Abstract
Background
Epidemiologic studies have shown a relationship between glycated hemoglobin levels and cardiovascular events in patients with type 2 diabetes. We investigated whether intensive therapy to target normal glycated hemoglobin levels would reduce cardiovascular events in patients with type 2 diabetes who had either established cardiovascular disease or additional cardiovascular risk factors.
Methods
In this randomized study, 10,251 patients (mean age, 62.2 years) with a median glycated hemoglobin level of 8.1% were assigned to receive intensive therapy (targeting a glycated hemoglobin level below 6.0%) or standard therapy (targeting a level from 7.0 to 7.9%). Of these patients, 38% were women, and 35% had had a previous cardiovascular event. The primary outcome was a composite of nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes. The finding higher mortality in the intensive-therapy group led to a discontinuation of intensive therapy after a mean of 3.5 years of follow-up.
Results
At 1 year, stable median glycated hemoglobin levels of 6.4% and 7.5% were achieved in the intensive-therapy group and the standard-therapy group, respectively. During follow-up, the primary outcome occurred in 352 patients in the intensive-therapy group, as compared with 371 in the standard-therapy group (hazard ratio, 0.90; 95% confidence interval [CI], 0.78 to 1.04; P = 0.16). At the same time, 257 patients in the intensive-therapy group died, as compared with 203 patients in the standard-therapy group (hazard ratio, 1.22; 95% CI, 1.01 to 1.46; P = 0.04). Hypoglycemia requiring assistance and weight gain of more than 10 kg were more frequent in the intensive-therapy group (P<0.001).
Conclusions
As compared with standard therapy, the use of intensive therapy to target normal glycated hemoglobin levels for 3.5 years increased mortality and did not significantly reduce major cardiovascular events. These findings identify a previously unrecognized harm of intensive glucose lowering in high-risk patients with type 2 diabetes. (ClinicalTrials.gov number, NCT00000620.)
TYPE 2 DIABETES MELLITUS IS A METABOLIC disease that is diagnosed on the basis of sustained hyperglycemia. People with type 2 diabetes are at elevated risk for a number of serious health problems, including cardiovascular disease, premature death, blindness, kidney failure, amputations, fractures, frailty, depression, and cognitive decline.1 In prospective epidemiologic studies, the incidence of many of these outcomes is directly associated with the degree of hyperglycemia, as measured by the plasma glucose or the glycated hemoglobin level, a measure of the mean blood glucose level during the previous 2 to 3 months. Thus, after adjustment for other risk factors, an increase of 1% in the glycated hemoglobin level is associated with an increase of 18% in the risk of cardiovascular events,2 an increase of 12 to 14% in the risk of death,3,4 and an increase of 37% in the risk of retinopathy or renal failure.4
The graded relationship between the glycated hemoglobin level and cardiovascular events and death suggested that a therapeutic strategy lower glycated hemoglobin levels might reduce these outcomes. This hypothesis was supported by findings from some but not all previous clinical trials.1 However, the hypothesis was not explicitly tested in adequately powered, randomized trials focusing on cardiovascular outcomes. Nevertheless, data from basic science, epidemiologic analysis, and limited trials have been used to support guideline recommendations to target near-normal levels of glycated hemoglobin and glucose in selected patients with type 2 diabetes mellitus,5-8 despite a paucity of evidence regarding the risks and benefits of doing so with currently available therapies.
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial was specifically designed to determine whether a therapeutic strategy targeting normal glycated hemoglobin levels (i.e., below 6.0%) would reduce the rate of cardiovascular events, as compared with a strategy targeting glycated hemoglobin levels from 7.0 to 7.9% in middle-aged and older people with type 2 diabetes mellitus and either established cardiovascular disease or additional cardiovascular risk factors. The finding of higher mortality in the intensive-therapy group led to a decision to terminate the intensive regimen in February 2008, 17 months before the scheduled end of the study. We report the effects of the intensive intervention on mortality and the primary composite outcome of major cardiovascular events in all patients and in prespecified subgroups.
METHODS
ELIGIBILITY AND STUDY DESIGN
The rationale and design of the trial and a description of the glycemia intervention have been reported previously.9,10 Briefly, the ongoing multi-center clinical study, which is sponsored by the National Heart, Lung, and Blood Institute (NHLBI), is being conducted in 77 clinical centers (aggregated within seven networks) across the United States and Canada. We recruited volunteers who had type 2 diabetes mellitus and a glycated hemoglobin level of 7.5% or more and who either were between the ages of 40 and 79 years and had cardiovascular disease or were between the ages of 55 and 79 years and had anatomical evidence of significant atherosclerosis, albuminuria, left ventricular hypertrophy, or at least two additional risk factors for cardiovascular disease (dyslipidemia, hypertension, current status as a smoker, or obesity).9 Key exclusion criteria included frequent or recent serious hypoglycemic events, unwillingness to do home glucose monitoring or inject insulin, a body-mass index (the weight in kilograms divided by the square of the height in meters) of more than 45, a serum creatinine level of more than 1.5 mg per deciliter (133 μmol per liter), or other serious illness.
All 10,251 patients were randomly assigned to receive comprehensive intensive therapy targeting a glycated hemoglobin level of less than 6.0% or to receive standard therapy targeting a level of 7.0 to 7.9%. With the use of a double two-by-two factorial design, 4733 patients were randomly assigned to lower their blood pressure by receiving either intensive therapy (systolic blood-pressure target, <120 mm Hg) or standard therapy (systolic blood-pressure target, <140 mm Hg). In addition, 5518 patients were randomly assigned to receive either fenofibrate or placebo while maintaining good control of low-density lipoprotein cholesterol with simvastatin.11 These blood-pressure and lipid trials are continuing, and results regarding them remain masked. The study protocol was approved by the institutional review board or ethics committee at each center, as well as by a review panel at the NHLBI. All patients provided written informed consent.
Patients received instructional materials and behavioral counseling regarding diabetes care and were provided with glucose-lowering medications (from a study-supervised formulary) and glucose-monitoring supplies. Any marketed anti-hyperglycemic therapy that was not provided by the formulary could also be prescribed to any patient but was not provided by study investigators. Therapeutic regimens were individualized at the discretion of the investigators and patients on the basis of study-group assignment and the response to therapy. Adverse effects of therapy were carefully audited both locally and centrally to ensure the safety of the patients.12
Patients in the intensive-therapy group attended monthly visits for the first 4 months and then every 2 months thereafter, with at least one interim phone call, with the aim of rapidly and safely reducing glycated hemoglobin levels to below 6.0%. Additional visits were scheduled as needed to achieve glycemic goals, as described previously.9,10 Patients in the standard-therapy group had glycemic-management visits every 4 months.
PRIMARY AND SECONDARY OUTCOMES
The prespecified primary outcome was the first occurrence of nonfatal myocardial infarction or nonfatal stroke or death from cardiovascular causes. The latter included death from myocardial infarction, heart failure, arrhythmia, invasive cardiovascular interventions, cardiovascular causes after noncardiovascular surgery, stroke, unexpected death presumed to be from ischemic cardiovascular disease occurring within 24 hours after the onset of symptoms, and death from other vascular diseases.9 Death from any cause was one of several prespecified secondary outcomes. Study investigators also measured the effect of the intervention on microvascular disease, hypoglycemia, cognition, and quality of life, although these outcomes are not reported here. Key outcomes were adjudicated by a central committee whose members were unaware of study-group assignments on the basis of predefined criteria.13 A central laboratory that was unaware of study-group assignments analyzed blood for glycated hemoglobin levels. Study investigators outside the coordinating center and NHLBI project office were unaware of the accumulating rates of study outcomes.
SAFETY AND EFFICACY ANALYSES
An independent, 10-member data and safety monitoring committee that was appointed by the NHLBI reviewed the interim results approximately every 6 months. The committee’s role was to monitor the primary outcome and deaths from any cause, ensure the safety of patients, make recommendations to continue or alter the study design, and advise the NHLBI if there was clear evidence of benefit or harm. After reviewing mortality trends for several months (and as part of a preplanned safety analysis), on January 8, 2008, the committee concluded that the harm associated with the increased rate of death from any cause in the intensive-therapy group, as compared with that in the standard-therapy group, out-weighed any potential benefits and recommended that the intensive regimen be discontinued for safety reasons. This recommendation was accepted by the NHLBI. Patients were informed of this decision on February 5, 2008, and were subsequently switched to standard glycemic therapy. The public was informed of the decision in a press release on February 6, 2008. This report is based on data that were submitted to the coordinating center through December 10, 2007, and that were used by the data and safety monitoring committee to make its recommendation. Some of the adjudications of the causes of death were completed after that date.
STATISTICAL ANALYSIS
The study was designed to have a power of 89% to detect a 15% reduction in the rate of the primary outcome for patients in the intensive-therapy group, as compared with the standard-therapy group, assuming a two-sided alpha level of 0.05, a primary-outcome rate of 2.9% per year in the standard-therapy group, and a planned average follow-up of approximately 5.6 years. The original number of patients and power determinations for each study group were made under the assumption that the blood-pressure and lipid interventions would produce the effect sizes for which they were designed.9
All statistical analyses were conducted at the coordinating center with the use of S-Plus software, version 8.0 (Insightful) or SAS software, version 9.1 (SAS Institute). Baseline characteristics were compared in the two study groups with the use of chi-square tests and two-sample t-tests. At each assessment visit, glycated hemoglobin levels were summarized with the use of medians and interquartile ranges. Exposure to glucose-lowering drugs was summarized according to study group as the number of patients who received a prescription for a medication and the total person-years of prescriptions. The incidence of key safety outcomes — including severe hypoglycemia, heart failure, motor vehicle accidents in which the patient was the driver, fluid retention, elevated aminotransferase levels, and weight gain — were compared with the use of Fisher’s exact test.
