Abstract
Tremendous progress has been made in understanding the functions of γ-tubulin and, in particular, its role in microtubule nucleation since the publication of its discovery in 1989. The structure of γ-tubulin has been determined, and the components of γ-tubulin complexes have been identified. Significant progress in understanding the structure of the γ-tubulin ring complex and its components has led to a persuasive model for how these complexes nucleate microtubule assembly. At the same time, data have accumulated that γ-tubulin has important but less well understood functions that are not simply a consequence of its function in microtubule nucleation. These include roles in the regulation of plus-end microtubule dynamics, gene regulation, and mitotic and cell cycle regulation. Finally, evidence is emerging that γ-tubulin mutations or alterations of γ-tubulin expression play an important role in certain types of cancer and in other diseases.
INTRODUCTION
For many years, the identity of components of microtubule-organizing centers (MTOCs) that nucleate microtubule assembly and establish microtubule polarity was a central unanswered question in the field of mitosis and the cytoskeleton. The discovery of γ-tubulin (Oakley and Oakley, 1989), the key finding that allowed this question to be answered, came from a genetic screen in the fungus Aspergillus nidulans designed to identify genes important for microtubule function (Weil et al., 1986). The existence of members of the tubulin superfamily beyond the microtubule proteins α- and β-tubulin was completely unexpected at the time, and the discovery of γ-tubulin is a powerful validation of the value of forward genetics, in which well-designed screens let the cell tell us what is important rather than us imposing our preconceived notions on the cell.
γ-TUBULIN COMPLEXES NUCLEATE MICROTUBULE ASSEMBLY FROM MICROTUBULE-ORGANIZING CENTERS
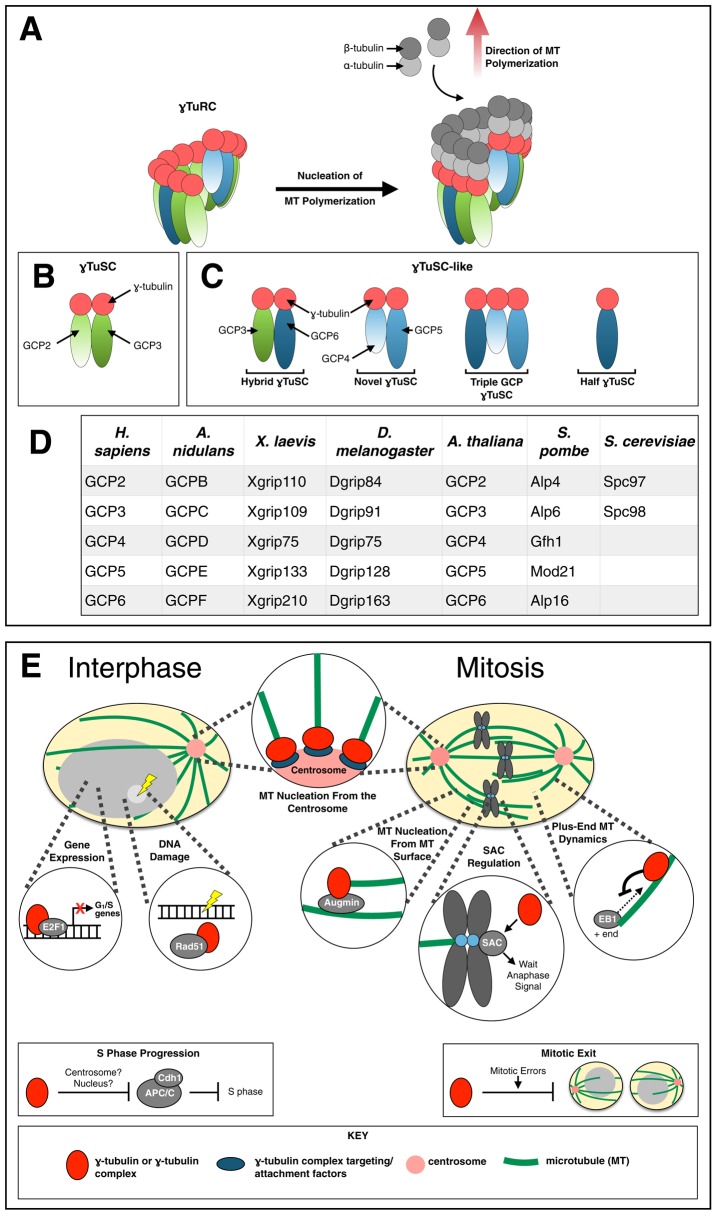
γ-Tubulin is ubiquitous in eukaryotes, and genome-sequencing projects have revealed that there are one to three γ-tubulin genes in eukaryotic genomes (Findeisen et al., 2014). It localizes to structurally diverse MTOCs and, with few exceptions, is required for microtubule nucleation (reviewed by Job et al., 2003). Ring-shaped structures called γTuRCs (γ-tubulin ring complexes) that contain γ-tubulin and associated proteins nucleate microtubule assembly in vitro (Zheng et al., 1995) and in vivo (Figure 1, A–D) (reviewed by Teixido-Travesa et al., 2010; Kollman et al., 2011; Lin et al., 2014). Proteins that associate with γ-tubulin have been identified and designated GRIPs (γ-tubulin ring proteins) or GCPs (γ-tubulin complex proteins) (Figure 1D). Five of these proteins, GCP2–6, are structurally related and contain conserved regions called GRIP motifs (Gunawardane et al., 2000; Guillet et al., 2011).
