Abstract
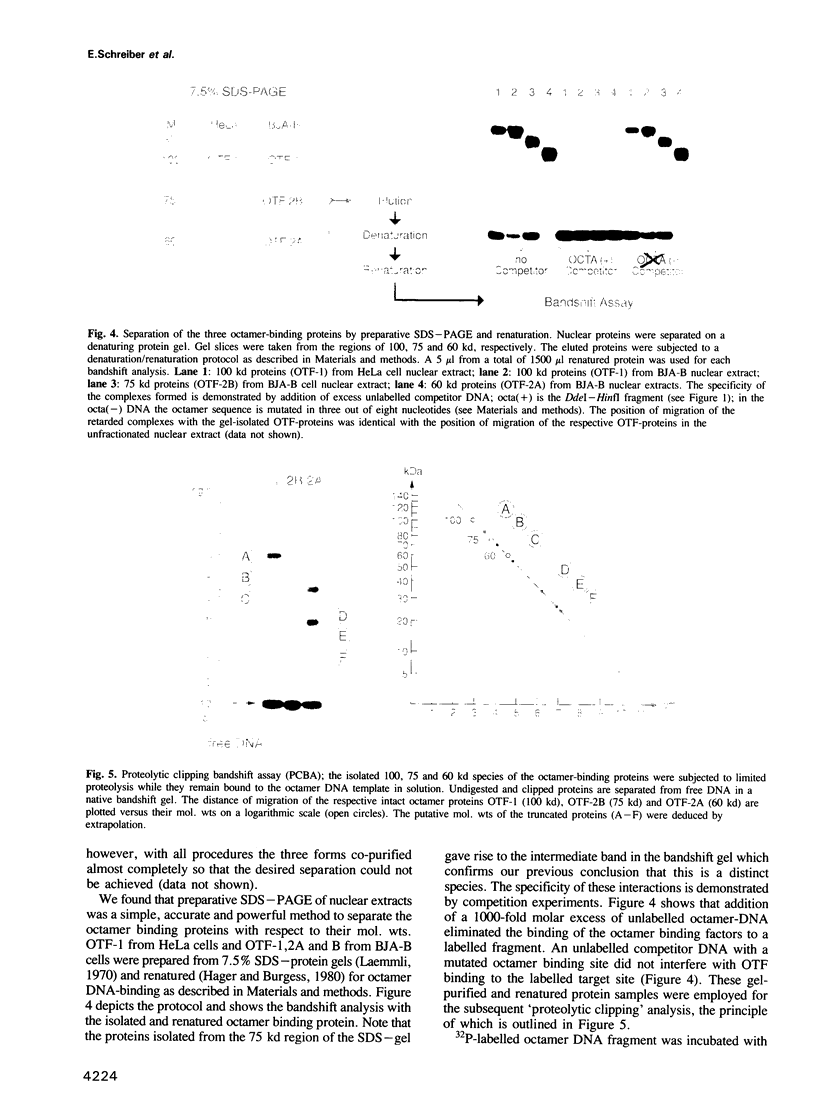
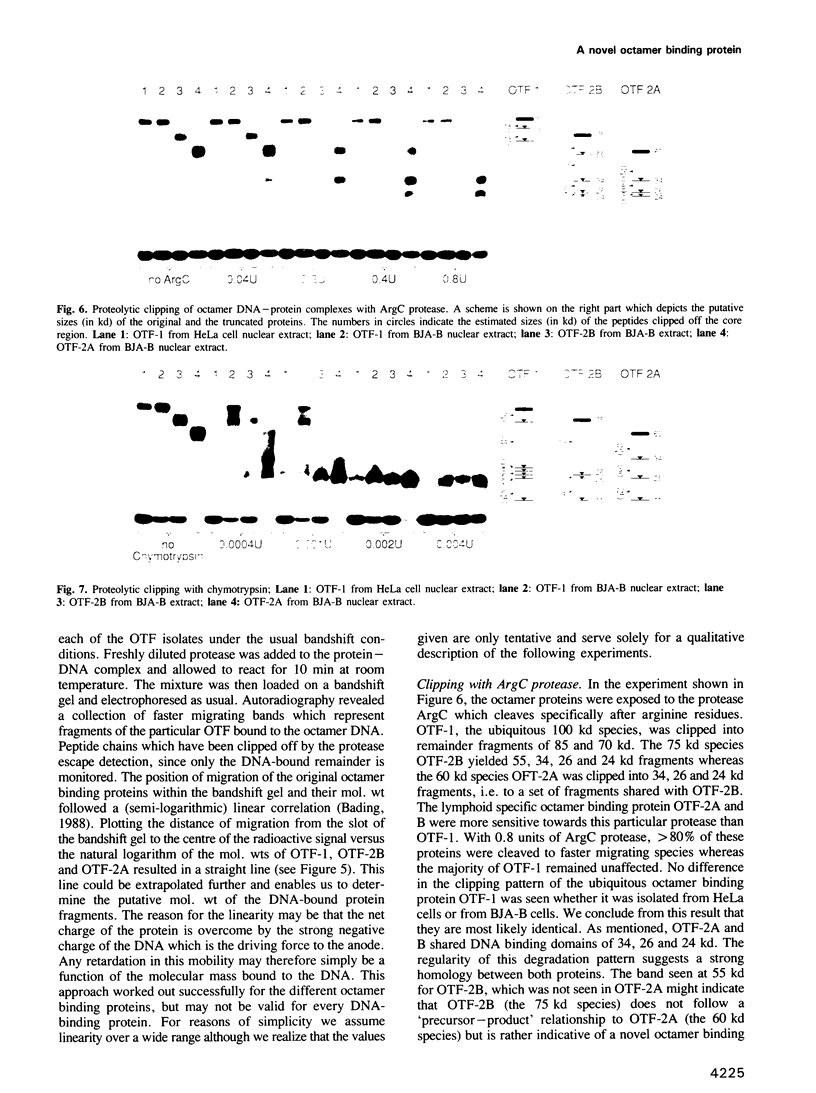
The octamer sequence ATGCAAAT is found in the promoters of immunoglobulin (Ig) heavy and light chain genes and in the heavy chain enhancer and is a major determinant of the cell type specific expression of Ig genes in B cells. An apparent paradox is that the same sequence serves as an upstream promoter or enhancer element in a variety of housekeeping genes such as the histone H2B and U snRNA genes. The differential usage of this regulatory sequence motif is thought to be mediated by different species of octamer binding proteins. One species of 100 kd, designated OTF-1, is present in all cell types and may exert its activating function only when it can interact with additional adjacent transcription factors. The lymphoid cell specific octamer binding protein of 60 kd (OTF-2A) specifically stimulates Ig promoters which consist essentially of a TATA-box and an octamer sequence upstream of it. Here we present evidence for yet another B cell specific octamer binding protein of 75 kd (OTF-2B). From several findings, including the absence of OTF-2B (but not OTF-2A) from a lymphocyte line that cannot respond to the IgH enhancer, we propose a role of the novel octamer factor in the long range activation by the IgH enhancer. We have used the proteolytic clipping bandshift assay (PCBA) technique to distinguish the three different forms found in B cells. This analysis indicates that the 75 kd-species OTF-2B is closely related to the 60 kd species OTF-2A.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bading H. Determination of the molecular weight of DNA-bound protein(s) responsible for gel electrophoretic mobility shift of linear DNA fragments examplified with purified viral myb protein. Nucleic Acids Res. 1988 Jun 24;16(12):5241–5248. doi: 10.1093/nar/16.12.5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard D. W., Bothwell A. Mutational analysis of the immunoglobulin heavy chain promoter region. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9626–9630. doi: 10.1073/pnas.83.24.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji J., Olson L., Schaffner W. A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell. 1983 Jul;33(3):729–740. doi: 10.1016/0092-8674(83)90015-6. [DOI] [PubMed] [Google Scholar]
- Bohmann D., Keller W., Dale T., Schöler H. R., Tebb G., Mattaj I. W. A transcription factor which binds to the enhancers of SV40, immunoglobulin heavy chain and U2 snRNA genes. Nature. 1987 Jan 15;325(6101):268–272. doi: 10.1038/325268a0. [DOI] [PubMed] [Google Scholar]
