Abstract
Background
The aim of this study was to evaluate the serum lipoxin A4 (LXA4) and neutrophil/lymphocyte (Ne/Ly) ratio in individuals with achieved systemic risk factors for periodontitis.
Material/Methods
One hundred and eighty volunteers (69 male, 111 female) who were categorized as systemically healthy control, diabetes, hyperlipidemia, obese and menopause were recruited for this cross-sectional study. Sociodemographic characteristics and oral health behaviors were recorded via questionnaire. Clinical periodontal parameters, including plaque index (PI), gingival index (GI), probing pocket depth (PD), clinical attachment level (CAL), sulcus bleeding index (SBI) and decayed, missing, and filled teeth index (DMFT), were assessed. Systemic parameters and LXA4 levels were evaluated in serum samples.
Results
Clinical periodontal parameters and DMFT were higher in subjects with achieved systemic risk factors than in healthy subjects. The systemically healthy with periodontitis group had higher serum LXA4 levels than the systemically healthy with non-periodontitis group (P<0.05). The Ne/Ly ratio was higher in the hyperlipidemic group with periodontitis than in the hyperlipidemic group with non-periodontitis (P<0.05). In the control group, serum LXA4 levels were positively correlated with the PD, CAL and SBI.
Conclusions
In the presence of periodontitis, an increase in LXA4 levels and the Ne/Ly ratio in hyperlipidemic patients could contribute to the hypothesis that these parameters could be an indicator in periodontitis and its systemic risk factors.
MeSH Keywords: Chronic Periodontitis, Lipoxins, Lymphocytes, Neutrophils, Risk Factors
Background
It is noteworthy that low-grade inflammation has a central role in several systemic diseases such as diabetes mellitus, hypertension, osteoporosis and cardiovascular disease (CVD) [1]. New therapeutic strategies related to host modulation are crucial to eliminate inappropriate inflammation, which can result in disease due to the current health situation or periodontitis due to gingivitis [1].
Recent studies have been shown that arachidonic acid (AA)-derived inflammatory mediators also facilitate the resolution of inflammation [2]. Lipoxin (LX) is one of these pro-resolving lipid mediators (PLMs) and is generated via 15-lipoxigenase from AA. Unresolved inflammation can be responsible for the development of periodontitis, and LX could have therapeutic value in periodontitis by decreasing neutrophil (Ne) production of reactive oxygen species and pro-inflammatory cytokines [3], which damage tissues, and increasing phagocytosis and the production of anti-inflammatory cytokines such as interleukin-10 [4].
It is well known that periodontitis is associated with achieved systemic risk factors and the presence of systemic disease leads to an increase in periodontal breakdown [5]. The risk factors related to periodontitis can be classified as unmodifiable or modifiable (achieved) risk factors [6]. The relationship between periodontitis and diabetes [7], hyperlipidemia [8], obesity [9] or menopause [10] has been demonstrated previously, and protective and treatment roles for PLMs have been reported in various systemic diseases [1].
Neutrophils have a key position in the pathogenesis of periodontal disease [11]. The Ne/lymphocyte (Ly) ratio (Ne/Ly) has been recently used as an inflammatory marker, and increases in this ratio have been related to various cancers and an elevated risk of death due to CVD [12].
To date, no studies have investigated serum levels of LXA4, one of the first PLMs recognized [4], in patients with achieved systemic risk factors for periodontitis. Therefore, the aim of this study was to evaluate the serum LXA4 levels and the Ne/Ly ratio in patients with periodontitis and its achieved systemic risk factors.
Material and Methods
Study population
This study was conducted in the Department of Internal Medicine at Süleyman Demirel University. The study protocol, which was performed in accordance with the ethical standards outlined in the 1964 Declaration of Helsinki as revised in 2000, was approved by the Local Ethics Committee of Süleyman Demirel University (protocol number 21.08.2013/179). Volunteers for routine medical controls from September 2013 to March 2014 were invited to participate in this cross-sectional study. Five hundred and fifty volunteers were evaluated, among which 180 were eligible for study inclusion. Oral and written consent forms were obtained from each patient.
The exclusion criteria were as follows: pregnancy or lactating at the time of the study; history of chemotherapy, radiotherapy or renal diseases; history of systemic antibiotic administration within the previous 3 months and hormone replacement therapy; history of periodontal treatment within the last 6 months.
Participants were requested to complete a questionnaire regarding their sociodemographics, systemic diseases and medications, oral health behaviors and smoking status. Body mass index (BMI) and waist circumferences (WC) were measured. BMI was calculated as the body weight (kilograms) divided by the height squared (square meters). A BMI ≥30 was defined as obese, and a WC ≥94 cm for men and ≥80 cm for women was classified as abdominal obese according to World Health Organization (WHO) criteria [13]. One hundred and eighty volunteers who met these inclusion criteria were categorized as follows:
no systemic disease or systemically healthy control (C);
type 2 diabetes mellitus (D);
hyperlipidemia (H);
obese (O);
postmenopause (Post/M). Women were subcategorized as premenopause (Pre/M).
Metabolic parameters
Venous blood samples were obtained to measure the fasting blood glucose (FBG), glycated hemoglobin (HbA1c), triglyceride (TRG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), creatinine, alkaline phosphatase (ALP), calcium (Ca), 25-hidroxy-vitamin D3 (Vit D) and Ne and Ly counts, which were determined in the Clinical Biochemistry Laboratory at Süleyman Demirel University Hospital, Isparta, Turkey.