FIGURE 1:
γ-Tubulin or γ-tubulin complexes in microtubule nucleation and beyond. (A) Nucleation of microtubule assembly by a γTuRC. The γTuRC consists of GCPs that bind to γ-tubulin and to each other, forming a complex in which a ring of γ-tubulin molecules effectively mimics the plus end of a microtubule. It forms a pre-existing nucleus for microtubule assembly. At physiological tubulin concentrations, spontaneous assembly of tubulin into microtubules is rare. Instead, almost all microtubule growth occurs from preformed nucleating structures, principally the γTuRC. (B) In S. cerevisiae only two GCPs exist, Spc97 and Spc98. They assemble with γ-tubulin to form γTuSCs. (C) The finding that GCP2–6 all bind to γ-tubulin raises the possibility that GCPs and γ-tubulin may assemble into alternative γTuSC-like structures (Kollman et al., 2011) that assemble along with γTuSCs to form the γTuRC. (D) Designations for GCPs in various organisms in which they have been studied extensively. (E) Functions of γ-tubulin. γ-Tubulin complexes nucleate microtubules from the centrosome and other MTOCs in both interphase and mitosis or from microtubule surfaces in mitosis. In interphase and mitosis, γ-tubulin has been shown to regulate microtubule plus-end dynamics. In mitosis, it plays a role in the spindle assembly checkpoint (SAC) and the control of mitotic exit. In interphase, γ-tubulin plays an important role in inhibiting APC/CCdh1, thereby promoting the transition from the G1 to the S phase. It also appears to regulate E2F1-mediated gene expression and complexes with Rad51, suggesting a role in the Rad51-mediated DNA damage response.
Beyond GCP2–6, γTuRCs contain three additional, more recently discovered, core subunit proteins, MOZART1 (mitotic spindle-organizing protein associated with a ring of γ-tubulin, or GCP9) and MOZART2A and MOZART2B (GCP8A and GCP8B), which are closely related to each other (Hutchins et al., 2010). MOZART1 is highly conserved in eukaryotes, except in Saccharomyces cerevisiae and close relatives (Hutchins et al., 2010; Lin et al., 2014), and it is essential for mitotic spindle assembly and function in humans and Schizosaccharomyces pombe (Hutchins et al., 2010; Masuda et al., 2013). Arabidopsis thaliana has two MOZART1 homologues, Gip1 and Gip2, that interact with GCP3. They are not essential individually, but Gip1/2 double mutants are embryonic lethal (Nakamura et al., 2012). MOZART2A and MOZART2B are apparently restricted to vertebrates, in which they play a role in recruitment of the γTuRC to the centrosome (Teixido-Travesa et al., 2010; Lin et al., 2014). In addition to the core γTuRC components, various phyla have proteins, unrelated in sequence to GCP2–9, that are involved in targeting γ-tubulin complexes to MTOCs (reviewed extensively by Lin et al., 2014).
THE STRUCTURE OF THE γTuRC SUGGESTS A MECHANISM FOR MICROTUBULE NUCLEATION
High-resolution structures of human γ-tubulin (Aldaz et al., 2005; Rice et al., 2008) and GCP4 (Guillet et al., 2011) and lower-resolution structures of small γ-tubulin complexes (γTuSCs, containing two molecules of γ-tubulin and one molecule each of GCP2 and 3) from S. cerevisiae (Kollman et al., 2008, 2010) have been determined, and these and other findings have led to a persuasive model for the structure of the γTuRC (Kollman et al., 2011). In this model, GCP2–6 each binds directly to γ-tubulin and assemble into a structure that contains 13 γ-tubulin molecules (perhaps containing five γTuSCs and one molecule each of GCP4-6 bound to γ-tubulin; Figure 1A). GCP3 contains a hinge region, and in the current model, movement about this hinge would alter the positioning of γ-tubulin molecules. In one position, the γ-tubulin molecules would be too far apart to nucleate microtubule assembly, but in the other position, the straight position, they would have exactly the same spacing as microtubule protofilaments and would form a perfect template for microtubule assembly. Control of the conformation of GCP3 could therefore regulate the ability of the γTuRC to nucleate microtubule assembly. Important unanswered questions in this area are the exact arrangement of GCP2–6 in the γTuRC and the positioning of MOZART1, 2A, and 2B in the complex. The hinge model, although persuasive, needs to be tested rigorously, and if it is correct, it raises the question of what controls the rotation of GCP3 around its hinge.
γ-TUBULIN COMPLEXES NUCLEATE MICROTUBULES FROM THE SIDES OF EXISTING MICROTUBULES
Although microtubule nucleation from MTOCs is extremely important, in many phyla MTOCs are not apparent. Even in vertebrate mitosis, centrosomes are not always required for the formation of a functional spindle (Khodjakov et al., 2000), and when centrosomes are present, many microtubules may be nucleated independent of the centrosome. Similarly MTOC-independent nucleation of cytoplasmic microtubules occurs in many phyla.
