Abstract
This review focuses on how blood flow to contracting skeletal muscles is regulated during exercise in humans. The idea is that blood flow to the contracting muscles links oxygen in the atmosphere with the contracting muscles where it is consumed. In this context, we take a top down approach and review the basics of oxygen consumption at rest and during exercise in humans, how these values change with training, and the systemic hemodynamic adaptations that support them. We highlight the very high muscle blood flow responses to exercise discovered in the 1980s. We also discuss the vasodilating factors in the contracting muscles responsible for these very high flows. Finally, the competition between demand for blood flow by contracting muscles and maximum systemic cardiac output is discussed as a potential challenge to blood pressure regulation during heavy large muscle mass or whole body exercise in humans. At this time, no one dominant dilator mechanism accounts for exercise hyperemia. Additionally, complex interactions between the sympathetic nervous system and the microcirculation facilitate high levels of systemic oxygen extraction and permit just enough sympathetic control of blood flow to contracting muscles to regulate blood pressure during large muscle mass exercise in humans.
I. INTRODUCTION
A. Major Theme
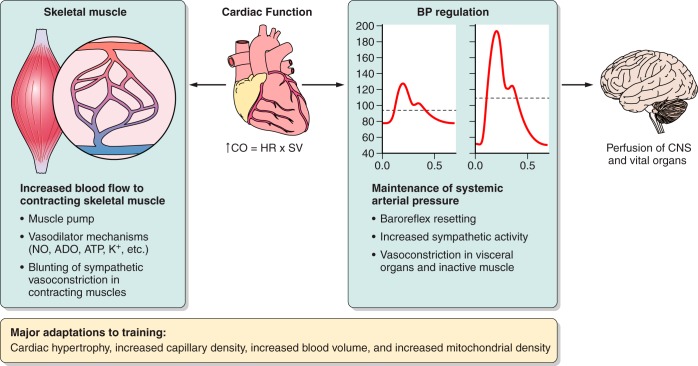
The major theme of this review is that during large muscle mass exercise like running or cycling there are two potentially competing physiological needs. First, because the metabolic costs of muscle contraction can be high and prolonged, skeletal muscle blood flow needs to be matched to the metabolic demands of the contracting muscles. Second, regulation of blood pressure is also needed to ensure there is adequate perfusion pressure to all organs. The idea that these two important physiological needs “compete” arises when the mass and vasodilator capacity of skeletal muscle are considered in the context of the maximum values for cardiac output seen during exercise. This raises the possibility that vasodilation in the contracting muscles might outstrip cardiac output and threaten blood pressure regulation (68, 301, 392, 393).
The potential competition between vasodilation and blood pressure regulation outlined above has emerged as a major new idea in integrative physiology over the last 30 or so years (392). However, there were hints that this was an issue as early as the 1960s (301). In this review we consider the many factors that contribute to the homeostatic and regulatory mechanisms operating to meet the two main physiological needs emphasized above. We also make the case that during heavy exercise sympathetic modulation of the peripheral circulation (including contracting skeletal muscle) operates in a way that 1) maintains arterial blood pressure at a minimal “acceptable” level of ∼100 mmHg, 2) facilitates the perfusion of a large mass of active muscle, and 3) increases oxygen extraction across the contracting skeletal muscles. These three points reflect an integrative perspective that we and others have been developing over the last 15 or so years (68, 392).
B. Structure of This Review
With our high level perspective as a background, we will work our way down from ideas related to oxygen consumption, cardiac output, skeletal muscle blood flow, and blood pressure regulation. To explore our first physiological need, we describe the range of oxygen consumption observed in humans and focus on the large increases in oxygen consumption that can occur during large muscle mass rhythmic exercise. We then discuss the magnitude of the cardiac output required to deliver this oxygen to the contracting muscles, and how blood flow is distributed to the microcirculation to meet the demand for oxygen by the active muscles. Our rationale for this top down approach is that in an era of reductionism, many scientists and trainees are less familiar with fundamental concepts related to whole body oxygen consumption, cardiac output, and blood flow distribution. Therefore, a synthesis of key facts and concepts is needed to frame the overall discussion of exercise hyperemia. We also start at the systemic level and “work down” because our own research has focused on integrated issues related to whole body oxygen consumption, skeletal muscle blood flow, and oxygen delivery to contracting muscles in conscious humans.
The second physiological need we identified is the ongoing need to regulate arterial pressure when the demand for oxygen by the exercising skeletal muscle is increased by several orders of magnitude, and as a result skeletal muscle blood flow is very high. So, in addition to considering the heart as a pump, the blood vessels as a delivery system, and the skeletal muscle as the end user, we must also consider the overall need of the organism to maintain an arterial pressure sufficient to perfuse the brain and other vital organs. This is especially important in humans who are upright and have a large brain located above heart level, which lowers cerebral perfusion pressure. Thus the autonomic nervous system serves as a regulator of blood pressure and is also critical in the regulation of skeletal muscle blood flow during exercise. The list below enumerates eight major questions. Additional key points and subsidiary questions will be used as needed to further frame our exploration of these issues.
What is the range of oxygen consumption in humans?
How is the oxygen delivery generated to meet the demands of the contracting muscles?
What fraction of cardiac output goes to skeletal muscle during exercise?
What are peak values for skeletal muscle blood flow?
How is blood pressure regulated when blood flow to contracting skeletal muscles is very high?
What are the local blood flow responses to muscle contraction, and what mechanisms cause them?
How does the sympathetic nervous system control blood flow to both inactive and contracting skeletal muscles?
Can this information be coherently integrated, and what perspectives do we have?
For each of these topics, we will emphasize data from studies in healthy humans. Complementary examples from animal models, comparative physiology, and human pathophysiology will be used as warranted to illuminate or reinforce key points. In general, we will also attempt to relate most elements of the review to rhythmic or dynamic exercise performed with a large mass of contracting muscles like running or cycling for a few minutes or more, which is typically referred to as aerobic exercise. While important insights can be gained by considering the physiological responses to small muscle mass exercise and static exercise, our overall goal is to present an integrated picture related to exercise as locomotion (16).
Of note, in preindustrial societies, prolonged movement including running was required for the purposes of hunting, foraging, herding, eluding predators, and muscle-powered agriculture. Along these lines, there are provocative arguments for evolutionary adaptations that favored the emergence of human endurance exercise capacity in the context of our traditional ways of life. For example, so-called persistence hunting requires continuous movement for many hours while running game animals to exhaustion (52, 279). More recently, there has also been a focus on the health consequences of inactivity and the powerful health benefits of regular aerobic exercise (50, 325).
While we work our way down from systemic responses to the factors in contracting skeletal muscle that cause blood flow to rise during exercise, these responses are so integrative it is unsatisfying to merely provide a linear catalog of them. Therefore, we adopted a narrative approach, and there may be digressions into topics and mechanisms that have already been covered in detail, or that will be covered in depth subsequently. This approach might seem unconventional, but exercise hyperemia is complex and our goal is to impart the readers with an appreciation of this complexity.
Before embarking on this intellectual journey, we would like to alert the readers' attention to six key publications that have informed our thinking and provide foundational integration and synthesis on the topics we are addressing. These include Handbook of Physiology chapters by Barcroft (25) and Shepherd (431), the seminal monographs by Shepherd (432) and Rowell (390, 391), and also a critical review article by Clausen (91). While our goal is to provide a comprehensive state-of-the-art survey of contemporary knowledge, equally important is the need to identify important unresolved issues related to muscle blood flow and exercise hyperemia. Ultimately, questions stimulate the generation of new knowledge and insight.
C. Foundational Concepts and Definitions
There are a number of concepts critical to integrating ideas about skeletal muscle blood flow and blood pressure regulation during exercise. Because they are essential for the extended discussion of the topics that follow, we will outline them here.
Exercise and electrically induced muscle contraction are not synonymous. In both cases, metabolic activity in the muscles increases. However, exercise is associated with a variety of parallel cardiovascular and respiratory responses associated with generating the effort required for the orderly recruitment of motor units that cause the muscles to contract (121, 390, 391). The most obvious examples are the increases in heart rate, blood pressure, and ventilation that can occur almost instantaneously at the start of exercise via so-called “central command.” With electrical stimulation of peripheral nerves, central command is bypassed, and the recruitment of motor units is either reversed or more random (157, 273, 465, 474). This may reflect in part lower input resistance for depolarization in large axons when external current is applied. When the muscle is stimulated directly, contraction can be caused by either direct electrical effects on the muscle cells or by stimulating branches of motor nerves in the muscle (318, 338). In both cases, the effects of electrical stimulation on how the contraction is evoked is dependent on the specifics of the experimental paradigm, and the general conclusion is that it differs from voluntary exercise. In the final analysis, exercise always includes contraction, but contractions can be generated in the absence of exercise.
Static and dynamic exercise are not the same (16). Static (sometimes called isometric) exercise usually refers to sustained contractions lasting seconds to minutes with limited muscle shortening. The prototypical static exercise is a sustained handgrip performed at some fraction of maximum voluntary contraction for a period of perhaps a minute or longer. The prototypical dynamic (or rhythmic) exercise is something like running or cycling that features brief contractions performed over and over again. Due to the ease of measuring forearm blood flow and the ability to give high doses of drugs locally via the brachial artery, rhythmic handgripping is also a frequently used model to study exercise hyperemia. This approach permits blood flow responses to be “pharmacodissected” without causing marked effects on systemic blood pressure that might engage cardiovascular reflexes and confound the local effects of the drug. In other words, the forearm can be used as an in vivo bioassay system in humans. In most of the studies we will cite, rhythmic forearm exercise means perhaps 20–30 contractions/min separated by 1–2 s between contractions. However, some studies have used longer periods of “static” contraction with a few seconds between (280, 511). Is this a model of static exercise or dynamic exercise? The dividing line is not always clear. Another important caveat is that blood flow and hence oxygen delivery to the contracting muscles can be restricted or absent during isometric or static contractions as contraction compresses the muscle vessels leading to a reliance on high-energy phosphate stores and glycolysis to generate ATP in support of the ongoing contractions (28, 470). This contrasts with the aerobic ATP production and corresponding requirement for increased blood flow during rhythmic contractions.
Large versus small muscle mass exercise needs to be considered when interpreting experimental results. Handgripping is obviously small muscle mass exercise since <1 kg (out of perhaps 20–30 kg) of muscle is being activated. The obvious problem with this model is that humans do not “run with their arms,” and the purpose of the forearm and hands are very different from the big locomotor muscles of the lower extremities. For example, many of the motor units in the hand and forearm may contain only a few muscle fibers consistent with the need for precision movements by the upper extremity (310). During one leg rhythmic knee extension (kicking) exercise, it is possible to isolate 2–3 kg of contracting muscle (408). In contrast, running and cycling are obviously large muscle mass exercise because they use ∼50% of total muscle mass. Activities like cross country skiing, rowing, and swimming might be characterized as whole body exercise. The points about small and large muscle mass exercise are critical for our later discussion about how skeletal muscle vasodilation is “managed” during heavy exercise to regulate mean arterial pressure. This is important because mean arterial pressure is “the” regulated variable in the cardiovascular system (301), and the brain is above heart level in upright humans. Finally, concerns about electrical stimulation aside, rhythmic handgripping and one leg kicking might have more in common with isolated muscle preparations than whole body exercise when considered in light of the mass of the contracting muscles.
The words peak or maximum are context specific during exercise (389). In general, maximum means the highest value recorded under any circumstance. For example, the highest oxygen consumption (V̇o2) value for most untrained or recreationally active humans can be observed during progressively faster walking or running uphill on a treadmill to exhaustion. Lower peak V̇o2 values (except in athletes like canoeists who are arm trained) are typically seen during incremental arm cranking. Thus the highest value obtained during a running-based test might be described as V̇o2max for that individual. In contrast, the highest value seen during incremental arm cranking would be the peak V̇o2 for that specific activity. Only in athletes with highly trained arms and legs (e.g., rowers and cross country skiers) is the addition of arm exercise to heavy leg exercise required to evoke V̇o2max, whereas in most humans running is a sufficient stimulus (36, 422, 459). Finally, during exercise at V̇o2max, the forces generated by the contracting muscle are not nearly maximal. For example, power outputs of ∼1,500 W on a cycle ergometer are possible during brief periods of sprinting (66), but values of 500–600 W would be exceptional during a V̇o2max test in an elite cyclist.
What is “maximum” muscle blood flow and under what circumstances does it occur? Similar values for flow to a given muscle can be obtained with various combinations of perfusion pressure and vascular tone. Given that blood flow is what is measured experimentally and carries oxygen to the contracting muscles, we are going to focus on blood flow values and assume under most circumstances that perfusion pressure is ∼100 mmHg. However, when is a response truly maximal? What happens when vasodilating substances are infused “on top” of a maximal physiological response? These questions will be discussed in detail later in several sections of this review. We raise them here to highlight some of the complexity of the phenomenon we are dealing with in this review.
1. Vascular resistance or vascular conductance as a calculated index of vascular tone?
Vascular tone can be expressed as either resistance (pressure/flow) or conductance (flow/pressure). From an analytical perspective, it is important to note that there is a curvilinear relationship between pressure and flow at a given resistance, whereas the relationship between pressure and flow at a given conductance is more linear (270, 342). These relationships will have important implications later when we discuss conceptual issues related to sympathetic vasoconstriction in contracting muscles and the effects of even modest vasoconstriction on arterial pressure. In both cases, these derived terms are used to understand what is happening to the caliber of the blood vessels in the muscle vascular bed. While some investigators report blood flow values, some vascular resistance, and some vascular conductance, we tend to favor the use of conductance because it is linearly related to flow. However, much of the literature is framed in the context of Darcy's law where pressure = flow/resistance, so some interpretive flexibility is required here (55).
2. Absolute or relative values for exercise related variables?
Under some circumstances we will discuss whole body oxygen consumption or skeletal muscle blood flow using absolute values typically expressed in terms of liters per minute. In other cases, these values will be either expressed as per kilogram of body weight or per 100 g of muscle. In some cases (especially when the fitness or training status of groups is different), things are expressed on a relative basis as a fraction of V̇o2max. In general, our goal in using absolute, normalized, or relative units is to highlight the values that provide the most physiological insight. We also seek to clarify issues that can be confused by factors like differences in body size. For example, the heart rate response to exercise at a given fraction of V̇o2max is generally similar for young healthy people independent of fitness status; in contrast, the heart rate response to a given absolute work load can vary dramatically and be much lower in trained versus untrained subjects (390, 391).
3. What do the categories untrained, exercise trained, and elite mean for exercise related studies?
For the purposes of this review, untrained (sometimes also called sedentary) refers to humans who participate in no formal leisure time exercise and also do not perform regular heavy physical labor. The term trained can include individuals who are casual exercisers perhaps doing 30 min/day of moderately vigorous activity most days. It can also include committed recreational athletes who participate in local competitions. Elite refers to highly competitive endurance athletes who typically train intensely for several hours per day year round for many years. Tables 1 and 2 provide a summary of estimates for so-called reference man and woman and also values for trained subjects and elite athletes.
Table 1.
Reference Vo2 and hemodynamic values at rest and during maximal exercise
| Reference Man |
Reference Woman |
|||
|---|---|---|---|---|
| Rest | Exercise (maximal) | Rest | Exercise (maximal) | |
| Vo2, ml·kg−1·min−1 | ||||
| Sed | 3.0–3.5 | <45 | ∼3.0 | <35 |
| Active | ∼3.5 | 50–60 | ∼3.5 | 40–50 |
| Elite | 3.5 | 70–85 | 3.5 | ∼60–73 |
| Heart rate, beats/min | ||||
| Sed | 70 | ∼200 | 70 | ∼200 |
| Active | 60 | ∼200 | 60 | ∼200 |
| Elite | 50 | ∼200 | 50 | ∼200 |
| CO, l/min | ||||
| Sed | ∼5 | ∼20 | ∼3.5–4 | ∼15 |
| Active | ∼5–6 | ∼25 | ∼3.5–4 | ∼20 |
| Elite | ∼5–6 | 30–40 | ∼3.5–4 | ∼25 |
| Stroke volume, ml | ||||
| Sed | ∼65 | ∼100 | ∼55 | ∼70 |
| Active | ∼90 | ∼125 | ∼60–70 | ∼100 |
| Elite | ∼110 | ∼150–200 | 70 | ∼125 |
Table 2.
Reference physical characteristic values
| Reference Man | Reference Woman | |
|---|---|---|
| Weight, kg | ||
| Sed | 70+ | 60+ |
| Active | 68 | 55 |
| Elite | 63 | 50 |
| Height, cm | 180 | 164 |
| Age, yr | 20–35 | 20–35 |
| Lean body weight, kg | 56 | 41 |
Lean body mass has a marked influence on all of the hemodynamic values (see Table 1).
Most of the preceding concepts reflect highly contrived laboratory situations. Much human activity might be described as intermittent with periods of fast and slow locomotion with occasional brief but high force efforts interrupted by periods of relative rest. This was certainly our history when more of our economic activity was labor intensive and our diversions were active as opposed to screen based (17, 49, 355). One reason there has been so much experimental emphasis on the physiological responses to running and cycling is because these activities are amenable to controlled laboratory-based studies with treadmills and cycle ergometers.
D. Muscle Blood Flow and Metabolism Are Closely Matched During Exercise
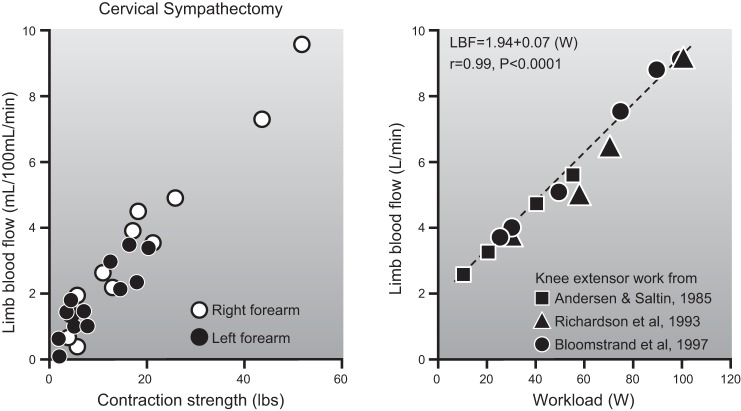
Muscle blood flow is closely matched to the metabolic demands of contraction. As shown in Figure 1, this matching occurs across a range of intensities from rest to heavy exercise and during both small and large muscle mass exercise. It is also seen in response to both single contractions and more prolonged exercise lasting for hours.
Figure 1.
Blood flow responses to single handgrip contractions of 0.33 s (left panel) and steady-state 1-leg kicking performed for a number of minutes (right panel). For both forms of exercise there is a linear relationship between exercise intensity and the blood flow response. This figure exemplifies the relationship between skeletal muscle metabolic demand and exercise hyperemia. The handgrip data also suggest that the rapid increases in blood flow to contracting muscles during exercise is due to vasodilation in the active muscles. The similarity of the responses in normal and sympathectomized limbs indicates that it is not dependent on sympathetic vasodilator nerves. For details, see Refs. 101 and 404.
The action of muscle contraction on bony levers generates movement, movement is a requirement of life, and energy is required to fuel this movement. In vertebrates, movement in general and locomotion in specific is caused by the recruitment of skeletal muscle motor units and contraction of skeletal muscle fibers with subsequent coordinated movement of the limbs (63, 142, 209). It is fueled by ATP, and ATP sources include high-energy phosphate stores, anaerobic metabolism, or the aerobic generation of ATP by the mitochondria (404). For periods of exercise lasting minutes or longer, aerobic generation of ATP by the mitochondria is critical and requires oxygen and substrate. There are substantial fuel stores in the form of carbohydrate and fat in skeletal muscle, and both glucose and free fatty acids from other tissues can be delivered to the muscle via the blood. However, the airborne source of oxygen is remote from the skeletal muscles and with the notable exception of some diving mammals, not much oxygen is stored in the muscles (322). So, fundamental questions for those interested in exercise, especially for more than a few minutes, relate to both how and how much oxygen gets from the air to the exercising muscles (146) via blood flow generated by the cardiovascular system linking the air/lung interface with the contracting muscles (501).
II. THE RANGE OF OXYGEN CONSUMPTION IN HUMANS
Resting oxygen consumption in humans averages 3–4 ml·kg−1·min−1. This means that in young healthy humans weighing 50–100 kg, somewhere between 0.15 and 0.4 liters of oxygen is being consumed per minute at rest. Most (∼80%) of the oxygen consumed in a resting human is used by the brain, heart, liver, and kidneys. The digestive tract does not consume much oxygen unless it is stimulated by eating and digestion (292), and likewise, skeletal muscle does not consume much oxygen unless the muscles are contracting. Bone and fat use only a small percentage of the oxygen consumed at rest.
In a larger context, resting metabolic rate in homoeothermic animals is dominated by the relationship between body surface area and body volume. This is because heat is lost from the body to the environment based on body surface area (234, 258). Surface area increases more slowly than body volume (area is a squared function and volume a cubed function), and resting metabolic rate is scaled to body volume to the 0.67–0.75 power. Thus large animals have lower resting metabolic rates expressed per kilogram than small animals. Figure 2 shows the classic scaling analysis of Kleiber (258), demonstrating the relationship between body heat production per day (a surrogate of oxygen consumption and basal metabolic rate) and body weight on a log scale among mammals.
Figure 2.
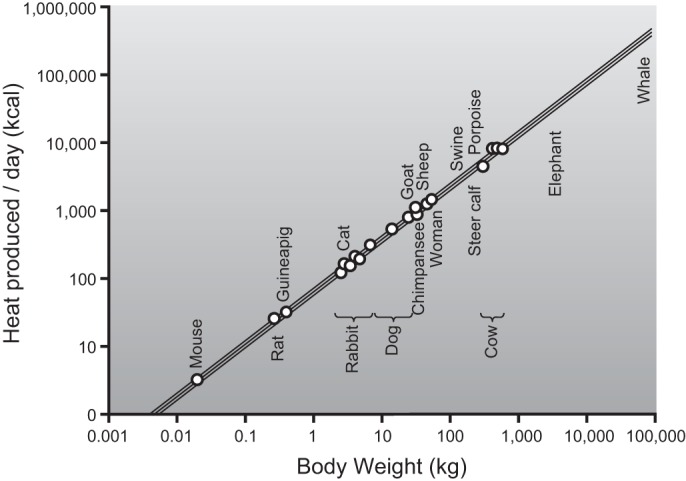
The relationship between body size on the x-axis and resting metabolic rate (heat production in kcal/day) on the y-axis. This figure shows that for every 10-fold (log) increase in body weight, the increase in resting metabolic rate is proportionally less than 1 log. This fundamental observation related to scaling helps explain why resting metabolic rate is lower on a per kilogram basis in larger animals. This type of analysis can also be used to compare exercise responses in elite human athletes of differing sizes. [Adapted from Kleiber (258).]
During exercise in young untrained subjects, oxygen consumption can increase 10- to 15-fold and reach maximal values of 30–50 ml·kg−1·min−1. The variability of V̇o2max is due to a number of factors such as the body composition of the subjects, level of physical activity, blood volume, hemoglobin mass, stroke volume, and poorly understood “genetic” factors. With intense aerobic exercise training, many healthy young men can achieve a maximal oxygen uptake near 60 ml·kg−1·min−1, provided the training is of sufficient intensity and duration to elicit a maximal adaptive response and that they become very lean. Of note, this maximal oxygen uptake value is similar to estimates for male hunter gatherers and pastoralists in nonmechanized cultures (102, 382, 499). In elite male endurance athletes, a V̇o2max in the 70–85 ml·kg−1·min−1 range is typically reported (361, 405). Early observations of these very high values were made in the 1930s on Donald Lash (the first man to break 9 min for 2 miles, ∼3,200 m) and other elite runners by Robinson and colleagues at the Harvard Fatigue Laboratory (380). Values in women are ∼10–15% lower than men as a result of having relatively less muscle mass and lower hematocrit and hemoglobin (139, 352, 405). Figure 3 shows the estimated distribution of maximal oxygen consumption (V̇o2max) for a group of 44,549 males (472) and also the relationship between whole body V̇o2max and total body hemoglobin (238).
Figure 3.
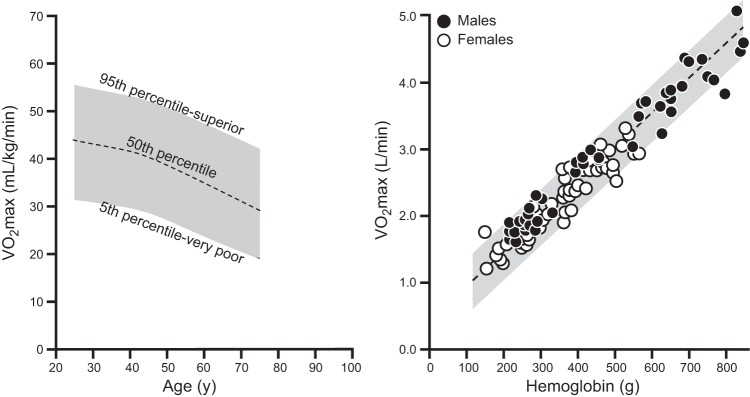
Population normal values for maximal oxygen consumption in ∼45,000 United States males expressed in ml·kg−1min−1 are shown in the left panel. For women, values and ranges 10–15% lower would typically be expected. The right panel demonstrates that total body hemoglobin is an important determinant of V̇o2max. This is because whole body hemoglobin content, along with maximum cardiac output, are major determinants of maximum oxygen delivery during exercise. This figure also shows the wide range of values for maximum oxygen uptake when considered in either relative (e.g., per kg; left panel) or absolute (l/min; right panel) terms. [Adapted from Joyner (238) and Trappe et al. (472).]
It should also be pointed out that V̇o2max is typically highly reproducible for a given individual assuming there are not major changes in physical activity or body composition (168, 444, 463). However, on average, V̇o2max typically declines by ∼10% per decade starting at age 30 (65, 188, 227, 362). The beginning of this decline can be delayed by a decade and its rate can be slowed with intense training (203, 362, 383). There are a number of physiological and gas exchange criteria used to assess what constitutes a maximal or peak response to exercise; however, the most compelling is the observation of a plateau in oxygen consumption in spite of an increased work load (212, 463). Figure 4 shows an example of oxygen consumption leveling off in a well-trained, but non-elite cyclist during an incremental maximal exercise test.
Figure 4.
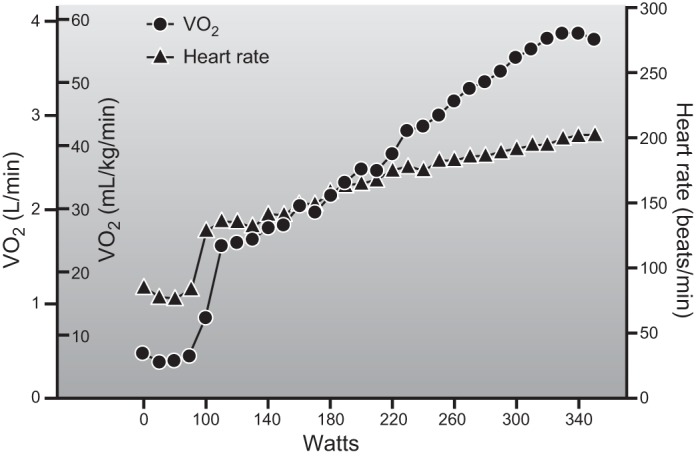
Individual record from a V̇o2max test in a well-trained but nonelite athletic male cyclist (18 yr of age) who weighed 67 kg. The x-axis is power output, and the y-axis is oxygen consumption both in l/min and scaled for body weight. There is a progressive increase in V̇o2 as power output increases with a leveling off at the highest work rates. This is accompanied by a linear increase in heart rate up to a value of ∼200 beats/min which is typical for a healthy young male. A V̇o2max value in the high 50s is typically attainable in young healthy lean male subjects who have participated in prolonged and intense exercise training. (Figure provided by Dr. Blair Johnson, unpublished observations.)
A. Summary
Absolute values for oxygen consumption at rest are largely dependent on body size. In healthy subjects, oxygen consumption can rise dramatically during exercise. The maximum value achieved during exercise is termed V̇o2max. It is a highly reproducible value and influenced by a number of factors including exercise training history of the individual.
III. CARDIAC OUTPUT AND PERIPHERAL OXYGEN EXTRACTION DURING EXERCISE: HOW THE OXYGEN DELIVERY NEEDED TO MEET MUSCLE'S DEMAND FOR OXYGEN IS GENERATED
A. Cardiac Output and Oxygen Extraction at Rest
According to the Fick principle, oxygen consumption = blood flow × arterial-venous O2 difference. When this principle is applied to the whole organism, it becomes oxygen consumption = cardiac output × systemic a-VO2 difference. At rest, textbook values in young healthy males weighing around 70 kg are typically ∼5 l/min for cardiac output (40, 431). This cardiac output is achieved via a combination of a heart rate of ∼70 beats per minute (bpm) and a stroke volume of ∼70 ml/beat. At sea level, hemoglobin values typically average ∼14–15 g/dl (∼9 mM) in young healthy men, and this hemoglobin is ∼98% saturated with oxygen. Because the oxygen-carrying capacity of a gram of hemoglobin is ∼1.34 ml (186), each liter of blood pumped by the heart carries ∼200 ml of oxygen. So, ∼1 liter of oxygen leaves the heart each minute in an average-sized healthy young male at rest. These values (see Tables 1 and 2) represent what was once referred to “reference man,” which typically means they were obtained in young healthy male undergraduate or medical students who were relatively lean, normally active, and weighed ∼70 kg (19, 33, 358). These values have likely declined on a population basis with the recent widespread increases in body fat and reductions in physical activity. However, the specific determinants of resting oxygen delivery cited above vary based on the size of the individual, blood pressure, and hemoglobin. For example, resting cardiac output is higher in anemic individuals or after normovolemic hemodilution (244, 503). Nonetheless, the convective transport of oxygen leaving the heart remains relatively constant under most circumstances at rest.
When measurements of mixed venous oxygen saturation are made in reference man, blood sampled in the pulmonary artery is typically ∼75% saturated (39, 40, 323). This means that 50 ml of oxygen is extracted from the peripheral circulation for each liter of blood leaving the heart per minute. Thus, when cardiac output is 5 l/min, resting oxygen consumption is ∼250 ml/min or ∼3.5 ml·kg−1·min−1. A key concept is that only a small fraction of the available oxygen delivered to the periphery by the arterial blood is in fact consumed. Additionally, oxygen extraction can vary by tissue, and ∼70% of available oxygen is extracted in the coronary circulation at rest and only ∼20–30% (or less) in the brain, kidney, and splanchnic circulations (44, 45, 86, 137, 265, 395). These patterns of organ-specific oxygen extraction at rest suggest that blood flow might be safely diverted away from tissues like the liver and kidney during exercise. However, blood and blood flow have other functions beyond gas exchange. These include important roles in metabolism and waste elimination along with fluid and electrolyte balance. Perhaps these functions are better supported by blood flow well in excess of that required to deliver the needed oxygen to specific resting tissues.
B. How Do Cardiac Output and Oxygen Extraction Change With Exercise?
During maximum exercise, heart rate increases to values of ∼200 bpm in young healthy humans. The classical view is that this increase in heart rate is accomplished by a combination of vagal withdrawal at the onset of exercise (up to a heart rate of ∼100 bpm) and increases in sympathetic nerve activity to the heart (379, 492). However, there is evidence from animal models that cardiac sympathetic nerve activity rises with the onset of exercise (246, 480). Additionally, some care needs to be taken when interpreting data from the human studies that use drugs to evaluate this issue because vagal withdrawal can be very fast and potentially occur within one heartbeat. The onset of sympathetic neural activity might also happen quickly, but the effects on heart rate might take several seconds or more to observe due to the more diffuse nature of sympathetic innervation and slower conduction velocity in the sympathetic nervous system compared with vagal control of the sinoatrial (SA) node.
Stroke volume also increases at the onset of exercise due to a complex interplay of factors including increased myocardial contractility and depending on posture (upright vs. supine) how much blood is returned to the central circulation via the skeletal muscle pump which acts to empty the veins in the lower extremities where blood pools due to gravity (40, 41, 274). Likewise, increases in respiration may also serve to improve venous return to the heart (321). In reference man, a stroke volume of ∼100 ml/beat would be typical, meaning that maximum cardiac output might reach a value of ∼20 l/min. Again, values vary based on body size, body composition, and sex of the subject. In general, cardiac and lung volumes are scaled on the basis of the allometric relationships discussed earlier (Figure 2). These relationships are most clearly seen when there are several log differences (10- to 100-fold or greater) in body size between species. However, in elite endurance athletes from disciplines that favor different-sized participants (e.g., small runners vs. large rowers), it is possible to see the impact of scaling on a number of variables related to oxygen uptake (231).
During maximal exercise in an untrained reference man, mixed venous oxygen saturation falls from ∼75% at rest to ∼25–30%. This means that ∼140–150 ml of oxygen is extracted by the peripheral tissues for each liter of cardiac output. Figure 5 shows the classic data from the 1950s on oxygen extraction at rest and during exercise from Mitchell et al. (323). With the use of the values outlined above, whole body oxygen consumption would be ∼3 l/min or 43 ml·kg−1·min−1 which is similar to the data reported by these investigators. Of note from this paper are the very low values for oxygen saturation (<20% in some subjects) of blood draining the femoral vein during heavy exercise. Venous saturation from the less active arms also fell from ∼50 to 25% during treadmill running consistent with the idea that blood flow is redistributed to the contracting muscles during heavy exercise. Under these conditions, oxygen consumption in the less active arms can be supported with reduced total blood flow and more oxygen extraction.
Figure 5.
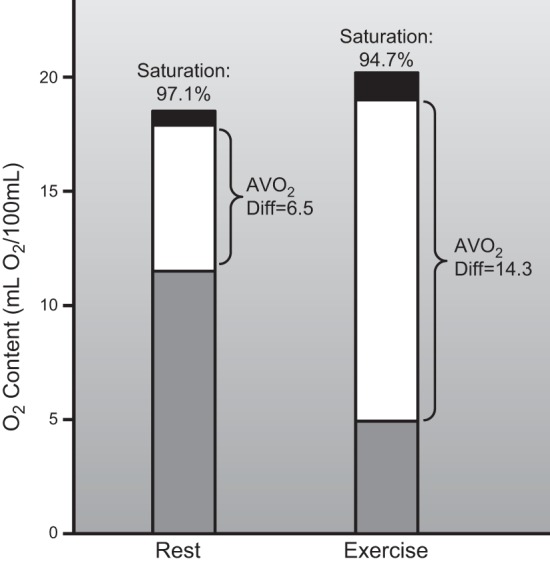
The classic data from Mitchell, Sproule, and Chapman, demonstrating oxygen-carrying capacity, arterial oxygen saturation, and mixed venous oxygen saturation in an untrained young healthy subject at rest and during maximal exercise. This figure emphasizes that while only ∼33% of the oxygen leaving the heart is extracted during rest, ∼70–75% can be extracted during heavy exercise in an untrained subject. The increase in whole body systemic oxygen extraction with exercise is facilitated by very high levels of extraction across the exercising muscle beds in conjunction with redistribution of blood flow from less active muscles and visceral organs. For details, see Ref. 323.
C. Blood Flow Redistribution
Blood flow redistribution also occurs in vascular beds other than the less active skeletal muscles. For example, as a result of sympathetic vasoconstriction, renal and splanchnic blood flow can both fall to ∼25% of resting values during heavy exercise in humans, but oxygen consumption in these tissues is preserved by marked increases in extraction (86, 395, 396). Blood flow to the kidneys at rest is ∼1.2 l/min, and the liver receives ∼1.6 l/min; this means that two liters of blood flow can be redirected from these vascular beds to the skeletal muscles during heavy exercise. Cerebral blood flow is ∼0.75 l/min or 15% of resting cardiac output. This absolute value does not change dramatically during exercise or perhaps increases slightly (21, 180). Coronary blood flow increases three- to fourfold from 0.15–0.20 to 0.5–0.8 l/min during maximum exercise driven primarily by the increased heart rate (137, 236, 254, 296, 351, 482). It is also important to point out that sympathetically mediated blood flow redistribution in visceral organs does not happen in all species. Dogs, for example, can perform prodigious feats of prolonged high-intensity endurance exercise while renal blood flow remains near values observed in resting animals (485).
