Abstract
Background
Non-sentinel lymph node (NSLN) status prediction with molecular biomarkers may make some sentinel lymph node (SLN) positive breast cancer patients avoid the axillary lymph node dissection, but the available markers remain limited.
Methods
SLN positive patients with and without NSLN invasion were selected, and genes differentially expressed or fused in SLN metastasis were screened by next-generation RNA sequencing.
Results
Six candidates (all ER/PR+, HER2−, Ki-67 <20 %) with metastatic SLNs selected from 305 patients were equally categorized as NSLN negative and positive. We identified 103 specifically expressed genes in the NSLN negative group and 47 in the NSLN positive group. Among them, FABP1 (negative group) and CYP2A13 (positive group) were the only 2 protein-encoding genes with expression levels in the 8th to 10th deciles. Using a false discovery rate threshold of <0.05, 62 up-regulated genes and 98 down-regulated genes were discovered in the NSLN positive group. Furthermore, 10 gene fusions were identified in this group with the most frequently fused gene being IGLL5.
Conclusions
The biomarkers screened in present study may broaden our understanding of the mechanisms of breast cancer metastasis to the lymph nodes and contribute to the axillary surgery selection for SLN positive patients.
Electronic supplementary material
The online version of this article (doi:10.1186/s12957-015-0642-2) contains supplementary material, which is available to authorized users.
Keywords: Breast cancer, Sentinel lymph node (SLN), Non-sentinel lymph node (NSLN), Axillary lymph node dissection (ALND), RNA sequencing
Background
Axillary lymph node dissection (ALND) was introduced as a standard surgical procedure for breast cancer in the 1800s and played a significant role in patients’ staging, prognosis assessment, regional disease control, and treatment direction [1, 2]. However, with the aid of new screening methods, more early stage patients with no invasion of axillary lymph nodes (ALN) have been able to be identified in recent years. For these patients, instead of reducing the incidence of recurrence or improving survival, ALND was found to be associated with increased risk of adverse effects such as lymphedema, limited mobility, neuropathic pain, numbness, and sensory loss [3–5]. To solve this dilemma, sentinel lymph node biopsy (SLNB), a less invasive surgery with equivalent clinical value, was developed and has readily become a routine surgery in early breast cancer patients [6, 7].
As the first site of tumor cell infiltration via lymphatic vessels, sentinel lymph nodes (SLN) with no detectable metastasis are seen as safety indicator, thus, making ALND unnecessary. On the other hand, if SLN is positive for metastasis, ALND is still recommended to clarify the status of the remaining non-sentinel lymph nodes (NSLNs) in the axilla [8, 9]. Nevertheless, it was reported that 40–70 % of SLN positive patients were actually free of metastasis in their NSLNs [10, 11]. In order to avoid the over-treatment suffering brought about by ALND, it has become imperative for breast cancer surgeons to find effective methods that can distinguish SLN positive patients with low probability of NSLN invasion from those with high probability of NSLN invasion.
Among these methods, predictive models based on retrospective analysis of patients’ clinical characteristics (e.g., age, histological type, tumor size, lymphovascular invasion, and hormone receptor status), such as the nomogram of Memorial Sloan-Kettering Cancer Center [12] and the scoring systems of MD Anderson [13], Tenon [14], Cambridge [15], and Stanford [16], were the most frequently mentioned ones. However, the routine clinical practice and patient characteristics varied among different hospitals, thereby, greatly influencing the accuracy, consistency, and repeatability of these models and hampering their extensive application. On the other hand, it was hypothesize that tumor with specific gene expression or fusion may have more invasive behavior and thus possess with higher possibility of metastasis in lymph node. Therefore, some scientists were dedicated to search for biomarkers that can predict NSLN status [17–23], but until recently, the available choices remained limited and their practical value still needed additional verification.
In present study, next-generation RNA sequencing (RNA-Seq) was utilized to compare gene expression level differences for breast cancer metastasized to the SLN between patients with and without NSLN invasion. To our knowledge, it is the first time that NSLN prediction markers were screened according to gene expression profiling of the SLN metastasis. Although further validation is required in the future, these markers could broaden our understanding of the mechanisms of breast cancer metastasis to the lymph nodes and might provide assistance in decision making when choosing appropriate surgery strategies for SLN positive breast cancer patients.
Methods
Patients
Treatment-naive breast cancer patients who received SLNB at our hospital were selected for the present study. Among them, patients with metastatic SLN were divided into NSLN positive and negative groups based on their ALND results. For traditional clinical indexes such as age, tumor size, histological type, and numbers of metastatic SLN and ALN, as well as estrogen receptor (ER), progesterone receptor (PR), HER2, Ki-67 status, and patients with greatly varying characteristics, were excluded from each group. For the remaining patients in the two refined groups, 10 slices (4–5 μm) of paraffin embedded SLN samples were collected for subsequent analysis. To participate in the study, all patients signed an informed consent form that was approved by the ethics and scientific committees at the affiliated hospital of Academy of Military Medical Sciences.
RNA extraction, library preparation, and sequencing
Using the delineation line drawn by the pathologist on the reverse side of each slice as a guide, the metastatic tumor in the SLN was scraped into a 1.5 ml RNase-free tube and sent for RNA extraction using the RNeasy FFPE kit (Qiagen, Germany) according to the manufacturer’s instructions. The obtained total RNA was measured using a NanoDrop 2000 (Thermo Scientific, USA) and stored at −80 °C until used. Libraries of mRNA derived from total RNA were constructed using the Illumina ®TruSeq™ RNA Sample Preparation Kit (USA) according to the manufacturer’s instructions. The concentration and size distribution of the libraries were determined using an Agilent 2100 Bioanalyzer (USA). The libraries were then sequenced using an Illumina Hiseq 2000 Genome Analyzer platform in paired-end 100-bp mode.
