Key Clinical Message
The granulocyte-colony-stimulating factor (G-CSF)-producing esophageal carcinosarcoma is extremely rare in esophageal cancer. In the present case, multidisciplinary therapy, which is surgical resection with preoperative chemotherapy, has been effectively treatment to granulocyte-colony-stimulating factor producing esophageal carcinosarcoma of the esophagus.
Keywords: Esophageal carcinosarcoma, granulocyte-colony-stimulating factor, neutrophilia, preoperative chemotherapy
Introduction
Carcinosarcoma is a rare malignant tumor that consists of both carcinomatous and sarcomatous elements. To make a definitive diagnosis, the histological identification of the coexistence of these elements in the tumor and an immunohistochemical analysis is needed 1–4.
Malignant tumors are sometimes accompanied by leukocytosis and other leukemoid symptoms due to the production of granulocyte-colony-stimulating factor (G-CSF), as in paraneoplastic syndromes 5,6. G-CSF belongs to a family of hemopoietic growth factors that regulate the production of granulocytes and macrophages. G-CSF-producing tumors have increasingly been noted to develop in a variety of organs, including the gingiva 7, lung 8, stomach 9, and pancreas 10.
We report an extremely rare case of a G-CSF-producing carcinosarcoma of the esophagus.
Case Report
A 69-year-old Japanese man was referred to our hospital for a further examination of his hmatological disorder. The patient presented with fatigue, swallowing disturbance, low-grade fever, and weight loss. There was no evidence of systemic infection or hmatological disease. Laboratory data showed leukocytosis, neutrophilia (leukocyte count 12,200/μL, 88.6% neutrophils), and elevated serum levels of C-reactive protein reaching 3.0 mg/dL. The level of serum G-CSF was elevated by 86.4 pg/mL (normal level; <39.0 pg/mL). Both radiography and endoscopy of the upper gastrointestinal tract revealed a tumor, 6 cm in diameter, on the posterior wall of the cervical esophagus (Figs.1 and 2). The histological findings of the biopsy specimens from the tumor suggested sarcomatoid squamous cell carcinoma, and a few cells were positive for antibodies against G-CSF (Fig.3). Neither metastasis nor invasion to the adjacent organs was detected by the computed tomography scan. Although the leukocytosis improved (leukocyte count 5700/μL, 72.4% neutrophils) after chemotherapy using cisplatin combined with 5-fluorouracil, the level of serum G-CSF was still abnormally high immediately prior to the operation (Fig.4). Video-assisted thoracoscopic esophagectomy and total pharyngo-laryngo-esophagectomy were performed at the same time. The macroscopic findings of the resected specimens revealed a lobulated polypoid tumor in the cervical esophagus (Fig.5). The histological findings revealed that the tumor consisted of a carcinomatous element of squamous cell carcinoma and a sarcomatous element of spindle-shaped and pleomorphic-shaped cells (Fig.6). In the immunohistochemical analysis, the protein vimentin was diffusely positive, whereas that of AE1/AE3 was focally positive. The expressions of Desmin, S100, and α-smooth muscle actin were negative. The sarcomatous element was found in most of the proper mucosal layer. No tumor cells had invaded the submucosal layer and metastasized to the dissected lymph nodes. According to the TNM classification, the final stage of the tumor was defined as Stage I (T1N0M0) 11. Though the tumor was mildly denatured after neoadjuvant chemotherapy, more than two-thirds of the tumor cells were viable. The serum level of G-CSF and the leukocyte count decreased to within the normal range after the resection (Fig.4). The patient’s recovery was uneventful, with neither tumor recurrence nor elevated serum levels of G-CSF occurring for 5 years after the surgery.
Figure 1.
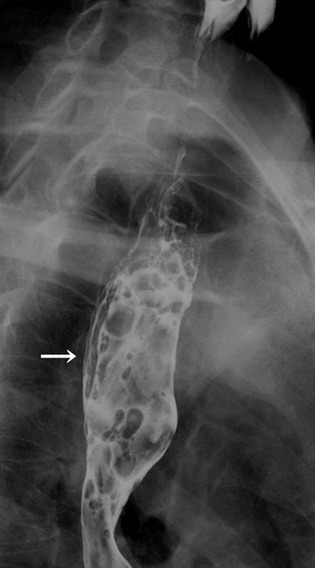
Radiography of the upper gastrointestinal tract. A polypoid tumor, 60 mm in size, was detected on the posterior wall of the cervical esophagus (arrow).
