Abstract
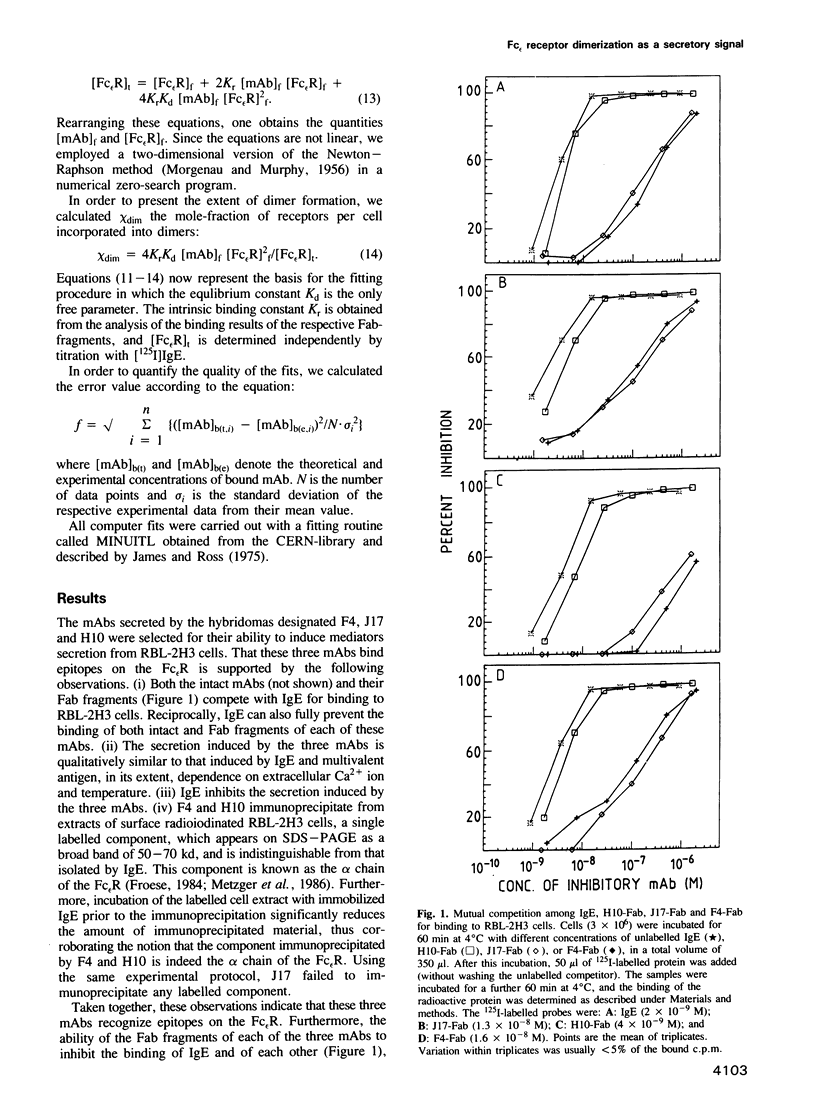
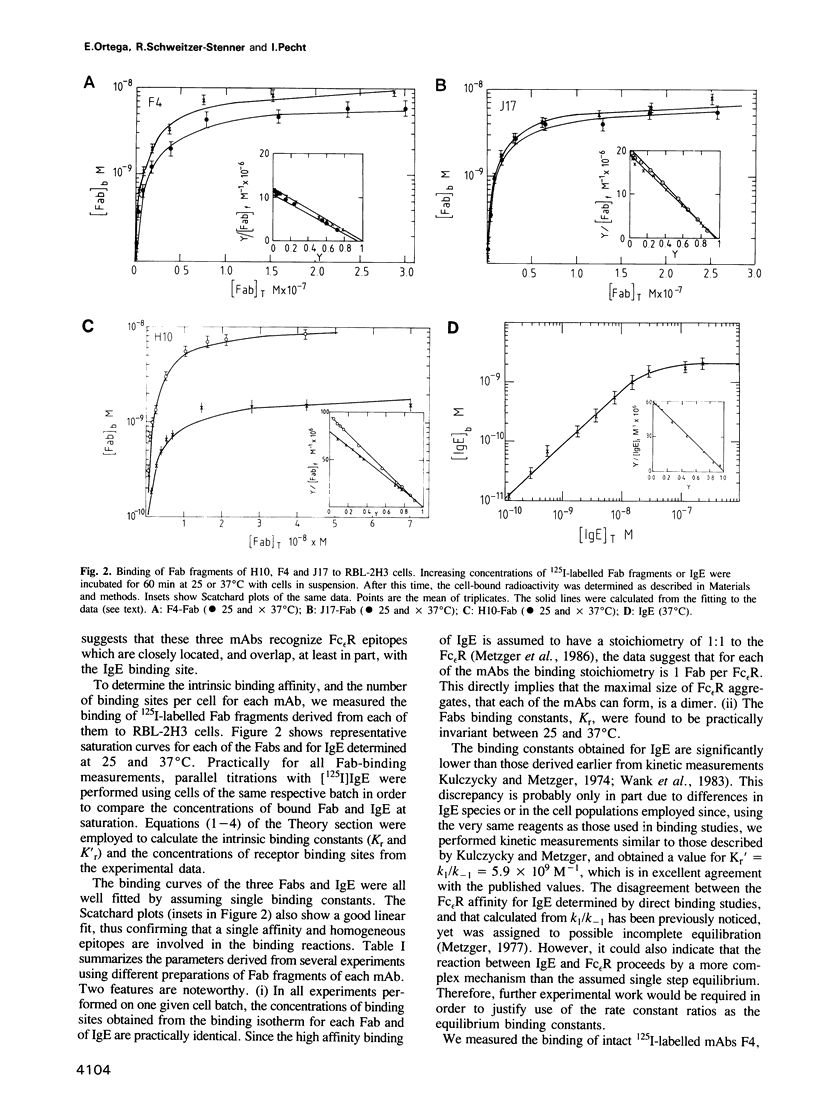
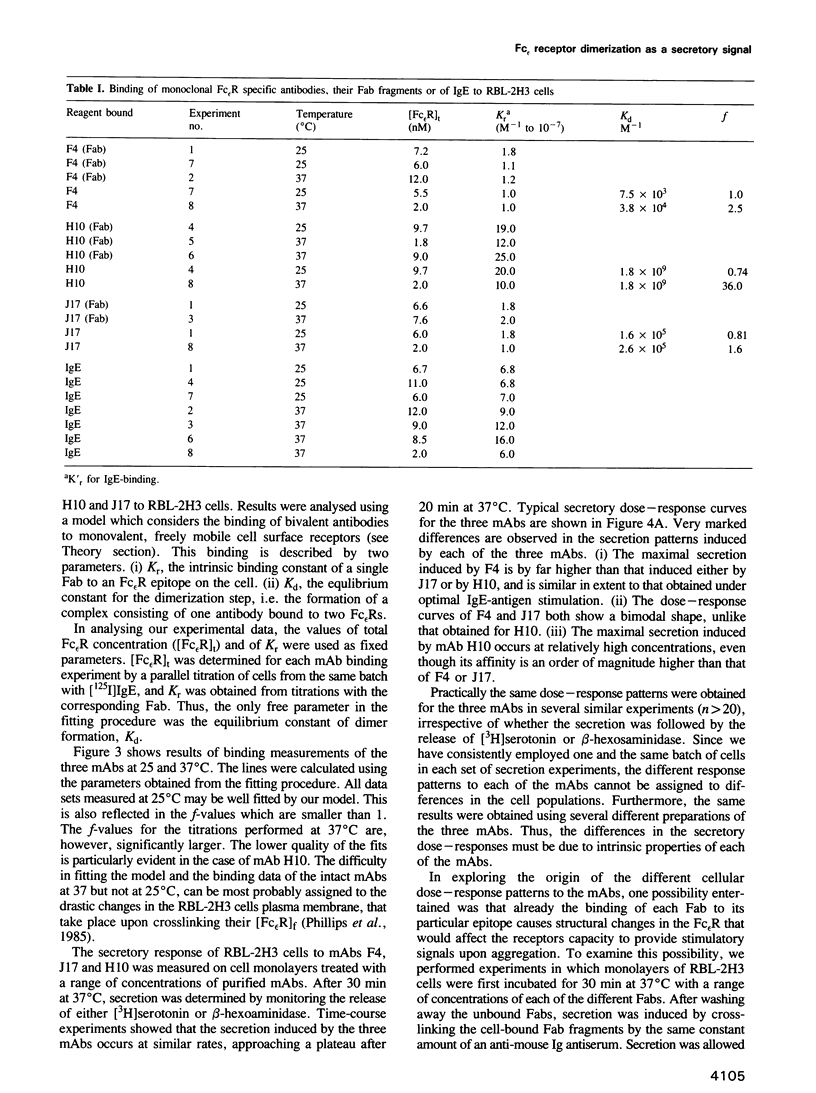
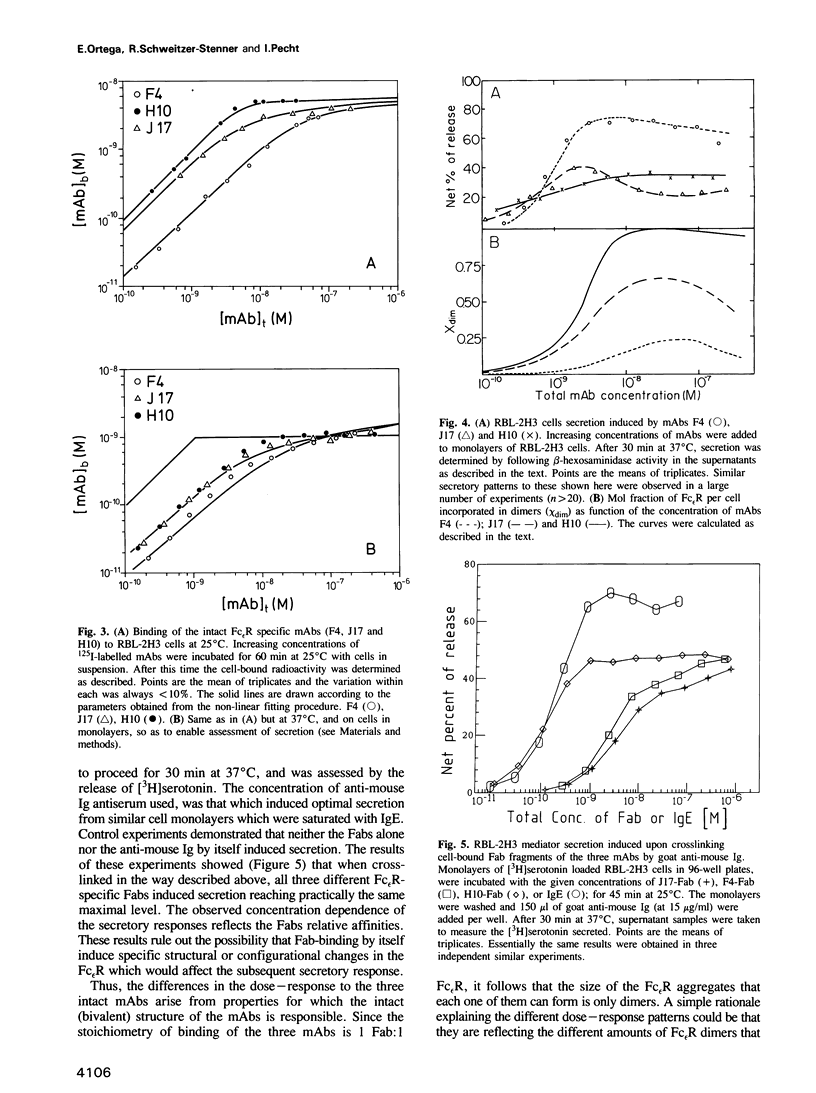
Three biologically active monoclonal antibodies (mAbs) specific for the monovalent, high-affinity membrane receptor for IgE (Fc epsilon R) were employed in analysing the secretory response of mast cells of the RBL-2H3 line to crosslinking of their Fc epsilon R. All three mAbs (designated F4, H10 and J17) compete with each other and with IgE for binding to the Fc epsilon R. Their stoichiometry of binding is 1 Fab:1 Fc epsilon R, hence, the intact mAbs can aggregate the Fc epsilon Rs to dimers only. Since all three mAbs induce secretion, we conclude that Fc epsilon R dimers constitute a sufficient 'signal element' for secretion of mediators for RBL-2H3 cells. The secretory dose-response of the cells to these three mAbs are, however, markedly different: F4 caused rather high secretion, reaching almost 80% of the cells' content, while J17 and H10 induced release of only 30-40% mediators content. Both the intrinsic affinities and equilibrium constants for the receptor dimerization were derived from analysis of binding data of the Fab fragments and intact mAbs. These parameters were used to compute the extent of Fc epsilon R dimerization caused by each of the antibodies. However, the different secretory responses to the three mAbs could not be rationalized simply in terms of the extent of Fc epsilon R dimerization which they produce. This suggests that it is not only the number of crosslinked Fc epsilon Rs which determines the magnitude of secretion-causing signal, but rather other constraints imposed by each individual mAb are also important.