Key Clinical Message
Maternal status epilepticus can cause fetal hypoxic ischemic encephalopathy that in turn results in acute polyhydramnios caused by fetal dysphagia; thus, acute polyhydramnios is a symptom that should lead to a suspicion of fetal dysphagia caused by hypoxic ischemic encephalopathy.
Keywords: Acute polyhydramnios, dysphagia, hypoxic ischemic encephalopathy, status epilepticus
Introduction
Acute polyhydramnios is defined as a condition fulfilling all the following criteria: (1) onset of excessive amniotic fluid within 1 week of diagnosis; (2) uterus large for dates; (3) pain due to enlarged uterus; and (4) respiratory symptoms 1. The prevalence rate of this condition was extremely low, at 0.04% (19/51,022) for singleton pregnancies 1. The reported causes of this condition include placental chorioangioma, cytomegalovirus (CMV) infection, and fetal malformation, such as anencephaly and esophageal atresia 2–5.
Meanwhile, to the best of our knowledge, there is no report that a maternal hypoxic episode caused fetal dysphagia, which in turn resulted in acute polyhydramnios.
Here, we report a case in which, because of maternal status epilepticus, a fetus developed hypoxic ischemic encephalopathy (HIE) and consequently dysphagia, which caused acute polyhydramnios.
Case
The patient was a 33-year-old female (gravida 1, para 1). At age 22, she underwent extirpation of intracranial arteriovenous malformations. At her initial presentation to our Department of Obstetrics, while she was being maintained on lamotrigine at a dose of 100 mg/day, she had no seizures and her pregnancy course had been uneventful. At 30 weeks and 3 days of gestation, she had a generalized seizure at home and, after more than 1 h from the onset of the seizure, she was rushed to our hospital. On arrival, she had disturbance of consciousness. Her level of consciousness was Glasgow coma scale 5 (E1V1M3), and the seizure persisted. Arterial blood gas analysis showed a pH of 7.038 and a base deficit of 19.2 mmol/L, indicating metabolic acidosis. Meanwhile, the fetal heart rate showed bradycardia in the range of 90–100 beats per minute (bpm). After the mother was placed on mechanical ventilation, the fetal heart rate increased to 110 bpm in 6 min, and 140 bpm in 8 min, showing recovery to the normal range. A computed tomography (CT) scan of the head did not show intracranial hemorrhage. She was diagnosed as having status epilepticus and managed in the intensive care unit (ICU). Meanwhile, fetal heart monitoring after admission to the ICU demonstrated loss of variability and tachycardia. Despite the nonreassuring fetal status, because resolution of the fetal hypoxic state was expected to be achieved by respiratory care of the mother, the pregnancy was not terminated. Fetal heart monitoring showed a normal pulse on the day after admission, and variability and acceleration were also detected on the fifth day after admission. However, the amniotic fluid gradually increased. The amniotic fluid index (AFI), which was 11 cm on admission, rose to 30 cm at 31 weeks and 5 days of gestation, showing acute polyhydramnios. Ultrasonography did not show signs of placental chorioangioma or fetal malformation. CMV infection was not found. Thus, we suspected that fetal dysphagia as a result of maternal status epilepticus caused acute polyhydramnios. Because she developed dyspnea caused by acute polyhydramnios, amniocentesis was performed at 33 weeks and 0 days of gestation. Five days later, labor rapidly progressed, and an infant was vaginally delivered approximately 2 h after the start of labor pain. The baby was male, weighing 1878 g, with Apgar scores of 3 and 8 at 1 and 5 min, respectively. Umbilical cord arterial blood gas analysis showed an arterial blood pH of 7.218 and a base deficit of 4.5 mmol/L. He was admitted to the neonatal intensive care unit. His respiration and circulation were stable, but he had severe dysphagia. A T1-weighted magnetic resonance imaging scan of the head performed on day 17 after birth showed high-intensity areas in the bilateral basal nuclei, thalamus, and pons basal ganglia, which indicated necrosis caused by chronic ischemic changes and corresponded to lesions formed during the fetal stage (Fig.1). In addition, an electroencephalogram showed decreased activity. Although neonatologists diagnosed the infant with a poor neurological prognosis, he is still alive.
Figure 1.
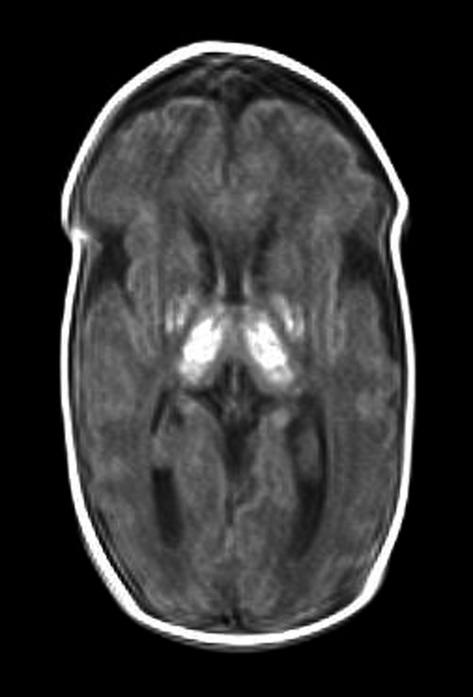
A T1-weighted MRI scan of the infant’s head on day 17 after birth. High-intensity areas are observed in the bilateral basal nuclei, both thalami, and an area from the posterior limb of the internal capsule to the dorsal pons. The volumes of both the cerebrum and cerebellum are small, and the cerebral sulcus is deep. Ventricular enlargement is also observed. These findings suggest fetal ischemia.
