Abstract
Microbial communities associated with marine sponges carry out nutrient transformations essential for benthic-pelagic coupling; however, knowledge about their composition and function is still sparse. We evaluated the richness and diversity of prokaryotic assemblages associated with three high-microbial-abundance (HMA) and three low-microbial-abundance (LMA) sympatric Mediterranean sponges to address their stability and uniqueness. Moreover, to examine functionality and because an imbalance between nitrogen ingestion and excretion has been observed for some of these species, we sequenced nitrogenase genes (nifH) and measured N2 fixation. The prokaryotic communities in the two sponge types did not differ in terms of richness, but the highest diversity was found in HMA sponges. Moreover, the discrete composition of the communities in the two sponge types relative to that in the surrounding seawater indicated that horizontal transmission and vertical transmission affect the microbiomes associated with the two sponge categories. nifH genes were found in all LMA species and sporadically in one HMA species, and about half of the nifH gene sequences were common between the different sponge species and were also found in the surrounding water, suggesting horizontal transmission. 15N2-enriched incubations showed that N2 fixation was measurable in the water but was not associated with the sponges. Also, the analysis of the isotopic ratio of 15N to 14N in sponge tissue indicated that N2 fixation is not an important source of nitrogen in these Mediterranean sponges. Overall, our results suggest that compositional and functional features differ between the prokaryotic communities associated with HMA and LMA sponges, which may affect sponge ecology.
INTRODUCTION
Marine sponges are sessile filter-feeder metazoans that dominate many hard-bottom benthic substrates around the world (1, 2). They filter large volumes of water and efficiently clear particles down to less than 2 μm in size (see reference 3 and references therein). Sponges host complex microbial communities that are generally specific, stable, and distinct in composition compared with the characteristics of the microbes in the surrounding seawater (4–7). Their acquisition can be through horizontal transmission from the surrounding water or by vertical transmission, where microbes are transferred from parental sponges to progeny (6, 8).
Sponge-associated microbes can supply limiting nutrients to the host and process metabolic waste from the host (4, 9, 10), but the rich taxonomy and functional genetic potential of the sponge-associated microbes suggest that they carry out additional functions (11). Sponges have been categorized as those with a high microbial abundance (HMA) and those with a low microbial abundance (LMA) (12), where HMA species harbor dense, diverse microbial communities and LMA species contain fewer and less diverse microorganisms (6, 13–15). Despite their distinct microbial compositions, HMA and LMA sponges show similar functional gene repertoires, which has been interpreted to be a functional convergence between the two microbiome types (10, 11, 16).
A previous study on sympatric LMA and HMA Mediterranean sponges showed a lack of balance between particulate organic nitrogen (PON) ingestion and dissolved inorganic nitrogen (DIN) excretion that could be explained only by the activity of sponge-associated microbes (17). Interestingly, it was shown that even though the amoA gene was present in both HMA and LMA species, a net excretion of nitrite and nitrate was measurable only in HMA sponges. In order to account for imbalances between PON and DIN inventories, N2 fixation has been proposed to be an important nitrogen source (17, 18). Reports on sponge-associated N2 fixation are, however, scarce and to our knowledge are constrained to a few studies of tropical sponges (19–21). In addition, diverse nifH genes, encoding nitrogenase reductase, of putative N2-fixing bacteria (diazotrophs) were found to be associated with two sponges from the Florida Keys, indicating that N2 fixation by sponge symbionts could be an important but neglected source of nitrogen in coral reefs (22, 23). The distribution and importance of N2 fixation in marine sponges are, however, far from resolved, and in particular, information from temperate marine regions is lacking.
In the present study, we expanded previous studies on the diversity and modes of acquisition of the microbes associated with HMA and LMA sponges by adding a temporal scale and asked the following questions: (i) are nifH genes present in HMA and LMA sponges, and if so, is the putative diazotrophy mediated by the same microbial groups in different sponge species? (ii) Is N2 fixation a significant source of nitrogen for marine sponges? To address these questions, we combined 16S rRNA and nifH gene sequencing with measurements of N2 fixation in sponges and surrounding water from the temperate Mediterranean Sea.
MATERIALS AND METHODS
Sample collection.
In May, August, and November 2009 and in February 2010, scuba divers collected two specimens of each of six sympatric sponge species from off the Montgri Coast (northwest Mediterranean Sea, 42°3′N, 3°13′E) at a 15-m depth. Three species were previously classified as HMA species (Agelas oroides, Chondrosia reniformis, and Petrosia ficiformis), and three were classified as LMA species (Dysidea avara, Axinella damicornis, Spirastrella cunctatrix) (3, 10, 24). The target species belong to six different orders and are dominant species at the study site. To remove food microbes or loosely associated microbes from the sponges, they were maintained in filtered (pore size, 0.2 μm) water in the dark for 4 to 5 h, before being frozen in liquid nitrogen. In parallel with sponge collection, 2 water samples (4 liters each) were collected from a location 2 m above the sponge community. Subsamples of 300 to 500 ml from each 4-liter sample were filtered in the laboratory through 0.2-μm-pore-size polycarbonate filters (Nuclepore, VWR, Barcelona, Spain), and the filters were then frozen in liquid nitrogen. The sponges and filters were stored at −80°C until DNA extraction.
DNA extraction.
Eight filters (4 months × 2 replicates) and 48 sponge tissue samples (∼2 mm3; 4 months × 6 species × 2 replicates) were dissected into small sections using a sterile scalpel. DNA was extracted using a DNeasy tissue kit (Qiagen, Valencia, CA) after incubation with lysis buffer overnight and a 100-μl elution volume (10).
Prokaryote composition in sponges.
To gain insight into the community composition of the prokaryotes associated with sponge tissue, 16S rRNA genes were PCR amplified using primers 515f and 806r (25) complemented with sample-specific bar codes for paired-end Illumina sequencing and MyTaq Red chemicals (Bioline). All samples were amplified in triplicate, quality checked (1% agarose), pooled, and purified with an Agencourt AMPure XP system (Beckman Coulter). The DNA concentrations were then quantified (PicoGreen; Invitrogen), and the samples were pooled in equimolar amounts and sequenced on a MiSeq Illumina platform at the Berlin Genome Center. The sequences were analyzed using mothur (v.1.32.0) software (26) according to the online protocol (http://www.mothur.org/wiki/MiSeq_SOP). It was checked that the paired sequences overlap correctly and also that there were no more than 7 nucleotides of the same type behind each other. Ambiguous base calls were excluded, and the sequences were also checked for the correct length. Singletons were removed from the data set, and sequences were clustered with a 97% threshold into operational taxonomic units (OTUs) using the alignment tool from ARB Silva (www.arb-silva.de), which was also used for the taxonomic assignments. Taxonomies were then verified and corrected (if required) using the tools of the Ribosomal Database Project (RDP) (http://rdp.cme.msu.edu). The normalized OTU table resulting from the analysis in mothur was used in Primer 6 software to calculate similarity matrices, for an analysis of similarity (ANOSIM), and for similarity percentage (SIMPER) analysis. Data were then visualized by nonmetric multidimensional scaling (MDS). A phylogenetic tree for the OTUs containing at least 50 sequences was constructed using the FastTree (http://www.microbesonline.org/fasttree/) and iTOL (http://itol.embl.de/) programs.
Analysis of community richness and diversity.
