Abstract
Background
Periodontal disease is a chronic destructive disease which occurs in adults, young people, and children. Periodontal disease and periodontal pathogens have been associated with several systemic diseases and more recently, several studies have suggested the relationship between periodontal disease and cancer. Studies with adjustment for the effect of smoking exposure, have found significant positive associations with different cancer sites. This review has outlined recent epidemiologic researches pointing to a possible role for tooth loss and periodontal disease in carcinogenesis.
Methods
In this review, articles were selected from PubMed between1995 and June 2010 including human. Amongst 5,984 articles identified from the electronic search, 17 articles were selected for a full-text reading based on the inclusion and the exclusion criteria.
Results
Nine out of 10 case-control studies reported a significant increase in the risk of oral cancer in patients with periodontitis and one with no significant association. Among 6 studies examining esophageal cancer and periodontal disease, 5 studies found a significant association between them and one study failed to find a significant increased risk of cancer. Also amongst 5 studies which focused on upper gastrointestinal, gastric cancer, and periodontal disease, 4 studies found an increased risk of cancer while one study did not report any relationship. In lung cancer evaluations, 3 out of 4 studies showed some levels of association between lung cancer and periodontal disease but after adjustment for smoking, no relationship were found. Three cohort studies have evaluated overall cancer rates in periodontal patients; two of them found small but significant association between cancers and periodontal disease.
Conclusion
The results indicate that there is a possible link between cancer and severe periodontal disease after adjustment for smoking and drinking habits.
Keywords: Periodontitis, Tooth loss, Oral hygiene, Mouth neoplasm
Introduction
Periodontal disease is a chronic destructive disease, which occurs in adults, young people and children [1]. Periodontal pathogens which are found in the dental biofilm cause gingival inflammation (gingivitis). Periodontitis occurs when periodontal tissue destruction and alveolar bone loss happen [2, 3]. Prevalence of the periodontitis depends on the geographic area and characteristics of people [1, 2]. The total prevalence of severe periodontitis ranges from 10 to 15 percent and most of them are due to mild periodontal disease like gingivitis [2, 4]. There are many risk factors for periodontal like gender, [2] tobacco, diabetes, nutrition [4], Body Mass Index (BMI) [5], socioeconomic status, and access to dental care [1].
Infections caused by periodontal pathogens like Porphyromonas gingivalis, Actinobacillus actinomycetemcomitans, Treponema denticola, and Tannerella forsythensis are contributed for periodontitis [4, 6, 7].
By the way, it seems that some systemic conditions such as cardiovascular disease [8], diabetes mellitus [9], preterm birth [10], osteoporosis [11, 12], respiratory disease [13], and systemic infections are related to periodontal status [14, 15]. Recently, some studies have reported an association between oral cancers[16-18], cancer of upper gastrointestinal system[19], lung, and periodontal disease [3, 20,21].The purpose of this paper is to review the studies which reported an association between tooth loss and periodontal disease as a risk factor for cancer in human, independent of other known risk factors.
Materials and Methods
To review the association between periodontal disease and cancer, five steps were undertaken: An electronic search was performed using Medline, PubMed from1995 to June 2010.
Step1
The appropriate terms were extracted from articles and books. These terms were as follows:
1. Periodontal disease and cancer 2. Periodontal disease and oral cancer 3. Periodontal disease and esophageal cancer 4. Periodontal disease and lung cancer 5. Periodontal disease and gastric cancer 6. Periodontal disease and upper gastrointestinal cancer
Step2
The terms above were searched separately and resulted in 5984 articles for periodontal disease and cancer terms, 2918 articles for periodontal disease and oral cancer terms, 34 articles for periodontal disease and esophageal cancer terms, 144 articles for periodontal disease and lung cancer terms, 51 articles for periodontal disease and gastric cancer terms and 16 articles for periodontal disease and upper gastrointestinal cancer.
Step3
The titles of articles were reviewed and the appropriate ones were selected. In this step 115 articles were selected.
Step4
Abstracts were reviewed and selecting the articles was based on the inclusion and exclusion criteria. In this step 17 articles were selected. Inclusive criteria: All articles which directly evaluated the relation between periodontal disease and cancer.
Exclusion Criteria: Studies which did not directly evaluate the relation between periodontal disease and cancer. Also Studies that were in the form of case series and case reports and studies with fewer than 100 patients and finally studies which were not adjusted for smoking and drinking habits.