Serum lipoxin levels
The remaining serum samples for routine biochemical parameters were collected into Eppendorf tubes and stored at −80°C until the laboratory analyses were performed. LXA4 levels were determined using a sandwich enzyme linked immunosorbent assay (ELISA) kit (Cusabio Biotech, Wuhan, China).
Periodontal and dental parameters
Only one calibrated dentist (B.D.) performed the periodontal and dental examination by measuring the probing pocket depth (PD), clinical attachment level (CAL), plaque index (PI) [14], gingival index (GI) [15], sulcus bleeding index (SBI) [16] and decayed, missing and filled teeth (DMFT) index [17]. All of the periodontal parameters were evaluated at four sites (mesio-buccal, mid-buccal, disto-buccal, and mid-lingual) around each tooth (including wisdom teeth) using a periodontal probe (Williams periodontal probe, Hu-Friedy, Chicago, IL). Participants were classified as periodontitis (P) or non-periodontitis (NP). The groups were assigned as follows: NP group, individuals with teeth having PD <5 mm; P group, individuals with ≥1 tooth with PD ≥5 mm and CAL ≥4 mm [18].
Statistical analyses
Statistical software was used to evaluate the study data (SPSS 20, IBM, Chicago, IL). The Kolmogorov-Smirnov test was used to identify the normality of the data, and Levene’s homogeneity test was used to assess the homogeneity of the variables. One-way ANOVA and t-tests were used for normally distributed metabolic and clinical periodontal parameters, and the chi-square test for non-parametric sociodemographic variables was performed. Correlations between serum and clinical periodontal parameters were determined by Pearson’s correlation analysis. A value of P<0.05 was considered significant. Multiple regression analyses were performed to examine the relationships between independent variables (systemic parameters) and dependent variables (periodontal parameters). All variables that showed a significant correlation with each dependent variable in correlation test were included in the multiple regression models.
Results
One hundred and eighty volunteers (111 females, 69 males) categorized as C (n=28), D (n=75), H (n=99), O (n=119) and Post/M (n=37). Sociodemographic characteristics of the study population are shown in Table 1. Individuals in the risk factor groups demonstrated an older age and higher BMI and abdominal obesity ratios with periodontitis but a lower education level compared to group C. There were a greater proportion of females in the risk factor groups, and the prevalence of osteoporosis increased with Post/M. In all of the groups, Post/M group had the highest DMFT value. There were no significant differences regarding smoking and oral hygiene habits among the study groups (P>0.05).
Table 1.
Sociodemographic characteristics (n [%]) of the study population.
| Variable | C | D | H | O | Post/M |
|---|---|---|---|---|---|
| Age (years)* | |||||
| 20–30 | 4 (%14.3) | 2 (%2.7) | 7 (%7.1) | 6 (%5) | 0 (%0) |
| 30–40 | 14 (%50) | 9 (%12) | 17 (%17.2) | 23 (%19.3) | 0 (%0) |
| 40–50 | 6 (%21.4) | 18 (%24) | 32 (%32.3) | 36 (%30.3) | 7 (%18.9) |
| 50–60 | 4 (%14.3) | 29 (%38.7) | 28 (%28.3) | 34 (%28.6) | 18 (%48.6) |
| 60–70 | 0 (%0) | 15 (%20) | 13 (%13.1) | 16 (%13.4) | 10 (%27) |
| >70 | 0 (%0) | 2 (%2.7) | 2 (%2) | 4 (%3.4) | 2 (%5.4) |
|
| |||||
| Sex* | |||||
| Male | 19 (%67.9) | 33 (%44) | 40 (%40.4) | 39 (%32.8) | 37 (%100) |
| Female | 9 (%32.1) | 42 (%56) | 59 (%59.6) | 80 (%67.2) | |
|
| |||||
| BMI (kg/m2)* | |||||
| <25 | 28 (%100) | 15 (%20) | 21 (%21.2) | 0 (%0) | 7 (%18.9) |
| 25–30 | 0 (%0) | 21 (%28) | 28 (%28.3) | 50 (%42) | 11 (%29.7) |
| >30 | 0 (%0) | 39 (%52) | 50 (%50.5) | 69 (%58) | 19 (%51.4) |
|
| |||||
| Abdominal obesity* | |||||
| No | 26 (%92.9) | 10 (%13.3) | 14 (%14.1) | 8 (%6.7) | 1 (%2.7) |
| Yes | 2 (7.1) | 65 (%86.7) | 85 (%85.9) | 111 (%93.3) | 36 (%97.3) |
|
| |||||
| Education* | |||||
| Primary school | 5 (%17.9) | 43 (%57.3) | 53 (%53.5) | 64 (%53.8) | 26 (%70.3) |
| High school | 2 (%7.1) | 20 (%26.7) | 31 (%31.3) | 40 (%33.6) | 7 (%18.9) |
| University | 21 (%75) | 12 (%16) | 15 (%15.2) | 15 (%12.6) | 4 (%10.8) |
|
| |||||
| Osteoporosis* | |||||
| No | 28 (100%) | 66 (88%) | 89 (89.9%) | 103 (86.6%) | 24 (64.9) |
| Yes | 0 (0%) | 9 (12%) | 10 (10.1%) | 16 (13.4%) | 13 (35.1) |
|
| |||||
| Smoking | |||||
| None | 23 (%82.1) | 46 (%61.3) | 60 (%60.6) | 77 (%64.7) | 30 (%81.1) |
| Former | 0 (%0) | 15 (%20) | 16 (%16.2) | 20 (%16.8) | 3 (%8.1) |
| <10 cigarettes/day | 5 (%17.9) | 5 (%6.7) | 10 (%10.1) | 9 (%7.6) | 2 (%5.4) |
| >10 cigarettes/day | 0 (%0) | 9 (%12) | 13 (%13.1) | 13 (%10.9) | 2 (%5.4) |
|
| |||||
| Tooth brushing | |||||
| 2–3 times/day | 4 (%14.3) | 7 (%9.3) | 11 (%11.1) | 11 (%9.2) | 6 (%16.2) |
| 1 time/day | 3 (%10.7) | 22 (%29.3) | 24 (%24.2) | 35 (%29.4) | 10 (%27) |
| Less 1 time/day | 21 (%75) | 46 (%61.3) | 64 (%64.6) | 73 (%61.3) | 21 (%21) |
|
| |||||
| Flossing | |||||
| No | 24 (%85.7) | 40 (%53.3) | 61 (%61.6) | 75 (%63) | 23 (%62.2) |
| Yes | 4 (%14.3) | 35 (%46.7) | 38 (%38.4) | 44 (%37) | 14 (%37.8) |
|
| |||||
| Periodontal diagnosis* | |||||
| Periodontitis | 13 (%46.4) | 40 (%53.3) | 59 (%59.6) | 67 (%56.3) | 37 (%100) |
| Non Periodontitis | 15 (%53.6) | 35 (%46.7) | 40 (%40.4) | 52 (%43.7) | 0 (%0) |
Statistically significant difference among the groups (P=0.000).