The indices were normalized on data sets subsampled to the least number of sequences obtained per sample (621 sequences). Richness, referring to the number of OTUs (species), was estimated by use of the Chao1 index and the estimated number of OTUs to yield a coverage of 100%. Diversity, which refers to species richness and evenness (the relative species abundances), was estimated from the number of OTUs needed to achieve an average similarity of 50% and by use of the Shannon and Simpson indices.
Composition of putative diazotrophs.
nifH genes were amplified using a nested PCR approach with primers nifH3 and nifH4 (27), followed by primers nifH1 and nifH2 (28). The PCRs were conducted with Pure Taq Ready-To-Go PCR beads (GE Healthcare), and negative control reactions were performed with pure water (Sigma). The PCR reagents were mixed in a UV-treated bench (VWR), and the DNA template was added in a separate UV-treated bench. For the initial PCR, 1 μl of a 1:10 dilution of the extracted DNA was used as the template, and 1 μl of the PCR product was transferred to the next PCR mixture. PCR products were gel purified (E.Z.N.A. gel extraction kit), cloned (TOPO TA cloning kit; Invitrogen), and sequenced commercially (GATC Biotech). A total of 451 nifH sequences were obtained; the average was 14 sequences per sample, and the range was 2 to 31 sequences per sample. For the negative-control samples, no bands were visible. Nevertheless, a gel slice with a product of the expected size (∼359 bp) was excised, and the product was purified and cloned. No nifH sequences were obtained from these negative controls.
For the obtained sequences, vector sequences were removed, and the sequences were loaded by use of ARB Project (www.arb-home.de) software into the Zehr lab nifH database (www.es.ucsc.edu/∼wwwzehr/research/database/), which was updated in April 2014. The sequences were translated and aligned in ARB Project software and added to the existing nifH phylogenetic tree from the database using the quick-add method with the maximum parsimony algorithm. Closely related sequences and sequences from this study were exported, and a phylogenetic tree was calculated using FastTree (29). The abundance patterns of the OTUs (sequences with ≥97% similarity) were used in Primer 6 (Primer-E Ltd.) to calculate the similarity matrices and for ANOSIM. The data were then visualized by MDS.
N2 fixation associated with sponges.
In July 2013, six specimens of each of the 3 HMA species and 3 LMA species were collected by scuba divers from the location and at the depth given above and transported to the laboratory. A piece from each sponge was immediately frozen, and the sponges were acclimatized in 125-liter aquaria with running seawater for 24 h. The sponges and seawater were then incubated in 250-ml Pyrex bottles submerged in flowing seawater at the in situ temperature and with the in situ day-night light cycle for 24 h. One set of 24 bottles [(6 bottles with sponge species + 1 bottle with seawater + 1 bottle with filtered seawater blank) × 3 replicates] was incubated with in situ water amended with 10 ml 15N2-enriched artificial seawater (see below). Incubations with seawater only were used to measure the planktonic N2 fixation rates. Incubations with filtered (pore size, 0.2 μm) seawater served as water blanks. As controls we used the same setup of 24 bottles, but incubation was with air-enriched seawater instead of 15N2-enriched seawater (see Table S1 in the supplemental material). To measure the natural abundance of 15N2 of the plankton, duplicate 200-ml samples were obtained from the mixture of water and 15N2-air-enriched seawater prior to incubation, filtered through a 0.2-μm-pore-size Nuclepore filter, and frozen at −80°C. 15N2-enriched water was made from artificial seawater (Cold Spring Harbor protocols), where 10 ml 15N2 gas (lot number MBBB0968V; Aldrich, Madrid, Spain) or air was added per 1 liter through a septum using a gas-tight syringe. The bottles were inverted 100 times and maintained for 24 h, at which time the bubble was no longer visible. This procedure should ensure 90 to 100% tracer equilibration (30). Ten milliliters enriched artificial seawater was then added to each 250-ml Pyrex bottle containing sponge material, and the mixture was incubated for 24 h.
To verify that the sponges were actively pumping, picoplankton (mainly Synechococcus sp.) abundance and inorganic nutrients were measured at the beginning and end of the incubation. For picoplankton counts, triplicate 2-ml water samples were fixed with 1% paraformaldehyde and 0.05% glutaraldehyde (final concentrations), frozen in liquid nitrogen, and stored at −80°C. Flow cytometry (FACSCalibur flow cytometer [Becton Dickinson] with a 488-nm excitation laser) and cell autofluorescence were used to enumerate the Synechococcus spp. as described by Gasol and Moran (31). Gating was done using Becton Dickinson CellQuest software. Samples for nutrient analysis were collected in acid-rinsed 50-ml plastic bottles and frozen. The levels of NH4+ (ammonium), NO3− and NO2− (nitrate plus nitrite, NOx−), and PO43− (phosphate) in the samples were measured with an Alliance autoanalyzer as described by Grasshoff et al. (32). The remaining ca. 230 ml from the incubation bottles was filtered through a precombusted Whatman GF/F filter. The filters and the sponges were kept frozen at −80°C until isotopic analysis (see Table S1 in the supplemental material).
Isotopic analysis.
The 15N/14N ratio (d15N) was calculated. It was measured for water samples (filters) and sponge tissue (see Table S1 in the supplemental material). The sponges and filters were dried in a lyophilizer, and the sponge tissue was then ground with a ceramic mortar and pestle until it was homogeneous. Tissue samples of ca. 2 mg and the filters were then analyzed in a Flash EA 1112 elemental analyzer (Thermo Fisher Scientific) coupled to a Delta C isotope ratio mass spectrometer through a Conflo III interface. Acetanilide of known isotopic composition was used as a reference.
Nucleotide sequence accession numbers.
16S rRNA gene sequences are available under NCBI BioProject accession number PRJNA269111 and BioSample accession numbers SAMN03252029 to SAMN03252084. nifH gene sequences are available from the GenBank database under accession numbers KP259999 to KP260449.
RESULTS
Prokaryotic community composition based on 16S rRNA gene sequencing.
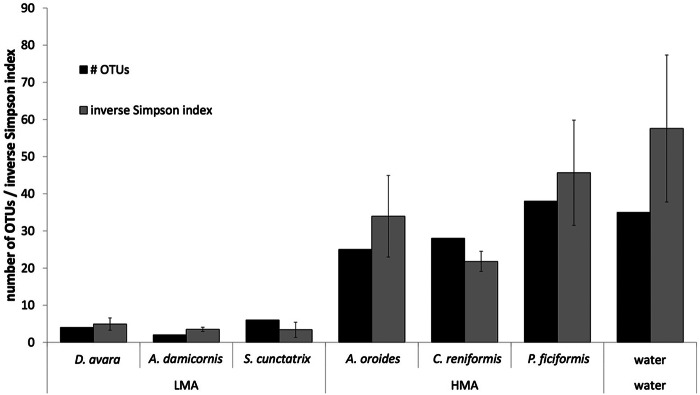
Quality filtering of 134,258 paired-end Illumina reads retained 131,555 high-quality sequences from the sponge and seawater samples. The normalization to 621 sequences resulted in average richness coverage rates of 87% and 90 to 98% in seawater and sponges, respectively. The estimated number of OTUs yielding a coverage of 100% did not differ significantly between the HMA and LMA sponges (Welch's t test, P = 0.522; Table 1). All sponge samples showed a lower species richness than the water samples according to the Chao1 index. There was no difference in richness between HMA and LMA species (Welch's t test, P = 0.536; Table 1). As for diversity, the number of OTUs needed to achieve an average similarity of 50% per sponge species differed between the HMA and LMA sponges (Welch's t test, P = 0.015, Fig. 1). Likewise, the alpha diversity differed between the HMA and LMA species, as calculated by the inverse Simpson index (Welch's t test, P = 0.049; Fig. 1) and the Shannon index (Welch's test, P < 0.001; Table 1), showing that the HMA species and water hosted a consistently higher diversity of microbial species than the LMA species.