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barsumian E. L., Isersky C., Petrino M. G., Siraganian R. P. IgE-induced histamine release from rat basophilic leukemia cell lines: isolation of releasing and nonreleasing clones. Eur J Immunol. 1981 Apr;11(4):317–323. doi: 10.1002/eji.1830110410. [DOI] [PubMed] [Google Scholar]
- Defize L. H., Moolenaar W. H., van der Saag P. T., de Laat S. W. Dissociation of cellular responses to epidermal growth factor using anti-receptor monoclonal antibodies. EMBO J. 1986 Jun;5(6):1187–1192. doi: 10.1002/j.1460-2075.1986.tb04345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmrich F., Strittmatter U., Eichmann K. Synergism in the activation of human CD8 T cells by cross-linking the T-cell receptor complex with the CD8 differentiation antigen. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8298–8302. doi: 10.1073/pnas.83.21.8298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Pol J. A. Epidermal growth factor receptor of A431 cells. Characterization of a monoclonal anti-receptor antibody noncompetitive agonist of epidermal growth factor action. J Biol Chem. 1985 Apr 25;260(8):5003–5011. [PubMed] [Google Scholar]
- Fewtrell C., Metzger H. Larger oligomers of IgE are more effective than dimers in stimulating rat basophilic leukemia cells. J Immunol. 1980 Aug;125(2):701–710. [PubMed] [Google Scholar]
- Froese A. Receptors for IgE on mast cells and basophils. Prog Allergy. 1984;34:142–187. [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Ishizaka K., Ishizaka T. Induction of erythema-wheal reactions by soluble antigen-gammaE antibody complexes in humans. J Immunol. 1968 Jul;101(1):68–78. [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K. Activation of mast cells for mediator release through IgE receptors. Prog Allergy. 1984;34:188–235. [PubMed] [Google Scholar]