Step 5
Reviewing of full text of the 17 selected articles and extracting relevant information. To categorize data, the included studies were ranked according to their design and sample size. Using these criteria, scientific validation was determined as follows:
• Scientifically and Clinically Validated (SCV): Systematic reviews of RCTs (Randomized clinical trials) or two or more RCTs + ≥ 100 patients or one RCT and two or more prospective studies + ≥150 patients.
Clinically Well Documented (CWD): One RCT and two or more prospective studies + ≥40 patients or no RCTs but at least three prospective studies + ≥60 patients or no RCTs but two or fewer prospective studies + ≥100 patients.
Clinically Documented (CD): No RCTs, at least two prospective + any retrospective studies + ≤ 40 patients- or no RCTs, retrospective studies + ≥ 60 patients.
Clinically Insufficiently Documented (CID): None of the above, expert opinion only, case report only [22].
Literature Review
The association between cancer, tooth loss, and periodontal disease has been evaluated by several studies [1]. Some found a significant increase in oral cancer risk in patients with periodontitis; while others reported tobacco and alcohol are the main risk factor in oral cancer. The following section will review 17 studies on the risk of tooth loss and periodontal disease in cancer.
In 1995 Bundgaard et al. reported results of a study which compared questionnaires of 161 cases with intra-oral squamous cell carcinoma and 400 healthy cases in Denmark [23]. The questionnaires included consumption of tobacco, alcohol, and periodontal status. After adjustment for duration and frequency of tobacco and alcohol consumption, subjects with 15 teeth and less showed a two-fold increase risk in cancer.
Dr Rezende designed a cross-sectional prospective study, comparing dental health and periodontal status (CPITN) of 50 untreated oral squamous cell carcinoma and 50 healthy subjects. After adjustment for age, sex, smoking and drinking habits; periodontal examination clarified that, 76% of subjects with cancer had periodontal pocket depths of 6 mm or greater but in the control group 10% of the subjects had periodontal packets [24].
In 2001, Garrote compared oral hygiene, smoking, and drinking history of 200 oral cancer cases with 200 control subjects in Cuba. Poor oral Periodontal Disease and Tooth Loss as Risks for Cancer conditions (Silness and Loe plaque index more than 40%) and the number of missing teeth were 4.6 times more in oral cancer compared with control subjects. Positive smoking and drinking history also was 2.6 fold more in cancer cases [25].
In another study, Guha et al. compared condition of mouth, toothbrush use, and daily mouthwash use in 924 head and neck cancer cases with 928 controls individuals. After sex, gender, alcohol, and smoking adjustment, the rate of poor oral hygiene was higher in test group compared to the control group. Also those who do not use toothbrush and daily mouthwash were more frequent in cancer cases. The results indicate that periodontal disease (which was indicated by a poor condition of mouth and missing teeth) and not using mouthwash may be independent causes of cancers of head, neck, and esophagus [26].
In a study by Hiraki 5,240 cancer subjects and 10,480 healthy controls were examined from 2000 to 2005. Tooth loss was categorized into four groups as follow group 1: number of remaining teeth ≥21; group 2: with 9 to 20 teeth; group 3: with 1 to 8 teeth; and group 4: with no remaining teeth. Paired in sex, age, tobacco, and alcohol consumption; the fewer number of remaining teeth were associated with increased risk of head, neck, esophageal, and lung cancers [27]. Marshall et al. examined relation between tobacco, alcohol, dental, dietary factors and oral cancer from 1975 to 1983. One control was matched to each of 290 oral cancers, with all information which was collected by interview. Loss of 11 or more teeth showed 2.7-fold increased risk of oral cancer after adjustment for alcohol and smoking [28].
The department of nutrition in Harvard University School of Public Health designed a prospective study with postal questionnaires. After adjusting of age, gender, fruit and vegetable intake, smoking, and drinking habit; no significant increase in risk of oral cancer were found but poor condition of mouth and missing 16 or more teeth were associated with oral cancer after smoking and alcohol control [29].
Rosenquist et al. analyzed oral status as risk factors for oral and oropharyngeal squamous cell carcinoma (OOSCC) in Southern Sweden [29]. This study was conducted in132 cases and 320 controls; examination had 2 parts: panoramic radiographs and a modified gingival bleeding index (GBI). According to the result of this study, poor oral hygiene and visible plaque have affected person's susceptibility for cancer, after adjustment for alcohol and smoking. Subjects, who had lost more than 20 teeth, had 3-fold increased risk for cancer [30].
Tezel in 2007 published a study using pre-existing data from admitted patients between 1999 and 2005. Tezel compared 51 men with SCC of tongue with 54 healthy subjects. After adjustment for age, sex, and smoking; assessment of panoramic radiographs for measurement of alveolar bone loss revealed each millimetre of alveolar bone loss lead to 5.23-fold increase in the risk of tongue cancer [31].