Murata et al. (2005) showed convincingly that cortical microtubules in higher plant cells are nucleated from the sides of existing microtubules at a characteristic angle of 42º with respect to existing microtubules, that γ-tubulin is at the branch points, and that lateral microtubule nucleation is γ-tubulin dependent. Similarly, Janson et al. (2005) demonstrated that microtubules are nucleated from γ-tubulin complexes at the sides of cytoplasmic microtubules in S. pombe. These data established the principle that γ-tubulin complexes function in nucleation of microtubules from the sides of existing microtubules. Data obtained over the intervening decade have identified the augmin complex as a key player in lateral microtubule nucleation.
Augmin is an eight-subunit complex that is important for localizing γ-tubulin to mitotic spindles and for centrosome-independent microtubule generation in mitotic and meiotic spindles (Goshima et al., 2008; Ho et al., 2011; Petry et al., 2011; Hsia et al., 2014). In higher plants, augmin colocalizes with GCPs, and they are corecruited to cortical microtubules. Knockdown of augmin with a microRNA reduces branching lateral nucleation of microtubules (Liu et al., 2014). In Xenopus extracts, augmin and γTuRCs along with the microtubule assembly factor TPX2 and the GTP-bound RanGTPase nucleate microtubule assembly, forming fan-like structures consistent with the possibility that the microtubules are in branched arrays (Petry et al., 2013). Microtubule nucleation persists if augmin or TPX2 are depleted, but fan-like arrays do not form. Monitoring the plus-end microtubule-tracking protein EB-1 revealed that new microtubules are nucleated from the sides of existing microtubules and that the newly nucleated microtubules have the same polarity as the microtubules from which they are nucleated. Such lateral nucleation of microtubule assembly in a mitotic apparatus would result in the proliferation of spindle microtubules but, importantly, would not disrupt the polarity of microtubules in the spindle. These and related findings support a persuasive although not completely proven model that the augmin complex binds to microtubule walls and recruits the γTuRC through NEDD1 or other proteins to initiate new microtubule nucleation (Uehara et al., 2009; Zhu et al., 2009; Johmura et al., 2011). The fact that augmin apparently plays a role in lateral microtubule nucleation in both plants and animal suggests that augmin-mediated lateral microtubule nucleation is widespread in eukaryotes. However, many organisms do not have homologues of all augmin subunits (Lin et al., 2014), and the fact that the polarity of microtubules nucleated from the sides of microtubules in S. pombe is opposite to that of augmin-mediated nucleation (Janson et al., 2005) suggests there are at least two lateral nucleation mechanisms.
The theme that has emerged from two decades of research is that γ-tubulin complexes are almost universally involved in microtubule nucleation. Mechanisms have evolved, however, to control and target these complexes, such that microtubule nucleation occurs when and where it is needed.
γ-TUBULIN BEYOND MICROTUBULE NUCLEATION
The traditional view has been that each protein has a single function, but increasingly, proteins have been shown to be multifunctional. In this regard, increasing evidence indicates that γ-tubulin has functions beyond microtubule nucleation (Cuschieri et al., 2007). In many ways, this makes sense, because γ-tubulin is at the hub of the microtubule cytoskeleton and, along with other MTOC components, is in a unique position to receive and send signal molecules transported by microtubule motors. The problem with interpreting some of these data, however, is that, because γ-tubulin has a critical role in mitosis and disruption of mitosis has many consequences, it is difficult to distinguish putative non–microtubule-nucleation functions of γ-tubulin from consequences of mitotic failure or alteration of microtubule nucleation. Nevertheless, we believe that there is ample evidence that γ-tubulin has important functions beyond microtubule nucleation (summarized in Figure 1E).
γ-TUBULIN PLAYS A ROLE IN THE REGULATION OF MICROTUBULE PLUS-END DYNAMICS
There have been repeated reports from multiple phyla that mutations or deficiencies in γ-tubulin or GCPs alter plus-end microtubule dynamics (Paluh et al., 2000; Vogel et al., 2001; Zimmerman and Chang, 2005; Bouissou et al., 2009) and that γ-tubulin alters the distribution of the plus end–tracking protein Bim1 in S. cerevisiae (Cuschieri et al., 2006) and its homologue, EB1, in Drosophila (Bouissou et al., 2014). This is surprising, since γ-tubulin is most obviously located at the minus end. Two non–mutually exclusive explanations have been proposed. Considering that there is constant bidirectional transport along microtubules, one idea is that γ-tubulin complexes at MTOCs could bind catastrophe or rescue factors or the motor molecules that transport them. This would facilitate loading of molecules that affect microtubule dynamics onto motor molecules, thereby affecting their transport to microtubule plus ends and, consequently, affecting microtubule dynamics at plus ends. Consistent with this idea, γ-tubulin mutations alter binding of motor proteins and/or microtubule stability factors (Vogel et al., 2001; Zimmerman and Chang, 2005; Cuschieri et al., 2006). For example, some γ-tubulin mutations alter the binding of kinesin-14 and kinesin-5 to γ-tubulin complexes, while others alter the interaction of cytoplasmic dynein with spindle pole bodies (SPBs; Li et al., 2005; Rodriguez et al., 2008; Olmsted et al., 2014), although the binding of type 5 and 14 kinesins may be involved in regulating microtubule nucleation rather than plus-end microtubule dynamics (Olmsted et al., 2014).