Analyses of primary and secondary outcomes were performed with the use of time-to-event methods according to the intention-to-treat principle, and occurrences of these outcomes in the two study groups were compared with the use of hazard ratios and 95% confidence intervals. Two-sided P values were obtained from likelihood-ratio tests from Cox proportional-hazards regression analyses. Our inspection of plots of survival estimates versus follow-up time indicated that the assumption of proportional hazards for the glycemia intervention appeared to be valid. The Cox models contained a term representing study-group assignments plus terms accounting for the following prespecified stratifying variables: assignment to either the blood-pressure trial or the lipid trial, assignment to the intensive blood-pressure intervention in the blood-pressure trial, assignment to receive fibrate in the lipid trial, the seven clinical-center networks, and the presence or absence of a previous cardiovascular event. An analysis of how sensitive the results were to inclusion of these variables as stratifying factors rather than as covariates in the Cox model was also performed. Event rates are expressed as the percentage of events per follow-up year, taking into account censoring of follow-up data. Kaplan–Meier estimates were used to obtain the proportion of patients who had an event during follow-up.
We assessed the consistency of the effect of study-group assignment on total mortality and the primary outcome among prespecified subgroups using statistical tests of interaction between the treatment effect and the subgroup within the Cox model. We report all nominal P values, un-adjusted for the multiplicity associated with the various tests performed for this study or monitoring of the primary and mortality end points by the data and safety monitoring committee. Since we conducted 15 statistical tests of hypotheses related to secondary end points and subgroups, there was a 54% chance (i.e., 1 – [1 – 0.05]15) that at least one of these tests would be statistically significant at an alpha level of 0.05, assuming independence between tests.
Post hoc exploratory analyses to identify factors associated with higher mortality in the intensive-therapy group examined baseline characteristics, hypoglycemic events, risk factors for hypoglycemic events, single medications prescribed, combinations of medications prescribed, cointerventions, changes in weight, achieved blood pressure, achieved glycated hemoglobin levels, and occurrence of a cardiovascular event during follow-up. Detailed analyses of these and other potential causal factors or mechanisms will be reported separately.
RESULTS
PATIENTS
A total of 10,251 men and women with a mean (±SD) age of 62.2±6.8 years and a median glycated hemoglobin level of 8.1% (interquartile range, 7.6 to 8.9) were randomly assigned to either the intensive-therapy group or the standard-therapy group (see the figure in the Supplementary Appendix, available with the full text of this article at www.nejm.org). Approximately 38% of the entire cohort of patients were women. Recruitment occurred in two phases: 1174 patients were recruited during a 20-week period from January to June 2001, and 9077 patients were recruited from February 2003 to October 2005.14 Key baseline characteristics were similar in the two study groups (Table 1). The mean duration of follow-up at the time the data and safety monitoring committee recommended the discontinuation of the intensive regimen was 3.5 years (median, 3.4).
Table 1. Characteristics of the Patients at Baseline.*.
| Variable | Intensive Therapy (N = 5128) | Standard Therapy (N = 5123) |
|---|---|---|
| Age (yr) | 62.2±6.8 | 62.2±6.8 |
| Female sex (%) | 38.7 | 38.4 |
| Median duration of diabetes (yr) | 10 | 10 |
| Previous cardiovascular event (%) | 35.6 | 34.8 |
| Previous congestive heart failure (%) | 4.9 | 4.8 |
| Race or ethnic group (%)† | ||
| White | 64.4 | 64.5 |
| Black | 19.7 | 18.9 |
| Hispanic | 7.0 | 7.4 |
| Education (%) | ||
| Less than high school | 15.7 | 14.0 |
| High-school graduate | 26.1 | 26.7 |
| Some college | 32.7 | 32.9 |
| College degree or higher | 25.5 | 26.4 |
| Cigarette-smoking status (%) | ||
| Current | 14.3 | 13.7 |
| Former | 44.4 | 44.0 |
| Never | 41.3 | 42.3 |
| Weight (kg) | 93.5±18.7 | 93.6±18.7 |
| Body-mass index | 32.2±5.5 | 32.2±5.5 |
| Waist circumference (cm) | 106.8±14.3 | 106.8±13.8 |
| Blood pressure (mm Hg) | ||
| Systolic | 136.2±17.0 | 136.5±17.2 |
| Diastolic | 74.8±10.6 | 75.0±10.7 |
| Medications (%) | ||
| Insulin | 34.1 | 35.7 |
| Metformin | 59.7 | 60.0 |
| Any sulfonylurea | 50.8 | 49.4 |
| Any thiazolidinedione | 19.5 | 19.2 |
| Any antihypertensive agent | 84.9 | 86.0 |
| Angiotensin-converting–enzyme inhibitor | 53.0 | 53.0 |
| Aspirin | 54.8 | 54.1 |
| Beta-blocker | 28.7 | 29.9 |
| Any thiazide diuretic | 26.5 | 26.4 |
| Statin | 61.7 | 62.4 |
| Glycated hemoglobin (%) | ||
| Mean | 8.3±1.1 | 8.3±1.1 |
| Median | 8.1 | 8.1 |
| Fasting serum glucose (mg/dl) | 174.9±56.0 | 175.7±56.5 |
| Cholesterol (mg/dl) | ||
| Total | 183.3±42.1 | 183.3±41.6 |
| Low-density lipoprotein | 104.9±34.0 | 104.9±33.8 |
| High-density lipoprotein | ||
| Women | 47.2±13.0 | 46.9±12.2 |
| Men | 38.4±9.5 | 38.8±9.8 |
| Median triglyceride (mg/dl) | 156 | 154 |
| Potassium (mg/dl) | 4.5±0.4 | 4.5±0.7 |
| Serum creatinine (mg/dl) | 0.9±0.2 | 0.9±0.2 |
Plus–minus values are means ±SD. There were no significant differences between the two study groups at baseline. The body-mass index is the weight in kilograms divided by the square of the height in meters. To convert the values for glucose to millimoles per liter, multiply by 0.05551. To convert the values for cholesterol to millimoles per liter, multiply by 0.02586. To convert the values for triglycerides to millimoles per liter, multiply by 0.01129. To convert the values for potassium to millimoles per liter, multiply by 0.2558. To convert the values for creatinine to micromoles per liter, multiply by 88.4.
Race was self-reported, and patients could check multiple categories.
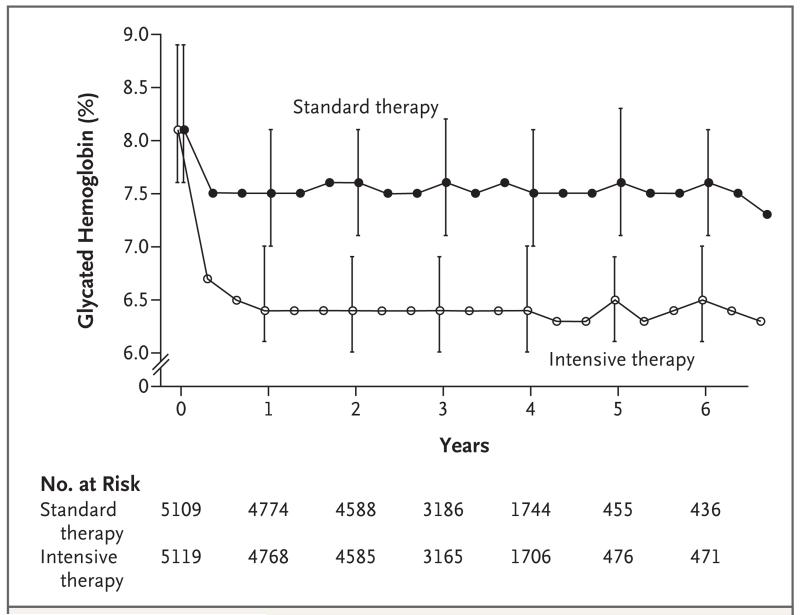
The two therapeutic strategies rapidly achieved different glycated hemoglobin levels (Fig. 1). Within 4 months after randomization, the median glycated hemoglobin level had fallen from 8.1% at baseline to 6.7% (interquartile range, 6.2 to 7.2) in the intensive-therapy group and to 7.5% (interquartile range, 7.0 to 8.2) in the standard-therapy group. Stable median levels of 6.4% (interquartile range, 6.1 to 7.0) and 7.5% (interquartile range, 7.0 to 8.1), respectively, were achieved in the two groups at 1 year and were maintained throughout the follow-up period.
Figure 1. Median Glycated Hemoglobin Levels at Each Study Visit.
I bars denote interquartile ranges.
The lower glycated hemoglobin levels in the intensive-therapy group were associated with a greater exposure to drugs from every class (Table 2). There were also more frequent changes in the dose or the number of drugs used. The glucose-lowering regimen was modified by adding or removing a drug or by increasing or decreasing the dose of an oral agent or insulin (by ≥10% of the previous dose) a mean number of 4.4 times per year in the intensive-therapy group and 2.0 times per year in the standard-therapy group.