Zheng et al. studied relationship between dentition status and the risk of oral cancer in Beijing, between 1989 and 1990. This study assessed questionnaires of 404 cases and controls who noted the number of their missing teeth [32]. The result was categorized by gender. Any tooth loss with and without tooth replacement, caused 2-3 fold increase in the risk of oral cancer for men and a 5-8 fold increased risk for women. A positive relation was observed between tooth lost, smoking, and alcohol drinking with more than 15 times increased risk of oral cancer [32].
Between 2003 and 2007, Abnet conducted a case-control study, examined the relation between tooth loss and esophageal squamous cell carcinoma (ESCC) in Iran. They assessed 283 ESCC cases and 560 controls adjusted in age and gender, alcohol consumption, and smoking. Subjects with ESCC had significantly higher DMFT (decayed, missing, or filled teeth) and received less dental care compared with controls [33].In another study, Abnet et al. categorized participants in a chemoprevention trial to three groups: 1.Subjects with tooth loss less than the median number of tooth lost for all subjects 2. Subjects with tooth loss greater than the median number of tooth lost for all subjects 3. Subjects with tooth loss equal to the median number of tooth lost for all subjects
According to this study,13% increased risk of total death was observed in subjects with greater than median number of tooth lost; 35% increased risk of upper gastrointestinal cancer death and 28% increased risk of heart disease death and no significantly increased risk of death from cancer in other sites. They also adjusted alcohol and smoking between three groups [34].
In the last study by Abnet et al. conducted in 49 esophageal SCC, 66 esophageal/gastric cardia adenocarcinoma, and 179 gastric non-cardia adenocarcinomas in1999. In subjects paired in age, sex, tobacco smoking results showed significantly increased risk for gastric non-cardia cancer. No statistically significant relations were found between tooth loss and esophageal SCC or esophageal/gastric cardia adenocarcinoma [35].
In a case-control study Watabe et al. compared 242 cases with gastric cancer and 484 controls, after adjustment for smoking and drinking habits. According to this study, the loss of 10 teeth and more resulted in a 2-fold increased risk for gastric cancer [36].
According to Tu et al. study the association between periodontal pathogens and lung cancer is controversial. In a recent study conducted in Scotland, after adjusted in age, gender, BMI, systolic blood pressure and father’s socioeconomic status in 12, 223 cases, no association was observed between tooth loss, with and without adjustment for baseline smoking status, and lung cancer [37].
Michaud et al. selected 1043 subjects with colorectal cancer, 698 melanoma, 678 lung cancers, and 541 advanced prostate cancers. Significant association was observed between the periodontal disease, kidney, pancreas, and hematological cancers after adjustment for alcohol and smoking, while there was no association for lung cancer [38].
Cabrera et al. studied the relation between tooth loss and cancer in 1462 women [39]. There was no significant association between tooth loss and cancer in this study after adjustment for alcohol and smoking. Also no association was reported between tooth loss and C-reactive protein in this study.
According to the above validation criteria, the level of evidence of the articles we reviewed about the association of periodontal disease and oral cancer as well as gastric cancer, lung cancer, upper GI, and esophageal cancer is placed in CWD. These data are summarized in table 1.
Table 1.
