Abstract
In marine sediments cathodic oxygen reduction at the sediment surface can be coupled to anodic sulfide oxidation in deeper anoxic layers through electrical currents mediated by filamentous, multicellular bacteria of the Desulfobulbaceae family, the so-called cable bacteria. Until now, cable bacteria have only been reported from marine environments. In this study, we demonstrate that cable bacteria also occur in freshwater sediments. In a first step, homogenized sediment collected from the freshwater stream Giber Å, Denmark, was incubated in the laboratory. After 2 weeks, pH signatures and electric fields indicated electron transfer between vertically separated anodic and cathodic half-reactions. Fluorescence in situ hybridization revealed the presence of Desulfobulbaceae filaments. In addition, in situ measurements of oxygen, pH, and electric potential distributions in the waterlogged banks of Giber Å demonstrated the presence of distant electric redox coupling in naturally occurring freshwater sediment. At the same site, filamentous Desulfobulbaceae with cable bacterium morphology were found to be present. Their 16S rRNA gene sequence placed them as a distinct sister group to the known marine cable bacteria, with the genus Desulfobulbus as the closest cultured lineage. The results of the present study indicate that electric currents mediated by cable bacteria could be important for the biogeochemistry in many more environments than anticipated thus far and suggest a common evolutionary origin of the cable phenotype within Desulfobulbaceae with subsequent diversification into a freshwater and a marine lineage.
INTRODUCTION
Filamentous Deltaproteobacteria of the Desulfobulbaceae family—so-called cable bacteria (1)—mediate an electron current that links spatially separated cathodic and anodic redox reactions in marine sediments (2). This process, which invokes electron transfer across centimeter distances, was first discovered in laboratory experiments with marine sediment (3). In these experiments, long-distance electron transfer was demonstrated from (i) an otherwise unexplainable formation of a sulfide- and oxygen-free zone, (ii) the presence of a distinct pH signature consistent with proton consumption by cathodic oxygen reduction (i.e., O2 + 4H+ + 4e− → 2H2O) and proton production by anodic sulfide oxidation (i.e., H2S + 4H2O → SO42− + 10H+ + 8e−), and (iii) rapid interaction between spatially separated H2S oxidation and O2 reduction (3). Subsequent studies demonstrated that an electric field, manifested as an electric potential gradient is associated with the microbially mediated long-distance electron transport between the anodic and cathodic reactions (4–6), as predicted by geobattery and biogeobattery theories (7, 8). The electric field drives an ionic current in the pore water of the same magnitude but opposite in direction to the electron current in the cable bacteria, thus closing the electric circuit. On the basis of the initial (3) and subsequent studies, it has been found that the major anodic process in marine sediments is the oxidation of sulfides (1, 6, 9, 10), whereas the cathodic process might be the reduction of either oxygen (2, 5, 9, 11) or nitrate (5, 12). The overall electrically coupled process has been termed electrogenic sulfur oxidation (e-SOx) (9).
At present, cable bacterial filaments, 16S rRNA gene sequences similar to cable bacterial sequences, and/or geochemical hallmarks of e-SOx have been found in a variety of marine environments. These include sulfide-rich coastal sediments (1, 2, 9, 12) and salt marsh sediments (9, 13), as well as seasonally hypoxic basins, subtidal coastal mud plains, hydrothermal vent areas, cold seep flats, and mangrove sediments (9). The common traits of these environments are high rates of sulfide generation and limited bioturbation (9) in combination with fairly high porewater conductivity (salinities > 20‰).
Thus far, cable bacteria have not been reported from outside the marine environment, but e-SOx has previously been suggested as a mechanism that contributes to the rapid recycling of sulfate often observed in freshwater sediments (14), while patterns of P-mobilization and pH distribution in sulfide-rich sediments from a volcanic freshwater lake have been suggested to indicate the presence of the process (15). The presence of electric fields around contaminated groundwater plumes has also been ascribed to processes involving microbial long-distance electron transport (7, 16), although the underlying biogeochemical processes, the microorganisms, rates, and spatial scales have not been investigated.
In the present study, we directly tested for the presence of cable bacteria and electrogenic oxidation processes involving microbially mediated long-distance electron transport in freshwater sediments. We applied microscale measurements of electric fields, oxygen, pH, and sulfide to demonstrate electrogenic activity in laboratory incubations of homogenized freshwater sediments. In addition, we measured the same parameters directly in the field to demonstrate the presence of microbial electrogenic activity in situ. Although this approach allows for the detection of general electrogenic oxidation activity, it does not allow for specification of the anodic reaction involved. To which extent this includes sulfide oxidation will be discussed. Fluorescence in situ hybridization (FISH), scanning electron microscopy (SEM), and 16S rRNA gene sequencing of single filaments were used to determine the abundance, morphology, and identity of freshwater cable bacteria.
MATERIALS AND METHODS
Laboratory experiments. (i) Sediment sampling and incubation.
Surface sediments were collected from the banks of Giber Å (56°3′53.87″N, 10°10′20.27″E), a lowland, hard-water stream in Eastern Jutland, Denmark. The sampling site was located 40 m above sea level and ∼5 km from the sea. Sampling was performed in August 2013 and April 2014.
Upon return to the laboratory, the sediment was sieved through a 5-mm-pore-size sieve, homogenized under an N2 atmosphere, and transferred to a glass tube (inner diameter, 45 mm; height, 68 mm). The sediment core was then transferred to a mini-aquarium (volume, 0.36 liter) which was inserted into a 60-liter Styrofoam-insulated temperature-controlled (15.9°C) tank with unfiltered tap water. The water in the tank was gently purged with atmospheric air from an air pump and circulated within the tank via a submersed water pump. The tap water and stream water had comparable chemistry as the stream water was mainly tertiary treated domestic wastewater and groundwater from the same sources as the tap water (for tap water, 4.4 mM [HCO3−], 0.8 mM [SO42−], and a conductivity of 0.1 S m−1; for stream water, 4.8 mM [HCO3−], 0.3 mM [SO42−], and a conductivity of 0.1 S m−1).