TABLE 1.
Estimated number of OTUs yielding a coverage of 100% and real coverage of subsampled 16S rRNA gene sequences, richness (Chao1 index), and Shannon-Wiener index of diversity per sponge species and seawater samplea
| Sample | Species | No. of OTUs | Coverage (%) | Chao1 index | H |
|---|---|---|---|---|---|
| LMA sponges | D. avara | 586 ± 161 | 92 ± 2 | 505 ± 152 | 2.44 ± 0.29 |
| A. damicornis | 200 ± 99 | 98 ± 1 | 229 ± 85 | 1.80 ± 0.17 | |
| S. cunctatrix | 679 ± 254 | 90 ± 4 | 628 ± 229 | 2.32 ± 0.92 | |
| HMA sponges | A. oroides | 521 ± 275 | 91 ± 3 | 483 ± 179 | 4.33 ± 0.40 |
| C. reniformis | 221 ± 75 | 97 ± 0.5 | 283 ± 69 | 3.69 ± 0.15 | |
| P. ficiformis | 370 ± 164 | 93 ± 0.5 | 439 ± 117 | 4.62 ± 0.16 | |
| Seawater | 1,443 ± 583 | 87 ± 3 | 1,000 ± 164 | 5.10 ± 0.34 |
Averages ± standard deviations (n = 8) are given. LMA, low microbial abundance; HMA, high microbial abundance; H, Shannon-Wiener index of diversity.
FIG 1.
Number of 16S rRNA gene-based OTUs contributing to 50% of the average similarity per sponge species or water calculated by SIMPER analysis and by use of the inverse Simpson index as a proxy for alpha diversity. Error bars show standard deviations (n = 8; i.e., duplicates from 4 months).
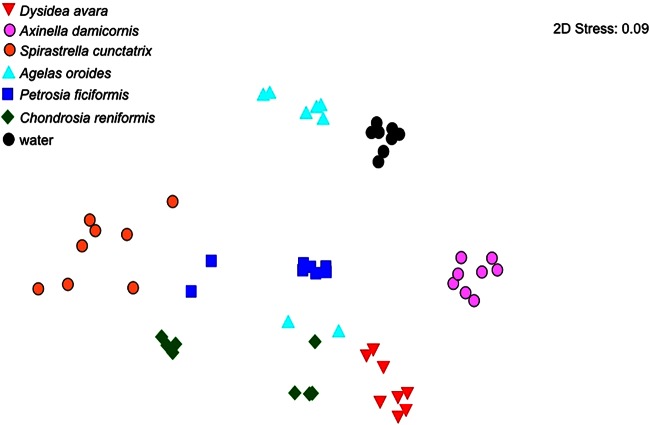
The prokaryotic community composition was compared along a temporal scale by analyzing the samples collected in May, August, November, and February. An ANOSIM between water and the two sponge types (HMA and LMA sponges) showed significant differences between the sponges (HMA and LMA sponges) and seawater, between HMA and LMA sponges, and between each group and seawater (Table 2). When all samples are considered, the month of collection did not affect the community composition (ANOSIM, R = 0.032; P = 0.845; Table 2). Thus, the community composition of the same sponge species sampled in different months clustered more closely than that of different sponge species; the community composition of single sponge species differed significantly from that of water (Table 3). Despite the lack of a temporal effect, MDS showed that the sequences of the HMA species from a particular month clustered separately from the sequences of the HMA species from other months, i.e., May for P. ficiformis, February for A. oroides, and May and August for C. reniformis (Fig. 2).
TABLE 2.
ANOSIM results per sponge type and water for 16S rRNA genes obtained by Illumina sequencinga
| Groups compared | R | P |
|---|---|---|
| Months | 0.032 | 0.846 |
| Water vs sponges | 0.141 | 0.006 |
| Sponges (including water) | 0.906 | 0.001 |
| HMA sponges vs LMA sponges | 0.220 | 0.001 |
| HMA sponges vs water | 0.306 | 0.001 |
| LMA sponges vs water | 0.282 | 0.003 |
Months, May, August, November, and February; HMA, high microbial abundance; LMA, low microbial abundance.
TABLE 3.
ANOSIM of 16S rRNA gene sequences obtained from sponge species and water samples
| Samplea | Species |
P value or R valueb |
||||||
|---|---|---|---|---|---|---|---|---|
| LMA |
HMA |
Water | ||||||
| D. avara | A. damicornis | S. cunctatrix | A. oroides | C. reniformis | P. ficiformis | |||
| LMA sponges | D. avara | 1.000 | 1.000 | 0.891 | 0.694 | 0.889 | 1.000 | |
| A. damicornis | 0.002 | 1.000 | 0.981 | 0.989 | 0.798 | 1.000 | ||
| S. cunctatrix | 0.001 | 0.002 | 0.990 | 0.705 | 0.897 | 1.000 | ||
| HMA sponges | A. oroides | 0.001 | 0.001 | 0.001 | 0.844 | 0.836 | 0.733 | |
| C. reniformis | 0.002 | 0.002 | 0.001 | 0.002 | 0.470 | 1.000 | ||
| P. ficiformis | 0.001 | 0.002 | 0.001 | 0.001 | 0.008 | 0.977 | ||
| Water | 0.001 | 0.001 | 0.002 | 0.001 | 0.001 | 0.002 | ||
HMA, high microbial abundance; LMA, low microbial abundance.
P values appear below the diagonal space, and R values appear above the diagonal space.
FIG 2.
Nonmetric MDS plot of the similarity of the 16S rRNA gene composition in samples from sponges and water on four sampling dates. The 8 symbols per sample represent 2 replicates and 4 months. 2D, two dimensional.
Most of the dominant OTUs containing at least 50 sequences represented Proteobacteria, primarily Alpha- and Gammaproteobacteria, followed by Bacteroidetes and Actinobacteria (Fig. 3; see also Fig. S1 in the supplemental material). Archaea, Chloroflexi, and Cyanobacteria were also present (see Fig. S1 in the supplemental material). Only one dominant OTU was represented in all sponge species but not in the water, and none was exclusively found in water. Twenty-four percent of the dominant OTUs were found only in one sponge species, and 8% were shared with water (Fig. 3; see also Fig. S2 in the supplemental material). Most (70%) of the dominant OTUs were found in 2 to 5 species, and 40% of these were exclusive to sponges (Fig. 3; see also Fig. S2 in the supplemental material).
FIG 3.
Phylogenetic tree including the 16S rRNA gene sequences representing the most abundant OTUs (>50 sequences per OTU). The presence of a 16S rRNA gene sequence in one or more samples of a sponge species or water is indicated by colored circles.
The HMA sponges had in common four dominant OTUs, which were not found in water (OTUs 24, 82, 111, and 200, representing unclassified Gammaproteobacteria, members of the Alteromonadaceae of the Gammaproteobacteria, unclassified Gammaproteobacteria, and Cyanobacteria, respectively). Three OTUs were found only in two HMA species and not in water (OTUs 67, 75, and 161, representing unclassified Bacteroidetes, unclassified Acidobacteria, and Planctomycetes, respectively; Fig. 3). Each of the HMA species hosted 2 or more species-specific OTUs not found in water.