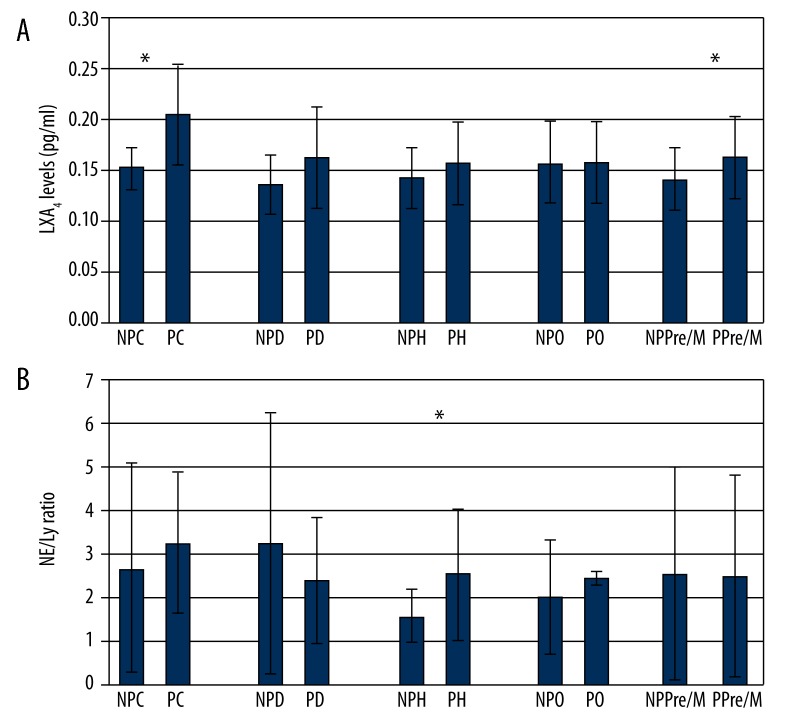
The groups with systemic achieved risk factors demonstrated higher clinical periodontal parameters and FBG levels and lower vitamin D levels compared with the C group (Table 2). The entire menopause groups had periodontitis. For the C and Pre/M groups, individuals with periodontitis had higher serum LXA4 levels than the non-periodontitis controls (Table 3 and Figure 1A). For the hyperlipidemic group, PD, CAL, FBG and the Ne/Ly ratio were higher in the periodontitis group than in the non-periodontitis group (Table 3 and Figure 1B).
Table 2.
Clinical periodontal and systemic parameters (mean ±SD).
| Variable | C n=28 |
D n=75 |
H n=99 |
O n=119 |
Post/M n=37 |
P |
|---|---|---|---|---|---|---|
| PI | 1.24±0.35 | 1.73±0.71 | 1.74±0.68 | 1.70±0.67 | 1.61±0.61 | * |
| GI | 1.11±0.24 | 1.43±0.45 | 1.40±0.37 | 1.42±0.39 | 1.48±0.48 | † |
| PD (mm) | 2.64±0.34 | 3.01±0.65 | 2.97±0.63 | 3.00±0.58 | 2.87±0.53 | ‡ |
| CAL (mm) | 2.66±0.37 | 3.38±0.93 | 3.28±0.89 | 3.31±0.85 | 3.27±0.83 | † |
| SBI | 1.82±0.45 | 2.47±0.80 | 2.41±0.69 | 2.47±0.71 | 2.58±0.78 | † |
| DMFT | 14.96±5.09 | 15.67±7.78 | 13.52±6.77 | 14.82±7.63 | 18.97±7.92 | § |
| FBG (mg/dl) | 88.41±6.60 | 202.01±96.51 | 160.13±91.03 | 150.54±81.30 | 139.34±61.77 | † |
| HbA1c (%) | 5.70 | 9.16±3.01 | 8.50±3.21 | 8.33±3.00 | 7.40±1.62 | N.S. |
| TC (mg/dl) | 169.84±12.58 | 199.01±61.19 | 190.82±53.47 | 189.15±49.18 | 199.87±48.10 | N.S. |
| TRG (mg/dl) | 93.42±5.23 | 218.49±139.20 | 208.30±123.25 | 204.95±127.37 | 194.72±125.91 | * |
| HDL (mg/dl) | 44.95±4.12 | 45.19±11.55 | 42.84±9.81 | 44.87±11.58 | 49.83±15.18 | N.S. |
| LDL (mg/dl) | 104.37±9.83 | 113.65±56.20 | 107.39±47.95 | 105.96±41.66 | 114.77±42.16 | N.S. |
| TC/HDL | 3.79±0.13 | 4.49±1.10 | 4.55±1.12 | 4.42±1.17 | 4.24±1.22 | N.S. |
| AST (U/L) | 20.88±5.11 | 26.54±16.17 | 23.65±13.87 | 25.21±13.21 | 27.63±14.90 | N.S. |
| ALT (U/L) | 42.44±23.57 | 30.79±23.16 | 25.87±19.00 | 28.05±20.49 | 27.16±19.37 | N.S. |
| BUN (mg/dl) | 13.38±1.60 | 13.62±5.82 | 13.01±5.73 | 13.49±5.72 | 13.49±5.54 | N.S. |
| Creatinine (mg/dl) | 0.93±0.11 | 0.96±0.26 | 0.94±0.27 | 0.93±0.27 | 0.93±0.26 | N.S. |
| ALP (U/L) | 79.17±15.71 | 95.65±28.37 | 84.46±20.91 | 88.62±27.70 | 111.57±39.50 | N.S. |
| Ne/Ly | 2.82±2.27 | 2.48±1.56 | 2.41±1.43 | 2.43±1.49 | 2.57±1.36 | N.S. |
| Ca (mg/dl) | 8.89±0.48 | 9.44±0.64 | 9.44±0.59 | 9.50±0.63 | 9.54±0.59 | N.S. |
| Vit D (nmol/l) | 67.23±28.42 | 28.32±22.20 | 29.00±21.07 | 30.90±25.23 | 36.75±27.07 | † |
| LXA4 (pg/ml) | 0.166±0.037 | 0.161±0.05 | 0.155±0.04 | 0.158±0.04 | 0.165±0.06 | N.S. |
Statistically significant difference among C and D, H, O groups (P<0.05);