(ii) Microsensor measurements.
Electric fields in the pore water were determined from depth profiles of electric potentials (EP) measured 2 days and 14 days after the transfer with an in-house made electric potential microelectrode (EPM) (4). The depth profiles of pH, O2 and H2S were obtained in parallel to the EP measurements, using in-house made microsensors for pH, O2, and H2S (17–19). A general-purpose reference electrode (REF201 Red Rod electrode; Radiometer Analytical, Denmark) was used as reference during the pH and EP measurements. To minimize turbulence-induced variations in tip potentials in the reference electrode, a short silicon tube closed with a paper stopper was filled with tank water and mounted on the tip of the reference electrode before it was placed in the tank. The EPM and the reference electrode were connected to a custom-made millivoltmeter with a resistance of >1014 Ω (Aarhus University, Denmark). The signal was digitized for PC-processing using a 16-bit A/D converter (ADC-216; Unisense A/S, Denmark). Microsensors for oxygen, H2S, and pH were connected to a four-channel Microsensor Multimeter (Unisense). For stepwise movement of the sensors, they were fixed in a two-dimensional microprofiling system (Unisense). The software SensorTrace PRO (Unisense) was used to control the movement of the microsensors and for logging sensor signals. Two calibration points were used for calibration of the O2 sensor: air-saturated tank water (100% air saturation) and the anoxic zone in the sediment (0% saturation). The H2S sensor was calibrated in a darkened calibration chamber containing O2-free HCl. A three-point calibration curve in the range 0 to 20 μM H2S was made by adding fixed amounts of dissolved Na2S from a ca. 10 mM stock solution. Sulfide concentrations in the calibration media were determined on Zn-acetate fixed subsamples by using the method of Cline (20). Total sulfide concentrations (ΣH2S = [H2S] + [HS−] + [S2−]) in the sediment were calculated from the pH and the H2S concentration, measured at the same depth, as described previously (17). The pH sensor was calibrated in AVS TITRINORM buffers (VWR Chemicals, Denmark) having pH values of 4.0, 7.0, 8.0, and 9.0, traceable to standard reference material from NIST (http://www.nist.gov). Since the pH sensor is a potentiometric electrode, it will capture also the EP present in the sediment, which may induce errors in the estimates of sediment pH. To correct for such an artifact, the EP profile was subtracted from the raw signal obtained with the pH sensor during profiling, and the residual values were used to calculate the pH at any depth from the calibration data obtained as described above.
(iii) Oxygen experiment.
The dependence of electric fields on O2 and hence their link to cathodic O2 reduction (5) was tested by examining the influence of O2 availability on the distribution of the electric potential in the core after 2 weeks of incubation. The mini aquarium with the core was covered with Parafilm (Sigma-Aldrich, USA) and raised so that the rim was positioned ∼2 cm above water level in the tank. The EPM, the reference electrode, the O2 sensor, and the gas exchange tubes were inserted through holes in the film. The reference electrode was furnished with a water-filled tube as described above. The O2 concentration in the overlying water was monitored continuously with the O2 sensor and controlled by flushing the overlying water and headspace with ambient air or a N2/CO2 mixture (400 ppm CO2). Depth profiles of the electric potential in the sediment were measured in the presence or absence of oxygen in the overlying water.
(iv) Cut experiment.
The presence of electron transport through a contiguous electron conductor (i.e., bacterial filaments) was tested through a cut experiment (2) that investigated EP distributions before and after a sediment cut was performed. The cut experiment was performed on the core after 2 weeks using a platinum wire (diameter, 125 μm) mounted on a turnable, two-branched fork (width, 44 mm) that was fixed in a micromanipulator. By inserting and rotating the fork manually, the sediment could be cut through horizontally without any visual disturbance of the upper part of the core. Three EP profiles were measured before and after the sediment core was cut 3 mm below the sediment surface.
(v) FISH sampling.
Samples for FISH-based identification of cable bacteria were collected with a cutoff syringe that was pushed 1 cm into the sediment. The samples were fixed in ethanol (final concentration, 50% [vol/vol]) and stored at −20°C until analysis.
In situ measurements and sampling.
In situ measurements of EP, O2, and pH were performed in October 2013 in the air-exposed waterlogged sediment 1 to 2 cm above the waterline of the Giber Å stream within a few meters from the sediment sampling location used for the laboratory experiments (Fig. 1a). A preliminary survey had indicated the presence of cable bacterial filaments here as macroscopic aggregations of particles trapped by invisible threads formed when the sediment was gently swirled around with water in a tray (Fig. 1b and c). Microsensors and setup were as described for the laboratory measurements, except that the stand for the micromanipulator was placed directly on the bank and the reference electrode was placed in the adjacent stream.
FIG 1.
Profiling with electric potential (EP) microsensor in situ and extraction of cable bacteria. (a) EP profiling in undisturbed sediment near the water edge in Giber Å. (b) After gentle washing in a tray, the sediment separates out into sand and centimeter-long rolls of litter fragments tied together by cable bacteria. (c) Tuft of cable bacteria after washing and removal of coarse litter (dark-field microscopy, ×100 magnification). (d) EP profiling after a horizontal cut ca. 3 mm below an area of 15 by 15 mm.
An in situ cut experiment was performed 1 day after the EP, O2, and pH profiling session. A set of EP profiles was measured in the sediment within a 15-by-15-mm area as described above, and then a thin nylon string that was mounted on a two-prong plastic fork was dragged horizontally ∼3 mm below the surface through the sediment area, without any visual disturbances of the surface (Fig. 1d). EP profiling was repeated within the cut area less than 5 min after the cut.
Samples for porosity determinations (n = 2) were collected in cutoff syringes near the spots where microprofiling had been performed. Porosity was determined gravimetrically in the lab. The conductivity of the sediment was determined from the conductivity of the Giber Å water and the porosity by using the formulas of Ullman and Aller (21) for muddy sediments. The conductivity of the Giber Å water was determined with a hand-held conductivity meter (SevenGo; Mettler Toledo, Denmark). The samples for identification and quantification of cable bacteria were collected in triplicate in plastic straws (inner diameter, 5 mm) at the spots where microprofiling had been performed.