Validation of studies on association of periodontal disease and cancers
| Cancer site | Number of study | Systematic reviews of RCTs | Randomized controlled trial | Prospective studies | Retrospective studies | Range of the sample sizes in studies | Scientific validation |
|---|---|---|---|---|---|---|---|
| Oral cancer | 10 | 0 | 0 | 4 | 6 | ≥100 | CWD |
| gastric cancer | 5 | 0 | 0 | 2 | 3 | ≥100 | CWD |
| Lung cancer | 3 | 0 | 0 | 1 | 2 | ≥100 | CWD |
| Upper GI and Esophageal Cancer | 7 | 0 | 0 | 2 | 5 | ≥100 | CWD |
| Total cancer | 3 | 0 | 0 | 0 | 3 | ≥100 | CWD |
CWD=Clinically Well Documented, RCTs=Randomized Clinical Trials
Results
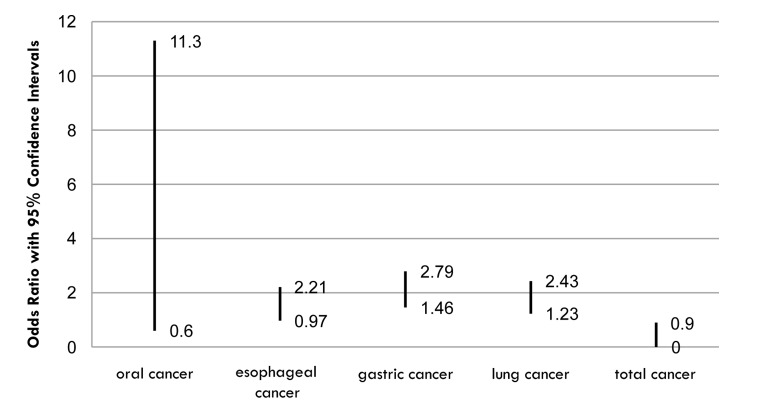
The results of this review are summarized in tables 2 and 3. The association between oral cancer and tooth loss or periodontal disease has been evaluated in several studies. Among 10 studies, which focused on oral cancer and periodontal disease or missing teeth, nine case-control studies reported a significant increase in risk of oral cancer (odds ratio (OR) ranging from0.6 to 11.3) while one case found no significant association between them. All studies were adjusted for alcohol and smoking habits. These results indicated significant increase in oral cancer risk in patients with tooth loss or other parameters of periodontal disease.
Table 2.
Studies support association cancer with periodontal disease.
| Cancer site | Author | Population | Study design | Risk estimate | Oral health status | Adjusted factor | Result |
|---|---|---|---|---|---|---|---|
| Oral cancer | Bundgaard et al. [23] | 161cases, 400 controls; Denmark Baseline age range: 45 to75 | Case-control | OR: 1.73 (1.23, 2.43) | Loss of teeth and dental condition by exam (good, average, poor) | Age, gender, tobacco and alcohol consumption | Significantly increased risk of esophageal cancer when missing between 6 and 15 teeth controlled for smoking |
| Oral cancer | De Rezende et al.[24] | 150 patients with oral and oropharyngeal squamous cell carcinoma | Case–control | No OR calculated | CPITNB and DMFTC | Age and sex, smoking and drinking habits | 76% of subjects in cancer group showed >6mm pockets compared to 10% of control group |
| Oral cancer | Garrote et al. [25] | 200 case/control pairs; Cuba Baseline age range: 25–91 | Case–control | OR: 2.74 (1.23, 6.12) <16 teeth lost | Missing teeth | Age, gender , education (years), smoking and drinking habits | Significantly increased risk of oral cancer for patients missing 16 or more teeth after smoking/ETOH adjustment |
| Oral and esophageal cancer | Guha et al .[26] | 924 cases head and neck and esophageal SCCA/928 controls in Europe | Case–control | Europe OR 2.89 | Dental condition by exam (good, average, Poor) | Sex , gender, alcohol and smoking | Poor oral condition significant increased risk of head and neck cancer |
| Oral, Lung, esophageal and gastric cancer | Hiraki et al. [27] | 429 cases head and neck cancer out of 5240 cancer patients and 10,480 control patients in Japan | Case–control | OR 1.68 for 0 remaining teeth | Loss of teeth | Sex, age, tobacco and alcohol consumption | Significantly increased risk of head and neck cancer to decreased number remaining teeth |
| Oral cancer | Marshall, et al. [28] | 290 case/control pairs; Western New York, USA | Case–control | OR: 2.7 (1.1, 6.5) <11 teeth lost | Missing teeth | Age, gender, smoking and alcohol consumption | Significantly increased risk of oral cancer with loss of 11 or more teeth after smoking and alcohol adjustment |
| Oral cancer | Rosenquist et al. [30] | 132 cases, 320 controls; Southern Sweden Baseline age range: 33–89 | Case–control | OR: 5.3 (2.5, 11.3) | Missing teeth, Panoramic radiograph | Age, gender, county, tobacco and alcohol consumption | Significantly increased risk of oral and oropharyngeal cancer for missing over 20 teeth after adjustment for smoking and EtOH |
| Oral cancer | Tezal et al. [31] | 151 cases and 54 controls in the United States | Case–control | OR :5.23 | mm of alveolar bone loss | Sex , age and smoking and alcohol consumption | Significantly increased risk of tongue Cancer with each mm of bone lost after sex, age and smoking adjustment |
| Oral cancer | Zheng et al. [32] | 404 case–control pairs; Beijing, Baseline age range: 18–80 | Case–control | OR: 2.7 (1.1, 6.5) | Missing teeth | Age, gender, smoking, alcohol consumption | Significantly increased risk of tongue cancer with each mm of bone lost after smoking adjustment |