The LMA sponges had five dominant OTUs in common (27, 47, 65, 83, and 546, representing Alpha- and Betaproteobacteria; Fig. 3), but they were also found in the water samples. Two of the LMA species (S. cunctatrix and A. damicornis) hosted two or more specific OTUs not found in the water. No species-specific OTUs were found in any LMA species.
Putative diazotrophs associated with sponges and water.
The composition and temporal persistence of nifH genes in all of the sponge species and in the surrounding water were analyzed (Table 4). Only the LMA species D. avara and A. damicornis contained consistently amplifiable nifH genes; 168 and 160 nifH gene sequences were obtained, respectively. For S. cunctatrix, nifH genes could be amplified only from samples collected in August; 14 sequences were obtained. Of the HMA species, only P. ficiformis showed a weak PCR product in the samples collected in August, resulting in 3 nifH sequences. A total of 106 nifH sequences were obtained from the water samples. Whereas the rarefaction curves for the LMA samples were saturated, the curves for water samples were not (data not shown). However, the duplicate sponge samples showed some variation (Table 4).
TABLE 4.
Number of sequences from sponge and water samples within specific nifH gene subclustersa
| Sample and speciesb | No. of sequences in the following subcluster: |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1A | 1E | 1G | 1K | 3E | 3G | 3H | 3I | 3J | 3L | 3P | 3Q | 3S | 4A | 4F | |
| Water | |||||||||||||||
| May 1 | 1 | 1 | 4 | 3 | 5 | ||||||||||
| May 2 | 2 | 4 | 3 | 3 | 2 | ||||||||||
| Aug 1 | 6 | 3 | 2 | 1 | 2 | ||||||||||
| Aug 2 | 5 | 2 | 1 | 1 | 1 | 4 | |||||||||
| Nov 1 | 11 | 2 | 1 | ||||||||||||
| Nov 2 | 5 | 2 | 1 | 2 | 1 | ||||||||||
| Feb 1 | 1 | 2 | 7 | 2 | |||||||||||
| Feb 2 | 1 | 6 | 3 | 3 | |||||||||||
| HMA sponge, P. ficiformis | |||||||||||||||
| Aug 1 | 2 | ||||||||||||||
| Aug 2 | 1 | ||||||||||||||
| LMA sponges | |||||||||||||||
| S. cunctatrix | |||||||||||||||
| Aug 1 | 7 | ||||||||||||||
| Aug 2 | 4 | 3 | |||||||||||||
| D. avara | |||||||||||||||
| May 1 | 7 | 9 | |||||||||||||
| May 2 | 3 | 20 | 3 | ||||||||||||
| Aug 1 | 24 | 1 | 6 | ||||||||||||
| Aug 2 | 14 | ||||||||||||||
| Nov 1 | 13 | 1 | 2 | ||||||||||||
| Nov 2 | 6 | 4 | 6 | ||||||||||||
| Feb 1 | 25 | 3 | |||||||||||||
| Feb 2 | 7 | 2 | 4 | ||||||||||||
| A. damicornis | |||||||||||||||
| May 1 | 4 | 1 | 3 | 6 | 7 | 4 | |||||||||
| May 2 | 2 | 3 | 4 | 17 | |||||||||||
| Aug 1 | 2 | 1 | 19 | ||||||||||||
| Aug 2 | 1 | 6 | 14 | 4 | 1 | ||||||||||
| Nov 1 | 2 | 9 | |||||||||||||
| Nov 2 | 17 | 1 | 3 | ||||||||||||
| Feb1 | 15 | ||||||||||||||
| Feb 2 | 9 | 1 | 7 | 4 | 1 | ||||||||||
The subclusters are defined in Fig. 4.
Duplicates for each sample (indicated by 1 and 2) are shown. HMA, high microbial abundance; LMA, low microbial abundance.
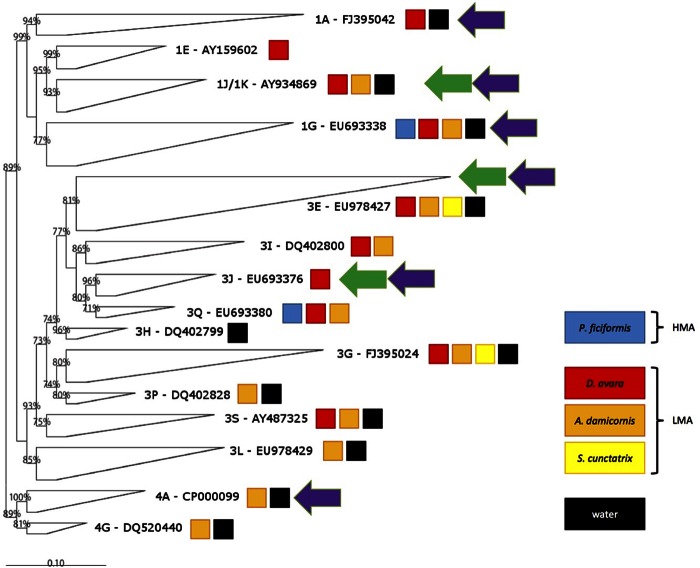
The nifH sequences obtained fell into canonical clusters I, III, and IV, including Proteobacteria and Cyanobacteria, anaerobic bacteria, and diverse nifH homologs found in methanogens, respectively (33, 34). Most of the sponge samples contained nifH sequences in more than one subcluster (Fig. 4). Only subcluster 3G contained sequences from the three LMA species and water. Certain subclusters included sequences from water and some sponge species: subclusters 1G, 1K, 3E, and 3S contained sequences from water, D. avara, and A. damicornis. Subcluster 1A contained sequences from water and D. avara, and subclusters 3L, 3P, 4A, and 4F contained sequences from water and A. damicornis (Table 4). Only 4 of the 15 subclusters were specific for sponges: subclusters 1E and 3J were specific for D. avara, and subclusters 3I and 3Q were specific for D. avara and A. damicornis. Subcluster 3H contained only sequences from water (Table 4).
FIG 4.
Phylogenetic tree (calculated on the basis of deduced amino acid sequences) of the nifH sequences obtained. The GenBank accession number of a sequence representing the closest relative to our sequences is provided for each subcluster. The colored boxes show the presence of sequences from the sampled sponge species and from the water in the subcluster. Green and purple arrows, subclusters also containing sequences from Caribbean sponges from references 48 and 23, respectively.
ANOSIM of the sequence composition of sponge species and water showed significant differences (R = 0.21, P = 0.005) because of the sequences from the water column (Fig. 5). However, there were no significant differences between the sponges if water was excluded. D. avara and A. damicornis showed significant differences by a two-way ANOSIM using the sponge species and sampling months as parameters (R = 0.531, P = 0.037). Overall, significant differences between months were not observed; however, there was a difference between May and August (R = 0.185, P = 0.05) and May and February (R = 0.22, P = 0.04) when all sponge samples were considered.
FIG 5.