- Davidson I., Fromental C., Augereau P., Wildeman A., Zenke M., Chambon P. Cell-type specific protein binding to the enhancer of simian virus 40 in nuclear extracts. Nature. 1986 Oct 9;323(6088):544–548. doi: 10.1038/323544a0. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfus M., Doyen N., Rougeon F. The conserved decanucleotide from the immunoglobulin heavy chain promoter induces a very high transcriptional activity in B-cells when introduced into an heterologous promoter. EMBO J. 1987 Jun;6(6):1685–1690. doi: 10.1002/j.1460-2075.1987.tb02418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephrussi A., Church G. M., Tonegawa S., Gilbert W. B lineage--specific interactions of an immunoglobulin enhancer with cellular factors in vivo. Science. 1985 Jan 11;227(4683):134–140. doi: 10.1126/science.3917574. [DOI] [PubMed] [Google Scholar]
- Falkner F. G., Zachau H. G. Correct transcription of an immunoglobulin kappa gene requires an upstream fragment containing conserved sequence elements. Nature. 1984 Jul 5;310(5972):71–74. doi: 10.1038/310071a0. [DOI] [PubMed] [Google Scholar]
- Fletcher C., Heintz N., Roeder R. G. Purification and characterization of OTF-1, a transcription factor regulating cell cycle expression of a human histone H2b gene. Cell. 1987 Dec 4;51(5):773–781. doi: 10.1016/0092-8674(87)90100-0. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerster T., Matthias P., Thali M., Jiricny J., Schaffner W. Cell type-specificity elements of the immunoglobulin heavy chain gene enhancer. EMBO J. 1987 May;6(5):1323–1330. doi: 10.1002/j.1460-2075.1987.tb02371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies S. D., Morrison S. L., Oi V. T., Tonegawa S. A tissue-specific transcription enhancer element is located in the major intron of a rearranged immunoglobulin heavy chain gene. Cell. 1983 Jul;33(3):717–728. doi: 10.1016/0092-8674(83)90014-4. [DOI] [PubMed] [Google Scholar]
- Grosschedl R., Baltimore D. Cell-type specificity of immunoglobulin gene expression is regulated by at least three DNA sequence elements. Cell. 1985 Jul;41(3):885–897. doi: 10.1016/s0092-8674(85)80069-6. [DOI] [PubMed] [Google Scholar]
- Hager D. A., Burgess R. R. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal Biochem. 1980 Nov 15;109(1):76–86. doi: 10.1016/0003-2697(80)90013-5. [DOI] [PubMed] [Google Scholar]
