Abstract
Background
Her2/neu is one of the epidermal growth factor receptors families and seems to have prognostic significance of some solid tumors. The objective of this study is to evaluate the possibility of Her2 expression in gastric cancers and the possible relationship of Her2 with tumor’s clinicopathologic parameters and also its prognostic role.
Methods
This study was performed on 100 cases of gastric carcinoma with stage I b to III (according to TNM staging). Survival, recurrence date of patients, grade and lymph nodes involvement were assessed. Her2/neu expression was determined by immunohistochemical method on received sample blocks. Survival of patients with or without Her2-neu expression were evaluated by Kaplan- Meier method and compared with the log-rank test followed by multivariate analysis using Cox regression.
Results
Seven cases were 3+ membranous Her2 reactivity, 5 cases were 2+ and13 cases were 1+; also 75% of cases demonstrated no reactivity. Regardingrelationship between tumor grade and membranous Her2 , all patients with poorly differentiated tumors were Her2 negative but patients with moderate and well differentiated tumor had 18.1% and 19.6% Her2 reactivity respectively; there were no significant difference between groups statistically(P>0.05). Median overall survival was 27.25 and 46 months in Her2 negative and her2 positive cases respectively; there were no significant difference between groups statistically as well (P>0.05).
Conclusion
Her2 reactivity has not relationship with tumor grade and lymph node involvement as well as tumor stage. From the other point of view no significant correlation is found between Her2 expression and disease free survival or overall survival of gastric cancer patients.
Keywords: Gastric cancer, Adenocarcinoma, Her2 neu peptide, Survival analysis
Introduction
Epidermal growth factor receptor (EGFR or HER1) and its homolog's HER2, HER3, and HER4 are glycoproteins that consist of extracellular domain and intracellular domain carrying Tyrosine Kinase Activity [1].
It seems that EGFR is important not only in cell proliferation but also in a number of varied processes which is important for tumor progression, such as cell motility, cell survival, and angiogenesis [2].
Expression of HER2 has been demonstrated with several methods including amplification, FISH, CISH, and immunohistochemical methods [3- 6].
There has been conflicting results for the hypothesis that the expression of EGFR2 may be a significant predictor of prognosis in gastric carcinoma. Some studies have reported that HER2 over expression is a poor prognostic factor [7, 8] while other studies have failed to find any association with prognosis whatsoever [9, 10].
In a study conducted by Ghaderi [11] HER1 expression was observed in 32% and HER2 in 16% of cases. Although significant positive correlations were observed between HER1 expression, tumor size, local invasion, lymph node involvement, and tumor stage; but a negative correlation was found between HER2 expression and tumor stage. A study by Garcia et al [12] showed that there is no significant correlation between high level HER2 content with tumor characteristics including stage, tumor grade, histological type, and lymph node involvement; however high levels of HER2 seems to be significantly associated with a shorter overall survival period .
According to these paradoxical results, this study was designed to detect the frequency of HER2/neu over expression in gastric cancer, the relation between HER2 over expression, stage, grade, and lymph node involvement as well as its relation with prognosis and survival in gastric cancers.
Materials and Methods
This multicentric historical cohort study was conducted in Valiasr , Emam Khomeini, and Ghods Hospitals ( Arak, Iran ) as well as Alzahra University Hospital ( Isfahan , Iran ) on 109 patients who had undergone curative total or partial gastrectomy for primary gastric carcinoma stage I b to III (TNM staging) between 2001 and 2008. In all cases the diagnosis had been confirmed at least 1 year prior to this study. In less than 2 months after surgery, they have been treated with the routine chemo-radiation therapy protocol (Standard Mayo Clinic protocol). 9 cases excluded from the study because 3 pathological blocks were not available and 6 cases were omitted due to incorrect demographic data for follow up.
Patients who underwent gastrectomy for lymphoma, gastrointestinal stromal tumor, peptic ulcer, and patients who had distant or peritoneal metastasis were excluded. All specimens were reevaluated with regard to histological subtype, grade, and stage (According to WHO criteria of TNM staging system) by two pathologists; and in February 2008, survival and recurrence date of patients were assessed; we checked their files available in hospitals or cancer registry site to gather data to record the pathology sample number and their phone numbers for following up.