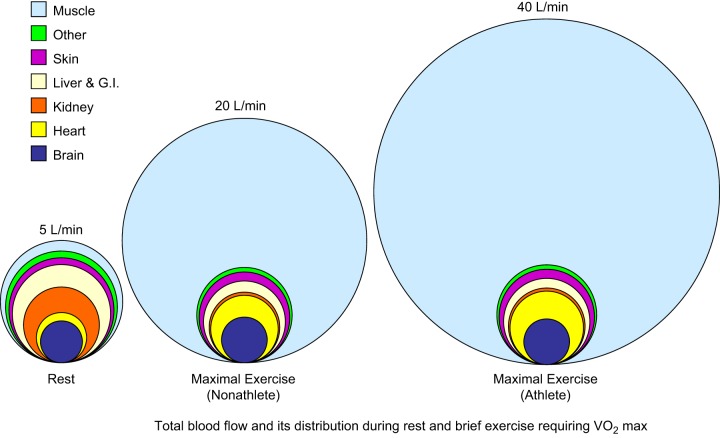
A key integrative point from the data above is that ∼4 l/min or ∼80% of cardiac output is directed to the brain, heart, kidneys, and liver in a human at rest. This means, as shown in Figure 6, that ∼1 l/min of blood flow, 200 ml of oxygen delivery, and perhaps 50 ml of oxygen consumption are sufficient to support resting metabolism in the other 80–90% of body tissues. The physiological implications and mechanisms responsible for the redistribution of blood flow during exercise will be discussed in several contexts in upcoming sections of this review. One puzzling element of these responses is that based on the low oxygen extraction across the brain, it might viewed as relatively “over perfused” at rest like the liver and kidney. However, unlike the liver and kidney, the brain is not subject to a marked reduction in blood flow during heavy exercise in humans.
Figure 6.
Schematic showing idealized distribution of blood flow at rest (left) and during maximum exercise in a healthy untrained young male subject (middle) and an elite endurance athlete (right). At rest, cardiac output is ∼5 l/min and rises to ∼20 l/min in the untrained subject during maximum exercise. Values of ∼40 l/min have been reported in elite endurance athletes. As cardiac output rises with exercise, brain blood flow remains constant (or increases slightly) while blood flow to the heart increases to meet the increased demands for myocardial blood flow that are primarily associated with exercise-induced increases in heart rate. Skeletal muscle blood flow increases dramatically, while blood flow to other tissues, especially the abdominal viscera and kidneys, is reduced. During heavy exercise, the vast increase in cardiac output is directed almost exclusively to contracting skeletal and cardiac muscles. Since maximum heart rate is similar in both young healthy subjects and elite athletes, the primary factor responsible for the very high cardiac outputs in the elite athlete is an extremely high stroke volume facilitated by a large compliant left ventricle. Ideas synthesized from Refs. 39, 67, 86, 91, 146, 292, 389–391, 394, 396.
Table 1 demonstrates a range of values for maximal oxygen uptake in young healthy subjects of both sexes (18). It also includes data from individuals who are not formally trained but are involved in high physical activity, nonmechanized lifestyles such as traditional hunting and gathering or muscle-powered farming. While there are many changes that occur in humans as a result of aging that can influence these values, the primary factor appears to be a reduction in maximal heart rate with age (344, 461, 473). The effects of aging on changes in stroke volume are confounded by reduced physical activity and coexisting diseases such as hypertension. However, stroke volume can be preserved in physically active otherwise healthy older subjects (203).
D. What Are the Effects of Endurance Training?
With endurance exercise training there can be an increase in left ventricular mass and chamber volume without wall thickening, a phenomenon known as eccentric cardiac hypertrophy (326, 447, 483). This adaptation augments stroke volume and thus maximum cardiac output (maximum heart rate does not change much) (10, 144, 274, 414). This increase in stroke volume is also facilitated by training-induced increases in blood volume that include both increases in red cell mass and plasma volume (94, 95). While healthy untrained subjects can increase their stroke volume by ∼20% with standard aerobic exercise training programs, at least some individuals have much more robust responses while others are likely less “trainable” (51, 514). Much greater training induced increases in stroke volume may also be possible with more intense and prolonged training (20, 144, 211).
In addition to changes in stroke volume, peripheral oxygen extraction can increase modestly with training. This is due to increased capillary density in the trained skeletal muscles that facilitates very high levels of oxygen extraction across exercising skeletal muscle vascular beds (5, 104, 143, 313, 314).
In parallel with these structural changes in the heart and the increase in skeletal muscle capillarity, there can be up to approximately twofold increases in skeletal muscle mitochondrial content with endurance training (216, 218). In the 1970s, this increase in mitochondrial content was thought to contribute to training-induced increases in V̇o2max. However, subsequent studies in rodents were able to dissociate changes in skeletal muscle mitochondrial content with training and V̇o2max (113, 217, 218). Parenthetically, skeletal muscle mitochondrial content and how it changes with training are major determinants of submaximal endurance performance. These changes also have important implications for substrate metabolism especially during prolonged exercise in both humans and other species (218). In contrast to the heart and skeletal muscle, in most cases the pulmonary system does not show major adaptive changes to endurance exercise training (184, 320, 406). However, lifetime exposure to high altitude can increase both lung volumes and diffusing capacity (87).
When typical adaptive values in response to prolonged endurance exercise training are considered, a 20% increase in stroke volume and cardiac output along with a 5–10% increase in oxygen extraction would lead to a V̇o2max value of ∼4 l/min in many young healthy male subjects (224). If this was accompanied by a 5–10% loss of body weight, it would appear that a V̇o2max value up to ∼60 ml·kg−1·min−1 is achievable in many young males. Maximum values ∼10–15% lower are possible in young healthy females who are highly trained but not elite endurance athletes. Such a highly trained but nonelite subject is shown in Figure 4, and as mentioned earlier, values in this range are similar to measurements and estimates made in hunter gatherer and pastoral populations.
E. What Are the Values in Elite Athletes?
Elite male athletes can typically have V̇o2max values between 70 and 85 ml·kg−1·min−1 and up to 6 or even 7 l/min (231, 361, 372). Almost all of this, compared with their well-trained but nonelite counterparts, is due to very large stroke volumes. Values of ∼200 ml/beat have been reported using invasive techniques in otherwise normal-sized men (146). Thus a maximum cardiac output of 35–40 l/min is possible in elite male endurance athletes. These values are consistent with the idea that some individuals respond impressively to training. When elite endurance athletes retire from training and become inactive, their V̇o2max drifts downward towards values similar to untrained controls (105, 106, 473).
While it is assumed that genetic factors may explain why some individuals have an impressive ability to increase their stroke volume in response to endurance exercise training, the evidence for a single or limited number of DNA variants explaining this phenomenon has not emerged. There is evidence that a suite of genetic markers can explain a significant portion of the variable increase in V̇o2max in response to fitness style training (469); however, it is not clear how these markers influence the stroke volume responses to training. Even less information is available concerning genetic factors that might influence physiological adaptations to the type of prolonged intense training performed by elite endurance athletes. Moreover, success in elite endurance athletics, like most human phenotypes, probably represents a combination of environmental exposures and behavioral factors (e.g., training) that operate in concert with a large number of gene variants and other epigenetic factors (287). There are data suggestive that genetic variability in angiotensin converting enzyme (ACE) might explain at least part of the very high stroke volumes and V̇o2max values seen in elite athletes. However, the evidence is not convincing for common ACE variants. Likewise, gene variants related to mitochondrial function do not explain the very high V̇o2max values seen in elite endurance athletes (372, 378).
The high values noted above also appear to be scaled for body size when comparisons are made between very large endurance athletes like rowers and smaller competitors like runners. In endurance sports like swimming and rowing, large body size can be advantageous (424). Elite male rowers who are typically 1.9–2.0 m tall and weigh 90–100 kg can have V̇o2max values in excess of 7 l/min (339). V̇o2max values for elite women are typically ∼10–15% lower on a per kilogram basis. Given that elite women are typically smaller than men, a V̇o2max value of 5 l/min would be remarkable (158, 226, 238, 339, 421).
An additional concept that underpins this entire discussion is that these high V̇o2max values can be achieved during activities like cycling or running that engage perhaps only 50% of whole body skeletal muscle mass. Under most circumstances and in most subject groups, adding arm exercise to leg exercise does not increase V̇o2max further. Exceptions to this general rule include rowers and cross-country skiers, who have highly trained arms and legs. In these subjects, adding arm exercise to leg exercise results in a V̇o2max that is 5–10% higher than the peak response seen during leg exercise alone (423, 459).
Finally, another important observation in elite male athletes is that some have a tendency to experience arterial hypoxemia (exercise-induced arterial hypoxemia, EIAH) during heavy whole body exercise (122). While EIAH has not been reported in untrained male subjects, it has been reported in untrained female subjects, and it may be more prevalent in elite female endurance athletes (133, 199, 222, 223). This hypoxemia is due to the complex interactions between the very high cardiac outputs detailed above, flow limitation at very high minute ventilations, ventilation perfusion matching, pulmonary diffusing capacity, and physiological shunts through the lung. The predominant mechanism or mechanisms accounting for this desaturation is a matter of ongoing debate in the pulmonary and exercise physiology communities (3, 118, 122, 198, 219, 233, 353, 366, 367). It is also important for the nonexpert to recognize that in male endurance elite athletes, minute ventilations in excess of 150 l/min are frequently seen (8, 493).
F. Summary
Oxygen consumption can increase 10- to 15-fold above values at rest during exercise in healthy young untrained humans with 20-fold or higher increments seen in highly trained elite endurance athletes. These increases in oxygen consumption are driven by a four- to eightfold increase in cardiac output resulting from acute increases in heart rate and stroke volume and in the case of trained subjects (especially elite athletes) structural changes in the left ventricle that increase stroke volume further. The increases in cardiac output are also accompanied by two- to threefold increases in whole body oxygen extraction. Increased whole body extraction is driven by high arterial-venous O2 differences in the exercising muscles and reduced blood flow to less active skeletal muscle and the renal and splanchnic vascular beds. Increases in capillary density in the trained skeletal muscles also augment oxygen extraction and contribute to the rise in V̇o2max with training.
It is also important to note that in certain “athletic” species including dogs and ponies, V̇o2max values in excess of 120 ml·kg−1·min−1 are seen with values of 140–150 ml·kg−1·min−1 reported in both foxhounds and thoroughbred horses (30, 332, 364). Incredibly, pronghorn antelopes are thought to have V̇o2maxs in excess of 200 ml·kg−1·min−1 based on the fact that they can run 11 km in 10 min (282). All of these athletic species have unusually high heart-to-body weight ratios (502) with scaled stoke volumes ∼1.6 times greater than those seen in less athletic animals. They also possess contractile spleens that store red blood cells which are mobilized during exercise to increase hematocrit and thus arterial oxygen carrying capacity (42, 43, 220, 221, 285, 455). Finally, there are reports of a V̇o2max of >90 ml·kg−1·min−1 in an Olympic medalist with a genetic mutation that caused him to have very high hematocrit and hemoglobin levels (245).
IV. TOTAL SKELETAL MUSCLE BLOOD FLOW
Because most of the oxygen consumed during exercise is used by the contracting skeletal muscles, we will now focus on maximal or peak skeletal muscle blood flow. To address the topic of maximal or peak skeletal muscle blood flow, we must first understand what fraction of cardiac output is directed to skeletal muscle and then how much skeletal muscle mass is contracting during heavy exercise. This will then set the stage for subsequent discussions about peak or maximum blood flow values and how these values interact with exercising muscle mass to generate the impressive increases in oxygen consumption outlined above. After we discuss the relevant concepts and data, the factors which contribute to the rise in skeletal muscle blood flow during exercise will be the next major topic for discussion.
On the basis of the concepts outlined earlier, if cardiac output was 20 l/min with 0.5 liters directed to the heart, 0.75 liters directed to the brain, and 0.7 liters directed to renal and splanchnic vascular beds, this would permit ∼18 l/min of blood flow be directed to skeletal muscle (see Figure 6). Parenthetically, blood flow to the skin is minimal unless the cutaneous vascular bed dilates for the purposes of thermoregulation. During severe thermal stress, total skin blood flow can reach 6–8 l/min in healthy young males. This makes exercise in warm (especially humid) environments perhaps the greatest overall challenge to the human cardiovascular system due to a three-way competition between blood pressure regulation and the need for high levels of both muscle and skin blood flow (389).
Thus, if we take the range of cardiac outputs in normal humans to be between 20 and 40 l/min for healthy young men, and 15–30 l/min for healthy young women (the upper limits in both sexes reflecting elite endurance athletes), then total muscle blood flow can reach 30–35 l/min in average-sized men who are elite endurance athletes. Hypothetically, even higher values might be possible in very large elite endurance athletes like rowers, with V̇o2max values in excess of 7 l/min (339). Figure 6 emphasizes these concepts and shows the regional distribution of cardiac output in a hypothetical resting normal young male of ∼70 kg and how this changes with maximal exercise. For comparison sake, it also shows similar estimates for a highly trained elite endurance athlete.
Another way to estimate total skeletal muscle blood flow is to assume that almost all of the increase in oxygen consumption caused by exercise is occurring in the contracting skeletal muscles. Additionally, most (∼90%) of the oxygen delivered to the leg muscles during maximum running or cycling is being extracted. Since oxygen consumption by nonexercising tissues does not increase, and based on the idea that each liter of arterial blood carries 200 ml of oxygen, it then takes ∼6 liters of cardiac output for every liter of whole body oxygen consumption (40). If all of the available oxygen were extracted, it would take ∼5 liters of skeletal muscle blood flow to generate 1 liter of additional whole body oxygen uptake during exercise. The values can vary depending on factors such as hematocrit and hemoglobin concentration, but estimates of total skeletal muscle blood flow generated on the basis of either cardiac output or increments in oxygen consumption are generally convergent. Both approaches emphasize that during heavy whole body exercise the vast majority of cardiac output goes to contracting muscles.
V. PEAK VALUES FOR SKELETAL MUSCLE BLOOD FLOW
Given the values for whole body skeletal muscle blood flow outlined above, the question then becomes what is peak or maximum blood flow per kilogram of contracting muscle? This question is obviously confounded by both the mass of active muscle and perfusion pressure because blood flow to a given vascular bed reflects both. However, in young healthy subjects, and especially in endurance-trained subjects, mean arterial pressure rises only modestly during heavy exercise as a result of the marked vasodilation in the skeletal muscles (92). This means that vasodilation in the contracting muscles far outstrips changes in blood pressure as the major determinant of exercise hyperemia in humans and most species under most circumstances.
A. Active Muscle Mass
Before we address the flow- and pressure-related issues, a few ideas about muscle mass are critical. Skeletal muscle typically comprises 40–50% of lean body mass in young humans, with the lower range reflecting women who have relatively less skeletal muscle mass than men (33). This means that for a 70 kg man with roughly 60 kg of lean body mass (see Tables 1 and 2), total muscle mass is ∼30 kg. For a 55 kg reference woman with ∼40 kg of lean body mass, there would be ∼20 kg in total muscle mass (these values assume 15 and 25% body fat for reference man and woman, respectively). Along these lines, for running and cycling, a reasonable assumption for contracting skeletal mass in young men might be 10–15 kg (33–50% of skeletal muscles mass) based on both imaging and anthropometric techniques (370). Additionally, the respiratory muscles are perhaps ∼2 kg and highly active during heavy exercise (191).
B. Mean Arterial Pressure
A second important point raised earlier is that mean arterial pressure typically remains ∼100 mmHg during aerobic whole body exercise and does not rise markedly above resting values in young healthy humans (91). For example, in the study on elite cross country skiers we discuss next, mean arterial pressure during whole body exercise was ∼95 mmHg (67, 68). While blood pressure does rise during exercise, the classic baroreceptor resetting studies of Donald and colleagues (315) in dogs show that during heavy exercise the operating point rises by about 15–20 mmHg. This means that the rise in pressure can be <20% while cardiac output is increasing four- to eightfold. Under these circumstances, the vast majority of cardiac output is going to the contracting skeletal muscles and the major factor accounting for this rise in flow is vasodilation. However, depending on the mode of exercise and subject group, a rise in blood pressure of 30–40% during dynamic exercise is not uncommon (6, 376, 422). In this case, the pressor response would amplify the increase in flow caused by the vasodilation by an amount proportional to the increase in pressure.
However, pulse pressure can increase dramatically during whole body exercise due to reductions in diastolic pressure caused by peripheral vasodilation in the contracting muscles and increases in systolic pressure caused by large stroke volumes ejected quickly into the aorta (397). This contrasts with the large increases in mean, systolic, and diastolic arterial pressure seen during even small muscle mass static exercise (2, 56) with even larger increases seen during heavy weight lifting (290). In this context, using an arbitrary value for mean arterial pressure of ∼100 mmHg during rhythmic exercise will permit us to focus on blood flow, which is typically the measured variable in most experimental paradigms and also the variable most closely linked to oxygen delivery to the contracting muscles.
C. What Do Cardiac Output, Total Muscle Blood Flow, and Active Muscle Mass Tell Us About Peak Muscle Blood Flow?
If blood flow were equally distributed to all 30 kg skeletal muscles in an untrained male with a peak cardiac output of 20 l/min and ∼18 l/min of total skeletal muscle blood flow during heavy exercise, muscle blood flow would be ∼60 ml·min−1·100 g muscle−1. These values would increase by ∼20% in highly trained subjects and might conceivably double in some elite endurance athletes. If 10–15 kg of muscle were active, then skeletal muscle blood flow would be in the range of 120–180 ml·min−1·100 g muscle−1 in young healthy untrained subjects and values 50–100% higher are possible elite athletes (275, 438). Since V̇o2max per kilogram of lean tissue (as opposed to total body weight) is generally similar in men and women across training and athletic status, these values usually apply to both sexes. Along these lines, measurements of leg blood flow in elite cross-country skiers during whole body exercise that elicited a cardiac output of 30 l/min report values of ∼20 l/min or roughly 200 ml·min−1·100 g−1. At the same time blood flow to the upper extremity in these athletes was ∼5 l/min (67).
The calculations, estimates, and experimental values from the skiers all relate to large muscle mass or even whole body exercise. Importantly, they are much higher than the traditional values for maximum muscle blood flow obtained using techniques including venous occlusion plethysmography or xenon (133Xe) clearance (88, 187, 241, 368, 457). In this context, perhaps “the” major advance in the field of exercise hyperemia over the last 30 years (since the Shepherd Handbook chapter of 1983, see Ref. 431) has been the observation that much higher skeletal muscle blood flows are possible in humans and other species.
Prior to the 1980s, maximum skeletal muscle blood flow in humans was thought to be in the range of 50–80 ml·min−1·100 g−1. Notably these values were also about twofold lower than those observed (∼100–150 ml·min−1·100 g−1) using various electrical stimulation paradigms to evoke muscle contractions in isolated perfused dog hindlimb preparations (24, 131, 225, 448).
D. Discovery of Much Higher Values
In the early and middle 1980s, several groups began to investigate the issue of maximum skeletal muscle blood flow during exercise in rats, dogs, ponies, and also humans (6, 14, 15, 293, 333). The animal studies used radiolabeled microsphere injections during treadmill running, and the human studies used continuous thermodilution (and later Doppler ultrasound; Ref. 500) during single leg kicking exercise to isolate blood flow to the contracting quadriceps muscles. This means that the animal studies were whole body while the human study focused on a limited mass (2–3 kg) of active muscle. This is an important point because compared with dogs and ponies, humans have relatively small hearts and limited peak or maximum cardiac output.
In both humans and animals, the revision of ideas about peak blood flow was due in part to technical advances. However, there was evidence in the 1970s for high peak muscle blood flow (200 ml·min−1·100 g−1) in the diaphragm muscles of the heat stressed panting greyhound (191). Indicator dilution techniques were used to measure leg blood flow in humans in the 1960s and 70s during large muscle mass exercise, making it hard to assess maximum flow in a limited mass of active muscle (235, 248, 495). A key issue for the animal studies was ensuring that there was adequate mixing and distribution of the radiolabeled microspheres so that the quantity of microspheres embedded in the tissues during exercise reflected the blood flow. For the human studies, a relatively small mass of active muscle that can perform rhythmic exercise needed to be identified. For thermodilution to work, the arterial inflow and venous outflow to the muscle had to have straightforward vascular anatomy that limited potential contamination from other vascular beds. Additionally, these vessels needed to be easily accessible for catheter placement.
Table 3 shows values expressed as milliliters of blood flow per minute per 100 g of muscle from a number of human and animal studies showing high values for skeletal muscle blood flow (6, 14, 15, 295, 297, 331, 333, 350, 376). Figure 7 shows the data from three early studies that led to the revision of ideas about peak blood flow values in contracting skeletal muscles during exercise (6, 15, 333). The data in Table 3 and Figure 7 show that values in the range of 200–300 ml·min−1·100 g tissue−1 are possible. The key point is that values are two- to fourfold higher than the previously widely accepted “peak” values of 50–100 ml·min−1·100 g−1. They are also substantially higher than values observed in isolated dog hindlimb preparations. It should be noted that there can also be large regional differences in peak blood flow associated with muscle fiber type in rodents (15, 269, 291, 334). Fiber type is highly compartmentalized in these animals so that in specific areas of a given muscle the fiber type composition is relatively homogeneous. However, in humans, most skeletal muscle has a “mixed” or mosaic pattern so regional differences are probably less pronounced (14, 15, 278).
Table 3.
Muscle blood flow during exercise in various species
| Author | Species | Exercise Mode | Muscle Group(s) | Blood Flow, ml·min−1·100 g−1 |
|---|---|---|---|---|
| Armstrong and Laughlin, 1983; Ref. 14 | Sprague-Dawley rats | Treadmill running (speed = 60 m/min) | Knee extensors | |
| Vastus intermedius | 396 ± 36 | |||
| Vastus medialis | 284 ± 42 | |||
| Vastus lateralis | ||||
| Red | 389 ± 50 | |||
| Middle | 224 ± 32 | |||
| White | 86 ± 18 | |||
| Rectus femoris | ||||
| Red | 312 ± 38 | |||
| White | 166 ± 16 | |||
| Armstrong and Laughlin, 1985; Ref. 15 | Sprague-Dawley rats | Treadmill running (speed = 105 m/min) | Knee extensors | |
| Vastus intermedius | 495 ± 73 | |||
| Vastus medialis | 325 ± 47 | |||
| Vastus lateralis | ||||
| Red | 495 ± 66 | |||
| Middle | 293 ± 50 | |||
| White | 102 ± 24 | |||
| Rectus femoris | ||||
| Red | 363 ± 47 | |||
| White | 173 ± 23 | |||
| Musch et al., 1987; Ref. 333 | Exercise-trained foxhounds | Treadmill running (maximal) | Gracilis | 252 ± 22 |
| Gastrocnemius | 255 ± 17 | |||
| Semimembranosus | 330 ± 26 | |||
| Semitendinosus | 128 ± 14 | |||
| Musch et al., 1987; Ref. 331 | Untrained mongrel dogs | Treadmill running (maximal) | Gracilis | 206 ± 12 |
| Gastrocnemius | 255 ± 17 | |||
| Semimembranosus | 342 ± 20 | |||
| Semitendinosus | 134 ± 12 | |||
| Parks and Manohar, 1983; Ref. 350 | Ponies | Treadmill running (32 km/h) | Diaphragm | ∼215 ± 20 |
| Manohar, 1986; Ref. 293 | Ponies | Treadmill running (32 km/h) | Diaphragm | 265 ± 36 |
| Gluteus medius | 253 ± 36 | |||
| Biceps femoris | 233 ± 29 | |||
| Triceps brachii | 227 ± 26 | |||
| Manohar, 1990; Ref. 295 | Ponies | Treadmill running (32 km/h at a 7% grade) | Diaphragm | ∼325 ± 25 |
| Andersen and Saltin, 1985; Ref. 6 | Humans | Single leg kicking (maximal; avg 54W) | Knee extensors | 247 ± 18 |
| Richardson et al., 1995; Ref. 376 | Exercise-trained (cyclists) humans | Single leg kicking (maximal; avg 99W) | Knee extensors | 386 ± 26 |
Values are means ± SE.
Figure 7.
Transformational findings from three of the landmark studies showing that blood flow to contracting skeletal muscles during exercise could be much higher than previously imagined. The left panel is data from rats during treadmill running, the middle panel is from dogs, and the right panel is from humans during 1-leg kicking. The rat data show blood flow responses in ml·min−1·100 g−1 in various compartments of the hindlimb as running speed increased (VI, vastus intermedius; VLR, VLM, VLW, red, middle, and white vastus lateralis, respectively). The dog data show hindlimb blood flow responses vs. percent of V̇o2max generated during treadmill running. The human data show the blood flow responses in l/min vs. power output during 1-leg kicking at a rate of 60 kicks/min with the quadriceps (2–3 kg). The rat and dog data were obtained using radiolabeled microspheres and the human data via thermodilution. [Adapted from Andersen and Saltin (6), Armstrong and Laughlin (15), and Musch et al. (333).]
These very high muscle blood flows are typically associated with only modest increases in blood pressure from rest. Additionally, the highest values in selected muscle groups are on the order of 350 ml·min−1·100 g−1, and flows of ∼385 ml·min−1·100 g−1 have been reported in elite cyclists during one-leg kicking (47, 377). Comparable flows have reported in the respiratory muscles of ponies during heavy exercise (294, 295). One caveat about the human measurements is that perfusion pressure in the lower extremities might be higher than systemic perfusion pressure. This is because the skeletal muscles are below heart level and gravity can augment perfusion pressure. This augmented perfusion pressure, in conjunction with the skeletal muscle pump, which would keep venous pressure extremely low, could contribute to the high blood flows seen in humans during leg kicking (267). Studies in normal humans, patients with valvular insufficiency, and patients with a congenital absence of venous valves all support this interpretation (41, 359, 360, 454). These studies indicate that the muscle pump operates to increase the perfusion pressure gradient across a dependent exercising limb by keeping venous pressure low. It also facilitates venous return and cardiac filling (41).
E. Why Were Values for Peak Blood Flow Before the 1980s so Low?
Given the fact that detailed measurements of oxygen consumption, cardiac output, and deep venous oxygen saturation were available in athletes by the later 1950s and during the 1960s, it is interesting to consider why the accepted values for peak or maximum skeletal muscle blood flow were so low for so long. There was also excellent V̇o2 and hemodynamic data on dogs running at high speed (485). In retrospect, estimates of the mass of contracting muscle consuming the oxygen along with the amount of blood flow and extraction required to support this level of oxygen consumption should have been possible. Such estimates would have raised serious questions regarding the values obtained using venous occlusion plethysmography in the forearm and calf or 133Xe washout in other muscles.
The low peak muscle blood flows measured using venous occlusion plethysmography have several explanations (241). First, the whole forearm or calf volume is the tissue denominator and the limbs include fat, bone, and skin as well as muscle. Second, for plethysmography to work, the limb needs to be above heart level. Additionally, during high flow states, venous congestion can increase very quickly during plethysmography and reduce perfusion pressure and flow. In the case of the leg, this has the effect of lowering perfusion pressure compared with the upright posture. Third, the measurements are made during brief pauses in contraction so any contribution of the muscle pump acting locally on the microcirculation to increase flow is lost (however, any impedance to flow caused by the contractions would also be minimized) (478). In the case of the calf, the muscle pump also minimizes venous pressure and ensures that any gravitational augmentation of arterial pressure that occurs in the upright posture can be fully expressed (359, 360). If these mechanisms each led to a 50% underestimation of peak muscle blood flow and they interacted, they could provide at least a partial explanation for the discrepancy between the older and more recent observations. For example, peak calf blood flow between ∼60 and 80 ml·min−1·100 g−1 after ischemic exercise was reported by Snell et al. (445) in untrained men and endurance athletes, respectively. If adjusted for the factors just outlined, these observations would translate to estimates of flow on the order of ∼200 ml·min−1·100 g−1. These estimates are still relatively low, but closer to the more recent values measured with thermodilution, by Doppler ultrasound, and in the case of animal microspheres.
Like venous occlusion plethysmography, there are issues associated with the 133Xe washout technique (88, 92, 187). In this technique, radioactive xenon is injected into a tissue and the rate of washout from the tissue is proportional to the blood flow. The time resolution of this technique is slow, the relationship between the external radiation counter and the xenon can be variable, and a host of assumptions about the tissue distribution of the label and washout are required. While it is easy to criticize this technique in retrospect, it provided key insights into cerebral blood flow and ventilation perfusion matching that were also applied diagnostically prior to the advent of other imaging techniques (266, 319, 520).
It is also interesting to note that peak values for muscle blood flow made using in situ dog hindlimb preparations with electrical stimulation protocols designed to elicit “maximal” responses are on the order of 100–150 ml·min−1·100 g−1 (225). This is in spite of the fact that the vast majority of blood flow in the dog hindlimb during contractions or exercise is directed to skeletal muscle. This raises questions about the role of normal exercise (vs. electrically stimulated contractions) in evoking a coordinated pattern of mechanical forces that augment skeletal muscle perfusion. Importantly, tetanic contractions that normally evoke “maximal” blood flow responses in preparations that are prevasodilated with adenosine and sodium nitroprusside actually lower hindlimb blood flow (131). Additionally, the values observed (∼170 ml·min−1·100 g−1) remain lower than those seen with microspheres during voluntary exercise as summarized in Table 3. The reasons for this “lower peak flow” during pharmacological vasodilation are not clear, but perhaps relate to a washout of dilating factors and loss of coordinated flow regulation at the level of the microcirculation. There could also be alterations in how the muscle pump affects flow during concurrent administration of vasodilating drugs. Finally, the contractions themselves might impede flow by compressing the intramuscular arterioles and venules (9, 28, 280, 470).
F. Is There a Maximum Value for Skeletal Muscle Blood Flow?
The surprising magnitude of the blood flow values described in the 1980s led to questions about the upper limits for skeletal muscle blood flow. In this context, blood flow to cardiac muscle in the left ventricle can equal ∼500 ml·min−1·100 g−1 in equines during heavy exercise (12, 296, 298, 351) with values of 300–400 ml·min−1·100 g−1 seen in other species including humans (150, 204, 264, 519). Estimates of skin blood flow equal to 6–8 l/min distributed in perhaps 2 kg of skin in heated humans would also seem to rival the per 100 g values seen in skeletal and cardiac muscle (389). When additional vasodilator stimuli (either drugs or hypoxia) are superimposed on heavy exercise, under some circumstances there can be further vasodilation. For example, addition of hypoxia during one-leg kicking in healthy untrained young male subjects augmented skeletal muscle blood flow from ∼270 to 300 ml·min−1·100 g−1. This ∼10% increase in muscle blood flow demonstrated that significant further vasodilation was possible in this subject group (399). In contrast, Manohar (297) infused adenosine into skeletal muscle vascular beds of ponies performing heavy exercise and found no additional increase in blood flow. Of note, in humans studies, with the exception of ATP (181, 329), infusions of high doses of potent vasodilators in the femoral artery at rest typically evoke peak blood flow responses somewhat lower than those seen during heavy exercise (330, 371).
G. Summary
Oxygen consumption can increase above resting values 10- to 15-fold during exercise in normal healthy young humans.
This peak value for oxygen uptake can increase 20–50% in most subjects as a result of prolonged intense exercise training and can be 20-fold or greater above resting in elite endurance athletes.
During exercise, each liter of oxygen consumption is typically associated with ∼5–6 l of cardiac output. This increase in cardiac output is a result of an increase in heart rate and stroke volume.
The primary effects of endurance exercise training relate to increases in cardiac output driven by an augmented stroke volume due to left ventricular hypertrophy and increases in blood volume. Elite athletes have remarkably high stroke volumes and large blood volumes (94, 95, 414). Cardiac output values of 40 l/min have been seen in elite male endurance athletes (146).
There can be an increase in capillary density in the vascular bed of the trained muscles which can further augment oxygen delivery and extraction. This is especially important during large muscle mass exercise when oxygen extraction can be 80–90%. During small muscle mass exercise including both handgripping and one-leg kicking deep venous saturation during heavy exercise remains at about 30% meaning that only 70% of the available oxygen is extracted. These high values for venous saturation have been interpreted to reflect either “luxury perfusion” for a given oxygen consumption or admixture of venous blood from noncontracting tissue in the limb including skin. In this context, older evidence suggests that deep venous samples from the forearm are relatively uncorrupted by skin blood flow (27, 93).
Based on a number of calculations and also derived from data collected primarily in the 1980s, our understanding of the actual upper limits of skeletal muscle blood flow has increased. Values on the order of 300–400 ml·min−1·100 g−1 are possible. These higher values were discovered due to advances in blood flow measurement techniques and insightful experimental designs.
VI. THE AUTONOMIC NERVOUS SYSTEM: BLOOD PRESSURE VERSUS BLOOD FLOW TO CONTRACTING SKELETAL MUSCLES
We now make the case that blood flow to the contracting muscles is normally restrained by the sympathetic nervous system during heavy large muscle mass or whole body exercise for the purposes of regulating arterial pressure (120, 343, 398). This has been characterized by Rowell (392) as what might be called the “sleeping giant hypothesis.” This characterization reflects the idea that the vast ability of skeletal muscle to vasodilate can potentially outstrip the ability of the heart to generate an adequate cardiac output and maintain a “reasonable” (∼100 mmHg) mean arterial pressure. Such a pressure is required to maintain blood flow to other organs including the brain. When blood pressure is not maintained during exercise as in cases of autonomic failure, blood pressure can fall low enough within a minute of exercise to evoke loss of consciousness due to cerebral hypoperfusion (301).
A. Blood Pressure Is Maintained in Cross-Country Skiers
During the review of systemic oxygen consumption, cardiac output, and values for peak skeletal muscle blood flow, one key discrepancy emerged. Values for skeletal muscle blood flow in quadrupeds, including dogs and ponies that are considered “athletic” animals, are higher than values for skeletal muscle blood flow seen during large muscle mass or whole body exercise in humans. However, they are similar to the values seen in humans during one-leg knee extension exercise. This suggests that blood flow to contracting human muscles is restrained during large muscle mass or whole body exercise. This is true even in elite human athletes with very high cardiac outputs. As shown in Figure 8, if blood flow to the arms and legs during whole body skiing had been similar to the values seen during either arm or leg only skiing, then mean arterial pressure would have fallen to ∼75 mmHg versus the observed ∼95 mmHg assuming no change in cardiac output. The observation that blood pressure did not fall in the skiers can be explained by restraint of blood flow to the contracting muscles under these circumstances. This discussion provides one line of evidence that there can be competition among systemic blood pressure regulation, cardiac output, and the demand by contracting skeletal muscles for blood flow during heavy large muscle mass or whole body exercise in humans (68).
Figure 8.

The distribution of blood flow to the arms and legs in a hypothetical elite cross-country skier during heavy exercise. The left panel shows that legs receive ∼20 l/min of blood flow during maximal diagonal skiing with only modest arm effort. Under these circumstances, cardiac output exceeds 30 l/min and mean arterial pressure (MAP) is ∼95 mmHg. The right panel shows what happens if the maximum values for double arm poling (DP) are added to the maximum values for the legs from diagonal skiing. If both beds dilated “maximally” and cardiac output remained constant, mean arterial pressure would fall to an estimated 75 mmHg. Under these circumstances, an additional ∼4 l/min of cardiac output would need to be generated to maintain mean arterial pressure at 95 mmHg. One implication of this figure is that at least some vasoconstriction in the contracting muscles is required to maintain arterial pressure during heavy exercise even in elite athletes who possess very high values for maximum cardiac output. [Adapted from Calbet et al. (67).]
B. Blood Pressure Falls During Exercise in Patients With Autonomic Failure
An early key observation with bearing on the concept that the sympathetic nervous system restrains blood flow to maintain arterial pressure is that arterial pressure falls during exercise in humans with autonomic nervous system failure (301). Figure 9 shows the blood pressure responses in a patient studied in the early 1960s who had undergone thoracolumbar sympathectomy to treat malignant hypertension. At that time and for a few decades before the advent of effective drug therapy for hypertension, various forms of surgical sympathectomy were used to treat severe hypertension. These procedures were frequently effective in lowering blood pressure, but orthostatic hypotension was a common side effect (301, 508). Even when this subject shown in Figure 9 exercised in the head-down position to maximize venous return and stroke volume, blood pressure still fell during exercise. This particular patient had the normal ability to increase heart rate with exercise because vagal and sympathetic innervation to the heart were intact. However, the blood pressure responses clearly show that unrestrained vasodilation in skeletal muscle outstripped the ability of cardiac output to keep up and generate adequate perfusion pressure for the level of exercise.