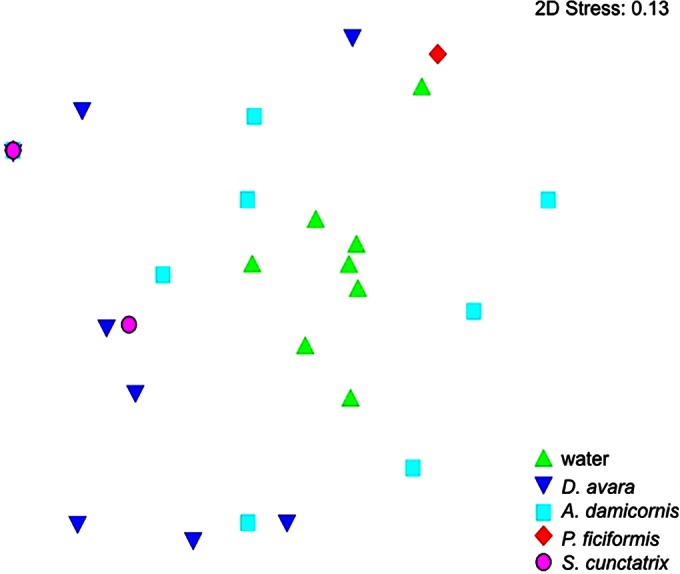
Nonmetric MDS plot based on the similarities of the nifH gene sequences obtained from water and sponge samples. The 8 symbols per sample represent 2 replicates for each 4 months; however, nifH genes were amplified in only 1 and 2 months for P. ficiformis and S. cunctatrix, respectively. (Note the overlapping of the symbols for S. cunctatrix, P. ficiformis, and A. damicornis at the left.)
Nitrogen fixation.
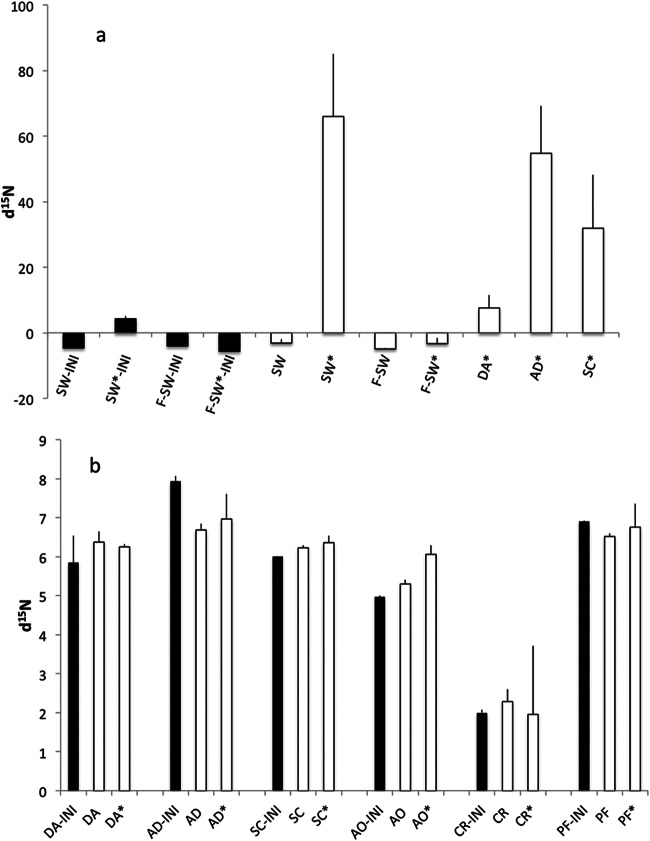
Sponge feeding was confirmed throughout the incubation by the depletion of Synechococcus cells in sponge bottles compared to the number of Synechococcus cells in control bottles (Welch's t test, P < 0.05 for all comparisons; see Fig. S3 in the supplemental material) as well as by the excretion of inorganic nutrients (Welsh's t test, P < 0.05 for all comparisons; see Fig. S4 in the supplemental material). The d15N signal in plankton (−4.6‰) before 15N2 addition (the initial value for seawater [SW-INI]) did not differ significantly from that in filtered water (−4‰ for the initial value for filtered seawater [F-SW-INI]; Fig. 6a). Addition of 15N2 produced a significant (Welsh's t test, P = 0.019) increase in the d15N signal to 4.29‰ (for the initial value for seawater under 15N2-enriched conditions [SW*-INI]) relative to that in the blank with filtered seawater (for the initial value for filtered seawater under 15N2-enriched conditions [F-SW*-INI]; Fig. 6a). After 24 h of incubation, the d15N in plankton from the bottles amended with 15N2 (seawater under 15N2-enriched conditions [SW*]; Fig. 6a) increased more than 10-fold (65‰; Welsh's t test, P < 0.0001), whereas no significant changes were detected in the plankton controls (seawater [SW]) and blanks (filtered seawater [F-SW], filtered seawater under 15N2-enriched conditions [F-SW*]). The plankton that survived the sponge incubation with 15N2-enriched water showed an increase in d15N to 7.6‰ in D. avara, which was not significantly different from the initial value of 4.3‰ for SW*-INI; however, there was a significant difference in A. damicornis (55‰; Welsh's t test, P < 0.0001) and S. cunctatrix (32‰; Welsh's t test, P = 0.006) (Fig. 6a).
FIG 6.
d15N (in parts per 1,000) for plankton (a) and sponge tissue (b). Black bars for plankton indicate the initial (INI) natural background levels in plankton in seawater (SW-INI) and filtered seawater (F-SW-INI) and d15N just after enrichment with 15N2 in seawater (SW*-INI) and filtered seawater (F-SW*-INI). For sponge tissue, black bars represent the natural d15N background level and white bars represent the d15N level after 24 h of incubation for nonenriched and 15N2-enriched conditions (*). Error bars are SEs. SW, seawater; F-SW, filtered seawater; DA, D. avara; AD, A. damicornis; SC, S. cunctatrix; AO, A. oroides; CR, C. reniformis; PF, P. ficiformis. Data are for three samples for all conditions.
The initial background level of d15N in the sponge tissue was 5‰ to 8‰ in all species except C. reniformis, which showed a significantly (Welsh's t test, P < 0.05) lower value of 2‰ (Fig. 6b). A. oroides was the only species showing an increased d15N when it was incubated with 15N2, reaching a mean value of 6‰, which was not significantly different from that for the control (Fig. 6b).
DISCUSSION
Diversity, richness, and composition of sponge-associated prokaryotes.
The use of Illumina sequencing revealed that the microbial communities associated with HMA sponges were more diverse than those associated with LMA sponges, as indicated by the Shannon and Simpson diversity indexes, emphasizing the rare and dominant OTUs, respectively. This is consistent with the findings of studies using other techniques (6, 13, 35). The Shannon diversity indexes were similar to those calculated for other LMA and HMA species from the Red Sea (36) but were slightly higher than those obtained in a previous study with the same Mediterranean sponge species (24), possibly reflecting differences in sequencing depth, as we detected 2 to 3 times higher numbers of OTUs in some of the species than the numbers detected in the previous study (24). Against expectations, we found no difference in richness between HMA and LMA sponges. While the exact reason for this is unclear, we suggest that our integration of samples over time, which included transiently associated microbes, may have contributed to the similar richness observed for the HMA and LMA sponge groups.
Our analysis of prokaryotes in sponges and the surrounding seawater showed that common features in HMA and LMA species were the existence of a specific microbial mixture composed of (i) microbes in one, a few, or most of the sponge species which were shared with the water and (ii) sponge-specific microbes which were not present in the water but which were found in single, a few, or many sponge species. The overlap between OTUs in the surrounding water and inside a sponge has been interpreted to be a sign of horizontal transmission (37), whereas sponge-specific microbes are likely an outcome of vertical transmission (6, 24, 35, 38). Interestingly, we found the highest proportion of OTUs potentially transmitted vertically in HMA sponges. In contrast, a higher proportion of the OTUs were acquired from the environment in the LMA sponges. These findings are consistent with a recent suggestion that the two acquisition mechanisms co-occur in sponges (39) but also document that the relative importance of the mechanisms differs between sponge types.