The second proposed explanation comes from work in Drosophila, in which γTuRC proteins localize along astral microtubules and are proposed to act as rescue factors that arrest microtubule disassembly (Bouissou et al., 2009, 2014). It is worth noting that the proposed rescue activity of γTuRCs distal to MTOCs might not be intrinsic to the γTuRC but a function of its binding to rescue factors, as discussed above. Another possibility that is unfortunately difficult to test is that γ-tubulin mutations alter the microtubule lattice and thereby alter microtubule dynamics.
γ-TUBULIN FUNCTIONS IN THE REGULATION OF MITOSIS AND THE CELL CYCLE
There have also been persuasive reports of perturbation of the regulation of mitotic exit by alterations of γ-tubulin or GCPs. Alanine-scanning mutants of human γ-tubulin expressed as the sole γ-tubulin in S. pombe allowed cells to go through anaphase and cytokinesis when spindle formation was disrupted (Hendrickson et al., 2001). Mutations in the GCP4 and GCP5 homologues of S. pombe caused a failure of mitotic arrest in the presence of the antimicrotubule agent thiabendazole (Vardy and Toda, 2000). Similarly, a γ-tubulin mutation in A. nidulans caused mitotic exit before successful completion of mitosis in a strain in which the establishment of spindle bipolarity was delayed by a type 14 kinesin deletion (Prigozhina et al., 2001). Another allele caused a premature mitotic exit even when the microtubule cytoskeleton was completely disassembled with benomyl (Prigozhina et al., 2004). Likewise, RNA interference (RNAi) of Dgrip84 (GCP2) in Drosophila caused an untimely mitotic exit in the presence of colchicine (Colombie et al., 2006). Thus genetic or RNAi perturbations of γ-tubulin or GCPs in three organisms and four laboratories all point to a microtubule nucleation-independent role for γ-tubulin complexes in the control of mitotic exit.
A possibly related area in which results from several labs have indicated a non–microtubule nucleation function for γ-tubulin is coordination of mitotic events and/or the spindle assembly checkpoint (SAC). In a systematic alanine mutagenesis screen, a number of γ-tubulin mutants were shown to cause premature anaphase entry and defective cytokinesis, suggesting that γ-tubulin regulates the SAC and orderly mitotic exit (Hendrickson et al., 2001). An A. nidulans γ-tubulin allele caused late mitotic events (chromosomal disjunction, spindle elongation, and mitotic exit) to become disordered (Prigozhina et al., 2004). The failure of chromosomal disjunction in this instance was not due to lack of microtubule assembly or force generation, because chromosomes were often stretched dramatically in anaphase, even though they did not disjoin. With respect to mitotic regulation and the SAC, it is worth noting that there are reports that the SAC/mitotic regulatory proteins CDC20 and BubR1 are in a complex with γ-tubulin in mammalian cells (Muller et al., 2006) and that Mad2 binds to Alp4p (GCP2) in S. pombe (Mayer et al., 2006).
Finally, there is incontrovertible evidence that γ-tubulin plays a role in the regulation of cell cycle progression in interphase. In A. nidulans, a γ-tubulin allele, mipAD159, causes failure of inactivation of the anaphase-promoting complex/cyclosome (APC/C) at the G1/S boundary (Nayak et al., 2010). This failure correlates with failure of the APC/C activator Cdh1 to dissociate from the SPB, and it can be overridden by deletion of Cdh1 (Edgerton-Morgan and Oakley, 2012). Importantly, this failure occurs if microtubules are present or if they are completely depolymerized, so it is not a microtubule nucleation phenomenon (Nayak et. al., 2010). This allele also causes mislocalization of the SAC proteins Bub1/R1 (A. nidulans has a single gene with functional domains of Bub1 and BubR1) and Mps1, thereby abrogating the SAC (Edgerton et al., 2015). Note also that analyses of an alp4 (GCP2) mutation indicate Alp4 has an essential function in G1 in S. pombe (Vardy and Toda, 2000). This mutation, moreover, can cause septation, even when mitosis is arrested, by allowing inappropriate recruitment of the Sid1 kinase to the SPB (Vardy et al., 2002). There are also reports that γ-tubulin modulates the activity of E2F transcription factors, which control the expression of a number of genes that are necessary for DNA replication and centrosome duplication (Hoog et al., 2011; Ehlen et al., 2012). The key challenge in understanding the various nonnucleation functions of γ-tubulin will be to move from phenomenology to molecular mechanism.
γ-TUBULIN IN DISEASE
Although the functional significance is not yet clear, there is strong and intriguing evidence of the association of γ-tubulin with tumor suppressor proteins, particularly those involved in DNA repair. It binds to Rad51 in nuclei in response to DNA damage (Lesca et al., 2005) and coimmunoprecipitates with ATR, BRCA1, and C53 from nuclei (Zhang et al., 2007; Hubert et al., 2011; Horejsi et al., 2012). BRCA1/BARD1 binds to and monoubiquitinates γ-tubulin, and there is evidence that this ubiquitination is important in the regulation of centrosome number (Hsu et al., 2001; Starita et al., 2004), a particularly interesting finding, since centrosome amplification is important to cancer progression.