- Hanke J. H., Landolfi N. F., Tucker P. W., Capra J. D. Identification of murine nuclear proteins that bind to the conserved octamer sequence of the immunoglobulin promoter region. Proc Natl Acad Sci U S A. 1988 May;85(10):3560–3564. doi: 10.1073/pnas.85.10.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hromas R., Van Ness B. Nuclear factors bind to regulatory regions of the mouse kappa immunoglobulin gene. Nucleic Acids Res. 1986 Jun 25;14(12):4837–4848. doi: 10.1093/nar/14.12.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübscher U. Double replica southwestern. Nucleic Acids Res. 1987 Jul 10;15(13):5486–5486. doi: 10.1093/nar/15.13.5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp D. J., Morahan G., Cowman A. F., Harris A. W. Production of RNA for secreted immunoglobulin mu chains does not require transcriptional termination 5' to the microM exons. Nature. 1983 Jan 6;301(5895):84–86. doi: 10.1038/301084a0. [DOI] [PubMed] [Google Scholar]
- LaBella F., Sive H. L., Roeder R. G., Heintz N. Cell-cycle regulation of a human histone H2b gene is mediated by the H2b subtype-specific consensus element. Genes Dev. 1988 Jan;2(1):32–39. doi: 10.1101/gad.2.1.32. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landolfi N. F., Capra J. D., Tucker P. W. Interaction of cell-type-specific nuclear proteins with immunoglobulin VH promoter region sequences. Nature. 1986 Oct 9;323(6088):548–551. doi: 10.1038/323548a0. [DOI] [PubMed] [Google Scholar]
- Landolfi N. F., Capra J. D., Tucker P. W. Protein-nucleotide contacts in the immunoglobulin heavy-chain promoter region. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3851–3855. doi: 10.1073/pnas.84.11.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolfi N. F., Yin X. M., Capra J. D., Tucker P. W. A conserved heptamer upstream of the IgH promoter region octamer can be the site of a coordinate protein-DNA interaction. Nucleic Acids Res. 1988 Jun 24;16(12):5503–5514. doi: 10.1093/nar/16.12.5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C., Levin A., Branton D. Copper staining: a five-minute protein stain for sodium dodecyl sulfate-polyacrylamide gels. Anal Biochem. 1987 Nov 1;166(2):308–312. doi: 10.1016/0003-2697(87)90579-3. [DOI] [PubMed] [Google Scholar]
- Lenardo M., Pierce J. W., Baltimore D. Protein-binding sites in Ig gene enhancers determine transcriptional activity and inducibility. Science. 1987 Jun 19;236(4808):1573–1577. doi: 10.1126/science.3109035. [DOI] [PubMed] [Google Scholar]
- Lennon G. G., Perry R. P. C mu-containing transcripts initiate heterogeneously within the IgH enhancer region and contain a novel 5'-nontranslatable exon. Nature. 1985 Dec 5;318(6045):475–478. doi: 10.1038/318475a0. [DOI] [PubMed] [Google Scholar]