Diverse phyla dominate microbial communities associated with LMA sponges from different ocean provinces (13, 36, 40). Indeed, our results support the idea that LMA sponges host diverse prokaryotic communities without general common dominant groups at a geographical scale. The HMA sponge communities were dominated by Proteobacteria, Cyanobacteria, Bacteroidetes, Acidobacteria, and Planctomycetes. However, the phyla and subphyla which have been reported to characterize HMA species (36, 40), such as Chloroflexi and Gamma- and Alphaproteobacteria, were found but were not abundant in our samples.
On the basis of the sponge species and water samples examined, no temporal differences in microbial community compositions were found; however, the MDS representation indicated monthly changes for the three HMA species, reflecting the transient predominance of diverse phylogenetic groups. Based on the findings of an earlier study (38) and also on our findings that LMA sponges shared a higher percentage of OTUs with the surrounding seawater than HMA sponges, we anticipated a higher proportion and abundance of temporary prokaryotic groups in LMA species than in HMA species, but this was not observed. In general, the temporal variability in sponge-associated prokaryotic communities has been attributed to OTUs that are low in abundance or transient over time, whereas dominant OTUs in sponge communities are assumed to be stable through the seasons (40–42), suggesting that the sponge hosts buffer against environmental fluctuations. Our finding, however, of abundant transient groups causing monthly differences in the microbial composition in HMA species contrasts with this generalization and highlights the importance of transient horizontally transmitted microbes in HMA sponges.
Putative diazotrophs associated with sponges and water.
Symbiosis with N2-fixing prokaryotes often confers a significant ecological advantage to a host that may allow survival in otherwise marginal habitats (43). For instance, N2 fixation, conceivably carried out in anoxic zones of sponge tissue (44), may maintain the nitrogen balance in marine sponges (17, 18). Indeed, N2 fixation has been detected in tropical marine sponges (19, 20, 45–47). Since planktonic primary production is at times nitrogen limited in the Mediterranean Sea, especially during summer (48, 49), N2 fixation would presumably be advantageous and, consequently, prevalent in the microbial communities hosted by the indigenous sponges. Surprisingly, we found nifH genes in all of the LMA species but in only one of the HMA species, which had 3 sequences, although a general presence of this functional gene was expected in LMA and HMA species on the basis of data from tropical regions (23, 47, 50). Our nifH sequences fell into 15 subclusters, of which only 6 contained sequences previously found in sponges from the Caribbean. Because the number of nifH sequences that were retrieved were similar to or slightly higher than the number retrieved from tropical species, e.g., Ircinia strobilina and Mycale laxissima (23, 47), the limited overlap with nifH sequences from the Caribbean likely reflects the high degree of diversity in temperate sponges but also an underexplored effort in terms of the number of sponge species and sites. In most subclusters, the related nonsponge sequences originated from microbial mats, lake sediments, or termites.
The fact that most nifH subclusters contained sequences from sponges and water samples may indicate that those microbes are transient and non-food-related members of the sponge microbiota acquired from the water. This is based on the premise that all the sponges cleaned their filtration systems of food-microbes because they were kept alive and active in filtered seawater for 4 to 5 h before tissue was fixed for analysis. The presence of nifH sequences from different sponge species in common subclusters was in contrast to the functional convergence (different microbial groups with the same function in each sponge species) found in previous analyses of sponge-associated amoA genes (10, 16). This finding points to the existence of potential differences in the coevolution or a lack of coevolution between sponges and microbes mediating different functions. Despite overlaps between nifH genes in the water and sponges, the diazotrophic communities differed significantly due to variability in the relative abundance of nifH phylotypes and clusters in water versus sponges. The extent to which this was caused by the poor coverage of nifH gene diversity in the water samples is, however, unknown. Nevertheless, the consistent presence of nifH genes in some LMA species and the lack of nifH genes in most HMA species may indicate that LMA species actively select for particular microbial phenotypes, as was suggested above for microbial phylotypes. The fact that nifH could be amplified from only some sponge species in summertime may be indicative of a seasonal pattern driven by exhaustion of nitrogen for plankton in summer (51). However, additional data are needed to test this hypothesis.
N2 fixation in sponges.
The experimental measurement of N2 fixation in the Mediterranean sponges was performed after the detection of nifH genes in some of the species. The experiments were done in July because low ambient concentrations of inorganic nutrients are usually found at this time (51), making N2 fixation in sponges most likely. The presence of nifH genes in the LMA species was, however, not accompanied by any measurable N2 fixation. The high concentrations of oxygen and NH3 could have suppressed N2 fixation, but our measurements of contemporary planktonic N2 fixation suggest that the experimental conditions were suitable for N2 fixation. It is, however, well-known that the mere presence of a functional gene does not imply that a process will be measurable at any time or under all conditions. Indeed, N2 fixation can be ephemeral in situ (as described in reference 52) and difficult to measure even in pure cultures of diazotrophs (e.g., see reference 53), and published rates from tropical sponges have been very low (21). Hence, we cannot out rule out the possibility of some potential N2 fixation in the examined Mediterranean sponges, but our data do indicate that N2 fixation is not a major source of nitrogen, at least not in July. Consequently, the imbalances between PON and DIN inventories found for these sponge species (17) are likely not driven by N2 fixation.
d15N as an indicator of N2 fixation.
A stable isotope composition has been used to investigate nutritional sources in marine symbioses, and it has been suggested that low d15N values of about 1.5‰ may be indicative of N2 fixation (54). Therefore, sponge tissue with this level of d15N would suggest N2 fixation by the microbial symbionts (22, 46). Accordingly, the high d15N values found for LMA sponges from the Florida Keys (46) suggest no or negligible N2 fixation in these sponges, a finding which falls in line with our findings of high d15N levels and no measurable N2 fixation in the Mediterranean LMA sponges. Of the sponges examined, only the HMA species C. reniformis showed a low d15N of 2‰, potentially indicating nitrogen import through N2 fixation. The lack of nifH amplification and direct measurements of N2 fixation in this species suggest, however, that stable isotope composition is not an ideal indicator of microbial N2 fixation in sponges. Indeed, the preferential uptake of isotopically light nitrate or ammonium from the ambient water or the preferential remineralization and uptake of isotopically light nitrogen from dissolved organic nitrogen and PON sources could generate sponge d15N values similar to those produced by microbial N2 fixation (47).
Conclusions.
This first in-depth investigation of combined 16S rRNA and nifH gene richness and diversity over different months in Mediterranean sponges shows that species of HMA and LMA sponges host unique associated prokaryotic communities that differ from those in the ambient water. These sponge types host microbiota with similar levels of richness, but the diversity is distinctly higher in HMA sponges. Our findings indicate that horizontal microbial transmission co-occurs with vertical transmission in both sponge categories but that the former is prevalent in LMA species. Most nifH subclusters were shared between the sponge species and the water samples, which may suggest that those microbes are transient members of the sponge microbiota. This is consistent with the d15N values and the results of 15N incorporation assays indicating that sponge-associated N2 fixation is an insignificant and/or ephemeral source of nitrogen in the sponges of the temperate Mediterranean Sea examined.
Supplementary Material
ACKNOWLEDGMENTS
We thank Paula Lopez-Sendino, Amaia Astarloa, and Jeanett Hansen for excellent laboratory assistance and Rafel Simó and Pilar Teixidor (CCiT-UB) for advice on isotope manipulation and analysis.