Data analysis
Sequenced reads were processed and aligned to the UCSC reference human genome (build hg19) using the Tophat software [24] default setting and were then fed to Cufflinks software [25] to assemble transcripts and estimate their abundances. To calculate gene expression levels, read counts were normalized to the number of fragments per kilobase of transcript per million mapped reads (FPKM) according to the gene length and total mapped reads. The unsupervised hierarchical clustering of gene expression levels from the selected samples and the final dendrogram visualization were performed using the R programming package. The Cuffcompare program was used to track the expression levels of each transcript within samples and to produce a combined gene expression file. This file was then run through the Cuffdiff program to test for differences in gene expression in breast cancer metastasized to SLN between patients with and without NSLN invasion. First, specifically expressed genes were identified as being expressed in the NSLN negative or positive group exclusively. They were divided into lowly (1st–3rd decile), moderately (4th–7th decile), and highly (8th–10th decile) expressed genes according to their expression levels. The non-specific genes were used to further filter down- and up-regulated genes with a false discovery rate (FDR) <0.05. Gene Ontology function classifications of regulated genes were assigned using DAVID (p ≤ 0.001) [26]. Fusion genes were searched using Tophat with “--fusion-search” specified during the process of read alignment [27]. A “supporting” read must map to both sides of a fusion by at least 13 bases. For intra-chromosomal fusions, the distance between the fusion points must be at least 100 kb. Reads or pairs that map to more than two places were ignored. The final fusion genes with ≥5 supporting reads and pairs were identified in the end.
Results
Patient characteristics
Sixty-nine SLN positive breast cancer patients were chosen from 305 patients who received SLNB between November 2010 and April 2013 at our hospital. Among them, 32 patients were NSLN positive and the other 37 patients were NSLN negative. Based on their clinical indexes, 3 patients were selected from each group for subsequent research. The characteristics of the 6 patients are listed in Table 1. Their backgrounds were generally the same: all were moderately differentiated invasive ductal carcinoma (IDC), with positive ER/PR, and negative HER2. For Ki-67, the requirements had to be broadened to ≤20 %, since there were insufficient patients in the NSLN negative group when the recommended cut-off point of 14 % was used [28].
Table 1.
The characteristics of the selected patients
| NSLN negative | NSLN positive | |||||
|---|---|---|---|---|---|---|
| Patient ID | 84816 | 94948 | 67161 | 76948 | 86923 | 94812 |
| Tumor type | IDC | IDC | IDC | IDC | IDC | IDC |
| Tumor grade | MD | MD | MD | MD | MD | MD |
| Tumor size (cm) | 1.5 | 1 | 3 | 3 | 2 | 2.5 |
| SLN (P/T) | 1/2 | 1/4 | 1/1 | 2/2 | 1/1 | 1/2 |
| ALN (P/T) | 0/18 | 0/16 | 0/18 | 1/21 | 2/21 | 1/28 |
| ER | 3+, >75 % | 3+, >75 % | 2+, 50–70 % | 2+, 50–75 % | 3+, >75 % | 3+, >75 % |
| PR | 3+, >75 % | 1+, ~15 % | 1+, 10–30 % | 1+, 25–50 % | 2+, 50–60 % | 3+, >75 % |
| HER2 | Negative | Negative | Negative | Negative | Negative | Negative |
| Ki-67 | 3–5 % | 20 % | 15–20 % | 10 % | 10 % | 5–10 % |
IDC invasive ductal carcinoma, MD moderate differentiated, P positive, T total
RNA extraction, library preparation, and sequencing
As showed in Additional file 1, the extracted RNA concentrations for each sample were all >100 ng/μl and their OD260/OD280 ratio ranged from 1.78 to 2.03, which ensured that the samples could be used for downstream experiments. We successfully generated cDNA libraries of 350–500 bp and obtained 18–27 million (range 18,549,392–27,137,861; mean 22,775,012) high-quality sequencing reads with a sequencing quality of >25 for each base in five samples and >20 in sample 67161 (Fig. 1). The raw sequence data has been deposited in a public repository (Gene Expression Omnibus (GEO)) with the access number GSE64850. After filtering the repetitive or very low complexity reads (0.16 % of the sequenced reads on average), we mapped an average of 52.27 % (range 19.57–67.22 %) of the reads to the human genome (UCSC version hg19) (Additional file 2).
Fig. 1.
Distribution of sequencing base quality. a Base quality of paired-end reads for NSLN negative group. b Base quality of paired-end reads for NSLN positive group. The base quality of sequencing reads was greater than 25 in five samples except for 67167 whose base quality was slightly lower (≥20)
Variation in gene expression
We used FPKM values to measure the gene expression level, which can compensate for biases between samples. Considering that the expression levels of a gene may not be accurately detected by RNA-Seq when its FPKM value is less than 1, only those genes with FPKM values ≥1 were considered for subsequent analysis. The unsupervised hierarchical clustering of gene expression levels clearly categorized the six patients into the NSLN negative or NSLN positive group in a manner consistent with their clinical traits (Fig. 2a). Furthermore, NSLN negative samples displayed highly similar gene expression profiles (Additional file 3) as supported by the Pearson correlation of gene expression values: 0.8, 0.72, and 0.83 for 67167 vs. 84816, 67167 vs. 94948, and 84816 vs. 94948, respectively. However, NSLN positive samples showed a much greater heterogeneity in gene expression than NSLN negative ones because of the more diverse sample 76948 (Pearson correlation coefficients were 0.63 for 76948 vs. 86923 and 0.44 for 76948 vs. 94812, respectively).