Figure 2.
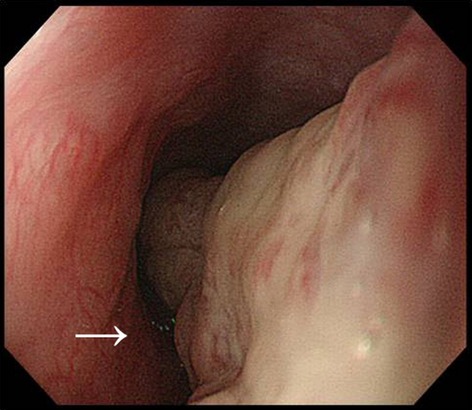
Endoscopy for the upper gastrointestinal tract. A polypoid and mobile tumor with an irregular surface was observed on the posterior wall of the cervical esophagus (arrow).
Figure 3.
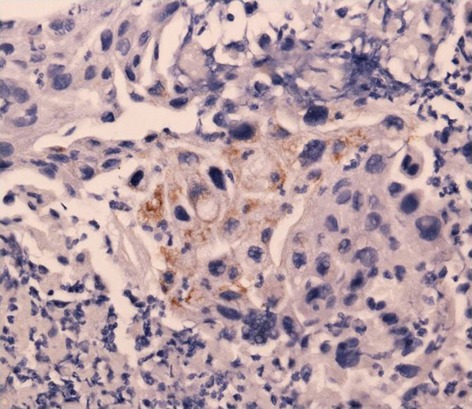
Immunohistochemical staining for the biopsy specimen was positive for granulocyte-colony-stimulating factor.
Figure 4.
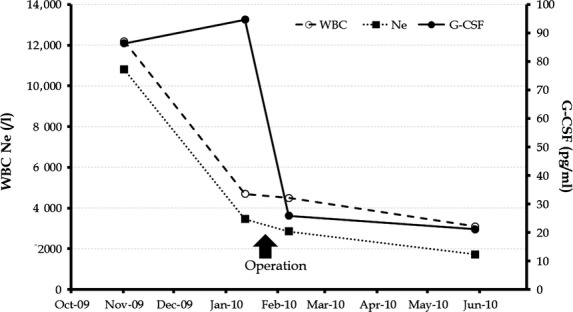
Changes in the serum levels of granulocyte-colony-stimulating factor (G-CSF) and in the counts of white blood cells (WBCs) and neutrophil WBCs (Ne).
Figure 5.
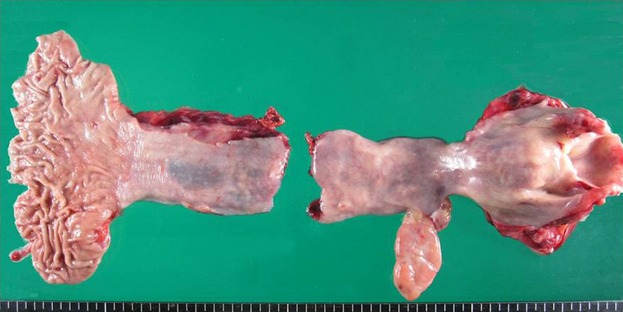
Macroscopic findings of the resected specimen showed a lobulated polypoid tumor that was 60 × 30 mm in diameter.
Figure 6.
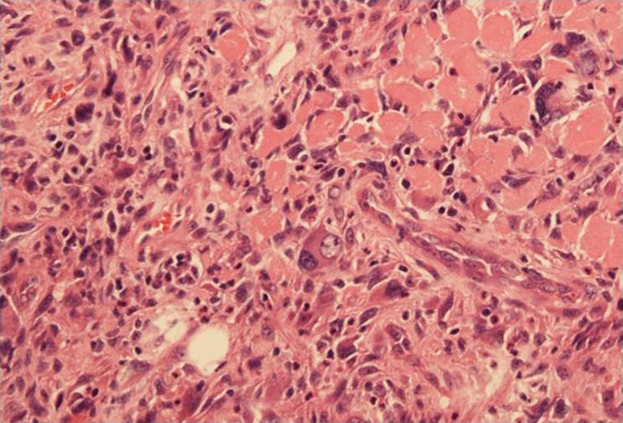
Histological findings after hematoxylin and eosin staining revealed a squamous cell carcinoma component and a sarcomtous component with spindle- and pleomorphic-shaped cells.