statistically significant difference among C and D, H, O, Post/M groups (P<0.05);
statistically significant difference among C and D, O groups (P<0.05);
statistically significant difference among Post/M and H, O groups (P<0.05); N.S. – not significant (P>0.05).
Table 3.
Intergroup and intragroup significant comparisons of periodontal and serum parameters between NP and P groups according to the systemic risk factors (mean ±SD).
| Variable | NPC n=15 |
PC n=13 |
P | NPD n=35 |
PD n=40 |
P | NPH n=40 |
PH n=59 |
P | NPO n=52 |
PO n=67 |
P | NPPre/M n=35 |
PPre/M n=39 |
P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GI | 1.14±0.31 | 1.45±0.04 | * | 1.07±0.35 | 1.38±0.38 | * | |||||||||
| PD (mm) | 2.68±0.34 | 3.02±0.65 | * | 2.50±0.47 | 3.05±0.06 | * | 2.60±0.38 | 3.10±0.64 | * | ||||||
| CAL (mm) | 2.56±023 | 3.04±0.54 | * | 2.52±0.39 | 3.44±0.92 | * | 2.68±0.34 | 3.39±0.92 | * | 2.50±0.47 | 3.40±0.83 | * | 2.60±0.38 | 3.28±0.76 | * |
| SBI | 1.92±0.56 | 2.53±0.07 | * | 1.76±0.69 | 2.44±0.77 | * | |||||||||
| DMFT† | 14.33±8.71 | 16.01±7.84 | 13.89±6.69 | 15.18±0.75 | |||||||||||
| FBG‡,§ (mg/dl) | 86.77±5.11 | 95.60±8.17 | * | 229.20±179.8 | 200.06±89.76 | 106.27±49.10 | 169.86±93.61 | * | 97.67±18.43 | 156.64±8.19 | * | 98.40±37.80 | 136.56±73.54 | * | |
| TRG& (mg/dl) | 93.42±5.23 | 228.33±80.16 | 162.00±88.57 | 171.50±103.11 | 129.31±82.08 | 181.29±81.59 | * | ||||||||
| Vit D¶ (nmol/l) | 69.45±29.31 | 24.65±2.18 | 33.84±12.65 | 31.01±18.03 | |||||||||||
| Ne/Ly | 2.05±1.31 | 2.56±1.50 | * | ||||||||||||
| LXA4 (pg/ml) | 0.153±0.02 | 0.205±0.05 | * | 0.141±0.03 | 0.163±0.04 | * |
Statistically significant difference between the groups P<0.05;
statistically significant difference among PH and PPost/M groups (P<0.05);
statistically significant difference among NPD and NPC, NPO, NPH groups (P<0.05);
statistically significant difference among PD and PO, PPost/M groups (P<0.05);
statistically significant difference among NPC and NPH, NPD groups (P<0.05);
statistically significant difference among NPC and NPH, NPO groups (P<0.05).
Figure 1.
(A) Intragroup comparisons of the LXA4 levels according to the periodontal diagnosis. (B) Intragroup comparisons of the Ne/Ly ratios according to the periodontal diagnosis. * Significant difference between groups (P<0.05).
Significant correlations between serum markers and clinical periodontal parameters are shown in Table 4. In the C group, TC/HDL was positively correlated with PI, PD and CAL. PI and GI were positively correlated with the Ne/Ly ratio for the D and H groups. For the C group, serum LXA4 levels were positively correlated with PD, CAL and SBI.
Table 4.