All sample blocks was referred to a centralized university lab for IHC examination. Anti-Her2-neu monoclonal antibody (Ao485-USA, DAKO) was applied to section from formalin-fixed paraffin- embedded specimens, using the envision + Detection system/DAB+. Paraffin sections were deparaffinized with Xylen and dehydrated through graduated alcohols up to 70%. Slides were incubated with antibody at a dilution of 1:50, using antibody diluents for 20 minute at room temperature, after being washed with PBS (PH=7.2), the slides were incubated with labeled polymer HRP for 30 minutes.
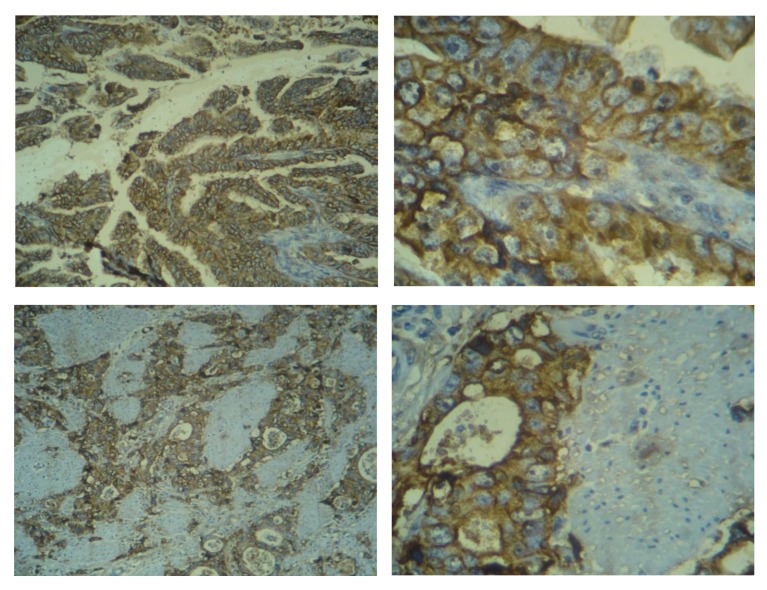
All HER2-neu marker slides were reviewed independently by two pathologists, Dr Chehrei and Dr Sanei. Information about patient’s prognosis including survival or recurrence was concealed during this part of study. A consensus meeting was held when there was any discrepancy. HER2-neu over expression was scored as follow, score 0: no staining or membrane staining was observed in less than 10% of tumor cells, score 1: a faint membrane staining in more than 10% of malignant cells and these cells were only stained in part of their membrane, score 2: a weak to moderate complete membrane staining in more than 10% of tumor cells, score 3: a strong complete membrane staining in more than 10% of tumor cells (Figure 1). Breast adenocarcinoma with well-established high expression of HER2 served as positive control.
Figure 1.
3+ Her2.neu staining in gastric adenocarcinoma
All data were analyzed by SPSS (SPSS Inc, Chicago IL, USA, version 16). Simple descriptive techniques were used to describe the variables among the participants. Overall survival of patients with gastric adenocarcinoma determined with life table method. Survival of patients with and without Her2-neu expression were evaluated by Kaplan- Meier method and compared by the log-Rank test then multi variants analysis was done with Cox regression. Chi square test were used to correlate between quantitative data. The level of significance is 0.05.
Results
Clinicopathological data
Amongst the 100 gastric cancer patients, 73% were male and 27% were female. The median age was 63.81 (61.31-66.31); 45% of tumors were in the body, 36% in antropyloric region and 19% in cardia. Partial gastrectomy was done for 62% of cases and total gastrectomy for 38%. In histopathology examination 71% of cases were of intestinal type, 23% were signet ring (diffuse type), and 6% were mucinous type. Stage evaluation revealed that 18% were in stage I, 37% stage II, and 45% stage III. Regarding grade, 56% had well differentiated histology, 22% moderately differentiated, and 22% poorly differentiated.