γ-Tubulin expression or localization pattern is altered in a variety of cancers, including some multiple myelomas, non–small cell lung cancers (Maounis et al., 2012; Dementyeva et al., 2013), ductal hyperplasia and breast cancer (Niu et al., 2009; Cho et al., 2010), gliomas and glioblastoma cell lines (Katsetos et al., 2006), and medulloblastomas and medulloblastoma cell lines (Caracciolo et al., 2010). Spontaneous mutations in one of the two human γ-tubulin genes, TUBG1, are associated with lissencephaly and microcephaly (Poirier et al., 2013; Bahi-Buisson et al., 2014). Further effort devoted to studies of both the microtubule nucleation-dependent and nucleation-independent functions of γ-tubulin in different cell and tissue contexts should shed light on the relevance of this cytoskeleton protein in health and disease.
Acknowledgments
The authors apologize for not being able to cite many studies of γ-tubulin due to space limitations. We thank Liz Oakley (University of Kansas) for her comments and proofreading. This work was supported by the Irving S. Johnson Fund of the University of Kansas Endowment (B.R.O.) and National Institutes of Health grants R01GM056312 and R01GM06023 (Y.Z.).
Abbreviations used:
- γTuRC
γ-tubulin ring complex
- γTuSC
γ-tubulin small complex
- APC/C
anaphase-promoting complex/cyclosome
- GCP
γ-tubulin complex protein
- GRIP
γ-tubulin ring protein
- MTOC
microtubule organizing center
- RNAi
RNA interference
- SAC
spindle assembly checkpoint
- SPB
spindle pole body.
Footnotes
REFERENCES
- Aldaz H, Rice LM, Stearns T, Agard DA. Insights into microtubule nucleation from the crystal structure of human γ-tubulin. Nature. 2005;435:523–527. doi: 10.1038/nature03586. [DOI] [PubMed] [Google Scholar]
- Bahi-Buisson N, Poirier K, Fourniol F, Saillour Y, Valence S, Lebrun N, Hully M, Bianco CF, Boddaert N, Elie C, et al. The wide spectrum of tubulinopathies: what are the key features for the diagnosis. Brain. 2014;137:1676–1700. doi: 10.1093/brain/awu082. [DOI] [PubMed] [Google Scholar]
- Bouissou A, Verollet C, de Forges H, Haren L, Bellafche Y, Perez F, Merdes A, Raynaud-Messina B. γ-Tubulin ring complexes and EB1 play antagonistic roles in microtubule dynamics and spindle positioning. EMBO J. 2014;33:114–128. doi: 10.1002/embj.201385967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouissou A, Verollet C, Sousa A, Sampaio P, Wright M, Sunkel CE, Merdes A, Raynaud-Messina B. γ-Tubulin ring complexes regulate microtubule plus end dynamics. J Cell Biol. 2009;187:327–334. doi: 10.1083/jcb.200905060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caracciolo V, D'Agostino L, Dráberová E, Sládková V, Crozier-Fitzgerald C, Agamanolis DP, de Chadarévian JP, Legido A, Giordano A, Dráber P, Katsetos CD. Differential expression and cellular distribution of γ-tubulin and βIII-tubulin in medulloblastomas and human medulloblastoma cell lines. J Cell Physiol. 2010;223:519–529. doi: 10.1002/jcp.22077. [DOI] [PubMed] [Google Scholar]
- Cho EH, Whipple RA, Matrone MA, Balzer EM, Martin SS. Delocalization of γ-tubulin due to increased solubility in human breast cancer cell lines. Cancer Biol Ther. 2010;9:66–76. doi: 10.4161/cbt.9.1.10451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombie N, Verollet C, Sampaio P, Moisand A, Sunkel C, Bourbon HM, Wright M, Raynaud-Messina B. The Drosophila γ-tubulin small complex subunit Dgrip84 is required for structural and functional integrity of the spindle apparatus. Mol Biol Cell. 2006;17:272–282. doi: 10.1091/mbc.E05-08-0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuschieri L, Miller R, Vogel J. γ-tubulin is required for proper recruitment and assembly of Kar9-Bim1 complexes in budding yeast. Mol Biol Cell. 2006;17:4420–4434. doi: 10.1091/mbc.E06-03-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuschieri L, Nguyen T, Vogel J. Control at the cell center: the role of spindle poles in cytoskeletal organization and cell cycle regulation. Cell Cycle. 2007;6:2788–2794. doi: 10.4161/cc.6.22.4941. [DOI] [PubMed] [Google Scholar]
- Dementyeva E, Kryukov F, Kubiczkova L, Nemec P, Sevcikova S, Ihnatova I, Jarkovsky J, Minarik J, Stefanikova Z, Kuglik P, Hajek R. Clinical implication of centrosome amplification and expression of centrosomal functional genes in multiple myeloma. J Transl Med. 2013;11:77. doi: 10.1186/1479-5876-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton H, Paolillo V, Oakley BR. Spatial regulation of the spindle assembly checkpoint and anaphase-promoting complex in Aspergillus nidulans. Mol Microbiol. 