Figure 9.
Blood pressure responses to supine and head-down tilt exercise in an individual with autonomic failure as a result of surgical sympathectomy of the thoracolumbar sympathetic chain. The fall in blood pressure during supine exercise highlights the need for the sympathetic nervous system to restrain blood flow to contracting skeletal muscles for the purposes of regulating blood pressure. The fact that this fall in blood pressure also occurred when venous return was maximized by 15% head-down tilt emphasizes this point. The x-axis in the figures represents time with each vertical line representing 10 s. For details, see Ref. 301.
C. Peak Cardiac Output and Peak Muscle Blood Flow Are Mismatched
Additional evidence for sympathetic restraint of blood flow to contracting muscles during large muscle mass or whole body exercise in humans is simply based on calculations (301, 346, 422). If peak cardiac output is 20 l/min in young healthy untrained men and perhaps 15 l/min in young healthy untrained women, this means that only ∼6 kg of skeletal muscle would be able to be maximally (∼300 ml·min−1·100 g−1) vasodilated in the men and perhaps 4 kg in women before arterial pressure would fall to values of <100 mmHg. Along these lines, it is likely that more muscle mass is contracting during large muscle mass exercise like running and cycling in humans.
D. Arm Exercise Added to Cycling Can Reduce Leg Blood Flow
When arm exercise of sufficient intensity is superimposed on ongoing leg exercise, there is some evidence that leg blood flow declines so that cardiac output is “conserved” when total blood flow demand by the exercising arms is increased (422). Likewise, maneuvers that increase the work of breathing and thus the demand for blood flow by the respiratory muscles have been shown to cause reductions in leg blood flow during heavy exercise (197). However, as noted earlier, the effects of adding arm exercise to ongoing leg exercise can be complex and contradictory. This is likely the result of differences in the study protocols and the extent to which the subjects had both highly trained arms and legs (67, 413, 422).
E. Additional Evidence for Sympathetic Restraint of Muscle Blood Flow
There are other lines of evidence consistent with the idea that the sympathetic nervous system normally restrains blood flow to contracting skeletal muscles. Highlights include the following observations: 1) when α-adrenergic blocking drugs are infused into the vascular beds of contracting skeletal muscles blood flow has been reported to increase in some studies (61, 193, 512). 2) When afferent baroreceptor traffic is increased via either carotid neck suction in humans or electrical stimulation of the carotid sinus nerve in animals during exercise (thus sending a false signal that arterial pressure has increased), there can be a reflex reduction in sympathetic outflow and blood flow to contracting muscles can increase (458, 489). 3) Skeletal muscle blood flow during small muscle mass exercise in healthy older men is generally similar to that seen in younger male subjects (134, 230). During larger muscle mass exercise, muscle blood flow is typically lower in older subjects (32, 134, 271, 365). The idea is that peak cardiac output is reduced with aging and thus more sympathetic restraint of blood flow to contracting skeletal muscles is required to maintain arterial pressure. 4) There are also excellent studies demonstrating this principle from both human patients and experimental animal models of congestive heart failure (195, 348, 369, 407, 460, 468).
F. Small Changes in Flow: Impact on Arterial Pressure
Another key concept stemming from the fact that the vast majority of cardiac output is directed toward skeletal muscle during exercise is that small changes in skeletal muscle blood flow can have a critical impact on arterial pressure. If 80% of cardiac output is directed toward skeletal muscle in a subject with a mean blood pressure of 100 mmHg, vasoconstriction that restrains skeletal muscle blood flow by 10% will increase mean arterial pressure by ∼8%. A 20% reduction will increase mean arterial pressure by 16%. These estimates are supported by the data on skeletal muscle blood flow and calculated mean arterial pressure in the cross-county skiers discussed above. It is also important to remember that in the discussions of peak skeletal muscle blood flow during small muscle mass exercise in humans venous saturation from blood draining these muscles typically remains ∼30% saturated. This saturation value is substantially higher than the ∼10% venous oxygen saturations seen during heavy large muscle mass or whole body exercise. Because skeletal muscle mitochondria can operate at a very low Po2 (174), this suggests there is a significant margin of safety that allows extraction to increase without threatening oxygen consumption by the contracting skeletal muscles. Thus reducing blood flow to contracting muscle by ∼20% to regulate arterial pressure can also explain the very low deep venous saturations in the blood-draining contracting skeletal muscles during heavy large muscle mass or whole body exercise in humans (67, 323, 369).
There are a number of caveats to what might be described as the luxury perfusion hypothesis. First, while skeletal muscle oxygen consumption might not be threatened during large muscle mass exercise by low mitochondrial Po2, maneuvers that cause small increases in blood flow or oxygen delivery can improve performance. Second, deep venous O2 saturation does not provide a detailed picture of conditions in the mitochondria of contracting muscles and how adequate oxygenation is deep in the muscle. However, our major point is that any “overperfusion” seen during small muscle mass exercise provides a margin of “excess” flow that can be constricted without too much compromise during large muscle mass exercise. This then “frees up” blood flow for a larger mass of active muscles and also facilitates the maintenance of arterial blood pressure. So, while the performance of one muscle might not be optimal, the collective perfusion of all of the active muscles during large muscle mass exercise might be optimized given the overall limitation of cardiac output on muscle blood flow and oxygen delivery that we have emphasized.
G. Baroreflex Restraint of Blood Flow to Regulate Arterial Pressure
The carotid neck suction and carotid sinus nerve stimulation data introduced above are consistent with the idea that arterial baroreflexes are ultimately responsible for mediating the competing demands between the contracting skeletal muscles for more blood flow with the need to regulate systemic arterial pressure during exercise. This interpretation is consistent with observations about blood pressure falling during exercise in patients with autonomic failure. Thus it is reasonable to introduce the concept of baroreflex resetting during exercise. The arterial baroreflexes are stretch-sensitive afferent receptor systems located primarily in the carotid sinus and aortic arch. These receptors respond to mechanical deformation. They are stimulated by stretch (classically caused by a rise in arterial pressure) and suppress sympathetic activity and activate vagally mediated bradycardia as part of the reflex blood pressure-lowering responses to the afferent signals (237). However, during exercise, blood pressure, sympathetic activity, and heart rate all increase.
For many years, exercise-related increases in blood pressure and heart rate were primarily attributed to baroreceptor inactivation during exercise. In other words, the baroreceptors were “turned off,” and blood pressure and heart rate were permitted to rise to meet the physiological challenges associated with exercise (237). In the 1960s and 1970s, studies in both humans and animals challenged this concept and instead showed that the baroreceptors were reset so that blood pressure and heart rate continued to be regulated, but at a slightly higher pressure during exercise (38, 39, 315, 489, 496, 497). This is shown graphically in Figure 10. Along these lines, Ogoh et al. (345), in an extremely clever experiment, used carotid neck suction to alter carotid baroreflex afferent input during exercise at heart rates of 90, 120, and 150 bpm. They showed that as heart rate increased, blood pressure was regulated less by changes in heart rate and cardiac output with more reliance on changes in vascular tone. Again, because so much blood flow is directed to skeletal muscle during heavy exercise, these observations indicate that skeletal muscle is a major target for vasoconstriction and thus blood pressure regulation during heavy exercise in humans.
Figure 10.
Demonstration in chronically instrumented dogs of baroreceptor resetting during exercise. This record was generated in animals that had undergone isolation of the carotid sinuses, permitting pressure in the carotid sinus to be controlled independently of arterial pressure. The input was carotid sinus pressure (x-axis); the output was systemic pressure measured in the whole animal (y-axis). During exercise, baroreceptor regulation of heart rate was reset to defend a higher arterial pressure, but the stimulus response curve to a given change in pressure was similar. Exercise was performed while running on a treadmill at 5.5 km/h up either a 7 or 21% grade. [Adapted from Joyner (237).]
H. Summary
We have presented a number of lines of evidence that the sympathetic nervous system normally acts to restrain blood flow to contracting skeletal muscle during large muscle mass or whole body exercise in humans. Key observations include 1) the lower blood flow values observed during large muscle mass or whole body exercise in humans relative to quadrupeds, especially athletic animals with large hearts. 2) The challenges to venous return and cerebral blood flow associated with the upright posture in humans versus the horizontal position in most quadrupeds. 3) The observation that blood pressure falls during supine or head down exercise in patients with autonomic failure. 4) Adding arm exercise to ongoing leg exercise can reduce blood flow to the leg muscles under certain circumstances in some subject groups. 5) In conditions like aging or disease states like congestive heart failure, blood flow to exercising muscles is restrained by the sympathetic nervous system to maintain blood pressure in the face of limited systemic cardiac output. 6) Observations showing that there is ongoing baroreceptor restraint of blood flow to contracting muscles during exercise in both humans and animals. The lines of evidence are also supported by calculations and modeling that use well-accepted blood flow, cardiac output, and blood pressure data. Having established that skeletal muscle blood flow can be very high and under some circumstances threaten arterial pressure, we now consider the mechanisms operating at the level of the contracting muscles that can cause blood flow to be so high.
VII. LOCAL BLOOD FLOW RESPONSES TO MUSCLE CONTRACTION
We have worked our way down from concepts related to oxygen transport, cardiac output, skeletal muscle blood flow, and blood pressure regulation and now begin to discuss the blood flow responses to contraction across the vascular bed of a single muscle or perhaps a limb. Figure 11 shows one of the earliest examples of the blood responses to contraction from Gaskell in 1877 (172). This record is from an in situ study of blood flow across the hindlimb of a dog during electrically stimulated muscle contractions. Blood flow was measured by collecting the venous effluent draining muscles electrically stimulated to contract. Adjacent to this tracing is a recent record from our lab of brachial artery blood flow after a single brief forearm contraction in a conscious human.
Figure 11.
The left panel is one of the original qualitative (no units provided) records of skeletal muscle blood flow (measured by timed collection of venous outflow) from the hindlimb of a dog whose skeletal muscle was stimulated to contract. Each tick mark on the x-axis represents a time interval of 5 s. The right panel shows a quantitative (ml/min) record of the brachial artery blood flow response to a single brief forearm contraction in a human more than 130 yr later. The key point is that with contraction there is an increase in venous effluent as blood is expelled from the muscle and veins. Arterial inflow stops or is attenuated as the contracting muscles compress the microcirculation of the skeletal muscle. With release of the electrical stimulation or voluntary handgrip contraction, there is a large increase in flow that declines rapidly. As is the case with the left panel in Figure 1, the marked and almost immediate rise in blood flow after the contraction or handgrip stops suggests that rapid vasodilation in the contracting skeletal muscles is a key determinant of exercise hyperemia. For details related to the left panel, see Ref. 172. Figure in the right panel are unpublished observations from our lab.
The blood flow responses shown in these two measurements made more than 130 years apart are strikingly similar, and from these records several things become clear. First, during contraction blood flow may stop temporarily when the contracting muscles compress the microcirculation and obstruct flow. Second, upon release of the contraction, there is a rapid increase in blood flow. Third, the flow then falls quickly and returns to baseline. This pattern demonstrates that the rise in flow in response to a brief contraction can be both large and fast. The increase in flow also occurs without a major increase in perfusion pressure, indicating that the response is generated within the skeletal muscle. It is also consistent with the idea that vasodilation in contracting muscle is the main local phenomenon driving the blood flow responses to exercise. The one problem with this interpretation is that vasodilation might take 5 s to occur (183, 300). However, the increase in flow is essentially immediate and seen after contractions lasting <1 s (324, 488).
As we work through the mechanisms in the contracting muscle that might contribute to exercise hyperemia, we will consider their ability to evoke both rapid and prolonged increases in blood flow, the extent to which these increases are caused by skeletal muscle vasodilation, and also the magnitude of the hyperemic response they can generate. Along these lines, Gaskell (172, 173) and Shepherd (431) identified broad classes of vasodilator mechanisms that might explain the rise in flow including substances released by nerves, substances carried in the blood, or substances released from the skeletal muscles. The major additions to these three factors since the original observations include the potential mechanical effects of contraction on blood flow both in terms of causing vasodilation and also augmenting perfusion pressure in skeletal muscles (131). Importantly, beginning in the 1980s, the role of the endothelium as a major site of vascular control emerged. It is also interesting to note that the observations of Gaskell in the 1870s and the primary role for vasodilation that we and others before us have favored were anticipated in the late 1700s by the Scottish surgeon Hunter who observed that “blood goes to where it is needed” (392). This simple statement also highlights the fundamental idea that metabolic demand and blood flow to contracting skeletal muscles is matched closely under most circumstances.
A. Site of the Vasodilation
Before we examine the mechanisms causing vasodilation in skeletal muscle, it is important to review where this dilation is occurring. The basic architecture of the skeletal muscle vasculature is well known (31, 356). The key point to remember is that compared with exercise, resting skeletal muscle is vasoconstricted. This means we have to identify the location in the microcirculation where most of this vasoconstriction is to coherently discuss where the vasodilation occurs. In this context, the “typical” skeletal muscle is perfused by a feed artery branching off a major conduit artery. There are then four to six branch orders before the terminal arterioles give rise to capillaries where the vast majority (but not all) of the gas exchange takes place in the skeletal muscles (356, 357). When the microcirculation is visualized using imaging and video techniques in a contracting skeletal muscle, there is marked dilation in all elements of the arteriolar tree, with the most pronounced dilation seen in the smallest arterioles (487, 488). Recent evidence also suggests that in many preparations up to ∼80% of capillaries are perfused at rest, challenging the older idea that capillary recruitment is a major phenomenon contributing to exercise hyperemia and gas exchange in contracting skeletal muscles (363).
Additionally, when measurements of pressure are made in these very small blood vessels, blood pressure in the feed arteries is similar to that observed in the conduit arteries (∼100 mmHg), and then falls to values of ∼50–75 mmHg or lower in the most distal elements of the arteriolar tree (115, 132, 169). Pressures of 25–40 mmHg are typically seen in the capillaries. Importantly, a major pressure drop is observed in the arterioles as they descend in size from ∼50 to 10 μm. This is consistent with the idea that these vessels are the chief sites of vascular resistance in resting skeletal muscle. However, these concepts are not uniform, and there are observations of pressure drops and hence resistance in the feed arteries of skeletal muscle. These vessels have also been shown to vasodilate in response to contractions (507, 513). Additionally, in some microvascular preparations, there can be a significant pressure drop in the feed arteries before entering the arteriolar network. Finally, with muscle contractions, the smallest arterioles that vasodilate most vigorously are relatively resistant to sympathetically mediated vasoconstriction (132, 487).
While large-conduit arteries such as the femoral or brachial arteries can vasodilate during exercise, this dilation is not functionally significant for the regulation of blood flow in exercising muscles (464). In the coronary circulation, there can be marked anatomic stenosis of large arteries of ∼70% before the stenosis limits the ability of downstream vasodilation to cause large increases in blood flow (71, 137, 138). To study parallel events in exercising skeletal muscle, we inflated an intra-arterial balloon in the brachial artery of healthy subjects. During these experiments, we were struck by the magnitude of balloon inflation (∼80% of brachial artery cross sectional area on visual inspection) needed to acutely reduce perfusion pressure and blood flow to contracting forearm vessels. However, a combination of downstream vasodilation and collateral circulation around the elbow permitted the blood flow responses to submaximal forearm exercise to recover rapidly (Figure 12). At steady state, they were remarkably normal (74, 75, 78, 79, 85).
Figure 12.
An individual record (compressed) demonstrating recovery of brachial artery blood velocity during acute reductions in perfusion pressure via intra-arterial balloon inflation during rhythmic handgripping at 20% maximal voluntary contraction. Exercise caused a rapid increase in blood velocity, while balloon inflation caused a fall in blood velocity that recovered within a couple of minutes. Breaks in velocity signal indicate times of image acquisition for diameter measurements, and breaks in arterial pressure tracing indicate times for blood sampling.
B. Conducted Vasodilation
Another important feature of the vasodilator response to muscle contractions is that these responses can be conducted upstream in the microcirculation (136, 161, 425, 426, 428). In other words, vasodilation that starts in the smallest arterioles closest to the capillaries in the contracting muscles can ascend to the larger elements of the arteriolar network including the feed arteries. This mechanism is dependent on an intact endothelium and appears to be an active process that includes Ca2+ and electrical signaling along the endothelium and between the smooth muscle cells and endothelial cells (151, 152, 427, 429, 506). This includes cell-to-cell communication between both homocellular gap junctions between cells of similar type and heterocellular gap junctions that facilitate communication between cell types (410). For example, endothelial and vascular smooth muscle cells. From a functional perspective, studies in animal models demonstrate that conducted (e.g., ascending) vasodilation can be blunted in conditions like aging that are associated with reduced blood flow responses to exercise in some models (34, 228). These responses are also subject to modulation by the sympathetic nervous system in ways that might contribute to both the regulation of arterial pressure and the matching of blood flow and metabolism in vivo (22, 200, 201).
C. Properties of Dilating Substances
There are a number of essential criteria that any vasodilating substance must possess to explain all or part of the local effects of contraction on skeletal muscle blood flow (431). Table 4 is updated from the 1983 Handbook of Physiology chapter by Shepherd and outlines the relevant properties. 1) A substance or its precursor should be present in skeletal muscles (or perhaps the nearby nerves, blood, or blood vessels). 2) The substances should have access to the muscle resistance vessels because this is where the critical vasodilation occurs. 3) The concentration of substances in the interstitial fluid or endothelium must be sufficient to cause vasodilation, and the concentration should be proportional to the skeletal muscle contractile activity. 4) Endogenous administration of the substance should be capable of causing prolonged and marked vasodilation without major sensations in humans. Exercise hyperemia can last for hours, is not “painful,” and generally does not evoke other sensations, so this is an essential property. 5) Pharmacological agents or other physiological maneuvers which modify the blood flow responses to exercise should also modify the vasodilator responses to any putative dilator substance given exogenously.
Table 4.
Criteria for vasodilator substances
| Criteria for Vasodilator Substances | |
|---|---|
| 1 | The substance or substances (or their precursors) thought to cause vasodilation should be present in the tissue or tissues thought to release them. These tissues could include skeletal muscle, vascular smooth muscle, the vascular endothelium, blood, or nerves. |
| 2 | The substance or substances should have access to the muscle resistance vessels. |
| 3 | The concentration of the substance in the interstitial fluid or at the vascular endothelium should be sufficient to cause dilation in a concentration-dependent manner that is related to contractile activity. |
| 4 | Exogenous administration of the substance or substances should be capable of causing prolonged dilation without sensations in humans. |
| 5 | Pharmacological agents or physiological maneuvers that alter the blood flow responses to exercise should have similar effects on the vasodilator responses to any putative substances given exogenously. |
These concepts were adapted from Shepherd (431). The major additions in these criteria since the original criteria of Shepherd include the discovery of the vascular endothelium as a key site of vascular control.
Along the lines of the updated Shepherd criteria listed in Table 4, it should be noted that basic ideas about both the mechanisms contributing to exercise hyperemia and the basic properties of these mechanisms have not changed much over many years. One major new discovery over the last 35 or so years has been the role of the vascular endothelium as a key site of blood flow regulation (170). The influence of this discovery and the many findings flowing from it are especially remarkable given the widespread prior belief that the vascular endothelium was primarily a barrier (170, 171). Additionally, in some quarters of the scientific community, it was widely believed that insights from physiological investigations would be limited in the era of molecular biology and reductionism (239).
D. Speed of the Vasodilation
This basic pattern of blood flow response to a single muscle contraction shown in Figure 11 demonstrates that the rise in blood flow in response to contraction can be both substantial and fast. The immediacy of the blood flow response has led to debate. The argument is that any dilator mechanism might take at least 5 s to be observed (183, 300). For example, this time frame seems reasonable for substances released by nerves that would be required to bind to receptors on vascular smooth muscle and then cause a series of intracellular events leading to vasodilation. Likewise, substances released by contracting muscles would have to diffuse out of the muscle fibers, avoid degradation or metabolism to survive in the interstitial space, and then activate either receptors on or second messenger systems in the vascular smooth muscle. The argument is that each step involved in either neural or metabolic vasodilation is associated with time delays precluding a major role for these mechanisms in the immediate rise in blood flow following skeletal muscle contractions (436). Evidence to support these concepts includes observations in the microcirculation that a 5- to 20-s delay after the onset of contraction is required to observe vasodilation (183, 300). These data are frequently cited as indirect evidence that the extremely rapid rise in flow almost certainly has to be mediated by the mechanical effects of muscle contraction on the skeletal muscle microcirculation.
More recently, it has been argued that vasodilation can occur almost immediately and that the mechanical effects of contraction only generate a trivial amount of blood flow (412, 443, 478). Other powerful evidence against a major role for mechanical factors dominating the early rise in muscle blood flow with contractions is shown in the left panel of Figure 1. It demonstrates that even a very brief contraction elicits a rise in flow that is graded with the strength of contraction. If the dilation were strictly mechanical, then it might be unrelated to the strength of contraction. Importantly, recent observations clearly show that there can be dilation within 1 s in the microcirculation (324, 488). Thus, since there is an increase in blood flow without an appreciable increase in perfusion pressure, the evidence for rapid vasodilation in the skeletal muscle microcirculation is strong and the question is what mechanisms explain this phenomenon and are they fast enough.
E. Rapid Neurally Mediated Vasodilation
The theory that there can be neurally mediated vasodilation in skeletal muscle is an old one with two potential sources of neural input. The first is dilation via activation of autonomic vasodilator nerves in skeletal muscle, and the second is vasodilation evoked by motor nerves. Neurally mediated dilation is attractive because it might explain the immediate rise in flow with exercise and, in the case of motor nerves, the matching of blood flow and contractile activity. In many species either behavioral stimuli in conscious animals or electrical stimulation of selected brain areas in anesthetized preparations can evoke a “defense reaction” (1, 96, 159, 213, 307, 308). This is a feed-forward adaptation that includes a rise in heart rate and blood pressure along with an increase in skeletal muscle blood flow that might “prepare” the animal for fight or flight. In awake animals instrumented to measure muscle blood flow on a beat-to-beat basis, there can also be a large increase in limb blood flow when the animals are anticipating exercise (485).
In the defense response sympathetic dilator nerves innervating the skeletal muscle are activated while sympathetic vasoconstriction is occurring elsewhere in the body (96, 160, 162, 484). Recent work by Matsukowa and colleagues (261, 262, 306) in conscious cats trained to weight lift showed that sympathetic vasodilator fibers can evoke increases in blood flow at the onset of contractions. There is also a history of human studies showing modest levels of forearm vasodilation in a resting limb when contractions are performed with the contralateral hand or other muscles. In some studies dilation in the resting forearm can be blocked by brachial artery administration of atropine (409). In this context, dilation in resting limbs during exercise has been interpreted by a number of investigators as demonstrating neurally mediated vasodilation at the onset of contraction in human muscles (147–149, 402, 409). However, others have argued that the dilation is due to small and difficult to detect unintended contractions in apparently resting limbs. Along these lines, when there is no electrical activity detected in the “resting” limbs, there is no vasodilation (103).
Neurally mediated skeletal muscle vasodilation has been ascribed to histamine, acetylcholine, and also nitric oxide. There is also some evidence suggesting it can be a β2-mediated vasodilator response to increased circulating epinephrine. In animals, the muscle blood flow responses seen during the defense reaction are sensitive to atropine and can be abolished by sympathectomy. There is also histological evidence for sympathetic cholinergic nerves to limb skeletal muscle in many species (185). More recently, it has been shown that the vasodilator response during the defense reaction or behavioral stress in a number of species can be blocked or attenuated by the administration of nitric oxide (NO) synthase inhibitors (261, 262). This raises the possibility of acetylcholine from sympathetic nerves causing NO release from adjacent vascular endothelium. Other possibilities include a population of nitroxidergic dilator nerves or the release of NO as a cotransmitter from the sympathetic cholinergic nerves (116, 309).
A pattern of hemodynamic responses similar to the defense reaction may be seen in humans exposed to experimental emotional stress (381). When the stress is severe, impressive increases in muscle blood flow are possible. In the late 1950s, prior to the advent of modern ethical review, Blair et al. (46), in one of the most notable human physiology studies ever conducted, instrumented medical students to measure forearm blood flow responses to emotional stress. In some subjects they placed a brachial artery catheter to locally infuse drugs and investigate the mechanisms contributing to vasodilator responses in the forearm. They also graphically described their experimental strategy for inducing emotional stress:
“For the present purposes we were interested in obtaining large responses rather than in the character and reproducibility of the emotional stimuli, and various stimuli were used. Before the experiments the subjects were told about the proposed measurements and injections, but they were not told that they would be emotionally stressed, since surprise was often an important feature of the stress. After the experiments a full explanation was given and the subjects were asked not to divulge this to other subjects.
Periods of emotional stress lasting 2–3 min were produced by the following stimuli. 1) The subject was told that he would shortly be examined orally in physiology or that he would be tested in mental arithmetic. He was then kept in suspense for 2–3 min before being told that the test would not be applied, and that he could relax. 2) Some of the medical students were given a grueling oral examination in physiology, and were severely criticized each time they gave wrong answers and sometimes when they gave correct answers. 3) Some subjects were tested in mental arithmetic. This has been found by several investigators to be a convenient emotional stimulus, capable of causing a considerable cardiovascular disturbance. . . . . . . 4) The subject was asked to worry himself by thinking of unpleasant things. 5) In some of the experiments on the normal subjects a needle was inserted into the brachial artery for the recording of arterial pressure or the infusion of atropine. In other experiments, when arterial puncture was not necessary, a needle was inserted subcutaneously, and the subject was led to believe that this was in an artery. The insertion of the needle itself caused stress and an increase in forearm blood flow in some subjects. In each case time was allowed for the forearm blood flow to revert to resting level for several minutes. The subject was then deliberately frightened in the following way. The operators pretended that blood was leaking around the intra-arterial needle, that a hematoma was forming, and that there was a considerable loss of blood. By their conversation and demeanor they tried to indicate to the subject that they were worried and alarmed, and were thinking of abandoning the experiment. About half the subjects were hoaxed successfully; these became alarmed and some even complained of pain in the arm and throbbing in the head. After a few minutes the subject was reassured and consoled, and the real purpose of the hoax was briefly explained. In every case anxiety was promptly relieved. Other subjects, some of whom were able to look at their arm and see for themselves that all was well, were not deluded by the acting and were not frightened. This particular stimulus, which is later referred to as ‘severe stress’ could, of course, be used only once for each subject.”
In response to the stressors described above, examples of 10-fold or greater increases in forearm blood flow were seen, and under some circumstances, the vasodilation could be blunted by atropine (see left panel Figure 13) or also nerve block. The dilation was also blunted or absent in the forearms of subjects who had undergone surgical sympathectomy to treat a variety of conditions. However, there have been competing nonneural explanations for this response in humans over the years, including a primary role for circulating epinephrine released from the adrenal medulla during emotional stress. Epinephrine evokes skeletal muscle vasodilation via β2-adrenergic receptors that are located on both the vascular endothelium where they evoke NO release and on vascular smooth muscle where they activate cAMP-mediated dilator pathways (486). Consistent with these observations, the forearm vasodilator responses to emotional stress are blunted or absent in subjects who have undergone regional or systemic β-adrenergic blockade (167, 176). Other studies in patients who had undergone upper extremity nerve blocks or sympathectomy sometimes demonstrated a normal forearm vasodilator response during mental stress (185, 215, 281). Finally, in some studies, the limb vasodilator responses to mental stress have been associated with acute sympathetic withdrawal, but such responses have been observed inconsistently (72, 192).
Figure 13.
Forearm blood flow responses to mental stress. The left panel is an individual record showing the responses to severe mental stress as outlined in the text and comes from the classic study of Blair et al. (46). Note that the rise in flow is not immediate and is attenuated by brachial artery administration of atropine. The right panel shows the forearm blood flow responses in a more controlled form of mental stress (Stroop color word test); the open circles show the control forearm, and the closed circles show the forearm treated with a brachial artery infusion of the nitric oxide synthase (NOS) inhibitor l-NMMA. When the l-NMMA was allowed to washout for an hour, the responses were similar in both forearms. This demonstrates that the vasodilator responses to mental stress can be eliminated in large part by administration of the NOS inhibitor l-NMMA. For many years it was assumed that this response was due primarily to activation of vasodilating sympathetic cholinergic nerves. However, anatomical studies have failed to reveal the existence of such nerves in humans, and the current explanation for this rise in flow centers on vasodilation evoked via activation of β2-adrenergic receptors and mechanical stimulation of the vascular endothelium. Both mechanisms have a likely NO component. [Adapted from Blair et al. (46) and Dietz et al. (126).]
In more recent studies our laboratory showed that brachial artery administration of the NO synthase (NOS) inhibitor NG-monomethyl-l-arginine (l-NMMA) blunted the dilator response in the human forearm to mental stress. The magnitude of this blunting was similar to or slightly greater than the effects brachial artery atropine (126). We initially postulated that acetylcholine from sympathetic cholinergic nerves caused release of NO from the vascular endothelium. In this context, at least some of the dilation evoked by activation of β2-adrenergic receptors also has an endothelial component that can be blunted by NOS inhibition (145, 486).
However, in a series of subsequent studies using nerve blocks along with other sympathoexcitatory stimuli, we began to doubt the existence of sympathetic vasodilator nerves in human skeletal muscle (125, 374). We now believe that the rise in blood pressure along with circulating epinephrine accounts for the forearm dilator response to mental stress (240). Our interpretation is that blood pressure response causes mechanical distension of the vascular endothelium and also activation of local cholinergic mechanisms that both cause the release of NO. These factors act in concert with the β2-mediated vasodilation caused by circulating epinephrine which can also have an NO-mediated component (302). While there has been strong histochemical evidence for the existence of sympathetic vasodilator fibers in the skeletal muscles of a number of species, such evidence is lacking humans (484). Additionally, either acute or chronic sympathectomy does not appreciably alter the rise in blood flow seen following a brief contraction or more prolonged periods of exercise in young adults (101). Likewise, the initial rise is muscle blood flow at the beginning of exercise is unaffected in acutely sympathectomized rats (354). Finally, in humans and other species, intra-arterial administration of atropine has little or no impact on the rise in muscle blood flow to either a single contraction or more prolonged periods of exercise (13, 54, 60, 441). All of these observations argue against a major role in humans for sympathetic vasodilator nerves in exercise hyperemia.
Conversely, there is evidence both for and against basal sympathetic activity constraining the initial vasodilation in contracting human muscle. We have previously demonstrated that acute increases in sympathetic activity (via lower body negative pressure) reduces the blood flow and vasodilator responses to a single muscle contraction in young adults, whereas it has no effect in older adults who presumably already have elevated basal levels of sympathetic activity (76). On the other hand, α-adrenergic blockade augments the blood flow and vasodilator responses following a single muscle contraction in older adults (76).
F. Acetylcholine Spillover From Skeletal Muscle
Another potential neural mechanism that can evoke rapid vasodilation in skeletal muscle centers on the idea that acetylcholine spills over from motor nerves and evokes vasodilation in blood vessels near those motor nerves (505). This is an attractive hypothesis because it would closely match blood flow with contractile activity. However, there are several problems with this hypothesis when it is extended to preparations beyond the microcirculation. First, as noted above, administration of atropine has little effect on the blood flow responses to contraction. Second, when the forearm muscles of humans are selectively paralyzed with neuromuscular blocking drugs in a way that permits them to fire and release their acetylcholine but not evoke contractions, marked vasodilation is not seen (140). These observations show that acetylcholine spillover from motor nerves is probably not a major contributor to exercise hyperemia in human skeletal muscle.
G. The Muscle Pump
The next major factor that might explain the rapid rise in blood flow seen in Figure 11 is the muscle pump (477). The general concept is that contraction compresses blood vessels (especially veins) in skeletal muscle and relaxation either causes suction or tethers the blood vessels open in a way that permits blood flow to rise dramatically. Any significant suction would effectively augment perfusion pressure. This mechanism is in addition to the role of the muscle pump in functionally augmenting perfusion pressure in the legs during upright exercise by emptying the veins in the lower extremity (267, 360, 430, 431, 434, 454). This keeps venous pressure very low and increases the perfusion pressure (mean arterial pressure minus mean venous pressure) to the dependent limb. Consistent with this interpretation is the observation that peak oxygen uptake is ∼7% higher in the upright versus supine position during cycling in humans (123). There is also evidence that individuals with venous incompetence have altered leg blood flow responses to exercise in the upright posture (335, 336).
Sherriff and Van Bibber (437) used a creative approach to demonstrate the potential for energy to be imparted by the contracting muscles on blood and the ability of this energy to generate flow. They bypassed the heart and surgically constructed an auto-pumping circuit of contracting skeletal muscles in pigs. With this preparation (Figure 14), a blood flow of ∼100 ml/min could be generated and sustained by the muscle pump compared with values of ∼450 ml/min when blood flow was being delivered by the heart. While total flow from the circuit was modest, direct comparisons with the normal heart-perfused preparation are difficult because of differences in both venous and arterial pressure.
Figure 14.
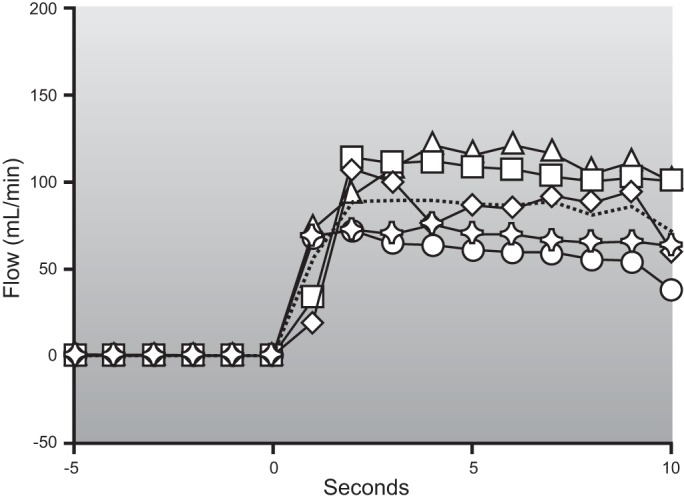
Skeletal muscle blood flow responses to auto-perfusion generated when a circuit connecting the hindlimbs of anesthetized pigs is isolated from the heart and the hindlimbs are electrically stimulated to contract. The pumping actions of the muscle contractions can increase blood flow in the absence of the heart and a normal arterial pressure. This shows that the skeletal muscle pump operating in isolation can generate some blood flow, but this increase in flow is modest at best compared with the large increases in skeletal muscle blood flow associated with exercise, but interpretation is not completely straightforward in the absence of normal perfusion pressure and gas exchange. The solid lines with various symbols represent the individual responses from each of the five pigs studied. The dashed line represents the averaged response for the five pigs (mean is based on 1 experiment from each of the 5 pigs). [Adapted from Sheriff and Van Bibber (437).]
To test the contribution of the isolated muscle pump in humans, Tschakovsky et al. (478) developed a cuff system that rhythmically squeezed the forearm while they measured brachial artery blood flow. The compression pattern and pressures generated by the external cuff were designed to mimic the pressures generated by the contracting muscles. As shown in Figure 15, when the forearm was at or above heart level, this rhythmic “pumping” of the forearm caused little increase in brachial artery blood flow. In contrast, when the arm was below heart level, there were modest increases in blood flow with rhythmic cuff pumping of the forearm. This is consistent with the idea that full but not empty veins can contribute to the hyperemic responses to exercise.
Figure 15.
The effects of rhythmic (1 s inflation/2 s deflation) external cuff compressions on forearm blood flow. The rhythmic inflation of an external cuff around the forearm was designed to mimic the mechanical effects of contraction. It causes a modest increase in forearm blood flow when the arm is below heart level. In contrast, when the arm is at heart level, there is little increase in blood flow. In either case, the increase in flow associated with cuff compression is very modest compared with what can be achieved with either a single contraction or more prolonged rhythmic hand gripping. This figure also shows that the effects of the muscle pump are dependent on limb position and also provides evidence that the muscle pump acting alone can generate only modest levels of blood flow. [Adapted from Tschakovsky et al. (478).]