Macroscopic or gross evaluation of tumors showed 73% had ulcerative surface, also the median size of lesions were 5.77 Cm (5.21-6.33), the median number of lymph nodes were 7 (6-8), and the median number of involved lymph nodes were 2.73 (2.09-3.37).
Survival analysis
The mean follow-up time was 22.7month, the minimum and maximum times were between 1 month and 78 months. Overall median survival was 23.09(16.23-29.76) months and the mean of survival was 29.52(23.67-35.36); the related data of survival are shown in table 1; the one and two years survival rate were 70% and 48% respectively; the five years survival was 19%. Median disease free survival (DFS) was 16.32 months. 72% of cases had recurrence. The death had occurred in 66%.
Table 1.
Life table survival function of 100 gastric cancer patients
| Interval time(months) | Number Entering interval | Number withdrawing during interval | Number exposed to risk | Number of Terminal Events | Proportion Terminating | Proportion Surviving | Cumulative proportion surviving at endof interval | SE*of cumulative proportion surviving at endof interval | Hazard Rate | SE of Hazard Rate |
|---|---|---|---|---|---|---|---|---|---|---|
| .0-12 | 100 | 0 | 100.000 | 30 | 0.30 | 0.70 | 0.70 | 0.05 | 0.03 | 0.01 |
| 12-24 | 70 | 17 | 61.500 | 19 | 0.31 | 0.69 | 0.48 | 0.05 | 0.03 | 0.01 |
| 24-36 | 34 | 7 | 30.500 | 13 | 0.43 | 0.57 | 0.28 | 0.05 | 0.05 | 0.01 |
| 36-48 | 14 | 2 | 13.000 | 4 | 0.31 | 0.69 | 0.19 | 0.05 | 0.03 | 0.01 |
| 48-60 | 8 | 2 | 7.000 | 0 | 0 | 1.00 | 0.19 | 0.05 | 0 | 0 |
| 60-72 | 6 | 4 | 4.000 | 0 | 0 | 1.00 | 0.19 | 0.05 | 0 | 0 |
| >72 | 2 | 2 | 1.000 | 0 | 0 | 1.00 | 0.19 | 0.05 | 0 | 0 |
SE: Standard Error
Her2/neu Score of reactivity
In evaluation of membranous Her2 reactivity according to mentioned criteria in methods, 7 cases were (+++), 5 (++), 13 (+), and 75 showed no reactivity (Negative).
Her2 score according to clinicopathological data and prognosis
Patients with stage I had not any her2 reactivity (Negative), but in stage III 13.3% of cases were her2 positive but these results had no significant difference statistically. Regarding to relation of tumor grade and membranous her2, all patients with poorly differentiated were her2 negative, while patients with moderate and well differentiated tumor had 18.1% and 19.6% her2 reactivity respectively; there were no statistically significant difference between groups(P>0.05) .
Correlation between her2 reactivity and lymph node involvement was investigated by univariate analysis which revealed cases with lymph node involvement and without involvement had 13.2% and 9.4% positive her2 reactivity respectively. There was no statistically significant difference between groups (P>0.05). Table 2 shows Her2 over expression according to clinicopathological data.
Table 2.