Fig. 2.
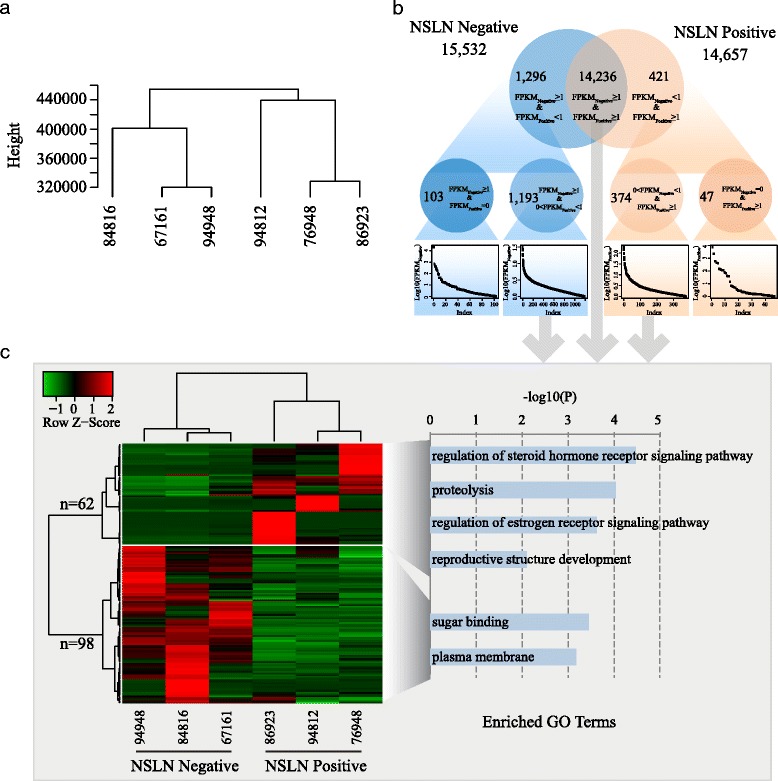
Gene expression variations between NSLN negative and positive groups. a Clustering of gene expression levels in the six samples categorized them in a manner consistent with their clinical traits. b Venn diagram of genes with FPKM ≥ 1 in the two groups. There are more NSLN negative group-specific genes than NSLN positive group-specific genes. c Heatmap of 62 up-regulated genes and 98 down-regulated genes in the NSLN positive group and their enriched GO terms
In order to identify the important and specifically expressed genes, we first classified genes with FPKM ≥ 1 in each group into lowly, moderately, and highly expressed genes (Additional file 4). Then we explored genes exclusively expressed in each group (Fig. 2b) and examined their expression category (Additional file 5). In the NSLN negative group, 103 genes were specifically expressed and 12 of them with highly expression (11 were RNA genes and 1 was a protein-coding gene, FABP1). In contrast, 47 genes were specifically expressed in the NSLN positive group, 13 of which with highly expression, including 12 non-coding RNA genes (4 Micro RNAs, 8 small nucleolar RNAs) and 1 protein-coding gene, CYP2A13 (see Table 2).
Table 2.
Specifically and highly expressed genes in the NSLN negative and positive groups
| Gene | FPKM | Description | Category |
|---|---|---|---|
| 13 NSLN-positive-specific | |||
| MIR3936 | 2210.6 | MicroRNA 3936 | RNA gene |
| MIR223 | 7256.4 | MicroRNA 223 | RNA gene |
| SNORA3 | 569 | Small nucleolar RNA, H/ACA box 3 | RNA gene |
| SNORA18 | 433.8 | Small nucleolar RNA, H/ACA box 18 | RNA gene |
| MIR941-3 | 409.7 | MicroRNA 941-3 | RNA gene |
| SNORA7B | 143.2 | Small nucleolar RNA, H/ACA box 7B | RNA gene |
| SNORA13 | 131.6 | Small nucleolar RNA, H/ACA box 13 | RNA gene |
| SNORA2A | 113.8 | Small nucleolar RNA, H/ACA box 2A | RNA gene |
| SCARNA11 | 112 | Small nucleolar RNA, H/ACA box 11 | RNA gene |
| MIR3907 | 76 | MicroRNA 3907 | RNA gene |
| SNORA84 | 66.7 | Small nucleolar RNA, H/ACA box 84 | RNA gene |
| CYP2A13 | 39.6 | Cytochrome P450, family 2, subfamily A, polypeptide 13 | Protein-coding |
| SNORA62 | 38.9 | Small nucleolar RNA, H/ACA box 62 | RNA gene |
| 12 NSLN-negative-specific | |||
| SNORD89 | 20306.9 | Small nucleolar RNA, C/D box 89 | RNA gene |
| MIR499A | 683.4 | MicroRNA 499a | RNA gene |
| SNORA51 | 452.6 | Small nucleolar RNA, H/ACA box 51 | RNA gene |
| SNORA40 | 419.3 | Small nucleolar RNA, H/ACA box 40 | RNA gene |
| SNORA46 | 281.1 | Small nucleolar RNA, H/ACA box 46 | RNA gene |
| SCARNA3 | 206.1 | Small Cajal body-specific RNA 3 | RNA gene |
| SNORA27 | 181.4 | Small nucleolar RNA, H/ACA box 27 | RNA gene |
| SNORA32 | 99.9 | Small nucleolar RNA, H/ACA box 32 | RNA gene |
| FABP1 | 84.2 | Fatty acid binding protein 1, liver | Protein-coding |
| MIR941-2 | 43.3 | MicroRNA 941-2 | RNA gene |
| MIR941-4 | 43.3 | MicroRNA 941-4 | RNA gene |
| SNORA1 | 43 | Small nucleolar RNA, H/ACA box 1 | RNA gene |
From the remaining 15,803 non-specific genes (Fig. 2b, 14,236 + 1193 + 374), we further identified 62 up-regulated and 98 down-regulated genes in the NSLN positive group using the Cuffdiff software with a threshold of FDR < 0.05 (Fig. 2c and Additional file 6). This was consistent with the overall expression profiling of all genes. Among the 160 genes, the top 10 of differentially expressed genes were listed in Table 3. Genes involved in reproductive structure development (p = 0.008), proteolysis (p = 9.19e-5), regulation of steroid hormone receptor signaling pathway (p = 3.4e-5), and regulation of estrogen receptor signaling pathway (p = 2.4e-4) were enriched in the up-regulated gene group, including four kallikrein-related peptidase (KLK) subfamily members (KLK10, KLK11, KLK12, and KLK13), whereas genes involved in sugar binding (p = 3.5e-4), the plasma membrane (p = 6.6e-4) (Fig. 2c), and the B cell receptor antigen signaling pathway (FDR = 3.63e-10) were enriched in the down-regulated gene group (Additional file 7).