Table 2. Prescribed Glucose-Lowering Drugs.*.
| Drug Class and Name | Intensive Therapy (N = 5128) | Standard Therapy (N = 5123) | ||
|---|---|---|---|---|
| no. of patients (%) | person-years | no. of patients (%) | person-years | |
| Single class | ||||
|
| ||||
| Metformin | 4856 (94.7) | 14,444 | 4452 (86.9) | 12,693 |
|
| ||||
| Secretagogue† | 4443 (86.6) | 12,021 | 3779 (73.8) | 10,059 |
|
| ||||
| Glimepiride | 4010 (78.2) | 9,142 | 3465 (67.6) | 8,955 |
|
| ||||
| Repaglinide | 2574 (50.2) | 4,447 | 908 (17.7) | 1,293 |
|
| ||||
| Thiazolidinedione‡ | 4702 (91.7) | 12,844 | 2986 (58.3) | 6,719 |
|
| ||||
| Rosiglitazone | 4677 (91.2) | 12,639 | 2946 (57.5) | 6,563 |
|
| ||||
| α-Glucosidase inhibitor§ | 1191 (23.2) | 941 | 263 (5.1) | 200 |
|
| ||||
| Incretin¶ | 911 (17.8) | 566 | 251 (4.9) | 175 |
|
| ||||
| Exenatide | 622 (12.1) | 415 | 204 (4.0) | 155 |
|
| ||||
| Any insulin | 3965 (77.3) | 11,902 | 2837 (55.4) | 7,842 |
|
| ||||
| Any bolus insulin | 2834 (55.3) | 6,806 | 1794 (35.0) | 4,336 |
|
| ||||
| Combination of classes | ||||
|
| ||||
| No. of classes without insulin | ||||
|
| ||||
| 1 or 2 | 2798 (54.6) | 2,011 | 3224 (62.9) | 6,612 |
|
| ||||
| 3 | 3030 (59.1) | 3,681 | 1681 (32.8) | 2,545 |
|
| ||||
| 4 or 5 | 539 (10.5) | 332 | 109 (2.1) | 67 |
|
| ||||
| No. of classes with insulin | ||||
|
| ||||
| 0 | 916 (17.9) | 829 | 892 (17.4) | 1,495 |
|
| ||||
| 1 or 2 | 3311 (64.6) | 6,603 | 2375 (46.4) | 5,284 |
|
| ||||
| 3 | 2668 (52.0) | 4,126 | 834 (16.3) | 1,027 |
|
| ||||
| 4 or 5 | 526 (10.3) | 344 | 64 (1.2) | 36 |
Metformin, glimepiride, repaglinide, rosiglitazone, acarbose, and exenatide were provided by a study-supervised formulary. Patients could receive more than one medication or combination of medications and may therefore be counted in more than one category. All individual medications that are listed were prescribed to at least 10% of patients in either group.
Patients received glimepiride, glyburide, gliclazide, repaglinide, or nateglinide.
Patients received rosiglitazone or pioglitazone.
All the patients in this category received acarbose except one who received miglitol.
Patients received exenatide or sitagliptin.
As compared with the standard-therapy group, the intensive-therapy group had significantly higher rates of hypoglycemia, weight gain, and fluid retention (Table 3). The annualized rate of hypoglycemic episodes requiring medical assistance was 3.1% in the intensive-therapy group and 1.0% in the standard-therapy group, and the mean weight gain at 3 years was 3.5 kg and 0.4 kg in the two groups, respectively. One death in each group was classified as probably related to hypoglycemia, according to the adjudicated analysis of causes of death. Patients in the two groups had similar exposure to cardiovascular protective interventions and had similar changes in non-glycemic characteristics associated with cardiovascular events (Table 3). Significantly fewer patients in the intensive-therapy group received an angiotensin-converting–enzyme inhibitor than in the standard-therapy group (69.7% vs. 71.9%, P = 0.02). However, blood-pressure levels were slightly lower in the intensive-therapy group.
Table 3. Adverse Events, Clinical Measures, Tobacco Use, and Use of Nonglycemic Medication after Randomization.*.
| Variable | Intensive Therapy (N = 5128) |
Standard Therapy (N = 5123) |
P Value† |
|---|---|---|---|
| Adverse events | |||
| Hypoglycemia — no. (%) | |||
|
| |||
| Requiring medical assistance | 538 (10.5) | 179 (3.5) | <0.001 |
|
| |||
| Requiring any assistance | 830 (16.2) | 261 (5.1) | <0.001 |
|
| |||
| Fatal or nonfatal heart failure — no. (%) | 152 (3.0) | 124(2.4) | 0.10 |
|
| |||
| Motor vehicle accident in which patient was driver — no./total no. (%) |
9/5033 (0.2) | 14/5036 (0.3) | 0.40 |
|
| |||
| Any nonhypoglycemic serious adverse event — no. (%) | 113 (2.2) | 82 (1.6) | 0.03 |
|
| |||
| Fluid retention — no./total no. (%)‡ | 3541/5053 (70.1) | 3378/5054 (66.8) | <0.001 |
|
| |||
| Clinical measures | |||
|
| |||
| Weight gain >10 kg since baseline — no./total no. (%) | 1399/5036 (27.8) | 713/5042 (14.1) | <0.001 |
|
| |||
| Alanine aminotransferase >3 times ULN — no./total no. (%)§ | 51/5065 (1.0) | 77/5061 (1.5) | 0.02 |
|
| |||
| Low-density lipoprotein cholesterol — mg/dl¶ | 90.8±33.5 | 90.6±34.0 | 0.74 |
|
| |||
| Blood pressure — mm Hg¶ | |||
|
| |||
| Systolic | 126.4±16.7 | 127.4±17.2 | 0.002 |
|
| |||
| Diastolic | 66.9±10.5 | 67.7±10.6 | <0.001 |
|
| |||
| Cigarette-smoking status — no. (%) ∥ | 0.54 | ||
|
| |||
| Current (previous 30 days) | 505 (9.8) | 508 (9.9) | |
|
| |||
| Former | 2524 (49.2) | 2467 (48.2) | |
|
| |||
| Never | 2093 (40.9) | 2143 (41.8) | |
|
| |||
| Missing data | 6 (0.1) | 5 (0.1) | |
|
| |||
| Use of nonglycemic medication — no./total no. (%) | |||
|
| |||
| Antihypertensive | 4664/5127 (91.0) | 4714/5123 (92.0) | 0.06 |
|
| |||
| Angiotensin-converting–enzyme inhibitor | 3512/5038 (69.7) | 3621/5037 (71.9) | 0.02 |
|
| |||
| Aspirin | 3736/4950 (75.5) | 3753/4970 (75.5) | 0.98 |
|
| |||
| Beta-blocker | 2395/5038 (47.5) | 2450/5037 (48.6) | 0.27 |
|
| |||
| Statin | 4432/5039 (88.0) | 4425/5054 (87.6) | 0.54 |
Plus–minus values are means ±SD. Data within categories are not mutually exclusive. Percentages may not total 100 because of rounding. ULN denotes upper limit of the normal range.
P values were calculated with the use of Fisher’s exact test or a two-sample t-test.
Of the patients with fluid retention, 89% had pretibial edema or ankle swelling, 30% had shortness of breath, 12% had congestive heart failure or pulmonary edema, and 24% had nocturia.
The ULN for alanine aminotransferase was 65 U per liter for men and 50 U per liter for women.
Data were obtained from last available measurement at the 12-month visit or later.
Smoking status was reported at the last annual visit.
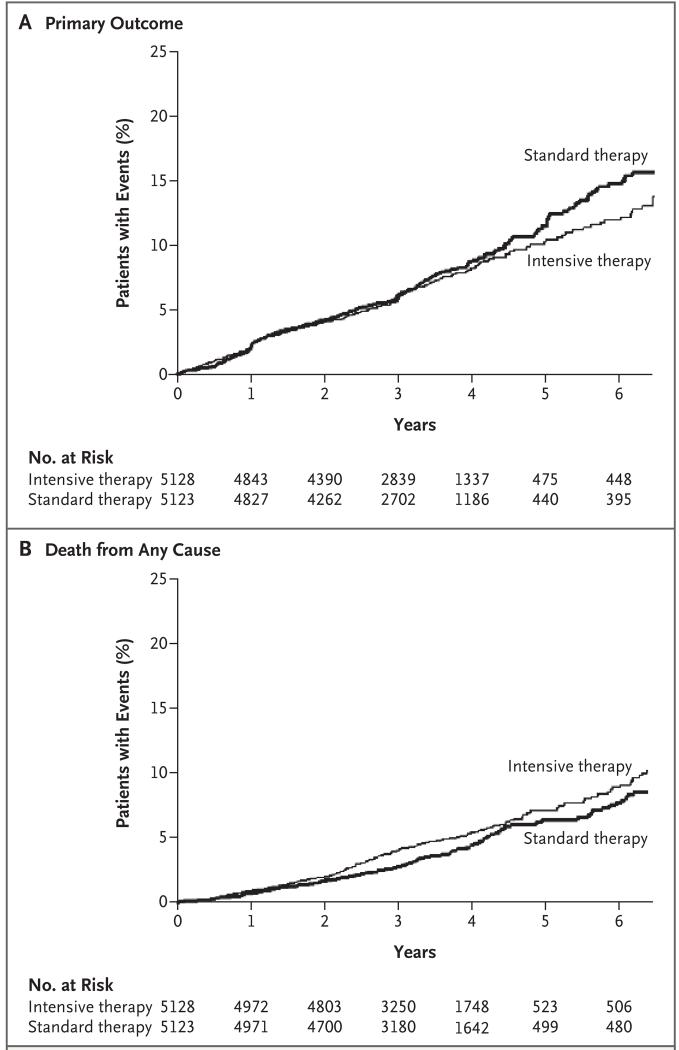
During the intervention period, the primary composite outcome of nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes occurred in 723 patients, and there were 460 deaths from any cause. In the data reviewed by the data and safety monitoring committee, vital status was ascertained in 97.8% of patients within the previous 12 months; 50 patients (0.5%, including 26 patients in the intensive-therapy group and 24 in the standard-therapy group) were lost to follow-up, and 162 patients (1.6%, including 84 in the intensive-therapy group and 78 in the standard-therapy group) withdrew consent. The mean follow-up time for patients who either withdrew consent or were lost to follow-up was 1.2 years in the intensive-therapy group and 1.0 year in the standard-therapy group (P = 0.22). There were fewer occurrences of the composite primary outcome in the intensive-therapy group, with rates of the primary outcome beginning to separate in the two study groups after 3 years (Table 4 and Fig. 2A). This trend was not significant, with rates of 6.9% in the intensive-therapy group and 7.2% in the standard-therapy group (hazard ratio, 0.90; 95% confidence interval [CI], 0.78 to 1.04; P = 0.16). In the intensive-therapy group, the rate of nonfatal myocardial infarction was lower than in the standard-therapy group (3.6% vs. 4.6%; hazard ratio, 0.76; 95% CI, 0.62 to 0.92; P = 0.004), and the rate of death from cardiovascular causes was higher (2.6% vs. 1.8%; hazard ratio, 1.35; 95% CI, 1.04 to 1.76; P = 0.02); there was no significant difference in the rate of nonfatal stroke (1.3% vs. 1.2%; hazard ratio, 1.06; 95% CI, 0.75 to 1.50; P = 0.74).