- Mason J. O., Williams G. T., Neuberger M. S. Transcription cell type specificity is conferred by an immunoglobulin VH gene promoter that includes a functional consensus sequence. Cell. 1985 Jun;41(2):479–487. doi: 10.1016/s0092-8674(85)80021-0. [DOI] [PubMed] [Google Scholar]
- Mocikat R., Falkner F. G., Mertz R., Zachau H. G. Upstream regulatory sequences of immunoglobulin genes are recognized by nuclear proteins which also bind to other gene regions. Nucleic Acids Res. 1986 Nov 25;14(22):8829–8844. doi: 10.1093/nar/14.22.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger M. S. Expression and regulation of immunoglobulin heavy chain gene transfected into lymphoid cells. EMBO J. 1983;2(8):1373–1378. doi: 10.1002/j.1460-2075.1983.tb01594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill E. A., Kelly T. J. Purification and characterization of nuclear factor III (origin recognition protein C), a sequence-specific DNA binding protein required for efficient initiation of adenovirus DNA replication. J Biol Chem. 1988 Jan 15;263(2):931–937. [PubMed] [Google Scholar]
- Parslow T. G., Blair D. L., Murphy W. J., Granner D. K. Structure of the 5' ends of immunoglobulin genes: a novel conserved sequence. Proc Natl Acad Sci U S A. 1984 May;81(9):2650–2654. doi: 10.1073/pnas.81.9.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petterson M., Schaffner W. A purine-rich DNA sequence motif present in SV40 and lymphotropic papovavirus binds a lymphoid-specific factor and contributes to enhancer activity in lymphoid cells. Genes Dev. 1987 Nov;1(9):962–972. doi: 10.1101/gad.1.9.962. [DOI] [PubMed] [Google Scholar]
- Picard D., Schaffner W. Cell-type preference of immunoglobulin kappa and lambda gene promoters. EMBO J. 1985 Nov;4(11):2831–2838. doi: 10.1002/j.1460-2075.1985.tb04011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruijn G. J., van Driel W., van Miltenburg R. T., van der Vliet P. C. Promoter and enhancer elements containing a conserved sequence motif are recognized by nuclear factor III, a protein stimulating adenovirus DNA replication. EMBO J. 1987 Dec 1;6(12):3771–3778. doi: 10.1002/j.1460-2075.1987.tb02712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosales R., Vigneron M., Macchi M., Davidson I., Xiao J. H., Chambon P. In vitro binding of cell-specific and ubiquitous nuclear proteins to the octamer motif of the SV40 enhancer and related motifs present in other promoters and enhancers. EMBO J. 1987 Oct;6(10):3015–3025. doi: 10.1002/j.1460-2075.1987.tb02607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidereit C., Heguy A., Roeder R. G. Identification and purification of a human lymphoid-specific