When various maneuvers like unloaded cycling or leg kicking, very light exercise, or measurements of the decay in blood flow immediately after exercise are examined, the relative contribution of the muscle pump can be variable (289, 439). During upright exercise, the rapid reduction in venous pressure can account for 67% of the rise in femoral blood flow seen during the first 10 s of cycling at a low power output (516). However, during more prolonged heavy exercise, the contribution of the muscle pump is more modest and accounts for only a small fraction of the flow, and during heavy exercise, the net effect of higher muscle forces impeding blood flow might offset the flow-promoting effects of the muscle pump (289).
Taken together, these observations suggest that skeletal muscle pump can facilitate exercise hyperemia and that the main contribution is to the immediate rise in skeletal muscle blood flow seen at the onset of exercise. As noted above, the skeletal muscle pump is clearly critical for the systemic hemodynamic responses in humans exercising while upright.
An important caveat related to how much flow the muscle pump can generate is that blood flow values above 150 ml·min−1·100 g−1 are rarely reported in studies using the isolated dog gastrocnemius plantaris preparation (23, 24, 225). This observation may reflect experimental conditions including how the arterial inflow to the muscle is isolated. However, with careful optimization of all experimental variables, blood flows of 200 ml·min−1·100 g−1 are possible (452; B. Gladden, personal communication). These values are still substantially lower than those reported by Musch and colleagues using microspheres in running dogs (331, 333). They are also lower than the peak flow of ∼300 ml·min−1·100 g−1 (or higher) seen in various species including humans (47, 376). Could the “additional” flow seen in vivo compared with the in situ results be due to the muscle pump? In this context, when voluntary exercise is superimposed on maximum pharmacological vasodilation in conscious dogs, there is no further increase in blood flow. At one level, this is another observation that argues against a major role for the muscle pump in exercise hyperemia, but it could also reflect the flow-impeding effects muscle contraction (194).
In several sections of this review, we have commented on the idea that in addition to their effects on veins, the forces generated by skeletal muscle contraction can impede blood flow to the muscle by compressing other elements of the circulation. One final caveat on this topic comes from an applied observation in competitive cyclists who appear to prefer faster pedaling frequencies than might be predicted based on measurements of oxygen consumption alone. The speculation is that more frequent and lower force contractions at a given power output interrupt blood flow less than slightly longer and higher force contractions at slower pedaling rates (190).
H. Chemical Vasodilation: General Comments
The previous sections have discounted the role of neurally mediated vasodilation and the muscle pump in exercise hyperemia. Thus, by default, we have arrived at the conclusion that the primary mechanism responsible for exercise hyperemia must be vasodilation in the microcirculation caused by chemical factors (Table 4). These could be released from the contracting muscles or the nearby endothelium, or perhaps carried in the blood. Before we discuss the many potential mechanisms that might cause chemical vasodilation, several important concepts outlined by Shepherd (431) need to be reviewed.
1) Any chemical factor or factors that initiate the vasodilation (or for that matter, blood flow in general) in contracting skeletal muscles during exercise may not be the same factors that sustain it. Prolonged intense exercise can occur in many species including humans. Therefore, skeletal muscle blood flow must be able to achieve very high levels for prolonged periods of time.
2) There is likely significant redundancy in chemical vasodilating factors. This makes studying these factors very difficult because pharmacological approaches that “block” a putative vasodilator substance, thus revealing its contribution, might be masked by an increased contribution from another chemical vasodilator. For example, if substance X is antagonized and the contribution of substance X to exercise hyperemia is eliminated, there might be a buildup of other dilating factors sufficient to either keep the blood flow response relatively normal or restore it after a brief fall in flow. So when blocking compounds are administered (before or during contractions), the temporal resolution of the flow measurements is a potentially important element of experimental design that requires careful consideration. Additionally, a drug given before exercise might not reach the vessels subject to dilation during the contractions, and redundant factors that might compensate for a missing dilator substance could be engaged right away. Likewise, the effects of a blocking drug given during contractions might be missed if blood flow measurements are discontinuous and any temporary reduction in flow is compensated for by another vasodilator mechanism prior to the next measurement of blood flow.
3) Many (in fact most) putative vasodilating substances also stimulate the vascular endothelium to release NO. Thus it is sometimes difficult to know whether the substance in question is contributing directly to the exercise hyperemia, or indirectly via stimulation of NO released from the vascular endothelium, or both. These general considerations can make the design and interpretation of experiments that attempt to “pharmacodissect” exercise hyperemia challenging. Human studies are further limited by the availability of approved pharmacological tools and safety concerns. While more drugs are available for use in animals, their specificity of action is subject to question.
In addition to these factors, muscle contractions can cause a brief compression and cessation of arterial inflow to the skeletal muscle. Additionally, even brief periods of ischemia lasting only a few seconds are typically sufficient to initiate a reactive hyperemia response (256). So, depending on how the contractions compress the skeletal muscle blood vessels, an element of reactive hyperemia might also be involved in exercise hyperemia.
Finally, there is an almost existential question about the continuing search for a single dilating substance that explains the vast majority of the exercise hyperemia response. Is it possible to design an experiment that will permit such a substance to be identified? Are there many examples of single mechanisms dominating important physiological responses? Are most critical physiological responses subject to redundant control so that a margin of safety exists in case one or more mechanism is absent or dysfunctional? While we and others have argued the search for a single dilator is likely to be futile, the evaluation of each new metabolic substance or mechanism that might contribute to exercise hyperemia is frequently framed in the context that it might be the “missing” dominant vasodilator substance (243).
I. Rapid Chemical Vasodilation
Numerous in vivo experiments have demonstrated that there is an immediate (within 1–2 s) increase in flow following a brief muscle contraction (54, 76, 85, 101, 249, 253, 337, 476, 478). Recent evidence from both human and animal models suggests that K+ channels in the vascular smooth muscle play an important role in this immediate dilation. Armstrong et al. (11) originally demonstrated that the initial vasodilation in the hamster cremaster muscle in response to muscle contractions was attenuated when 1) release of K+ from voltage-dependent K+ channels in skeletal muscle were inhibited, 2) inwardly rectifying K+ (KIR) channels in smooth muscle were blocked, or 3) Na+-K+ pump in smooth muscle was inhibited. More recently, inhibition of K+-mediated vascular hyperpolarization in the human forearm effectively attenuates the peak and total vasodilator response to various intensities of single muscle contractions (107).
Emerging evidence in young humans also suggests a potential role of NO in contraction-induced rapid vasodilation (54, 85, 107). NOS inhibition alone (85), in combination with muscarinic receptor blockade (54), or cyclooxygenase inhibition (107) significantly reduces the peak and total postcontraction vasodilation. It is important to note that muscarinic receptor blockade (54) or cyclooxygenase inhibition (442) alone has little to no effect on the rapid vasodilator response to muscle contractions. Lastly, as shown in Figure 16, inhibition of NO and prostaglandin synthesis in combination with administration of substances that block or inhibit K+-mediated vascular hyperpolarization nearly abolishes the immediate vasodilation following a single muscle contraction (107).
Figure 16.
The vasodilator response to a single forearm contraction at 20% of maximum is reduced by ∼80% when nitric oxide, prostaglandin, and K+-mediated hyperpolarization vasodilator pathways are inhibited. Blockade of NO and PGs alone can reduce this response ∼50%, and blockade of K+-mediated hyperpolarization alone can reduce it by ∼60%. At this time, it is not known if blockade of all of these pathways in combination will affect the steady-state blood flow responses to exercise. This is perhaps the most complete blunting of a vasodilator response to contractions currently available and highlights the idea that the vasodilator responses to contractions are highly redundant. It is also interesting to note that the immediate vasodilator responses to contractions can be blunted in conditions like aging (76, 85, 253). (Figure provided by Drs. Anne Crecelius and Frank Dinenno.)
The immediate vasodilator responses are also facilitated by a temporary loss of sympathetic tone in the contracting muscles, and during whole body exercise a brief reduction in sympathetic outflow (242, 354). This latter effect might occur as blood is translocated from the periphery and activates both cardiopulmonary and arterial baroreflexes which in turn cause a brief reflex reduction in sympathetic outflow to muscle (70). Conversely, increases in sympathetic outflow can limit the rapid vasodilator responses to forearm contractions in humans (76, 119), and stimulation of α-adrenergic receptors with norepinephrine in the microvascular resistance networks of mice blunts rapid-onset vasodilation and restricts blood flow following a single contraction (228).
J. Blood Flow and Metabolism Matching During Ongoing Exercise
As noted repeatedly in this review, skeletal muscle oxygen consumption and blood flow appear to be tightly linked. This relationship suggests that some signal or signals proportional to the metabolic demand of the contractions are the primary drivers of skeletal muscle vasodilation. In this context, two major ideas related to flow and metabolism matching have predominated. The first is that some factor or factors is released by the contracting muscles and linked to muscle activity. An example might be K+ associated with skeletal muscle repolarization. The second is that some metabolite(s) is released from the contracting skeletal muscles in proportion to metabolic activity that might match blood flow and metabolism. Historically, much interest has focused on adenosine.
K. Substances Released by the Contracting Skeletal Muscles
1. Potassium and osmolarity
Potassium ions and changes in osmolarity associated with muscle contraction have been proposed as potential mediators of exercise hyperemia and, as noted above, appear important to the immediate vasodilation seen at the onset of contractions. These mechanisms might fulfill the general criteria that their concentration is linked to contractile activity in a graded way, but experiments over many years have cast doubt over their role as major contributors to ongoing exercise hyperemia. In the case of K+, it is clear that the concentration can rise sufficiently (>10 mM) to evoke marked dilation via activation of KIR channels and Na+-K+-ATPase with hyperpolarization of affected cells (64, 232). However, exogenous administration of potassium does not cause marked dilation, and it is painful in humans. In a notable experiment, potassium depletion in dogs did affect the vasodilation seen at the onset of contractions. However, it did not disrupt the relationship between oxygen consumption and blood flow during more prolonged bouts of contraction even though the ability of the skeletal muscle to produce force in the potassium-depleted animals was reduced (202). These observations argue against an obligatory role for K+ in the sustained vasodilation seen during exercise.
Like K+, the concentrations of phosphorus and also the osmolarity around the resistance vessels in the contracting muscles can increase. However, these increases are transient, and while they might contribute to vasodilation at the onset of contractions, they are probably not essential to sustain it. Additionally, the range of changes in potassium and osmolarity are probably not great enough to explain the range of blood flow increases seen in contracting muscles during exercise (316, 317, 431, 446). Similar issues apply to other electrolytes (26).
Early studies on metabolites that might cause vasodilation focused on the possibility that lactate or lactic acid might be the metabolic vasodilator (214). However, mild and moderate exercise are not associated with much if any lactic acid release from contracting muscles, so it is difficult to see how either lactate ion or H+ might be obligatory. Additionally, exogenous administration of lactate does not cause appreciable dilation (247). Finally, patients with McArdle's disease (myophosphorylase deficiency) do not produce lactic acid even in response to heavy exercise (189, 276, 277). These patients also show a hyperdynamic circulatory response to exercise, indicating that their vasodilator responses to exercise are excessive in the absence of lactic acid production. These observations highlight the evidence against a major role for lactate or lactic acid in the sustained vasodilation seen during exercise.
L. Adenosine
The most commonly discussed potential vasodilator substances released by contracting skeletal muscle include the adenine nucleotides adenosine and also ADP and ATP. Along these lines, the so-called adenosine hypothesis is a milestone in thinking about how cardiac muscle contractile activity is linked to blood flow (37), and has been adapted for skeletal muscle (299). In this hypothesis, a mismatch between flow and metabolism leads to the buildup and release of adenosine as either high energy stores in the muscle are depleted or there is increased ATP turnover in the contracting muscles. This then leads to a rise in skeletal muscle blood flow proportional to the contractile activity and metabolic demand.
The adenosine hypothesis and its offspring have generated a host of ideas and experimental evidence on exercise hyperemia. However, it is difficult to test because adenosine has a very short half-life in blood, obtaining accurate measurements of its interstitial concentration is very challenging, and values in blood when they are obtained may or may not reflect concentrations in the interstitial space (29). While some progress has been made measuring concentrations of vasoactive substances including adenosine in the interstitial fluid using microdialysis, there are concerns with this technique (208). 1) The time resolution of microdialysis measurements is slow, meaning that very brief changes in the concentration of one or more putative vasodilating substances would be difficult to detect. 2) Muscle damage with catheter insertion might contaminate the microdialysate. 3) Many putative vasodilating substances have exceedingly short half-lives, and this is usually related to sampling substances directly or some breakdown product of them needs to be considered. In this context, care needs to be taken when interpreting data from microdialysis studies. However, in many cases it is the best data available at this time.
There are four additional caveats concerning a role for adenosine in exercise hyperemia in humans. 1) While exogenous administration of adenosine can evoke marked vasodilation in the leg, the dilation is less than that seen during heavy knee extension exercise, so the argument is that if adenosine is a major factor in exercise hyperemia it must do so in conjunction with other factors (330). 2) There are responders and nonresponders to exogenous adenosine administration, and both groups have marked hyperemic (and vasodilator) responses to exercise (303–305). 3) In the responders, adenosine-mediated vasodilation can be significantly blunted by administration of NOS inhibitors; however, exercise hyperemia is not (304). 4) Administration of compounds that block adenosine deaminase do not cause a major increase in blood flow to contracting muscles (303). There are also parallel observations from a number of animal studies on these issues. For example, addition of adenosine deaminase which should enhance the breakdown of adenosine does not reduce blood flow in running rats (257).
In a particularly insightful experiment, Hester et al. (210) performed a series of contractions in an isolated canine skeletal muscle preparation before and during the infusion of high doses of adenosine over 3 h. Figure 17 shows that while adenosine alone could produce marked vasodilation, this vasodilation waned with time. This finding indicates that the skeletal muscle blood vessels had become desensitized to its vasodilator effects. However, when the muscle was stimulated to contract after the vasodilator responses to adenosine were absent, the hyperemic responses to contraction were similar to pre-adenosine infusion values (210).
Figure 17.
Effects of prolonged adenosine infusion on blood flow on an isolated dog hindlimb preparation. Prior to adenosine infusion, electrically evoked skeletal muscle contraction caused an increase in blood flow. Adenosine was then infused for 150 min, and the vasodilator response to adenosine waned over that time. After the dilator response waned, the increase in blood flow to contractions was still present. The tachyphylaxis to adenosine indicated that skeletal muscle blood vessels had become desensitized to its vasodilator effects. The continued vasodilation with contractions after vasodilator responsiveness to adenosine had waned indicates that adenosine is not obligatory to generate a normal vasodilator response to contractions. [Adapted from Hester et al. (210).]
The observations related to adenosine noted above suggest that either adenosine is not normally participating in skeletal muscle vasodilation during exercise or it is not “obligatory.” They also highlight a number of the general problems associated with identifying a single major dilating substance that accounts for most of the vasodilator responses seen in contracting muscles during exercise. One area where evidence for the participation of adenosine in the regulation of blood flow to contracting muscles is stronger is when there is reduced oxygen delivery to contracting muscles via a restriction of arterial inflow (75, 255, 260). This is also the case for adenosine in the regulation of coronary blood flow (272). Additionally, in pigs during heavy exercise, administration of an adenosine deaminase blocker lowers blood pressure during heavy exercise (268). This observation suggests that adenosine is being released from the muscle and that in this model skeletal muscle is “underperfused.” If so, it might be contributing to the regulation of blood flow in the contracting skeletal muscles. However, based on a synthesis of results highlighted above, it is difficult to make a case that adenosine is obligatory for exercise hyperemia under most circumstances. Consistent with these ideas, we have shown that adenosine plays a key role in regulating skeletal muscle blood flow responses to acute hypoperfusion (75).
One important question about adenosine, especially when comparing small and large muscle mass exercise, is the relatively high levels of saturation seen in the venous effluent during small muscle mass exercise. As noted earlier, this suggests that the muscle might be overperfused and that adenosine release might not be occurring. However, during heavy large muscle mass or whole body exercise, when almost all of the oxygen is being extracted across the active muscle, is the muscle “underperfused” and does adenosine contribute to the vasodilator responses?
M. ATP
During the first wave of enthusiasm for adenosine as a vasodilator substance linked to exercise hyperemia in the 1960s and early 1970s, the possibility that either ADP or ATP was the major vasodilating metabolite released by the contracting muscles also emerged (163–165). Many of the issues associated with determining adenosine concentrations in or near contracting skeletal muscles also apply to ADP and ATP. Like adenosine, they can cause marked skeletal muscle vasodilation, and like adenosine, they would directly link metabolism and blood flow. Finally, there are a number of potential sources of ATP and sites of action that include both vasodilator and vasoconstrictor responses (283).
One particularly attractive property of ATP is that it can evoke levels of vasodilation seen in human limbs during heavy exercise when given exogenously (181, 327). To date, it is the only exogenously administered vasodilator with this property. However, the role of NO release as a contributor to ATP-mediated vasodilation is controversial with some studies reporting that NO release is not involved in ATP mediated vasodilation in human limbs and other studies showing a contribution (109, 327, 384, 440). If NO is a major part of the ATP-mediated vasodilator response, then the question “why NOS inhibition does not cause a bigger decrement in exercise hyperemia?” applies to ATP as well as to adenosine. Thus the main arguments in favor of ATP as a key mediator of skeletal muscle vasodilation during exercise are the magnitude of the flow it can evoke and also its ability to interfere with sympathetic vasoconstriction (403). However, many of the general concerns related to adenosine and exercise hyperemia also apply to ATP and ADP. Finally, we will reexamine the contribution of ATP during the subsequent discussion of new ideas about blood-borne factors that might contribute to skeletal muscle vasodilation during exercise, because there is more than one source of its release (283).
N. Substances Released From the Endothelium
The discovery in the 1980s that skeletal muscle blood flow was roughly an order of magnitude greater than previously thought was a major milestone in exercise hyperemia. However, the emergence of the vascular endothelium as a site of vasomotor control had even more widespread implications. Prior to that time it was assumed that the vascular endothelium served primarily a barrier function, separating the blood from the tissues. Additionally, the discovery of endothelial dysfunction as a major risk factor for cardiovascular disease has had important implications for the pathophysiology of a number of diseases (53, 114, 170, 171, 207). Perhaps even more fundamentally, the emergence of NO and a new class of gas transmitter/signaling mechanisms was of even more importance. In this context, it is interesting to note that the fundamental observations on the role of the vascular endothelium as a major site of vascular control was made using classic organ bath studies using physiological and pharmacological approaches as opposed to reductionist molecular techniques (170).
It is also interesting to speculate about the discovery of the vasoactive properties of the endothelium in retrospect. It had been known for many years that arterial infusions of acetylcholine could cause dilation in vivo but not the relaxation of vessel strips or rings. Based on the assumption that the strips and rings provide more insight into the basic behavior of blood vessels, the in vivo response was seen as paradoxical, and efforts to explain it were made (170). One idea was that the acetylcholine temporarily interfered with sympathetic neurotransmission and thus caused acetylcholine-mediated vasodilation (433). However, the increases in flow with temporary loss of sympathetic tone are modest compared with acetylcholine-mediated vasodilation. In view of this history, it is tempting to ask what might have happened if the question had instead been “what is wrong” with the responses observed in vitro? Attempting to reconcile an in vivo observation with in vitro findings is a fundamentally different intellectual proposition than attempting to reconcile the in vitro findings with results from an intact animal or human. If the latter perspective had been more widespread, would the discovery of the endothelium as a site of vascular control come earlier? How many other phenomena are being missed or poorly explained in an effort to reconcile observations from whole animals with ever more granular reductionist preparations? At what point for any given problem might reversing the order of the attempted reconciliation raise new perspectives or questions and lead to additional insight?
With the emergence of the vascular endothelium as a site of blood flow regulation, three important questions emerged. First, what substances are released by the vascular endothelium that might contribute to the regulation of skeletal muscle blood flow? Second, do these factors contribute to exercise hyperemia? Third, how might other putative vasodilator substances interact with the vascular endothelium? These general topics have been touched on briefly above as needed to discuss other potential vasodilating mechanisms covered earlier highlighting the integrated nature of the response.
The main vasodilating substances released by the vascular endothelium are NO and prostaglandins. Depending on the model used, blockade of endothelial NOS with arginine analogs can reduce skeletal muscle blood flow on the order of 10–30% (141, 417, 419). In most human studies, the upper limit appears to be ∼20%. Inhibition of prostaglandin synthesis leads to smaller reductions in flow than NOS inhibition (328, 419, 442). Additionally, at least some of the vasodilation associated with prostaglandins is due to prostaglandin-mediated NO release (330, 340, 373, 411), further complicating the interpretation of many pharmacological studies. During double blockade, with few exceptions, reductions in blood flow on the order of 20% or less are seen and typically not greater than the reduction seen with NOS inhibition alone (330, 417).
Along these lines, aging can be associated with reduced endothelial function (420) and the effects of NOS inhibition on the blood flow responses in contracting muscles are generally less in older versus younger humans (84, 111, 416). This suggests a blunted contribution of NO to exercise hyperemia in older humans, but the marked dilator responses seen in the contracting muscles of older subjects again highlights the modest role and nonobligatory role of NO in exercise hyperemia. Another fundamental issue in evaluating the role of NO is the extent to which changes in muscle blood flow reflect reductions in blood flow to the resting muscles. In many studies, the increase above resting or baseline during exercise after administration of NOS inhibitors remains similar to control conditions.
The situation during whole body exercise is also complicated by the systemic effects of NOS inhibition on blood pressure and cardiovascular reflexes. In humans, leg blood flow during a whole body eNOS inhibition during cycle ergometer exercise is relatively constant, but vascular resistance is higher because blood pressure is higher (166). The rise in blood pressure with NOS inhibition also leads to a reflex reduction in sympathetic activity, clouding the interpretation of blood flow and vasodilator responses because the loss of an endothelial vasodilator signal might be obscured by the loss of a sympathetic vasoconstriction due to baroreceptor-mediated sympathetic withdrawal (89).
In dogs exercising after ganglionic blockade to eliminate systemic vasoconstrictor activity, NOS inhibition causes a ∼30% reduction in blood flow to contracting skeletal muscles (435). In addition to these observations in humans and chronically instrumented animals performing voluntary exercise, there is also evidence from a number of sources suggesting that endothelial NO contributes deferentially to the regulation of exercise hyperemia and different fiber types (98). Under some circumstances, fast-twitch skeletal muscle is more dependent on NO-mediated vasodilation than slow-twitch skeletal muscle (100). There are also observations suggesting the opposite pattern of involvement for NO, and this has to do with the exercise intensity and whether the NOS inhibitor was given before or during exercise (334). However, the relevance of these findings to humans is unclear because, unlike rodents that have highly compartmentalized skeletal muscle containing predominantly fast, slow, or intermediate fiber types, human skeletal muscle is mixed (14, 15, 278). However, an important finding from these studies is that blockade of neuronal NOS can influence the blood flow response to exercise, indicating that in addition to the vascular endothelium, NO might also be released by the contracting skeletal muscles (97, 99, 102).
The other major issue with NO-mediated vasodilation is that as noted several places above at least some element of the vasodilator responses to many substances (adenosine, ATP, β2-receptor agonists, and prostaglandins) have a major NO component. So, the question for any putative dilator substance thought to play a major role in exercise hyperemia is that, if substance X is a major contributor to exercise hyperemia, why doesn't inhibition of endothelial responses have a bigger impact on exercise hyperemia? For some substances, this question is specific to NOS, whereas for other substances it is specific to combined inhibition of both NO- and PG-mediated effects. Finally, there is emerging evidence that NO interacts with superoxide to generate what has been characterized as a “bang-bang” vasodilator mechanism (177, 178). The extent to which this mechanism might contribute to exercise hyperemia is unclear.
O. Blood-Borne Vasodilator Substances
Two obvious blood-borne vasodilator candidates are oxygen and CO2. Studies conducted over many years have thoroughly evaluated both CO2 and hypoxia per se as vasodilator factors, and while both can cause small increases in blood flow, the magnitude of the dilation they evoke pales compared with that seen with exercise. It is also not easy to determine how changes in their partial pressure might be directly related to changes in flow over a wide range of blood flow values. We and others have systematically manipulated oxygen delivery to contracting muscles using hypoxia, several forms of hyperoxia, and inflation of balloons in the brachial artery to reduce perfusion pressure. In general, changes in arterial oxygen content evoke either increases (hypoxia) or decreases (hyperoxia) in blood flow with the net effect of maintaining constant oxygen delivery to the contracting muscles (73, 80–83, 182, 399, 504, 509, 510). A similar compensatory vasodilator response is seen when oxygen delivery is reduced by lowering perfusion pressure via balloon inflation to the contracting muscles (74, 75, 77–79).
However, other experimental approaches to induce hypoperfusion in contracting muscles have produced conflicting results with regards to flow restoration. Partial flow restoration or compensation has been reported to occur when external positive pressure is used to reduce blood flow in the contracting muscles of the arm and leg (112, 400). In both of these studies the partial flow restoration was attributed to an augmented systemic pressor response. Conversely, minimal flow recovery was observed when local forearm perfusion pressure was acutely reduced via positional changes (i.e., forearm above heart level) (498). A lack of flow restoration with positional perfusion lowering is further supported by the idea that muscle force production is reduced and fatigue can be increased by reductions in perfusion pressure (517, 518). Taken together, the magnitude of flow restoration or compensation and mechanisms involved appear to be dependent on the experimental approach used to induce hypoperfusion in contracting skeletal muscle.
In a number of studies we have evaluated metabolic, endothelial, and neural mechanisms that contribute to compensatory vasodilation. These include the contribution of vasodilators like NO and adenosine and interactions between sympathetic vasoconstriction and metabolic vasodilation. In general, our findings demonstrate that NO contributes to the compensatory dilator responses during both hypoxia and hypoperfusion (77, 82). Adenosine appears to contribute only during hypoperfusion (75, 83). β-Adrenergic receptor mechanisms appear to contribute during lower intensity hypoxic exercise (509), but much of this contribution is likely due to β-adrenergic-mediated NO release (73, 117). This response again highlights the general observation that many dilator substances involved in exercise hyperemia have effects both on vascular smooth muscle and the vascular endothelium. At higher hypoxic exercise intensities, it is likely there is another cause of NO release.
It is also interesting to note that when arterial oxygen content is increased ∼25–30% during exercise in hyperbaric hyperoxia, the blood flow responses are reduced by a similar amount (80, 81). The mechanisms of this blunting and relative vasoconstriction are currently poorly understood, but an enhanced α-adrenergic vasoconstriction does not explain the majority of the large reductions in blood flow during exercise in hyperbaric hyperoxia (81). Preliminary data from our laboratory suggest that oxidative stress associated with the hyperoxia inactivating a key dilator substance like NO is not involved. Overall, these findings are another example of the tight linkage between oxygen demand and delivery seen during exercise under most circumstances. They also reinforce the challenge of identifying a dominant factor responsible for the changes in blood flow and vasodilator responses in contracting muscles evoked by a specific intervention. In contrast to the vasoconstriction observed during forearm exercise noted above with hyperbaric hyperoxia, experimentally increasing oxygen delivery to contracting forearm muscles by elevating mean arterial and perfusion pressure does not lead to compensatory vasoconstriction and normalization of blood flow (475).
While oxygen and CO2 per se are not major blood-borne factors that directly cause vasodilation in the active muscles, two new concepts have emerged related to blood-borne substances and vasodilation. One idea is that ATP is released from deoxygenating red blood cells (35, 179, 229) and that this release evokes vasodilation in areas of the skeletal muscle that are consuming oxygen. ATP can also be released via mechanical deformation of red blood cells (108, 449, 450). In both cases, this mechanism would meet a number of criteria associated with so-called “blood flow metabolism matching.” Importantly, as discussed above, ATP has a number of properties that “mimic” the vasodilator responses associated with exercise including the ability to generate a large increase in skeletal muscle blood flow and also to interfere with sympathetic vasodilation from the contracting skeletal muscles. However, at this time, the primary evidence that ATP released from RBCs is perhaps the major cause of exercise hyperemia is primarily indirect or correlational.
Arguments against it include the previously mentioned observation that at least some portion of the dilator response to exogenous ATP administration has an endothelial component that is at the upper end of that seen for exercise. Additionally, there is some evidence that the ATP release is defective in RBCs from patients with cystic fibrosis (449); however, patients with cystic fibrosis have a relatively normal blood flow response to forearm handgripping (418). Finally, ATP release from RBCs might also be blunted or absent in healthy older patients (250) and in diabetics (451), and in both groups contraction can still evoke marked increases in blood flow.
Similar to the ATP-RBC interactions noted, there are also several potential sites of interaction between NO, hemoglobin, and red blood cells. These interactions occur in a way that might also permit NO to be stored in the form of S-nitrosohemoglobin and then released during deoxygenation of hemoglobin to facilitate the matching of blood flow with metabolic demand (124, 453). However, NOS inhibition via local infusions can reduce blood flow modestly consistent with the idea that endothelial (or perhaps skeletal muscle) sources of NO are the main contributors during exercise, particularly under hypoxic conditions (82, 110). This interpretation seems reasonable since local infusions of small amounts of NOS inhibitors should not disrupt the red blood cell-mediated NO metabolism or the recently identified eNOS found within erythrocytes (515). Of note, during whole body NOS inhibition, the blood flow responses to exercise are remarkably normal as well, even when doses of NOS inhibitors sufficient to reduce whole body NO synthesis by ∼70% are administered (166). The idea that local infusion of a NOS inhibitor reduces the vasodilator and blood flow response during hypoxic exercise in humans also argues against the idea that nitrite and/or formation of erythrocyte nitroso species is a key vasodilator in this response (135, 175, 471). If nitrite were responsible, either directly or indirectly (via reduction to NO), the compensatory vasodilation would have been maintained despite NOS inhibition. However, while these observations show a role for endothelial sources of NO, they do not rule out a contribution of NO from other sources including the RBC.
P. The Challenge of Redundancy
Figure 18 shows vasodilator responses in the coronary circulation of dogs under control conditions and during triple blockade when NO, adenosine, and potassium ATP channels are blocked. Figure 19 shows analogous data from human forearm studies. In general, efforts to block multiple vasodilator pathways in skeletal muscle have demonstrated that the blood flow response to prolonged contractions is incredibly robust and difficult to reduce by more than ∼20% under most circumstances (286, 415). Figure 19 also demonstrates the critical importance of both the time resolution of the blood flow measurements in evaluating pharmacological approaches to blocking one or more dilator pathways and issues related to individual variability.
Figure 18.
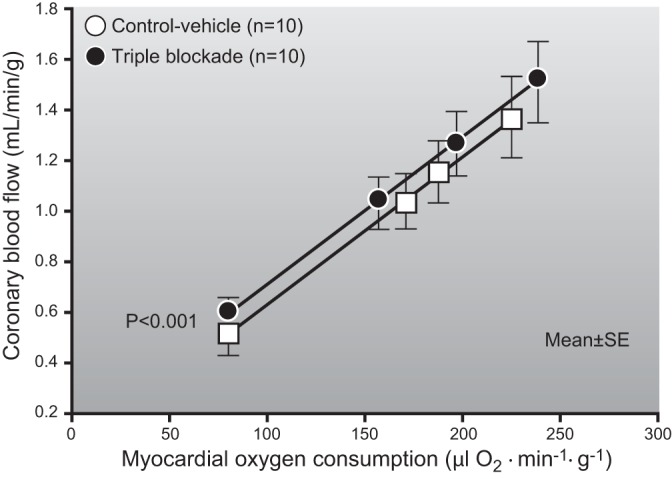
Vasodilator responses in coronary circulation of dogs during exercise, under control conditions, and after triple blockade when nitric oxide, adenosine, and calcium ATP channels are all blocked using various pharmacological antagonists. Note that the rise in coronary blood flow in response to an increase in myocardial oxygen consumption generated by graded treadmill exercise is similar in both conditions. This is an example of vasodilator redundancy in the coronary circulation that is similar to the redundancy seen in many human and animal studies on the regulation of blood flow in contracting human and animal muscles. It is seen in both isolated preparations and during exercise in conscious animals. While there are key differences between the coronary and skeletal muscle circulations, the concept that redundant vasodilator mechanisms govern the blood flow responses to exercise is a key commonality, as are the many putative dilator substances and mechanisms that might be involved. [Adapted from Tune et al. (481).]
Figure 19.

Individual record showing the critical importance of time resolution and redundant vasodilator pathways in evaluating blood flow responses to pharmacological blockade in contracting skeletal muscles. In this forearm handgripping study (rhythmic contractions performed at 10% of maximal voluntary contraction for 20 min), addition of the KATP channel blocker glibenclamide after combined blockade of nitric oxide and prostaglandin synthesis (l-NAME + Ketorolac) caused an impressive but temporary reduction in blood flow to contracting forearm muscles. This was followed by a compensatory hyperemic response and return to steady-state values. One possible interpretation is that the blood flow response to contractions became more dependent on KATP channels during blockade of nitric oxide and prostaglandin synthesis. Thus it fell dramatically when glibenclamide was administered. This fall in flow then caused a mismatch between oxygen delivery and metabolism in the contracting muscles that evoked adenosine release. This caused a brief hyperemic response above the normal blood flow value associated with contractions and then a return toward the steady-state value seen before any intervention. These observations emphasize the need for rapid time course measurements of flow (beat-to-beat) when pharmacological blocking agents were given during contractions. Otherwise, it might be possible to miss effects of the drug on the pathway of interest. The individual responses seen in the larger experiment were variable, highlighting the possibility that there might be significant individual variability in the pathways normally recruited to evoke exercise hyperemia. [Adapted from Schrage et al. (415).]
In the study shown in Figure 19, sequential additions of pharmacological blocking agents led to a temporary reduction in blood flow followed by a rebound and then return to steady state. One possibility is that as one pathway was blocked the blood flow response became more dependent on the remaining intact mechanisms or that other mechanisms were recruited. As these remaining pathways were inhibited there was a marked reduction in flow followed by a buildup of perhaps adenosine during a period of hypoperfusion and then a restoration of flow. If blood flow values had not been recorded on a beat-to-beat basis, some or all of this response might have been missed. A key question then is why compensatory vasodilation is not seen generally when any putative dilating substance or pathway is blocked. As we have emphasized before, small muscle mass exercise is associated with relatively high levels of deep venous oxygen extraction. If one accepts the argument that perfusion is higher than required to meet the metabolic demands of the contracting muscles, then a 10–20% reduction in flow might not trigger a sufficient buildup of other dilating factors to return flow to baseline levels. Thus, for compensatory vasodilation to be seen, we hypothesize that the reduction in flow must be large enough to cause a buildup of other dilating substances. This might only happen when the combination of exercise intensity and the insult to flow is sufficient to cause metabolic stress in the contracting muscles. There could also be individual differences between subjects that influence the metabolic responses to reductions in “bulk” flow. Additionally, factors like the structure of the microcirculation and collateral flow might also influence the response in the case of mechanical obstruction. Importantly, compensatory vasodilation might not be observed even if it is occurring without continuous measurements of blood flow.
This interpretation is supported by our studies that reduced perfusion pressure and blood flow in the contracting forearm via balloon inflation in the brachial artery. Figure 20 shows that when blood flow is reduced using this approach, a role for adenosine in regulating vascular tone to the active muscles emerges. The fact that the pattern of response seen in Figure 19 is not uniform in all subjects is also consistent with observations we have made concerning the possibility of individual variability in the contribution of various pathways to skeletal muscle vasodilation (304, 305). These observations and this discussion highlight the idea that a large number of redundant control mechanisms contribute to skeletal muscle vasodilation during exercise (243).
Figure 20.
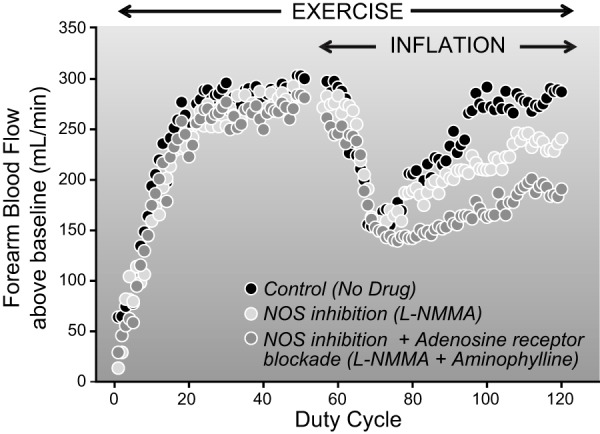
Blood flow responses to temporary reductions in blood flow and perfusion pressure to the rhythmically contracting forearm muscles (20 contractions/min at 20% of maximum) evoked by inflation of a balloon in the brachial artery. Under control conditions, downstream vasodilation as contractions continued caused a complete recovery of blood flow over 2–3 min. This recovery of flow was delayed and blunted by NOS inhibition with l-NMMA and further blunted by the adenosine antagonist aminophylline. While adenosine does not appear obligatory in the vasodilator responses to contractions under many circumstances, its role becomes more critical when blood flow to the contracting muscles is restricted.
