Abstract
SWR/J--RF/J hybrid mice spontaneously acquire new germline ecotropic proviruses at high frequency. We have performed ovarian transplantation and in situ hybridization studies to delineate the mechanism and developmental stage of germline provirus acquisition. In addition, we have developed a novel, efficient and simple method to introduce single copy proviruses into the mouse germline. The results reported here have direct implications for understanding how proviruses are acquired in the germline, for using murine leukemia viruses as insertional mutagens, and for using retroviral vectors to introduce foreign genes into the mouse germline.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramczuk J., Sawicki W. Pronuclear synthesis of DNA in fertilized and parthenogenetically activated mouse eggs. Exp Cell Res. 1975 May;92(2):361–371. doi: 10.1016/0014-4827(75)90390-0. [DOI] [PubMed] [Google Scholar]
- Baranska W., Sawicki W., Koprowski H. Infection of mammalian unfertilized and fertilized ova with oncogenic viruses. Nature. 1971 Apr 30;230(5296):591–592. doi: 10.1038/230591a0. [DOI] [PubMed] [Google Scholar]
- Bautch V. L. Genetic background affects integration frequency of ecotropic proviral sequences into the mouse germ line. J Virol. 1986 Nov;60(2):693–701. doi: 10.1128/jvi.60.2.693-701.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler C. E., Staal S. P., Rowe W. P., Martin M. A. Variation in the number of copies and in the genomic organization of ecotropic murine leukemia virus proviral sequences in sublines of AKR mice. J Virol. 1982 Aug;43(2):629–640. doi: 10.1128/jvi.43.2.629-640.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lander M. R., Rands E., Lowy D. R. Structure of endogenous murine leukemia virus DNA in mouse genomes. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5774–5778. doi: 10.1073/pnas.77.10.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland N. G., Cooper G. M. Transfection by exogenous and endogenous murine retrovirus DNAs. Cell. 1979 Feb;16(2):347–356. doi: 10.1016/0092-8674(79)90011-4. [DOI] [PubMed] [Google Scholar]