- Kinet J. P., Metzger H., Hakimi J., Kochan J. A cDNA presumptively coding for the alpha subunit of the receptor with high affinity for immunoglobulin E. Biochemistry. 1987 Jul 28;26(15):4605–4610. doi: 10.1021/bi00389a002. [DOI] [PubMed] [Google Scholar]
- Kulczycki A., Jr, Metzger H. The interaction of IgE with rat basophilic leukemia cells. II. Quantitative aspects of the binding reaction. J Exp Med. 1974 Dec 1;140(6):1676–1695. doi: 10.1084/jem.140.6.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Kappler J. The antigen-specific, major histocompatibility complex-restricted receptor on T cells. Adv Immunol. 1986;38:1–30. doi: 10.1016/s0065-2776(08)60005-x. [DOI] [PubMed] [Google Scholar]
- Menon A. K., Holowka D., Baird B. Small oligomers of immunoglobulin E (IgE) cause large-scale clustering of IgE receptors on the surface of rat basophilic leukemia cells. J Cell Biol. 1984 Feb;98(2):577–583. doi: 10.1083/jcb.98.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon A. K., Holowka D., Webb W. W., Baird B. Cross-linking of receptor-bound IgE to aggregates larger than dimers leads to rapid immobilization. J Cell Biol. 1986 Feb;102(2):541–550. doi: 10.1083/jcb.102.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger H., Alcaraz G., Hohman R., Kinet J. P., Pribluda V., Quarto R. The receptor with high affinity for immunoglobulin E. Annu Rev Immunol. 1986;4:419–470. doi: 10.1146/annurev.iy.04.040186.002223. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Hodgdon J. C., Hussey R. E., Protentis J. P., Schlossman S. F., Reinherz E. L. Antigen-like effects of monoclonal antibodies directed at receptors on human T cell clones. J Exp Med. 1983 Sep 1;158(3):988–993. doi: 10.1084/jem.158.3.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J. A. Interaction of divalent antibody with cell surface antigens. Biochemistry. 1979 Jan 23;18(2):264–269. doi: 10.1021/bi00569a004. [DOI] [PubMed] [Google Scholar]