2015;95:442–457. doi: 10.1111/mmi.12871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton-Morgan H, Oakley BR. γ-Tubulin plays a key role in inactivating APC/CCdh1 at the G1-S boundary. J Cell Biol. 2012;198:785–791. doi: 10.1083/jcb.201203115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlen A, Rossello CA, von Stedingk K, Hoog G, Nilsson E, Pettersson HM, Jirstrom K, Alvarado-Kristensson M. Tumors with nonfunctional retinoblastoma protein are killed by reduced γ-tubulin levels. J Biol Chem. 2012;287:17241–17247. doi: 10.1074/jbc.M112.357038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findeisen P, Muhlhausen S, Dempewolf S, Hertzog J, Zietlow A, Carlomagno T, Kollmar M. Six subgroups and extensive recent duplications characterize the evolution of the eukaryotic tubulin protein family. Genome Biol Evol. 2014;6:2274–2288. doi: 10.1093/gbe/evu187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima G, Mayer M, Zhang N, Stuurman N, Vale RD. Augmin: a protein complex required for centrosome-independent microtubule generation within the spindle. J Cell Biol. 2008;181:421–429. doi: 10.1083/jcb.200711053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillet V, Knibiehler M, Gregory-Pauron L, Remy MH, Chemin C, Raynaud-Messina B, Bon C, Kollman JM, Agard DA, Merdes A, Mourey L. Crystal structure of γ-tubulin complex protein GCP4 provides insight into microtubule nucleation. Nat Struct Mol Biol. 2011;18:915–919. doi: 10.1038/nsmb.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardane RN, Martin OC, Cao K, Zhang L, Dej K, Iwamatsu A, Zheng Y. Characterization and reconstitution of Drosophila γ-tubulin ring complex subunits. J Cell Biol. 2000;151:1513–1524. doi: 10.1083/jcb.151.7.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson TW, Yao J, Bhadury S, Corbett AH, Joshi HC. Conditional mutations in γ-tubulin reveal its involvement in chromosome segregation and cytokinesis. Mol Biol Cell. 2001;12:2469–2481. doi: 10.1091/mbc.12.8.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho CM, Hotta T, Kong Z, Zeng CJ, Sun J, Lee YR, et al. Augmin plays a critical role in organizing the spindle and phragmoplast microtubule arrays in Arabidopsis. Plant Cell. 2011;23:2606–2618. doi: 10.1105/tpc.111.086892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoog G, Zarrizi R, von Stedingk K, Jonsson K, Alvarado-Kristensson M. Nuclear localization of γ-tubulin affects E2F transcriptional activity and S-phase progression. FASEB J. 2011;25:3815–3827. doi: 10.1096/fj.11-187484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horejsi B, Vinopal S, Sladkova V, Draberova E, Sulimenko V, Sulimenko T, Vosecka V, Philimonenko A, Hozak P, Katsetos CD, Draber P. Nuclear γ-tubulin associates with nucleoli and interacts with tumor suppressor protein C53. J Cell Physiol. 2012;227:367–382. doi: 10.1002/jcp.22772. [DOI] [PubMed] [Google Scholar]
- Hsia KC, Wilson-Kubalek EM, Dottore A, Hao Q, Tsai KL, Forth S, Shimamoto Y, Milligan RA, Kapoor TM. Reconstitution of the augmin complex provides insights into its architecture and function. Nat Cell Biol. 2014;16:852–863. doi: 10.1038/ncb3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu LC, Doan TP, White RL. Identification of a γ-tubulin-binding domain in BRCA1. Cancer Res. 2001;61:7713–7718. [PubMed] [Google Scholar]
- Hubert T, Vandekerckhove J, Gettemans J. Cdk1 and BRCA1 target γ-tubulin to microtubule domains. Biochem Biophys Res Commun. 2011;414:240–245. doi: 10.1016/j.bbrc.2011.09.064. [DOI] [PubMed] [Google Scholar]
- Hutchins JR, Toyoda Y, Hegemann B, Poser I, Heriche JK, Sykora MM, Augsburg M, Hudecz O, Buschhorn BA, Bulkescher J, et al. Systematic analysis of human protein complexes identifies chromosome segregation proteins. Science. 2010;328:593–599. doi: 10.1126/science.1181348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janson ME, Setty TG, Paoletti A, Tran PT. Efficient formation of bipolar microtubule bundles requires microtubule-bound γ-tubulin complexes. J Cell Biol. 2005;169:297–308. doi: 10.1083/jcb.200410119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job D, Valiron O, Oakley B. Microtubule nucleation. Curr Opin Cell Biol. 2003;15:111–117. doi: 10.1016/s0955-0674(02)00003-0. [DOI] [PubMed] [Google Scholar]
- Johmura Y, Soung NK, Park JE, Yu LR, Zhou M, Bang JK, Kim BY, Veenstra TD, Erikson RL, Lee KS. Regulation of microtubule-based microtubule nucleation by mammalian polo-like kinase 1. Proc Natl Acad Sci USA. 2011;108:11446–11451. doi: 10.1073/pnas.1106223108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsetos CD, Reddy G, Dráberová E, Smejkalová B, Del Valle L, Ashraf Q, Tadevosyan A, Yelin K, Maraziotis T, Mishra OP, et al. Altered cellular distribution and subcellular sorting of γ-tubulin in diffuse astrocytic gliomas and human glioblastoma cell lines. J Neuropathol Exp Neurol. 2006;65:465–477. doi: 10.1097/01.jnen.0000229235.20995.6e. [DOI] [PubMed] [Google Scholar]
- Khodjakov A, Cole RW, Oakley BR, Rieder CL. Centrosome-independent mitotic spindle formation in vertebrates. Curr Biol. 2000;10:59–67. doi: 10.1016/s0960-9822(99)00276-6. [DOI] [PubMed] [Google Scholar]