The other possibility is that some un- or ill-defined and undiscovered vasodilator substance might explain a large percentage of the exercise hyperemia response. Based on the fact that the vascular biology world was generally surprised at the emergence of the vascular endothelium as a major site of vascular regulation and the observation that it explained a number of unexplained vasodilator responses, the possibility of additional vasodilating mechanisms should not be discounted. However, based on the repeated demonstrations of redundancy to date, the idea that there is a missing factor that might explain most or all of the vasodilator responses to exercise should viewed with caution.
Q. Summary
The mechanisms acting within contracting skeletal muscles that cause blood flow to rise are complex. While neural and mechanical mechanisms can both cause blood flow to rise in skeletal muscle, the magnitude of these increases is small compared with the vast increases in flow seen during exercise. In contrast, a number of chemical and metabolic factors can evoke large increases in flow. Many of these factors meet some or all of the criteria enumerated in Table 4. Importantly, chemical and metabolic vasodilation can also occur rapidly. At this time no one substance has emerged that can explain most of the vasodilator response seen in contracting skeletal muscles during exercise, and redundant control of this phenomenon via a host of substances seems likely.
VIII. METABOLIC VERSUS SYMPATHETIC CONTROL OF BLOOD FLOW IN SKELETAL MUSCLES
A. Functional Sympatholysis
As part of our discussion on the human hemodynamic responses to whole body exercise, we pointed out that under some circumstances it is possible for the marked vasodilation in the contracting muscles to potentially “threaten” blood pressure regulation and highlighted the competition between skeletal muscle vasodilation and systemic blood pressure regulation. These themes have run throughout this review. Now we turn our attention to how the factors that cause vasodilation in skeletal muscle interact with the sympathetic nervous system to ultimately regulate both skeletal muscle blood flow and mean arterial pressure during exercise in humans.
B. Contraction Blunts Sympathetic Vasoconstriction
While Figure 9 clearly shows the impact of metabolic vasodilation in contracting skeletal muscles on the blood pressure responses to exercise in the absence of sympathetic vasoconstriction, what happens to skeletal muscle blood flow when the sympathetic nervous system is activated? In another foundational study conducted in the early 1960s, hindlimb skeletal muscles in the dog were pump perfused at varying flow rates at rest and during contractions. Thus flow was controlled at fixed levels in contrast to the usual hindlimb preparation that permits blood flow to rise in response to contractions. Figure 21 shows that at rest, carotid occlusion, a maneuver that stimulates sympathetic outflow to the muscle, caused an increase in pressure in the perfusion circuit consistent with the interpretation that there was vasoconstriction in the hindlimb (375).
Figure 21.
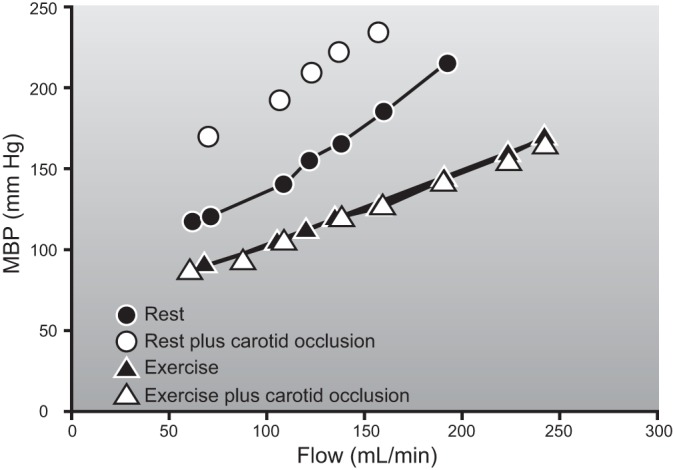
The effects of sympathetic stimulation (carotid occlusion) on perfusion pressure (MBP) in an isolated hindlimb preparation perfused with a roller pump at different rates of flow. As flow increases at rest, there is a rise in pressure in the roller pump circuit that increases further when sympathetic activity is increased by activation of carotid baroreflexes. This observation is consistent with the idea that the skeletal muscle vessels are being vasoconstricted. During contractions, pressure in the circuit is lower and rises less as flow is increased as a result of vasodilation in the muscles. Additionally, sympathetic stimulation has no effect on perfusion pressure consistent with the idea that the sympathetic nerves' ability to evoke vasoconstriction is blunted in contracting skeletal muscles. This effect has been termed “functional sympatholysis,” a controversial term as discussed further in the text. While this figure shows a complete blunting of the sympathetic constrictor responses during contractions, the results were variable, and in some animals, the constrictor responses were not fully abolished (Mitchell, personal communication). For details, see Ref. 375.
During contraction, blood pressure was lower at a given rate of flow consistent with the interpretation that contraction induced vasodilation in the muscles. Importantly, there was also no increase in pressure in the circuit when the sympathetic nervous system was stimulated by carotid occlusion. This lack of a rise in perfusion pressure demonstrated that the ability of the sympathetic nerves to evoke vasoconstriction in contracting skeletal muscles was either blunted or eliminated by contractions. This phenomenon was termed “functional sympatholysis” (375).
The term functional sympatholysis has been the matter of some debate because some investigators believe that implicit in this term is the absolute elimination of all sympathetic control of blood flow to contracting skeletal muscle. There are also arguments about whether or not the magnitude of any blunting is dependent on whether vascular resistance or vascular conductance is used as the primary outcome variable (393).
This debate also emphasizes the general problem of how to compare blood flow and hemodynamic responses at vastly differing levels of blood flow prior to an intervention, in this case activation of the sympathetic nerves. For example, if blood flow to a resting skeletal muscle has a hypothetical arbitrary value of 50 units and sympathetic activation reduces the blood flow to 25 units, is the magnitude of vasoconstriction the same or different if the blood flow during contraction is 500 arbitrary units and sympathetic activation reduces the blood flow to 475 units? The answer to this question can depend on the analysis used. In terms of absolute flow, perhaps constriction is the same. In terms of percent reduction in flow, the constriction during exercise is lower assuming arterial pressure is similar under both circumstances. Differences in the answer will also be obtained if resistance versus conductance is used. However, the fundamental observations in Figures 9 and 21 show apparently divergent results indicating that at least some sympathetic control of blood flow to contracting muscles is essential during whole body exercise in humans to maintain the arterial pressure, but at the same time under some circumstances sympathetic control of blood flow can be completely eliminated in contracting muscles. Is it possible to reconcile these findings?
In an attempt to resolve this confusion we performed a series of experiments using brachial artery administration of tyramine to evoke norepinephrine release from the perivascular nerves in the forearm (479). Tyramine was used to avoid a number of issues associated with brachial artery administration of norepinephrine including stimulation of luminal and nonjunctional adrenergic receptors. We then had subjects perform rhythmic handgrip exercise at varying intensities and infused the tyramine to see if the vasoconstriction was the same or different than that observed at rest. As part of our experimental strategy, we used brachial artery infusions of sodium nitroprusside and adenosine to serve as high flow controls and adjusted the doses of tyramine to account for the higher flows. This approach was done to ensure the concentration of the tyramine was not diluted by higher brachial artery blood flows during either exercise or the drug infusions. Using this approach we were able to generate dose-response curves to tyramine that caused graded reductions up to ∼70% of forearm blood flow during the drug induced high flow control experiments. In contrast, the effects of tyramine on blood flow during contractions were markedly attenuated, but not eliminated, during exercise intensities that closely matched the blood flow increases caused by the drug infusions. For example, tyramine infusions caused reductions in blood flow of ∼10–15% during heavy rhythmic handgrip exercise (81, 128, 130, 385, 386, 479). These findings are summarized and illustrated in Figure 22.
Figure 22.
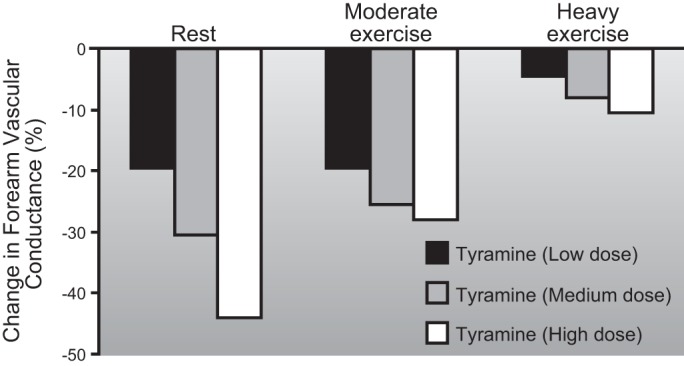
The effects of tyramine infusion on brachial artery vasodilator responses at rest and during rhythmic forearm exercise (20 contractions/min for 8 min). Tyramine is the prototypical indirectly acting sympathomimetic amine that causes release of norepinephrine from the sympathetic nerves. At rest and during both moderate (6.4 kg) and heavy exercise (12.1 kg), increasing doses of tyramine (2, 4, and 8 μg·dl forearm volume−1·min−1) caused dose-dependent vasoconstriction. This constriction was blunted by moderate exercise and attenuated more during heavy exercise. The tyramine infusions were adjusted to account for differences in blood flow to ensure a similar concentration under each condition. Importantly, when brachial artery flow was raised with either sodium nitroprusside or adenosine in the absence of exercise, there was no blunting of the constrictor responses (data not shown). The results highlighted in this figure show that contractions blunt sympathetic vasoconstriction in humans but that some constrictor tone is still present. This tone is critical to restrain blood flow to the contracting muscles for the purposes of blood pressure regulation during large muscle mass or whole body exercise. [Adapted from Tschakovsky et al. (479).]
There are two important points from these experimental results. First, our experimental strategy bypassed many of the data analysis issues related to baseline flow and provided clear evidence that the sympathetic nerves evoked vasoconstriction in contracting skeletal muscles is blunted in humans. Second, we also demonstrated that this blunting was not absolute and that at least some adrenergic vasoconstriction was possible in contracting skeletal muscles during heavy exercise. These points now provide a springboard for further discussions on sympatholysis.
Many of the metabolic vasodilating substances discussed previously have been shown in one preparation or another to reduce norepinephrine release from the sympathetic nerves innervating blood vessels (prejunctional sympatholysis) or interfere with vasoconstriction (postjunctional sympatholysis) evoked by either sympathetic nerve stimulation or administration of α-adrenergic agonists (490, 491). In addition, pH may also play an important role in this response.
In a series of beautifully executed studies, Faber and colleagues evaluated the interaction of local metabolic environment in skeletal muscle on α1- and α2-mediated vasoconstriction in rat skeletal muscles (7, 153, 154, 341, 347). In these muscles, postjunctional α1 receptors predominate in larger arterioles while postjunctional α2 receptors predominate in the smaller arteriolar elements of the microcirculation. Most importantly, reductions in pH interfere with α2 mediated postjunctional vasoconstriction but α1-mediated vasoconstrictor responses are intact (311, 312, 462). Parenthetically, there is also substantial and generally underappreciated postjunctional α2 tone in humans that accounts for perhaps 50% of overall adrenergic tone at rest (127), and a similar fraction is also seen in dogs (284).
In support of these observations, VanTeeffelen and Segal (487) evaluated the ability of sympathetic nerve stimulation to cause vasoconstriction in different sized arterioles during several levels of contraction in hamster skeletal muscles. Consistent with the ideas from the Faber group, they demonstrated as shown in Figure 23 that contraction caused almost complete sympatholysis in the smallest arterioles with diameters of ∼10–20 μm. The ability of the sympathetic nerves to evoke vasoconstriction upstream in the first- and second-order arterioles and feed arteries with diameters between ∼30 and 80 μm was preserved during contractions. In addition to this retained sympathetic control during contractions upstream in the arteriolar tree, sympathetic nerve stimulation also appears to eliminate or attenuate conducted vasodilator responses (200, 263, 429). Together, these findings demonstrate a significant sympatholytic effect in the smallest blood vessels, but with the possibility for substantial sympathetic control of vascular resistance upstream. From a hemodynamic perspective, this is important because it implies that the chief site of vascular control tends to drift upward towards larger vessels in vasodilated tissues.
Figure 23.
The effects of sympathetic nerve activation (SNA) on the diameter of a feed artery (FA) and 3A arteriole in a hamster skeletal muscle. Measurements were made at rest and during contractions. A shows the FA response. During rest, sympathetic simulation caused constriction. During contractions, the diameter of the FA increased by 50%, but this dilation was reversed and essentially eliminated by sympathetic stimulation. In contrast, B shows the response in a much smaller 3A arteriole. The impressive constriction seen during sympathetic stimulation at rest is followed by a doubling of diameter during contractions. This dilation is unaffected when sympathetic stimulation is superimposed. This figure illustrates that sympatholysis is most pronounced in the smallest resistance vessels closest to the capillaries, while the larger arterial elements of the skeletal muscle microcirculation remain subject to sympathetic vasoconstriction. If sympathetic vasoconstriction eliminates relatively upstream vasodilation in contracting muscles, this would restrain total muscle blood flow. However, sympatholysis in the smallest vessels might serve to optimize the distribution of flow towards the most metabolically active or stressed elements of the contracting muscle(s). The overall effects of such responses would be seen at the systemic level where such sympathetic restraint is critical to regulate mean arterial pressure and also explains the almost total extraction of oxygen across exercising skeletal muscle vascular beds under some circumstances including heavy large muscle mass or whole body exercise. [Adapted from Van Teeffelen and Segal (487).]
Conceptually, these findings in the microcirculation also reinforce the systemic hemodynamic observations we have emphasized. They are consistent with the idea that at least some sympathetic control of blood flow to contracting muscles is required to maintain a mean arterial pressure of at least 90–100 mmHg during large muscle mass exercise. First, constriction in larger elements of feed arteries and proximal arterioles might be sufficient to provide the overall level of vasoconstriction needed to generate or ensure this blood pressure. Second, vasodilation in the most metabolically stressed areas of the microcirculation would be relatively preserved because the α2 receptors located in the smallest arterioles lose their ability to evoke vasoconstriction during exercise. Third, while total blood flow to a given muscle might be reduced by perhaps 10–20%, a differential pattern of vasoconstriction within the muscle might also have the effect of matching blood flow and metabolism across the vascular bed by preferentially reducing flow to areas of the muscle under less metabolic stress. Such a pattern of constriction might also explain the increases in oxygen extraction seen during large muscle mass exercise compared with small muscle mass exercise.
This interpretation and working hypothesis is also consistent with the early ideas promulgated by Strandell and Shepherd (457) who showed continued sympathetic control of blood flow to contracting muscles was possible. Consistent with this hypothesis, it is interesting to note that infusions of vasodilating substances into the limbs of humans performing heavy exercise do not improve oxygen consumption. In fact, vasodilator infusions during heavy exercise seem to disrupt the matching of blood flow and metabolism that might result from the interactions of metabolic vasodilation and sympathetic vasoconstriction described above (69, 288, 401). Our interpretation of these observations is that when total limb flow is already very high and parts of the muscle are maximally dilated, infusions of exogenous vasodilators would dilate regions with little demand for oxygen and blood flow. Under these circumstances, this would cause a classic “steal syndrome” with the drugs causing dilation in parts of the limb that are relatively constricted and not consuming much oxygen thus diverting or “stealing” flow from areas of the contracting muscle where demand is high.
Thus it appears possible to reconcile the interactions between metabolic vasodilating factors and sympathetic vasoconstriction in contracting skeletal muscles both at the local level, and in terms of the need to regulate mean arterial pressure during heavy exercise. This conclusion is also supported by observations that sympathetic control of exercise hyperemia is relatively preserved in aging where it might be critical to restrain vasodilation more compared with young subjects during heavy exercise when maximum cardiac output is limited (130, 259, 349).
There are several other features of sympatholysis that warrant discussion. First, in rodent models, sympatholysis appears to be more pronounced in contracting highly glycolytic versus highly oxidative skeletal muscle fibers (466). Again, the relevance of this observation to humans is not clear, because humans have mixed skeletal muscle versus the highly compartmentalized skeletal muscle of rodents. Second, as is the case with the mixture of skeletal muscle fibers in human muscles versus the more compartmentalized muscle in rodents, the effects of contraction on α1- vs. α2-mediated responses might not be as pronounced in humans and vasoconstriction caused by both receptor subtypes appears to be blunted by exercise (385). Third, as is the case for metabolic vasodilation in general, there has also been a search for the substance principally responsible for sympatholysis. In animal models and some human studies, NO has emerged as a mediator of sympatholysis, and there is evidence to both support and reject a role for it (90, 128, 129, 155, 467, 479). However, administration of exogenous nitrovasodilators does not cause sympatholysis (81, 386). To date, exogenous administration of ATP does mimic the sympatholytic effects of contraction on sympathetic vasoconstriction (252, 387, 388), and this is also one of the primary arguments favoring ATP as a major player in exercise hyperemia. Fourth, in conditions like hypertension, aging, and perhaps congestive heart failure, there is evidence that sympatholysis is attenuated (130, 156, 251, 494). In other words, the blood vessels in contracting muscles under these conditions remain subject to substantial sympathetic control. This has the potential to reduce skeletal muscle blood flow and perhaps limit exercise capacity in individuals with these conditions.
In addition to norepinephrine, it is also possible that cotransmitters including ATP and neuropeptide Y (NPY) released from the sympathetic nerves also can evoke vasoconstriction in contracting muscles. The idea is that as sympathetic nerve firing rates increase, more ATP and NPY are released from the sympathetic nerve and then bind on postjunctional P2x and Y1 receptors where they can evoke vasoconstriction. Studies that have tested this hypothesis in dogs support some role for those mechanisms (57–59, 62), but the primary competition between vasodilation and vasoconstriction in contracting muscle during exercise is likely one between the vasodilator mechanisms and norepinephrine.
There are also interactions between metabolic vasodilation and α-adrenergic vasoconstriction that influence the blood flow responses to hypoxia, hyperoxia, and hypoperfusion. As discussed earlier, muscle blood flow is augmented during exercise under hypoxic conditions. This augmentation occurs despite an increase in sympathetic vasoconstrictor activity directed at the skeletal muscle (196), which is capable of restraining the increase in blood flow to the contracting muscle during hypoxia (456, 509). So there is competition between hypoxic dilation and sympathetic constriction and evidence that hypoxia can attenuate vasoconstrictor responses to sympathetic nerve activation and exogenous norepinephrine in resting skeletal muscle of animals and humans (48, 205, 206). However, we have found that the increased blood flow during hypoxic exercise (relative to normoxic conditions) is due to enhanced vasodilator mechanisms as opposed to reduced postjunctional α-adrenergic vasoconstrictor responsiveness (e.g., sympatholysis) (510). In contrast, the magnitude of vasodilation in hypoperfused contracting muscle is also under some degree of sympathetic vasoconstrictor restraint, as α-adrenergic blockade unmasks a greater flow recovery (74).
C. Summary
The functional sympatholysis narrative has emerged as a classic example of how local and systemic responses work together to optimize physiological function (see Figure 24). Retained vasoconstriction in larger blood vessels permits total muscle blood flow to remain under some sympathetic control while sympatholysis especially in the smallest arterioles ensures that the available flow is distributed to the most metabolically stressed areas of the active muscles. Together, these mechanisms permit arterial blood pressure to be maintained while maximizing the extraction of oxygen across the exercising skeletal muscle during conditions when 80–90% of cardiac output is directed to contracting skeletal muscles. These observations also explain the very high levels of systemic oxygen extraction outlined at the outset of this review and demonstrated in Figure 5.
Figure 24.
Summary figure of the relationships between the local factors causing blood flow to rise in contracting skeletal muscles, cardiac output, and the need to regulate arterial blood pressure to ensure the perfusion of the central nervous system (CNS) and other vital organs. As emphasized throughout this review, factors released by the contracting muscles act locally to evoke vasodilation and blunt sympathetic vasoconstriction (functional sympatholysis). These events require an increase in cardiac output that is also facilitated by the systemic actions of the muscle pump to augment venous return. At the same time, it operates to increase perfusion pressure and amplify the effects of the vasodilating substances in the skeletal muscles. The cardiac hypertrophy and increases in blood volume caused by training also permit higher levels of muscle blood flow in the trained state. All of these acute and chronic adaptions are balanced by the autonomic nervous system in a way the permits arterial blood pressure to be maintained.
IX. INTEGRATION, PERSPECTIVES, AND KEY QUESTIONS
There can be vast increases in blood flow to contracting skeletal muscles during exercise in humans and other species. These increases in skeletal muscle blood flow are essential to meet the demands of the contracting skeletal muscle for oxygen, and for exercise to be prolonged. On a systemic level, the key determinant of these overall responses is the generation of a cardiac output as a result of the increases in heart rate and stroke volume that can both meet the demands for oxygen by the contracting muscles and perfusion pressure by other organs. In humans, these demands are also met by the diversion of blood flow away from less active skeletal muscle and other tissues so that the vast majority of cardiac output is directed toward the exercising skeletal muscles. These responses and adaptations are at their most impressive in elite highly trained endurance athletes.
At the same time these systemic hemodynamic and gas exchange events are occurring, there is a competition at the level of the contracting skeletal muscles between mechanisms that cause local vasodilation and reflex sympathetic vasoconstrictor mechanisms that maintain systemic arterial pressure. Vasodilating factors operating within the skeletal muscle limit sympathetic vasoconstriction in the arterioles closest to the contracting skeletal muscles while permitting continued vasoconstriction upstream. This interplay between dilator and constrictor mechanisms improves the extraction of oxygen across the muscle and provides enough vascular tone in the skeletal muscles so the arterial blood pressure does not fall or increases modestly. When this balance is lost, as in autonomic failure, blood pressure falls during large muscle mass exercise. When it is excessive, there can be a hypertensive response to exercise, limited skeletal muscle blood flow, and exercise intolerance (494).
Importantly, while neural and mechanical factors might contribute modestly to the rise in skeletal muscle blood flow, during exercise the main driver is vasodilation in the active muscles by a combination of chemical or metabolic factors that remain obscure. To date, no single factor has been shown to account for much more than 20–30% of the vasodilator responses to ongoing exercise in experimental conditions that favor a key role for that substance. Attempts to block one or more dilating factor alone or in combination either before or during contractions are typically ineffective in identifying a single or even several major factors that could explain the majority of vasodilator responses we have discussed.
Does this mean there are key mechanisms similar to the endothelial-derived factors discovered in the 1980s, yet to be discovered that might explain the vasodilator responses to exercise? Are the number of potential vasodilating substances simply so numerous that blockade of one or more of them will evoke alterations in the concentration of other factors to generate a relatively intact response? Are studies in either isolated human muscles or contracting muscles in animal models fundamentally flawed because unlike whole body exercise, oxygen extraction across the vascular bed even during heavy exercise is incomplete and blood flow is relatively “luxuriant”? Are the current experimental tools, especially the pharmacological agents, simply inadequate to fully explore what is likely happening in the interstitial space at the interface between the contracting muscles and resistance vessels? Do genetically modified animals offer a solution or will lifelong alterations of a key pathway or mechanism merely evoke compensatory phenotypic plasticity in other pathways so that the overall response remains intact? Finally, have we engaged in futile effort to reconcile complex responses with observations made in simpler systems versus using simple systems to understand complex responses? In either case, the experimental approaches and data may be similar, but perhaps the intellectual perspective and thus the conclusions different.
At the end of this review we clearly know, as Hunter stated in the 1700s (392), that blood goes where it is needed, and this principle certainly applies to contracting skeletal muscles during exercise in humans and other species. However, beyond this simple principle, how much do we really know? The vasodilating substances from one or more sources acting in combination under various circumstances to cause exercise hyperemia remain elusive. However, before we become too focused on what has not been learned in the last 30 or so years, perhaps we should review what has been learned in that time.
Blood flow to contracting muscles can be much higher than previously imagined.
The vascular endothelium is a major site of vascular regulation.
We can assert, with some confidence, that neurally mediated vasodilation and the mechanical effects of the muscle on the blood vessels do not drive the vast majority of blood flow to contracting skeletal muscles during exercise.
The interactions of the autonomic nervous system, sympathetic vasoconstriction, and metabolic vasodilation have been explored, and during large muscle mass rhythmic exercise in humans, the sympathetic nerves can cause some vasoconstriction in the active muscles. While this constriction is attenuated compared with rest, it is critical to maintain arterial blood pressure. Additionally, the distribution of the retained vasoconstrictor tone within the contracting muscles seems to operate in a manner that optimizes or maximizes oxygen extraction within the muscles.
Progress about the factors including K+-mediated mechanisms in conjunction with endothelial factors appear to play a major role in initiating the vasodilation at the onset of contractions have been made. Additionally, blood-borne sources of ATP and NO that might sustain vasodilation during exercise have been proposed and are being explored. Understanding the contribution of the factors that initiate the vasodilation and the blood-borne substances that contribute to vasodilation as exercise continues would seem like an especially ripe area for further research because older studies suggest that K+-mediated mechanisms are not obligatory for vasodilation and exercise hyperemia during prolonged periods of rhythmic contractions.
For almost all of the mechanisms and interactions outlined above, insight into how they are altered in diseases like heart failure and hypertension along with conditions like aging have been made. In these cases the contribution of endothelial factors and functional sympatholysis to exercise hyperemia are blunted. These observations may explain some of the peripheral factors associated with exercise intolerance in these groups and also have therapeutic implications (91, 468, 494).
The conclusion in the early 1980s was that the topic of muscle blood flow contained “a wealth of hidden information for those with the ability to find it” (431). The summary above clearly shows the fruits of the research conducted since that time. Thus the conclusion from the early 1980s might be modified to read that the topic of exercise hyperemia contains a wealth of integrative challenges for those with the curiosity, creativity, and ability to explore them.
GRANTS
This work was supported by National Institutes of Health Grants HL46493, AR55819, HL105467, and UL1TR000135, along with the prior Clinical and Translational Science Award and General Clinical Research Center grants; the Mayo Foundation; and the Frank and Shari Caywood Professorship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
acknowledgments
We thank Kathryn Thomas, a summer student in the lab, and Maja Johnson for their resolute assistance in preparing this manuscript including work on the figures and references. Bryce Bergene provided superb artistic support for many of the final figures. Drs. Jill Barnes, Sushant Ranadive, Jacqueline Limberg, Blair Johnson, and Donal O'Leary provided helpful and critical comments on the manuscript as did Ronée Harvey, a Mayo medical student. Drs. Steve Segal and Tuhin Roy provided insights on the nuances of the microcirculation. Dr. Bruce Gladden had a number of key comments on the many papers that have used in situ preparations to study blood flow to contracting muscles. Shelly Roberts, R.N., Chris Johnson, Branton Walker, and Pam Engrav have supported studies on muscle blood flow conducted in the Joyner lab for more than a decade. Drs. John Eisenach and Tim Curry remain incredibly committed long-term clinical collaborators who are also outstanding physiologists. Janet Beckman has provided superb secretarial assistance to M. J. Joyner for more than 25 years. Our own studies could not have been conducted without the enthusiastic participation of our many volunteer subjects.
Address for reprint requests and other correspondence: M. J. Joyner, Dept. of Anesthesiology, Mayo Clinic, 200 First St. SW, Rochester, MN 55905 (e-mail: joyner.michael@mayo.edu).