Table 3.
Top ten of differentially expressed genes in the NSLN positive group
| Gene | NSLN negative | NSLN positive | Log2(FC) | FDR | Description |
|---|---|---|---|---|---|
| Up-regulated genes | |||||
| KLK11 | 0.93 | 185.48 | 7.64 | 0.00015 | Kallikrein-related peptidase 11 |
| SCGB3A1 | 8.06 | 1231.39 | 7.26 | 4.89E-05 | Secretoglobin, family 3A, member 1 |
| CLEC3A | 0.98 | 143.59 | 7.19 | 2.96E-06 | C-type lectin domain family 3, member A |
| CYP2A6 | 0.45 | 63.31 | 7.12 | 0.00029 | Cytochrome P450, family 2, subfamily A, polypeptide 6 |
| KLK10 | 0.17 | 19.11 | 6.80 | 0.0034 | Kallikrein-related peptidase 10 |
| KLK12 | 0.89 | 94.90 | 6.73 | 0.0011 | Kallikrein-related peptidase 12 |
| KLK13 | 0.57 | 49.98 | 6.45 | 7.42E-05 | Kallikrein-related peptidase 13 |
| CYP2A7 | 0.33 | 26.37 | 6.32 | 0.0052 | Cytochrome P450, family 2, subfamily A, polypeptide 7 |
| OBP2B | 0.43 | 33.39 | 6.29 | 0.0391 | Odorant binding protein 2B |
| KCNC2 | 0.32 | 21.60 | 6.09 | 0.0040 | Potassium voltage-gated channel, Shaw-related subfamily, member 2 |
| Down-regulated genes | |||||
| KRT20 | 17.19 | 0.07 | −7.95 | 0.00019 | Keratin 20 |
| KRT4 | 6.42 | 0.05 | −7.14 | 0.048 | Keratin 4 |
| VPREB1 | 25.93 | 0.19 | −7.10 | 0.012 | Pre-B lymphocyte 1 |
| RBP2 | 31.24 | 0.58 | −5.75 | 0.0014 | Retinol binding protein 2, cellular |
| ALDOB | 4.64 | 0.09 | −5.66 | 0.011 | Aldolase B, fructose-bisphosphate |
| BIRC7 | 7.80 | 0.16 | −5.57 | 0.017 | Baculoviral IAP repeat containing 7 |
| FIBCD1 | 7.75 | 0.19 | −5.38 | 0.00091 | Fibrinogen C domain containing 1 |
| MUC13 | 8.85 | 0.23 | −5.24 | 0.0022 | Mucin 13, cell surface associated |
| FCAMR | 50.50 | 1.38 | −5.19 | 4.97E-12 | Fc receptor, IgA, IgM, high affinity |
| DHRS2 | 19.04 | 0.55 | −5.10 | 0.0085 | Dehydrogenase/reductase (SDR family) member 2 |
FC fold change of (NLSN positive/negative)
Fusion gene
A total of 10 different gene fusions were identified in the NSLN positive group, including 7 fusions taking place only in 94812, 2 fusions happening only in 76948, and 1 fusion occurring only in 86923 (Table 4). The intra-chromosome gene fusion WAC-DNAJC1 that occurred only in 94812 was located between a part of exon 3 of WAC and the whole of exon 10 of DNAJC1 (Fig. 3a). The inter-chromosome gene fusion CACNG4-RANBP3 that occurred in 86923 was located in the intron sequence between exons 1 and 2 of both CACNG4 and RANBP3 (Additional file 8). The PDE3A-SLCO5A1 gene fusion in 76948 was also localized between the introns of both genes (Additional file 8). The remaining 7 gene fusions were formed through fusion of one formal gene and an ensemble gene (Table 4). Interestingly, the most frequently fused gene was IGLL5 (immunoglobulin lambda-like polypeptide 5) that fused with four variants of the IGLV1 (partial mRNA for immunoglobulin lambda light chain) gene. These four variants were located in a 75 kb region about 445 to 520 kb upstream of IGLL5 (Fig. 3b) and exhibit similar gene structure (Fig. 3c). The fusion point for IGLL5 was in the intron region of one transcript and the exon 2 region of the other transcript, while fusion points for the IGLV1 variants were located in the exon 2 regions (Fig. 3d).
Table 4.