Table 4. Primary and Secondary Outcomes.*.
| Outcome | Intensive Therapy (N = 5128) | Standard Therapy (N = 5123) | Hazard Ratio (95% CI) |
P Value | ||
|---|---|---|---|---|---|---|
| no. of patients (%) | % per yr | no. of patients (%) | % per yr | |||
| Primary outcome | 352 (6.9) | 2.11 | 371 (7.2) | 2.29 | 0.90 (0.78–1.04) | 0.16 |
| Secondary outcome | ||||||
| Death | ||||||
| Any cause | 257 (5.0) | 1.41 | 203 (4.0) | 1.14 | 1.22 (1.01–1.46) | 0.04 |
| Cardiovascular causes | 135 (2.6) | 0.79 | 94 (1.8) | 0.56 | 1.35 (1.04–1.76) | 0.02 |
| Nonfatal myocardial infarction | 186 (3.6) | 1.11 | 235 (4.6) | 1.45 | 0.76 (0.62–0.92) | 0.004 |
| Nonfatal stroke | 67 (1.3) | 0.39 | 61 (1.2) | 0.37 | 1.06 (0.75–1.50) | 0.74 |
| Fatal or nonfatal congestive heart failure |
152 (3.0) | 0.90 | 124 (2.4) | 0.75 | 1.18 (0.93–1.49) | 0.17 |
| Causes of death | ||||||
| Any | 257 (5.0) | 1.41 | 203 (4.0) | 1.14 | 1.22 (1.01–1.46) | 0.04 |
| Unexpected or presumed cardio- vascular disease† |
86 (1.7) | 67 (1.3) | ||||
| Fatal myocardial infarction† | 19 (0.4) | 13 (0.3) | ||||
| Fatal congestive heart failure† | 23 (0.4) | 16 (0.3) | ||||
| Fatal procedure† | ||||||
| For cardiovascular disease | 10 (0.2) | 3 (0.1) | ||||
| For noncardiovascular disease | 1 (<0.1) | 3 (0.1) | ||||
| Fatal arrhythmia† | 4 (0.1) | 10 (0.2) | ||||
| Fatal stroke† | 9 (0.2) | 11 (0.2) | ||||
| Other cardiovascular disease† | 8 (0.2) | 10 (0.2) | ||||
| Cancer | 65 (1.3) | 63 (1.2) | ||||
| Condition other than cancer or cardiovascular disease‡ |
50 (1.0) | 35 (0.7) | ||||
| Undetermined | 7 (0.1) | 11 (0.2) | ||||
The primary outcome was the first occurrence of nonfatal myocardial infarction or nonfatal stroke or death from cardiovascular causes. Data within categories are not mutually exclusive, and patients who were classified as having more than one possible cause of death are listed in the relevant categories. Hazard ratios are for the intensive-therapy group as compared with the standard-therapy group.
This condition was a component of the outcome of fatal cardiovascular disease.
Additional details are provided in the Supplementary Appendix.
Figure 2. Kaplan–Meier Curves for the Primary Outcome and Death from Any Cause.
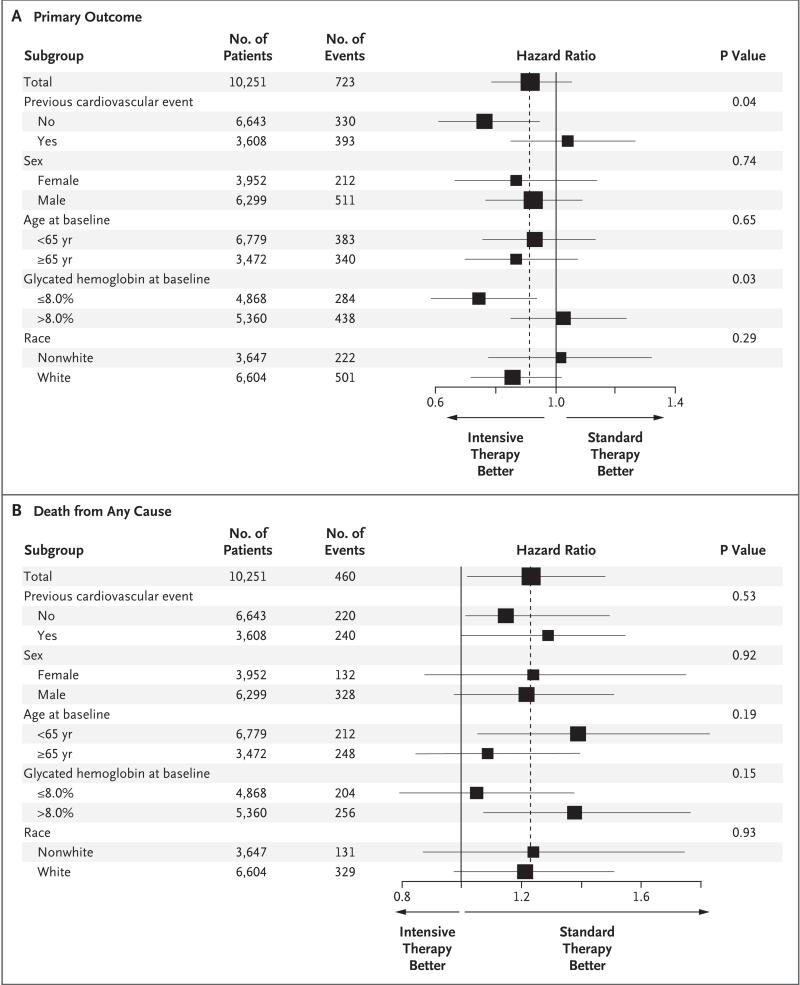
The rate of death from any cause was higher in the intensive-therapy group than in the standard-therapy group (5.0% vs. 4.0%; hazard ratio, 1.22; 95% CI, 1.01 to 1.46; P = 0.04). In sensitivity analyses of the Cox model for death from any cause that included variables as stratifying factors rather than as covariates, the estimated hazard ratio for mortality in the intensive-therapy group, as compared with that in the standard-therapy group, was stable to the second decimal place. Rates of death in the two study groups began to separate after 1 year, and the differences persisted throughout the follow-up period (Fig. 2B). The effect on mortality was consistent within the subgroups with no evidence of heterogeneity (Fig. 3) and persisted in models adjusting for differences in the receipt of medications for blood pressure and lipids.
Figure 3. Hazard Ratios for the Primary Outcome and Death from Any Cause in Prespecified Subgroups.
Data regarding glycated hemoglobin levels at baseline are presented for 10,288 patients because a baseline level was not available for 23 patients. Horizontal bars represent the 95% confidence interval, and vertical dashed lines indicate the overall hazard ratio. The size of each square is proportional to the number of patients.
For the primary outcome, there was some evidence of heterogeneity among prespecified subgroups, which suggested that patients in the intensive-therapy group who had not had a cardiovascular event before randomization (P for interaction = 0.04) or whose baseline glycated hemoglobin level was 8.0% or less (P for interaction = 0.03) may have had fewer fatal or nonfatal cardiovascular events than did patients in the standard-therapy group (Fig. 3). Preliminary nonprespecified exploratory analyses of episodes of severe hypoglycemia after randomization and differences in the use of drugs (including rosiglita-zone), weight change, and other factors did not identify an explanation for the mortality finding.
DISCUSSION
We conducted this study because previous clinical trials had not established the effects of intensive glucose lowering on cardiovascular events or mortality in patients with type 2 diabetes mellitus. For example, in the United Kingdom Prospective Diabetes Study,15 an intensive glucose-lowering regimen significantly reduced a composite outcome of seven diabetes-related events, as compared with conventional therapy. However, the effects on cardiovascular events and mortality were not significant. Conversely, in the Veterans Affairs Diabetes Feasibility Trial,16 intensive glucose lowering was associated with a nonsignificant increase in cardiovascular events and no difference in mortality, and in the University Group Diabetes Program,17,18 the group that received a sulfonylurea (tolbutamide) had higher mortality.
Our findings indicate that a comprehensive, customized, therapeutic strategy targeting glycated hemoglobin levels below 6.0% increased the rate of death from any cause after a mean of 3.5 years, as compared with a strategy targeting levels of 7.0 to 7.9% in patients with a median glycated hemoglobin level of 8.1% and either previous cardiovascular events or multiple cardiovascular risk factors. Patients in both groups had lower mortality than reported in epidemiologic studies of similar patients.19,20 However, as compared with the standard-therapy group, the intensive-therapy group had a relative increase in mortality of 22% and an absolute increase of 1.0% during this follow-up period, with similar differences in death from cardiovascular causes. This increase in mortality is equivalent to one extra death for every 95 patients who were treated for 3.5 years.
This study was not designed to test the components of the intervention strategy. Analyses that we have performed to date have not identified and clear explanation for this higher mortality. In the intensive-therapy group, a median glycated hemoglobin level of 6.4% was rapidly achieved and maintained by a combination of behavioral and pharmacologic approaches. The standard-therapy group had fewer study visits and used fewer drugs and drug combinations. Thus, the higher rate of death in the intensive-therapy group may be related to factors associated with the various strategies. These factors include but are not limited to differences in the achieved glycated hemoglobin level of 6.4% in the intensive-therapy group, as compared with 7.5% in the standard-therapy group; in the magnitude of the reduction in glycated hemoglobin levels in the two study groups; in the speed of the reduction in glycated hemoglobin levels, with reductions of approximately 1.4% in the intensive-therapy group and 0.6% in the standard-therapy group within the first 4 months after randomization; in changes in drug regimens and in the rate of hypoglycemia; in adverse effects due to an undetected interaction of the various drug classes used at high doses; or in some combination of these or many other possibilities, perhaps in combination with the clinical characteristics of the patients in the study.
Differences in mortality emerged 1 year to 2 years after randomization. It is notable that after about 3 years, a nonsignificant decrease in the rate of the primary outcome emerged in the intensive-therapy group (Fig. 2A), due to significantly fewer nonfatal myocardial infarctions, despite more deaths from cardiovascular causes and a similar number of strokes (Table 4). These patterns with respect to mortality and the primary outcome suggest that if there is any benefit associated with intensive glucose lowering, it may take several years to emerge, during which time there is an increased risk of death. This intriguing possibility can be answered only by further research.