Discussion
Esophageal carcinosarcoma tends to assume a polypoid shape more frequently than does squamous cell carcinoma. The clinical symptoms and signs are similar to those of squamous cell carcinoma, including dysphagia, odynophagia, chest pain, and weight loss. The mean age of patients at diagnosis falls within their seventh decade, and a strong male preponderance exists 12. Immunohistochemical analysis is the gold standard for the diagnosis of carcinosarcoma. CEA, EMA, pancreatin, chromogranin A, CD 56, and synaptophysin are highly specific markers for carcinomatous elements, whereas desmin, vimentin, and smooth muscle/sarcomeric actin signal sarcomatous elements 4,13,14.
There are two types of carcinosarcoma: the true carcinosarcoma and the so-called carcinosarcoma 15. Three major theories have been proposed for the pathogenesis of carcinosarcoma. The first theory is that the spindle cell component develops in reaction to the carcinosarcoma. The second posits that two individual stem cells independently or simultaneously transform into malignant cells, progressing thereafter into separate tumors (true carcinosarcoma). The third theory suggests that the individual components are derived from the same malignant stem cell (so-called carcinosarcoma) 15. This third theory has been genetically demonstrated by the TP53 mutation analysis of carcinosarcoma 16. In the present case, the tumor was composed of both carcinomatous and sarcomatous elements. The transitional zone of those elements was observed, and no differentiated cells were detected in the sarcomatous elements, suggesting that both the carcinoma and sarcoma elements had the same origin. Thus, this case was diagnosed as a so-called carcinosarcoma of the esophagus.
Although several treatment modalities are available, including surgical resection, endoscopic resection, and chemoradiotherapy, no specific clinical management was recommended at the time 3,5,17,18. Iyomasa et al. reported that the prognosis of esophageal carcinosarcoma was poorer than that of squamous cell carcinoma in the esophagus due to the former’s more frequent hematogenous metastasis 19. Early detection and surgical resection may improve survive in a localized esophageal carcinosarcoma.
G-CSF-producing tumors have been reported in various organs since 1950 20. The diagnostic criteria for G-CSF-producing tumors include (1) marked leukocytosis, (2) a high serum concentration of G-CSF, (3) normalization of the leukocyte count after tumor resection, and (4) evidence of G-CSF production in tumor cells 8. The present case contains a G-CSF-producing esophageal carcinosarcoma that meets all of these diagnostic criteria. To the best of our knowledge, five cases of G-CSF-producing esophageal carcinosarcomas, including the present case, have been reported in English (Table1) 21–24. All cases of the G-CSF-producing esophageal carcinosarcoma were reported in Japan. The serum levels of G-CSF in the six reported cases ranged from 48 to 286 pg/mL, and the tumor diameters ranged from 40 to 85 mm. Regarding to prognosis, the survival period was 4 months to more than 60 months.
Table 1.
Clinicopathological findings of granulocyte– colony-stimulating factor producing esophageal carcinosarcoma.
| Author | Year | Age (year) | Sex | G-CSF (pg/ml) | Size (mm) | Type | Depth | LN metastasis | Surgery | Chemotherapy | Prognosis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ota 21 | 1998 | 62 | Male | 286 | 40 | 0-I | sm | Unknown | Resection | Unknown | Unknown |
| Maejima 22 | 2007 | 80 | Male | 111 | 60 | 1 | U | Unknown | No resection | None | Dead in 4 months |
| Sasaki 23 | 2007 | 62 | Male | 108 | 85 | 1 | mp | Positive | Resection | Performed | Dead in 5 months |
| Miyamoto 24 | 2008 | 51 | Male | 48 | 80 | 1 | Ad | Positive | Resection | Performed | 23 months alive |
| Our case | 2014 | 69 | Male | 86.4 | 50 | 0-Ip | mm | Negative | Resection | Performed1 | 60 months alive |
LN, lymph node; sm, submucosa; mp, muscularis propria; Ad, adventitia; mm, muscularis mucosa.
