Abstract
Background
Although cerebral lesions ≥3mm on imaging are associated with incident stroke, lesions < 3mm are typically ignored.
Objective
To examine stroke risks associated with subclinical brain lesions by size (< 3 mm only, lesions ≥3 mm only, both < 3 mm and ≥3 mm) and white matter hyperintensities (WMH).
Design
Community cohort, Atherosclerosis Risk in Communities (ARIC) Study
Setting
Two ARIC sites with magnetic resonance imaging (MRI) data (1993–95)
Participants
1,884 (99%) adults (50–73 years, 40% men; 50% black) with MRI and no prior stroke; average 14.5 years follow-up.
Measurements
MRI lesions: none (n=1611), < 3 mm only (n=50), ≥3 mm only (n=185), or both < 3 and ≥3 mm lesions (n=35); WMH score (0–9 scale). Outcomes: incident stroke (n=157), overall mortality (n=576), stroke mortality (n=50). Hazard Ratios (HR) estimated with proportional hazards models.
Results
Compared to no lesions, stroke risk was tripled with lesions < 3mm only (HR=3.47, 95% CI:1.86-6.49), doubled with lesions ≥3 mm only (HR=1.94, 95% CI:1.22-3.07), and was 8-fold higher with both < 3 mm and ≥3 mm-sized lesions (HR=8.59, 95% CI:4.69-15.73). Stroke risk doubled with WMH ≥3 (HR=2.14, 95% CI:1.45-3.16). Stroke mortality risk tripled with lesions < 3 mm only (HR=3.05, 95% CI:1.04-8.94), doubled with lesions ≥3 mm (HR=1.9, 95% CI:1.48-2.44) and was seven-times higher with both lesion sizes (HR=6.97, 95% CI:2.03-23.93).
Limitations
Few stroke events (n=147), especially hemorrhagic (n=15); limited numbers of participants with only lesions ≤3mm (n=50) or with both lesions ≤3mm and 3–20mm (n=35).
Conclusions
Very small cerebrovascular lesions may be associated with increased risks of stroke and mortality; having both < 3 mm and ≥3 mm lesions may represent a particularly striking risk increase. Larger studies are needed to confirm findings and provide more precise estimates.
Introduction
Subclinical brain infarcts (SBI) are standardly defined as lesions > 3 mm on brain imaging (1, 2) in persons with no history of clinical stroke, and both SBI and white matter hyperintensities (WMH) have been associated with increased risk of stroke and mortality, mainly in older people.(3–14) Brain structural abnormalities may be objective markers of stroke risk, yet lesions < 3 mm are typically ignored in clinical and research settings due to potential misclassification of presumed non-vascular lesions, such as Virchow-Robin spaces, as vascular lesions, and lack of data regarding associations with outcomes. However, even very small lesions may be mediated through vascular processes such as infarcts, leukoaraiosis, and endothelial dysfunction;(15–18) the STRIVE consortium recently included small lesions, including potential perivascular spaces as a possible form of cerebral small vessel disease.(18) The relationship of lesions < 3mm to important clinical outcomes is unknown. If even very small lesions < 3mm are associated with stroke and mortality, these may identify at-risk persons early on and in whom targeted preventive measures may be warranted.
Ethnic minorities, including non-Hispanic blacks, are more likely than their white counterparts to suffer strokes, strokes at earlier ages, stroke-related disability, and stroke deaths.(19),(20) Yet, most studies of brain structural abnormalities and stroke risk have been in older and primarily white populations.(5, 6, 10, 13, 14, 21) Increased stroke risk associated with brain vascular lesions has been observed in the younger Framingham Offspring cohort,(6) as have increased stroke and mortality in a middle-to-older aged Japanese population(4) but studies in middle-aged persons and minorities including blacks are limited. Identifying early markers of at-risk individuals could significantly impact the public health burden of cerebrovascular disease in all ethnic groups, given associations with cognitive decline/dementia, gait impairment, and stroke.(13, 22–26)
The purpose of this study was to examine the associations of incident stroke, stroke-related mortality, and all-cause mortality with SBI lesions < 3mm, lesions ≥3mm, the combination of < 3mm and ≥3mm-sized lesions, and WMH in a middle-aged biracial population.