| Esophageal and gastric cancer | Abnet et al. [34] | 283 esophageal SCCA and 560 Controls in Iran | Cohort | OR 2.10 | DMFT and poor hygiene, Loss of teeth | Age, gender tobacco and alcohol consumption | Significantly increased risk of esophageal SCCA with 32 DMFT compared to <15 Also found significant risk with poor oral hygiene and increasing numbers of teeth lost |
| Esophageal cancer | Abnet et al. [35] | 28,790 person cohort; People’s Republic of China 2,625 upper GI deaths Baseline age range: 40–69 | Cohort | OR: 1.35 (1.14, 1.59) Upper GI cancer mortality | Missing teeth | Age, gender, tobacco (never vs. Ever used regularly for 6 months)drinking habits | Significant increase in risk of upper GI deaths with increasing loss of teeth especially in a younger age controlled for smoking and EtOH |
| Upper GI and Gastric cancer | Watabe et al. [36] | 242 cases, 484 controls; Japan Baseline age range: 40–79 | Case–control | OR: 1.73 (1.23, 2.43) <10 teeth lost | Missing teeth | Age, gender, residential area, smoking and drinking habits | Significant increased risk of gastric cancer with more than 20 teeth lost when compared to none lost |
| Total cancer, Lung cancer, esophageal and Gastric cancer | Michaud et al. [38] | 11,328 person cohort; USA 191 lung and bronchus cancer deaths Baseline age range: 25–74 | Cohort | OR: 1.64 (1.19, 2.26) History of Periodontal disease | Missing teeth and history of periodontal disease | Age, smoking and drinking habits profession, race, geographic location, physical activity, | Significantly increased risk of overall cancer for patients with a history of periodontal disease after controlling for smoking |
DMFT = Decayed, missing, or filled teeth, mm= millimeter, SCCA=Squamous cell carcinoma, GI=Gastrointestinal , OR= Odd Ratio
Table 3.
Studies did not support association cancer with periodontal disease
| Cancer site | Author | Population | Study design | Risk estimate | Oral health status | Adjusted factor | Result |
|---|---|---|---|---|---|---|---|
| Oral cancer | Talamini et al. [29] | 132 cases, 148 controls; Italy Baseline age range: 27–86 | Case–control | OR: 1.4 (0.6, 3.1) <16 teeth lost | Missing teeth | Age, gender, fruit and vegetable intake, smoking and drinking habits | No significant increase in risk of oral cancer to increased missing 16 or more teeth found but poor condition of mouth was associated after smoking and EtOH control |
| Esophageal and gastric cancer | Abnet et al. [33] | 179 gastric noncardia cases 66 esophageal/gastric cardia cases ,49 esophageal cases Baseline age range: 50–69 | Cohort | OR: 1.46 (0.97, 2.21) | Missing teeth | Age, education, smoking and drinking habits | No significant association seen for esophageal or gastric cardia cancer with increased loss of teeth |
| Lung cancer and Total cancer | Tu et al.[37] | 12,223 person cohort; Glasgow, Scotland 549 cancer deaths Baseline age range: B30 years | Cohort | OR: 1.00 (0.98, 1.02) | Missing teeth Missing teeth and history of periodontal disease Missing teeth | Age, year of birth, gender, smoking and drinking habits | No significant increase in risk between cancer deaths and increased missing teeth |
| Total cancer | Cabrera et al. [39] | 1,462 women; Gothenburg, Sweden 68 cancer deaths Baseline age range: 38–60 | Cohort | OR: 1.16 (0.90, 1.49) | Missing teeth | Age, smoking, age at first birth, parity, smoking and drinking habits | No significant association between cancer mortality and increased number of missing teeth |
Amongst 6 studies examining esophageal cancer and periodontal disease or tooth loss, 5 cases found a significant association (OR from 0.97 to 2.21), and one study failed to find a significant increase in cancer risk. This means that the evidence did not yet support a specific association between esophageal cancer, periodontal disease with overall poor oral condition (Silnss and Loe plaque index more than 40%), and increased number of missing teeth.
In review of upper gastrointestinal, gastric cancer, and periodontal disease or tooth loss studies, 4 studies found an increased risk of cancer (OR ranging from 1.46 to 2.79), and one study did not find any relationship. Again the evidence did not yet support a specific association between upper gastrointestinal, gastric cancer, and periodontal diseases. In lung cancer evaluations, three out of four studies showed some levels of association between lung cancer and periodontal disease or tooth loss (OR ranged from 1.23 to 2.43) but the relation did not remain after adjustment for smoking, suggesting that smoking is a very important interfering factor, so more studies are needed to clarify this association.