This work was supported by the Spanish Ministerio de Economia y Competitividad through grants CGL2010-18466 and CGL2013-43106-R to R.C. and M.R.; grants 09-066396 and 11-105450 from the Danish Council for Independent Research, Natural Sciences, to L.R.; and a Marie Curie Fellowship from the EU (project PIEF-GA-2011-299810) to C.D. This is a contribution from the Marine Biogeochemistry and Global Change research group, funded by the Generalitat de Catalunya (Catalan Government) through grant 2014SGR1029.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01320-15.
REFERENCES
- 1.Hooper JNA, van Soest RWM. 2002. Systema Porifera: a guide to the classification of sponges. Kluwer Academic/Plenum Publishers, New York, NY. [Google Scholar]
- 2.Díaz MC, Rutzler K. 2001. Sponges: an essential component of Caribbean coral reefs. Bull Mar Sci 69:535–546. [Google Scholar]
- 3.Maldonado M, Ribes M, van Duyl FC. 2012. Nutrient fluxes through sponges: biology, budgets, and ecological implications. Adv Mar Biol 62:113–182. doi: 10.1016/B978-0-12-394283-8.00003-5. [DOI] [PubMed] [Google Scholar]
- 4.Taylor MW, Radax R, Steger D, Wagner M. 2007. Sponge-associated microorganisms: evolution, ecology, and biotechnological potential. Microbiol Mol Biol Rev 71:295–347. doi: 10.1128/MMBR.00040-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee OO, Wang Y, Yang J, Lafi FF, Al-Suwailem A, Qian P-Y. 2011. Pyrosequencing reveals highly diverse and species-specific microbial communities in sponges from the Red Sea. ISME J 5:650–664. doi: 10.1038/ismej.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmitt S, Tsai P, Bell J, Fromont J. 2012. Assessing the complex sponge microbiota: core, variable and species-specific bacterial communities in marine sponges. ISME J 6:564–576. doi: 10.1038/ismej.2011.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Easson CG, Thacker RW. 2014. Phylogenetic signal in the community structure of host-specific microbiomes of tropical marine sponges. Front Microbiol 5:532. doi: 10.3389/fmicb.2014.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steger D, Ettinger-Epstein P, Whalan S, Hentschel U, de Nys R, Wagner M, Taylor MW. 2008. Diversity and mode of transmission of ammonia-oxidizing archaea in marine sponges. Environ Microbiol 10:1087–1094. doi: 10.1111/j.1462-2920.2007.01515.x. [DOI] [PubMed] [Google Scholar]
- 9.Siegl A, Bayer K, Kozytska S, Hentschel U, Schmitt S. 2008. Sponges and microbes—new frontiers in ancient symbiosis. Vie Milieu 58:165–174. [Google Scholar]
- 10.Ribes M, Jiménez E, Yahel G, López-Sendino P, Diez B, Massana R, Sharp JH, Coma R. 2012. Functional convergence of microbes associated with temperate marine sponges. Environ Microbiol 14:1224–1239. doi: 10.1111/j.1462-2920.2012.02701.x. [DOI] [PubMed] [Google Scholar]
- 11.Bayer K, Moitinho-Silva L, Brümmer F, Cannistraci CV, Ravasi T, Hentschel U. 2014. GeoChip-based insights into the microbial functional gene repertoire of marine sponges (high microbial abundance, low microbial abundance) and seawater. FEMS Microbiol Ecol 90:832–843. doi: 10.1111/1574-6941.12441. [DOI] [PubMed] [Google Scholar]
- 12.Hentschel U, Usher KM, Taylor MW. 2006. Marine sponges as microbial fermenters. FEMS Microbiol Ecol 55:167–177. doi: 10.1111/j.1574-6941.2005.00046.x. [DOI] [PubMed] [Google Scholar]
- 13.Giles EC, Kamke J, Moitinho-Silva L, Taylor MW, Hentschel U, Ravasi T, Schmitt S. 2013. Bacterial community profiles in low microbial abundance sponges. FEMS Microbiol Ecol 83:232–241. doi: 10.1111/j.1574-6941.2012.01467.x. [DOI] [PubMed] [Google Scholar]
- 14.Erwin PM, Olson JB, Thacker RW. 2011. Phylogenetic diversity, host-specificity and community profiling of sponge-associated bacteria in the northern Gulf of Mexico. PLoS One 6:e26806. doi: 10.1371/journal.pone.0026806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gloeckner V, Wehrl M, Moitinho-Silva L, Gernert C, Schupp P, Pawlik JR, Lindquist NL, Erpenbeck D, Wörheide G, Hentschel U. 2014. The HMA-LMA dichotomy revisited: an electron microscopical survey of 56 sponge species. Biol Bull 227:78–88. [DOI] [PubMed] [Google Scholar]
- 16.Fan L, Reynolds D, Liu M, Stark M, Kjelleberg S, Webster NS, Thomas T. 2012. Functional equivalence and evolutionary convergence in complex communities of microbial sponge symbionts. Proc Natl Acad Sci U S A 109:E1878–E1887. doi: 10.1073/pnas.1203287109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiménez E, Ribes M. 2007. Sponges as a source of dissolved inorganic nitrogen: nitrification mediated by temperate sponges. Limnol Oceanogr 52:948–958. doi: 10.4319/lo.2007.52.3.0948. [DOI] [Google Scholar]
- 18.Díaz M, Ward B. 1997. Sponge-mediated nitrification in tropical benthic communities. Mar Ecol Prog Ser 156:97–107. doi: 10.3354/meps156097. [DOI] [Google Scholar]
- 19.Wilkinson C, Fay P. 1979. Nitrogen fixation in coral reef sponges with symbiotic cyanobacteria. Nature 279:527–529. doi: 10.1038/279527a0. [DOI] [Google Scholar]
- 20.Shieh W, Lin Y. 1994. Association of heterotrophic nitrogen-fixing bacteria with a marine sponge of Halichondria sp. Bull Mar Sci 54:557–564. [Google Scholar]
- 21.Southwell M. 2007. Sponge impacts on coral reef nitrogen cycling, Key Largo, Florida: PhD dissertation University of North Carolina at Chapel Hill. [Google Scholar]
- 22.Mohamed NM, Cicirelli EM, Kan J, Chen F, Fuqua C, Hill RT. 2008. Diversity and quorum-sensing signal production of Proteobacteria associated with marine sponges. Environ Microbiol 10:75–86. doi: 10.1111/j.1462-2920.2007.01431.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhang F, Vicente J, Russell TH. 2014. Temporal changes in the diazotrophic bacterial communities associated with Caribbean sponges Ircinia strobilina and Mycale laxissima. Front Microbiol 5:561. doi: 10.3389/fmicb.2014.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blanquer A, Uriz MJ, Galand PE. 2013. Removing environmental sources of variation to gain insight on symbionts vs. transient microbes in high and low microbial abundance sponges. Environ Microbiol 15:3008–3019. doi: 10.1111/1462-2920.12261. [DOI] [PubMed] [Google Scholar]