METHODS
Population
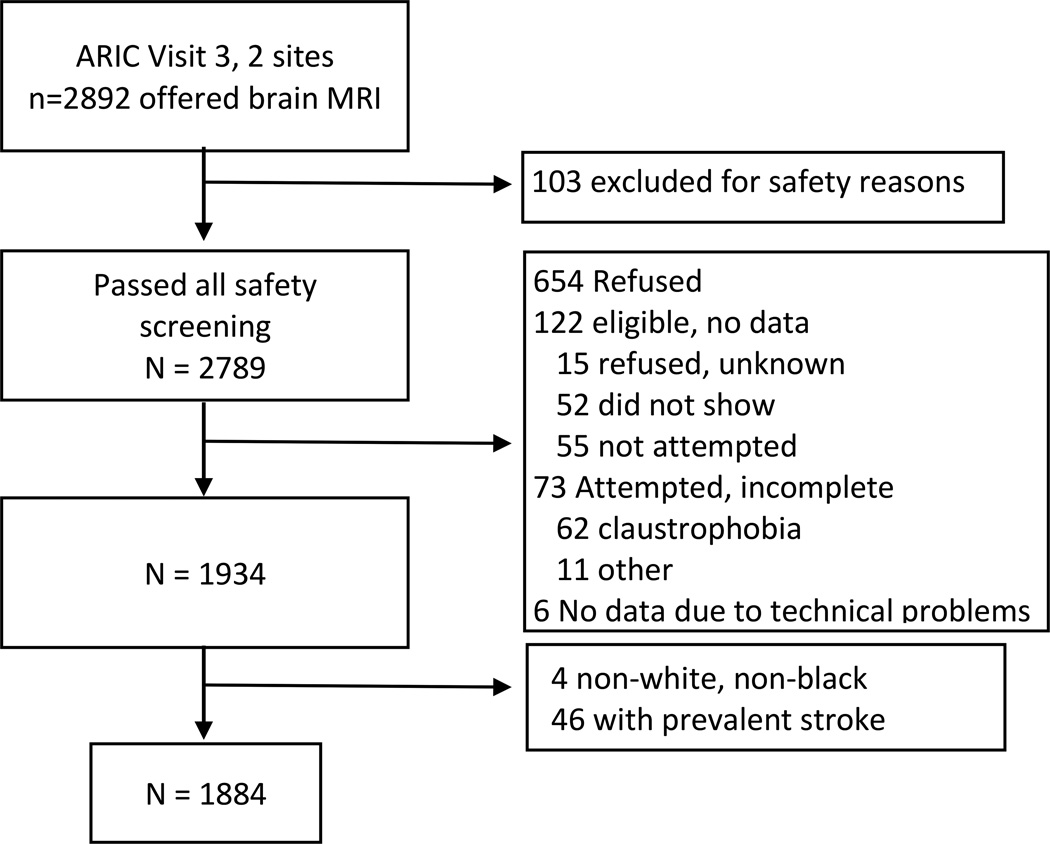
The ARIC study cohort has been previously described.(27) Participants ≥55 years from Forsyth County, NC and Jackson, MS were invited to undergo brain magnetic resonance imaging (MRI) at ARIC visit 3 (1993–95, n=2,892). Of these, 103 were ineligible for safety reasons; 654 refused; 122 did not initially refuse but did not undergo MRI exam; 73 attempted but did not complete and 6 completed MRI forms but had no data. (Online Appendix Figure 1) Participants who underwent MRI were older 62 vs 59 years) but otherwise similar to those without MRI. (Online Appendix Table 1) We obtained MRI data on 1,934 participants, excluded 46 with prevalent strokes and four who reported non-white, non-black ethnicity, leaving 1,884 for this analysis. Institutional Review Boards approved study protocols; all participants provided informed consent.
Brain Imaging
Protocols for brain MRI have been described in detail and were identical to those used in the Cardiovascular Health Study (CHS).(28, 29) Briefly, 5 mm contiguous axial whole brain T1, T2, and proton density weighted images were obtained using 1.5-T scanners. SBI were defined by shape, absence of mass effect, and hyperintensity to gray matter on proton density and T2-weighted images, in contrast to perivascular spaces which demonstrate intensity similar to cerebrospinal fluid and show typical locations and morphology.(30) SBI within cerebral white matter were also required to be hypointense on T1-weighted images. The maximal right-to-left and anterior-to-posterior lesions dimensions were recorded with an electronic cursor. The superior-to-inferior dimension was reported by the number of 5 mm axial sections on which the lesion appeared. Lesions < 3 mm on right-to-left or anterior-to-posterior measurements were recorded as “less than 3 mm.”(28, 31) Images were double read independently by two neuroradiologists and discrepant cases were adjudicated by consensus among three or more readers.
We included cortical and non-cortical lesions. Lacunes were defined as non-cortical lesions 3–20 mm in the basal ganglia, brain stem, thalamus, internal capsule, or cerebral white matter. Non-lacunar lesions were 3–20 mm lesions outside these areas or > 20 mm in any area on right-to-left or anterior-to-posterior measurements. The number of lesions < 3 mm were recorded as 0, 1–2, or > 2, and lesions ≥3 mm were recorded as 0, 1, or ≥2 (up to 5).(28, 31)
Periventricular and subcortical WMH were graded on a scale from 0 (no white matter signal abnormalities) to 9 (extensive confluent white matter involvement), based on pattern matching to a set of reference standards.(29, 32)
Incident Stroke and Mortality Ascertainment and Criteria
Adjudicated nonfatal and fatal hospitalized clinical strokes were identified through yearly phone interviews and surveillance methods that additionally included hospital record reviews and medical chart abstraction.(33) Deaths were identified through contacts with next of kin, hospital records, state death records, and the National Death Index. Stroke mortality was adjudicated by expert stroke reviewers. Follow-up was complete through 2010.
Stroke was defined based on the National Survey of Stroke criteria and required evidence of sudden or rapid onset of neurological symptoms that persisted for > 24 hours or led to death with no other apparent cause such as trauma, tumor, infection, or anticoagulation therapy.(7, 33, 34) Of hospitalized strokes, 99% underwent a diagnostic CT or MRI.(7) Symptoms plus acute infarctions or absence of hemorrhage on imaging defined ischemic strokes. Hemorrhagic strokes met 1 of the following criteria: (1) CT or MRI with intraparenchymal hematoma; (2) demonstration at autopsy or surgery; or (3) at least 1 major or 2 minor neurological deficits; a bloody spinal fluid on lumbar puncture; and no CT or MRI, with or without cerebral angiography demonstrating an avascular mass effect and no evidence of aneurysm or arteriovenous malformation.(7, 21, 33)
Covariates
All covariates were measured at ARIC Visit 3 when the MRI was conducted. Body mass index (BMI) was calculated as kilograms/meters2. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or use of antihypertensive medication during the past 2 weeks. Diabetes mellitus was defined as a fasting blood glucose ≥ 126 mg/dL, nonfasting blood glucose > 200 mg/dL, antidiabetic medication use during the past two weeks, or a physician diagnosis of diabetes. Standardized questionnaires were used to ascertain medical history, smoking, and alcohol consumption. Smoking and alcohol status were categorized as never, former, or current. Educational attainment was categorized as < 12 years, 12–16 years, > 16 years. Additional covariates from the Framingham Stroke Risk Factors were not included due to reductions in the sample size and low prevalence (e.g. atrial fibrillation < 1% [n=4] and LVH 4% [n=78]).