Her2 over expression according to clinicopathological data
| Clinicopathologic Data | Subgroup(n) | Her2 positive group |
Her2 negative |
Significant | ||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Stage | I(18) | 0 | 0 | 18 | 100 | 0.2 |
| II(37) | 6 | 16.2 | 31 | 83.8 | ||
| III(45) | 6 | 86.7 | 39 | 13.7 | NS | |
| Grade | Poorly(22) | 0 | 0 | 22 | 10 | 0.1 |
| Moderate(22) | 4 | 18.1 | 18 | 81.9 | ||
| Well(56) | 11 | 19.6 | 45 | 8.4 | NS | |
| Lymph nodes involvement | Involved (68) | 59 | 86.8 | 9 | 13.2 | 0.4 |
| Non involved(32) | 29 | 90.6 | 3 | 9.4 | NS | |
NS : No significant
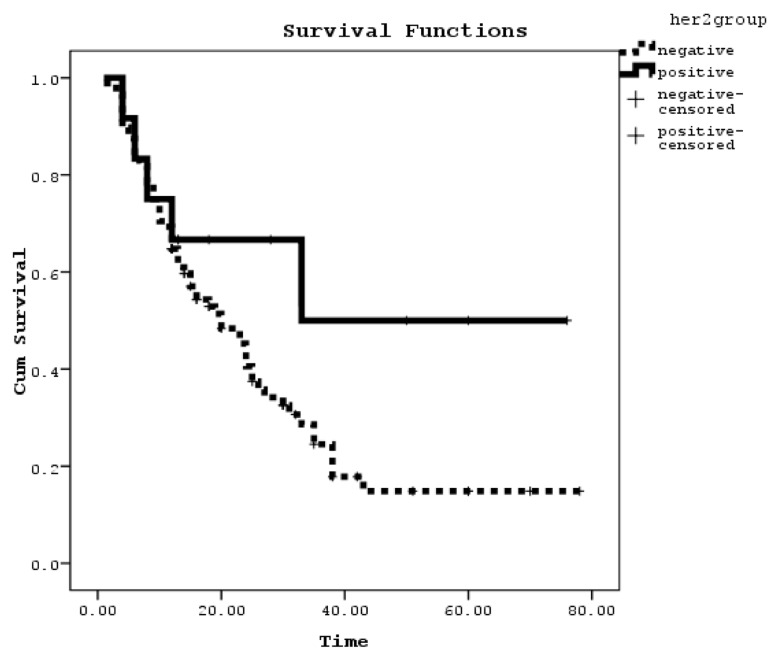
According to the collected data, the median overall survival was 27.25(21.43-33.06) months in membranous her2 negative and 46(26.34-65.65) months in her2 positive. There were no statistically significant differences between these groups (P>0.05) (Figure2). Also by Cox regression analysis her2 reactivity was not an independent prognostic factor.
Figure 2.
Overall survivals as a function of membranous Her2 reactivity
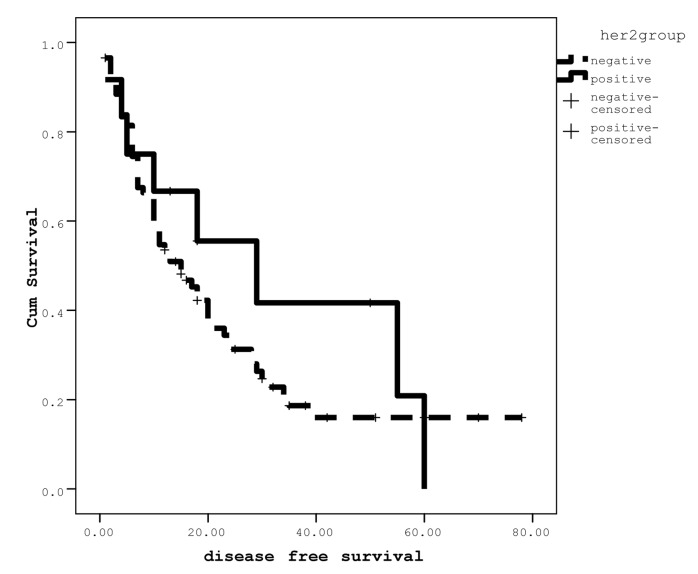
The median disease free survival (DFS) in the group with negative membranous her2 was 22.41(18.38-30.44) months and in positive her2 was 31.65(16.40-46.90) months; there was no statistically significant difference between groups (P>0.05) (Figure 3). Cox Regression analysis was performed to find out the effect of grade and stage but again no significant difference was detected between her2 reactivity status and DFS.
Figure 3.