REFERENCES
- 1.Abrahams VC, Hilton SM. The role of active muscle vasodilatation in the alerting stage of the defence reaction. J Physiol 171: 189–202, 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol 89: 372–383, 1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amann M. Pulmonary system limitations to endurance exercise performance in humans. Exp Physiol 97: 311–318, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen P, Adams RP, Sjogaard G, Thorboe A, Saltin B. Dynamic knee extension as model for study of isolated exercising muscle in humans. J Appl Physiol 59: 1647–1653, 1985. [DOI] [PubMed] [Google Scholar]
- 5.Andersen P, Henriksson J. Capillary supply of the quadriceps femoris muscle of man: adaptive response to exercise. J Physiol 270: 677–690, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol 366: 233–249, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson KM, Faber JE. Differential sensitivity of arteriolar alpha 1- and alpha 2-adrenoceptor constriction to metabolic inhibition during rat skeletal muscle contraction. Circ Res 69: 174–184, 1991. [DOI] [PubMed] [Google Scholar]
- 8.Anholm JD, Stray-Gundersen J, Ramanathan M, Johnson RL Jr. Sustained maximal ventilation after endurance exercise in athletes. J Appl Physiol 67: 1759–1763, 1989. [DOI] [PubMed] [Google Scholar]
- 9.Anrep GV, von Saalfeld E. The blood flow through the skeletal muscle in relation to its contraction. J Physiol 85: 375–399, 1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, Thomas JD, Palmer D, Levine BD. Effect of aging and physical activity on left ventricular compliance. Circulation 110: 1799–1805, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong ML, Dua AK, Murrant CL. Potassium initiates vasodilatation induced by a single skeletal muscle contraction in hamster cremaster muscle. J Physiol 581: 841–852, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armstrong RB, Essen-Gustavsson B, Hoppeler H, Jones JH, Kayar SR, Laughlin MH, Lindholm A, Longworth KE, Taylor CR, Weibel ER. O2 delivery at V̇o2max and oxidative capacity in muscles of standardbred horses. J Appl Physiol 73: 2274–2282, 1992. [DOI] [PubMed] [Google Scholar]
- 13.Armstrong RB, Laughlin MH. Atropine: no effect on exercise muscle hyperemia in conscious rats. J Appl Physiol 61: 679–682, 1986. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong RB, Laughlin MH. Blood flows within and among rat muscles as a function of time during high speed treadmill exercise. J Physiol 344: 189–208, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armstrong RB, Laughlin MH. Rat muscle blood flows during high-speed locomotion. J Appl Physiol 59: 1322–1328, 1985. [DOI] [PubMed] [Google Scholar]
- 16.Asmussen E. Similarities and dissimilarities between static and dynamic exercise. Circ Res 48: I3–10, 1981. [PubMed] [Google Scholar]
- 17.Astrand I, Astrand PO, Christensen EH, Hedman R. Intermittent muscular work. Acta Physiol Scand 48: 448–453, 1960. [DOI] [PubMed] [Google Scholar]
- 18.Astrand PO. Human physical fitness with special reference to sex and age. Physiol Rev 36: 307–335, 1956. [DOI] [PubMed] [Google Scholar]
- 19.Astrand PO, Rodahl K. Textbook of Work Physiology: Physiological Bases of Exercise, edited by Van Dalen DB. New York: McGraw-Hill, 1977, p. 143, 147, 180–190. [Google Scholar]
- 20.Bacon AP, Carter RE, Ogle EA, Joyner MJ. V̇o2max trainability and high intensity interval training in humans: a meta-analysis. PloS One 8: e73182, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bada AA, Svendsen JH, Secher NH, Saltin B, Mortensen SP. Peripheral vasodilatation determines cardiac output in exercising humans: insight from atrial pacing. J Physiol 590: 2051–2060, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bagher P, Segal SS. Regulation of blood flow in the microcirculation: role of conducted vasodilation. Acta Physiol 202: 271–284, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barclay JK. Physiological determinants of Qmax in contracting canine skeletal muscle in situ. Med Sci Sports Exerc 20: S113–118, 1988. [DOI] [PubMed] [Google Scholar]
- 24.Barclay JK, Boulianne CM, Wilson BA, Tiffin SJ. Interaction of hyperoxia and blood flow during fatigue of canine skeletal muscle in situ. J Appl Physiol 47: 1018–1024, 1979. [DOI] [PubMed] [Google Scholar]
- 25.Barcroft H. Circulation in skeletal muscle. In: Handbook of Physiology. Circulation. Washington, DC: Am. Physiol. Soc., 1963, sect. 2, vol. II, p. 1353–1385. [Google Scholar]
- 26.Barcroft H, Foley TH, McSwiney RR. Experiments on the liberation of phosphate from the muscles of the human forearm during vigorous exercise and on the action of sodium phosphate on forearm muscle blood vessels. J Physiol 213: 411–420, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barcroft H, Greenwood B, Whelan RF. Blood flow and venous oxygen saturation during sustained contraction of the forearm muscles. J Physiol 168: 848–856, 1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barcroft H, Millen JL. The blood flow through muscle during sustained contraction. J Physiol 97: 17–31, 1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bassingthwaighte JB. Interstitial adenosine: the measurement, the interpretation. J Mol Cell Cardiol 24: 337–350, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bayly WM, Hodgson DR, Schulz DA, Dempsey JA, Gollnick PD. Exercise-induced hypercapnia in the horse. J Appl Physiol 67: 1958–1966, 1989. [DOI] [PubMed] [Google Scholar]
- 31.Bearden SE, Payne GW, Chisty A, Segal SS. Arteriolar network architecture and vasomotor function with ageing in mouse gluteus maximus muscle. J Physiol 561: 535–545, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beere PA, Russell SD, Morey MC, Kitzman DW, Higginbotham MB. Aerobic exercise training can reverse age-related peripheral circulatory changes in healthy older men. Circulation 100: 1085–1094, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Behnke AB, Wilmore JH. Evaluation and Regulation of Body Build and Composition: International Research Monograph Series in Physical Education. Englewood Cliffs, NJ: Prentice-Hall, 1974, p. 236. [Google Scholar]
- 34.Behringer EJ, Shaw RL, Westcott EB, Socha MJ, Segal SS. Aging impairs electrical conduction along endothelium of resistance arteries through enhanced Ca2+-activated K+ channel activation. Arteriosclerosis Thrombosis Vasc Biol 33: 1892–1901, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res 26: 40–47, 1992. [DOI] [PubMed] [Google Scholar]
- 36.Bergh U, Kanstrup IL, Ekblom B. Maximal oxygen uptake during exercise with various combinations of arm and leg work. J Appl Physiol 41: 191–196, 1976. [DOI] [PubMed] [Google Scholar]
- 37.Berne RM. The role of adenosine in the regulation of coronary blood flow. Circ Res 47: 807–813, 1980. [DOI] [PubMed] [Google Scholar]
- 38.Bevegard BS, Shepherd JT. Circulatory effects of stimulating the carotid arterial stretch receptors in man at rest and during exercise. J Clin Invest 45: 132–142, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bevegard BS, Shepherd JT. Regulation of the circulation during exercise in man. Physiol Rev 47: 178–213, 1967. [DOI] [PubMed] [Google Scholar]
- 40.Bevegard S. Studies on the regulation of the circulation in man. With special reference to the stroke volume and the effect of muscular work, body position and artificially induced variations of the heart rate. Acta Physiol Scand Suppl 57: 1–36, 1962. [PubMed] [Google Scholar]
- 41.Bevegard S, Lodin A. Postural circulatory changes at rest and during exercise in five patients with congenital absence of valves in the deep veins of the legs. Acta Med Scand 172: 21–29, 1962. [DOI] [PubMed] [Google Scholar]
- 42.Bishop CM. Heart mass and the maximum cardiac output of birds and mammals: implications for estimating the maximum aerobic power input of flying animals. Philos Trans Roy Soc Lond B 352: 447–456, 1997. [Google Scholar]
- 43.Bishop CM, Spivey RJ. Integration of exercise response and allometric scaling in endotherms. J Theoret Biol 323: 11–19, 2013. [DOI] [PubMed] [Google Scholar]
- 44.Bishop JM, Donald KW, Taylor SH, Wormald PN. Changes in arterial-hepatic venous oxygen content difference during and after supine leg exercise. J Physiol 137: 309–317, 1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bishop JM, Donald KW, Wade OL. Changes in the oxygen content of hepatic venous blood during exercise in patients with rheumatic heart disease. J Clin Invest 34: 1114–1125, 1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blair DA, Glover WE, Greenfield AD, Roddie IC. Excitation of cholinergic vasodilator nerves to human skeletal muscles during emotional stress. J Physiol 148: 633–647, 1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blomstrand E, Radegran G, Saltin B. Maximum rate of oxygen uptake by human skeletal muscle in relation to maximal activities of enzymes in the Krebs cycle. J Physiol 501: 455–460, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boegehold MA, Johnson PC. Periarteriolar and tissue Po2 during sympathetic escape in skeletal muscle. Am J Physiol Heart Circ Physiol 254: H929–H936, 1988. [DOI] [PubMed] [Google Scholar]
- 49.Booth FW, Chakravarthy MV, Spangenburg EE. Exercise and gene expression: physiological regulation of the human genome through physical activity. J Physiol 543: 399–411, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Booth FW, Gordon SE, Carlson CJ, Hamilton MT. Waging war on modern chronic diseases: primary prevention through exercise biology. J Appl Physiol 88: 774–787, 2000. [DOI] [PubMed] [Google Scholar]
- 51.Bouchard C, Sarzynski MA, Rice TK, Kraus WE, Church TS, Sung YJ, Rao DC, Rankinen T. Genomic predictors of the maximal O2 uptake response to standardized exercise training programs. J Appl Physiol 110: 1160–1170, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bramble DM, Lieberman DE. Endurance running and the evolution of Homo. Nature 432: 345–352, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Brevetti G, Schiano V, Chiariello M. Endothelial dysfunction: a key to the pathophysiology and natural history of peripheral arterial disease? Atherosclerosis 197: 1–11, 2008. [DOI] [PubMed] [Google Scholar]
- 54.Brock RW, Tschakovsky ME, Shoemaker JK, Halliwill JR, Joyner MJ, Hughson RL. Effects of acetylcholine and nitric oxide on forearm blood flow at rest and after a single muscle contraction. J Appl Physiol 85: 2249–2254, 1998. [DOI] [PubMed] [Google Scholar]
- 55.Brown GO. Henry Darcy and the making of a law. Water Resour Res 38: 2002. [Google Scholar]
- 56.Buck JA, Amundsen LR, Nielsen DH. Systolic blood pressure responses during isometric contractions of large and small muscle groups. Med Sci Sports Exerc 12: 145–147, 1980. [PubMed] [Google Scholar]
- 57.Buckwalter JB, Hamann JJ, Clifford PS. Neuropeptide Y1 receptor vasoconstriction in exercising canine skeletal muscles. J Appl Physiol 99: 2115–2120, 2005. [DOI] [PubMed] [Google Scholar]
- 58.Buckwalter JB, Hamann JJ, Clifford PS. Vasoconstriction in active skeletal muscles: a potential role for P2X purinergic receptors? J Appl Physiol 95: 953–959, 2003. [DOI] [PubMed] [Google Scholar]
- 59.Buckwalter JB, Hamann JJ, Kluess HA, Clifford PS. Vasoconstriction in exercising skeletal muscles: a potential role for neuropeptide Y? Am J Physiol Heart Circ Physiol 287: H144–H149, 2004. [DOI] [PubMed] [Google Scholar]
- 60.Buckwalter JB, Mueller PJ, Clifford PS. Autonomic control of skeletal muscle vasodilation during exercise. J Appl Physiol 83: 2037–2042, 1997. [DOI] [PubMed] [Google Scholar]
- 61.Buckwalter JB, Mueller PJ, Clifford PS. Sympathetic vasoconstriction in active skeletal muscles during dynamic exercise. J Appl Physiol 83: 1575–1580, 1997. [DOI] [PubMed] [Google Scholar]
- 62.Buckwalter JB, Taylor JC, Hamann JJ, Clifford PS. Do P2X purinergic receptors regulate skeletal muscle blood flow during exercise? Am J Physiol Heart Circ Physiol 286: H633–H639, 2004. [DOI] [PubMed] [Google Scholar]
- 63.Burke RE. Motor unit properties and selective involvement in movement. Exerc Sport Sci Rev 3: 31–81, 1975. [PubMed] [Google Scholar]
- 64.Burns WR, Cohen KD, Jackson WF. K+-induced dilation of hamster cremasteric arterioles involves both the Na+/K+-ATPase and inward-rectifier K+ channels. Microcirculation 11: 279–293, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buskirk ER, Hodgson JL. Age and aerobic power: the rate of change in men and women. Federation Proc 46: 1824–1829, 1987. [PubMed] [Google Scholar]
- 66.Calbet JA, De Paz JA, Garatachea N, Cabeza de Vaca S, Chavarren J. Anaerobic energy provision does not limit Wingate exercise performance in endurance-trained cyclists. J Appl Physiol 94: 668–676, 2003. [DOI] [PubMed] [Google Scholar]
- 67.Calbet JA, Jensen-Urstad M, van Hall G, Holmberg HC, Rosdahl H, Saltin B. Maximal muscular vascular conductances during whole body upright exercise in humans. J Physiol 558: 319–331, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Calbet JA, Joyner MJ. Disparity in regional and systemic circulatory capacities: do they affect the regulation of the circulation? Acta Physiol 199: 393–406, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Calbet JA, Lundby C, Sander M, Robach P, Saltin B, Boushel R. Effects of ATP-induced leg vasodilation on Vo2 peak and leg O2 extraction during maximal exercise in humans. Am J Physiol Regul Integr Comp Physiol 291: R447–R453, 2006. [DOI] [PubMed] [Google Scholar]
- 70.Callister R, Ng AV, Seals DR. Arm muscle sympathetic nerve activity during preparation for and initiation of leg-cycling exercise in humans. J Appl Physiol 77: 1403–1410, 1994. [DOI] [PubMed] [Google Scholar]
- 71.Canty JM Jr, Smith TP Jr. Adenosine-recruitable flow reserve is absent during myocardial ischemia in unanesthetized dogs studied in the basal state. Circ Res 76: 1079–1087, 1995. [DOI] [PubMed] [Google Scholar]
- 72.Carter JR, Ray CA. Sympathetic neural responses to mental stress: responders, nonresponders and sex differences. Am J Physiol Heart Circ Physiol 296: H847–H853, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Casey DP, Curry TB, Wilkins BW, Joyner MJ. Nitric oxide-mediated vasodilation becomes independent of beta-adrenergic receptor activation with increased intensity of hypoxic exercise. J Appl Physiol 110: 687–694, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Casey DP, Joyner MJ. α-Adrenergic blockade unmasks a greater compensatory vasodilation in hypoperfused contracting muscle. Front Physiol 3: 271, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Casey DP, Joyner MJ. Contribution of adenosine to compensatory dilation in hypoperfused contracting human muscles is independent of nitric oxide. J Appl Physiol 110: 1181–1189, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Casey DP, Joyner MJ. Influence of alpha-adrenergic vasoconstriction on the blunted skeletal muscle contraction-induced rapid vasodilation with aging. J Appl Physiol 113: 1201–1212, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Casey DP, Joyner MJ. NOS inhibition blunts and delays the compensatory dilation in hypoperfused contracting human muscles. J Appl Physiol 107: 1685–1692, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Casey DP, Joyner MJ. Prostaglandins do not contribute to the nitric oxide-mediated compensatory vasodilation in hypoperfused exercising muscle. Am J Physiol Heart Circ Physiol 301: H261–H268, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Casey DP, Joyner MJ. Skeletal muscle blood flow responses to hypoperfusion at rest and during rhythmic exercise in humans. J Appl Physiol 107: 429–437, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Casey DP, Joyner MJ, Claus PL, Curry TB. Hyperbaric hyperoxia reduces exercising forearm blood flow in humans. Am J Physiol Heart Circ Physiol 300: H1892–H1897, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Casey DP, Joyner MJ, Claus PL, Curry TB. Vasoconstrictor responsiveness during hyperbaric hyperoxia in contracting human muscle. J Appl Physiol 114: 217–224, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Casey DP, Madery BD, Curry TB, Eisenach JH, Wilkins BW, Joyner MJ. Nitric oxide contributes to the augmented vasodilatation during hypoxic exercise. J Physiol 588: 373–385, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Casey DP, Madery BD, Pike TL, Eisenach JH, Dietz NM, Joyner MJ, Wilkins BW. Adenosine receptor antagonist and augmented vasodilation during hypoxic exercise. J Appl Physiol 107: 1128–1137, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Casey DP, Walker BG, Curry TB, Joyner MJ. Ageing reduces the compensatory vasodilatation during hypoxic exercise: the role of nitric oxide. J Physiol 589: 1477–1488, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Casey DP, Walker BG, Ranadive SM, Taylor JL, Joyner MJ. Contribution of nitric oxide in the contraction-induced rapid vasodilation in young and older adults. J Appl Physiol 115: 446–455, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Castenfors J. Renal function during prolonged exercise. Ann NY Acad Sci 301: 151–159, 1977. [DOI] [PubMed] [Google Scholar]
- 87.Cerny FC, Dempsey JA, Reddan WG. Pulmonary gas exchange in nonnative residents of high altitude. J Clin Invest 52: 2993–2999, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cerretelli P, Marconi C, Pendergast D, Meyer M, Heisler N, Piiper J. Blood flow in exercising muscles by xenon clearance and by microsphere trapping. J Appl Physiol 56: 24–30, 1984. [DOI] [PubMed] [Google Scholar]
- 89.Charkoudian N, Joyner MJ, Barnes SA, Johnson CP, Eisenach JH, Dietz NM, Wallin BG. Relationship between muscle sympathetic nerve activity and systemic hemodynamics during nitric oxide synthase inhibition in humans. Am J Physiol Heart Circ Physiol 291: H1378–H1383, 2006. [DOI] [PubMed] [Google Scholar]
- 90.Chavoshan B, Sander M, Sybert TE, Hansen J, Victor RG, Thomas GD. Nitric oxide-dependent modulation of sympathetic neural control of oxygenation in exercising human skeletal muscle. J Physiol 540: 377–386, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Clausen JP. Circulatory adjustments to dynamic exercise and effect of physical training in normal subjects and in patients with coronary artery disease. Prog Cardiovasc Dis 18: 459–495, 1976. [DOI] [PubMed] [Google Scholar]
- 92.Clausen JP, Lassen NA. Muscle blood flow during exercise in normal man studied by the 133Xenon clearance method. Cardiovasc Res 5: 245–254, 1971. [DOI] [PubMed] [Google Scholar]
- 93.Coles DR, Cooper KE, Mottram RF, Occleshaw JV. The source of blood samples withdrawn from deep forearm veins via catheters passed upstream from the median cubital vein. J Physiol 142: 323–328, 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Convertino VA. Blood volume response to physical activity and inactivity. Am J Med Sci 334: 72–79, 2007. [DOI] [PubMed] [Google Scholar]
- 95.Convertino VA. Blood volume: its adaptation to endurance training. Med Sci Sports Exerc 23: 1338–1348, 1991. [PubMed] [Google Scholar]
- 96.Coote JH, Hilton SM, Zbrozyna AW. The ponto-medullary area integrating the defence reaction in the cat and its influence on muscle blood flow. J Physiol 229: 257–274, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Copp SW, Hirai DM, Ferguson SK, Musch TI, Poole DC. Role of neuronal nitric oxide synthase in modulating microvascular and contractile function in rat skeletal muscle. Microcirculation 18: 501–511, 2011. [DOI] [PubMed] [Google Scholar]
- 98.Copp SW, Hirai DM, Hageman KS, Poole DC, Musch TI. Nitric oxide synthase inhibition during treadmill exercise reveals fiber-type specific vascular control in the rat hindlimb. Am J Physiol Regul Integr Comp Physiol 298: R478–R485, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Copp SW, Hirai DM, Schwagerl PJ, Musch TI, Poole DC. Effects of neuronal nitric oxide synthase inhibition on resting and exercising hindlimb muscle blood flow in the rat. J Physiol 588: 1321–1331, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Copp SW, Holdsworth CT, Ferguson SK, Hirai DM, Poole DC, Musch TI. Muscle fibre-type dependence of neuronal nitric oxide synthase-mediated vascular control in the rat during high speed treadmill running. J Physiol 591: 2885–2896, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Corcondilas A, Koroxenidis GT, Shepherd JT. Effect of a brief contraction of forearm muscles on forearm blood flow. J Appl Physiol 19: 142–146, 1964. [DOI] [PubMed] [Google Scholar]
- 102.Cordain L, Gotshall RW, Eaton SB, Eaton SB 3rd. Physical activity, energy expenditure and fitness: an evolutionary perspective. Int J Sports Med 19: 328–335, 1998. [DOI] [PubMed] [Google Scholar]
- 103.Cotzias C, Marshall JM. Vascular and electromyographic responses evoked in forearm muscle by isometric contraction of the contralateral forearm. Clin Auton Res 3: 21–30, 1993. [DOI] [PubMed] [Google Scholar]
- 104.Coyle EF. Integration of the physiological factors determining endurance performance ability. Exerc Sport Sci Rev 23: 25–63, 1995. [PubMed] [Google Scholar]
- 105.Coyle EF, Hemmert MK, Coggan AR. Effects of detraining on cardiovascular responses to exercise: role of blood volume. J Appl Physiol 60: 95–99, 1986. [DOI] [PubMed] [Google Scholar]
- 106.Coyle EF, Martin WH 3rd, Sinacore DR, Joyner MJ, Hagberg JM, Holloszy JO. Time course of loss of adaptations after stopping prolonged intense endurance training. J Appl Physiol 57: 1857–1864, 1984. [DOI] [PubMed] [Google Scholar]
- 107.Crecelius AR, Kirby BS, Luckasen GJ, Larson DG, Dinenno FA. Mechanisms of rapid vasodilation after a brief contraction in human skeletal muscle. Am J Physiol Heart Circ Physiol 305: H29–H40, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Crecelius AR, Kirby BS, Richards JC, Dinenno FA. Mechanical effects of muscle contraction increase intravascular ATP draining quiescent and active skeletal muscle in humans. J Appl Physiol 114: 1085–1093, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Crecelius AR, Kirby BS, Richards JC, Garcia LJ, Voyles WF, Larson DG, Luckasen GJ, Dinenno FA. Mechanisms of ATP-mediated vasodilation in humans: modest role for nitric oxide and vasodilating prostaglandins. Am J Physiol Heart Circ Physiol 301: H1302–H1310, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Crecelius AR, Kirby BS, Voyles WF, Dinenno FA. Augmented skeletal muscle hyperaemia during hypoxic exercise in humans is blunted by combined inhibition of nitric oxide and vasodilating prostaglandins. J Physiol 589: 3671–3683, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Crecelius AR, Kirby BS, Voyles WF, Dinenno FA. Nitric oxide, but not vasodilating prostaglandins, contributes to the improvement of exercise hyperemia via ascorbic acid in healthy older adults. Am J Physiol Heart Circ Physiol 299: H1633–H1641, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Daley JC 3rd, Khan MH, Hogeman CS, Sinoway LI. Autonomic and vascular responses to reduced limb perfusion. J Appl Physiol 95: 1493–1498, 2003. [DOI] [PubMed] [Google Scholar]
- 113.Davies KJ, Packer L, Brooks GA. Biochemical adaptation of mitochondria, muscle, and whole-animal respiration to endurance training. Arch Biochem Biophys 209: 539–554, 1981. [DOI] [PubMed] [Google Scholar]
- 114.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation 109: III27–32, 2004. [DOI] [PubMed] [Google Scholar]
- 115.Davis MJ, Ferrer PN, Gore RW. Vascular anatomy and hydrostatic pressure profile in the hamster cheek pouch. Am J Physiol Heart Circ Physiol 250: H291–H303, 1986. [DOI] [PubMed] [Google Scholar]
- 116.Davisson RL, Possas OS, Murphy SP, Lewis SJ. Neurogenically derived nitrosyl factors mediate sympathetic vasodilation in the hindlimb of the rat. Am J Physiol Heart Circ Physiol 272: H2369–H2376, 1997. [DOI] [PubMed] [Google Scholar]
- 117.Dawes M, Chowienczyk PJ, Ritter JM. Effects of inhibition of the l-arginine/nitric oxide pathway on vasodilation caused by beta-adrenergic agonists in human forearm. Circulation 95: 2293–2297, 1997. [DOI] [PubMed] [Google Scholar]
- 118.Delmonico MJ, Kostek MC, Johns J, Hurley BF, Conway JM. Can dual energy X-ray absorptiometry provide a valid assessment of changes in thigh muscle mass with strength training in older adults? Eur J Clin Nutr 62: 1372–1378, 2008. [DOI] [PubMed] [Google Scholar]
- 119.DeLorey DS, Wang SS, Shoemaker JK. Evidence for sympatholysis at the onset of forearm exercise. J Appl Physiol 93: 555–560, 2002. [DOI] [PubMed] [Google Scholar]
- 120.Delp MD, O'Leary DS. Integrative control of the skeletal muscle microcirculation in the maintenance of arterial pressure during exercise. J Appl Physiol 97: 1112–1118, 2004. [DOI] [PubMed] [Google Scholar]
- 121.Dempsey JAJB. Wolffe memorial lecture. Is the lung built for exercise? Med Sci Sports Exerc 18: 143–155, 1986. [PubMed] [Google Scholar]
- 122.Dempsey JA, Hanson PG, Henderson KS. Exercise-induced arterial hypoxaemia in healthy human subjects at sea level. J Physiol 355: 161–175, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Diaz FJ, Hagan RD, Wright JE, Horvath SM. Maximal and submaximal exercise in different positions. Med Sci Sports Exerc 10: 214–217, 1978. [PubMed] [Google Scholar]
- 124.Diesen DL, Hess DT, Stamler JS. Hypoxic vasodilation by red blood cells: evidence for an S-nitrosothiol-based signal. Circ Res 103: 545–553, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dietz NM, Engelke KA, Samuel TT, Fix RT, Joyner MJ. Evidence for nitric oxide-mediated sympathetic forearm vasodiolatation in humans. J Physiol 498: 531–540, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dietz NM, Rivera JM, Eggener SE, Fix RT, Warner DO, Joyner MJ. Nitric oxide contributes to the rise in forearm blood flow during mental stress in humans. J Physiol 480: 361–368, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dinenno FA, Eisenach JH, Dietz NM, Joyner MJ. Post-junctional alpha-adrenoceptors and basal limb vascular tone in healthy men. J Physiol 540: 1103–1110, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dinenno FA, Joyner MJ. Blunted sympathetic vasoconstriction in contracting skeletal muscle of healthy humans: is nitric oxide obligatory? J Physiol 553: 281–292, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dinenno FA, Joyner MJ. Combined NO and PG inhibition augments alpha-adrenergic vasoconstriction in contracting human skeletal muscle. Am J Physiol Heart Circ Physiol 287: H2576–H2584, 2004. [DOI] [PubMed] [Google Scholar]
- 130.Dinenno FA, Masuki S, Joyner MJ. Impaired modulation of sympathetic alpha-adrenergic vasoconstriction in contracting forearm muscle of ageing men. J Physiol 567: 311–321, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dobson JL, Gladden LB. Effect of rhythmic tetanic skeletal muscle contractions on peak muscle perfusion. J Appl Physiol 94: 11–19, 2003. [DOI] [PubMed] [Google Scholar]
- 132.Dodd LR, Johnson PC. Diameter changes in arteriolar networks of contracting skeletal muscle. Am J Physiol Heart Circ Physiol 260: H662–H670, 1991. [DOI] [PubMed] [Google Scholar]
- 133.Dominelli PB, Foster GE, Dominelli GS, Henderson WR, Koehle MS, McKenzie DC, Sheel AW. Exercise-induced arterial hypoxaemia and the mechanics of breathing in healthy young women. J Physiol 591: 3017–3034, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Donato AJ, Uberoi A, Wray DW, Nishiyama S, Lawrenson L, Richardson RS. Differential effects of aging on limb blood flow in humans. Am J Physiol Heart Circ Physiol 290: H272–H278, 2006. [DOI] [PubMed] [Google Scholar]
- 135.Dufour SP, Patel RP, Brandon A, Teng X, Pearson J, Barker H, Ali L, Yuen AH, Smolenski RT, Gonzalez-Alonso J. Erythrocyte-dependent regulation of human skeletal muscle blood flow: role of varied oxyhemoglobin and exercise on nitrite, S-nitrosohemoglobin, and ATP. Am J Physiol Heart Circ Physiol 299: H1936–H1946, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Duling BR, Berne RM. Propagated vasodilation in the microcirculation of the hamster cheek pouch. Circ Res 26: 163–170, 1970. [DOI] [PubMed] [Google Scholar]
- 137.Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev 88: 1009–1086, 2008. [DOI] [PubMed] [Google Scholar]
- 138.Duncker DJ, Bache RJ. Regulation of coronary vasomotor tone under normal conditions and during acute myocardial hypoperfusion. Pharmacol Ther 86: 87–110, 2000. [DOI] [PubMed] [Google Scholar]
- 139.Durstine JL, Pate RR, Sparling PB, Wilson GE, Senn MD, Bartoli WP. Lipid, lipoprotein, and iron status of elite women distance runners. Int J Sports Med 8 Suppl 2: 119–123, 1987. [DOI] [PubMed] [Google Scholar]
- 140.Dyke CK, Dietz NM, Lennon RL, Warner DO, Joyner MJ. Forearm blood flow responses to handgripping after local neuromuscular blockade. J Appl Physiol 84: 754–758, 1998. [DOI] [PubMed] [Google Scholar]
- 141.Dyke CK, Proctor DN, Dietz NM, Joyner MJ. Role of nitric oxide in exercise hyperaemia during prolonged rhythmic handgripping in humans. J Physiol 488: 259–265, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Edgerton R. The nervous system and movement. In: ACSM's Advanced Exercise and Physiology. Philadelphia, PA: Lippincott Williams & Wilkins, 2012. [Google Scholar]
- 143.Egginton S. Invited review: activity-induced angiogenesis. Pflügers Arch 457: 963–977, 2009. [DOI] [PubMed] [Google Scholar]
- 144.Ehsani AA, Hagberg JM, Hickson RC. Rapid changes in left ventricular dimensions and mass in response to physical conditioning and deconditioning. Am J Cardiol 42: 52–56, 1978. [DOI] [PubMed] [Google Scholar]
- 145.Eisenach JH, Clark ES, Charkoudian N, Dinenno FA, Atkinson JL, Fealey RD, Dietz NM, Joyner MJ. Effects of chronic sympathectomy on vascular function in the human forearm. J Appl Physiol 92: 2019–2025, 2002. [DOI] [PubMed] [Google Scholar]
- 146.Ekblom B, Hermansen L. Cardiac output in athletes. J Appl Physiol 25: 619–625, 1968. [DOI] [PubMed] [Google Scholar]
- 147.Eklund B, Kaijser L. Blood flow in the resting forearm during prolonged contralateral isometric handgrip at maximal effort. J Physiol 277: 359–366, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Eklund B, Kaijser L. Effect of regional alpha- and beta-adrenergic blockade on blood flow in the resting forearm during contralateral isometric handgrip. J Physiol 262: 39–50, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Eklund B, Kaijser L, Knutsson E. Blood flow in resting (contralateral) arm and leg during isometric contraction. J Physiol 240: 111–124, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ekstrom-Jodal B, Haggendal E, Malmberg R, Svedmyr N. The effect of adrenergic-receptor blockade on coronary circulation in man during work. Acta Med Scand 191: 245–248, 1972. [PubMed] [Google Scholar]
- 151.Emerson GG, Segal SS. Electrical activation of endothelium evokes vasodilation and hyperpolarization along hamster feed arteries. Am J Physiol Heart Circ Physiol 280: H160–H167, 2001. [DOI] [PubMed] [Google Scholar]
- 152.Emerson GG, Segal SS. Electrical coupling between endothelial cells and smooth muscle cells in hamster feed arteries: role in vasomotor control. Circ Res 87: 474–479, 2000. [DOI] [PubMed] [Google Scholar]
- 153.Faber JE. In situ analysis of alpha-adrenoceptors on arteriolar and venular smooth muscle in rat skeletal muscle microcirculation. Circ Res 62: 37–50, 1988. [DOI] [PubMed] [Google Scholar]
- 154.Faber JE, Meininger GA. Selective interaction of alpha-adrenoceptors with myogenic regulation of microvascular smooth muscle. Am J Physiol Heart Circ Physiol 259: H1126–H1133, 1990. [DOI] [PubMed] [Google Scholar]
- 155.Fadel PJ, Farias Iii M, Gallagher KM, Wang Z, Thomas GD. Oxidative stress and enhanced sympathetic vasoconstriction in contracting muscles of nitrate-tolerant rats and humans. J Physiol 590: 395–407, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fadel PJ, Wang Z, Watanabe H, Arbique D, Vongpatanasin W, Thomas GD. Augmented sympathetic vasoconstriction in exercising forearms of postmenopausal women is reversed by oestrogen therapy. J Physiol 561: 893–901, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Feiereisen P, Duchateau J, Hainaut K. Motor unit recruitment order during voluntary and electrically induced contractions in the tibialis anterior. Exp Brain Res 114: 117–123, 1997. [DOI] [PubMed] [Google Scholar]
- 158.Fiskerstrand A, Seiler KS. Training and performance characteristics among Norwegian international rowers 1970–2001. Scand J Med Sci Sports 14: 303–310, 2004. [DOI] [PubMed] [Google Scholar]
- 159.Folkow B. Physiological aspects of the “defence” and “defeat” reactions. Acta Physiol Scand Suppl 640: 34–37, 1997. [PubMed] [Google Scholar]
- 160.Folkow B, Haeger K, Uvnas B. Cholinergic vasodilator nerves in the sympathetic outflow to the muscles of the hind limbs of the cat. Acta Physiol Scand 15: 401–411, 1948. [DOI] [PubMed] [Google Scholar]
- 161.Folkow B, Sonnenschein RR, Wright DL. Loci of neurogenic and metabolic effects on precapillary vessels of skeletal muscle. Acta Physiol Scand 81: 459–471, 1971. [DOI] [PubMed] [Google Scholar]
- 162.Folkow B, Uvnas B. The distribution and functional significance of sympathetic vasodilators to the hind limps of the cat. Acta Physiol Scand 15: 389–400, 1948. [DOI] [PubMed] [Google Scholar]
- 163.Forrester T. A case of serendipity. Purinergic Signal 4: 93–100, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Forrester T. An estimate of adenosine triphosphate release into the venous effluent from exercising human forearm muscle. J Physiol 224: 611–628, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Forrester T, Lind AR. Identification of adenosine triphosphate in human plasma and the concentration in the venous effluent of forearm muscles before, during and after sustained contractions. J Physiol 204: 347–364, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Frandsenn U, Bangsbo J, Sander M, Hoffner L, Betak A, Saltin B, Hellsten Y. Exercise-induced hyperaemia and leg oxygen uptake are not altered during effective inhibition of nitric oxide synthase with N(G)-nitro-l-arginine methyl ester in humans. J Physiol 531: 257–264, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Freyschuss U, Hjemdahl P, Juhlin-Dannfelt A, Linde B. Cardiovascular and sympathoadrenal responses to mental stress: influence of beta-blockade. Am J Physiol Heart Circ Physiol 255: H1443–H1451, 1988. [DOI] [PubMed] [Google Scholar]
- 168.Froelicher VF Jr, Brammell H, Davis G, Noguera I, Stewart A, Lancaster MC. A comparison of the reproducibility and physiologic response to three maximal treadmill exercise protocols. Chest 65: 512–517, 1974. [DOI] [PubMed] [Google Scholar]
- 169.Fronek K, Zweifach BW. Microvascular pressure distribution in skeletal muscle and the effect of vasodilation. Am J Physiol 228: 791–796, 1975. [DOI] [PubMed] [Google Scholar]
- 170.Furchgott RF. The 1996 Albert Lasker Medical Research Awards. The discovery of endothelium-derived relaxing factor and its importance in the identification of nitric oxide. JAMA 276: 1186–1188, 1996. [PubMed] [Google Scholar]
- 171.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288: 373–376, 1980. [DOI] [PubMed] [Google Scholar]
- 172.Gaskell WH. The changes of the blood-stream in muscles through stimulation of their nerves. J Anat Physiol 11: 360–402, 1877. [PMC free article] [PubMed] [Google Scholar]
- 173.Gaskell WH. On the tonicity of the heart and blood vessels. J Physiol 3: 48–92, 1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gayeski TE, Connett RJ, Honig CR. Minimum intracellular Po2 for maximum cytochrome turnover in red muscle in situ. Am J Physiol Heart Circ Physiol 252: H906–H915, 1987. [DOI] [PubMed] [Google Scholar]
- 175.Gladwin MT. Evidence mounts that nitrite contributes to hypoxic vasodilation in the human circulation. Circulation 117: 594–597, 2008. [DOI] [PubMed] [Google Scholar]