- Kollman JM, Merdes A, Mourey L, Agard DA. Microtubule nucleation by γ-tubulin complexes. Nat Rev Mol Cell Biol. 2011;12:709–721. doi: 10.1038/nrm3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollman JM, Polka JK, Zelter A, Davis TN, Agard DA. Microtubule nucleating γ-TuSC assembles structures with 13-fold microtubule-like symmetry. Nature. 2010;466:879–882. doi: 10.1038/nature09207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollman JM, Zelter A, Muller EG, Fox B, Rice LM, Davis TN, Agard DA. The structure of the γ-tubulin small complex: implications of its architecture and flexibility for microtubule nucleation. Mol Biol Cell. 2008;19:207–215. doi: 10.1091/mbc.E07-09-0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesca C, Germanier M, Raynaud-Messina B, Pichereaux C, Etievant C, Emond S, Burlet-Schiltz O, Monsarrat B, Wright M, Defais M. DNA damage induce γ-tubulin-RAD51 nuclear complexes in mammalian cells. Oncogene. 2005;24:5165–5172. doi: 10.1038/sj.onc.1208723. [DOI] [PubMed] [Google Scholar]
- Li S, Oakley CE, Chen G, Han X, Oakley BR, Xiang X. Cytoplasmic dynein's mitotic spindle pole localization requires a functional anaphase-promoting complex, γ-tubulin, and NUDF/LIS1 in Aspergillus nidulans. Mol Biol Cell. 2005;16:3591–3605. doi: 10.1091/mbc.E04-12-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TC, Neuner A, Schiebel E. Targeting of γ-tubulin complexes to microtubule organizing centers: conservation and divergence. Trends Cell Biol. 2014;25:296–307. doi: 10.1016/j.tcb.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Liu T, Tian J, Wang G, Yu Y, Wang C, Ma Y, Zhang X, Xia G, Liu B, Kong Z. Augmin triggers microtubule-dependent microtubule nucleation in interphase plant cells. Curr Biol. 2014;24:2708–2713. doi: 10.1016/j.cub.2014.09.053. [DOI] [PubMed] [Google Scholar]
- Maounis NF, Draberova E, Mahera E, Chorti M, Caracciolo V, Sulimenko T, Riga D, Trakas N, Emmanouilidou A, Giordano A, et al. Overexpression of γ-tubulin in non-small cell lung cancer. Histol Histopathol. 2012;27:1183–1194. doi: 10.14670/HH-27.1183. [DOI] [PubMed] [Google Scholar]
- Masuda H, Mori R, Yukawa M, Toda T. Fission yeast MOZART1/Mzt1 is an essential γ-tubulin complex component required for complex recruitment to the microtubule organizing center, but not its assembly. Mol Biol Cell. 2013;24:2894–2906. doi: 10.1091/mbc.E13-05-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer C, Filopei J, Batac J, Alford L, Paluh JL. An extended anaphase signaling pathway for Mad2p includes microtubule organizing center proteins and multiple motor-dependent transitions. Cell Cycle. 2006;5:1456–1463. doi: 10.4161/cc.5.13.2912. [DOI] [PubMed] [Google Scholar]
- Muller H, Fogeron ML, Lehmann V, Lehrach H, Lange BM. A centrosome-independent role for γ-TuRC proteins in the spindle assembly checkpoint. Science. 2006;314:654–657. doi: 10.1126/science.1132834. [DOI] [PubMed] [Google Scholar]
- Murata T, Sonobe S, Baskin TI, Hyodo S, Hasezawa S, Nagata T, Horio T, Hasebe M. Microtubule-dependent microtubule nucleation based on recruitment of γ-tubulin in higher plants. Nat Cell Biol. 2005;7:961–968. doi: 10.1038/ncb1306. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Yagi N, Kato T, Fujita S, Kawashima N, Ehrhardt DW, Hashimoto T. Arabidopsis GCP3-interacting protein 1/MOZART 1 is an integral component of the γ-tubulin-containing microtubule nucleating complex. Plant J. 2012;71:216–225. doi: 10.1111/j.1365-313X.2012.04988.x. [DOI] [PubMed] [Google Scholar]
- Nayak T, Edgerton-Morgan H, Horio T, Xiong Y, De Souza CP, Osmani SA, Oakley BR. γ-Tubulin regulates the anaphase-promoting complex/cyclosome during interphase. J Cell Biol. 2010;190:317–330. doi: 10.1083/jcb.201002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Y, Liu T, Tse GM, Sun B, Niu R, Li HM, Wang H, Yang Y, Ye X, Wang Y, Yu Q, Zhang F. Increased expression of centrosomal α, γ-tubulin in atypical ductal hyperplasia and carcinoma of the breast. Cancer Sci. 2009;100:580–587. doi: 10.1111/j.1349-7006.2008.01075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley CE, Oakley BR. Identification of γ-tubulin, a new member of the tubulin superfamily encoded by mipA gene of Aspergillus nidulans. Nature. 1989;338:662–664. doi: 10.1038/338662a0. [DOI] [PubMed] [Google Scholar]