Disease free survivals as a function of membranous Her2 reactivity
Discussion
EGFR and its ligands are frequently over expressed in human cancers. EGFR over expression may result from gene amplification or mutation, transcriptional abnormalities or autocrine stimulation by enhanced expression of the ligands EGF and plays a critical role in tumor progression by stimulating cell cycle progression, invasion, and metastasis [13].
Prognosis of gastric cancers is poor and the patients respond poorly to the conventional treatment. The most important prognostic factor in gastric tumors is TNM staging but histological subtype also has prognostic significance according to Laurens classification which divides gastric tumors to diffuse and intestinal type [14, 15, and 16]. There is high degree of inconsistency in different studies regarding other important prognostic factors in gastric cancer; in some studies a high level of expression of epidermal growth factor family members is reported [17].
The over expression of this molecule in gastric cancer and its correlation with the outcome of the disease has been a subject of interest over the last decade. Over expression of Her2 in gastric cancers varies from 8.2% to 62.5% in different reports [18].
In breast cancer, HER2 over expression has been documented in 10-34% of invasive cancers and has been associated with poor prognosis. [19]
HER2/neu amplification and over expression are currently attracting attention because these factors can be used for prognostic and predictive markers in breast cancer [20]. As a prognostic marker, HER2 is used to predict the course and outcome of the disease and as a predictive marker, HER2 is used to foresee the patient’s therapeutic response to adjuvant chemotherapy [21], endocrine therapy, and also to select patients for treatment with antiHER2 monoclonal antibody immunotherapy. Favorable clinical results of antiHER2 antibodies in breast cancer have led to the analysis of HER2 expression in other solid tumors.
The only proven, potentially curative treatment for gastric cancer is surgical resection of all gross and microscopic disease. Even after what is felt to be a curative gastrectomy, disease recurs in both regional and/or distant sites in the majority of patients. Efforts to improve these poor results have developed the effective pre and postoperative systemic and regional adjuvant therapies [18, 22].
In this study we investigated the expression of one member of the epidermal growth factor receptor family (Her2/neum) in 100 gastric cancer patients who underwent curative partial or total gastrectomy. According to our findings, 12% of patients expressed HER2/neu protein by IHC and there was no significant relationship between Her2 over expression and clinicopathologic manifestation; also the prognosis has no direct correlation with this marker.
In contrast to our study, in a Korean study at Kangbuk Samsung Hospital, HER2 over expression and gene amplification were determined with semi- quantitative standard immunohistochemical staining, chromogenic in situ hybridization (CISH), and fluorescence in situ hybridization (FISH) in 182 gastric cancer patients who underwent curative surgery. Amongst whom 15.9% expressed HER2/neu protein by IHC. Intestinal-type cancer exhibited higher rates of HER2 amplification than did diffuse-type cancer. Survival analysis was performed on 182 patients who had lived for more than 4 weeks after surgery. Tumors with HER2 amplification were associated with poor mean survival rate (922 vs 3243 days) as well as 3-year survival rate (42.9 VS 74%: P<0.05). Tumor associated with HER2 over expression also exhibited poor mean survival rate (1240 VS 2405 days) and 3year survival rate (51.7 VS 65.8% P<0.05) [3].
On the other hand a research was performed at Guy’s hospital in London which C-erbB-2 expression was investigated in 93 routinely processed cases of gastric carcinoma. C-erbB-2 membrane immunoreactivity was observed in 11% (10/93) of tumors by immunohistochemical method, all of them were well differentiated intestinal type (p< 0.01). Overall, patients with tumors expressing this proto- oncogene had significantly better prognosis (p<0.05). Amongst intestinal-type tumors, those who were c-erbB-2 positive formed a distinct sub- population and had a better prognosis (p<0.02) [23].
Another study was conducted by Grabsch H and coworkers on 924 gastric carcinoma patients in Germany and England; C-erb-B2 expression was evaluated by immunohistochemical method and the results were similar to our study. No significant correlation between 5-year survival rate and expression of c-erbB-2 protein was found. In poorly differentiated carcinoma with c-erbB-2 protein positive, overall survival was significantly shorter than in those without protein expression (p<0.01). They concluded that c-erbB-2 protein is not a useful prognostic indicator in gastric carcinoma [18].