Cable bacterium enumeration, identification, and SEM.
Immediately upon return to the laboratory, the plastic straws were sliced in 6-mm sections down to 18 mm. From each section, the filaments were gently separated out from coarse sediment particles as a tuft and split with one half or quarter going to immediate enumeration and the rest being preserved with ethanol.
The subsamples for filament length density determination were spread in a drop of water on microscope slides and sealed with a coverslip ensuring that all filaments were within the area of the coverslip. By dark-field and phase-contrast light microscopy the number of intercepts with filaments along randomized search lines was counted. From that and knowing the length of search lines, the area of the coverslip, and the volume of the sediment slices, the meters of filament per cubic centimeter of sediment were calculated as described before for roots (22) and Desulfobulbaceae and Thioploca filaments (2, 23). A few filaments of Beggiatoa sp., easily recognized by their sulfur inclusions, were encountered and excluded from the enumeration. The identity of the cable bacteria was confirmed by FISH on ethanol-preserved subsamples.
Samples for FISH from the lab incubations and the field were diluted 1:5 in phosphate-buffered saline, and 10 μl of this mixture was filtered onto polycarbonate membrane filters (type GTTP; pore size, 0.2 μm; Millipore). FISH and DAPI (4′,6′-diamidino-2-phenylindole) staining were performed on the filter according to previously published protocols (24) using probe DSB706 specific for the Desulfobulbaceae family (25), together with probe EUB-I-III (26) targeting almost all bacteria.
For SEM analysis tufts of filaments where transferred from the ethanol preserved sampled to silicon wafers, washed, and air dried. Essentially as described before (2), SEM of the filaments was obtained by using a NanoSEM scanning electron microscope (Nova 600 NanoSEM; FEI) operated in low-vacuum (60 Pa) and 5-kV mode.
For phylogenetic identification, single filaments were picked from sediment subsamples with glass hooks under the microscope as described previously (1, 2). The filaments were rinsed by three consecutive washes in a drop of sterile artificial seawater (salinity, 25‰, pH 8; Reef Crystal) and subsequently lysed by ultrasonic bead beating (glass bead diameter, 0.1 mm) with a Sonoplus HD2070 ultrasonic homogenizer for 3 min at a power of approximately 21 W. The extracted DNA was amplified by using a GenomePlex whole-genome amplification kit (Sigma-Aldrich). Whole-genome sequencing was performed on an Ion Torrent PGM (Life Technologies, USA) using 316v2 chips and 400-bp chemistry according to the manufacturer's protocols. Raw sequencing reads were quality trimmed using the prinseq-lite.pl script (27) with the parameters listed in Table S1 in the supplemental material. GenomePlex adapters were clipped, and reads shorter than 150 bp were removed by using Seqclean (https://sourceforge.net/projects/seqclean/). The trimmed and clipped reads were assembled using gsAssembler version 2.6 (Roche 454 Life Sciences, Branford, CT) with 10 different configurations: minimum overlap settings of 50 or 100 bp, respectively, and minimum similarity values of 96 to 100% with 1% steps. In parallel, reads were assembled using SPAdes version 3.1.1 (28). Sequences featuring a sequence identity of at least 85% compared to the 16S rRNA sequence of Desulfobulbus propionicus DSM 2032 (GenBank accession number CP002364; locus tag, Despr_R0039) were extracted from the assemblies using BLASTn (29) and a custom Perl script. Extracted sequences were aligned using the SINA online tool (30) and inspected for different ribotypes using ARB (31). All ribotypes were manually checked for chimeras using BLASTn and the GenBank 16S rRNA database (version 28 May 2015) (32). The longest representatives of each ribotype represented full-length 16S rRNA gene sequences and were used for subsequent phylogenetic analysis. The full-length 16S rRNA gene sequences were aligned using the SINA online tool and added to the Silva Release 115 SSU Ref database (33) using ARB. A phylogenetic tree was calculated based on nearly full-length sequences and marine cable bacterial reference sequences with more than 500 nucleotides, using the maximum-likelihood method (RAxML) implemented in ARB with a GTR+G substitution model, a position variability filter for Bacteria, and rapid bootstrap analysis (1,000 iterations). Pairwise sequence similarities were also calculated in ARB.
For characterizing the diversity of Desulfobulbaceae contained in the single filament data sets, trimmed Ion Torrent reads were mapped against the SILVA nonredundant 16S rRNA reference database, Release 119 using BBmap 34.94 (http://sourceforge.net/projects/bbmap/). All reads showing a sequence identity of at least 90% to a reference sequence were extracted. The extracted reads were taxonomically classified using the SILVAngs server (33). Reads classified as Desulfobulbaceae were extracted and mapped onto the retrieved Desulfobulbaceae full-length 16S rRNA gene sequences (see above) using gsMapper version 2.6 (Roche).
GenBank accession numbers.
Sequences obtained in the present study were deposited at GenBank under accession numbers KP728462 to KP728465.
RESULTS
Laboratory investigations.
At the first microsensor profiling session 2 days after the initiation of the April 2014 incubations, sulfide was present throughout the anoxic sediment column at ΣH2S concentrations between 0.5 and 2 μM (Fig. 2a). pH dropped steadily with depth, most steeply in the ∼1-mm-wide oxic zone, as expected when oxygen is consumed by noncathodic oxidation processes, such as metal oxidation, nitrification, or organotrophic microbial respiration. An electric field was present, as seen from the increase in electric potential by 6 mV with depth. As judged from the voltage gradient, the magnitude of the field was 0.31 V m−1 in the upmost part of the anoxic zone.
FIG 2.