Data Analysis
Associations of participant characteristics with lesion types (no lesions, lesions < 3mm only, lesions ≥3 mm only, and both < 3 mm and ≥3 mm lesions) were examined using Fisher’s exact tests, Kruskal-Wallis tests and multinomial regression to estimate relative risk ratios (RRR).
Cumulative incidence curves show incidence of stroke and stroke mortality outcomes over time by lesion type and WMH (standardized curves are provided in the online Appendix Figure 2). Cox proportional hazard models were used to estimate hazard ratios (HR) for associations of MRI predictors with adjudicated event outcomes (incident stroke, stroke subtype, all-cause mortality, and stroke-mortality). We examined the associations of having none vs. very small only (< 3mm) vs. larger only (≥3mm) vs. both (< 3mm and ≥3mm) lesion types in a single model. We then examined presence of very small lesions and numbers of very small lesions (dose-response effects) in separate models (e.g. associations of having very small lesions regardless of larger lesion presence), in order to preserve cell sizes when examining numbers of lesions. Presence of larger lesions, lacunar\non-lacunar infarcts, and number of lacunar infarcts were similarly examined in separate models, again to preserve cell sizes. WMH was examined using ordinal WMH (grade 0–9) and dichotomous WMH (grade < 3 vs. ≥3).(14, 29)
Models were adjusted for a priori chosen covariates including age, sex, a 3-level race–center variable (black–Forsyth, black–Jackson, white–Forsyth), education, BMI, smoking, alcohol use, diabetes, systolic and diastolic blood pressure, hypertension medications, heart disease, statins, high-density lipoprotein and low-density lipoprotein (LDL) cholesterol, and triglycerides. Alternative adjustment models (including only the first three, four and seven adjustors) provided quantitatively similar results with the same conclusions, as did additional adjustments for aspirin use; unadjusted models provided the same conclusions with larger effect sizes. Proportional hazards assumptions did not appear violated via diagnostic log-log-survival plots and Schoenfeld residual tests (p=0.692 for stroke). Effect modifications by sex, race, and age were unsupported. Stata version 13 (StataCorp LP, College Station, TX) was used.
Role of the Funding Source
The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
RESULTS
Over an average follow-up of 14.5 years, there were 157 clinical strokes, 50 stroke-related deaths, and 576 all-cause deaths. Among those without stroke events, 90% had over 10 years of follow-up.
Table 1 shows the visit 3 population characteristics (50% black, 60% women, and median age 63 [range 50–73]), stratified by presence and size of lesion. A majority of participants (64%) were < 65 years at the MRI exam. Lesion type (< 3 mm only, ≥3 mm only, or both) was associated with typical cerebrovascular disease risk factors, including older age, black race, lower education, smoking, diabetes, hypertension, hypertension medication, systolic and diastolic blood pressure, and alcohol.
Table 1.
Characteristics at Visit 3, Concurrent with Imaging, Stratified by Lesion Size*
| Total (N=1884) |
No Lesions (N=1611) |
Lesions <3mm Only (N=50) |
Lesions ≥3mm Only (N=185) |
Both (N=35) |
P value† | |
|---|---|---|---|---|---|---|
| Age, years | 62.37 (4.50) | 62.09 (4.48) | 63.54 (4.19) | 64.11 (4.35) | 64.03 (4.59) | <0.001 |
| Men | 747 (40%) | 639 (40%) | 20 (40%) | 70 (38%) | 16 (46%) | 0.840 |
| Black | 934 (50%) | 761 (47%) | 33 (66%) | 111 (60%) | 29 (83%) | <0.001 |
| Race – Site | ||||||