- Olmsted ZT, Colliver AG, Riehlman TD, Paluh JL. Kinesin-14 and kinesin-5 antagonistically regulate microtubule nucleation by γ-TuRC in yeast and human cells. Nat Commun. 2014;5:5339. doi: 10.1038/ncomms6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paluh JL, Nogales E, Oakley BR, McDonald K, Pidoux AL, Cande WZ. A mutation in γ-tubulin alters microtubule dynamics and organization and is synthetically lethal with the kinesin-like protein pkl1p. Mol Biol Cell. 2000;11:1225–1239. doi: 10.1091/mbc.11.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry S, Groen AC, Ishihara K, Mitchison TJ, Vale RD. Branching microtubule nucleation in Xenopus egg extracts mediated by augmin and TPX2. Cell. 2013;152:768–777. doi: 10.1016/j.cell.2012.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry S, Pugieux C, Nedelec FJ, Vale RD. Augmin promotes meiotic spindle formation and bipolarity in Xenopus egg extracts. Proc Natl Acad Sci USA. 2011;108:14473–14478. doi: 10.1073/pnas.1110412108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier K, Lebrun N, Broix L, Tian G, Saillour Y, Boscheron C, Parrini E, Valence S, Pierre BS, Oger M, et al. Mutations in TUBG1, DYNC1H1, KIF5C and KIF2A cause malformations of cortical development and microcephaly. Nat Genet. 2013;45:639–647. doi: 10.1038/ng.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigozhina NL, Oakley CE, Lewis A, Nayak T, Osmani SA, Oakley BR. γ-Tubulin plays an essential role in the coordination of mitotic events. Mol Biol Cell. 2004;15:1374–1386. doi: 10.1091/mbc.E03-06-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigozhina NL, Walker RA, Oakley CE, Oakley BR. γ-Tubulin and the C-terminal motor domain kinesin-like protein, KLPA, function in the establishment of spindle bipolarity in Aspergillus nidulans. Mol Biol Cell. 2001;12:3161–3174. doi: 10.1091/mbc.12.10.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice LM, Montabana EA, Agard DA. The lattice as allosteric effector: structural studies of αβ- and γ-tubulin clarify the role of GTP in microtubule assembly. Proc Natl Acad Sci USA. 2008;105:5378–5383. doi: 10.1073/pnas.0801155105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez AS, Batac J, Killilea AN, Filopei J, Simeonov DR, Lin I, Paluh JL. Protein complexes at the microtubule organizing center regulate bipolar spindle assembly. Cell Cycle. 2008;7:1246–1253. doi: 10.4161/cc.7.9.5808. [DOI] [PubMed] [Google Scholar]
- Starita LM, Machida Y, Sankaran S, Elias JE, Griffin K, Schlegel BP, Gygi SP, Parvin JD. BRCA1-dependent ubiquitination of γ-tubulin regulates centrosome number. Mol Cell Biol. 2004;24:8457–8466. doi: 10.1128/MCB.24.19.8457-8466.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixido-Travesa N, Villen J, Lacasa C, Bertran MT, Archinti M, Gygi SP, Caelles C, Roig J, Luders J. The γeixi revisited: a comparative analysis of interphase and mitotic human γTuRC redefines the set of core components and identifies the novel subunit GCP8. Mol Biol Cell. 2010;21:3963–3972. doi: 10.1091/mbc.E10-05-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara R, Nozawa RS, Tomioka A, Petry S, Vale RD, Obuse C, Goshima G. The augmin complex plays a critical role in spindle microtubule generation for mitotic progression and cytokinesis in human cells. Proc Natl Acad Sci USA. 2009;106:6998–7003. doi: 10.1073/pnas.0901587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardy L, Fujita A, Toda T. The γ-tubulin complex protein Alp4 provides a link between the metaphase checkpoint and cytokinesis in fission yeast. Genes Cells. 2002;7:365–373. doi: 10.1046/j.1365-2443.2002.00530.x. [DOI] [PubMed] [Google Scholar]
- Vardy L, Toda T. The fission yeast γ-tubulin complex is required in G1 phase and is a component of the spindle assembly checkpoint. EMBO J. 2000;19:6098–6111. doi: 10.1093/emboj/19.22.6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J, Drapkin B, Oomen J, Beach D, Bloom K, Snyder M. Phosphorylation of γ-tubulin regulates microtubule organization in budding yeast. Dev Cell. 2001;1:621–631. doi: 10.1016/s1534-5807(01)00073-9. [DOI] [PubMed] [Google Scholar]
- Weil CF, Oakley CE, Oakley BR. Isolation of mip (microtubule-interacting protein) mutations of Aspergillus nidulans. Mol Cell Biol. 1986;6:2963–2968. doi: 10.1128/mcb.6.8.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Hemmerich P, Grosse F. Centrosomal localization of DNA damage checkpoint proteins. J Cell Biochem. 2007;101:451–465. doi: 10.1002/jcb.21195. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Wong ML, Alberts B, Mitchison T. Nucleation of microtubule assembly by a γ-tubulin-containing ring complex. Nature. 1995;378:578–583. doi: 10.1038/378578a0. [DOI] [PubMed] [Google Scholar]
- Zhu H, Fang K, Fang G. FAM29A, a target of Plk1 regulation, controls the partitioning of NEDD1 between the mitotic spindle and the centrosomes. J Cell Sci. 2009;122:2750–2759. doi: 10.1242/jcs.048223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman S, Chang F. Effects of γ-tubulin complex proteins on microtubule nucleation and catastrophe in fission yeast. Mol Biol Cell. 2005;16:2719–2733. doi: 10.1091/mbc.E04-08-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]