Three cohort studies have evaluated overall cancer rates in periodontal patients indicating two of them found small but significant association between them (OR ranged 0.9 to 2.26). These suggest that while missing teeth may not be an independent risk factor for overall cancer risk, periodontal disease may play a significant role.
Discussion
There are some interfering factors in the assessment of relation between periodontal diseases and cancer [33]. The studies have used different criteria in the measurement of periodontal disease. Some used tooth loss as a marker and others used patient history, clinical, and radiographic findings as criteria for periodontal patients [28]. It should be noted that teeth can be lost due to caries, trauma, or periodontal disease; so it is difficult to eliminate caries and trauma as confounding elements [25].
On the other hand, some environmental factors also appeared to be confounding factor among these studies [17]. Age, sex, gender, genetic, and socioeconomic status are considered as risk factors for both cancer and periodontal disease. In fact studies which are adjusted for these factors can be used for the assessment of periodontal disease as an independent risk factor of cancer [25-27].
In summary, this article showed the possible link between periodontal disease and oral cancer. There is also correlation for esophageal and gastric cancer as well as overall cancer rates. According to this review, periodontal disease can be considered as a risk factor in cancer but additional studies are needed to clarify these suggested associations.
Conclusion
With limitation of this study, additional studies are needed to confirm periodontal disease as a risk factor for various types of cancer and support the possible association between periodontal disease and cancer rates. Consistent and standard criteria of periodontal disease (such as amount of alveolar bone loss) and consistent control of the interfering factors such as smoking and alcohol use will be helpful to identify a possible link between periodontal disease and cancer and it is important to confirm this association’s mechanism.
Figure 1.
Odds ratio ranges of cancers
Acknowledgments
This study was performed in Khorasgan Azad University with kind assistance of periodontology department.
Footnotes
Conflicts of Interest
The authors declare that they have no conflict of interest in this article.
Authors' Contribution
MS proposed the topic and collected initial article, ShA collected all articles about this topic, and MS reviewed these article and wrote the article main structure.
REFERENCES
- 1.Papapanou PN. Periodontal diseases: epidemiology. Ann Periodontol . 1996;1:1–36. doi: 10.1902/annals.1996.1.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Irfan UM, Dawson DV, Bissada NF. Epidemiology of periodontal disease: a review and clinical perspectives. JInt Acad Periodontol . 2001;3:14–21. [PubMed] [Google Scholar]
- 3.Michaud DS, Joshipura K, Giovannucci E, Fuchs CS. A prospective study of periodontal disease and pancreatic cancer in US male health professionals. J Natl Cancer Inst. 2007;99:171–5. doi: 10.1093/jnci/djk021. [DOI] [PubMed] [Google Scholar]
- 4.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;36:1809–20. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 5.Genco RJ, Grossi SG, Ho A, Nishimura F, Murayama Y. A proposed model linking inflammation to obesity, diabetes, and periodontal infections. J Periodontol . 2005;76:2075–84. doi: 10.1902/jop.2005.76.11-S.2075. [DOI] [PubMed] [Google Scholar]
- 6.Oringer RJ. Modulation of the host response in periodontal therapy. J Periodontol. 2002;73:460–70. doi: 10.1902/jop.2002.73.4.460. [DOI] [PubMed] [Google Scholar]
- 7.Gibson FC, Yumoto H, Takahashi Y, Chou HH, Genco CA. InnateimmunesignalingandPorphyromonas gingivalis-accelerated atherosclerosis. J Dent Res. 2006;85:106–21. doi: 10.1177/154405910608500202. [DOI] [PubMed] [Google Scholar]