- Herr W., Gilbert W. Germ-line MuLV reintegrations in AKR/J mice. Nature. 1982 Apr 29;296(5860):865–868. doi: 10.1038/296865a0. [DOI] [PubMed] [Google Scholar]
- Hooper M., Hardy K., Handyside A., Hunter S., Monk M. HPRT-deficient (Lesch-Nyhan) mouse embryos derived from germline colonization by cultured cells. Nature. 1987 Mar 19;326(6110):292–295. doi: 10.1038/326292a0. [DOI] [PubMed] [Google Scholar]
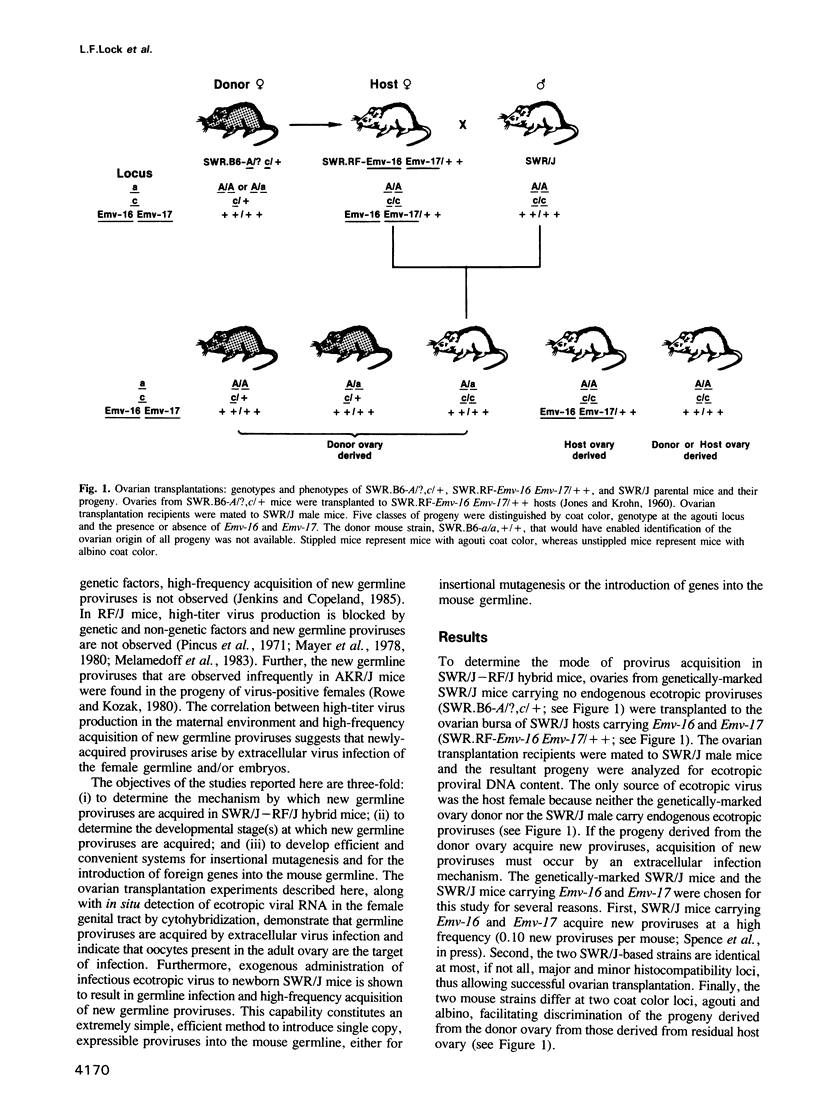
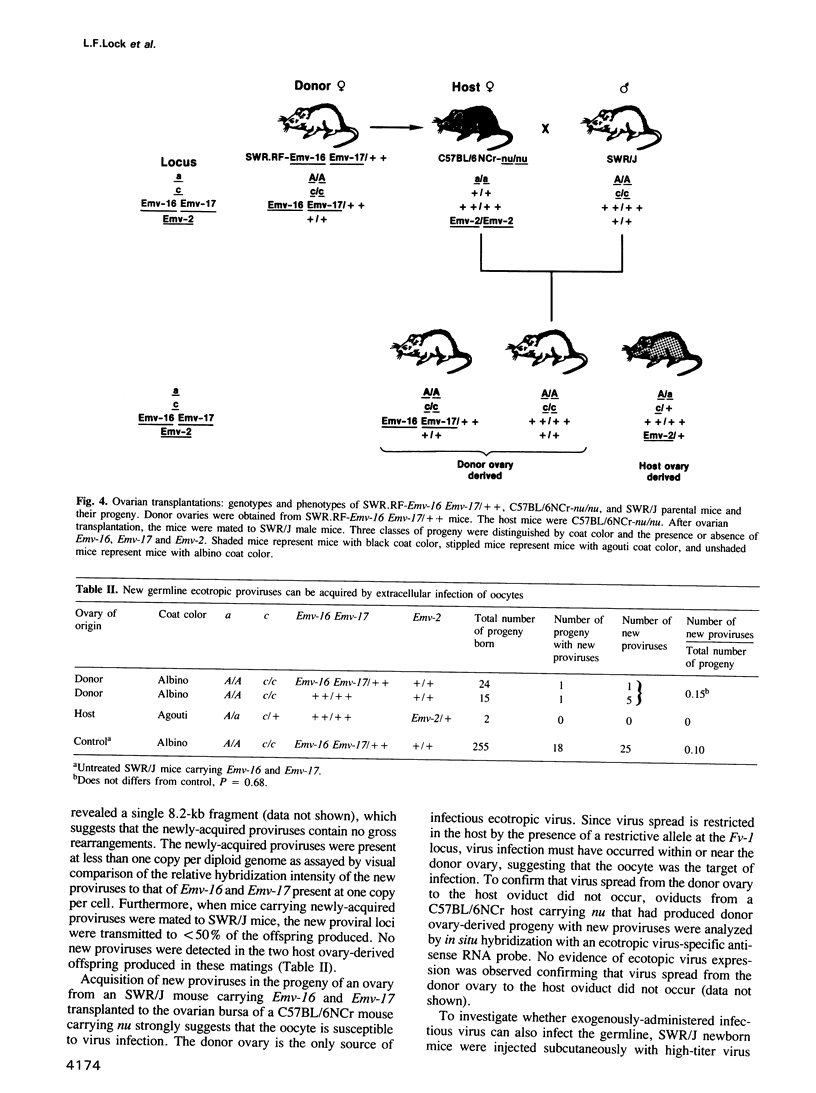
- JONES E. C., KROHN P. L. Orthotopic ovarian transplantation in mice. J Endocrinol. 1960 Apr;20:135–146. doi: 10.1677/joe.0.0200135. [DOI] [PubMed] [Google Scholar]
- Jaenisch R. Germ line integration and Mendelian transmission of the exogenous Moloney leukemia virus. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1260–1264. doi: 10.1073/pnas.73.4.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R., Harbers K., Schnieke A., Löhler J., Chumakov I., Jähner D., Grotkopp D., Hoffmann E. Germline integration of moloney murine leukemia virus at the Mov13 locus leads to recessive lethal mutation and early embryonic death. Cell. 1983 Jan;32(1):209–216. doi: 10.1016/0092-8674(83)90511-1. [DOI] [PubMed] [Google Scholar]
- Jaenisch R. Retroviruses and embryogenesis: microinjection of Moloney leukemia virus into midgestation mouse embryos. Cell. 1980 Jan;19(1):181–188. doi: 10.1016/0092-8674(80)90399-2. [DOI] [PubMed] [Google Scholar]
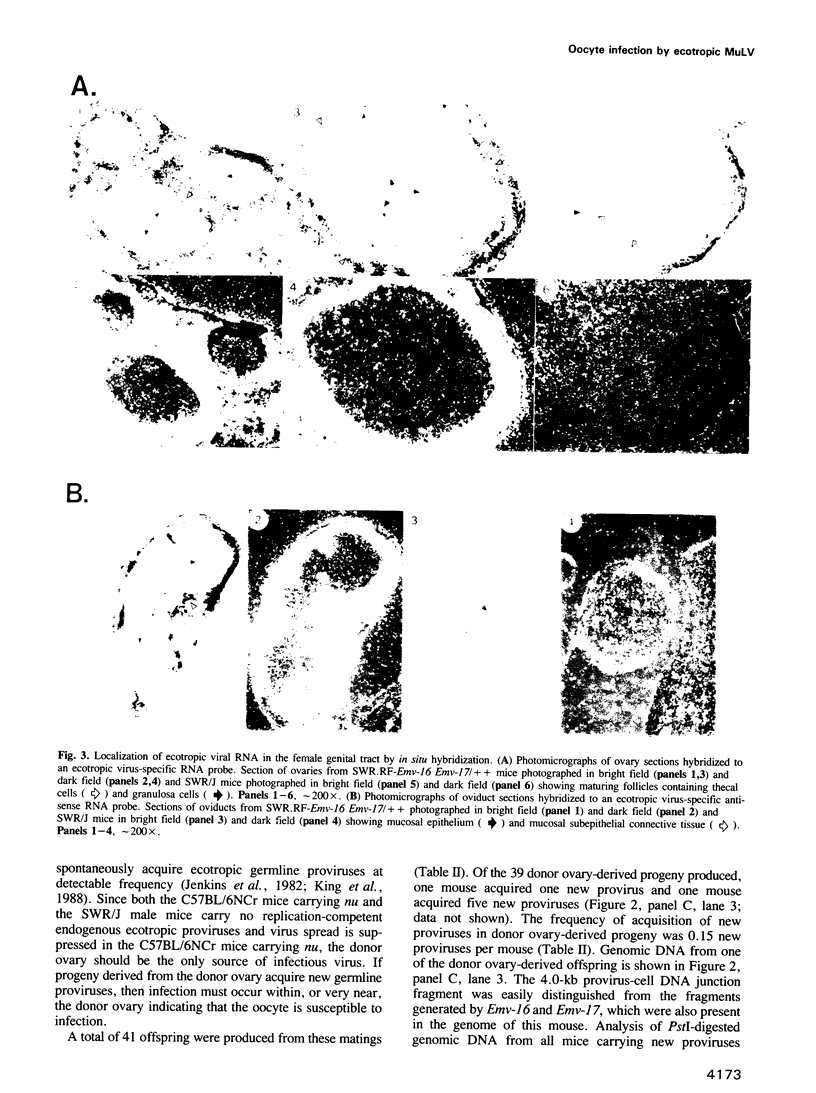
- Jenkins N. A., Copeland N. G. High frequency germline acquisition of ecotropic MuLV proviruses in SWR/J-RF/J hybrid mice. Cell. 1985 Dec;43(3 Pt 2):811–819. doi: 10.1016/0092-8674(85)90254-5. [DOI] [PubMed] [Google Scholar]
- Jenkins N. A., Copeland N. G., Taylor B. A., Lee B. K. Dilute (d) coat colour mutation of DBA/2J mice is associated with the site of integration of an ecotropic MuLV genome. Nature. 1981 Oct 1;293(5831):370–374. doi: 10.1038/293370a0. [DOI] [PubMed] [Google Scholar]