| Forsyth – White | 945 (50%) | 845 (53%) | 17 (34%) | 74 (40%) | 6 (17%) | <0.001 |
| Forsyth – Black | 113 (6%) | 96 (6%) | 4 (8%) | 10 (5%) | 3 (9%) | |
| Jackson – Black | 821 (44%) | 665 (41%) | 29 (58%) | 101 (55%) | 26 (74%) | |
| Education | ||||||
| <12 years | 508 (27%) | 408 (25%) | 19 (38%) | 61 (33%) | 20 (61%) | <0.001 |
| 12–16 years | 640 (34%) | 558 (35%) | 15 (30%) | 58 (31%) | 8 (24%) | |
| >16 years | 733 (39%) | 644 (40%) | 16 (32%) | 66 (36%) | 5 (15%) | |
| Body Mass Index kg/m2 | 27.98 (5.2) | 27.96 (5.20) | 28.05 (4.19) | 28.13 (5.63) | 28.18 (5.01) | 0.678 |
| Current smoker | 342 (18%) | 277 (17%) | 7 (14%) | 44 (24%) | 13 (38%) | 0.003 |
| Diabetes | 326 (17%) | 264 (17%) | 12 (24%) | 39 (22%) | 11 (32%) | 0.021 |
| Hypertension | 900 (48%) | 722 (45%) | 34 (68%) | 114 (63%) | 29 (85%) | <0.001 |
| Systolic BP (mmHg) | 128.16 (20.7) | 126.70 (19.63) | 138.34 (17.43) | 136.05 (26.33) | 139.06 (20.81) | <0.001 |
| Diastolic BP (mmHg) | 72.14 (11.1) | 71.73 (10.77) | 73.96 (11.86) | 74.48 (12.98) | 75.89 (12.75) | 0.005 |
| Hypertension Medication | 802 (43%) | 636 (40%) | 27 (54%) | 110 (59%) | 28 (80%) | <0.001 |
| Heart Disease | 103 (6%) | 82 (5%) | 3 (6%) | 14 (8%) | 3 (9%) | 0.327 |
| Total cholesterol, mg/dL‡ | 209.14 (38.2) | 209.62 (38.04) | 207.36 (36.86) | 208.66 (40.34) | 193.00 (36.10) | 0.183 |
| HDL cholesterol mg/dL‡ | 54.92 (19.8) | 54.94 (19.80) | 57.72 (17.19) | 54.14 (19.86) | 55.09 (21.04) | 0.345 |
| LDL cholesterol, mg/dL‡ | 127.22 (35.1) | 127.50 (34.76) | 126.50 (36.05) | 126.99 (37.44) | 116.92 (34.76) | 0.488 |
| Triglycerides, mg/dL‡ | 135.33 (90.2) | 136.35 (91.32) | 115.70 (57.68) | 137.41 (93.05) | 104.97 (33.38) | 0.129 |
| Statin Use | 76 (4%) | 66 (4%) | 0 (0%) | 9 (5%) | 0 (0%) | 0.360 |
| Alcohol Use | ||||||
| Current drinker | 705 (38%) | 628 (39%) | 11 (22%) | 56 (31%) | 10 (29%) | 0.002 |
| Former drinker | 443 (24%) | 354 (22%) | 16 (32%) | 58 (32%) | 14 (41%) | |
| Never drinker | 725 (39%) | 621 (39%) | 23 (46%) | 69 (38%) | 10 (29%) | |
Values are unadjusted mean (SD) or N (%) from visit 3, considered the baseline visit for the analysis;
Abbreviations: BP = blood pressure; HDL = high-density lipoprotein; LDL = low-density lipoprotein
P value for test of difference among lesion type; Kruskal-Wallis test for continuous variables, Fisher's exact test for categorical variables
To convert values to mmol/L, multiply values for total, HDL, and LDL cholesterol by 0.0259; multiply values for triglycerides by 0.0113
Note: Four (4) participants had atrial fibrillation, 11 were on anticoagulants, 148 were taking lipid-lowering medications, 988 (52%) were taking aspirin-containing medications.
Risk of Stroke, All-cause Mortality, and Stroke-Mortality by Lesion Size
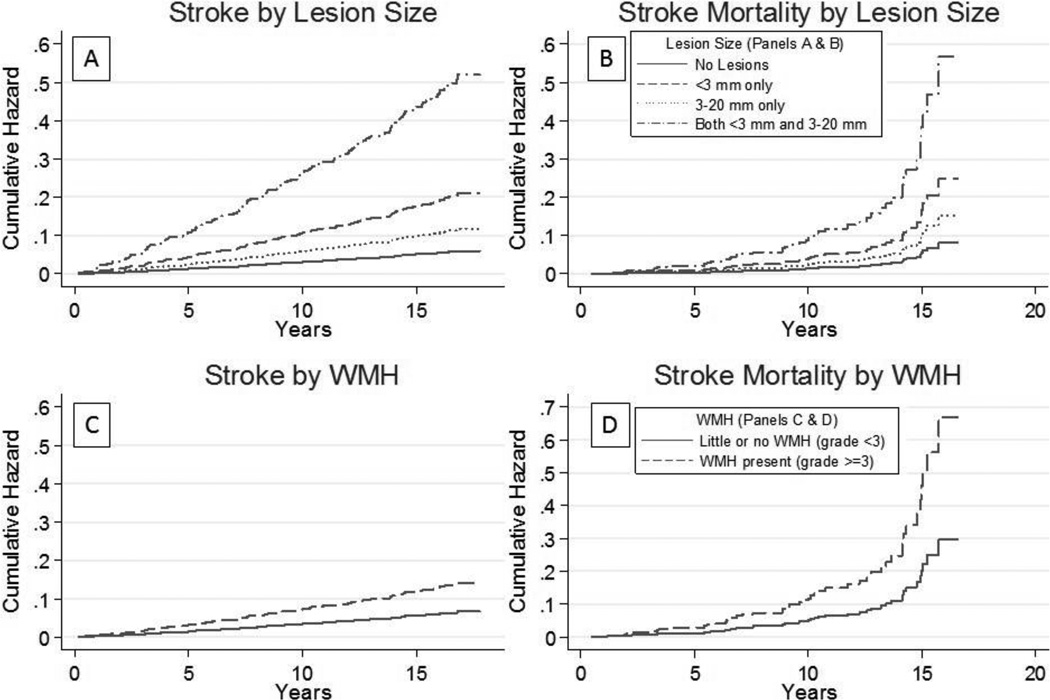
Most strokes (89%) were ischemic, with similar estimates reported for any stroke and ischemic stroke. Cumulative incidence curves associated with lesion size are shown in Figure 1 A-B and adjusted hazard ratios (HR) in Table 2. Lesions < 3 mm only were associated with a three-fold increased risk of incident stroke (HR=3.47, 95% CI: 1.86, 6.49) compared to no lesions as were lesions ≥3 mm only (HR=1.94, 95% CI: 1.22, 3.07). Having both lesion sizes, < 3 mm and ≥3 mm, was associated with a nearly nine-fold increased risk of incident stroke (HR=8.59, 95% CI: 4.69, 15.73). Lesions < 3 mm only (HR=17.25, 95% CI: 4.05, 73.46) and having both lesions < 3 mm and ≥3 mm (HR=26.67, 95% CI: 5.21, 136.46) were associated with increased risk of hemorrhagic stroke (Table 2), although estimates were less precise due to infrequent events.