Discussion
This case highlights two important clinical issues, namely that maternal status epilepticus can cause encephalopathy due to hypoxia in the infant and that acute polyhydramnios can be a symptom leading to suspected fetal dysphagia caused by HIE.
First, our case demonstrates that maternal status epilepticus can cause encephalopathy due to hypoxia in the infant. In the past, it was believed that status epilepticus during pregnancy was associated with a high fetal and maternal mortality. In fact, a review of 29 cases found nine maternal deaths and 14 fetal deaths 6. However, this review is considered to be limited by publication bias. A large-scale prospective study on pregnancy complicated by epilepsy (conducted by the EURAP Study Group) shows that in 36 cases with status epilepticus, there was no case of either stillbirth or maternal death that was directly caused by status epilepticus 7. Although neurocognitive developmental disorder in fetuses was not considered in these 36 cases, status epilepticus is currently not recognized as life-threatening in mothers and fetuses, as previously considered. In our case, because the umbilical arterial pH and base deficit were within the normal ranges at the time of delivery, we concluded that maternal hypoxia due to status epilepticus caused fetal HIE, which resulted in fetal dysphagia. Thus, women with a history of status epilepticus should be informed at prenatal counseling of the effects of maternal hypoxia on an infant.
Secondly, acute polyhydramnios can be a symptom of fetal dysphagia caused by HIE. Desmedt et al. 1 reported that fetal malformations, such as anencephaly and esophageal atresia, were observed in 11 of 19 cases with acute polyhydramnios, and that the perinatal mortality was 73.1% (14/19), indicating extremely poor prognosis. However, none of the 19 cases showed polyhydramnios induced by an episode of maternal hypoxia. In fact, central dysphagia caused by HIE in a fetus can result from not only diseases that induce maternal hypoxia, such as status epilepticus, as seen in our case, but also umbilical cord compression during pregnancy 8. Thus, the presence of fetal dysphagia should be considered in acute polyhydramnios.
The traditional obstetric teaching is that, when managing a seizure in a pregnant patient, every attempt should be made to stabilize the mother and resuscitate the fetus in utero before making a decision about delivery 9. However, it is unknown how long the time interval is between recovery of the maternal general condition and delivery or whether the time interval should be changed depending on gestational age at the time of seizure onset 9.
In this case, the maternal general condition was improved, and the fetal heart rate was concurrently normalized. Thus, the pregnancy was not terminated to avoid complications of preterm delivery. It is unknown whether the fetus should have been delivered by cesarean section soon after recovery of the maternal general condition. This issue awaits resolution through further accumulation of cases.
Conflict of Interest
The authors have no conflicts of interest to declare.
References
- Desmedt E, Henry OA, Steinberg LH. Beischer NA. Acute and subacute polyhydramnios in singleton pregnancies. Aust. N. Z. J. Obstet. Gynaecol. 1990;30:191–195. doi: 10.1111/j.1479-828x.1990.tb03210.x. [DOI] [PubMed] [Google Scholar]
- Monni G, Paladini D, Ibba RM, Teodoro A, Zoppi MA, Lamberti A, et al. Prenatal ultrasound diagnosis of congenital cystic adenomatoid malformation of the lung: a report of 26 cases and review of the literature. Ultrasound Obstet. Gynecol. 2000;16:159–162. doi: 10.1046/j.1469-0705.2000.00195.x. [DOI] [PubMed] [Google Scholar]
- Bornstein E, Barnhard Y, Ferber A, Segarra P. Divon MY. Acute progression of a unilateral fetal ovarian cyst to complex bilateral cysts causing acute polyhydramnios. J. Ultrasound Med. 2006;25:523–526. doi: 10.7863/jum.2006.25.4.523. [DOI] [PubMed] [Google Scholar]
- Panaccione JL, Esposito WJ. Haller JO. Acute polyhydramnios associated with chorioangioma. A case report. J. Reprod. Med. 1991;36:210–212. [PubMed] [Google Scholar]
- Bondagji N, Manning FA, Martel J, Harman CR. Morrison I. Complete resolution of CMV-associated acute hydramnios by single large volume reduction amniocentesis and maternal indomethacin. A case report. Fetal Diagn. Ther. 1996;11:345–347. doi: 10.1159/000264339. [DOI] [PubMed] [Google Scholar]
- Teramo K. Hiilesmaa VK. Pregnancy and fetal complications in epileptic pregnancies: review of the literature. In: Janz D, Dam M, Bossi L, Helge H, Richens A, Schmidt D, editors; Epilepsy, pregnancy, and the child. New York: Raven Press; 1982. pp. 53–59. [Google Scholar]
- EURAP Study Group. Seizure control and treatment in pregnancy: observations from the EURAP epilepsy pregnancy registry. Neurology. 2006;66:354–360. doi: 10.1212/01.wnl.0000195888.51845.80. [DOI] [PubMed] [Google Scholar]
- Perlitz Y, Ben-Shlomo I. Ben-Ami M. Acute polyhydramnios in term pregnancy may be caused by multiple nuchal cord loops. Ultrasound Obstet. Gynecol. 2010;35:253–254. doi: 10.1002/uog.7543. [DOI] [PubMed] [Google Scholar]
- Kaplan PW, Norwitz ER, Ben-Menachem E, et al. Obstetric risks for women with epilepsy during pregnancy. Epilepsy Behav. 2007;11:283–291. doi: 10.1016/j.yebeh.2007.08.012. [DOI] [PubMed] [Google Scholar]