- 8.Wactawski-Wende J. Periodontal diseases and osteoporosis: association and mechanisms. Ann Periodontol. 2001;6:197–208. doi: 10.1902/annals.2001.6.1.197. [DOI] [PubMed] [Google Scholar]
- 9.Saygun I, Kubar A, Ozdemir A, Slots J. Periodontitis lesions are a source of salivary cytomegalovirus andEpstein-Barr virus. J Periodontal Res. 2005;40:187–91. doi: 10.1111/j.1600-0765.2005.00790.x. [DOI] [PubMed] [Google Scholar]
- 10.Teng YT, Taylor GW, Scannapieco F. Periodontal health and systemic disorders. J Can Dent Assoc. 2002;68:188–92. [PubMed] [Google Scholar]
- 11.Scannapieco FA, Ho AW. Potential associations between chronic respiratory disease and periodontaldisease: analysis of National Health and NutritionExamination Survey III. J Periodontol. 2001;72:50–6. doi: 10.1902/jop.2001.72.1.50. [DOI] [PubMed] [Google Scholar]
- 12.Moutsopoulos NM, Madianos PN. Low-grade inflammation in chronic infectious diseases: paradigm ofperiodontal infections. Ann N Y Acad Sci. 2006;1088:251–64. doi: 10.1196/annals.1366.032. [DOI] [PubMed] [Google Scholar]
- 13.Haraszthy VI, Zambon J, Trevisan M, Zeid M, Genco RJ. Identification of periodontal pathogens in athermanous plaques. J Periodontol. 2000;71:1554–60. doi: 10.1902/jop.2000.71.10.1554. [DOI] [PubMed] [Google Scholar]
- 14.Southerland JH, Taylor GW, Moss K, Beck JD, Offenbacher S. Commonality in chronic inflammatorydiseases: periodontitis, diabetes, and coronary artery disease. J Periodontol. 2006;40:130–43. doi: 10.1111/j.1600-0757.2005.00138.x. [DOI] [PubMed] [Google Scholar]
- 15.Teng YT, Taylor GW, Scannapieco F. Periodontal health and systemic disorders. J Can Dent Assoc. 2002;68:188–92. [PubMed] [Google Scholar]
- 16.Stolzenberg-Solomon RZ, Dodd KW, Blaser MJ, Virtamo J, Taylor PR. Tooth loss, pancreatic cancer, andHelicobacter pylori. Am J Clin Nutr. 2003;78:176–81. doi: 10.1093/ajcn/78.1.176. [DOI] [PubMed] [Google Scholar]
- 17.Hujoel PP, Drangsholt M, Spiekerman C, Weiss NS. An exploration of the periodontitis-cancer association . Ann Epidemiol. 2003;13:312–6. doi: 10.1016/s1047-2797(02)00425-8. [DOI] [PubMed] [Google Scholar]
- 18.Abnet CC, Qiao YL, Mark SD, Dong ZW, Taylor PR. Prospective study of tooth loss and incident esophagealand gastric cancers in China. Cancer Causes Control. 2001;12:847–54. doi: 10.1023/a:1012290009545. [DOI] [PubMed] [Google Scholar]
- 19.Abnet CC, Qiao YL, Dawsey SM, Dong ZW, Taylor PR, Mark SD. Tooth loss is associated with increased risk oftotal death and death from upper gastrointestinal cancer, heart disease, and stroke in a Chinese population-based cohort. Int J Epidemiol. 2005;34:467–74. doi: 10.1093/ije/dyh375. [DOI] [PubMed] [Google Scholar]
- 20.Velly AM, Franco EL, Schlecht N. Relationship between dental factors and risk of upper aerodigestive tract cancer. Oral Oncol. 1998;34:284–91. [PubMed] [Google Scholar]
- 21.Rosenquist K, Wennerberg J, Schildt EB, Bladstrom A, Goran Hansson B, Andersson G. Oral status, oralinfections and some lifestyle factors as risk factors for oral and oropharyngeal squamous cell carcinoma. Acta Otolaryngol. 2005;125:1327–36. doi: 10.1080/00016480510012273. [DOI] [PubMed] [Google Scholar]
- 22.Gallucci GO, Morton D, Weber HP. Loading protocols for dental implants in edentulous patients. Int JOral Maxillofac Implants. 2009;24 Suppl:132. [PubMed] [Google Scholar]
- 23.Bundgaard T, Wildt J, Frydenberg M, Elbrond O, Nielsen JE. Case-control study of squamous cell cancer of the oral cavity in Denmark. Cancer Causes Control. 1995;6:57–67. doi: 10.1007/BF00051681. [DOI] [PubMed] [Google Scholar]