Fusion genes identified in NSLN positive samples
| Gene | Chrom | Sample ID | Reads # |
|---|---|---|---|
| WAC-DNAJC1 | chr10-chr10 fr | 94812 | 43 |
| ENSG00000211648(IGLV1-47)-IGLL5 | chr22-chr22 ff | 94812 | 55 |
| ENSG00000211651(IGLV1-44)-IGLL5 | chr22-chr22 ff | 94812 | 110 |
| ENSG00000211653(IGLV1-40)-IGLL5 | chr22-chr22 ff | 94812 | 65 |
| ENSG00000211655(IGLV1-36)-IGLL5 | chr22-chr22 ff | 94812 | 70 |
| ENSG00000230613(HM13-AS1)-HM13 | chr20-chr20 rf | 94812 | 20 |
| SSB-ENSG00000236852(RP11-3D23.1) | chr2-chrX rr | 94812 | 28 |
| PDE3A-SLCO5A1 | chr12-chr8 rr | 76948 | 9 |
| ENSG00000226958(CTD-2328D6.1)-HFM1 | chrX-chr1 rr | 76948 | 50 |
| CACNG4-RANBP3 | chr17-chr19 ff | 86923 | 24 |
fr stands for fusion occurring between forward strand in the first chromosome with reverse strand in the second chromosome. ff stands for fusion occurring between forward strands in both chromosomes. rr stands for fusion occurring between reverse strands in both chromosomes
Fig. 3.
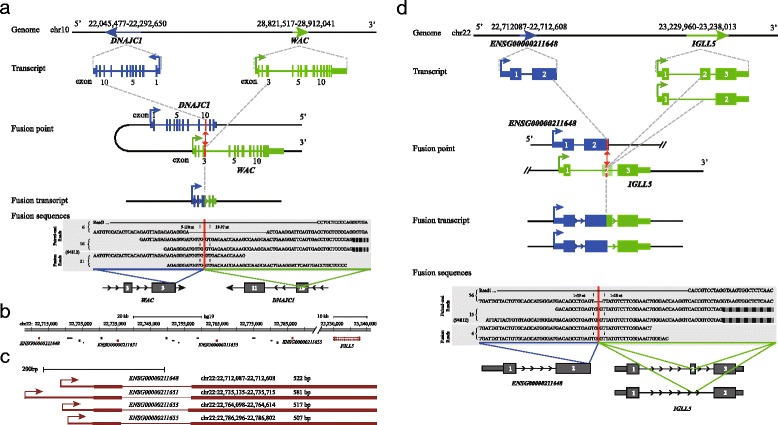
Examples of fusion genes in NSLN positive samples. a The WAC-DNAJC1 fusion identified only in 94812 was revealed by 21 fusion-spanning reads, 16 pair-end reads (with one read spanning the fusion point), and 6 pair-end reads. This fusion was between one-third of exon 3 of WAC (blue) and the whole of exon 10 of DNAJC1 (green). The break point was shown in a red vertical line. The numbers of reliable pair-end and fusion-spanning reads in each sample are indicated to the right of each read. The sample ID is showed in parentheses. b Schematic diagram of genomic locations of IGLL5 and four ensemble non-coding genes fused with IGLL5. Five genes with the fusion are shown in red. c The similar structure for four IGLV1 variables fused with IGLL5. These four genes were aligned to the right and the name, position, and length were shown above each gene. d One example of IGLL5 fusion with a variable of IGLV1, ENSG00000211648 that was only identified in 94812. There are two transcripts of IGLL5 gene and two fused transcripts with ENSG00000211648 correspondingly
Discussion
Because of growing evidence for its benefits and its minor side effects in patients, SLNB has readily replaced ALND and has become the routine procedure for surgical axillary staging in early breast cancer patients [6, 7]. For SLN negative patients, it is now widely accepted that ALND can be omitted [8, 9]. Because 40–70 % of SLN positive patients were reported to be free of metastasis in their NSLN, ALND in these patients remains controversial [10, 11]. In order to avoid the physical discomfort and potential complications associated with ALND in these patients, an effective method to predict the status of NSLN has become the urgent demand for breast surgeon. In contrast to the existing predictive models that are based on retrospective analysis of patients’ clinical characteristics [12–16], molecular tests may hold significant promise because they are more objective, more standardized, and easier to popularize [17–23]. Unfortunately, currently available markers remain limited and their practical value still needs additional verification.
Recently, the utilization of RNA-Seq in breast cancer has illustrated its power in revealing the variation landscape of the breast transcriptome and in finding regulatory interactions among cancer-related molecules [29, 30]. As a powerful next-generation sequencing technology, RNA-Seq can profile a full set of transcripts including mRNAs, small RNAs, and other non-coding RNAs qualitatively and quantitatively, providing a snapshot of gene expression patterns and regulatory elements in a cell, tissue, or organism. Compared with microarrays, RNA-Seq possesses the advantages of being high-throughput, cost effective, and of having superior accuracy. In addition, without relying on prior sequence information, RNA-Seq can profile gene expression based on the entire transcript (not a few segments). It can also identify novel isoforms and exons, allele-specific expression, mutations, and fusion transcripts [31]. These advantages make it ideal for studying complex diseases, particularly cancer. Despite its growing application in breast cancer, to the best of our knowledge, the present study is the first one using RNA sequencing to screen for potential markers predicting NSLN status in patients with metastatic SLN.