Figure 1.
Cumulative incidence of stroke and stroke mortality by lesion size and white matter hyperintensity (WMH) grade
Table 2.
Association of Lesions and White Matter Hyperintensities (WMH) with Incident Stroke, Stroke Subtypes, Mortality, and Stroke Among ARIC Participants*
| Stroke (ANY) N=157 (8%) |
Ischemic Stroke N=140 (7%) |
Hemorrhagic Stroke N=15 (1%) |
All-cause mortality N=576 (31%) |
Stroke Mortality N=50 (10%) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lesion Type | ||||||||||
| None (N=1611, 86%) |
-ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- |
| Very small (<3mm Only) (N=50, 3%) |
3.47 (1.86, 6.49) | <0.001 | 3.10 (1.57, 6.12) | 0.001 | 17.25 (4.05, 73.46) | <0.001 | 1.34 (0.84, 2.13) | 0.217 | 3.05 (1.04, 8.94) | 0.043 |
| Larger (≥3mm Only) (N=185, 10%) |
1.94 (1.22, 3.07) | 0.005 | 1.83 (1.12, 2.99) | 0.015 | 2.60 (0.47, 14.49) | 0.276 | 1.90 (1.48, 2.44) | <0.001 | 1.87 (0.83, 4.23) | 0.132 |
| Both (Very Small & Larger) (N=35, 2%) |
8.59 (4.69, 15.73) | <0.001 | 6.88 (3.58, 13.22) | <0.001 | 26.67 (5.21, 136.46) | <0.001 | 1.89 (1.14, 3.13) | 0.013 | 6.97 (2.03, 23.93) | 0.002 |
| Any Lesions <3mm (N=85, 5%) vs no lesions |
4.94 (3.08, 7.92) | <0.001 | 4.17 (2.51, 6.94) | <0.001 | 21.64 (6.19, 75.73) | <0.001 | 1.49 (1.04, 2.13) | 0.031 | 5.14 (2.08, 12.73) | <0.001 |
| Count of Lesions <3mm | ||||||||||
| 0 (N=1611, 95%) | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- |
| 1 to 2 (N=73, 4%) | 4.87 (2.99, 7.93) | <0.001 | 4.03 (2.37, 6.85) | <0.001 | 20.27 (5.55, 73.97) | <0.001 | 1.39 (0.94, 2.06) | 0.095 | 5.87 (2.33, 14.78) | <0.001 |
| >2 (N=12, 1%) | 5.72 (1.64, 19.94) | 0.006 | 5.73 (1.62, 20.24) | 0.007 | § | § | 2.13 (0.98, 4.66) | 0.058 | § | § |
| Any Lesions ≥3mm (N=220, 12%) vs no lesions |
2.54 (1.70, 3.79) | <0.001 | 2.35 (1.54, 3.60) | <0.001 | 6.42 (1.68, 24.44) | 0.006 | 1.88 (1.48, 2.37) | <0.001 | 2.63 (1.25, 5.52) | 0.011 |
| Any Non-lacunes ≥3mm† (N=32, 2%) |
4.92 (2.31, 10.49) | <0.001 | 5.41 (2.52, 11.61) | <0.001 | 16.40 (1.52, 177.32) | 0.021 | 3.90 (2.35, 6.46) | <0.001 | § | § |
| Any Lacunes 3–20mm† (N=188, 10%) |
2.30 (1.49, 3.55) | <0.001 | 2.04 (1.28, 3.25) | 0.003 | 7.14 (1.63, 31.34) | 0.009 | 1.69 (1.31, 2.17) | <0.001 | 3.33 (1.57, 7.05) | 0.002 |
| # of Lacunes 3–20mm‡ | ||||||||||
| 0 (N=1611, 90%) | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- |
| 1 (N=132, 7%) | 1.81 (1.06, 3.08) | 0.029 | 1.83 (1.05, 3.16) | 0.032 | 2.46 (0.26, 23.71) | 0.436 | 1.47 (1.09, 1.98) | 0.012 | 1.94 (0.78, 4.86) | 0.155 |
| ≥2 (N=56, 3%) | 3.64 (1.98, 6.69) | <0.001 | 2.58 (1.26, 5.28) | 0.009 | 23.24 (3.96, 136.48) | <0.001 | 2.36 (1.59, 3.50) | <0.001 | 11.71 (4.10, 33.47) | <0.001 |
| WMH (Grades 0–9) | 1.30 (1.15, 1.46) | <0.001 | 1.30 (1.14, 1.47) | <0.001 | 1.42 (0.99, 2.03) | 0.054 | 1.20 (1.12, 1.29) | <0.001 | 1.35 (1.10, 1.66) | 0.004 |
| WMH Grade (high vs low) | ||||||||||
| Low <3 (N=1658, 88%) | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- |
| High ≥3 (N=223, 12%) | 2.14 (1.45, 3.16) | <0.001 | 2.12 (1.41, 3.20) | <0.001 | 3.13 (0.93, 10.54) | 0.066 | 1.78 (1.42, 2.23) | <0.001 | 2.47 (1.25, 4.87) | 0.009 |
Values in table are HRs (95% CI) from Cox PHMs adjusted for age, sex, race-site, education, BMI, smoking, alcohol, diabetes, systolic and diastolic blood pressures, hypertension medication use, heart disease, HDL, LDL, triglycerides, statin use; all estimates compared to reference group of “no lesions” (n=1611) except WMH.