Neoadjuvant chemotherapy.
G-CSF-producing tumors are considered to have a poor prognosis because the autocrine and paracrine mechanisms of G-CSF via the JAK2/STAT3 pathway contribute to the migration and proliferation of cancer cells 25–28. G-CSF, therefore, accelerates the clinical progression of the disease 7,29.
In experiments on G-CSF-expressing tumor cells both in vitro and in vivo, G-CSF dose-dependently stimulated the proliferation and migration of tumor cells. These cells also demonstrated increases in angiogenesis and the recruitment of neutrophils and macrophages 28. Strong neovascularization causes rapid tumor growth and leads to the recruitment of many inflammatory cells 26–28. In addition, reactive oxygen species (ROS) and nitric oxide (NO), which is produced by infiltrated neutrophils, caused DNA damage and genomic alterations in the epithelial cells 30–32. Moreover, the invasive progression of the tumor and remodeling of the extracellular matrix are associated with the infiltration of inflammatory cells 32.
There is no feasible evidence of using neoadjuvant chemotherapy for esophageal carcinosarcoma. In the present case, neoadjuvant chemotherapy with 5-fluorouracil plus cisplatin was performed because the Japan Clinical Oncology Group (JCOG) 9907 study recommended neoadjuvant chemotherapy followed by surgery as a standard treatment for localized advanced squamous cell carcinomas of the thoracic esophagus 33,34. In addition, G-CSF-producing tumors have an aggressive clinical course 7,9,10,35. Chemotherapy with 5-fluorouracil plus cisplatin seemed to be partially effective in the present case, as the leukocytosis was improved, though the serum level of G-CSF was not decreased. Laryngo-pharyngo-esophagectomy was performed in a good condition. Moreover, the pathological findings of the resected specimen revealed that the tumor was mildly denatured. Generally, the prognosis of the G-CSF-producing tumors is poor. Therefore, early detection and multidisciplinary therapy are important for curing G-CSF-producing esophageal carcinosarcomas, due to the current lack of a specific approach. Recently, an in vitro study showed that expressing the JAK2/STAT3 pathway of G-CSF tumors contributed to chemo-resistance against cisplatin and paclitaxel 27. Targeted therapies to inhibit the JAK2/STAT3 pathway are expected to increase the sensitivity of chemotherapy to G-CSF-expressing tumors in the future.
In conclusion, the present report summarizes our experience in treating a patient with an extremely rare G-CSF-producing esophageal carcinosarcoma. In such cases, it is important to make an early diagnosis and determination of a therapeutic strategy, especially to improve the prognosis of a patient with such a tumor.
Conflict of Interest
None declared.
References
- Shimada K, Iwase K, Aono T, Nakai S, Takeda S, Fujii M, et al. Carcinosarcoma of the gallbladder producing alpha-fetoprotein and manifesting as leukocytosis with elevated serum granulocyte colony-stimulating factor: report of a case. Surg. Today. 2009;39:241–246. doi: 10.1007/s00595-008-3833-4. [DOI] [PubMed] [Google Scholar]
- Lokesh V, Naveen T. Pawar YS. Spindle cell sarcoma of esophagus: a rare case presentation. J. Cancer. Res. Ther. 2010;6:100–101. doi: 10.4103/0973-1482.63558. [DOI] [PubMed] [Google Scholar]