In conclusion, our data indicated that Her2 over expression is not related to growth, metastasis, and prognosis of gastric cancer. One important restrictive problem in all studies is that IHC methods can potentially be affected by host variables including tissue fixation, processing, and choice of primary antibodies, detection systems, and methods of antigen retrieval.
Furthermore, as the suggested scoring system for IHC is subjective, its interpretation may vary amongst observers. So, we recommend corresponding to different results from other studies and a larger multi-centric study or meta-analysis on results of previous studies; and also implementations of more accurate methods such as CISH and FISH for evaluation of Her2 amplification are needed.
Acknowledgments
The authors wish to thank Dr. M Sedighi, Dr. A Hassan-poor, Mr. Hajian and Mr. Mohajerani for their excellent assistance and encouragement during the course of this study.
Footnotes
Conflicts of Interest
The authors have no conflict of interests in this article.
Authors' Contribution
AJ designed the study, reviewed the literature and wrote the article. CA contributed to data collection, slide review, and analysis. AM and AS had a role in data collection, and SMH conducted the IHC staining and reviewed the slides.
REFERENCES
- 1.Klapper LN, Kirchbaum MH, Sela M, Yarden Y. Biochemical and clinical implications of the ErbB/ HER signaling network of growth factor receptors. Adv. Cancer Res. 2000;77:25–79. [PubMed] [Google Scholar]
- 2.Woodburn JR. The epidermal growth factor receptor and its inhi-bition in cancer therapy. Pharmacol.Ther. 1999;82:241–50. doi: 10.1016/s0163-7258(98)00045-x. [DOI] [PubMed] [Google Scholar]
- 3.Park DI, Yun JW, Park JH, Oh SJ, Kim HJ, Cho YK, et al. HER -2/neu Amplification Is an Independent Prognostic Factor in Gastric Cancer . Dig Dis Sci. 2006;51:1371–9. doi: 10.1007/s10620-005-9057-1. [DOI] [PubMed] [Google Scholar]
- 4.Cátia M, Ana R, Fernanda M, Fátima C, Raquel S, Gianpaolo S. Epidermal growth factor receptor structural alterations in gastric cancer. BioMed Central Ltd. 2008;8:10. doi: 10.1186/1471-2407-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anzai H. expression of metastasis related genes in surgical specimens of human gastric cancer can predict disease recurrence. Eur J Cancer. 1998;34:558–65. doi: 10.1016/s0959-8049(97)10075-2. [DOI] [PubMed] [Google Scholar]
- 6.Garcia I, Vizoso F, Andicoechea A, Fernandez P, Suarez C, García-Muñz JL, et al. C-erbB-2 oncoprotein content in gastric cancer and in adjacent mucosa. Int. J. Biol. Markers. 2000;15:231–4. doi: 10.1177/172460080001500305. [DOI] [PubMed] [Google Scholar]
- 7.Allgayer H, Babic R, Gruetzner KU, Tarabichi A, Schildberg FW. C-erbB -2 is of independent prognostic relevance in gastric cancer and is associated with the expression of tumor - associated protease systems. J Clin Oncol. 2000;18:2201–9. doi: 10.1200/JCO.2000.18.11.2201. [DOI] [PubMed] [Google Scholar]
- 8.Armando GD, Claudio DF, Letica QM, Edgardo RG, Dan G, Arturo AA, et al. Epidermal growth factor receptor expression correlates with poor survival in gastric adenocarcinoma from Mexican patients. Mod. Pathol. 2004;17:579–87. doi: 10.1038/modpathol.3800085. [DOI] [PubMed] [Google Scholar]
- 9.Gurel S, Dolar E, Yerci O, Samli B, Oztürk H, Nak SG, et al. The relationship between c- erb B -2 oncogene expression and clinicopatholigical factors in gastric cancer. J. Int. Med. Res. 1999;27:74. doi: 10.1177/030006059902700203. [DOI] [PubMed] [Google Scholar]