- Jenkins N. A., Copeland N. G., Taylor B. A., Lee B. K. Organization, distribution, and stability of endogenous ecotropic murine leukemia virus DNA sequences in chromosomes of Mus musculus. J Virol. 1982 Jul;43(1):26–36. doi: 10.1128/jvi.43.1.26-36.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury A. T., Hanafusa H. Synethesis and integration of viral DNA in chicken cells at different time after infection with various multiplicities of avian oncornavirus. J Virol. 1976 May;18(2):383–400. doi: 10.1128/jvi.18.2.383-400.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King S. R., Berson B. J., Risser R. Mechanism of interaction between endogenous ecotropic murine leukemia viruses in (BALB/c X C57BL/6) hybrid cells. Virology. 1988 Jan;162(1):1–11. doi: 10.1016/0042-6822(88)90388-1. [DOI] [PubMed] [Google Scholar]
- Kuehn M. R., Bradley A., Robertson E. J., Evans M. J. A potential animal model for Lesch-Nyhan syndrome through introduction of HPRT mutations into mice. Nature. 1987 Mar 19;326(6110):295–298. doi: 10.1038/326295a0. [DOI] [PubMed] [Google Scholar]
- Langdon W. Y., Theodore T. S., Buckler C. E., Stimpfling J. H., Martin M. A., Morse H. C., 3rd Relationship between a retroviral germ line reintegration and a new mutation at the ashen locus in B10.F mice. Retroviral integration and an ashen mutation. Virology. 1984 Feb;133(1):183–190. doi: 10.1016/0042-6822(84)90437-9. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Chattopadhyay S. K., Teich N. M., Rowe W. P., Levine A. S. AKR murine leukemia virus genome: frequency of sequences in DNA of high-, low-, and non-virus-yielding mouse strains. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3555–3559. doi: 10.1073/pnas.71.9.3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Rands E., Chattopadhyay S. K., Garon C. F., Hager G. L. Molecular cloning of infectious integrated murine leukemia virus DNA from infected mouse cells. Proc Natl Acad Sci U S A. 1980 Jan;77(1):614–618. doi: 10.1073/pnas.77.1.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A., Duran-Reynals M. L., Lilly F. Fv-1 regulation of lymphoma development and of thymic ecotropic and xenotropic MuLV expression in mice of the AKR/J x RF/J cross. Cell. 1978 Oct;15(2):429–435. doi: 10.1016/0092-8674(78)90012-0. [DOI] [PubMed] [Google Scholar]