- Xu F, Zou WB, Li XP, Xu YM, Qi XF, Hu LH, et al. Multiple carcinosarcomas of the esophagus and stomach. Oncol. Lett. 2013;5:1017–1021. doi: 10.3892/ol.2012.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KW, Lee WY, Hong SW, Chang YG, Lee B. Lee HK. Carcinosarcoma of the stomach: a case report. J. Gastric. Cancer. 2013;13:69–72. doi: 10.5230/jgc.2013.13.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson WA. Granulocytosis in neoplasia. Ann. N. Y. Acad. Sci. 1974;230:212–218. doi: 10.1111/j.1749-6632.1974.tb14451.x. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Sato K, Ohkawa H, Ueyama Y, Okabe T, Sato N, et al. Association of hypercalcemia with tumors producing colony-stimulating factor(s) Cancer Res. 1983;43:2368–2374. [PubMed] [Google Scholar]
- Kobayashi J, Miyazaki A, Yamamot T, Nakamori K, Suzuki R, Kaneko T, et al. Granulocyte colony-stimulating factor-producing squamous cell carcinoma of the lower gingiva: a case report. Head Neck Oncol. 2012;4:35. doi: 10.1186/1758-3284-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano S, Urabe A, Okabe T, Sato N. Kondo Y. Demonstration of granulopoietic factor(s) in the plasma of nude mice transplanted with a human lung cancer and in the tumor tissue. Blood. 1977;49:845–852. [PubMed] [Google Scholar]
- Kawaguchi M, Asada Y, Terada T, Takehara A, Munemoto Y, Fujisawa K, et al. Aggressive recurrence of gastric cancer as a granulocyte-colony-stimulating factor-producing tumor. Int. J. Clin. Oncol. 2010;15:191–195. doi: 10.1007/s10147-010-0023-3. [DOI] [PubMed] [Google Scholar]
- Nakajima A, Takahashi H, Inamori M, Abe Y, Kobayashi N, Kubota K, et al. Anaplastic carcinoma of the pancreas producing granulocyte-colony stimulating factor: a case report. J. Med. Case Rep. 2008;2:391. doi: 10.1186/1752-1947-2-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie H, Sobin MKG. Wittekind C. TNM classification of malignant tumours. 7th ed. New York, NY: Wiley-Blackwell; 2009. [Google Scholar]
- Ziauddin MF, Rodriguez HE, Quiros ED, Connolly MM. Podbielski FJ. Carcinosarcoma of the esophagus–pattern of recurrence. Dig. Surg. 2001;18:216–218. doi: 10.1159/000050133. [DOI] [PubMed] [Google Scholar]
- Bansal M, Kaneko M. Gordon RE. Carcinosarcoma and separate carcinoid tumor of the stomach. A case report with light and electron microscopic studies. Cancer. 1982;50:1876–1881. doi: 10.1002/1097-0142(19821101)50:9<1876::aid-cncr2820500937>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Teramachi K, Kanomata N, Hasebe T, Ishii G, Sugito M. Ochiai A. Carcinosarcoma (pure endocrine cell carcinoma with sarcoma components) of the stomach. Pathol. Int. 2003;53:552–556. doi: 10.1046/j.1440-1827.2003.01508.x. [DOI] [PubMed] [Google Scholar]
- Madan AK, Long AE, Weldon CB. Jaffe BM. Esophageal carcinosarcoma. J. Gastrointest. Surg. 2001;5:414–417. doi: 10.1016/s1091-255x(01)80071-8. [DOI] [PubMed] [Google Scholar]
- Amatya VJ, Takeshima Y, Kaneko M. Inai K. Esophageal carcinosarcoma with basaloid squamous carcinoma and rhabdomyosarcoma components with TP53 mutation. Pathol. Int. 2004;54:803–809. doi: 10.1111/j.1440-1827.2004.01759.x. [DOI] [PubMed] [Google Scholar]
- Hung JJ, Li AF, Liu JS, Lin YS. Hsu WH. Esophageal carcinosarcoma with basaloid squamous cell carcinoma and osteosarcoma. Ann. Thorac. Surg. 2008;85:1102–1104. doi: 10.1016/j.athoracsur.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Pesenti C, Bories E, Danisi C, Monges G. Giovannini M. Endoscopic treatment of esophageal carcinosarcoma: report of a case. Endoscopy. 2004;36:95. doi: 10.1055/s-2004-814125. [DOI] [PubMed] [Google Scholar]
- Iyomasa S, Kato H, Tachimori Y, Watanabe H, Yamaguchi H. Itabashi M. Carcinosarcoma of the esophagus: a twenty-case study. Jpn. J. Clin. Oncol. 1990;20:99–106. [PubMed] [Google Scholar]
- Hughes WF. Higley CS. Marked leukocytosis resulting from carcinomatosis. Ann. Intern. Med. 1952;37:1085–1088. doi: 10.7326/0003-4819-37-5-1085. [DOI] [PubMed] [Google Scholar]