The major function and most distinctive feature of RNA-Seq is measuring gene expression variance, which captures the genetic differences among patients. The most interesting observation in our study is that four KLK subfamily members (KLK10, KLK11, KLK12, and KLK13) were up-regulated in the NSLN positive group, suggesting their potential role in lymph node metastasis. The KLK gene family includes 15 highly conserved secreted serine proteases with similar structural characteristics, whose dysregulation was reported to be closely associated with endocrine-related cancer, such as prostate, breast, and ovarian cancers [32]. Although previous studies have demonstrated the crucial role of KLK10 and KLK11 in breast cancer patients’ relapse, disease progression and shorter survival rates [32, 33], a potential role for the KLK gene family in lymph node metastasis was first proposed in the present study. More studies are required to further confirm these results.
On the other hand, for the down-regulated genes in the NSLN positive group, B cell antigen receptor (BCR) signaling pathway, including some B cell surface molecules (CD22, CD72, Igα, Igβ, CD19, and CD21) and a few downstream regulated genes (SYK, LYN, BTK, and PTPN6), may be worthy of further attention. It is known that the BCR signal pathway is vital for the development and survival of B lymphocytes and that defective BCR signaling can result not only in impaired B cell development and immunodeficiency but also in a predisposition to autoimmunity [34]. Although the BCR signaling pathway was previously reported to play significant roles in chronic lymphocytic leukemia [35], this is the first time that it is linked with NSLN metastasis in breast cancer.
In contrast to the down- and up- regulated genes, the presence of specifically expressed genes and fusion genes may be more useful to the breast cancer surgeon, because they are relatively easier to analyze and their detection could be carried out during surgery, thereby, facilitating the implementation of appropriate surgery strategies for breast cancer patients in a timelier manner. For specifically expressed genes, two protein-coding genes, FABP1 and CYP2A13 which were expressed in the NSLN negative and positive groups, respectively, were worthy of further investigation. FABP1 was reported to correlate with non-alcoholic fatty liver disease [36], and CYP2A13 was found to be involved in the development and progression of lung adenocarcinoma [37]; however, neither of them was previously associated with NSLN metastasis. For fusion genes, the most frequently seen in the NSLN positive group was IGLL5, which was identified as one of the best predictors for relapse-free survival with >85 % accuracy in breast cancer patients [38]. This observation suggests that those rearrangements occurring in IGLL5 might be linked to the process of metastasis.
As a well-known biomarker for cell proliferation, Ki-67 plays a significant role in prognosis prediction [39] and has been routinely used in the subtyping of breast cancer [28]. However, we could not screen enough patients in the NSLN negative group using the recommended cut-off of 14 % [28]. Taking into consideration that such a cut-off was arbitrarily determined and still needed further confirmation, we broadened the requirement to 20 %. Even so, only six patients were finally screened, which may inevitably influence the strength of our results. Therefore, further verification in subsequent studies is required. Moreover, other subtypes of breast cancer (such as HER2 positive) were not evaluated in the present study and may need additional investigation, since their intrinsic metastatic mechanism may be completely different.
Lastly, we should note that predicting NSLN status with molecular biomarkers is based on the hypothesis that tumor with specific gene expression or fusion may have more invasive behavior and thus possess with higher possibility of metastasis in lymph node. However, specific gene expression or fusion in SLN does not necessarily mean the invasion of NSLN and that merely represents some kind of possibility. Therefore, as regards for the practical value of the biomarkers that screened in present study, additional verification should be warranted in the future.
Conclusions
In summary, this is the first time that molecular markers for NSLN status prediction in SLN positive breast cancer patients were identified using transcriptome sequencing. These markers could broaden our understanding of the mechanisms of breast cancer metastasis to the lymph nodes. More importantly, the specifically expressed genes (FABP1 and CYP2A13) and the fused gene (IGLL5) identified in our study may be integrated into an intro-operative diagnostic method, which could facilitate the implementation of appropriate surgery strategies in a timely manner.
Acknowledgements
This work was supported by a National Natural Science Foundation of China grant (81272914), the “Strategic Priority Research Program” of the Chinese Academy of Sciences, a Stem Cell and Regenerative Medicine Research grant (XDA01040405), a National Programs for High Technology Research and Development grant (863 Projects, 2012AA022502), and the Youth Innovation Promotion Association of Chinese Academy of Sciences (2014085).
Abbreviations
- ALN
axillary lymph nodes
- ALND
axillary lymph node dissection
- BCR
B cell antigen receptor
- ER
estrogen receptor
- FDR
false discovery rate
- FPKM
fragments per kilobase of transcript per million mapped reads
- IDC
invasive ductal carcinoma
- KLK
kallikrein-related peptidase
- NSLN
non-sentinel lymph node
- PR
progesterone receptor
- RNA-Seq
RNA sequencing
- SLN
sentinel lymph node
- SLNB
sentinel lymph node biopsy
Additional files
The results of RNA extraction. A table listing the quality of RNA extraction.
Statistic information of sequencing reads in selected patients. A table showing how the sequencing reads mapped.
Scatter plots of gene expression values between samples. A figure illustrating how similar of genes expressed among 6 samples. Panels a, b, and c show comparisons among 67161, 94948, and 84816 from the NSLN negative group. These three samples display very similar gene expression levels based on Pearson correlations. Panels d, e, and f show comparisons among 76948, 86923, and 94812 from the NSLN positive group. Gene expression levels in 76948 are quite dissimilar compared with those in 86923 and 94812 based on their Pearson correlations.
Classification of genes based on the expression levels. A table showing how the gene expression levels were classified.
Specifically expressed genes in the NSLN negative and –positive groups. A table showing the FPKM values of specifically expressed genes in two groups.
Expression levels of 98 down-regulated genes and 62 up-regulated genes in the NSLN positive group. A table showing the expression values of regulated genes.