- 25.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, Gormley N, Gilbert JA, Smith G, Knight R. 2012. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 6:1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, van Horn DJ, Weber CF. 2009. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zehr JP, McReynolds LA. 1989. Use of degenerate oligonucleotides for amplification of the nifH gene from the marine cyanobacterium Trichodesmium thiebautii. Appl Environ Microbiol 55:2522–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zani S, Mellon MT, Collier JL, Zehr JP. 2000. Expression of nifH genes in natural microbial assemblages in Lake George, New York, detected by reverse transcriptase PCR. Appl Environ Microbiol 66:3119–3124. doi: 10.1128/AEM.66.7.3119-3124.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price MN, Dehal PS, Arkin AP. 2010. Fast Tree 2—approximately maximum-likelihood trees for large alignments. PLoS One 5:e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohr W, Großkopf T, Wallace DWR, LaRoche J. 2010. Methodological underestimation of oceanic nitrogen fixation rates. PLoS One 5:e12583. doi: 10.1371/journal.pone.0012583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gasol JM, Moran XAG. 1999. Effects of filtration on bacterial activity and picoplankton community structure as assessed by flow cytometry. Aquat Microb Ecol 16:251–264. doi: 10.3354/ame016251. [DOI] [Google Scholar]
- 32.Grasshoff K, Ehrhardt M, Kremling K. 1983. Methods on sea-water analysis, 2nd ed Verlag Chemie, Weinheim, Germany. [Google Scholar]
- 33.Chien YT, Zinder SH. 1996. Cloning, functional organization, transcript studies, and phylogenetic analysis of the complete nitrogenase structural genes (nifHDK2) and associated genes in the archaeon Methanosarcina barkeri 227. J Bacteriol 178:143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zehr JP, Jenkins BD, Short SM, Steward GF. 2003. Nitrogenase gene diversity and microbial community structure: a cross-system comparison. Environ Microbiol 5:539–554. doi: 10.1046/j.1462-2920.2003.00451.x. [DOI] [PubMed] [Google Scholar]
- 35.Erwin PM, López-Legentil S, González-Pech R, Turon X. 2012. A specific mix of generalists: bacterial symbionts in Mediterranean Ircinia spp. FEMS Microbiol Ecol 79:619–637. doi: 10.1111/j.1574-6941.2011.01243.x. [DOI] [PubMed] [Google Scholar]
- 36.Moitinho-Silva L, Bayer K, Cannistraci CV, Giles EC, Ryu T, Seridi L, Ravasi T, Hentschel U. 2014. Specificity and transcriptional activity of microbiota associated with low and high microbial abundance sponges from the Red Sea. Mol Ecol 23:1348–1363. doi: 10.1111/mec.12365. [DOI] [PubMed] [Google Scholar]
- 37.Webster NS, Taylor MW, Behnam F, Lücker S, Rattei T, Whalan S, Horn M, Wagner M. 2010. Deep sequencing reveals exceptional diversity and modes of transmission for bacterial sponge symbionts. Environ Microbiol 12:2070–2082. doi: 10.1111/j.1462-2920.2009.02065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bjork J, Diez-Vives C, Coma R, Ribes M, Montoya J. 2013. Specificity and temporal dynamics of complex bacteria—sponge symbiotic interactions. Ecology 94:2781–2791. doi: 10.1890/13-0557.1. [DOI] [PubMed] [Google Scholar]
- 39.Sipkema D, de Caralt S, Morillo JA, Abu Al-Soud W, Sorensen SJ, Smidt H, Uriz MJ. 2 March 2015. Similar sponge-associated bacteria can be acquired via both vertical and horizontal transmission. Environ Microbiol; doi: 10.1111/1462-2920.12827. [DOI] [PubMed] [Google Scholar]
- 40.Simister R, Taylor MW, Rogers KM, Schupp PJ, Deines P. 2013. Temporal molecular and isotopic analysis of active bacterial communities in two New Zealand sponges. FEMS Microbiol Ecol 85:195–205. doi: 10.1111/1574-6941.12109. [DOI] [PubMed] [Google Scholar]
- 41.Anderson SA, Northcote PT, Page MJ. 2010. Spatial and temporal variability of the bacterial community in different chemotypes of the New Zealand marine sponge Mycale hentscheli. FEMS Microbiol Ecol 72:328–342. doi: 10.1111/j.1574-6941.2010.00869.x. [DOI] [PubMed] [Google Scholar]
- 42.Erwin PM, Pita L, Lopez-Legentil S, Turon X. 2012. Stability of sponge-bacteria symbioses over large seasonal shifts in temperature and irradiance. Appl Environ Microbiol 78:7358–7368. doi: 10.1128/AEM.02035-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fiore CL, Jarett JK, Olson ND, Lesser MP. 2010. Nitrogen fixation and nitrogen transformations in marine symbioses. Trends Microbiol 18:455–463. doi: 10.1016/j.tim.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Hoffmann F, Radax R, Woebken D, Holtapples M, Lavik G, Rapp HT, Schläppy ML, Schleper C, Kuypers MMM. 2009. Complex nitrogen cycling in the sponge Geodia barretti. Environ Microbiol 11:2228–2243. doi: 10.1111/j.1462-2920.2009.01944.x. [DOI] [PubMed] [Google Scholar]
- 45.Wilkinson C, Summons E, Evans E. 1999. Nitrogen-fixation in symbiotic marine sponges: ecological significance and difficulties in detection. Mem Queensl Museum 44:667–673. [Google Scholar]
- 46.Weisz JB, Hentschel U, Lindquist N, Martens CS. 2007. Linking abundance and diversity of sponge-associated microbial communities to metabolic differences in host sponges. Mar Biol 152:475–483. doi: 10.1007/s00227-007-0708-y. [DOI] [Google Scholar]
- 47.Mohamed NM, Colman AS, Tal Y, Hill RT. 2008. Diversity and expression of nitrogen fixation genes in bacterial symbionts of marine sponges. Environ Microbiol 10:2910–2921. doi: 10.1111/j.1462-2920.2008.01704.x. [DOI] [PubMed] [Google Scholar]
- 48.Garcia N, Raimbault P, Gouze E, Sandroni V. 2006. Nitrogen fixation and primary production in western Mediterranean. C R Biol 329:742–750. doi: 10.1016/j.crvi.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 49.Rahav E, Herut B, Stambler N, Bar Zeev E, Mulholland MR, Berman-Frank I. 2013. Uncoupling between dinitrogen fixation and primary productivity in the eastern Mediterranean Sea. J Geophys Res Biogeosci 118:195–202. doi: 10.1002/jgrg.20023. [DOI] [Google Scholar]
- 50.Mohamed NM, Saito K, Tal Y, Hill RT. 2010. Diversity of aerobic and anaerobic ammonia-oxidizing bacteria in marine sponges. ISME J 4:38–48. doi: 10.1038/ismej.2009.84. [DOI] [PubMed] [Google Scholar]
- 51.Bahamón N, Cruzado A. 2003. Modelling nitrogen fluxes in oligotrophic environments: NW Mediterranean and NE Atlantic. Ecol Model 163:223–244. doi: 10.1016/S0304-3800(03)00007-3. [DOI] [Google Scholar]
- 52.Bentzon-Tilia M, Traving SJ, Mantikci M, Knudsen-Leerbeck H, Hansen JL, Markager S, Riemann L. 2015. Significant N2 fixation by heterotrophs, photoheterotrophs and heterocystous cyanobacteria in two temperate estuaries. ISME J 9:273–285. doi: 10.1038/ismej.2014.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Farnelid H, Harder J, Bentzon-Tilia M, Riemann L. 2014. Isolation of heterotrophic diazotrophic bacteria from estuarine surface waters. Environ Microbiol 16:3072–3082. doi: 10.1111/1462-2920.12335. [DOI] [PubMed] [Google Scholar]
- 54.Sigman D, Casciotti K. 2001. Nitrogen isotopes in the ocean, p 1884–1894. In Steele JH, Thorpe SA, Turekian KK (ed), Encyclopedia of ocean sciences. Academic Press, San Diego, CA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.