Lacunes = 3–20 mm lesions in the basal ganglia, brain stem, thalamus, internal capsule, or cerebral white matter (including those who may also have small infarcts); non-lacunar lesions were any lesions 3–20 mm not located in these areas or >20 mm in any area on right-to-left or anterior-to-posterior measurements
Includes 31 participants who also had a very small lesion (<3mm)
Too few data to support estimates
Lesions < 3 mm were associated with a three-fold increased risk of stroke-mortality (HR=3.05, 95% CI: 1.04-8.94), but not all-cause mortality (HR=1.34, 95% CI: 0.84, 2.13). Conversely, lesions ≥3 mm were associated with a nearly two-fold increased risk of all-cause mortality (HR=1.90, 95% CI: 1.48, 2.44) but were not statistically associated with risk of stroke-mortality (HR=1.87, 95% CI: 0.83, 4.23). The presence of both lesion sizes was associated with increased risk of stroke mortality (HR=6.97, 95% CI: 2.03, 23.93) and all-cause mortality (HR=1.89, 95% CI: 1.14, 3.13). (Table 2
Risk of Stroke, All-cause Mortality, and Stroke-Mortality by Presence and Number of Lesions < 3 mm
Having any lesion < 3 mm, including concomitant lesions ≥3mm, was associated with increased risk of any stroke (HR=4.94, 95% CI: 3.08, 7.92), stroke mortality (HR=5.14, 95% CI: 2.08, 12.73) and all-cause mortality (HR=1.49, 95% CI: 1.04, 2.13). (Table 2) The presence of 1–2 lesions < 3 mm versus no lesions conveyed an approximate 5-fold increased risk of stroke (HR=4.87, 95% CI: 2.99, 7.93) and stroke mortality (HR=5.87, 95% CI: 2.33, 14.78). Having more than 2 lesions < 3 mm in size was similarly associated with a more than 5-fold increased risk of stroke (HR= 5.72, 95% CI: 1.64, 19.94). (Table 2) Stroke-mortality events were too rare to estimate relationships.
Risk of Stroke, All-cause and Stroke Mortality by Presence and Number of Lesions ≥3 mm
The presence of any lesion ≥3 mm was associated with an increased risk of incident stroke (HR=2.54, 95% CI: 1.70-3.79), with similar results when limited to non-lacunes (HR=4.92, 95% CI: 2.31, 10.49) or lacunes (HR=2.30, 95% CI: 1.49, 3.55). (Table 2) Having only one versus no lacunes was associated with an almost two-fold increased risk of stroke (HR=1.81, 95% CI: 1.06, 3.08) while having two or more lacunes was associated with a nearly four-fold risk of stroke (HR=3.64, 95% CI: 1.98, 6.69).
Similar associations were found between any lesions ≥3 mm and ischemic and hemorrhagic stroke (HR= 2.35, 95% CI: 1.54, 3.60; HR=6.42, 95% CI: 1.68, 24.44). (Table 2) Lacunes and non-lacunes were associated with risk of ischemic and hemorrhagic stroke. The data were also suggestive of a dose response when considering ischemic stroke risk and number of lacunes versus no lesions (1 lacune: HR=1.83, 95% CI: 1.05, 3.16; ≥2 lacunes: HR=2.58, 95% CI: 1.26, 5.28).
The presence of any lesion ≥3 mm was associated with stroke mortality (HR=2.63, 95% CI: 1.25, 5.52) and all-cause mortality (HR=1.88, 95% CI: 1.48, 2.37). Similarly, lacunes were associated with a three-fold increased risk of stroke mortality (HR=3.33, 95% CI: 1.57, 7.05), and nearly two-fold increased risk of all-cause mortality (HR=1.69, 95% CI: 1.31, 2.17); greater numbers of lacunes were associated with all-cause mortality (1 lacune: HR=1.47, 95% CI: 1.09, 1.98; ≥2 lacunes: HR=2.36, 95% CI: 1.59, 3.50), while ≥2 lacunes was associated with stroke mortality (1 lacune: HR=1.94, 95% CI: 0.78, 4.86; ≥2 lacunes: HR=11.71, 95% CI: 4.10, 33.47). Non-lacunes were also associated with all-cause mortality (HR=3.90, 95% CI: 2.35, 6.46). (Table 2)
Risk of Stroke, All-cause Mortality, and Stroke-Mortality by WMH
WMH were associated with incident stroke as an ordinal (0–9 scale, HR=1.30 per unit, 95% CI: 1.15, 1.46) and categorical (0–3 vs ≥3, HR=2.14, 95% CI: 1.45, 3.16) variable. (Table 2) The ordinal and categorical WMH were also associated with all-cause mortality (HR=1.20 per unit, 95% CI: 1.12, 1.29; HR=1.78, 95% CI: 1.42, 2.23) and stroke mortality (HR=1.35 per unit, 95% CI: 1.10, 1.66; HR=2.47, 95% CI: 1.25, 4.87).
Risk Factors of Lesion Occurrence
The primary characteristic associated with having only very small lesions (< 3mm) was hypertension (RRR=2.17, 95%CI: 1.14, 4.13) (Table 3). Having only larger lesions (≥3mm), was associated with hypertension (RRR=1.71, 95%CI: 1.20, 2.43) as well as older age, black race and current smoking status. Consistent with established risk factors of cerebrovascular disease (10, 13, 22), older age, black race, smoking, and hypertension (RRR=6.01, 95%CI: 2.01, 17.99), were associated with having both lesion sizes, while higher education was protective. Interaction terms between lesions or WMH and sex, race, or age in separate models did not support differential relationships between MRI abnormalities and stroke/mortality outcomes though sample sizes were limited for effect modification investigations.