Significant correlations between serum markers and clinical periodontal parameters.
| Variable | C | D | H | O | Post/M | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | r | P | |
| PI-Ne/Ly | 0.278 | 0.018* | 0.233 | 0.022* | ||||||
| PI-HDL | −0.542 | 0.016* | ||||||||
| PI-TC/HDL | 0.977 | 0.000† | ||||||||
| GI-DMFT | 0.491 | 0.008† | 0.457 | 0.000† | 0.351 | 0.000† | 0.389 | 0.000† | 0.573 | 0.000† |
| GI-Ne/Ly | 0.291 | 0.013* | 0.225 | 0.027* | ||||||
| GI-TC | 0.925 | 0.000† | ||||||||
| GI-TG | 0.781 | 0.000† | ||||||||
| GI-LDL | 0.574 | 0.010† | ||||||||
| PD-HDL | −0.617 | 0.005† | ||||||||
| PD-TC/HDL | 0.978 | 0.000† | ||||||||
| CAL-HDL | −0.617 | 0.005† | ||||||||
| CAL-TC/HDL | 0.0978 | 0.000† | ||||||||
| SBI-DMFT | 0.471 | 0.011* | 0.461 | 0.000† | 0.271 | 0.007† | 0.371 | 0.000† | 0.632 | 0.000† |
| SBI-TC | 0.811 | 0.000† | ||||||||
| SBI-TG | 0.753 | 0.000† | ||||||||
| LXA4-PD | 0.564 | 0.004† | ||||||||
| LXA4-CAL | 0.609 | 0.002† | ||||||||
| LXA4-SBI | 0.461 | 0.023* | ||||||||
P<0.05;
P<0.01.
The significant coefficients of the variables included in the model after multiple regression analyses are shown in Table 5. In D and O groups, TC/HDL was significantly associated with SBI. While in the D group, Ne/Ly was significantly correlated with GI, in the O group, LXA4 levels were significantly negative correlated with PD. In the H group, TC and LDL were significantly associated with GI. There was also significantly association between Ne/Ly and PI in the H group. In the Post/M group, TC/HDL and Ne/Ly were significantly associated with CAL.
Table 5.
Multiple regression analyses of systemic and periodontal parameters.
| Groups | Variable | Constant | PI (β) | GI (β) | PD (β) | CAL (β) | SBI (β) | R2 | P |
|---|---|---|---|---|---|---|---|---|---|
| C | TC/HDL | 1.016 | – | – | 1.060 | – | – | 0.57 | 0.000* |
| LX | 0.015 | – | – | – | 0.057 | – | 0.371 | 0.027* | |
| H | TC | 232.45 | – | 29.75 | – | – | – | 0.04 | 0.04* |
| LDL | 146.12 | – | 27.83 | – | – | – | 0.05 | 0.039* | |
| Ne/Ly | 1.56 | 0.49 | – | – | – | – | 0.06 | 0.02* | |
| D | TC/HDL | 5.45 | – | – | – | – | 0.39 | 0.08 | 0.03* |
| Ne/Ly | 1.016 | – | 1.01 | – | – | – | 0.085 | 0.013* | |
| O | TC/HDL | 5.72 | – | – | – | – | 0.52 | 0.093 | 0.004* |
| LX | 0.0207 | – | – | −0.016 | – | – | 0.05 | 0.018* | |
| Post/M | TC/HDL | 6.67 | – | – | 1.658 | 0.679 | – | 0.433 | 0.002* |
| Ne/Ly | 0.221 | – | – | – | 0.718 | – | 0.194 | 0.007* |
β – partial standardized regression coefficent; ‘−’ – not applicable. Significant coefficients of the variables included in the model after multiple regression analyses (significant level – P<0.05).
P<0.05 (significant level of the model).
Discussion
To the best our knowledge, this is the first study to investigate serum LXA4 and the Ne/Ly ratio in patients with achieved systemic risk factors for periodontitis.
Multiple chronic diseases are very important in the determination of health costs or health-related outcomes, and the available evidence highlights the significance of achieved systemic risk factors [19]. The relationships between periodontitis and common systemic risk factors for periodontitis such as diabetes [7], hyperlipidemia [8], obesity [9], and postmenopausal osteoporosis [20] are well known. Thus, the achieved systemic risk factors evaluated in our study are based on the results from extensive epidemiologic and clinical studies that are comparatively common in the population [7–10]. Both CVD and metabolic syndrome are complicated diseases characterized by complex risk factors related to morbidity and mortality rather than a single disease, and it is clear that all of the risk factors evaluated in our study are predictable risk factors for CVD or metabolic syndrome.
The prevalence of systemic diseases such as diabetes mellitus, hyperlipidemia, obesity and osteoporosis has been shown to increase with age [21], and our study provided compatible results. It has been stated that periodontitis risk ratio increases from 1, 4 to 5 times in smokers, and use of the cessation protocol contributes to periodontal healing [22]. In our present study, almost the entire population consisted of non-smokers, and therefore, the results were interpreted as independently of the effects of smoking. The contributions of education level to oral and systemic health have been shown, and oral care constitutes a part of systemic health [23]. In accordance with those results our study groups with systemic diseases had low education level and poor oral hygiene habits.
Obesity is a global risk factor for various diseases including diabetes, hyperlipidemia and hypertension [24]. Additionally, obesity related to low vitamin D levels lead to increase in the risk of CVD [25]. Similarly, our study results demonstrated that the risk factor groups had higher BMI [24,25], and abdominal obesity ratios and lower vitamin D levels compared with the control group. Clinical investigations have demonstrated statistically significant correlations between periodontitis and BMI [9,26,27]. Our current findings revealed that the risk factor groups had a higher BMI and periodontal parameters. Thus, our results are consistent with other studies demonstrating positive correlations between periodontitis and obesity [9,26]. According to our findings, significant correlation between TC/HDL and SBI in the obese group could also provide an important support the results of our present study and other researches [9,26,27].