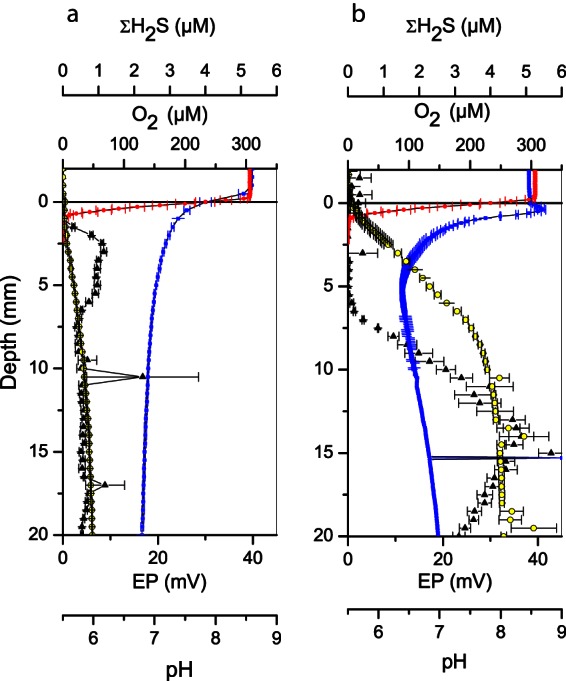
Oxygen (red), sulfide (black), pH (blue), and electric potential (yellow) profiles measured in laboratory incubated sediment 2 days (a) and 14 days (b) after transfer of homogenized sediment to glass core tubes. Error bars represent standard errors of the mean. The number of replicate profiles was three.
After 2 weeks of incubation, sulfide was no longer detectable in the top 5 to 6 mm of the anoxic zone, while ΣH2S concentrations below reached ∼5 μM (Fig. 2b). A pH peak was present in the ∼1-mm-deep oxic zone, and a pH minimum was detected in the anoxic zone near the sulfide front, indicating cathodic and anodic half-reactions, respectively. In accordance with these results, a substantial electric field had developed with a voltage range of 32 mV and a maximum strength of 3.6 V m−1 in the upper 7 mm of the core. We note here that the voltage range of the field corresponded to more than a half pH unit (the pH sensor used had a response of 54 mV per pH unit), and ignoring the interference of the electric field on the potentiometric pH sensor would have led to significant underestimation of the pH in the anoxic sediment layers and consequently to an underestimation of the concentration of ΣH2S.
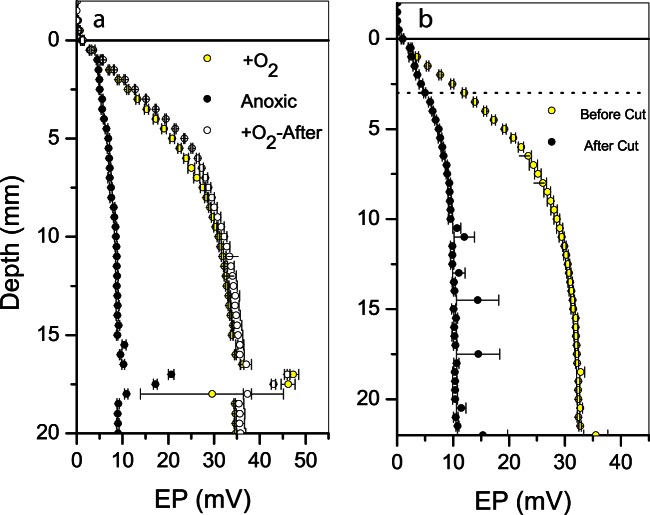
Removing oxygen from the water column led to an instant and reversible collapse of the electric field (Fig. 3a). The residual voltage gradient at anoxia was similar in magnitude to the voltage gradient observed in the initial phase of the experiment (Fig. 2a). Passing a thin wire horizontally through the sediment 3 mm below the sediment surface also led to a collapse of the electric field (Fig. 3b). The remaining EP gradient was similar in magnitude to the voltage gradient observed in the initial phase of the experiment and remained stable for at least 40 min after the cut. All of these trends were observed also in the August 2013 incubations. FISH analysis demonstrated the presence of filamentous Desulfobulbaceae in both cases (data not shown).
FIG 3.
Laboratory oxygen and cut experiments. (a) Oxygen experiment. EP profiles measured in the presence of an oxygenated water column (yellow) and EP profiles measured in the presence of an oxygen-free water column (black) are shown. EP profiles measured again 3 min after air saturation of the overlying was reestablished (white). (b) Cut experiment. The EP profile measured before the cut was performed (yellow) and the EP profile measured after passing a 125-μm-thick platinum wire horizontally through the sediment 3 mm below the sediment surface (black). The dashed line illustrates the vertical position of the cut. Error bars represent the standard errors of the mean. The number of replicate profiles at each treatment was three.
Field studies.
In situ measurements of pH and oxygen at the air-exposed banks of Giber Å revealed the presence of a distinct pH peak in the 2-mm-wide oxic zone, ∼0.15 mm below the sediment surface (Fig. 4a). The oxygen profile indicated exclusively the consumption of oxygen and thus the absence of microphytobenthic net photosynthesis, which otherwise could have generated a pH peak in the oxic zone due to CO2 fixation. Measurements of the EP distribution in the sediments showed that electric fields were present, since the electric potential increased from 1 mm below the sediment to deeper strata (Fig. 4a). The magnitude of the field was 0.75 ± 0.06 V m−1, or 20% of the maximum field strength observed in laboratory-incubated sediment. As in the laboratory, dragging a thin string horizontally through the sediment ca. 3 mm below the surface led to an almost 100% collapse of the electric field (Fig. 4b).
FIG 4.
