hagedorn C, Hansen DT, Simonson GH. 1978. Survival and movement of fecal indicator bacteria in soil under conditions of saturated flow. J Environ Qual 7:55–59. doi: 10.2134/jeq1978.00472425000700010011x. [DOI] [Google Scholar]
- 49.Sinton LW, Braithwaite RR, Hall CH, Mackenzie ML. 2007. Survival of indicator and pathogenic bacteria in bovine feces on pasture. Appl Environ Microbiol 73:7917–7925. doi: 10.1128/AEM.01620-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang L, Mankin KR, Marchin GL. 2004. Survival of fecal bacteria in dairy cow manure. Trans ASAE 47:1239–1246. doi: 10.13031/2013.16574. [DOI] [Google Scholar]
- 51.Solo-Gabriele HM, Wolfert M, Desmarais T, Palmer C. 2000. Sources of Escherichia coli in a coastal subtropical environment. Appl Environ Microbiol 66:230–237. doi: 10.1128/AEM.66.1.230-237.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Howell JM, Coyne MS, Cornelius PL. 1996. Effect of sediment particle size and temperature on fecal bacteria mortality rates and the fecal coliform/fecal streptococci ratio. J Environ Qual 25:1216–1220. doi: 10.2134/jeq1996.00472425002500060007x. [DOI] [Google Scholar]
- 53.Kibbey HJ, Hagedorn C, McCoy EL. 1978. Use of fecal streptococci as indicators of pollution in soil. Appl Environ Microbiol 35:711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Byappanahalli MN. 2000. Assessing the persistence and multiplication of fecal indicator bacteria in Hawai'i soil environment. Ph.D. thesis Water Resources Research Center, University of Hawaii at Manoa, Manoa, HI. [Google Scholar]
- 55.Ishii S, Sadowsky MJ. 2008. Escherichia coli in the environment: implications for water quality and human health. Microbes Environ 23:101–108. doi: 10.1264/jsme2.23.101. [DOI] [PubMed] [Google Scholar]
- 56.Byappanahalli MN, Nevers MB, Korajkic A, Staley ZR, Harwood VJ. 2012. Enterococci in the environment. Microbiol Mol Biol Rev 76:685–706. doi: 10.1128/MMBR.00023-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhai Q, Coyne MS, Barnhisel RI. 1995. Mortality rates of fecal bacteria in subsoil amended with poultry manure. Bioresour Technol 54:165–169. doi: 10.1016/0960-8524(95)00126-3. [DOI] [Google Scholar]
- 58.Cools D, Merckx R, Vlassak K, Verhaegen J. 2001. Survival of E. coli and Enterococcus spp. derived from pig slurry in soils of different texture. Appl Soil Ecol 17:53–62. doi: 10.1016/S0929-1393(00)00133-5. [DOI] [Google Scholar]
- 59.Semenov AV, Van Bruggen AHC, Van Overbeek L, Termorshuizen AJ, Semenov AM. 2007. Influence of temperature fluctuations on Escherichia coli O157:H7 and Salmonella enterica serovar Typhimurium in cow manure. FEMS Microbiol Ecol 60:419–428. doi: 10.1111/j.1574-6941.2007.00306.x. [DOI] [PubMed] [Google Scholar]
- 60.Asadishad B, Ghoshal S, Tufenkji N. 2013. Role of climate and freeze-thaw on the survival, transport, and virulence of Yersinia enterocolitica. Environ Sci Technol 47:14169–14177. doi: 10.1021/es403726u. [DOI] [PubMed] [Google Scholar]
- 61.Winfield MD, Groisman EA. 2003. Role of nonhost environments in the lifestyles of Salmonella and Escherichia coli. Appl Environ Microbiol 69:3687–3694. doi: 10.1128/AEM.69.7.3687-3694.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]