- 176.Glover WE, Greenfield AD, Shanks RG. The contribution made by adrenaline to the vasodilatation in the human forearm during emotional stress. J Physiol 164: 422–429, 1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Golub AS, Pittman RN. Bang-bang model for regulation of local blood flow. Microcirculation 20: 455–483, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Golub AS, Pittman RN. A paradigm shift for local blood flow regulation. J Appl Physiol 116: 703–705, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gonzalez-Alonso J. ATP as a mediator of erythrocyte-dependent regulation of skeletal muscle blood flow and oxygen delivery in humans. J Physiol 590: 5001–5013, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gonzalez-Alonso J, Dalsgaard MK, Osada T, Volianitis S, Dawson EA, Yoshiga CC, Secher NH. Brain and central haemodynamics and oxygenation during maximal exercise in humans. J Physiol 557: 331–342, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gonzalez-Alonso J, Mortensen SP, Jeppesen TD, Ali L, Barker H, Damsgaard R, Secher NH, Dawson EA, Dufour SP. Haemodynamic responses to exercise, ATP infusion and thigh compression in humans: insight into the role of muscle mechanisms on cardiovascular function. J Physiol 586: 2405–2417, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gonzalez-Alonso J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res 91: 1046–1055, 2002. [DOI] [PubMed] [Google Scholar]
- 183.Gorczynski RJ, Klitzman B, Duling BR. Interrelations between contracting striated muscle and precapillary microvessels. Am J Physiol Heart Circ Physiol 235: H494–H504, 1978. [DOI] [PubMed] [Google Scholar]
- 184.Green DJ, Naylor LH, George K, Dempsey JA, Stickland MK, Katayama K. Cardiovascular and pulmonary adaptations to exercise training. In: Physiological Bases of Human Performance During Work and Exercise, edited by Taylor NAS, Groeller H, and McLennan PL. Oxford, UK: Churchill Livingstone, 2008, p. 49–70. [Google Scholar]
- 185.Greenfield AD. Survey of the evidence for active neurogenic vasodilatation in man. Federation Proc 25: 1607–1610, 1966. [PubMed] [Google Scholar]
- 186.Gregory IC. The oxygen and carbon monoxide capacities of fetal and adult blood. J Physiol 236: 625–634, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Grimby G, Haggendal E, Saltin B. Local xenon 133 clearance from the quadriceps muscle during exercise in man. J Appl Physiol 22: 305–310, 1967. [DOI] [PubMed] [Google Scholar]
- 188.Hagberg JM. Effect of training on the decline of V̇o2max with aging. Federation Proc 46: 1830–1833, 1987. [PubMed] [Google Scholar]
- 189.Hagberg JM, Coyle EF, Carroll JE, Miller JM, Martin WH, Brooke MH. Exercise hyperventilation in patients with McArdle's disease. J Appl Physiol 52: 991–994, 1982. [DOI] [PubMed] [Google Scholar]
- 190.Hagberg JM, Mullin JP, Giese MD, Spitznagel E. Effect of pedaling rate on submaximal exercise responses of competitive cyclists. J Appl Physiol 51: 447–451, 1981. [DOI] [PubMed] [Google Scholar]
- 191.Hales JRS, Dampney RAL. Redistribution of cardiac-output in dog during heat-stress. J Therm Biol 1: 29–34, 1975. [Google Scholar]
- 192.Halliwill JR, Lawler LA, Eickhoff TJ, Dietz NM, Nauss LA, Joyner MJ. Forearm sympathetic withdrawal and vasodilatation during mental stress in humans. J Physiol 504: 211–220, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hamann JJ, Buckwalter JB, Valic Z, Clifford PS. Sympathetic restraint of muscle blood flow at the onset of dynamic exercise. J Appl Physiol 92: 2452–2456, 2002. [DOI] [PubMed] [Google Scholar]
- 194.Hamann JJ, Valic Z, Buckwalter JB, Clifford PS. Muscle pump does not enhance blood flow in exercising skeletal muscle. J Appl Physiol 94: 6–10, 2003. [DOI] [PubMed] [Google Scholar]
- 195.Hammond RL, Augustyniak RA, Rossi NF, Lapanowski K, Dunbar JC, O'Leary DS. Alteration of humoral and peripheral vascular responses during graded exercise in heart failure. J Appl Physiol 90: 55–61, 2001. [DOI] [PubMed] [Google Scholar]
- 196.Hanada A, Sander M, Gonzalez-Alonso J. Human skeletal muscle sympathetic nerve activity, heart rate and limb haemodynamics with reduced blood oxygenation and exercise. J Physiol 551: 635–647, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Harms CA, Babcock MA, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Dempsey JA. Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol 82: 1573–1583, 1997. [DOI] [PubMed] [Google Scholar]
- 198.Harms CA, McClaran SR, Nickele GA, Pegelow DF, Nelson WB, Dempsey JA. Effect of exercise-induced arterial O2 desaturation on V̇o2max in women. Med Sci Sports Exerc 32: 1101–1108, 2000. [DOI] [PubMed] [Google Scholar]
- 199.Harms CA, McClaran SR, Nickele GA, Pegelow DF, Nelson WB, Dempsey JA. Exercise-induced arterial hypoxaemia in healthy young women. J Physiol 507: 619–628, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Haug SJ, Segal SS. Sympathetic neural inhibition of conducted vasodilatation along hamster feed arteries: complementary effects of alpha1- and alpha2-adrenoreceptor activation. J Physiol 563: 541–555, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Haug SJ, Welsh DG, Segal SS. Sympathetic nerves inhibit conducted vasodilatation along feed arteries during passive stretch of hamster skeletal muscle. J Physiol 552: 273–282, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hazeyama Y, Sparks HV. Exercise hyperemia in potassium-depleted dogs. Am J Physiol Heart Circ Physiol 236: H480–H486, 1979. [DOI] [PubMed] [Google Scholar]
- 203.Heath GW, Hagberg JM, Ehsani AA, Holloszy JO. A physiological comparison of young and older endurance athletes. J Appl Physiol 51: 634–640, 1981. [DOI] [PubMed] [Google Scholar]
- 204.Heiss HW, Barmeyer J, Wink K, Hell G, Cerny FJ, Keul J, Reindell H. Studies on the regulation of myocardial blood flow in man. I. Training effects on blood flow and metabolism of the healthy heart at rest and during standardized heavy exercise. Basic Res Cardiol 71: 658–675, 1976. [DOI] [PubMed] [Google Scholar]
- 205.Heistad DD, Abboud FM, Mark AL, Schmid PG. Effect of hypoxemia on responses to norepinephrine and angiotensin in coronary and muscular vessels. J Pharmacol Exp Ther 193: 941–950, 1975. [PubMed] [Google Scholar]
- 206.Heistad DD, Wheeler RC. Effect of acute hypoxia on vascular responsiveness in man. I. Responsiveness to lower body negative pressure and ice on the forehead. II. Responses to norepinephrine and angiotensin 3. Effect of hypoxia and hypocapnia. J Clin Invest 49: 1252–1265, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 104: 2673–2678, 2001. [DOI] [PubMed] [Google Scholar]
- 208.Hellsten Y, Maclean D, Radegran G, Saltin B, Bangsbo J. Adenosine concentrations in the interstitium of resting and contracting human skeletal muscle. Circulation 98: 6–8, 1998. [DOI] [PubMed] [Google Scholar]
- 209.Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurons. J Neurophysiol 28: 560–580, 1965. [DOI] [PubMed] [Google Scholar]
- 210.Hester RL, Guyton AC, Barber BJ. Reactive and exercise hyperemia during high levels of adenosine infusion. Am J Physiol Heart Circ Physiol 243: H181–H186, 1982. [DOI] [PubMed] [Google Scholar]
- 211.Hickson RC, Bomze HA, Holloszy JO. Linear increase in aerobic power induced by a strenuous program of endurance exercise. J Appl Physiol 42: 372–376, 1977. [DOI] [PubMed] [Google Scholar]
- 212.Hill AV, Lupton H. Muscular exercise, lactic acid, and the supply and utilization of oxygen. Q J Med 16: 135–171, 1923. [Google Scholar]
- 213.Hilton SM. The defence-arousal system and its relevance for circulatory and respiratory control. J Exp Biol 100: 159–174, 1982. [DOI] [PubMed] [Google Scholar]
- 214.Hilton SM. Experiments on the post contraction hyperaemia of skeletal muscle. J Physiol 120: 230–245, 1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Holling HE. Effect of embarrassment on blood flow to skeletal muscle. Trans Am Clin Climatol Assoc 76: 49–59, 1964. [PMC free article] [PubMed] [Google Scholar]
- 216.Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem 242: 2278–2282, 1967. [PubMed] [Google Scholar]
- 217.Holloszy JO. Biochemical adaptations to exercise: aerobic metabolism. Exerc Sport Sci Rev 1: 45–71, 1973. [PubMed] [Google Scholar]
- 218.Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol 56: 831–838, 1984. [DOI] [PubMed] [Google Scholar]
- 219.Holmberg HC, Rosdahl H, Svedenhag J. Lung function, arterial saturation and oxygen uptake in elite cross country skiers: influence of exercise mode. Scand J Med Sci Sports 17: 437–444, 2007. [DOI] [PubMed] [Google Scholar]
- 220.Holt JP, Rhode EA, Kines H. Ventricular volumes and body weight in mammals. Am J Physiol 215: 704–715, 1968. [DOI] [PubMed] [Google Scholar]
- 221.Holt JP, Rhode EA, Peoples SA, Kines H. Left ventricular function in mammals of greatly different size. Circ Res 10: 798–806, 1962. [DOI] [PubMed] [Google Scholar]
- 222.Hopkins SR, Barker RC, Brutsaert TD, Gavin TP, Entin P, Olfert IM, Veisel S, Wagner PD. Pulmonary gas exchange during exercise in women: effects of exercise type and work increment. J Appl Physiol 89: 721–730, 2000. [DOI] [PubMed] [Google Scholar]
- 223.Hopkins SR, Harms CA. Gender and pulmonary gas exchange during exercise. Exerc Sport Sci Rev 32: 50–56, 2004. [DOI] [PubMed] [Google Scholar]
- 224.Hopper MK, Coggan AR, Coyle EF. Exercise stroke volume relative to plasma-volume expansion. J Appl Physiol 64: 404–408, 1988. [DOI] [PubMed] [Google Scholar]
- 225.Horstman DH, Gleser M, Delehunt J. Effects of altering O2 delivery on V̇o2 of isolated, working muscle. Am J Physiol 230: 327–334, 1976. [DOI] [PubMed] [Google Scholar]
- 226.Hunter GR, Weinsier RL, McCarthy JP, Enette Larson-Meyer D, Newcomer BR. Hemoglobin, muscle oxidative capacity, and V̇o2max in African-American and Caucasian women. Med Sci Sports Exerc 33: 1739–1743, 2001. [DOI] [PubMed] [Google Scholar]
- 227.Jackson AS, Wier LT, Ayers GW, Beard EF, Stuteville JE, Blair SN. Changes in aerobic power of women, ages 20–64 yr. Med Sci Sports Exerc 28: 884–891, 1996. [DOI] [PubMed] [Google Scholar]
- 228.Jackson DN, Moore AW, Segal SS. Blunting of rapid onset vasodilatation and blood flow restriction in arterioles of exercising skeletal muscle with ageing in male mice. J Physiol 588: 2269–2282, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Jagger JE, Bateman RM, Ellsworth ML, Ellis CG. Role of erythrocyte in regulating local O2 delivery mediated by hemoglobin oxygenation. Am J Physiol Heart Circ Physiol 280: H2833–H2839, 2001. [DOI] [PubMed] [Google Scholar]
- 230.Jasperse JL, Seals DR, Callister R. Active forearm blood flow adjustments to handgrip exercise in young and older healthy men. J Physiol 474: 353–360, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Jensen K, Johansen L, Secher NH. Influence of body mass on maximal oxygen uptake: effect of sample size. Eur J Appl Physiol 84: 201–205, 2001. [DOI] [PubMed] [Google Scholar]
- 232.Jin CZ, Kim HS, Seo EY, Shin DH, Park KS, Chun YS, Zhang YH, Kim SJ. Exercise training increases inwardly rectifying K+ current and augments K+-mediated vasodilatation in deep femoral artery of rats. Cardiovasc Res 91: 142–150, 2011. [DOI] [PubMed] [Google Scholar]
- 233.Johnson BD, Saupe KW, Dempsey JA. Mechanical constraints on exercise hyperpnea in endurance athletes. J Appl Physiol 73: 874–886, 1992. [DOI] [PubMed] [Google Scholar]
- 234.Johnson DE. Contributions of animal nutrition research to nutritional principles: energetics. J Nutr 137: 698–701, 2007. [DOI] [PubMed] [Google Scholar]
- 235.Jorfeldt L, Wahren J. Leg blood flow during exercise in man. Clin Sci 41: 459–473, 1971. [DOI] [PubMed] [Google Scholar]
- 236.Jorgensen CR, Gobel FL, Taylor HL, Wang Y. Myocardial blood flow and oxygen consumption during exercise. Ann NY Acad Sci 301: 213–223, 1977. [DOI] [PubMed] [Google Scholar]
- 237.Joyner MJ. Baroreceptor function during exercise: resetting the record. Exp Physiol 91: 27–36, 2006. [DOI] [PubMed] [Google Scholar]
- 238.Joyner MJ. V̇o2max, blood doping, erythropoietin. Br J Sports Med 37: 190–191, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Joyner MJ. Why physiology matters in medicine. Physiology 26: 72–75, 2011. [DOI] [PubMed] [Google Scholar]
- 240.Joyner MJ, Dietz NM. Sympathetic vasodilation in human muscle. Acta Physiol Scand 177: 329–336, 2003. [DOI] [PubMed] [Google Scholar]
- 241.Joyner MJ, Dietz NM, Shepherd JT. From Belfast to Mayo and beyond: the use and future of plethysmography to study blood flow in human limbs. J Appl Physiol 91: 2431–2441, 2001. [DOI] [PubMed] [Google Scholar]
- 242.Joyner MJ, Lennon RL, Wedel DJ, Rose SH, Shepherd JT. Blood flow to contracting human muscles: influence of increased sympathetic activity. J Appl Physiol 68: 1453–1457, 1990. [DOI] [PubMed] [Google Scholar]
- 243.Joyner MJ, Proctor DN. Muscle blood flow during exercise: the limits of reductionism. Med Sci Sports Exerc 31: 1036–1040, 1999. [DOI] [PubMed] [Google Scholar]
- 244.Julius S, Pascual AV, Sannerstedt R, Mitchell C. Relationship between cardiac output and peripheral resistance in borderline hypertension. Circulation 43: 382–390, 1971. [DOI] [PubMed] [Google Scholar]
- 245.Juvonen E, Ikkala E, Fyhrquist F, Ruutu T. Autosomal dominant erythrocytosis caused by increased sensitivity to erythropoietin. Blood 78: 3066–3069, 1991. [PubMed] [Google Scholar]
- 246.Kadowaki A, Matsukawa K, Wakasugi R, Nakamoto T, Liang N. Central command does not decrease cardiac parasympathetic efferent nerve activity during spontaneous fictive motor activity in decerebrate cats. Am J Physiol Heart Circ Physiol 300: H1373–H1385, 2011. [DOI] [PubMed] [Google Scholar]
- 247.Keller CJ, Loeser A, Rein H. The physiology of the skeletal-muscle-blood circulation. Z Biol Munich 90: 260–298, 1930. [Google Scholar]
- 248.Kilbom A, Brundin T. Circulatory effects of isometric muscle contractions, performed separately and in combination with dynamic exercise. Eur J Appl Physiol Occup Physiol 36: 7–17, 1976. [DOI] [PubMed] [Google Scholar]
- 249.Kirby BS, Carlson RE, Markwald RR, Voyles WF, Dinenno FA. Mechanical influences on skeletal muscle vascular tone in humans: insight into contraction-induced rapid vasodilatation. J Physiol 583: 861–874, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kirby BS, Crecelius AR, Voyles WF, Dinenno FA. Impaired skeletal muscle blood flow control with advancing age in humans: attenuated ATP release and local vasodilation during erythrocyte deoxygenation. Circ Res 111: 220–230, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Kirby BS, Crecelius AR, Voyles WF, Dinenno FA. Modulation of postjunctional alpha-adrenergic vasoconstriction during exercise and exogenous ATP infusions in ageing humans. J Physiol 589: 2641–2653, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Kirby BS, Voyles WF, Carlson RE, Dinenno FA. Graded sympatholytic effect of exogenous ATP on postjunctional alpha-adrenergic vasoconstriction in the human forearm: implications for vascular control in contracting muscle. J Physiol 586: 4305–4316, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Kirby BS, Voyles WF, Simpson CB, Carlson RE, Schrage WG, Dinenno FA. Endothelium-dependent vasodilatation and exercise hyperaemia in ageing humans: impact of acute ascorbic acid administration. J Physiol 587: 1989–2003, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Kitamura K, Jorgensen CR, Gobel FL, Taylor HL, Wang Y. Hemodynamic correlates of myocardial oxygen consumption during upright exercise. J Appl Physiol 32: 516–522, 1972. [DOI] [PubMed] [Google Scholar]
- 255.Klabunde RE. Conditions for dipyridamole potentiation of skeletal muscle active hyperemia. Am J Physiol Heart Circ Physiol 250: H62–H67, 1986. [DOI] [PubMed] [Google Scholar]
- 256.Klabunde RE, Johnson PC. Reactive hyperemia in capillaries of red and white skeletal muscle. Am J Physiol Heart Circ Physiol 232: H411–H417, 1977. [DOI] [PubMed] [Google Scholar]
- 257.Klabunde RE, Laughlin MH, Armstrong RB. Systemic adenosine deaminase administration does not reduce active hyperemia in running rats. J Appl Physiol 64: 108–114, 1988. [DOI] [PubMed] [Google Scholar]
- 258.Kleiber M. Body size and metabolic rate. Physiol Rev 27: 511–541, 1947. [DOI] [PubMed] [Google Scholar]
- 259.Koch DW, Leuenberger UA, Proctor DN. Augmented leg vasoconstriction in dynamically exercising older men during acute sympathetic stimulation. J Physiol 551: 337–344, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Koch LG, Strick DM, Britton SL, Metting PJ. Reflex versus autoregulatory control of hindlimb blood flow during treadmill exercise in dogs. Am J Physiol Heart Circ Physiol 260: H436–H444, 1991. [DOI] [PubMed] [Google Scholar]
- 261.Komine H, Matsukawa K, Murata J, Tsuchimochi H, Shimizu K. Forelimb vasodilatation induced by hypothalamic stimulation is greatly mediated with nitric oxide in anesthetized cats. Jpn J Physiol 53: 97–103, 2003. [DOI] [PubMed] [Google Scholar]
- 262.Komine H, Matsukawa K, Tsuchimochi H, Nakamoto T, Murata J. Sympathetic cholinergic nerve contributes to increased muscle blood flow at the onset of voluntary static exercise in conscious cats. Am J Physiol Regul Integr Comp Physiol 295: R1251–R1262, 2008. [DOI] [PubMed] [Google Scholar]
- 263.Kurjiaka DT, Segal SS. Interaction between conducted vasodilation and sympathetic nerve activation in arterioles of hamster striated muscle. Circ Res 76: 885–891, 1995. [DOI] [PubMed] [Google Scholar]
- 264.Laaksonen MS, Kalliokoski KK, Luotolahti M, Kemppainen J, Teras M, Kyrolainen H, Nuutila P, Knuuti J. Myocardial perfusion during exercise in endurance-trained and untrained humans. Am J Physiol Regul Integr Comp Physiol 293: R837–R843, 2007. [DOI] [PubMed] [Google Scholar]
- 265.Lassen NA. Cerebral blood flow and oxygen consumption in man. Physiol Rev 39: 183–238, 1959. [DOI] [PubMed] [Google Scholar]
- 266.Lassen NA. Control of cerebral circulation in health and disease. Circ Res 34: 749–760, 1974. [DOI] [PubMed] [Google Scholar]
- 267.Laughlin MH. Skeletal muscle blood flow capacity: role of muscle pump in exercise hyperemia. Am J Physiol Heart Circ Physiol 253: H993–H1004, 1987. [DOI] [PubMed] [Google Scholar]
- 268.Laughlin MH, Klabunde RE, Delp MD, Armstrong RB. Effects of dipyridamole on muscle blood flow in exercising miniature swine. Am J Physiol Heart Circ Physiol 257: H1507–H1515, 1989. [DOI] [PubMed] [Google Scholar]
- 269.Laughlin MH, Ripperger J. Vascular transport capacity of hindlimb muscles of exercise-trained rats. J Appl Physiol 62: 438–443, 1987. [DOI] [PubMed] [Google Scholar]
- 270.Lautt WW. Resistance or conductance for expression of arterial vascular tone. Microvasc Res 37: 230–236, 1989. [DOI] [PubMed] [Google Scholar]
- 271.Lawrenson L, Poole JG, Kim J, Brown C, Patel P, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol 285: H1023–H1031, 2003. [DOI] [PubMed] [Google Scholar]
- 272.Laxson DD, Homans DC, Bache RJ. Inhibition of adenosine-mediated coronary vasodilation exacerbates myocardial ischemia during exercise. Am J Physiol Heart Circ Physiol 265: H1471–H1477, 1993. [DOI] [PubMed] [Google Scholar]
- 273.Lertmanorat Z, Durand DM. Electrode array for reversing the recruitment order of peripheral nerve stimulation: a simulation study. Conf Proc IEEE Eng Med Biol Soc 6: 4145–4148, 2004. [DOI] [PubMed] [Google Scholar]
- 274.Levine BD. VO2max: what do we know, and what do we still need to know? J Physiol 586: 25–34, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Levine JA, Abboud L, Barry M, Reed JE, Sheedy PF, Jensen MD. Measuring leg muscle and fat mass in humans: comparison of CT and dual-energy X-ray absorptiometry. J Appl Physiol 88: 452–456, 2000. [DOI] [PubMed] [Google Scholar]
- 276.Lewis SF, Haller RG. The pathophysiology of McArdle's disease: clues to regulation in exercise and fatigue. J Appl Physiol 61: 391–401, 1986. [DOI] [PubMed] [Google Scholar]
- 277.Lewis SF, Haller RG, Cook JD, Blomqvist CG. Metabolic control of cardiac output response to exercise in McArdle's disease. J Appl Physiol 57: 1749–1753, 1984. [DOI] [PubMed] [Google Scholar]
- 278.Lexell J, Downham DY. The occurrence of fibre-type grouping in healthy human muscle: a quantitative study of cross-sections of whole vastus lateralis from men between 15 and 83 years. Acta Neuropathol 81: 377–381, 1991. [DOI] [PubMed] [Google Scholar]
- 279.Lieberman DE, Bramble DM. The evolution of marathon running: capabilities in humans. Sports Med 37: 288–290, 2007. [DOI] [PubMed] [Google Scholar]
- 280.Lind AR, Williams CA. The control of blood flow through human forearm muscles following brief isometric contractions. J Physiol 288: 529–547, 1979. [PMC free article] [PubMed] [Google Scholar]
- 281.Lindqvist M, Davidsson S, Hjemdahl P, Melcher A. Sustained forearm vasodilation in humans during mental stress is not neurogenically mediated. Acta Physiol Scand 158: 7–14, 1996. [DOI] [PubMed] [Google Scholar]
- 282.Lindstedt SL, Hokanson JF, Wells DJ, Swain SD, Hoppeler H, Navarro V. Running energetics in the pronghorn antelope. Nature 353: 748–750, 1991. [DOI] [PubMed] [Google Scholar]
- 283.Lohman AW, Billaud M, Isakson BE. Mechanisms of ATP release and signalling in the blood vessel wall. Cardiovasc Res 95: 269–280, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Lohmeier TE, Hildebrandt DA, Dwyer TM, Iliescu R, Irwin ED, Cates AW, Rossing MA. Prolonged activation of the baroreflex decreases arterial pressure even during chronic adrenergic blockade. Hypertension 53: 833–838, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Longhurst JC, Musch TI, Ordway GA. O2 consumption during exercise in dogs—roles of splenic contraction and alpha-adrenergic vasoconstriction. Am J Physiol Heart Circ Physiol 251: H502–H509, 1986. [DOI] [PubMed] [Google Scholar]
- 286.Lopez MG, Silva BM, Joyner MJ, Casey DP. Roles of nitric oxide and prostaglandins in the hyperemic response to a maximal metabolic stimulus: redundancy prevails. Eur J Appl Physiol 113: 1449–1456, 2013. [DOI] [PubMed] [Google Scholar]
- 287.Lucia A, Gomez-Gallego F, Chicharro JL, Hoyos J, Celaya K, Cordova A, Villa G, Alonso JM, Barriopedro M, Perez M, Earnest CP. Is there an association between ACE and CKMM polymorphisms and cycling performance status during 3-week races? Int J Sports Med 26: 442–447, 2005. [DOI] [PubMed] [Google Scholar]
- 288.Lundby C, Boushel R, Robach P, Moller K, Saltin B, Calbet JA. During hypoxic exercise some vasoconstriction is needed to match O2 delivery with O2 demand at the microcirculatory level. J Physiol 586: 123–130, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Lutjemeier BJ, Miura A, Scheuermann BW, Koga S, Townsend DK, Barstow TJ. Muscle contraction-blood flow interactions during upright knee extension exercise in humans. J Appl Physiol 98: 1575–1583, 2005. [DOI] [PubMed] [Google Scholar]
- 290.MacDougall JD, Tuxen D, Sale DG, Moroz JR, Sutton JR. Arterial blood pressure response to heavy resistance exercise. J Appl Physiol 58: 785–790, 1985. [DOI] [PubMed] [Google Scholar]
- 291.Mackie BG, Terjung RL. Blood flow to different skeletal muscle fiber types during contraction. Am J Physiol Heart Circ Physiol 245: H265–H275, 1983. [DOI] [PubMed] [Google Scholar]
- 292.Madsen JL, Sondergaard SB, Moller S. Meal-induced changes in splanchnic blood flow and oxygen uptake in middle-aged healthy humans. Scand J Gastroenterol 41: 87–92, 2006. [DOI] [PubMed] [Google Scholar]
- 293.Manohar M. Blood flow to the respiratory and limb muscles and to abdominal organs during maximal exertion in ponies. J Physiol 377: 25–35, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Manohar M. Diaphragmatic perfusion heterogeneity during exercise with inspiratory resistive breathing. J Appl Physiol 68: 2177–2181, 1990. [DOI] [PubMed] [Google Scholar]
- 295.Manohar M. Inspiratory and expiratory muscle perfusion in maximally exercised ponies. J Appl Physiol 68: 544–548, 1990. [DOI] [PubMed] [Google Scholar]
- 296.Manohar M. Transmural coronary vasodilator reserve and flow distribution during maximal exercise in normal and splenectomized ponies. J Physiol 387: 425–440, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Manohar M. Vasodilator reserve in respiratory muscles during maximal exertion in ponies. J Appl Physiol 60: 1571–1577, 1986. [DOI] [PubMed] [Google Scholar]
- 298.Manohar M, Goetz TE, Hutchens E, Coney E. Atrial and ventricular myocardial blood flows in horses at rest and during exercise. Am J Vet Res 55: 1464–1469, 1994. [PubMed] [Google Scholar]
- 299.Marshall JM. The roles of adenosine and related substances in exercise hyperaemia. J Physiol 583: 835–845, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Marshall JM, Tandon HC. Direct observations of muscle arterioles and venules following contraction of skeletal muscle fibres in the rat. J Physiol 350: 447–459, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Marshall RJ, Schirger A, Shepherd JT. Blood pressure during supine exercise in idiopathic orthostatic hypotension. Circulation 24: 76–81, 1961. [DOI] [PubMed] [Google Scholar]
- 302.Martin CM, Beltran-Del-Rio A, Albrecht A, Lorenz RR, Joyner MJ. Local cholinergic mechanisms mediate nitric oxide-dependent flow-induced vasorelaxation in vitro. Am J Physiol Heart Circ Physiol 270: H442–H446, 1996. [DOI] [PubMed] [Google Scholar]
- 303.Martin EA, Nicholson WT, Curry TB, Eisenach JH, Charkoudian N, Joyner MJ. Adenosine transporter antagonism in humans augments vasodilator responsiveness to adenosine, but not exercise, in both adenosine responders and non-responders. J Physiol 579: 237–245, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Martin EA, Nicholson WT, Eisenach JH, Charkoudian N, Joyner MJ. Bimodal distribution of vasodilator responsiveness to adenosine due to difference in nitric oxide contribution: implications for exercise hyperemia. J Appl Physiol 101: 492–499, 2006. [DOI] [PubMed] [Google Scholar]
- 305.Martin EA, Nicholson WT, Eisenach JH, Charkoudian N, Joyner MJ. Influences of adenosine receptor antagonism on vasodilator responses to adenosine and exercise in adenosine responders and nonresponders. J Appl Physiol 101: 1678–1684, 2006. [DOI] [PubMed] [Google Scholar]
- 306.Matsukawa K, Ishii K, Liang N, Endo K. Have we missed that neural vasodilator mechanisms may contribute to exercise hyperemia at onset of voluntary exercise? Front Physiol 4: 23, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Matsukawa K, Nakamoto T, Liang N. Electrical stimulation of the mesencephalic ventral tegmental area evokes skeletal muscle vasodilatation in the cat and rat. J Physiol Sci 61: 293–301, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Matsukawa K, Shindo T, Shirai M, Ninomiya I. Direct observations of sympathetic cholinergic vasodilatation of skeletal muscle small arteries in the cat. J Physiol 500: 213–225, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Matsukawa K, Shindo T, Shirai M, Ninomiya I. Nitric oxide mediates cat hindlimb cholinergic vasodilation induced by stimulation of posterior hypothalamus. Jpn J Physiol 43: 473–483, 1993. [DOI] [PubMed] [Google Scholar]
- 310.McComas AJ. 1998. ISEK Congress Keynote Lecture: Motor units: how many, how large, what kind? Int Soc Electrophysiol Kinesiol Soc J Electromyography Kinesiol 8: 391–402, 1998. [DOI] [PubMed] [Google Scholar]
- 311.McGillivray-Anderson KM, Faber JE. Effect of acidosis on contraction of microvascular smooth muscle by alpha 1- and alpha 2-adrenoceptors. Implications for neural and metabolic regulation. Circ Res 66: 1643–1657, 1990. [DOI] [PubMed] [Google Scholar]
- 312.McGillivray-Anderson KM, Faber JE. Effect of reduced blood flow on alpha 1- and alpha 2-adrenoceptor constriction of rat skeletal muscle microvessels. Circ Res 69: 165–173, 1991. [DOI] [PubMed] [Google Scholar]
- 313.McGuire BJ, Secomb TW. Estimation of capillary density in human skeletal muscle based on maximal oxygen consumption rates. Am J Physiol Heart Circ Physiol 285: H2382–H2391, 2003. [DOI] [PubMed] [Google Scholar]
- 314.McGuire BJ, Secomb TW. A theoretical model for oxygen transport in skeletal muscle under conditions of high oxygen demand. J Appl Physiol 91: 2255–2265, 2001. [DOI] [PubMed] [Google Scholar]
- 315.Melcher A, Donald DE. Maintained ability of carotid baroreflex to regulate arterial pressure during exercise. Am J Physiol Heart Circ Physiol 241: H838–H849, 1981. [DOI] [PubMed] [Google Scholar]
- 316.Mellander S. Tissue osmolality as a mediator of exercise hyperemia. Scand J Clin Lab Invest 29: 139–144, 1972. [DOI] [PubMed] [Google Scholar]
- 317.Mellander S, Lundvall J. Role of tissue hyperosmolality in exercise hyperemia. Circ Res 28 Suppl 1: 39–45, 1971. [PubMed] [Google Scholar]
- 318.Merton PA, Hill DK, Morton HB. Indirect and direct stimulation of fatigued human muscle. Ciba Found Symp 82: 120–129, 1981. [DOI] [PubMed] [Google Scholar]
- 319.Milic-Emili J, Henderson JA, Dolovich MB, Trop D, Kaneko K. Regional distribution of inspired gas in the lung. J Appl Physiol 21: 749–759, 1966. [DOI] [PubMed] [Google Scholar]
- 320.Miller JD, Dempsey JA. The Effects of healthy ageing and COPD. In: Lung Development and Regeneration (Lung Biology in Health and Disease Series), edited by Massaro DJ, Massaro GD, Chambon P. New York: Marcell Decker, 2004, p. 483–524. [Google Scholar]
- 321.Miller JD, Pegelow DF, Jacques AJ, Dempsey JA. Skeletal muscle pump versus respiratory muscle pump: modulation of venous return from the locomotor limb in humans. J Physiol 563: 925–943, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Mirceta S, Signore AV, Burns JM, Cossins AR, Campbell KL, Berenbrink M. Evolution of mammalian diving capacity traced by myoglobin net surface charge. Science 340: 1234192, 2013. [DOI] [PubMed] [Google Scholar]
- 323.Mitchell JH, Sproule BJ, Chapman CB. The physiological meaning of the maximal oxygen intake test. J Clin Invest 37: 538–547, 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Moore AW, Bearden SE, Segal SS. Regional activation of rapid onset vasodilatation in mouse skeletal muscle: regulation through alpha-adrenoreceptors. J Physiol 588: 3321–3331, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Moore SC, Patel AV, Matthews CE, Berrington de Gonzalez A, Park Y, Katki HA, Linet MS, Weiderpass E, Visvanathan K, Helzlsouer KJ, Thun M, Gapstur SM, Hartge P, Lee IM. Leisure time physical activity of moderate to vigorous intensity and mortality: a large pooled cohort analysis. PLoS Med 9: e1001335, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Morganroth J, Maron BJ, Henry WL, Epstein SE. Comparative left ventricular dimensions in trained athletes. Ann Internal Med 82: 521–524, 1975. [DOI] [PubMed] [Google Scholar]
- 327.Mortensen SP, Gonzalez-Alonso J, Bune LT, Saltin B, Pilegaard H, Hellsten Y. ATP-induced vasodilation and purinergic receptors in the human leg: roles of nitric oxide, prostaglandins, and adenosine. Am J Physiol Regul Integr Comp Physiol 296: R1140–R1148, 2009. [DOI] [PubMed] [Google Scholar]
- 328.Mortensen SP, Gonzalez-Alonso J, Damsgaard R, Saltin B, Hellsten Y. Inhibition of nitric oxide and prostaglandins, but not endothelial-derived hyperpolarizing factors, reduces blood flow and aerobic energy turnover in the exercising human leg. J Physiol 581: 853–861, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Mortensen SP, Gonzalez-Alonso J, Nielsen JJ, Saltin B, Hellsten Y. Muscle interstitial ATP and norepinephrine concentrations in the human leg during exercise and ATP infusion. J Appl Physiol 107: 1757–1762, 2009. [DOI] [PubMed] [Google Scholar]
- 330.Mortensen SP, Nyberg M, Thaning P, Saltin B, Hellsten Y. Adenosine contributes to blood flow regulation in the exercising human leg by increasing prostaglandin and nitric oxide formation. Hypertension 53: 993–999, 2009. [DOI] [PubMed] [Google Scholar]
- 331.Musch TI, Friedman DB, Pitetti KH, Haidet GC, Stray-Gundersen J, Mitchell JH, Ordway GA. Regional distribution of blood flow of dogs during graded dynamic exercise. J Appl Physiol 63: 2269–2277, 1987. [DOI] [PubMed] [Google Scholar]
- 332.Musch TI, Haidet GC, Ordway GA, Longhurst JC, Mitchell JH. Dynamic exercise training in foxhounds. I. Oxygen consumption and hemodynamic responses. J Appl Physiol 59: 183–189, 1985. [DOI] [PubMed] [Google Scholar]
- 333.Musch TI, Haidet GC, Ordway GA, Longhurst JC, Mitchell JH. Training effects on regional blood flow response to maximal exercise in foxhounds. J Appl Physiol 62: 1724–1732, 1987. [DOI] [PubMed] [Google Scholar]
- 334.Musch TI, McAllister RM, Symons JD, Stebbins CL, Hirai T, Hageman KS, Poole DC. Effects of nitric oxide synthase inhibition on vascular conductance during high speed treadmill exercise in rats. Exp Physiol 86: 749–757, 2001. [DOI] [PubMed] [Google Scholar]
- 335.Nadland IH, Wesche J, Sheriff DD, Toska K. Does the great saphenous vein stripping improve arterial leg blood flow during exercise? Eur J Vasc Endovasc Surg 41: 697–703, 2011. [DOI] [PubMed] [Google Scholar]
- 336.Nadland IH, Wesche J, Sheriff DD, Toska K. Does venous insufficiency impair the exercise-induced rise in arterial leg blood flow? Phlebology 26: 326–331, 2011. [DOI] [PubMed] [Google Scholar]
- 337.Naik JS, Valic Z, Buckwalter JB, Clifford PS. Rapid vasodilation in response to a brief tetanic muscle contraction. J Appl Physiol 87: 1741–1746, 1999. [DOI] [PubMed] [Google Scholar]
- 338.Nepveu ME, Donati F, Fortier LP. Train-of-four stimulation for adductor pollicis neuromuscular monitoring can be applied at the wrist or over the hand. Anesth Analg 100: 149–154, 2005. [DOI] [PubMed] [Google Scholar]
- 339.Nevill A, Brown D, Godfrey R, Johnson P, Romer L, Stewart AD, Winter EM. Modeling maximum oxygen uptake of elite endurance athletes. Med Sci Sports Exerc 35: 488–494, 2003. [DOI] [PubMed] [Google Scholar]
- 340.Nicholson WT, Vaa B, Hesse C, Eisenach JH, Joyner MJ. Aging is associated with reduced prostacyclin-mediated dilation in the human forearm. Hypertension 53: 973–978, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Nishigaki K, Faber JE, Ohyanagi M. Interactions between alpha-adrenoceptors and adenosine receptors on microvascular smooth muscle. Am J Physiol Heart Circ Physiol 260: H1655–H1666, 1991. [DOI] [PubMed] [Google Scholar]
- 342.O'Leary DS. Regional vascular resistance vs. conductance: which index for baroreflex responses? Am J Physiol Heart Circ Physiol 260: H632–H637, 1991. [DOI] [PubMed] [Google Scholar]
- 343.O'Leary DS, Robinson ED, Butler JL. Is active skeletal muscle functionally vasoconstricted during dynamic exercise in conscious dogs? Am J Physiol Regul Integr Comp Physiol 272: R386–R391, 1997. [DOI] [PubMed] [Google Scholar]
- 344.Ogawa T, Spina RJ, Martin WH 3rd Kohrt WM, Schechtman KB, Holloszy JO, Ehsani AA. Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation 86: 494–503, 1992. [DOI] [PubMed] [Google Scholar]
- 345.Ogoh S, Fadel PJ, Nissen P, Jans O, Selmer C, Secher NH, Raven PB. Baroreflex-mediated changes in cardiac output and vascular conductance in response to alterations in carotid sinus pressure during exercise in humans. J Physiol 550: 317–324, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Ogoh S, Fisher JP, Dawson EA, White MJ, Secher NH, Raven PB. Autonomic nervous system influence on arterial baroreflex control of heart rate during exercise in humans. J Physiol 566: 599–611, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Ohyanagi M, Nishigaki K, Faber JE. Interaction between microvascular alpha 1- and alpha 2-adrenoceptors and endothelium-derived relaxing factor. Circ Res 71: 188–200, 1992. [DOI] [PubMed] [Google Scholar]
- 348.Olson TP, Joyner MJ, Dietz NM, Eisenach JH, Curry TB, Johnson BD. Effects of respiratory muscle work on blood flow distribution during exercise in heart failure. J Physiol 588: 2487–2501, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Parker BA, Smithmyer SL, Jarvis SS, Ridout SJ, Pawelczyk JA, Proctor DN. Evidence for reduced sympatholysis in leg resistance vasculature of healthy older women. Am J Physiol Heart Circ Physiol 292: H1148–H1156, 2007. [DOI] [PubMed] [Google Scholar]
- 350.Parks CM, Manohar M. Distribution of blood flow during moderate and strenuous exercise in ponies (Equus caballus). Am J Vet Res 44: 1861–1866, 1983. [PubMed] [Google Scholar]