Menopause, which causes decreased estrogen levels, is one of the most important factors in osteoporosis, and postmenopausal women have an increased risk of developing periodontitis [28]. We confirmed that the prevalence of osteoporosis increased with menopause, and the entire menopause group presented periodontitis. In our study Pre/M with periodontitis had higher serum levels of LXA4. When taking into account that LXA4 has significant clinical effects as an estrogen receptor regulator, this knowledge may be useful for describing the anti-inflammatory effects of estrogen [29]. Thus, it can be speculated that LXA4 may have a protective role in the development of periodontitis in premenopausal women according to our present findings.
In this present study, most of the population had hyperlipidemia, which is also an important risk factor for many systemic diseases [24]. The association between serum lipids and periodontitis in systemically healthy [30,31] and hyperlipidemic patients [32,33] has already been reported. Our findings have been confirmed by Fentoğlu et al. [8,33,34] reporting significant positive correlations between the TC/HDL ratio, which is more useful for CVD risk assessment [35], and clinical periodontal parameters in the hyperlipidemic populations. The present results suggested that the TC/HDL was significantly correlated with PI, PD and CAL in the C group. Additionally, while clinical periodontal parameters were positively correlated with serum lipids, there were negative significant correlations between HDL and clinical periodontal parameters. These findings are also consistent with other results obtained for non-hyperlipidemic populations [30,36].
Currently, the Ne/Ly ratio has been used as a marker of inflammation, and it is suggested that this parameter should be used in the CVD risk evaluation [37]. It has been reported previously that leukocyte counts increase in patients with periodontitis [38] and decrease with periodontal treatment [39]. To date, no studies have been conducted to investigate the Ne/Ly ratio in both periodontitis and its achieved systemic risk factors. The present results demonstrated that the Ne/Ly ratio was higher in the PH group than the NPH group. The increased Ne/Ly ratio in the PH group and positive correlations between clinical periodontal parameters and the Ne/Ly ratio in the hyperlipidemic group collectively suggest that the inflammatory response related to periodontitis can be aggravated by hyperlipidemia. In fact, hypercholesterolemia contributes to the monocytic activity associated with increased Ne functions [8].
The present findings demonstrated that the Ne/Ly ratio was correlated positively with PI and GI in the D group, and it can be speculated that it facilitates an inflammatory response in both diabetes and periodontitis. Likewise, it is clear that diabetes leads to an increase in the risk of periodontal diseases [40]. In addition to our findings for diabetes, the increased Ne/Ly ratio in periodontitis patient with hyperlipidemia also can be supported by literature highlighting the role of hyperlipidemia rather than hyperglycemia in the increase in Ne functions [8].
As stated above, this is the first clinical study to investigate the serum levels of LXA4 in patient with periodontitis and its achieved systemic risk factors. According to the present results, systemically healthy and premenopausal subjects with periodontitis had higher serum LXA4 levels than healthy controls with periodontitis. These findings are supported by Russell et al. [29], who reported that LX had considerable effects as an estrogen receptor modulator. Several studies have investigated the protective roles of LXs in periodontitis [41–43]. Elabdeen et al. [43] reported that patients with aggressive periodontitis have higher levels of LXA4 but lower ω-3/ω-6 ratios than healthy controls without periodontal disease. In this present study, increased LXA4 levels were observed in periodontitis groups with systemically healthy. This situation could be clarified as decreased desaturase activity reduces the production of PLMs, whereas inflammation itself increases PLMs activity [43].
A strong positive correlation between inflammation and oxidative stress in periodontitis has been demonstrated [41]. It has been suggested that LX and aspirin-triggered LX have antioxidant effects [42]. Considering the antioxidant effects of LX, the present findings reinforce the hypothesis that increased LXA4 levels due to periodontitis can be associated with an increased oxidant status to limit periodontal destruction. In fact, an increase in oxidant activity in periodontitis has been reported [44]. Our present finding which introduce that there was significantly negative correlation between LXA4 levels and PD after further analyses in the obese group might strengthen our hypothesis regarding LXs.
This study has some limitations, such as a cross-sectional study design, which could introduce a challenge in terms of elucidating the essential role of LXA4 in achieved systemic risk factors of periodontitis, a lack of diet analyses and a rational comparison of PLMs and inflammatory mediators. Also, numerous factors have been shown to affect LXA4 levels and the metabolism of other essential fatty acids. Therefore, dietary habits, drugs and other systemic diseases can modify production levels of LXA4 and Ne/Ly ratio [45].
Conclusions
Within the limitations of this study, increased LXA4 levels in systemically healthy groups with periodontitis could suggest the importance of PLMs in the link between periodontitis and systemic diseases. Increased Ne/Ly ratio in the hyperlipidemic group with periodontitis could provide an important contribution to the role of hyperlipidemia in the increase in Ne function, which has major effects on the progression of periodontitis. An understanding of the role of endogenic PLMs such as LXA4 in the achieved systemic risk factors for periodontitis could also lead to new therapeutic strategies (such as the use of resolvins, protectins, statins, and ω-3, among others) in patients with periodontitis and its achieved systemic risk factors. Thus, further controlled longitudinal clinical studies are needed to clarify the potential inflammatory mechanisms underlying periodontitis and its systemic risk factors.
Acknowledgments
The authors thank Mr. Hakan DOĞANGÖNÜL for performing the laboratory analyses.