- 24.De Rezende CP, Ramos MB, Daguila CH, Dedivitis RA, Rapoport A. Oral health changes in patients with oral and oropharyngeal cancer. Braz J Otorhinolaryngol. 2008;74:596–600. doi: 10.1016/S1808-8694(15)30609-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandez-Garrote L, Herrero R, Ortiz-Reyes RM, Vaccarella S, Lence-Anta J, Ferbeye L. Risk factors forcancer of the oral cavity and oro-pharynx in Cuba. Br J Cancer. 2001;85:46–54. doi: 10.1054/bjoc.2000.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guha N, Boffetta P, Filho VW, Neto JE, Shangina O, Zaridze D. Oral health and risk of squamous cellcarcinoma of the head and neck and esophagus: results of two multicentric case-control studies. Am J Epidemiol. 2007;166:1159–73. doi: 10.1093/aje/kwm193. [DOI] [PubMed] [Google Scholar]
- 27.Hiraki A, Matsuo K, Suzuki T, Kawase T, Tajima K. Teeth loss and risk of cancer at 14 common sites in Japanese. Cancer Epidemiol Biomarkers Prev. . 2008;17:1222–7. doi: 10.1158/1055-9965.EPI-07-2761. [DOI] [PubMed] [Google Scholar]
- 28.Marshall JR, Graham S, Haughey BP, Shedd D, O’Shea R, Brasure J, et al. Smoking, alcohol, dentition anddiet in the epidemiology of oral cancer. Eur J Cancer B Oral Oncol. 1992;28B:9–15. doi: 10.1016/0964-1955(92)90005-l. [DOI] [PubMed] [Google Scholar]
- 29.Talamini R, Vaccarella S, Barbone F. Oral hygiene, dentition, sexual habits and risk of oral cancer. Br JCancer. 2000;83:1238–42. doi: 10.1054/bjoc.2000.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenquist K, Wennerberg J, Schildt EB, Bladstrom A, Goran Hansson B, Andersson G. Oral status, oral infections and some lifestyle factors as risk factors for oral and oropharyngeal squamous cell carcinoma. Acta Otolaryngol. 2005;125:1327–36. doi: 10.1080/00016480510012273. [DOI] [PubMed] [Google Scholar]
- 31.Tezel M, Sullivan MA, Reid ME, Marshall JR, Hyland A, Loree T. Chronic periodontitis and the risk for tonguecancer. Arch Otolaryngol Head Neck Surg. 2007;133:450–4. doi: 10.1001/archotol.133.5.450. [DOI] [PubMed] [Google Scholar]
- 32.Zheng TZ, Boyle P, Hu HF, Duan J, Jian PJ, Ma DQ, et al. Dentition, oral hygiene, and risk of oral cancer: a case- control study in Beijing, People’s Republic of China. Cancer Causes Control. 1990;1:235–41. doi: 10.1007/BF00117475. [DOI] [PubMed] [Google Scholar]
- 33.Abnet CC, Kamangar F, Islami F, Nasrollahzadeh D, Brennan P, Aghcheli K, et al. Tooth loss and lack of regular oral hygiene are associated with higher risk of esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2008;17:3062–8. doi: 10.1158/1055-9965.EPI-08-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abnet CC, Qiao YL, Dawsey SM, Dong ZW, Taylor PR, Mark SD. Tooth loss is associated with increased risk ofVol 4, No 4, Autumn 2011197total death and death from upper gastrointestinal cancer, heart disease, and stroke in a Chinese population-based cohort. Int J Epidemiol. 2005;34:467–74. doi: 10.1093/ije/dyh375. [DOI] [PubMed] [Google Scholar]
- 35.Abnet CC, Kamangar F, Dawsey SM, Stolzenberg-Solomon RZ, Albanes D, Pietinen P, et al. Tooth loss isassociated with increased risk of gastric non-cardia adenocarcinoma in a cohort of Finnish smokers. Scand J Gastroenterol. 2005;40:681–7. doi: 10.1080/00365520510015430. [DOI] [PubMed] [Google Scholar]
- 36.Watabe K, Nishi M, Miyake H, Hirata K. Lifestyle and gastric cancer: a case-control study. Oncol Rep. 1998;5:1191–4. doi: 10.3892/or.5.5.1191. [DOI] [PubMed] [Google Scholar]
- 37.Tu YK, Galobardes B, Smith GD, McCarron P, Jeffreys M, Gilthorpe MS. Associations between tooth lossand mortality patterns in the Glasgow Alumni Cohort. Heart. 2007;93:1098–103. doi: 10.1136/hrt.2006.097410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michaud DS, Liu Y, Meyer M, Giovannucci E, Joshipura K. Periodontal disease, tooth loss, and cancer risk in male health professionals: a prospective cohort study. Lancet Oncol. 2008;9:550–8. doi: 10.1016/S1470-2045(08)70106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cabrera C, Hakeberg M, Ahlqwist M, et al. Can the relation between tooth loss and chronic disease beexplained by socio-economic status? A 24-year follow-up from the population study of women in Gothenburg, Sweden. Eur J Epidemiol. 2005;20:229–36. doi: 10.1007/s10654-004-5961-5. [DOI] [PubMed] [Google Scholar]