- 351.Parks CM, Manohar M. Transmural coronary vasodilator reserve and flow distribution during severe exercise in ponies. J Appl Physiol 54: 1641–1652, 1983. [DOI] [PubMed] [Google Scholar]
- 352.Pate RR, Sparling PB, Wilson GE, Cureton KJ, Miller BJ. Cardiorespiratory and metabolic responses to submaximal and maximal exercise in elite women distance runners. Int J Sports Med 8 Suppl 2: 91–95, 1987. [DOI] [PubMed] [Google Scholar]
- 353.Pedersen PK, Mandoe H, Jensen K, Andersen C, Madsen K. Reduced arterial O2 saturation during supine exercise in highly trained cyclists. Acta Physiol Scand 158: 325–331, 1996. [DOI] [PubMed] [Google Scholar]
- 354.Peterson DF, Armstrong RB, Laughlin MH. Sympathetic neural influences on muscle blood flow in rats during submaximal exercise. J Appl Physiol 65: 434–440, 1988. [DOI] [PubMed] [Google Scholar]
- 355.Pinto Pereira SM, Ki M, Power C. Sedentary behaviour and biomarkers for cardiovascular disease and diabetes in mid-life: the role of television-viewing and sitting at work. PloS One 7: e31132, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Pittman RN. Influence of microvascular architecture on oxygen exchange in skeletal muscle. Microcirculation 2: 1–18, 1995. [DOI] [PubMed] [Google Scholar]
- 357.Pittman RN. Oxygen transport and exchange in the microcirculation. Microcirculation 12: 59–70, 2005. [DOI] [PubMed] [Google Scholar]
- 358.Plowman SA, Smith DL. Exercise Physiology For Health, Fitness, and Performance. Baltimore, MD: Lippincott Williams & Wilkins, 2014. [Google Scholar]
- 359.Pollack AA, Taylor BE, Myers TT, Wood EH. The effect of exercise and body position on the venous pressure at the ankle in patients having venous valvular defects. J Clin Invest 28: 559–563, 1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Pollack AA, Wood EH. Venous pressure in the saphenous vein at the ankle in man during exercise and changes in posture. J Appl Physiol 1: 649–662, 1949. [DOI] [PubMed] [Google Scholar]
- 361.Pollock ML. Submaximal and maximal working capacity of elite distance runners. Part I: Cardiorespiratory aspects. Ann NY Acad Sci 301: 310–322, 1977. [DOI] [PubMed] [Google Scholar]
- 362.Pollock ML, Foster C, Knapp D, Rod JL, Schmidt DH. Effect of age and training on aerobic capacity and body composition of master athletes. J Appl Physiol 62: 725–731, 1987. [DOI] [PubMed] [Google Scholar]
- 363.Poole DC, Copp SW, Ferguson SK, Musch TI. Skeletal muscle capillary function: contemporary observations and novel hypotheses. Exp Physiol 98: 1645–1658, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Poole DC, Erickson HH. Highly athletic terrestrial mammals: horses and dogs. Comprehensive Physiol 1: 1–37, 2011. [DOI] [PubMed] [Google Scholar]
- 365.Poole JG, Lawrenson L, Kim J, Brown C, Richardson RS. Vascular and metabolic response to cycle exercise in sedentary humans: effect of age. Am J Physiol Heart Circ Physiol 284: H1251–H1259, 2003. [DOI] [PubMed] [Google Scholar]
- 366.Powers SK, Dodd S, Lawler J, Landry G, Kirtley M, McKnight T, Grinton S. Incidence of exercise induced hypoxemia in elite endurance athletes at sea level. Eur J Appl Physiol Occup Physiol 58: 298–302, 1988. [DOI] [PubMed] [Google Scholar]
- 367.Powers SK, Martin D, Cicale M, Collop N, Huang D, Criswell D. Exercise-induced hypoxemia in athletes: role of inadequate hyperventilation. Eur J Appl Physiol Occup Physiol 65: 37–42, 1992. [DOI] [PubMed] [Google Scholar]
- 368.Proctor DN, Halliwill JR, Shen PH, Vlahakis NE, Joyner MJ. Peak calf blood flow estimates are higher with Dohn than with Whitney plethysmograph. J Appl Physiol 81: 1418–1422, 1996. [DOI] [PubMed] [Google Scholar]
- 369.Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, Loeffler DL, Joyner MJ. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J Appl Physiol 85: 68–75, 1998. [DOI] [PubMed] [Google Scholar]
- 370.Radegran G, Blomstrand E, Saltin B. Peak muscle perfusion and oxygen uptake in humans: importance of precise estimates of muscle mass. J Appl Physiol 87: 2375–2380, 1999. [DOI] [PubMed] [Google Scholar]
- 371.Radegran G, Saltin B. Nitric oxide in the regulation of vasomotor tone in human skeletal muscle. Am J Physiol Heart Circ Physiol 276: H1951–H1960, 1999. [DOI] [PubMed] [Google Scholar]
- 372.Rankinen T, Wolfarth B, Simoneau JA, Maier-Lenz D, Rauramaa R, Rivera MA, Boulay MR, Chagnon YC, Perusse L, Keul J, Bouchard C. No association between the angiotensin-converting enzyme ID polymorphism and elite endurance athlete status. J Appl Physiol 88: 1571–1575, 2000. [DOI] [PubMed] [Google Scholar]
- 373.Ray CJ, Abbas MR, Coney AM, Marshall JM. Interactions of adenosine, prostaglandins and nitric oxide in hypoxia-induced vasodilatation: in vivo and in vitro studies. J Physiol 544: 195–209, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Reed AS, Tschakovsky ME, Minson CT, Halliwill JR, Torp KD, Nauss LA, Joyner MJ. Skeletal muscle vasodilatation during sympathoexcitation is not neurally mediated in humans. J Physiol 525: 253–262, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Remensnyder JP, Mitchell JH, Sarnoff SJ. Functional sympatholysis during muscular activity. Observations on influence of carotid sinus on oxygen uptake. Circ Res 11: 370–380, 1962. [DOI] [PubMed] [Google Scholar]
- 376.Richardson RS, Kennedy B, Knight DR, Wagner PD. High muscle blood flows are not attenuated by recruitment of additional muscle mass. Am J Physiol Heart Circ Physiol 269: H1545–H1552, 1995. [DOI] [PubMed] [Google Scholar]
- 377.Richardson RS, Poole DC, Knight DR, Kurdak SS, Hogan MC, Grassi B, Johnson EC, Kendrick KF, Erickson BK, Wagner PD. High muscle blood flow in man: is maximal O2 extraction compromised? J Appl Physiol 75: 1911–1916, 1993. [DOI] [PubMed] [Google Scholar]
- 378.Rivera MA, Wolfarth B, Dionne FT, Chagnon M, Simoneau JA, Boulay MR, Song TM, Perusse L, Gagnon J, Leon AS, Rao DC, Skinner JS, Wilmore JH, Keul J, Bouchard C. Three mitochondrial DNA restriction polymorphisms in elite endurance athletes and sedentary controls. Med Sci Sports Exerc 30: 687–690, 1998. [DOI] [PubMed] [Google Scholar]
- 379.Robinson BF, Epstein SE, Beiser GD, Braunwald E. Control of heart rate by the autonomic nervous system. Studies in man on the interrelation between baroreceptor mechanisms and exercise. Circ Res 19: 400–411, 1966. [DOI] [PubMed] [Google Scholar]
- 380.Robinson S, Edwards HT, Dill DB. New records in human power. Science 85: 409–410, 1937. [DOI] [PubMed] [Google Scholar]
- 381.Roddie IC. Human responses to emotional stress. Irish J Med Sci 146: 395–417, 1977. [DOI] [PubMed] [Google Scholar]
- 382.Rode A, Shephard RJ. Cardiorespiratory fitness of an Arctic community. J Appl Physiol 31: 519–526, 1971. [DOI] [PubMed] [Google Scholar]
- 383.Rogers MA, Hagberg JM, Martin WH 3rd, Ehsani AA, Holloszy JO. Decline in V̇o2max with aging in master athletes and sedentary men. J Appl Physiol 68: 2195–2199, 1990. [DOI] [PubMed] [Google Scholar]
- 384.Rongen GA, Smits P, Thien T. Characterization of ATP-induced vasodilation in the human forearm vascular bed. Circulation 90: 1891–1898, 1994. [DOI] [PubMed] [Google Scholar]
- 385.Rosenmeier JB, Dinenno FA, Fritzlar SJ, Joyner MJ. Alpha1- and alpha2-adrenergic vasoconstriction is blunted in contracting human muscle. J Physiol 547: 971–976, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Rosenmeier JB, Fritzlar SJ, Dinenno FA, Joyner MJ. Exogenous NO administration and alpha-adrenergic vasoconstriction in human limbs. J Appl Physiol 95: 2370–2374, 2003. [DOI] [PubMed] [Google Scholar]
- 387.Rosenmeier JB, Hansen J, Gonzalez-Alonso J. Circulating ATP-induced vasodilatation overrides sympathetic vasoconstrictor activity in human skeletal muscle. J Physiol 558: 351–365, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Rosenmeier JB, Yegutkin GG, Gonzalez-Alonso J. Activation of ATP/UTP-selective receptors increases blood flow and blunts sympathetic vasoconstriction in human skeletal muscle. J Physiol 586: 4993–5002, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev 54: 75–159, 1974. [DOI] [PubMed] [Google Scholar]
- 390.Rowell LB. Human Cardiovascular Control. New York: Oxford Univ. Press, 1993, p. 500. [Google Scholar]
- 391.Rowell LB. Human Circulation: Regulation During Physical Stress. New York: Oxford Univ. Press, 1986, p. 432. [Google Scholar]
- 392.Rowell LB. Ideas about control of skeletal and cardiac muscle blood flow (1876–2003): cycles of revision and new vision. J Appl Physiol 97: 384–392, 2004. [DOI] [PubMed] [Google Scholar]
- 393.Rowell LB. Neural control of muscle blood flow: importance during dynamic exercise. Clin Exp Pharmacol Physiol 24: 117–125, 1997. [DOI] [PubMed] [Google Scholar]
- 394.Rowell LB, Blackmon JR, Bruce RA. Indocyanine green clearance and estimated hepatic blood flow during mild to maximal exercise in upright man. J Clin Invest 43: 1677–1690, 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Rowell LB, Blackmon JR, Kenny MA, Escourrou P. Splanchnic vasomotor and metabolic adjustments to hypoxia and exercise in humans. Am J Physiol Heart Circ Physiol 247: H251–H258, 1984. [DOI] [PubMed] [Google Scholar]
- 396.Rowell LB, Blackmon JR, Martin RH, Mazzarella JA, Bruce RA. Hepatic clearance of indocyanine green in man under thermal and exercise stresses. J Appl Physiol 20: 384–394, 1965. [DOI] [PubMed] [Google Scholar]
- 397.Rowell LB, Brengelmann GL, Blackmon JR, Bruce RA, Murray JA. Disparities between aortic and peripheral pulse pressures induced by upright exercise and vasomotor changes in man. Circulation 37: 954–964, 1968. [DOI] [PubMed] [Google Scholar]
- 398.Rowell LB, O'Leary DS. Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. J Appl Physiol 69: 407–418, 1990. [DOI] [PubMed] [Google Scholar]
- 399.Rowell LB, Saltin B, Kiens B, Christensen NJ. Is peak quadriceps blood flow in humans even higher during exercise with hypoxemia? Am J Physiol Heart Circ Physiol 251: H1038–H1044, 1986. [DOI] [PubMed] [Google Scholar]
- 400.Rowell LB, Savage MV, Chambers J, Blackmon JR. Cardiovascular responses to graded reductions in leg perfusion in exercising humans. Am J Physiol Heart Circ Physiol 261: H1545–H1553, 1991. [DOI] [PubMed] [Google Scholar]
- 401.Roy TK, Secomb TW. Functional sympatholysis and sympathetic escape in a theoretical model for blood flow regulation. Front Physiol 5: 192, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Rusch NJ, Shepherd JT, Webb RC, Vanhoutte PM. Different behavior of the resistance vessels of the human calf and forearm during contralateral isometric exercise, mental stress, and abnormal respiratory movements. Circ Res 48: I118–130, 1981. [PubMed] [Google Scholar]
- 403.Saltin B. In search of a vasodilator: is ATP the answer? J Physiol 590: 5261–5262, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Saltin B. Metabolic fundamentals in exercise. Med Sci Sports 5: 137–146, 1973. [PubMed] [Google Scholar]
- 405.Saltin B, Astrand PO. Maximal oxygen uptake in athletes. J Appl Physiol 23: 353–358, 1967. [DOI] [PubMed] [Google Scholar]
- 406.Saltin B, Blomqvist G, Mitchell JH, Johnson RL Jr, Wildenthal K, Chapman CB. Response to exercise after bed rest and after training. Circulation 38: VII1–78, 1968. [PubMed] [Google Scholar]
- 407.Saltin B, Mortensen SP. Inefficient functional sympatholysis is an overlooked cause of malperfusion in contracting skeletal muscle. J Physiol 590: 6269–6275, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Saltin B, Radegran G, Koskolou MD, Roach RC. Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiol Scand 162: 421–436, 1998. [DOI] [PubMed] [Google Scholar]
- 409.Sanders JS, Mark AL, Ferguson DW. Evidence for cholinergically mediated vasodilation at the beginning of isometric exercise in humans. Circulation 79: 815–824, 1989. [DOI] [PubMed] [Google Scholar]
- 410.Sandow SL, Looft-Wilson R, Doran B, Grayson TH, Segal SS, Hill CE. Expression of homocellular and heterocellular gap junctions in hamster arterioles and feed arteries. Cardiovasc Res 60: 643–653, 2003. [DOI] [PubMed] [Google Scholar]
- 411.Saunders NR, Dinenno FA, Pyke KE, Rogers AM, Tschakovsky ME. Impact of combined NO and PG blockade on rapid vasodilation in a forearm mild-to-moderate exercise transition in humans. Am J Physiol Heart Circ Physiol 288: H214–H220, 2005. [DOI] [PubMed] [Google Scholar]
- 412.Saunders NR, Tschakovsky ME. Evidence for a rapid vasodilatory contribution to immediate hyperemia in rest-to-mild and mild-to-moderate forearm exercise transitions in humans. J Appl Physiol 97: 1143–1151, 2004. [DOI] [PubMed] [Google Scholar]
- 413.Savard GK, Richter EA, Strange S, Kiens B, Christensen NJ, Saltin B. Norepinephrine spillover from skeletal muscle during exercise in humans: role of muscle mass. Am J Physiol Heart Circ Physiol 257: H1812–H1818, 1989. [DOI] [PubMed] [Google Scholar]
- 414.Sawka MN, Convertino VA, Eichner ER, Schnieder SM, Young AJ. Blood volume: importance and adaptations to exercise training, environmental stresses, and trauma/sickness. Med Sci Sports Exerc 32: 332–348, 2000. [DOI] [PubMed] [Google Scholar]
- 415.Schrage WG, Dietz NM, Joyner MJ. Effects of combined inhibition of ATP-sensitive potassium channels, nitric oxide, and prostaglandins on hyperemia during moderate exercise. J Appl Physiol 100: 1506–1512, 2006. [DOI] [PubMed] [Google Scholar]
- 416.Schrage WG, Eisenach JH, Joyner MJ. Ageing reduces nitric-oxide- and prostaglandin-mediated vasodilatation in exercising humans. J Physiol 579: 227–236, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Schrage WG, Joyner MJ, Dinenno FA. Local inhibition of nitric oxide and prostaglandins independently reduces forearm exercise hyperaemia in humans. J Physiol 557: 599–611, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Schrage WG, Wilkins BW, Dean VL, Scott JP, Henry NK, Wylam ME, Joyner MJ. Exercise hyperemia and vasoconstrictor responses in humans with cystic fibrosis. J Appl Physiol 99: 1866–1871, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Schrage WG, Wilkins BW, Johnson CP, Eisenach JH, Limberg JK, Dietz NM, Curry TB, Joyner MJ. Roles of nitric oxide synthase and cyclooxygenase in leg vasodilation and oxygen consumption during prolonged low-intensity exercise in untrained humans. J Appl Physiol 109: 768–777, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Seals DR, Moreau KL, Gates PE, Eskurza I. Modulatory influences on ageing of the vasculature in healthy humans. Exp Gerontol 41: 501–507, 2006. [DOI] [PubMed] [Google Scholar]
- 421.Secher NH. Physiological and biomechanical aspects of rowing. Implications for training. Sports Med 15: 24–42, 1993. [DOI] [PubMed] [Google Scholar]
- 422.Secher NH, Clausen JP, Klausen K, Noer I, Trap-Jensen J. Central and regional circulatory effects of adding arm exercise to leg exercise. Acta Physiol Scand 100: 288–297, 1977. [DOI] [PubMed] [Google Scholar]
- 423.Secher NH, Ruberg-Larsen N, Binkhorst RA, Bonde-Petersen F. Maximal oxygen uptake during arm cranking and combined arm plus leg exercise. J Appl Physiol 36: 515–518, 1974. [DOI] [PubMed] [Google Scholar]
- 424.Secher NH, Vaage O. Rowing performance, a mathematical model based on analysis of body dimensions as exemplified by body weight. Eur J Appl Physiol Occup Physiol 52: 88–93, 1983. [DOI] [PubMed] [Google Scholar]
- 425.Segal SS. Microvascular recruitment in hamster striated muscle: role for conducted vasodilation. Am J Physiol Heart Circ Physiol 261: H181–H189, 1991. [DOI] [PubMed] [Google Scholar]
- 426.Segal SS, Damon DN, Duling BR. Propagation of vasomotor responses coordinates arteriolar resistances. Am J Physiol Heart Circ Physiol 256: H832–H837, 1989. [DOI] [PubMed] [Google Scholar]
- 427.Segal SS, Duling BR. Conduction of vasomotor responses in arterioles: a role for cell-to-cell coupling? Am J Physiol Heart Circ Physiol 256: H838–H845, 1989. [DOI] [PubMed] [Google Scholar]
- 428.Segal SS, Duling BR. Flow control among microvessels coordinated by intercellular conduction. Science 234: 868–870, 1986. [DOI] [PubMed] [Google Scholar]
- 429.Segal SS, Welsh DG, Kurjiaka DT. Spread of vasodilatation and vasoconstriction along feed arteries and arterioles of hamster skeletal muscle. J Physiol 516: 283–291, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Shepherd JT. Behavior of resistance and capacity vessels in human limbs during exercise. Circ Res 20: I70, 1967. [Google Scholar]
- 431.Shepherd JT. Circulation to skeletal muscle. In: Handbook of Physiology. The Cardiovascular System. Peripheral Circulation and Organ Blood Flow. Bethesda, MD: Am. Physiol. Soc., 1983, sect. 2, vol. 3, p. 319–370. [Google Scholar]
- 432.Shepherd JT. Physiology of the Circulation in Human Limbs in Health and Disease. Philadelphia, PA: Saunders, 1963. [Google Scholar]
- 433.Shepherd JT, Lorenz RR, Tyce GM, Vanhoutte PM. Acetylcholine–inhibition of transmitter release from adrenergic nerve terminals mediated by muscarinic receptors. Federation Proc 37: 191–194, 1978. [PubMed] [Google Scholar]
- 434.Sheriff D. Point: the muscle pump raises muscle blood flow during locomotion. J Appl Physiol 99: 371–375, 2005. [DOI] [PubMed] [Google Scholar]
- 435.Sheriff DD, Nelson CD, Sundermann RK. Does autonomic blockade reveal a potent contribution of nitric oxide to locomotion-induced vasodilation? Am J Physiol Heart Circ Physiol 279: H726–H732, 2000. [DOI] [PubMed] [Google Scholar]
- 436.Sheriff DD, Rowell LB, Scher AM. Is rapid rise in vascular conductance at onset of dynamic exercise due to muscle pump? Am J Physiol Heart Circ Physiol 265: H1227–H1234, 1993. [DOI] [PubMed] [Google Scholar]
- 437.Sheriff DD, Van Bibber R. Flow-generating capability of the isolated skeletal muscle pump. Am J Physiol Heart Circ Physiol 274: H1502–H1508, 1998. [DOI] [PubMed] [Google Scholar]
- 438.Shih R, Wang Z, Heo M, Wang W, Heymsfield SB. Lower limb skeletal muscle mass: development of dual-energy X-ray absorptiometry prediction model. J Appl Physiol 89: 1380–1386, 2000. [DOI] [PubMed] [Google Scholar]
- 439.Shiotani I, Sato H, Yokoyama H, Ohnishi Y, Hishida E, Kinjo K, Nakatani D, Kuzuya T, Hori M. Muscle pump-dependent self-perfusion mechanism in legs in normal subjects and patients with heart failure. J Appl Physiol 92: 1647–1654, 2002. [DOI] [PubMed] [Google Scholar]
- 440.Shiramoto M, Imaizumi T, Hirooka Y, Endo T, Namba T, Oyama J, Hironaga K, Takeshita A. Role of nitric oxide towards vasodilator effects of substance P and ATP in human forearm vessels. Clin Sci 92: 123–131, 1997. [DOI] [PubMed] [Google Scholar]
- 441.Shoemaker JK, Halliwill JR, Hughson RL, Joyner MJ. Contributions of acetylcholine and nitric oxide to forearm blood flow at exercise onset and recovery. Am J Physiol Heart Circ Physiol 273: H2388–H2395, 1997. [DOI] [PubMed] [Google Scholar]
- 442.Shoemaker JK, Naylor HL, Pozeg ZI, Hughson RL. Failure of prostaglandins to modulate the time course of blood flow during dynamic forearm exercise in humans. J Appl Physiol 81: 1516–1521, 1996. [DOI] [PubMed] [Google Scholar]
- 443.Shoemaker JK, Tschakovsky ME, Hughson RL. Vasodilation contributes to the rapid hyperemia with rhythmic contractions in humans. Can J Physiol Pharmacol 76: 418–427, 1998. [DOI] [PubMed] [Google Scholar]
- 444.Skinner JS, Wilmore KM, Jaskolska A, Jaskolski A, Daw EW, Rice T, Gagnon J, Leon AS, Wilmore JH, Rao DC, Bouchard C. Reproducibility of maximal exercise test data in the HERITAGE family study. Med Sci Sports Exerc 31: 1623–1628, 1999. [DOI] [PubMed] [Google Scholar]
- 445.Snell PG, Martin WH, Buckey JC, Blomqvist CG. Maximal vascular leg conductance in trained and untrained men. J Appl Physiol 62: 606–610, 1987. [DOI] [PubMed] [Google Scholar]
- 446.Sparks HV Jr, Belloni FL. The peripheral circulation: local regulation. Annu Rev Physiol 40: 67–92, 1978. [DOI] [PubMed] [Google Scholar]
- 447.Spence AL, Naylor LH, Carter HH, Buck CL, Dembo L, Murray CP, Watson P, Oxborough D, George KP, Green DJ. A prospective randomised longitudinal MRI study of left ventricular adaptation to endurance and resistance exercise training in humans. J Physiol 589: 5443–5452, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Sperry JS, Meinzer FC, McCulloh KA. Safety and efficiency conflicts in hydraulic architecture: scaling from tissues to trees. Plant Cell Environ 31: 632–645, 2008. [DOI] [PubMed] [Google Scholar]
- 449.Sprague RS, Ellsworth ML, Stephenson AH, Kleinhenz ME, Lonigro AJ. Deformation-induced ATP release from red blood cells requires CFTR activity. Am J Physiol Heart Circ Physiol 275: H1726–H1732, 1998. [DOI] [PubMed] [Google Scholar]
- 450.Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ. Participation of cAMP in a signal-transduction pathway relating erythrocyte deformation to ATP release. Am J Physiol Cell Physiol 281: C1158–C1164, 2001. [DOI] [PubMed] [Google Scholar]
- 451.Sprague RS, Goldman D, Bowles EA, Achilleus D, Stephenson AH, Ellis CG, Ellsworth ML. Divergent effects of low-O2 tension and iloprost on ATP release from erythrocytes of humans with type 2 diabetes: implications for O2 supply to skeletal muscle. Am J Physiol Heart Circ Physiol 299: H566–H573, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Stainsby WN, Andrew GM. Maximal blood flow and power output of dog muscle in situ. Med Sci Sports Exerc 20: S109–112, 1988. [DOI] [PubMed] [Google Scholar]
- 453.Stamler JS, Jia L, Eu JP, McMahon TJ, Demchenko IT, Bonaventura J, Gernert K, Piantadosi CA. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science 276: 2034–2037, 1997. [DOI] [PubMed] [Google Scholar]
- 454.Stegall HF. Muscle pumping in dependent leg. Circ Res 19: 180, 1966. [Google Scholar]
- 455.Stewart IB, McKenzie DC. The human spleen during physiological stress. Sports Med 32: 361–369, 2002. [DOI] [PubMed] [Google Scholar]
- 456.Stickland MK, Smith CA, Soriano BJ, Dempsey JA. Sympathetic restraint of muscle blood flow during hypoxic exercise. Am J Physiol Regul Integr Comp Physiol 296: R1538–R1546, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Strandell T, Shepherd JT. The effect in humans of increased sympathetic activity on the blood flow to active muscles. Acta Med Scand Suppl 472: 146–167, 1967. [DOI] [PubMed] [Google Scholar]
- 458.Strange S, Rowell LB, Christensen NJ, Saltin B. Cardiovascular responses to carotid sinus baroreceptor stimulation during moderate to severe exercise in man. Acta Physiol Scand 138: 145–153, 1990. [DOI] [PubMed] [Google Scholar]
- 459.Stromme SB, Ingjer F, Meen HD. Assessment of maximal aerobic power in specifically trained athletes. J Appl Physiol 42: 833–837, 1977. [DOI] [PubMed] [Google Scholar]
- 460.Sullivan MJ, Knight JD, Higginbotham MB, Cobb FR. Relation between central and peripheral hemodynamics during exercise in patients with chronic heart failure. Muscle blood flow is reduced with maintenance of arterial perfusion pressure. Circulation 80: 769–781, 1989. [DOI] [PubMed] [Google Scholar]
- 461.Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol 37: 153–156, 2001. [DOI] [PubMed] [Google Scholar]
- 462.Tateishi J, Faber JE. Inhibition of arteriole alpha 2- but not alpha 1-adrenoceptor constriction by acidosis and hypoxia in vitro. Am J Physiol Heart Circ Physiol 268: H2068–H2076, 1995. [DOI] [PubMed] [Google Scholar]
- 463.Taylor HL, Buskirk E, Henschel A. Maximal oxygen intake as an objective measure of cardio-respiratory performance. J Appl Physiol 8: 73–80, 1955. [DOI] [PubMed] [Google Scholar]
- 464.Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 300: H2–H12, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Thomas CK, Nelson G, Than L, Zijdewind I. Motor unit activation order during electrically evoked contractions of paralyzed or partially paralyzed muscles. Muscle Nerve 25: 797–804, 2002. [DOI] [PubMed] [Google Scholar]
- 466.Thomas GD, Hansen J, Victor RG. Inhibition of alpha 2-adrenergic vasoconstriction during contraction of glycolytic, not oxidative, rat hindlimb muscle. Am J Physiol Heart Circ Physiol 266: H920–H929, 1994. [DOI] [PubMed] [Google Scholar]
- 467.Thomas GD, Victor RG. Nitric oxide mediates contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Physiol 506: 817–826, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Thomas GD, Zhang W, Victor RG. Impaired modulation of sympathetic vasoconstriction in contracting skeletal muscle of rats with chronic myocardial infarctions: role of oxidative stress. Circ Res 88: 816–823, 2001. [DOI] [PubMed] [Google Scholar]
- 469.Timmons JA, Knudsen S, Rankinen T, Koch LG, Sarzynski M, Jensen T, Keller P, Scheele C, Vollaard NB, Nielsen S, Akerstrom T, MacDougald OA, Jansson E, Greenhaff PL, Tarnopolsky MA, van Loon LJ, Pedersen BK, Sundberg CJ, Wahlestedt C, Britton SL, Bouchard C. Using molecular classification to predict gains in maximal aerobic capacity following endurance exercise training in humans. J Appl Physiol 108: 1487–1496, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Tonnesen KH. Blood-flow through muscle during rhythmic contraction measured by 133-xenon. Scand J Clin Lab Invest 16: 646–654, 1964. [DOI] [PubMed] [Google Scholar]
- 471.Totzeck M, Hendgen-Cotta UB, Luedike P, Berenbrink M, Klare JP, Steinhoff HJ, Semmler D, Shiva S, Williams D, Kipar A, Gladwin MT, Schrader J, Kelm M, Cossins AR, Rassaf T. Nitrite regulates hypoxic vasodilation via myoglobin-dependent nitric oxide generation. Circulation 126: 325–334, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Trappe S, Hayes E, Galpin A, Kaminsky L, Jemiolo B, Fink W, Trappe T, Jansson A, Gustafsson T, Tesch P. New records in aerobic power among octogenarian lifelong endurance athletes. J Appl Physiol 114: 3–10, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Trappe SW, Costill DL, Vukovich MD, Jones J, Melham T. Aging among elite distance runners: a 22-yr longitudinal study. J Appl Physiol 80: 285–290, 1996. [DOI] [PubMed] [Google Scholar]
- 474.Trimble MH, Enoka RM. Mechanisms underlying the training effects associated with neuromuscular electrical stimulation. Physical Ther 71: 273–280, 1991. [DOI] [PubMed] [Google Scholar]
- 475.Tschakovsky ME, Hughson RL. Ischemic muscle chemoreflex response elevates blood flow in nonischemic exercising human forearm muscle. Am J Physiol Heart Circ Physiol 277: H635–H642, 1999. [DOI] [PubMed] [Google Scholar]
- 476.Tschakovsky ME, Rogers AM, Pyke KE, Saunders NR, Glenn N, Lee SJ, Weissgerber T, Dwyer EM. Immediate exercise hyperemia in humans is contraction intensity dependent: evidence for rapid vasodilation. J Appl Physiol 96: 639–644, 2004. [DOI] [PubMed] [Google Scholar]
- 477.Tschakovsky ME, Sheriff DD. Immediate exercise hyperemia: contributions of the muscle pump vs. rapid vasodilation. J Appl Physiol 97: 739–747, 2004. [DOI] [PubMed] [Google Scholar]
- 478.Tschakovsky ME, Shoemaker JK, Hughson RL. Vasodilation and muscle pump contribution to immediate exercise hyperemia. Am J Physiol Heart Circ Physiol 271: H1697–H1701, 1996. [DOI] [PubMed] [Google Scholar]
- 479.Tschakovsky ME, Sujirattanawimol K, Ruble SB, Valic Z, Joyner MJ. Is sympathetic neural vasoconstriction blunted in the vascular bed of exercising human muscle? J Physiol 541: 623–635, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Tsuchimochi H, Matsukawa K, Komine H, Murata J. Direct measurement of cardiac sympathetic efferent nerve activity during dynamic exercise. Am J Physiol Heart Circ Physiol 283: H1896–H1906, 2002. [DOI] [PubMed] [Google Scholar]
- 481.Tune JD, Richmond KN, Gorman MW, Feigl EO. KATP channels, nitric oxide, and adenosine are not required for local metabolic coronary vasodilation. Am J Physiol Heart Circ Physiol 280: H868–H875, 2001. [DOI] [PubMed] [Google Scholar]
- 482.Tune JD, Richmond KN, Gorman MW, Feigl EO. Role of nitric oxide and adenosine in control of coronary blood flow in exercising dogs. Circulation 101: 2942–2948, 2000. [DOI] [PubMed] [Google Scholar]
- 483.Utomi V, Oxborough D, Whyte GP, Somauroo J, Sharma S, Shave R, Atkinson G, George K. Systematic review and meta-analysis of training mode, imaging modality and body size influences on the morphology and function of the male athlete's heart. Heart 99: 1727–1733, 2013. [DOI] [PubMed] [Google Scholar]
- 484.Uvnas B. Cholinergic vasodilator nerves. Federation Proc 25: 1618–1622, 1966. [PubMed] [Google Scholar]
- 485.Van Citters RL, Franklin DL. Cardiovascular performance of Alaska sled dogs during exercise. Circ Res 24: 33–42, 1969. [DOI] [PubMed] [Google Scholar]
- 486.Vanhoutte PM. Endothelial adrenoceptors. J Cardiovasc Pharmacol 38: 796–808, 2001. [DOI] [PubMed] [Google Scholar]
- 487.VanTeeffelen JW, Segal SS. Interaction between sympathetic nerve activation and muscle fibre contraction in resistance vessels of hamster retractor muscle. J Physiol 550: 563–574, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.VanTeeffelen JW, Segal SS. Rapid dilation of arterioles with single contraction of hamster skeletal muscle. Am J Physiol Heart Circ Physiol 290: H119–H127, 2006. [DOI] [PubMed] [Google Scholar]
- 489.Vatner SF, Franklin D, Van Citters RL, Braunwald E. Effects of carotid sinus nerve stimulation on blood-flow distribution in conscious dogs at rest and during exercise. Circ Res 27: 495–503, 1970. [DOI] [PubMed] [Google Scholar]
- 490.Verhaeghe RH, Shepherd JT. Effect of nitroprusside on smooth muscle and adrenergic nerve terminals in isolated blood vessels. J Pharmacol Exp Ther 199: 269–277, 1976. [PubMed] [Google Scholar]
- 491.Verhaeghe RH, Vanhoutte PM, Shepherd JT. Inhibition of sympathetic neurotransmission in canine blood vessels by adenosine and adenine nucleotides. Circ Res 40: 208–215, 1977. [DOI] [PubMed] [Google Scholar]
- 492.Victor RG, Seals DR, Mark AL. Differential control of heart rate and sympathetic nerve activity during dynamic exercise. Insight from intraneural recordings in humans. J Clin Invest 79: 508–516, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Vogiatzis I, Athanasopoulos D, Habazettl H, Kuebler WM, Wagner H, Roussos C, Wagner PD, Zakynthinos S. Intercostal muscle blood flow limitation in athletes during maximal exercise. J Physiol 587: 3665–3677, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Vongpatanasin W, Wang Z, Arbique D, Arbique G, Adams-Huet B, Mitchell JH, Victor RG, Thomas GD. Functional sympatholysis is impaired in hypertensive humans. J Physiol 589: 1209–1220, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Wahren J, Jorfeldt L. Determination of leg blood flow during exercise in man: an indicator-dilution technique based on femoral venous dye infusion. Clin Sci Mol Med 45: 135–146, 1973. [DOI] [PubMed] [Google Scholar]
- 496.Walgenbach SC, Donald DE. Inhibition by carotid baroreflex of exercise-induced increases in arterial pressure. Circ Res 52: 253–262, 1983. [DOI] [PubMed] [Google Scholar]
- 497.Walgenbach SC, Shepherd JT. Role of arterial and cardiopulmonary mechanoreceptors in the regulation of arterial pressure during rest and exercise in conscious dogs. Proc Mayo Clinic 59: 467–475, 1984. [DOI] [PubMed] [Google Scholar]
- 498.Walker KL, Saunders NR, Jensen D, Kuk JL, Wong SL, Pyke KE, Dwyer EM, Tschakovsky ME. Do vasoregulatory mechanisms in exercising human muscle compensate for changes in arterial perfusion pressure? Am J Physiol Heart Circ Physiol 293: H2928–H2936, 2007. [DOI] [PubMed] [Google Scholar]
- 499.Walker R, Hill K. Modeling growth and senescence in physical performance among the ache of eastern Paraguay. Am J Hum Biol 15: 196–208, 2003. [DOI] [PubMed] [Google Scholar]
- 500.Walloe L, Wesche J. Time course and magnitude of blood flow changes in the human quadriceps muscles during and following rhythmic exercise. J Physiol 405: 257–273, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Weibel ER. The Pathway for Oxygen. Structure and Function in the Mammalian Respiratory System. Cambridge, MA: Harvard Univ. Press, 1984. [Google Scholar]
- 502.Weibel ER, Taylor CR, Hoppeler H. The concept of symmorphosis: a testable hypothesis of structure-function relationship. Proc Natl Acad Sci USA 88: 10357–10361, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Weiskopf RB, Viele MK, Feiner J, Kelley S, Lieberman J, Noorani M, Leung JM, Fisher DM, Murray WR, Toy P, Moore MA. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA 279: 217–221, 1998. [DOI] [PubMed] [Google Scholar]
- 504.Welch HG, Bonde-Petersen F, Graham T, Klausen K, Secher N. Effects of hyperoxia on leg blood flow and metabolism during exercise. J Appl Physiol 42: 385–390, 1977. [DOI] [PubMed] [Google Scholar]
- 505.Welsh DG, Segal SS. Coactivation of resistance vessels and muscle fibers with acetylcholine release from motor nerves. Am J Physiol Heart Circ Physiol 273: H156–H163, 1997. [DOI] [PubMed] [Google Scholar]
- 506.Welsh DG, Segal SS. Endothelial and smooth muscle cell conduction in arterioles controlling blood flow. Am J Physiol Heart Circ Physiol 274: H178–H186, 1998. [DOI] [PubMed] [Google Scholar]
- 507.Welsh DG, Segal SS. Muscle length directs sympathetic nerve activity and vasomotor tone in resistance vessels of hamster retractor. Circ Res 79: 551–559, 1996. [DOI] [PubMed] [Google Scholar]
- 508.Whitelaw GP, Kinsey D, Smithwick RH. Factors influencing the choice of treatment in essential hypertension. Surgical, medical or a combination of both. Am J Surg 107: 220–231, 1964. [DOI] [PubMed] [Google Scholar]
- 509.Wilkins BW, Pike TL, Martin EA, Curry TB, Ceridon ML, Joyner MJ. Exercise intensity-dependent contribution of beta-adrenergic receptor-mediated vasodilatation in hypoxic humans. J Physiol 586: 1195–1205, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Wilkins BW, Schrage WG, Liu Z, Hancock KC, Joyner MJ. Systemic hypoxia and vasoconstrictor responsiveness in exercising human muscle. J Appl Physiol 101: 1343–1350, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Williams CA, Mudd JG, Lind AR. The forearm blood flow during intermittent hand-grip isometric exercise. Circ Res 48: I110–117, 1981. [PubMed] [Google Scholar]
- 512.Williams CA, Mudd JG, Lind AR. Sympathetic control of the forearm blood flow in man during brief isometric contractions. Eur J Appl Physiol Occup Physiol 54: 156–162, 1985. [DOI] [PubMed] [Google Scholar]
- 513.Williams DA, Segal SS. Feed artery role in blood flow control to rat hindlimb skeletal muscles. J Physiol 463: 631–646, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Wilmore JH, Stanforth PR, Gagnon J, Rice T, Mandel S, Leon AS, Rao DC, Skinner JS, Bouchard C. Cardiac output and stroke volume changes with endurance training: the HERITAGE Family Study. Med Sci Sports Exerc 33: 99–106, 2001. [DOI] [PubMed] [Google Scholar]
- 515.Wood KC, Cortese-Krott MM, Kovacic JC, Noguchi A, Liu VB, Wang X, Raghavachari N, Boehm M, Kato GJ, Kelm M, Gladwin MT. Circulating blood endothelial nitric oxide synthase contributes to the regulation of systemic blood pressure and nitrite homeostasis. Arteriosclerosis Thrombosis Vasc Biol 33: 1861–1871, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Wray DW, Donato AJ, Uberoi A, Merlone JP, Richardson RS. Onset exercise hyperaemia in humans: partitioning the contributors. J Physiol 565: 1053–1060, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Wright JR, McCloskey DI, Fitzpatrick RC. Effects of muscle perfusion pressure on fatigue and systemic arterial pressure in human subjects. J Appl Physiol 86: 845–851, 1999. [DOI] [PubMed] [Google Scholar]
- 518.Wright JR, McCloskey DI, Fitzpatrick RC. Effects of systemic arterial blood pressure on the contractile force of a human hand muscle. J Appl Physiol 88: 1390–1396, 2000. [DOI] [PubMed] [Google Scholar]
- 519.Wyss CA, Koepfli P, Mikolajczyk K, Burger C, von Schulthess GK, Kaufmann PA. Bicycle exercise stress in PET for assessment of coronary flow reserve: repeatability and comparison with adenosine stress. J Nuclear Med 44: 146–154, 2003. [PubMed] [Google Scholar]
- 520.Zardini P, West JB. Topographical distribution of ventilation in isolated lung. J Appl Physiol 21: 794–802, 1966. [DOI] [PubMed] [Google Scholar]