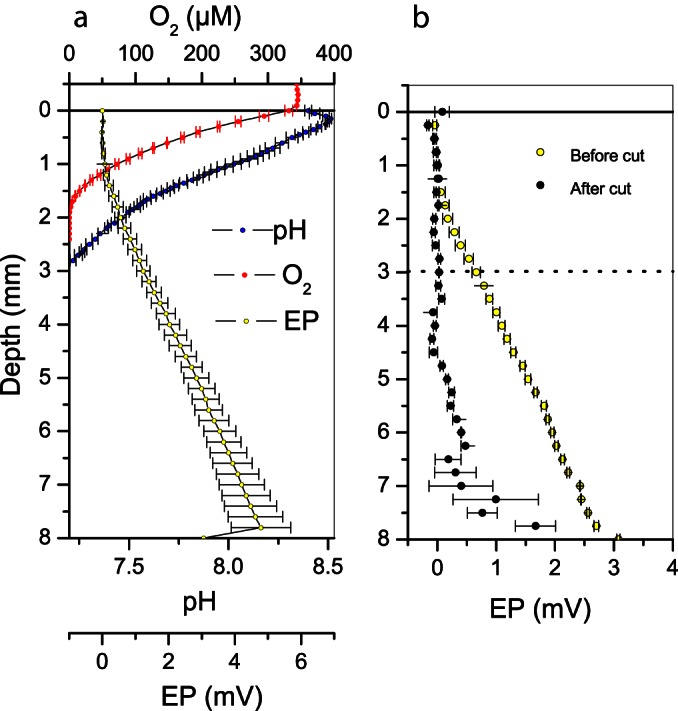
Depth distribution of EP, oxygen, and pH at the exposed bank of Giber Å. (a) In situ profiles of EP (yellow), oxygen (red), and pH (blue). (b) In situ cut experiment. EP profiles measured before the cut was performed (green), EP profiles measured after dragging a nylon string horizontally through the sediment 3 mm below the sediment surface (black). The dashed line illustrates the vertical position of the cut. Error bars represent standard errors of the mean for three replicate profiles.
Filamentous bacteria in Giber Å sediments.
Bacterial filaments were present at the field site down to at least 18 mm below the sediment surface (Fig. 5a), with maximum densities in the upper 6 mm. The depth-integrated abundance was highly variable within the area sampled: the central mean was 40.3 m cm−2 and the standard error was 23.6 m cm−2 for three replicate samples. FISH analysis of subsamples from each depth interval showed that all EUB-positive filaments had also hybridized with the DSB706 probe, meaning that all confirmed bacterial filaments belonged to the Desulfobulbaceae family. Approximately 30% of the filaments in the samples hybridized neither with the EUB-I-III probe nor with the DSB706 probe, probably because their ribosome content was too low to be detected by ordinary FISH. Either these filaments were already inactive or dead when sampled from the field site, or their ribosomes were degraded during storage. Their identity remains unknown, and in theory they could even represent filamentous Archaea. Due to these uncertainties the filament abundances obtained from light microscopy should be considered maximum estimates of viable Desulfobulbaceae filaments. The diameter of the filaments was 0.8 to 1.7 μm (mean 1.5 μm), while the length of single cells within filaments ranged between 2.7 and 5 μm (mean, 3.7 μm), according to measurements performed on FISH-positive filaments and filaments inspected by light microscopy. Ridges along the entire length of the bacterial filaments were observed with SEM (Fig. 5c) and between filaments the number of ridges varied from fewer than 28 to more than 34.
FIG 5.
Cable bacteria in Giber Å sediment. (a) Depth profile of Desulfobulbaceae filaments abundance at the exposed bank of Giber Å. (b) Filamentous Desulfobulbaceae in Giber Å sediment identified by FISH targeting 16S rRNA. The filaments appear yellow/orange from overlay with the DSB706 probe (red) and the EUB-I-III probe (green) and DAPI. A filament that did not hybridize with the DSB706 probe or the EUB-I-III probe was visualized with DAPI stain (blue). Scale bar, 10 μm. (c) Scanning electron micrograph of Desulfobulbaceae filaments from Giber Å sediment. The inset shows the joint between two neighboring cells. Scale bar, 2 μm.
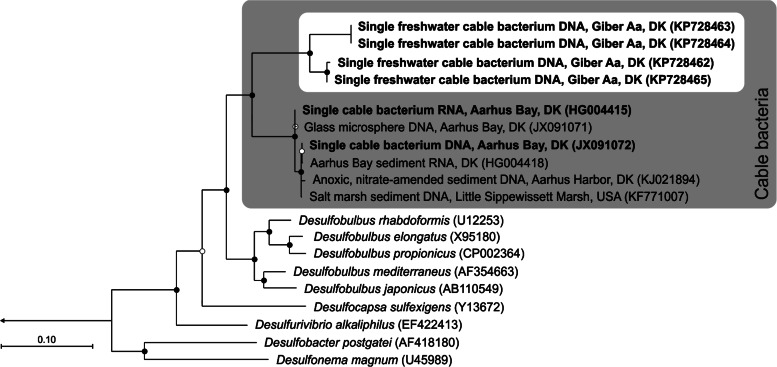
From four of the five single filaments collected, genomic DNA could be amplified, sequenced, and full-length 16S rRNA genes assembled. All four single filament data sets were strongly enriched for DNA sequences of Desulfobulbaceae (see Table S2 in the supplemental material). Only a single Desulfobulbaceae ribotype was retrieved from each of the four single filament assembly data sets. Read mapping further supported the presence of only a single Desulfobulbaceae ribotype in these data sets (see Table S2 in the supplemental material). The four freshwater cable 16S rRNA gene sequences clustered into clearly distinct groups. Two of the sequences were 100% identical and distinct (98% identity) from the other two sequences, which were 99.7% identical. Phylogenetic analysis placed the freshwater filaments as a sister group to the known marine cable bacteria, so that all cable bacteria form a monophyletic lineage, with the genus Desulfobulbus as the closest relatives (Fig. 6). Sequence similarities between freshwater and marine cable bacteria were 91.7 to 92.5% (based on a comparison of 533 positions, since there are no full-length sequences available for the marine group). Sequence similarities to the genus Desulfobulbus were 87.7 to 92.0% (based on comparison of 1,440 positions).
FIG 6.
Phylogenetic affiliation of freshwater cable bacteria based on 16S rRNA gene sequences. Maximum-likelihood tree with 1,000 bootstraps. Filled and open circles indicate bootstrap support of ≥90 and ≥50%, respectively. The tree is rooted (arrow) to Desulfovibrio desulfuricans (GenBank accession no. AF192153). The bar represents 10% estimated sequence divergence. Sequences obtained from single filaments are displayed in boldface. GenBank accession numbers are given in parentheses.