The strengths of our study include the random assignment of patients to study groups and follow-up of a large number of high-risk patients according to a common protocol, a high rate of follow-up, achievement and maintenance of an absolute difference in glycated hemoglobin levels of 1.1% for 3.5 years, implementation within clinics that routinely treat patients in the community, adjudication of outcomes by a committee unaware of study-group assignment, a factorial design in which blood-pressure and lipid interventions continue to be tested, and safety auditing by an independent committee. However, our study did not address the risks and benefits of various approaches to lowering glycated hemoglobin levels (including what rate of glucose lowering is optimal), the prevention of increased glycated hemoglobin levels in patients with type 2 diabetes mellitus who have glycated hemoglobin levels below 7.5%, the prevention of diabetes altogether, and the lowering of glycated hemoglobin levels in people who do not have cardio-vascular disease or additional cardiovascular risk factors. Indeed, the suggestion of a greater benefit in the primary outcome for patients with a lower glycated hemoglobin level or those without cardiovascular disease raises the possibility that certain subgroups of patients may benefit from intensive glucose lowering. However, our study was not designed to test these possibilities. Finally, glucose-lowering strategies were adjusted for each patient in an open fashion on the basis of their study-group assignment, their subsequent glycemic response to a drug or drug combination, and the development of clinical symptoms, such as hypoglycemia. This linkage of the study-group assignment to post-randomization exposures means that analyses to discern which aspects of the therapeutic strategy contributed to the observed outcomes were unlikely to clearly identify or exclude a cause. Thus, nonprespecified analyses of possible causes of the higher mortality in the intensive-therapy group can be only exploratory and require prospective testing.
Follow-up of the patients after they were switched from intensive therapy to standard therapy may provide crucial information regarding the long-term rates of death and cardiovascular events after a 3.5-year period of intensive therapy. Other trials investigating the long-term outcome of intensive glucose lowering21-25 will report the effect of a variety of therapeutic approaches on mortality and cardiovascular events in patients with type 2 diabetes mellitus and a range of clinical characteristics. Regardless of the results of these other trials, our study has identified a previously unrecognized harm of intensive glucose lowering in high-risk patients with type 2 diabetes mellitus and high glycated hemoglobin levels. This harm may be due either to the approach used for rapidly lowering glycated hemoglobin levels or to the levels that were achieved. Our findings highlight the importance of conducting trials with sufficient statistical power to assess commonly used approaches on clinically relevant outcomes.
Supplementary Material
Acknowledgments
Supported by grants (N01-HC-95178, N01-HC-95179, N01-HC-95180, N01-HC-95181, N01-HC-95182, N01-HC-95183, N01-HC-95184, IAA-Y1-HC-9035, and IAA-Y1-HC-1010) from the National Heart, Lung, and Blood Institute; by other components of the National Institutes of Health, including the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Aging, and the National Eye Institute; by the Centers for Disease Control and Prevention; and by General Clinical Research Centers. The following companies provided study medications, equipment, or supplies: Abbott Laboratories, Amylin Pharmaceutical, AstraZeneca, Bayer HealthCare, Closer Healthcare, GlaxoSmithKline, King Pharmaceuticals, Merck, Novartis, Novo Nordisk, Omron Healthcare, Sanofi-Aventis, and Schering-Plough.
Dr. Gerstein reports receiving consulting fees from Sanofi-Aventis, GlaxoSmithKline, Merck, Abbott, Novo Nordisk, Novartis, and Lilly, lecture fees from Sanofi-Aventis, GlaxoSmithKline, Merck, and Lilly, and grant support from Sanofi-Aventis, GlaxoSmithKline, King, and Merck and holding a patent that is completely assigned to Sanofi-Aventis, for which he receives no royalties or benefit; Dr. Goff, receiving grant support from Merck; Dr. Bigger, receiving grant support from McMaster University; Dr. Buse, having an equity interest in Insulet, MicroIslet, and dLife and receiving grant support from Bristol-Myers Squibb, Novartis, Pfizer, Novo Nordisk, Amylin, Eli Lilly, and Medtronic; Dr. Cushman, receiving consulting fees from Novartis, King, Takeda, and Sanofi-Aventis, lecture fees from Novartis, and grant support from Novartis, Hamilton Health, and Abbott; Dr. Genuth, receiving consulting fees from Merck, Mannkind, Sanofi-Aventis, and Novartis and lecture fees from Lilly and having an equity interest in Bristol-Myers Squibb; Dr. Grimm, receiving lecture fees from Merck, Pfizer, and Novartis; and Dr. Probstfield, receiving consulting fees from King and grant support from King and Sanofi-Aventis.
APPENDIX
Members of the ACCORD data and safety monitoring committee were A.M. Gotto (chair), K. Bailey, D. Gohdes, S. Haffner, R. Hiss, K. Jamerson, K. Lee, D. Nathan, J. Sowers, L. Walters. Members of the ACCORD study group were as follows: Steering Committee: W.T. Friedewald (chair), J.B. Buse (vice chair), J.T. Bigger, R.P. Byington, W.C. Cushman, H.C. Gerstein, H.N. Ginsberg, D.C. Goff, Jr., R. H. Grimm, Jr., F. Ismail-Beigi, J.L. Probstfield, D.G. Simons-Morton. Asterisks denote persons who are no longer affiliated with the study unit, and daggers persons who are deceased. Clinical Center Networks and Clinical Sites — Canada: Population Health Research Institute, Hamilton General Hospital, Canadian Diabetes Outcome Researchers, Hamilton, ON: H.C. Gerstein, S. Yusuf, Z. Punthakee, R. Russo, S. Anand, B. Cracknell, T. Cukierman-Yaffe, A. Gafni, G. Guyatt,* S. Hall, J. Kaszyca, E. Lonn,* D. McLeod, K. Read,* V. Reiding, N. Shehadeh,* B. Tadeson,* K. Thompson,* M. Vallis, V. Vasudeva, I. Wilderman.* Canadian Clinical Sites: McMaster Medical Centre, Hamilton, ON: Z. Punthakee, A. Smith, I. Stanton, S. Capes,* S. Danby, W. Harper, P. Harvey, D. Hunt, P. Manjoo,* A. Moroso, R. Otto, A. Prebtani, T. Valla. Six Nations Health Services, Ohsweken, ON: Z. Punthakee, A. Davis, S. Capes,* K.L. Hill, V. McCarthy. Diabetes, Hypertension and Cholesterol Centre, University of Calgary, Calgary, AB: A.L. Edwards, D.J. Mitchell, M.A. Clearwaters, C. Dielissen, M. Gillam, B. Hammond,* H. Jensen,* A. Kherani, D. Lau, V. Pringle, D. Rabi, R. Sigal, C. Smith,* M. Walker,* G. Williams.* Memorial University of Newfoundland, St. John’s: C. Joyce, M. Parsons, B. Rowe, J. Burton,* V. Chandurkar, S. Coady-McDonald,* D. Gibbons,* C. Kovacs, B. Murphy, R. Smart, S. Varghese. University of Alberta, Edmonton: L. Mereu, E. Ryan, P. Senior, P. Kirkland, J. Abe,* K. Dalton, W. Gendall, J. Germsheid,* D. Hartman,* A. Jeffrys,* C. MacDonald, N. Makhani, S. Mawani,* F. Morales, B. Paty,* M. Pick,* B. Schwanke, A. Stark, M. Tennant, S. Varma,* P. Werbiski-Wood, B. Woloschuk, W. Zimmerman.* Centre de Recherche Clinique de Laval, Laval, QC: A. Bélanger, S. Gauthier, G. Bahsali, C. Barbeau, E. Caponi, R. Duchesne,* R. Dumas, P. Gauthier, J. Girouard, N. Kandalaft, M. Labbé, J. Palardy, M. Pilon, J. Raymond, A. Schiffrin. St. Joseph’s Health Care London, London, ON: I. Hramiak, S. Tereschyn, M. Driscoll, M. Gehring, J. Gonder, C. Lincoln, W. McBeth, C. McDonald, T. McDonald,* P. Pauli,* T. Paul, S. Powers,* N. Ronald, V. Trinh, L. Vancer, G. Walsh.