Table 3.
Relative Risk Ratios (RRR) of Lesion Type Associated with Characteristics (from multinomial logistic regression with reference category of “No Lesions”)
| Predictor | Lesions <3 mm Only (N=50) |
Lesions ≥3 mm Only (N=185) |
Both (N=35) |
|||
|---|---|---|---|---|---|---|
| Age, years | 1.07 (1.00, 1.14) | 0.051 | 1.12 (1.08, 1.17) | <0.001 | 1.16 (1.06, 1.27) | 0.002 |
| Male vs. Female | 1.07 (0.58, 2.00) | 0.820 | 0.83 (0.58, 1.18) | 0.298 | 1.03 (0.47, 2.27) | 0.941 |
| Black vs. White | 1.79 (0.91, 3.51) | 0.092 | 1.62 (1.12, 2.36) | 0.011 | 2.68 (1.01, 7.08) | 0.047 |
| Hypertension | 2.17 (1.14, 4.13) | 0.018 | 1.71 (1.20, 2.43) | 0.003 | 6.01 (2.01, 17.99) | 0.001 |
| Body Mass Index kg/m2 | 0.96 (0.90, 1.02) | 0.198 | 0.98 (0.95, 1.02) | 0.334 | 0.97 (0.90, 1.05) | 0.463 |
| Diabetes | 1.29 (0.64, 2.58) | 0.478 | 1.28 (0.85, 1.93) | 0.244 | 1.83 (0.81, 4.17) | 0.149 |
| LDL mg/dL | 1.00 (0.99, 1.01) | 0.675 | 1.00 (0.99, 1.00) | 0.761 | 0.99 (0.98, 1.01) | 0.325 |
| Education | ||||||
| <12 years | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- |
| 12–16 years | 0.79 (0.38, 1.62) | 0.517 | 0.99 (0.65, 1.52) | 0.980 | 0.45 (0.18, 1.13) | 0.088 |
| >16 years | 0.78 (0.38, 1.60) | 0.500 | 1.06 (0.70, 1.62) | 0.768 | 0.32 (0.11, 0.92) | 0.034 |
| Alcohol Use | ||||||
| Current drinker | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- |
| Former drinker | 1.93 (0.85, 4.38) | 0.116 | 1.49 (0.97, 2.29) | 0.068 | 1.52 (0.59, 3.93) | 0.385 |
| Never drinker | 1.63 (0.74, 3.59) | 0.223 | 0.95 (0.62, 1.44) | 0.799 | 0.72 (0.26, 2.03) | 0.537 |
| Current smoker | 0.84 (0.36, 1.94) | 0.680 | 1.62 (1.08, 2.43) | 0.019 | 2.97 (1.29, 6.84) | 0.010 |
DISCUSSION
Our study demonstrates that, among asymptomatic middle-aged to older persons with no history of clinical stroke, even very small lesions in the brain were associated with risks of future stroke and mortality, similar to lesions of typical clinically-defined size thresholds (3–20mm), as were WMH in both white and black participants. Risk was greater if both sized lesions, i.e. < 3mm and those 3–20 mm, were present, even after adjusting for many potential confounders.
Although clinicians and researchers tend to be dismissive of very small cerebral lesions, the current findings provide evidence that this practice could warrant reconsideration. It is unclear if lesions of this size represent vascular pathology or perhaps dilated perivascular spaces called Virchow-Robin spaces.(30) However, even perivascular spaces are increasingly recognized as potentially pathologic. Lesions of presumed vascular origin and perivascular spaces share similar risk profiles, and perivascular spaces are associated with cerebral small vessel disease severity,(15–17) stroke and cognitive decline,(35) WMH(36, 37) and symptomatic lacunar infarcts,(16, 36) in support of a vascular pathology. In this study, expert neuroradiologists used a standard protocol and were aware of distinctions between infarcts and perivascular spaces (e.g. location, shape, intensity). Although we have high confidence in our classification of perivascular spaces versus silent infarcts, we acknowledge the distinction becomes more difficult for infarcts < 3mm since signal intensity becomes more subject to partial voluming (the lesion is small relative to the slice or pixel volume) and the morphology becomes more difficult to characterize. However, perivascular spaces are now largely considered a form of vascular disease, potentially due to endothelial dysfunction.(18) Regardless of etiology of the lesions, our study demonstrates increased risk for stroke and mortality associated with even the very small lesions, adding to a growing corpus of literature supporting associations between very small lesions and cardiovascular risk factors (31), clinical disease including atrial fibrillation,(38) and now, stroke and mortality.
Smaller cerebrovascular lacunar lesions (typically defined as up to 7mm) and larger (typically 5–20mm) lacunar lesions are thought to be pathogenically and clinically different, with the smaller ones developing from lipohyalinosis and potentially endothelial dysfunction (18) while the larger ones are more likely to be symptomatic and due to microatheromatous disease.(1) Our robust associations of lesions < 3mm with stroke and stroke mortality support the hypothesis that very small lesions are pathologic and of clinical importance; we extend previous work in the ARIC cohort which identified diabetes and hemoglobin A1C levels as risk factors for lesions < 7 mm, while lesions > 7 were associated with LDL cholesterol,(31) consistent with Fisher’s hypothesis(1) and previous studies on risk factors for silent lesions in the brain.(2, 21, 22, 32, 39) Our study extends these findings by examining lesions < 3mm separately from those ≤7mm. The most robust association of a risk factor with lesions < 3mm was hypertension followed by age and black race, all of which are well-known risk factors for stroke and cardiovascular disease.(2, 22, 29) The associations with cardiovascular risk factors further support the hypothesis that these lesions represent early microvascular disease.