DISCUSSION
Cable bacteria and electrogenic oxidation in freshwater sediment.
The results of our study demonstrate that cable bacteria and electrogenic processes are present in natural freshwater sediment environments. The Desulfobulbaceae filaments found in sediment from Giber Å are closely affiliated and monophyletic with the marine cable bacteria (Fig. 6) and also, morphologically, the freshwater filaments show similarities to their marine counterpart, most notably a ring of ridges that runs along the length of the filament (Fig. 5c) (2, 9). Since the ridges of the marine cable bacteria show enhanced charge mobility and consist of fibers inside a continuous periplasmic space (2), they have been proposed to function as wires, enabling electron transport along the length of the bacterial filament and thereby facilitating the electrogenic oxidation process (2, 10). This hypothesis is further strengthened by the presence of similar ridges in freshwater stains. The phylogeny of the cable bacteria suggests that this mode of life has evolved once within the Desulfobulbaceae; subsequently, the cable bacteria diversified into a freshwater and a marine group. Based on 16S rRNA sequence identities of ca. 92%, the freshwater and the marine cable bacterial lineages are distinct genera (34) and are clearly distinct from the closest cultured genus, Desulfobulbus.
Signatures of electrogenic oxidation processes were found in both laboratory-incubated sediment and in situ at the exposed water-saturated banks of Giber Å. The pH peak observed in the oxic zone of laboratory incubated sediment (Fig. 2b) and in situ (Fig. 4a) in the absence of net photosynthesis is indicative for proton consumption by cathodic oxygen reduction (3), while the electric fields seen in both the laboratory cores and in situ (Fig. 2b and 4a) suggest electron transfer between vertically separated anodic and cathodic half-reactions (5–7). That the electric field was primarily associated with electrogenic processes driven by cathodic oxygen consumption via a contiguous electron conductor was confirmed by two lines of evidence: more than 90% of the electric field disappeared when (i) oxygen was removed in the laboratory incubated sediment (Fig. 3a) and (ii) when the sediment was cut below the oxic zone in situ and in the laboratory (Fig. 3b and 4b). The small residual electric fields observed were possibly contributed by diffusion potentials or streaming potentials (35, 36).
Activity and niche of freshwater cable bacteria.
The density of the cable bacteria and the electrogenic oxidation activity at the Giber Å site were very similar to the findings reported from natural marine sediments: Malkin et al. (9) reported cable bacterial length densities of 82 to 122 m cm−3 in coastal marine sediments at the North Sea, which is only 2 to 3 times greater than the ∼40 m cm−3 observed at the Giber Å site (Fig. 5a). The electron current density at the anoxic-oxic interface, a measure of electrogenic oxidation (3, 5), at the Giber Å site was 14.4 ± 2 mA m−2 when estimated as described previously (4, 5) from the electric fields (Fig. 4a) and the conductivity of the sediment (0.02 S m−1, as estimated from the sediment porosity [0.6] and the conductivity of the Giber Å water [0.1 S m−1] according to Ullman and Aller [21]). This lies well within the 5- to 30-mA m−2 range reported from the North Sea sites (9). Assuming a vertical orientation of the filaments and no curls, the filament length density at the Giber Å site corresponds to a maximum abundance of 4 × 107 filaments m−2 in the upper part of the sediment. With the given current density at the anoxic oxic interface, each filament then supports an average current of ca 0.35 nA. Filaments at the marine sites support a current in the range 0.20 to 0.36 nA according to the data reported by Malkin et al. (9) and Meysman et al. (10). These similarities suggest that freshwater sediments represent niches that are favorable for cable bacteria and electrogenic processes as the marine environment and, as a consequence, suggest that the much lower sulfate concentrations in freshwater systems and the possible higher dissipative loss of energy due to a higher ohmic resistance of the porewater are not major limitations.
That low sulfate concentration is not a major limitation for freshwater cable bacteria might at first suggest that the electrogenic oxidation process does not rely on sulfide but is based on other electron donors such as short-chain fatty acids, Fe2+, or methane. Traditionally, iron reduction and methanogenesis, and not sulfate reduction, have been supposed to prevail in freshwater sediments due to low concentrations of sulfate (e.g., <1 mM in Giber Å compared to >28 mM in seawater at salinities of 35‰), implying an excess of reduced iron and carbon compared to sulfide. However, compilations of actual measurements have shown sulfate reduction to be a major pathway for anaerobic mineralization in many types of freshwater sediments (14, 37), and rates reported from both oligotrophic and eutrophic freshwater sediments (37) seem to be sufficient to support electrogenic oxidation of sulfide at rates higher than those that can be inferred from the current density measurements at Giber Å. The current density of 14.4 ± 2 mA m−2 estimated for the Giber Å site would correspond to an oxidation of 1.6 mmol of H2S m−2 day−1 if the anodic reaction was based solely on sulfide oxidation. Sulfate reduction rates in freshwater sediments with concentrations below 0.3 mM can reach 20 mmol m−2 day−1 (14, 37).
The high rates of sulfate reduction in freshwater sediments are associated with rapid reoxidation of sulfide, with turnover times in the range of hours for the sulfate pool. Typically, sulfide oxidation mediated by iron reduction has been suggested as an important route in the anoxic zone (14). In principle, an electrogenic oxidation process involving sulfide oxidation (i.e., e-SOx) could substitute this route through its inherent generation of sulfate (6), and e-SOx could thereby be a component in an iron-independent, carbon-fueled cryptic sulfur cycle, where sulfate is reduced to sulfide by sulfate-reducing bacteria and regenerated by colocalized cable bacteria. Such an interaction would allow the occurrence of highly active, sulfide-oxidizing cable bacterium communities in low sulfate environments. Sulfur mass balance studies (6) and further studies of the metabolic capability of freshwater cable bacteria are, however, required to test this hypothesis.