* Ottawa Hospital, Division of Endocrinology and Metabolism, Ottawa: H. Lochnan, T.C. Ooi, J. Maranger, R. Bate,* L. Bradley,* R. Buhrmann, M. Cyr,* C. Gilchrist, B. Hanlon, M. Harley, K. Jay,* T. Leech, J. Malcolm, M. McLean,* E. Parker, R. Sigal,* K. Sullivan. Royal Victoria Hospital, Montreal: J.F. Yale, S.A. Segal, N. Renouf, N. Allaire,* M.A.M.A. Alawadhi,* B. Belfer,* D.W. Blank, F. Bouchard, S. Buoy-Phang,* J. Carter, L. Coppin,* D. Dalpe,* I. Delpech,* P.M. Doran, F. Emmian,* S. Fortin,* N. Garfield, M. Gosselin,* S. Horan,* M. Kalergis,* S. Koutelias,* C. Légaré, A. Lombardo, J.A. Morais, M. Quigley, N. Renouf, C. Riopel,* S. Riopel, J.A. Rivera, G. Rochon,* M. Roy, M. Salera,* M.H. Sherman, M. Shingler, H.E. Staples,* L. Ulyatt,* Z. Yared.* St. Michael’s Hospital, Toronto: L.A. Leiter, G. Booth, L. Sparrow, H. Choi, D.C. Bedard, A. Berger, L.A. Berndl, A. Cheng,* V. Evalmplev,* J. Goguen, A. Hanna, R.G. Josse, J.A. Kalas, S. Perry, M. Pike.* Vancouver General Hospital, Vancouver, BC: T. Elliott, K. Dawson, J. Kong, M. Inducil, R. Al Amoudi,* T. Broughton,* L. Hall,* B. Harrison,* N. Hirvi,* R. Lee,* E. Norman,* B. Paty, M. Potter, D. Stevenson, A. Vafadaran. Health Sciences Centre Diabetes Research Group, Winnipeg, MB: V. Woo, L. Berard, T. Anderlic, K. Austman, A. Bernard, D. Catte,* P. Darvill, D. Hak, K. Hutchison, L. Janzen, T. Klopak, C. Mandock, M. Mathen, S. Mawani,* A. Mawani,* L. Murphy,* G. Nyomba, B. Penner, S. Pockett, S. Russell, F. Stockl, J. Studney,* R. Sukkau. Queen Elizabeth II Health Sciences Centre, Halifax, NS: C. Abbott, E. Ur, M. Yuille, M. Archibald,* A. Cruess, N. Davis, H. Fong,* S. Frizzell,* B. Hanway,* A. Hoskin-Mott, A. Imran, C. Ingraham,* G. McCarthy, H. Murdock,* T. Palmer, A.M. Patterson,* T. Ransom, D. Shu,* J. Tuttle. Western Clinical Center Network: University of Washington, Seattle: J.L. Probstfield, C. Kingry, A.S. Line, M.A. Corson, R. Knopp, E. Lipkin, M.D. Sullivan, J. Johnson, C. Griswold, K. Liebert, A. Brown,* D. Juliano,* E.M. Kurashige,* S. Moberg.* Western Clinical Sites: Northridge Hospital Medical Center, Cardiovascular Center, Northridge, CA: K. Ariani, K. Karunaratne, M. Azizad, C. Chow, H. Gutierrez, J. Partamian, J. Toven, J. Mular,* S. Sanders.* White Memorial Medical Center, Clinical Hypertension Services, Los Angeles: L.J. Haywood, V. Kamdar, D.L. DeQuattro, L. Wang, Z. Song, C. Miller, L. Becerra, A. Qi Cai, C. Pruitt, V. DeQuattro.† University of Washington Medical Center at Roosevelt, Family Medical Center, Seattle: R. Failor, A. Ellsworth, N. Jackson, C. Miller, D. Britt, S. Dobie, I. Hirsch, D. Khakpour, R. Quaempts, W. Stoffel, T. Wilcox, W. Neighbor,* K. Cappocia.* Idaho State University, Department of Family Medicine, Pocatello: R. Force, M. Macdonald, S. Lusk, C. Liday, E. Borzadek, S. Koester, T. Pettinger, R. Solbrig, C. Waldron, K. Pettingill,* W. Woodhouse,* B. Hoover.* Naval Medical Center San Diego, Cardiology Division, San Diego, CA: P.E. Linz, P.V. Pepper, J. Kozlowski, C. Chase, D. Samuelson, P. Gutierrez, C. Gonzales, M. Engle,* J. Coopersmith,* S. Griffin,* R. Lammers,* J. Leon.* Oregon Health & Science University, Section of Diabetes, Portland: M.C. Riddle, K.A. Hanavan, P.A. McDaniel, R. Swift, A.J. Ahmann, S.C. Gammell-Matthews, D.M. Karl, V. Burden, B. MacNeil, M. MacMurray, J. Weiss, C. Carlson,* S.K. DesRochers,* D. Negreanu,* E.A. Stephens.* Washington State University, Spokane: C. Wysham, D. Weeks, L. Kuntsmann, L. Maxwell, S. Yedinak, H. Pena,* J. White.* Kaiser Endocrine Clinic, San Diego, CA: J. Dudl, L. Lyons, B. House, M. Murray, P. Wu, A. Palma, S. Briere, T. Wilson, D. Becker,* K. Harden,* C. Hawley,* R. Stevenson.* Whittier Institute for Diabetes, Clinical Trials Department, La Jolla, CA: G. Dailey, M. Baron, A. Gianella, M. Jacobson, E. Farro, A. Philis-Tsimikas, A. Banares, A. Bravo-Medina, J. Horne,* E. Esquer,* R. Morrissey.* Minnesota–Iowa Clinical Center Network: Berman Center for Outcomes and Clinical Research, Minneapolis: R.H. Grimm, Jr., B.R. Kirpach, M.M. Bartkoske, C.M. Boyce,* N. Druckman,* A.M. Gillett,* J.A. Levin, G.J. Livingston, A.M. Murray, H. Wood,* HealthPartners Research Foundation, Minneapolis: K.L. Margolis. Minnesota–Iowa Clinical Sites: Berman Center Clinic, Minneapolis: S. Kempainen, M. Madden, M. Tariq Fareed,* K. Hall,* R. Moor, K. Wood. International Diabetes Center, Minneapolis: R. Bergenstal, R. Cuddihy, B. Davick, J. Hokanson,* M. Johnson, D. Kendall,* M. Lausch, S. List, A. Monk, R. Robinson,* K. Smith,* D. Whipple, G. Damberg, R. Hahn,* V. Koenig, M. Magadan, S. Sabin-Smith,* P. Stewart, E. Strock, D. Peremislov, K. Gunyou, R. Passi. University of Minnesota, Minneapolis: E.R. Seaquist, M.V. Mech, L.E. Benedict,* D.J. Demmon,* A.F. Kumar, S.M. Martinson,* S.A. Miller, C. Pease,* J.P. Rao,* J.B. Redmon, J.E. Swanson,† J.K. Wimmer.* University of Minnesota, Phalen Village Clinic, St. Paul: K. Peterson, L.A. Seaquist, C. Boese,* M. Cruciani, E. Dodds, F. Parenteau Ek,* J.L. Feldman, P. Fontaine, C.J. Lange, T.J. Mendenhall,* A.M. Peterson, A. Rudelt, T.M. Schrock,* D.P. Spielman,* S. Velasco,* J.C. Weinhandl. Riverside Health Partners Clinic, Department of Endocrinology, Minneapolis: J.M. Sperl-Hillen, P.J. O’Connor, M.E. Busch, A. Chung, B.K. Klein, N. Krugen, T. Bunkers-Lawson,* H.L. Ekstrom,* H.S. Gunderson,* B.M. Johnson,* J.H. MacIndoe,* D.J. Prewedo,* J.L. Rawl,* C.M. Roethke,* Mary Spencer. University of Iowa, Health Care Diabetes Clinical Research and Programs, Iowa City: W.I. Sivitz, S.M. Wayson, T.A. Lower,* L. Larson, L.A. Ahrens,* M. Bayless, S.E. Beck,* J. Chahal, C. Chenard, G.C. Doelle, V.M. Guzman, U.M. Kabadi,* K.A. Ochs,* A. Rahhal, R.G. Spanheimer,* L. Snetselaar,* K. Smith,* D. Wells. Ohio–Michigan Clinical Center Network: Case Western Reserve University, Division of Clinical and Molecular Endocrinology, Cleveland: F. Ismail-Beigi, S. Genuth, M. Thibonnier,* L. Vargo,* C. Kelly,* T. Bongorno,* A. Dolish,* L. Pavlik, M. Tiktin, S. Isteitieh. Ohio–Michigan Clinical Sites: University Hospitals of Cleveland, Division of Endocrinology, and University Hospitals Westlake Medical, Cleveland: F. Ismail-Beigi, A. Krikorian, L. Moore, L. Richardson, E. Coles-Herman, K. Yee, J. Frankino, M. Jing, A. Sood, L. Hustak,* M. Julius,* L. Pavlik,* T. Ross,* L. Long,* W. Schwing,* M. Tiktin,* M.K. Sullivan,* L. Strauss,* K. Behm,* F. Eskandari,* C. Hall,* D. Hayes,* K. Horowitz, S. Isteitieh,* Z. Madhun,* E. Seeholzer,* J. Shina,* H. Taylor,* A. Schnall,* S. Huang, M. Heeg, J. Tang, J. Belkin,* M.S. Lee,* T. Joly.* St. Vincent Charity Hospital, Lipid Research Center, Cleveland: L.S. Sadler, M. Griffith,* A. Hornsby,* K. Klyn, E. Ospelt, L. Long, M. DeSmit,* P. McCann, N.P. Schmidt,* C. Gottfried, T. Kulow, J. Zaletal, M.S. Kapadia. University Suburban Health Center, South Euclid, OH: A.M. Schnall, L. Dragmen, R. Ellert,* J. Smith, J. Leksan, T. Sussman, S. Huang, M. Heeg, J. Tang, J. Belkin,* M.S. Lee,* T. Joly.* Cleveland Veterans Affairs (VA) Medical Center, Department of Medicine, and Ravenna Community Based Outpatient Clinic, Cleveland: F. Ismail-Beigi, L. Hustak, M. Julius,* W. Schwing, M. Tiktin,* J. Anselmo,* F. Eskandari,* S. Daeumeyer,* C. Hall, D. Hayes,* K. Horowitz, S. Isteitieh,* C. Johnson,* E. Kern, M.A. Richmond,* L. Richardson, K. Roberts,* J. Shina,* A. Sood, P. Suhan,* H. Taylor, S. Watts,* J. Martin, L. Moore,* B. Burtch,* S. Ober, G.J. Strauss, A. Leone, J. Belkin,* S. Huang,* K. Frank,* D. Stephens,* M.S. Lee,* T. Joly.* The Cleveland Clinic Foundation, Cleveland: B.J. Hoogwerf, J. Brakeman, M. Matzinger,* J. Newsome,* J. Becker,* S. Bizjack,* B. Clingman,* S. Curtas, G. Depietro,* R. Ellert,* C. Horner, G. Bunae,* A. Hamrahian,* A. Hawkins, T. Head, S. Iannica, L. Jones, P. Kaiser, R. McCoy, A. Mehta, L. Olansky, A. Orasko, S. Reddy,* D. Ross,* L. Shockley,* E. Siraj,* M. Williams,* R. Zimmerman. Your Diabetes Endocrine Nutrition Group, Mentor, OH: D. Weiss, K.A. Fagan, T.M. Hanslik, J. Farrell, P. Brys, M. Oligny, K. Prokop, K. Lenardic, T. Karapanzcik, S. Huang, M. Heeg, J. Tang, J. Belkin,* M.S. Lee,* T. Joly.* Medical University of Ohio, Department of Medicine, Ruppert Health Center, Toledo: B. Akpunonu, R. Franco-Saenz,† J. Gilmore, M. Gilmore, L. Godfrey, P. Ross, B. Bauer, M. Chrisstie,* A. Lopez, P. Mulrow, C. Peters,* R. Pop-Busui,* J. Roman,* C. Smith,* J. Bick,* Z. Blust,* P.T. Nelsen, D. Marcus.