- Mayer A., Struuck F. D., Duran-Reynals M. L., Lilly F. Maternally transmitted resistance to lymphoma development in mice of reciprocal crosses of the RF/J and AKR/J strains. Cell. 1980 Feb;19(2):431–436. doi: 10.1016/0092-8674(80)90517-6. [DOI] [PubMed] [Google Scholar]
- McCubrey J., Horowitz J. M., Risser R. Structure and expression of endogenous ecotropic murine leukemia viruses in RF/J mice. J Exp Med. 1982 Nov 1;156(5):1461–1474. doi: 10.1084/jem.156.5.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamedoff M., Lilly F., Duran-Reynals M. L. Suppression of endogenous murine leukemia virus by maternal resistance factor. J Exp Med. 1983 Aug 1;158(2):506–514. doi: 10.1084/jem.158.2.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panthier J. J., Condamine H., Jacob F. Inoculation of newborn SWR/J females with an ecotropic murine leukemia virus can produce transgenic mice. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1156–1160. doi: 10.1073/pnas.85.4.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus T., Hartley J. W., Rowe W. P. A major genetic locus affecting resistance to infection with murine leukemia viruses. I. Tissue culture studies of naturally occurring viruses. J Exp Med. 1971 Jun 1;133(6):1219–1233. doi: 10.1084/jem.133.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Hartley J. W. Studies of genetic transmission of murine leukemia virus by AKR mice. II. Crosses with Fv-1 b strains of mice. J Exp Med. 1972 Nov 1;136(5):1286–1301. doi: 10.1084/jem.136.5.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Kozak C. A. Germ-line reinsertions of AKR murine leukemia virus genomes in Akv-1 congenic mice. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4871–4874. doi: 10.1073/pnas.77.8.4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Rowe W. P. Studies of genetic transmission of murine leukemia virus by AKR mice. I. Crosses with Fv-1 n strains of mice. J Exp Med. 1972 Nov 1;136(5):1272–1285. doi: 10.1084/jem.136.5.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki W., Abramczuk J., Blaton O. DNA synthesis in the second and third cell cycles of mouse preimplantation development. A cytophotometric study. Exp Cell Res. 1978 Mar 1;112(1):199–205. doi: 10.1016/0014-4827(78)90540-2. [DOI] [PubMed] [Google Scholar]