A KEGG pathway figure for the enriched pathway B cell receptor signaling pathway in down-regulated genes. A figure illustrating the pathway of down-regulated genes enriched. The genes colored with green were down-regulated in NSLN positive group.
The schematic diagram of other fused genes. A figure showing how the rest of gene fusions occurred. The two genes that fused together were shown in blue and green. There fused point was shown in a vertical red line.
Footnotes
Feng Liang Hongzhu Qu and Qiang Lin contributed equally to this work.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
FL analyzed the patients’ clinical data and collected the SLN samples. HQ analyzed the sequencing data and drafted the manuscript. QL collected the SLN samples and participated in RNA extraction. YY participated in data alignment and uploaded the raw data. XR constructed the cDNA library for sequencing. BZ drew the line delineating tumor position on the reverse side of each slide. YL drafted the manuscript. CY, HZ, XF, and XH participated in the design and coordination of the study. All authors have read and approved the final manuscript.
Contributor Information
Feng Liang, Email: lfpeakcool@126.com.
Hongzhu Qu, Email: quhongzhu@big.ac.cn.
Qiang Lin, Email: 812983585@qq.com.
Yadong Yang, Email: yangyd@big.ac.cn.
Xiuyan Ruan, Email: ruanxy@big.ac.cn.
Bo Zhang, Email: 13436660521@qq.com.
Yi Liu, Email: liuyi790114@163.com.
Chengze Yu, Email: yuchengze@sina.com.
Hongyan Zhang, Phone: +86 1084008502, Email: roninzhy@126.com.
Xiangdong Fang, Phone: +86 1084097495, Email: fangxd@big.ac.cn.
Xiaopeng Hao, Phone: +86 1066947382, Email: hxp307@sina.com.
References
- 1.Halsted WS. The results of operations for the cure of cancer of the breast performed at the Johns Hopkins Hospital from June, 1889, to January, 1894. Ann Surg. 1894;20(5):497–555. doi: 10.1097/00000658-189407000-00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jatoi I, Hilsenbeck SG, Clark GM, Osborne CK. Significance of axillary lymph node metastasis in primary breast cancer. J Clin Oncol. 1999;17:2334–40. [DOI] [PubMed]
- 3.Fisher B, Jeong JH, Anderson S, Bryant J, Fisher ER, Wolmark N. Twenty-five year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med. 2002;347:567–75. [DOI] [PubMed]
- 4.Rao R, Euhus D, Mayo HG, Balch C. Axillary node interventions in breast cancer: a systematic review. JAMA. 2013;310(13):1385–94. [DOI] [PubMed]
- 5.Noguchi M, Morioka E, Ohno Y, Noguchi M, Nakano Y, Kosaka T. The changing role of axillary lymph node dissection for breast cancer. Breast Cancer. 2013;20(1):41–6. [DOI] [PubMed]
- 6.Lyman GH, Temin S, Edge SB, Newman LA, Turner RR, Weaver DL, et al. Sentinel lymph node biopsy for patients with early-stage breast cancer: American society of clinical oncology clinical practice guideline update. J Clin Oncol. 2014;32(13):1365–83. [DOI] [PubMed]
- 7.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology. Breast Cancer Version 3.2014. http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- 8.D’Angelo-Donovan DD, Dickson-Witmer D, Petrelli NJ. Sentinel lymph node biopsy in breast cancer: a history and current clinical recommendations. Surg Oncol. 2012;21(3):196–200. doi: 10.1016/j.suronc.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Jafferbhoy S, McWilliams B. Clinical significance and management of sentinel node micrometastasis in invasive breast cancer. Clin Breast Cancer. 2012;12(5):308–12. doi: 10.1016/j.clbc.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Abdessalam SF, Zervos EE, Prasad M, Farrar WB, Yee LD, Walker MJ, et al. Predictors of positive axillary lymph nodes after sentinel lymph node biopsy in breast cancer. Am J Surg. 2001;182(4):316–20. [DOI] [PubMed]
- 11.Chen JJ, Wu J. Management strategy of early-stage breast cancer patients with a positive sentinel lymph node: with or without axillary lymph node dissection. Crit Rev Oncol Hematol. 2011;79(3):293–301. doi: 10.1016/j.critrevonc.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Van Zee KJ, Manasseh DM, Bevilacqua JL. A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol. 2003;10(10):1140–51. doi: 10.1245/ASO.2003.03.015. [DOI] [PubMed] [Google Scholar]
- 13.Hwang RF, Krishnamurthy S, Hunt KK, Mirza N, Ames FC, Feig B, et al. Clinicopathologic factors predicting involvement of nonsentinel axillary nodes in women with breast cancer. Ann Surg Oncol. 2003;10(3):248–54. [DOI] [PubMed]
- 14.Barranger E, Coutant C, Flahault A, Delpech Y, Darai E, Uzan S. An axilla scoring system to predict non-sentinel lymph node status in breast cancer patients with sentinel lymph node involvement. Breast Cancer Res Treat. 2005;91(2):113–9. [DOI] [PubMed]
- 15.Pal A, Provenzano E, Duffy SW, Pinder SE, Purushotham AD. A model for predicting non-sentinel lymph node metastatic disease when the sentinel lymph node is positive. Br J Surg. 2008;95(3):302–9. [DOI] [PubMed]