* The Ohio State University Medical Center, Division of Endocrinology, Diabetes and Metabolism, Columbus: K. Osei, E.A. Dziengelewski,* H. Breedlove, D. Boland,* C. Casey Boyer, S. Cataland, P.A. Green, J.E. Irwin, D.P. Schuster, J.L. Varga-Spangler, T. Bowles, K. Weiland, K. Arnold, T. Evans,* J. Bouttamy, A. Letson, E. Craig, F. Davidorf. University of Cincinnati/VA Medical Center, Research Service, Cincinnati: R.M. Cohen, K. Burton, J. Craig, B. Carter,* J. Harrer,* R. Hurd,* D. Lopez-Stickney,* C. Pritchard,* A. Pfefferman,* B.A. Ramlo-Halsted,* C. McCormick, C. Riley, M. Strominger,* A. Knittel, G. Groff, C. Bailey, A. Howald, N. Anderson, J. Laver Bierschbach, M. Tyzinski,* B. Smith,* S. Krug, V. Hershberger,* R.K. Hutchins,* L.A. Raymond.* Henry Ford Health System–New Center One, Detroit: D.M. Kahkonen,* T. Cushman, M. Roman, A.M. Stys, A. Thomas, K. White, M. Austin,* C. Chatterton, J.K. Francis,* C. Jones,* D. Kruger, A. McLellan,* F. Whitehouse, E. Higgins,* S. Levy, A. Schoenherr,* P. Edwards. Grunberger Diabetes Institute, Bloomfield Hills, MI: G. Grunberger, L.C. Aman,* A.H. Bandagi,* K.M. Russell, C. Tucker, Y. Abidova, A. Amirikia. Northeastern Clinical Center Network: Columbia University College of Physicians and Surgeons, New York: J.T. Bigger, C.R. Lopez-Jimenez, R. Bornholdt, L. Busacca, H.N. Ginsberg, P. Gonzales, D. Gosh,* P. Love,† A. Kosok,* E. Robinson,* R. Steinman, C. Watson, G. Reyes. Northeastern Clinical Sites: Jacobi Medical Center, Bronx, NY: U.K. Schubart, M. Mendoza, G. Goswami, A. Laufer,* J. Russo, N. Vincenty. Albert Einstein General Clinical Research Center, Bronx, NY: M.H. Alderman, L. Carroll, M.J. Sanguily, J.U. Gorkin, A.C. Mayer, L. Ramos, V. Sessoms, A. Fritts Stewart.* Cornell Internal Medicine Associates, New York: D. Brillon, J. Cordero, M.A. Richardson, E. Wei, F. Ganz, B.R. Meyer, J. Paley,* S. Anderson,* C. Charles,* A. Dwoskin,* R. Chiong, K. Hyams. The Diabetes Care and Information Center of New York, Flushing: D.L. Lorber, T. Arenstein, P. Depree, A.A. Elmorsy,* J.M. Wendel, L.L. Zintl, P. August, M. Beck,* M.D. Goldberg, M.J. Hofacker,* M. Marotta-Kollarus,* E.J.L. Ocampo, C.A. Resta, J.M. Tibaldi. The Cooper Health System, Cherry Hill, NJ: A. Bastien,* S. Grudzinski, P. Niblack, L. Abreu, T. Brobyn, K. Brown,* M. Casale,* D. Dougherty,* G. Haddad, K. Heintz, M. Kelly,* D. Linneman,* C. Olivia, M.A. Salvador,* P. Zee,* D. Hyman. Great Lakes Medical Clinic Research, Westfield, NY: D.F. Brautigam, R. Fischer, J.M. Chiarot, D.M. Scharf, B. Nunn,* J. Carlson, C. Flanders,* M.R. Hagen, S. Newman, T.A. Gordon. Naomi Berrie Diabetes Center, New York: R. Goland, C.H. Tuck,† P. Kringas, J. Hey-Hadavi,* J. Montes,* J. Vargas-Jerez, J. Salas-Spiegel. Ambulatory Care Network at Columbia University, New York: A. Getaneh, J. Ramirez,* E.F. Vasquez,* G. Kranwinkel. Irving Diabetes Research Unit, New York: D.S. Donovan, G. Febres,* C. Hernandez,* M.A. Jonaitis, L. Mesa. State University of New York Downstate Medical Center, Brooklyn: M.A. Banerji, M. Norton, P. Patel, V. Daly, S. Hirsch, C. Jazmin, R. Khillan, D. Mendonca, A. Relingado, E. Sandoval, M. Tiewala. Kings County, Brooklyn, NY: M.A. Banerji, M. Norton, P. Patel, V. Daly, S. Hirsch, C. Jazmin, R. Khillan, D. Mendonca, A. Relingado, E. Sandoval, M. Tiewala. Southeastern Clinical Center Network: Wake Forest University School of Medicine, Department of Public Health Sciences, Winston-Salem, NC: D.C. Goff, Jr., J.H. Summerson, L. Crago, C.S. Blackwell,* A. Bertoni,* R.L. Blaine, J.K. Kirk, R.L. Spach, J. Williamson, J. Calles, J. Katula, D.B. Wishnietsky.* Southeastern Clinical Sites: Duke University Medical Center, Durham, NC: M.N. Feinglos, J. Jones, M.B. Mason, M.A. Furst, W.J. Bean,* G. Gedon-Lipscomb, J.B. Green, T. Parham,* B.M. Satterwhite,* C.R. Thacker. Constant Care, Inc., Valdosta, GA: D. Padhiar, R. Noel,* N. Padhiar, S. West,* J. Braden, A. Francis.* Wake Forest University School of Medicine, Department of Geriatrics/Gerontology, Winston-Salem, NC: H.H. Atkinson, M. Dibari,* J. Allen, J. Stanfield, T. Delvalle-Fagan, L.J. Gordineer, L. Gordon, M. Gordon,* S.L. Smith,* H. Yates.* Downtown Health Plaza, Winston-Salem, NC: C.F. Pedley, G. Zurek, M. Baird, B. Dunn,* W. Kinder,* S. Mauney. University of North Carolina, Diabetes Care Center, Chapel Hill: J.B. Buse, M.D. Duclos, R.E. Kirby,* J.F. Largay, N.M. McDermott,* A. Goley, S.S. Braithwaite, M. Busby, J.M. Dostou, E.A. Fasy,* D.C. Kelly,* B. MacIntosh, C.E. Metz,* J. Jeffries, D. Rubin.* Holston Medical Group, Kingsport, TN: J.L. Miller, S.M. Norton, J. Weatherly,* S. Bishop,* B. Cross, K. Nuss, M. Surgenor, Y. Wood. Carolinas Medical Center Family Practice, Charlotte, NC: K. Andrews,* T. Barringer, C. Hoffman,* J. Konen,* C. Morris, P. Tochiki,* G. Reinblatt, P. Bruner.* Robeson Health Care Corporation, Fairmont Clinic, Fairmont, NC: R. Peace, D.O. Stuart,* J. Strickland, L. Cummings, D. Craig,* J. Stanfield.* Robeson Health Care, Julian T. Pierce Clinic, Pembroke, NC: R. Peace, D.O. Stuart,* J. Strickland, L. Cummings, D. Craig,* J. Stanfield.* Wake Forest University School of Medicine, Departments of Internal Medicine and Endocrinology, Winston-Salem, NC: J.R. Crouse, L. Menon, S. Marion,* D. Davis,* B. Cabrera,* J. Calles, T. Chandler, J. Ellis, E. Kouba, P. Riddle, E. Myers.* Tulane University Health Science Center, New Orleans: V. Fonseca, R.H. McDuffie, N.O. Asafu-Adjaye, S.M. Leger, P. Reilly, G. Afner, F. Arrey,* S. Asnani, E. Borshard,* D. Boyd,* A. Cemo, S. Chennur,* P. Dupart, R. Garg,* G.P. Girindra,* B. Gouda,* W. Itoua-N’Ganongo,* I. Innocent-Ituah,* C. Johnson,* N. Kuhadiya, M. Kukreja,* I. Mangan-Mbondi,* S. Mason,* C. McLain, J. Naylyanya,* K. Nazereth,* S. Nazereth,* S. Singh, T. Thethi, K. Varnado,* R. Williams.* Kaiser Permanente, Clinic Atlanta Crescent Medical Center, Tucker, GA: J.I. Barzilay, M. Eley,* K. Bader, D. Curry-Ball, S. Goodman,* M. Stevens. Veterans Affairs (VA) Clinical Center Network: Memphis VA Medical Center, Memphis, TN: W.C. Cushman, T.S. Geraci, S.M. Walsh, L.G. Coley, M.B. Elam, C.M. Huff, D.I. Pickering,* P. Massimino.* VA Clinical Sites: Memphis VA Medical Center, Hypertension/Lipid Research Clinic, Memphis, TN: M.B. Elam, C.W. Thompson, L. Lichtermann, S. Peeples, J. Turner-Bates,* M. Heimberg, D. Childress, S. Solomon, J. Turner, J. Jasper, R. Pfeifer, J. Coley. Baltimore VA Medical Center, Baltimore: B.P. Hamilton, J. Hamilton, G. Kuzbida, D. Bannerman-Wood,* W. Hatten, Jr., A. Lancaster, H. Jang. Carl T. Hayden VA Medical Center, Phoenix, AZ: J. Felicetta, M. Bourne-Collo, M.E. Svoboda, D. Clothier, M. Deitz,† C. Flaugher,* P. Hayward,* T. Scheibe,* D. Heritage,* S. Velarde, S. Heritage, J.P. Nelson. Atlanta VA Medical Service, Decatur, GA: M.E. Sweeney, D. Harrelson, S. McConnell, C.R. Rice,* F. Watson, R. Johnson, L. Whittington, M. Nanes, M. Salles. Ralph H. Johnson VA Medical Center, Primary Care, Charleston, SC: J. Basile, D.B. Ham, B. North-Lee, H.A. Baig, S.U. Rehman, J. Mixson, D. Nelson.* G.V. Montgomery VA Medical Center, Research Department, Jackson, MS: K.A. Kirchner, B.S. Ross, M. Kazi,* J. Subauste,* L.A. Hinton, L. Mack, B. James,* A. Spencer, L. Henegar,* A. Jones. VA NY Harbor Healthcare System, New York: L. Katz, E.A. Richardson, A.G. Goldberg,* A. Nieves, J.E. Russo,* S.A. Sochalski, D. Hoffman. Washington VA Medical Center, Washington, DC: V. Papademetriou, P. Narayan,* D. Wojciechowski, B. Gregory, R. Alignay,* E. Nylen, B. Rajendran, R. Hodges, A. Ross,* A. Notargiacomo.