- Siracusa L. D., Russell L. B., Eicher E. M., Corrow D. J., Copeland N. G., Jenkins N. A. Genetic organization of the agouti region of the mouse. Genetics. 1987 Sep;117(1):93–100. doi: 10.1093/genetics/117.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siracusa L. D., Russell L. B., Jenkins N. A., Copeland N. G. Allelic variation within the Emv-15 locus defines genomic sequences closely linked to the agouti locus on mouse chromosome 2. Genetics. 1987 Sep;117(1):85–92. doi: 10.1093/genetics/117.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P., Gridley T., Jaenisch R. Retroviruses and insertional mutagenesis in mice: proviral integration at the Mov 34 locus leads to early embryonic death. Genes Dev. 1987 Jun;1(4):366–375. doi: 10.1101/gad.1.4.366. [DOI] [PubMed] [Google Scholar]
- Steffen D. L., Taylor B. A., Weinberg R. A. Continuing germ line integration of AKV proviruses during the breeding of AKR mice and derivative recombinant inbred strains. J Virol. 1982 Apr;42(1):165–175. doi: 10.1128/jvi.42.1.165-175.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephension J. R., Reynolds R. K., Tronick S. R., Aaronson S. A. Distribution of three classes of endogenous type-C RNA viruses among inbred strains of mice. Virology. 1975 Oct;67(2):404–414. doi: 10.1016/0042-6822(75)90442-0. [DOI] [PubMed] [Google Scholar]
- Van Beveren C., Rands E., Chattopadhyay S. K., Lowy D. R., Verma I. M. Long terminal repeat of murine retroviral DNAs: sequence analysis, host-proviral junctions, and preintegration site. J Virol. 1982 Feb;41(2):542–556. doi: 10.1128/jvi.41.2.542-556.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Vogt P. K., Bishop J. M. Integration of deoxyribonucleic acid specific for Rous sarcoma virus after infection of permissive and nonpermissive hosts. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3067–3071. doi: 10.1073/pnas.70.11.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]