That the dissipative loss of energy due to ohmic resistance of the pore water is not a major restriction for freshwater cable bacteria and electrogenic processes is evident from the electric potentials measured in the sediments. The cable bacterial sediment can be considered a “biogeobattery” (7) where cable bacteria act as both electron conductor and catalyzer of the associated anodic and cathodic reactions, while the surrounding water-saturated sediment acts as a passive electrolytic conductor with a given electric resistance. The measured electric potential differences in the sediment are then direct measures of the energy dissipation per charge unit transported in the sediment porewater. For a given current density the electric potential scales as the inverse of the electrical conductivity (7), and therefore energy dissipation should be much higher in freshwater than in marine environments. However, while the highest recorded difference of 32 mV (Fig. 2b) is indeed high compared to the ∼1 mV recorded in marine sediment with similar current densities (6), it only represents ca. 3.2% of the ∼1,000 mV available from the sulfide oxidation process (38), leaving a considerable surplus for microbial activity.
Conclusions.
In the present study, we demonstrated the presence of cable bacteria and associated electrogenic processes in freshwater sediments. Our findings extend the known habitats of cable bacteria and suggest a widespread occurrence at oxic-anoxic interfaces of rivers, lakes, wetlands, aquifers and soils. Our findings also suggest a common evolutionary origin of the cable phenotype within Desulfobulbaceae with subsequent diversification into a freshwater and a marine lineage. It is possible that the freshwater lineage, like the marine lineage, is associated with the e-SOx reaction. Cable bacteria in freshwater sediments may then provide a mechanism for recycling scarce resources of sulfate, stimulating sulfate reduction. The exact nature of the interactions between cable bacteria and the cycling of sulfur in freshwater sediment warrants further investigations.
Supplementary Material
ACKNOWLEDGMENTS
We thank Lars B. Petersen and Preben G. Sørensen for the construction of oxygen, H2S, and pH microsensors and Jeanette Johansen and Karina Bomholt Henriksen for chemical analyses of water samples. We thank Trine Bech Søgaard for FISH analyses, Britta Poulsen for Ion Torrent sequencing, and Jie Song for operating the SEM.
We received funding from the European Research Council under the European Union's Seventh Framework Programme (FP/2007-2013)/ERC grant agreement 291650, the Danish National Research Foundation (DNRF104), and The Danish Council for Independent Research in the Natural Sciences.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01064-15.
REFERENCES
- 1.Schauer R, Risgaard-Petersen N, Kjeldsen KU, Bjerg JJT, Jørgensen BB, Schramm A, Nielsen LP. 2014. Succession of cable bacteria and electric currents in marine sediment. ISME J 8:1314–1322. doi: 10.1038/ismej.2013.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfeffer C, Larsen S, Song J, Dong MD, Besenbacher F, Meyer RL, Kjeldsen KU, Schreiber L, Gorby YA, El-Naggar MY, Leung KM, Schramm A, Risgaard-Petersen N, Nielsen LP. 2012. Filamentous bacteria transport electrons over centimetre distances. Nature 491:218–221. doi: 10.1038/nature11586. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen LP, Risgaard-Petersen N, Fossing H, Christensen PB, Sayama M. 2010. Electric currents couple spatially separated biogeochemical processes in marine sediment. Nature 463:1071–1074. doi: 10.1038/nature08790. [DOI] [PubMed] [Google Scholar]
- 4.Damgaard LR, Risgaard-Petersen N, Nielsen LP. 2014. Electric potential microelectrode for studies of electrobiogeophysics. J Geophys Res Biogeosci 119:1906–1917. doi: 10.1002/2014JG002665. [DOI] [Google Scholar]
- 5.Risgaard-Petersen N, Damgaard LR, Revil A, Nielsen LP. 2014. Mapping electron sources and sinks in a marine biogeobattery. J Geophys Res Biogeosci 119:1475–1486. doi: 10.1002/2014JG002673. [DOI] [Google Scholar]
- 6.Risgaard-Petersen N, Revil A, Meister P, Nielsen LP. 2012. Sulfur, iron-, and calcium cycling associated with natural electric currents running through marine sediment. Geochim Cosmochim Acta 92:1–13. doi: 10.1016/j.gca.2012.05.036. [DOI] [Google Scholar]
- 7.Revil A, Mendonca CA, Atekwana EA, Kulessa B, Hubbard SS, Bohlen KJ. 2010. Understanding biogeobatteries: where geophysics meets microbiology. J Geophys Res Biogeosci 115:G00G02. doi: 10.1029/2009JG001065. [DOI] [Google Scholar]
- 8.Sato M, Mooney HM. 1960. The electrochemical mechanism of sulfide self-potentials. Geophysics 1:226–249. [Google Scholar]
- 9.Malkin SY, Rao AMF, Seitaj D, Vasquez-Cardenas D, Zetsche E-M, Hidalgo-Martinez S, Boschker HTS, Meysman FJR. 2014. Natural occurrence of microbial sulphur oxidation by long-range electron transport in the seafloor. ISME J 8:1843–1854. doi: 10.1038/ismej.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meysman FJR, Risgaard-Petersen N, Malkin SY, Nielsen LP. 2015. The geochemical fingerprint of microbial long-distance electron transport in the seafloor. Geochim Cosmochim Acta 152:122–142. doi: 10.1016/j.gca.2014.12.014. [DOI] [Google Scholar]