- Rivnay B., Rossi G., Henkart M., Metzger H. Reconstitution of the receptor for immunoglobulin E into liposomes. Reincorporation of purified receptors. J Biol Chem. 1984 Jan 25;259(2):1212–1217. [PubMed] [Google Scholar]
- Rudolph A. K., Burrows P. D., Wabl M. R. Thirteen hybridomas secreting hapten-specific immunoglobulin E from mice with Iga or Igb heavy chain haplotype. Eur J Immunol. 1981 Jun;11(6):527–529. doi: 10.1002/eji.1830110617. [DOI] [PubMed] [Google Scholar]
- Segal D. M., Taurog J. D., Metzger H. Dimeric immunoglobulin E serves as a unit signal for mast cell degranulation. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2993–2997. doi: 10.1073/pnas.74.7.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seldin D. C., Adelman S., Austen K. F., Stevens R. L., Hein A., Caulfield J. P., Woodbury R. G. Homology of the rat basophilic leukemia cell and the rat mucosal mast cell. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3871–3875. doi: 10.1073/pnas.82.11.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu A., Tepler I., Benfey P. N., Berenstein E. H., Siraganian R. P., Leder P. Human and rat mast cell high-affinity immunoglobulin E receptors: characterization of putative alpha-chain gene products. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1907–1911. doi: 10.1073/pnas.85.6.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wank S. A., DeLisi C., Metzger H. Analysis of the rate-limiting step in a ligand-cell receptor interaction: the immunoglobulin E system. Biochemistry. 1983 Feb 15;22(4):954–959. doi: 10.1021/bi00273a038. [DOI] [PubMed] [Google Scholar]