- 10.Nakajima M, Sawada H, Yamada Y, Watanabe A, Tatsumi M, Yamashita J, et al. The prognostic signif - icance of amplification and over expression of c- met and c-erb B -2 in human gastric carcinomas. Cancer. 1999;85:1894. doi: 10.1002/(sici)1097-0142(19990501)85:9<1894::aid-cncr3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 11.Ghaderi A, Vasei M, Maleck-Hosseini SA, Gharesi-fard B, Khodami M, Doroudchi M, et al. The Expression of c-erbB - 1 and c-erbB -2 in Iranian Patients with Gastric Carcinoma. Path. Oncol. Research. 2002;8:522–6. doi: 10.1007/BF03036740. [DOI] [PubMed] [Google Scholar]
- 12.García I, Vizoso F, Martín A, Sanz L, Abdel-Lah O, Raigoso P, et al. Clinical Significance of the Epidermal Growth Factor Receptor and HER2 Receptor in Resectable Gastric Cancer. Annals of surgical oncology . 2003;10(3):234–41. doi: 10.1245/aso.2003.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Lui VW. Grandis GR, EGFR-mediated cell cycle regulation. Anticancer Res. 2002;22(1A):1–11. [PubMed] [Google Scholar]
- 14.Lau M, Le A, El-Serag HB. Noncardia gastric adenocarcinoma remains an important and deadly cancer in the United States: secular trends in incidence and survival. Am J Gastroenterol. 2006;101:2485. doi: 10.1111/j.1572-0241.2006.00778.x. [DOI] [PubMed] [Google Scholar]
- 15.Peng J, Wang Y. Epidemiology, pathology and clinical management of multiple gastric cancers: A mini- review. Surg Oncol. 2010;227:102–103. doi: 10.1016/j.suronc.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Setala LP, Kosma VM, Marin S, Lipponen PK, Eskelinen MJ, Syrjänen KJ, et al. Prognostic factors in gastric cancer: the value of vascular invasion, mitotic rate and lymphop- lasmacytic infiltration. Br J Cancer. 1996;74:766. doi: 10.1038/bjc.1996.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oshima CT, Lanzoni VP, Iriya K, Forones NM. C-erb B -2 onco-proteins in gastric carcinoma: correlation with clinical stage and prognosis. Int J Biol Markers. 2001;16:250–4. doi: 10.1177/172460080101600405. [DOI] [PubMed] [Google Scholar]
- 18.Grabsch H, Sivakumar S, Gray S, Gabbert HE, Müller W. HER2 expression in gastric cancer: Rare, heterogeneous and of no prognostic value - conclusions from 924 cases of two independent series. Cell Oncol. 2010;32(1-2):57–65. doi: 10.3233/CLO-2009-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaptain S, Tan LK, Chen B. Her-2/ neu and breast cancer. Diagn Mol Pathol. 2001;10:139. doi: 10.1097/00019606-200109000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Slamon DJ, Leyland Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, et al. Use of chemotherapy plus a mon - oclonal antibody against HER 2 for metastatic breast cancer that over expresses HER2 . N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 21.Pritchard KI, Shepherd LE, O'Malley FP, Andrulis IL, Tu D, Bramwell VH, et al. HER2 and Responsiveness of Breast Cancer to Adjuvant Chemotherapy. N Eng J Med. 2006;354:2103–11. doi: 10.1056/NEJMoa054504. [DOI] [PubMed] [Google Scholar]
- 22.Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, et al. Efficacy and safety of trastuzumab as a single agent in first - line treatment of HER2 - over expressing metastatic breast cancer .J. Clin. Oncol. 2002;20:719. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 23.Jain S, Filipe MI, Gullic WJ, Linehan J, Morris RW. CerbB-2 proto-oncogen expression and its relationship to survival in gastric carcinoma: an immunohistochemical study on archival material. Int J Cancer. 1991;48(5):668–71. doi: 10.1002/ijc.2910480506. [DOI] [PubMed] [Google Scholar]