octamer-binding protein (OTF-2) that activates transcription of an immunoglobulin promoter in vitro. Cell. 1987 Dec 4;51(5):783–793. doi: 10.1016/0092-8674(87)90101-2. [DOI] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986 Aug 29;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Siebenlist U., Gilbert W. Contacts between Escherichia coli RNA polymerase and an early promoter of phage T7. Proc Natl Acad Sci U S A. 1980 Jan;77(1):122–126. doi: 10.1073/pnas.77.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H., Sen R., Baltimore D., Sharp P. A. A nuclear factor that binds to a conserved sequence motif in transcriptional control elements of immunoglobulin genes. Nature. 1986 Jan 9;319(6049):154–158. doi: 10.1038/319154a0. [DOI] [PubMed] [Google Scholar]
- Sive H. L., Roeder R. G. Interaction of a common factor with conserved promoter and enhancer sequences in histone H2B, immunoglobulin, and U2 small nuclear RNA (snRNA) genes. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6382–6386. doi: 10.1073/pnas.83.17.6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudt L. M., Singh H., Sen R., Wirth T., Sharp P. A., Baltimore D. A lymphoid-specific protein binding to the octamer motif of immunoglobulin genes. Nature. 1986 Oct 16;323(6089):640–643. doi: 10.1038/323640a0. [DOI] [PubMed] [Google Scholar]
- Sturm R., Baumruker T., Franza B. R., Jr, Herr W. A 100-kD HeLa cell octamer binding protein (OBP100) interacts differently with two separate octamer-related sequences within the SV40 enhancer. Genes Dev. 1987 Dec;1(10):1147–1160. doi: 10.1101/gad.1.10.1147. [DOI] [PubMed] [Google Scholar]
- Vinson C. R., LaMarco K. L., Johnson P. F., Landschulz W. H., McKnight S. L. In situ detection of sequence-specific DNA binding activity specified by a recombinant bacteriophage. Genes Dev. 1988 Jul;2(7):801–806. doi: 10.1101/gad.2.7.801. [DOI] [PubMed] [Google Scholar]
- Wang J. Y., Nishiyama K., Araki K., Kitamura D., Watanabe T. Purification of an octamer sequence (ATGCAAAT)-binding protein from human B cells. Nucleic Acids Res. 1987 Dec 23;15(24):10105–10116. doi: 10.1093/nar/15.24.10105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiher H., König M., Gruss P. Multiple point mutations affecting the simian virus 40 enhancer. Science. 1983 Feb 11;219(4585):626–631. doi: 10.1126/science.6297005. [DOI] [PubMed] [Google Scholar]
- Wirth T., Staudt L., Baltimore D. An octamer oligonucleotide upstream of a TATA motif is sufficient for lymphoid-specific promoter activity. Nature. 1987 Sep 10;329(6135):174–178. doi: 10.1038/329174a0. [DOI] [PubMed] [Google Scholar]