- 16.Kohrt HE, Olshen RA, Bermas HR, Goodson WH, Wood DJ, Henry S, et al. New models and online calculator for predicting non-sentinel lymph node status in sentinel lymph node positive breast cancer patients. BMC Cancer. 2008;8:66. [DOI] [PMC free article] [PubMed]
- 17.Espinosa-Bravo M, Sansano I, Pérez-Hoyos S, Ramos M, Sancho M, Xercavins J, et al. Prediction of non-sentinel lymph node metastasis in early breast cancer by assessing total tumoral load in the sentinel lymph node by molecular assay. Eur J Surg Oncol. 2013;39(7):766–73. [DOI] [PubMed]
- 18.Eiró N, González LO, Atienza S, González-Quintana JM, Beridze N, Fernandez-Garcia B, et al. Prediction of metastatic breast cancer in non-sentinel lymph nodes based on metalloprotease-1 expression by the sentinel lymph node. Eur J Cancer. 2013;49(5):1009–17. [DOI] [PubMed]
- 19.Sauer G, Schneiderhan-Marra N, Muche R, Koretz K, Kazmaier C, Kreienberg R, et al. Molecular indicators of non-sentinel node status in breast cancer determined in preoperative biopsies by multiplexed sandwich immunoassays. J Cancer Res Clin Oncol. 2011;137(8):1175–84. [DOI] [PMC free article] [PubMed]
- 20.Nogi H, Suzuki M, Kamio M, Kato K, Kawase K, Toriumi Y, et al. Impact of CD44 + CD24- cells on non-sentinel axillary lymph node metastases in sentinel node-positive breast cancer. Oncol Rep. 2011;25(4):1109–15. [DOI] [PubMed]
- 21.Reyal F, Rouzier R, Depont-Hazelzet B, Bollet MA, Pierga JY, Alran S, et al. The molecular subtype classification is a determinant of sentinel node positivity in early breast carcinoma. PLoS One. 2011;6(5):e20297. [DOI] [PMC free article] [PubMed]
- 22.Kwon Y, Ro J, Kang HS, Kim SK, Hong EK, Khang SK, et al. Clinicopathological parameters and biological markers predicting non-sentinel node metastasis in sentinel node-positive breast cancer patients. Oncol Rep. 2011;25(4):1063–71. [DOI] [PubMed]
- 23.Tvedskov TF, Bartels A, Jensen MB, Paaschburg B, Kroman N, Balslev E, et al. Evaluating TIMP-1, Ki67, and HER2 as markers for non-sentinel node metastases in breast cancer patients with micrometastases to the sentinel node. APMIS. 2011;119(12):844–52. [DOI] [PubMed]
- 24.Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25(9):1105–11. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28(5):511–5. [DOI] [PMC free article] [PubMed]
- 26.da Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 27.Kim D, Salzberg SL. TopHat-Fusion: an algorithm for discovery of novel fusion transcripts. Genome Biol. 2011;12(8):R72. doi: 10.1186/gb-2011-12-8-r72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ, et al. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22(8):1736–47. [DOI] [PMC free article] [PubMed]
- 29.Eswaran J, Cyanam D, Mudvari P, Reddy SD, Pakala SB, Nair SS, et al. Transcriptomic landscape of breast cancers through mRNA sequencing. Sci Rep. 2012;2:264. [DOI] [PMC free article] [PubMed]
- 30.Horvath A, Pakala SB, Mudvari P, Reddy SD, Ohshiro K, Casimiro S, et al. Novel insights into breast cancer genetic variance through RNA sequencing. Sci Rep. 2013;3:2256. [DOI] [PMC free article] [PubMed]
- 31.Kaur H, Mao S, Shah S, Gorski DH, Krawetz SA, Sloane BF, et al. Next-generation sequencing: a powerful tool for the discovery of molecular markers in breast ductal carcinoma in situ. Expert Rev Mol Diagn. 2013;13(2):151–65. [DOI] [PMC free article] [PubMed]
- 32.Avgeris M, Mavridis K, Scorilas A. Kallikrein-related peptidases in prostate, breast, and ovarian cancers: from pathobiology to clinical relevance. Biol Chem. 2012;393(5):301–17. doi: 10.1515/hsz-2011-0260. [DOI] [PubMed] [Google Scholar]
- 33.Sano A, Sangai T, Maeda H, Nakamura M, Hasebe T, Ochiai A. Kallikrein 11 expressed in human breast cancer cells releases insulin-like growth factor through degradation of IGFBP-3. Int J Oncol. 2007;30(6):1493–8. [PubMed]
- 34.Gauld SB, Dal Porto JM, Cambier JC. B cell antigen receptor signaling: roles in cell development and disease. Science. 2002;296(5573):1641–2. doi: 10.1126/science.1071546. [DOI] [PubMed] [Google Scholar]
- 35.Robak T, Robak P. BCR signaling in chronic lymphocytic leukemia and related inhibitors currently in clinical studies. Int Rev Immunol. 2013;32(4):358–76. doi: 10.3109/08830185.2013.786711. [DOI] [PubMed] [Google Scholar]
- 36.Thumser AE, Moore JB, Plant NJ. Fatty acid binding proteins: tissue-specific functions in health and disease. Curr Opin Clin Nutr Metab Care. 2014;17(2):124–9. doi: 10.1097/MCO.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 37.Sun L, Fan X. Expression of cytochrome P450 2A13 in human non-small cell lung cancer and its clinical significance. J Biomed Res. 2013;27(3):202–7. doi: 10.7555/JBR.27.20120019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ascierto ML, Kmieciak M, Idowu MO, Manjili R, Zhao Y, Grimes M, et al. A signature of immune function genes associated with recurrence-free survival in breast cancer patients. Breast Cancer Res Treat. 2012;131(3):871–80. [DOI] [PMC free article] [PubMed]
- 39.Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 2010;11(2):174–83. [DOI] [PubMed]