- Ota S, Kato A, Kobayashi H, Yonezumi M, Yamaguchi J, Musashi M, et al. Monoclonal origin of an esophageal carcinosarcoma producing granulocyte-colony stimulating factor: a case report. Cancer. 1998;82:2102–2111. doi: 10.1002/(sici)1097-0142(19980601)82:11<2102::aid-cncr4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Maejima K, Watanabe M, Komine O, Mizutani S, Bou H, Tokunaga A, et al. Granulocyte-colony stimulating factor-producing esophageal carcinosarcoma: a case report. Esophagus. 2007;4:117–120. [Google Scholar]
- Sasaki K, Natsugoe S, Higashi M, Okumura H, Matsumoto M, Hanazono K, et al. Esophageal carcinosarcoma with granulocyte colony-stimulating factor: a case report. Esophagus. 2007;4:129–134. [Google Scholar]
- Miyamoto K, Shibata S. Kawasaki H. Carcinosarcoma of the esophagus producing granulocyte-colony stimulating factor: report of a case. Esophagus. 2008;5:171–175. [Google Scholar]
- Tachibana M, Miyakawa A, Tazaki H, Nakamura K, Kubo A, Hata J, et al. Autocrine growth of transitional cell carcinoma of the bladder induced by granulocyte-colony stimulating factor. Cancer Res. 1995;55:3438–3443. [PubMed] [Google Scholar]
- Gutschalk CM, Herold-Mende CC, Fusenig NE. Mueller MM. Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor promote malignant growth of cells from head and neck squamous cell carcinomas in vivo. Cancer Res. 2006;66:8026–8036. doi: 10.1158/0008-5472.CAN-06-0158. [DOI] [PubMed] [Google Scholar]
- Kumar J, Fraser FW, Riley C, Ahmed N, McCulloch DR. Ward AC. Granulocyte colony-stimulating factor receptor signalling via Janus kinase 2/signal transducer and activator of transcription 3 in ovarian cancer. Br. J. Cancer. 2014;110:133–145. doi: 10.1038/bjc.2013.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazawa H, Okada F, Kobayashi T, Tada M, Mori Y, Une Y, et al. Infiltration of neutrophils is required for acquisition of metastatic phenotype of benign murine fibrosarcoma cells: implication of inflammation-associated carcinogenesis and tumor progression. Am. J. Pathol. 2003;163:2221–2232. doi: 10.1016/S0002-9440(10)63580-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuzuki H, Fujieda S, Sunaga H, Noda I. Saito H. Expression of granulocyte colony-stimulating factor receptor correlates with prognosis in oral and mesopharyngeal carcinoma. Cancer Res. 1998;58:794–800. [PubMed] [Google Scholar]
- Weitzman SA, Weitberg AB, Clark EP. Stossel TP. Phagocytes as carcinogens: malignant transformation produced by human neutrophils. Science. 1985;227:1231–1233. doi: 10.1126/science.3975611. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Wu HH, Momose H, Rademaker A. Oyasu R. Marked enhancement of rat urinary bladder carcinogenesis by heat-killed Escherichia coli. Cancer Res. 1992;52:5329–5333. [PubMed] [Google Scholar]
- Coussens LM. Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi K, Koizumi W, Tanabe S, Sasaki T, Katada C, Azuma M, et al. Current management of esophageal squamous-cell carcinoma in Japan and other countries. Gastrointest. Cancer Res. 2009;3:153–161. [PMC free article] [PubMed] [Google Scholar]
- Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907) Ann. Surg. Oncol. 2012;19:68–74. doi: 10.1245/s10434-011-2049-9. [DOI] [PubMed] [Google Scholar]
- Shimakawa T, Asaka S, Usuda A, Yamaguchi K, Yoshimatsu K, Shiozawa S, et al. Granulocyte-colony stimulating factor (G-CSF)-producing esophageal squamous cell carcinoma: a case report. Int. Surg. 2014;99:280–285. doi: 10.9738/INTSURG-D-13-00265.1. [DOI] [PMC free article] [PubMed] [Google Scholar]