Footnotes
Source of support: Departmental sources
References
- 1.Das UN. Lipoxins, resolvins, protectins, maresins and nitrolipids, and their clinical implications with specific reference to diabetes mellitus and other diseases: part II. Clin Lipidol. 2013;8(4):465–80. [Google Scholar]
- 2.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8(5):349–61. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borgeson E, Lonn J, Bergstrom I, et al. Lipoxin A(4) inhibits porphyromonas gingivalis-induced aggregation and reactive oxygen species production by modulating neutrophil-platelet interaction and CD11b expression. Infect Immun. 2011;79(4):1489–97. doi: 10.1128/IAI.00777-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serhan CN. Novel lipid mediators and resolution mechanisms in acute inflammation: to resolve or not? Am J Pathol. 2010;177(4):1576–91. doi: 10.2353/ajpath.2010.100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim J, Amar S. Periodontal disease and systemic conditions: a bidirectional relationship. Odontology. 2006;94(1):10–21. doi: 10.1007/s10266-006-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borrell LN, Papapanou PN. Analytical epidemiology of periodontitis. J Clin Periodontol. 2005;32(Suppl 6):132–58. doi: 10.1111/j.1600-051X.2005.00799.x. [DOI] [PubMed] [Google Scholar]
- 7.Preshaw PM, Alba AL, Herrera D, et al. Periodontitis and diabetes: a two-way relationship. Diabetologia. 2012;55(1):21–31. doi: 10.1007/s00125-011-2342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fentoglu O, Bozkurt FY. The Bi-Directional Relationship between Periodontal Disease and Hyperlipidemia. Eur J Dent. 2008;2(2):142–46. [PMC free article] [PubMed] [Google Scholar]
- 9.Akman PT, Fentoglu O, Yilmaz G, Arpak N. Serum plasminogen activator inhibitor-1 and tumor necrosis factor-alpha levels in obesity and periodontal disease. J Periodontol. 2012;83(8):1057–62. doi: 10.1902/jop.2011.110548. [DOI] [PubMed] [Google Scholar]
- 10.Genco RJ, Borgnakke WS. Risk factors for periodontal disease. Periodontol, 2000. 2013;62(1):59–94. doi: 10.1111/j.1600-0757.2012.00457.x. [DOI] [PubMed] [Google Scholar]
- 11.Kantarci A, Oyaizu K, Van Dyke TE. Neutrophil-mediated tissue injury in periodontal disease pathogenesis: findings from localized aggressive periodontitis. J Periodontol. 2003;74(1):66–75. doi: 10.1902/jop.2003.74.1.66. [DOI] [PubMed] [Google Scholar]
- 12.Gibson PH, Croal BL, Cuthbertson BH, et al. Preoperative neutrophil-lymphocyte ratio and outcome from coronary artery bypass grafting. Am Heart J. 2007;154(5):995–1002. doi: 10.1016/j.ahj.2007.06.043. [DOI] [PubMed] [Google Scholar]
- 13.Organization WH. Obesity:preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894(i–xii):1–253. [PubMed] [Google Scholar]
- 14.Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condtion. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 15.Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 16.Muhlemann HR, Son S. Gingival sulcus bleeding – a leading symptom in initial gingivitis. Helv Odontol Acta. 1971;15(2):107–13. [PubMed] [Google Scholar]
- 17.Organisation WH. Oral Health Surveys: Basic Methods. 5th. World Health Organisation; Geneva: 2013. [Google Scholar]
- 18.Cakmak O, Alkan BA, Ozsoy S, et al. Association of gingival crevicular fluid cortisol/dehydroepiandrosterone levels with periodontal status. J Periodontol. 2014;85(8):e287–94. doi: 10.1902/jop.2014.130787. [DOI] [PubMed] [Google Scholar]
- 19.Reynolds MA. Modifiable risk factors in periodontitis: at the intersection of aging and disease. Periodontol 2000. 2014;64(1):7–19. doi: 10.1111/prd.12047. [DOI] [PubMed] [Google Scholar]
- 20.Jeffcoat MK, Lewis CE, Reddy MS, et al. Post-menopausal bone loss and its relationship to oral bone loss. Periodontol 2000. 2000;23:94–102. doi: 10.1034/j.1600-0757.2000.2230109.x. [DOI] [PubMed] [Google Scholar]
- 21.Chung HY, Lee EK, Choi YJ, et al. Molecular inflammation as an underlying mechanism of the aging process and age-related diseases. J Dent Res. 2011;90(7):830–40. doi: 10.1177/0022034510387794. [DOI] [PubMed] [Google Scholar]
- 22.Warnakulasuriya S, Dietrich T, Bornstein MM, et al. Oral health risks of tobacco use and effects of cessation. Int Dent J. 2010;60(1):7–30. [PubMed] [Google Scholar]
- 23.Herd P, Goesling B, House JS. Socioeconomic position and health: the differential effects of education versus income on the onset versus progression of health problems. J Health Soc Behav. 2007;48(3):223–38. doi: 10.1177/002214650704800302. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan PW, Ghushchyan VH, Ben-Joseph R. The impact of obesity on diabetes, hyperlipidemia and hypertension in the United States. Qual Life Res. 2008;17(8):1063–71. doi: 10.1007/s11136-008-9385-7. [DOI] [PubMed] [Google Scholar]
- 25.Lee JH, O’Keefe JH, Bell D, et al. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? J Am Coll Cardiol. 2008;52(24):1949–56. doi: 10.1016/j.jacc.2008.08.050. [DOI] [PubMed] [Google Scholar]