- 11.Malkin SY, Meysman FJ. 2015. Rapid redox signal transmission by cable bacteria beneath a photosynthetic biofilm. Appl Environ Microbiol 81:948–956. doi: 10.1128/aem.02682-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marzocchi U, Trojan D, Larsen S, Meyer RL, NP R, Schramm A, Nielsen LP, Risgaard-Petersen N. 2014. Electric coupling between distant nitrate reduction and sulfide oxidation in marine sediment. ISME J 8:1682–1690. doi: 10.1038/ismej.2014.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larsen S, Nielsen LP, Schramm A. 2015. Cable bacteria associated with long distance electron transport in New England salt marsh sediment. Environ Microbiol Rep 7:175–179. doi: 10.1111/1758-2229.12216. [DOI] [PubMed] [Google Scholar]
- 14.Pester M, Knorr K-H, Friedrich MW, Wagner M, Loy A. 2012. Sulfate-reducing microorganisms in wetlands: fameless actors in carbon cycling and climate change. Front Microbiol 3:1–19. doi: 10.3389/fmicb.2012.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ribeiro DC, Martins G, Nogueira R, Brito AG. 2014. Mineral cycling and pH gradient related with biological activity under transient anoxic–oxic conditions: effect on P mobility in volcanic lake sediments. Environ Sci Technol 48:9205–9210. doi: 10.1021/es501037g. [DOI] [PubMed] [Google Scholar]
- 16.Naudet V, Revil A, Bottero JY, Begassat P. 2003. Relationship between self-potential (SP) signals and redox conditions in contaminated groundwater. Geophys Res Lett 30:2091. doi: 10.1029/2003GL018096. [DOI] [Google Scholar]
- 17.Jeroschewski P, Steuckart C, Kuhl M. 1996. An amperometric microsensor for the determination of H2S in aquatic environments. Anal Chem 68:4351–4357. doi: 10.1021/ac960091b. [DOI] [Google Scholar]
- 18.Revsbech NP. 1989. An oxygen microsensor with a guard cathode. Limnol Oceanogr 34:474–478. doi: 10.4319/lo.1989.34.2.0474. [DOI] [Google Scholar]
- 19.Revsbech NP, Jørgensen BB. 1986. Microelectrodes: their use in microbial ecology. Adv Microb Ecol 9:293–352. doi: 10.1007/978-1-4757-0611-6_7. [DOI] [Google Scholar]
- 20.Cline JD. 1969. Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol Oceanogr 14:454. doi: 10.4319/lo.1969.14.3.0454. [DOI] [Google Scholar]
- 21.Ullman WJ, Aller RC. 1982. Diffusion coefficients in nearshore marine sediments. Limnol Oceanogr 27:552–556. doi: 10.4319/lo.1982.27.3.0552. [DOI] [Google Scholar]
- 22.Newman EI. 1966. A method of estimating the total length of root in a sample. J Appl Ecol 3:139–145. doi: 10.2307/2401670. [DOI] [Google Scholar]
- 23.Høgslund S, Nielsen JL, Nielsen LP. 2010. Distribution, ecology, and molecular identification of Thioploca from Danish brackish water sediments. FEMS Microbiol Ecol 73:110–120. doi: 10.1111/j.1574-6941.2010.00878.x. [DOI] [PubMed] [Google Scholar]
- 24.Pernthaler J, Glockner FO, Schonhuber W, Amann R. 2001. Fluorescence in situ hybridization (FISH) with rRNA-targeted oligonucleotide probes. Method Microbiol 30:207–226. doi: 10.1016/S0580-9517(01)30046-6. [DOI] [Google Scholar]
- 25.Manz W, Amann R, Ludwig W, Wagner M, Schleifer KH. 1992. Phylogenetic oligodeoxynucleotide probes for the major subclasses of proteobacteria: problems and solutions. Syst Appl Microbiol 15:593–600. doi: 10.1016/S0723-2020(11)80121-9. [DOI] [Google Scholar]
- 26.Daims H, Brühl A, Amann R, Schleifer K-H, Wagner M. 1999. The domain-specific probe EUB338 is insufficient for the detection of all Bacteria: development and evaluation of a more comprehensive probe set. Syst Appl Microbiol 22:434–444. doi: 10.1016/S0723-2020(99)80053-8. [DOI] [PubMed] [Google Scholar]
- 27.Schmieder R, Edwards R. 2011. Quality control and preprocessing of metagenomic datasets. Bioinformatics 27:863–864. doi: 10.1093/bioinformatics/btr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pruesse E, Peplies J, Glöckner FO. 2012. SINA: accurate high-throughput multiple sequence alignment of rRNA genes. Bioinformatics 28:1823–1829. doi: 10.1093/bioinformatics/bts252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ludwig W, Strunk O, Westram R, Richter L, Meier H, Yadhukumar, Buchner A, Lai T, Steppi S, Jobb G, Förster W, Brettske I, Gerber S, Ginhart AW, Gross O, Grumann S, Hermann S, Jost R, König A, Liss T, Lüßmann R, May M, Nonhoff B, Reichel B, Strehlow R, Stamatakis A, Stuckmann N, Vilbig A, Lenke M, Ludwig T, Bode A, Schleifer KH. 2004. ARB: a software environment for sequence data. Nucleic Acids Res 32:1363–1371. doi: 10.1093/nar/gkh293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Wheeler DL. 2005. GenBank. Nucleic Acids Res 33:D34–D38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. 2013. The SILVA rRNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yarza P, Yilmaz P, Pruesse E, Glockner FO, Ludwig W, Schleifer K-H, Whitman WB, Euzeby J, Amann R, Rossello-Mora R. 2014. Uniting the classification of cultured and uncultured bacteria and archaea using 16S rRNA gene sequences. Nat Rev Microbiol 12:635–645. doi: 10.1038/nrmicro3330. [DOI] [PubMed] [Google Scholar]
- 35.Bockris JO, Reddy AK. 1998. Modern electrochemistry 1: ionics, 2nd ed, vol 1 Plenum Press, Inc, New York, NY. [Google Scholar]
- 36.Overbeek JTG. 1952. Electrochemistry of the double layer, p 115–193. In Kruyt HR. (ed), Colloid science: irreversible system. Elsevier, New York, NY. [Google Scholar]
- 37.Holmer M, Storkholm P. 2001. Sulphate reduction and sulphur cycling in lake sediments: a review. Freshw Biol 46:431–451. doi: 10.1046/j.1365-2427.2001.00687.x. [DOI] [Google Scholar]
- 38.Thauer RK, Jungermann K, Decker K. 1977. Energy conservation in chemotropic anaerobic bacteria. Bacteriol Rev 41:100–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.