* St. Louis VA Medical Center, St. Louis: S. Giddings, E. Clark, A. Pittler, R. Davis, P. Harris, T. Hofmeister. Central Arkansas Clinic Healthcare System, Little Rock: D.L. Simmons, J.J. Cooper,* K. Dishongh,* P. Choksi,* S. Elbein, F. Faas, Z. Hamid, J. Johnson, A. Mayo,* M.S. Moriarty, D. Rani,* N. Rasouli, K. Watson, A. Makdissi. ACCORD Steering Committee Chair: W.T. Friedewald, Mailman School of Public Health and Columbia University College of Physicians and Surgeons, New York. Coordinating Center: Wake Forest University School of Medicine, Winston-Salem, NC: R.P. Byington, W.T. Ambrosius, R.T. Anderson,* J. Barnes, J. Beal, C. Bell, D.E. Bonds, S. Burton,* C. Collins, D. Cook, B. Craven, T. Craven, D. Dunbar, G.W. Evans, P. Feeney, C.D. Furberg, C.M. Greven, J. Griffin, L. Harvin, J. Hepler, L. Howard,* L.T. Howard-Perdue, M. Hough, W. Hwang, A. Kimel, D. Lefkowitz, A. Lopina,* J. Lovato, L.C. Lovato, M.E. Miller, D. Reboussin,* S. Rushing, L. Sanders, L. Sims, C. Stowe, M. Walkup,* S. Wilmoth, K. Wilson, N. Woolard. Drug Distribution Center: Albuquerque VA Medical Center, Albuquerque, NM: D. Raisch, R. Ringer, M. Sather, B. DelCurto, D. Garnand. ECG Reading Center: Wake Forest University School of Medicine, Winston-Salem, NC: R. Prineas, C. Campbell, Z. Zhang, L. Selph, S. Hall,* S. Hensley, Y. Li, M. Mills. Central Chemistry Laboratory: Northwest Lipid Research Laboratories, Seattle: S. Marcovina, J. Chmielewski, K. Gadbois, V. Gaur, G. Strylewicz, M. Ramirez, S. Waddell, M. Mehan.* ACCORD–MIND MRI Reading Center: University of Pennsylvania, Philadelphia: R.N. Bryan, C. Davatzkios, G. Moonis, L. Desiderio, S. D’Arcy.* Fundus Photograph Reading Center: University of Wisconsin Medical School, Madison: M. Davis, R. Danis, S. Johnson,* N. Robinson, L. Hubbard, B. Esser, D. Thayer, M. Neider, K. Glader, M. Burger. Project Office: National Heart, Lung, and Blood Institute, Bethesda, MD: D.G. Simons-Morton, L. Cooper,* M. Domanski, C. Nwachuku,* Y. Rosenberg, M. Salive,* P. Savage, J.L. Fleg, J.A. Cutler, N. Geller, D. Follmann,* M. Proschan,* C. Jennings, E. Schaeffer,* P. Mills,* J. Bittner,* R. Kirby, P. Frommer,† L. Fine, J. Chan. National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD: J. Fradkin, S. Malozowski, C. Meyers, T. Hostetter.* National Institute on Aging, Bethesda, MD: L. Launer. National Eye Institute, Bethesda, MD: E.Y. Chew. Centers for Disease Control and Prevention, Atlanta: A. Albright, K.M.V. Narayan, M. Engelgau,* P. Zhang.
Footnotes
No other potential conflict of interest relevant to this article was reported.
References
- 1.Goff DC, Jr, Gerstein HC, Ginsberg HN, et al. Prevention of cardiovascular disease in persons with type 2 diabetes mellitus: current knowledge and rationale for the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol. 2007;99:4i–20i. doi: 10.1016/j.amjcard.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Selvin E, Marinopoulos S, Berkenblit G, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004;141:421–31. doi: 10.7326/0003-4819-141-6-200409210-00007. [DOI] [PubMed] [Google Scholar]
- 3.Gerstein HC, Pogue J, Mann JF, et al. The relationship between dysglycaemia and cardiovascular and renal risk in diabetic and non-diabetic participants in the HOPE study: a prospective epidemiological analysis. Diabetologia. 2005;48:1749–55. doi: 10.1007/s00125-005-1858-4. [DOI] [PubMed] [Google Scholar]
- 4.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–12. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Diabetes Association Standards of medical care in diabetes — 2008. Diabetes Care. 2008;31(Suppl 1):S12–S54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- 6.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee Canadian Diabetes Association 2003 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2003;23(Suppl 2):S1–S152. doi: 10.1016/j.jcjd.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Rydén L, Standl E, Bartnik M, et al. Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. Eur Heart J. 2007;28:88–136. doi: 10.1093/eurheartj/ehl260. [DOI] [PubMed] [Google Scholar]
- 8.AACE Diabetes Mellitus Clinical Practice Guidelines Task Force American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract. 2007;13(Suppl 1):1–68. doi: 10.4158/EP.13.S1.1. [DOI] [PubMed] [Google Scholar]
- 9.Buse JB, Bigger JT, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol. 2007;99:21i–33i. doi: 10.1016/j.amjcard.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Gerstein HC, Riddle MC, Kendall DM, et al. Glycemia treatment strategies in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol. 2007;99:34i–43i. doi: 10.1016/j.amjcard.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Ginsberg HN, Bonds DE, Lovato LC, et al. Evolution of the lipid trial protocol of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol. 2007;99:56i–67i. doi: 10.1016/j.amjcard.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 12.Bonds DE, Kurashige EM, Bergenstal R, et al. Severe hypoglycemia monitoring and risk management procedures in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol. 2007;99:80i–89i. doi: 10.1016/j.amjcard.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 13. [Accessed May 19, 2008];ACCORD Web site. at http://www.accordtrial.org.
- 14.Kingry C, Bastien A, Booth G, et al. Recruitment strategies in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol. 2007;99:68i–79i. doi: 10.1016/j.amjcard.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 15.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–53. Erratum, Lancet 1999;354: 602. [PubMed] [Google Scholar]
- 16.Abraira C, Colwell JA, Nuttall F, et al. Cardiovascular events and correlates in the Veterans Affairs Diabetes Feasibility Trial: Veterans Affairs Cooperative Study on Glycemic Control and Complications in Type II Diabetes. Arch Intern Med. 1997;157:181–8. [PubMed] [Google Scholar]
- 17.Meinert CL, Knatterud GL, Prout TE, Klimt CR. A study of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. II. Mortality results. Diabetes. 1970;19(Suppl):789–830. [PubMed] [Google Scholar]
- 18.Genuth S. Exogenous insulin administration and cardiovascular risk in non-insulin-dependent and insulin-dependent diabetes mellitus. Ann Intern Med. 1996;124:104–9. doi: 10.7326/0003-4819-124-1_part_2-199601011-00005. [DOI] [PubMed] [Google Scholar]
- 19.Booth GL, Kapral MK, Fung K, Tu JV. Relation between age and cardiovascular disease in men and women with diabetes compared with non-diabetic people: a population-based retrospective cohort study. Lancet. 2006;368:29–36. doi: 10.1016/S0140-6736(06)68967-8. [DOI] [PubMed] [Google Scholar]
- 20.Thomas RJ, Palumbo PJ, Melton LJ, III, et al. Trends in the mortality burden associated with diabetes mellitus: a population-based study in Rochester, Minn, 1970-1994. Arch Intern Med. 2003;163:445–51. doi: 10.1001/archinte.163.4.445. [DOI] [PubMed] [Google Scholar]
- 21.Abraira C, Duckworth W, McCarren M, et al. Design of the cooperative study on glycemic control and complications in diabetes mellitus type 2: Veterans Affairs Diabetes Trial. J Diabetes Complications. 2003;17:314–22. doi: 10.1016/s1056-8727(02)00277-5. [DOI] [PubMed] [Google Scholar]
- 22.Study rationale and design of ADVANCE: action in diabetes and vascular disease — preterax and diamicron MR controlled evaluation. Diabetologia. 2001;44:1118–20. doi: 10.1007/s001250100612. [DOI] [PubMed] [Google Scholar]
- 23.Milicevic Z, Raz I, Strojek K, et al. Hyperglycemia and its effect after acute myocardial infarction on cardiovascular outcomes in patients with Type 2 diabetes mellitus (HEART2D) Study design. J Diabetes Complications. 2005;19:80–7. doi: 10.1016/j.jdiacomp.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 24.ORIGIN Trial Investigators. Gerstein H, Yusuf S, Riddle MC, Ryden L, Bosch J. Rationale, design, and baseline characteristics for a large international trial of cardiovascular disease prevention in people with dysglycemia: the ORIGIN Trial (Outcome Reduction with an Initial Glargine Intervention) Am Heart J. 2008;155:26–32. doi: 10.1016/j.ahj.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Magee MF, Isley WL. Rationale, design, and methods for glycemic control in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Trial. Am J Cardiol. 2006;97:20G–30G. doi: 10.1016/j.amjcard.2006.02.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.