- 26.Boesing F, Patino J, Da Silva V, Moreira E. The interface between obesity and periodontitis with emphasis on oxidative stress and inflammatory response. Obes Rev. 2009;10(3):290–97. doi: 10.1111/j.1467-789X.2008.00555.x. [DOI] [PubMed] [Google Scholar]
- 27.Suvan J, D’Aiuto F, Moles DR, et al. Association between overweight/obesity and periodontitis in adults. A systematic review. Obes Rev. 2011;12(5):e381–404. doi: 10.1111/j.1467-789X.2010.00808.x. [DOI] [PubMed] [Google Scholar]
- 28.Haas AN, Rosing CK, Oppermann RV, et al. Association among menopause, hormone replacement therapy, and periodontal attachment loss in southern Brazilian women. J Periodontol. 2009;80(9):1380–87. doi: 10.1902/jop.2009.090082. [DOI] [PubMed] [Google Scholar]
- 29.Russell R, Gori I, Pellegrini C, et al. Lipoxin A4 is a novel estrogen receptor modulator. FASEB J. 2011;25(12):4326–37. doi: 10.1096/fj.11-187658. [DOI] [PubMed] [Google Scholar]
- 30.Cutler CW, Shinedling EA, Nunn M, et al. Association between periodontitis and hyperlipidemia: cause or effect? J Periodontol. 1999;70(12):1429–34. doi: 10.1902/jop.1999.70.12.1429. [DOI] [PubMed] [Google Scholar]
- 31.Losche W, Karapetow F, Pohl A, et al. Plasma lipid and blood glucose levels in patients with destructive periodontal disease. J Clin Periodontol. 2000;27(8):537–41. doi: 10.1034/j.1600-051x.2000.027008537.x. [DOI] [PubMed] [Google Scholar]
- 32.Noack B, Jachmann I, Roscher S, et al. Metabolic diseases and their possible link to risk indicators of periodontitis. J Periodontol. 2000;71(6):898–903. doi: 10.1902/jop.2000.71.6.898. [DOI] [PubMed] [Google Scholar]
- 33.Fentoglu O, Oz G, Tasdelen P, et al. Periodontal status in subjects with hyperlipidemia. J Periodontol. 2009;80(2):267–73. doi: 10.1902/jop.2009.080104. [DOI] [PubMed] [Google Scholar]
- 34.Fentoglu O, Koroglu BK, Hicyilmaz H, et al. Pro-inflammatory cytokine levels in association between periodontal disease and hyperlipidaemia. J Clin Periodontol. 2011;38(1):8–16. doi: 10.1111/j.1600-051X.2010.01644.x. [DOI] [PubMed] [Google Scholar]
- 35.Naito HK. The association of serum lipids, lipoproteins, and apolipoproteins with coronary artery disease assessed by coronary arteriography. Ann NY Acad Sci. 1985;454:230–38. doi: 10.1111/j.1749-6632.1985.tb11862.x. [DOI] [PubMed] [Google Scholar]
- 36.Losche W, Marshal GJ, Apatzidou DA, et al. Lipoprotein-associated phospholipase A2 and plasma lipids in patients with destructive periodontal disease. J Clin Periodontol. 2005;32(6):640–44. doi: 10.1111/j.1600-051X.2005.00725.x. [DOI] [PubMed] [Google Scholar]
- 37.Varol E, Aksoy F, Ozaydin M, et al. Association between neutrophil-lymphocyte ratio and mitral annular calcification. Blood Coagul Fibrinolysis. 2014;25(6):557–60. doi: 10.1097/MBC.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 38.Shi D, Meng H, Xu L, et al. Systemic inflammation markers in patients with aggressive periodontitis: a pilot study. J Periodontol. 2008;79(12):2340–46. doi: 10.1902/jop.2008.080192. [DOI] [PubMed] [Google Scholar]
- 39.Christan C, Dietrich T, Hagewald S, et al. White blood cell count in generalized aggressive periodontitis after non-surgical therapy. J Clin Periodontol. 2002;29(3):201–6. doi: 10.1034/j.1600-051x.2002.290303.x. [DOI] [PubMed] [Google Scholar]
- 40.Mealey BL, Oates TW. Diabetes mellitus and periodontal diseases. J Periodontol. 2006;77(8):1289–303. doi: 10.1902/jop.2006.050459. [DOI] [PubMed] [Google Scholar]
- 41.Chapple IL, Matthews JB. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol 2000. 2007;43:160–232. doi: 10.1111/j.1600-0757.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 42.Zhou XY, Wu P, Zhang L, et al. Effects of lipoxin A(4) on lipopolysaccharide induced proliferation and reactive oxygen species production in RAW264.7 macrophages through modulation of G-CSF secretion. Inflamm Res. 2007;56(8):324–33. doi: 10.1007/s00011-007-7012-7. [DOI] [PubMed] [Google Scholar]
- 43.Elabdeen HR, Mustafa M, Szklenar M, et al. Ratio of pro-resolving and pro-inflammatory lipid mediator precursors as potential markers for aggressive periodontitis. PloS One. 2013;8(8):e70838. doi: 10.1371/journal.pone.0070838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trivedi S, Lal N, Mahdi AA, et al. Evaluation of antioxidant enzymes activity and malondialdehyde levels in patients with chronic periodontitis and diabetes mellitus. J Periodontol. 2014;85(5):713–20. doi: 10.1902/jop.2013.130066. [DOI] [PubMed] [Google Scholar]
- 45.Das UN. Essential fatty acids and their metabolites could function as endogenous HMG-CoA reductase and ACE enzyme inhibitors, anti-arrhythmic, anti-hypertensive, anti-atherosclerotic, anti-inflammatory, cytoprotective, and cardioprotective molecules. Lipids Health Dis. 2008;7:37. doi: 10.1186/1476-511X-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]