Associations with stroke appear stronger for lesions < 3mm than for larger lesions. There are several potential explanations for this findings. First, the events were few with overlapping confidence intervals for lesions < 3mm and those > 3mm, so the true association could also be smaller or similar to lesions > 3mm. Larger lesions could have led to strokes, resulting in deaths, drop-outs, or exclusion from the analysis due to prevalent stroke. However, persons with strokes at the baseline visit were excluded and deaths or loss-to-follow-up due to strokes seems unlikely since the follow-up was extremely high.
Our data supported a dose response mechanism which adds support for causal associations; risk for stroke and mortality was increased with either size lesion but was highest among those with both sized lesions and no other brain abnormalities (HR=14.76, 95% CI: 6.29, 34.60; Appendix Table 2). Furthermore, stroke risk increased with increasing numbers of lesions, regardless of lesion type. These findings are especially timely given evidence that statins may prevent incident subclinical infarcts.(40) Future studies are needed to examine the impact of preventive treatment in asymptomatic persons, including those with even the smallest abnormalities.
Strengths of this study include a middle-aged cohort at baseline with a large representation of African Americans, standardized measures of MRI lesions, and a validated ascertainment of incident stroke and stroke subtypes. Limitations included the infrequent events, particularly hemorrhagic stroke (n=15) and stroke-mortality (n=50) events. Despite these small samples, strong associations were observed. Studies with larger numbers of stroke events are needed to provide more stable and precise estimates of the relative risks associated with very small lesions. Use of 1.5T MRI and potential misclassification of lesions < 3mm due to lower pixel resolution may be considered limitations as higher resolution imaging is now available; this was not available at visit 3. We expect most misclassification of lesions < 3mm to result in non-infarcts being labeled as infarcts and bias results to null findings. Thus, we believe our findings are conservative. Newer high definition imaging might improve classification of lesions < 3 mm and should be examined in future studies to confirm our results. Although recent recommendations consider lesions of presumed vascular origin to be 3–15mm in size,(30) we used an established definition of 3–20mm at the time of this study. However, the focus of this paper was on risk associated with lesions < 3mm and the definition used is consistent with previous studies.(2, 12, 29, 41)
In conclusion, we found that middle-aged and older black and white adults with lesions < 3 mm only and lesions ≥3 mm only, as well as WMH, may be at heightened risk for incident stroke and mortality. The simultaneous presentation of both < 3mm and ≥3mm lesions may represent a particularly marked increase in risk. Larger studies are needed to confirm these findings, provide more precise estimates, and investigate physiologic pathways.
Acknowledgement
The authors thank the staff and participants of the ARIC study for their important contributions and Mr. Seth Lirette for analytical assistance during the revision of the manuscript.
Funding/Support: The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, HHSN268201100012C) with the ARIC carotid MRI examination funded by U01HL075572-01.
Appendix
Appendix Figure 1.
Study Flow Diagram
Appendix Figure 2. Standardized Cumulative Hazards of stroke and stroke mortality by lesion size and white matter hyperintensity (WMH) grade.
(Figures shown with conditional standardization based on adjustor variables in primary models reported in Table 2 set at mean and mode values for continuous/categorical variables respectively. Results are similar to those shown in the unadjusted KM plots in Figure 1).
Appendix Table 1. Comparison of Visit 3 Characteristics between those with MRI (N=4,035), those without MRI (N=1,935) at the Forsyth and Jackson ARIC sites, as well as those included in the current study (N=1,884/1,934).
Characteristics were similar in general with the exception of age; participants with completed MRIs were slightly older.
| Visit 3 Characteristics |
All (N=5,969) |
V3: No MRI (N=4,035) |
V3: MRI (N=1,934) |
Included (N=1,884) |
|---|---|---|---|---|
| Age (yrs) | 59.71 (5.83) | 58.43 (5.96) | 62.39 (4.50) | 62.37 (4.50) |
| Male (%) | 3495 (59%) | 2342 (58%) | 1153 (60%) | 1132 (60%) |
| Black (%) | 2469 (41%) | 1693 (42%) | 776 (40%) | 747 (40%) |
| BMI (m/kg2) | 3009 (50%) | 2040 (51%) | 969 (50%) | 945 (50%) |
| Smoker (%) | 2955 (50%) | 1995 (49%) | 960 (50%) | 934 (50%) |
| Diabetes (%) | 28.61 (5.90) | 28.90 (6.18) | 28.01 (5.19) | 27.98 (5.21) |
| HTN (%) | 4687 (79%) | 3118 (78%) | 1569 (82%) | 1530 (82%) |
| HTN Meds (%) | 1238 (21%) | 885 (22%) | 353 (18%) | 342 (18%) |
| Prev CHD (%) | 4820 (82%) | 3255 (82%) | 1565 (82%) | 1539 (83%) |
| Tot Chol (SI) | 1085 (18%) | 735 (18%) | 350 (18%) | 326 (17%) |
| HDL (SI) | 3165 (53%) | 2187 (54%) | 978 (51%) | 967 (52%) |
| Trigs (SI) | 2772 (47%) | 1834 (46%) | 938 (49%) | 900 (48%) |
| Statin Med (%) | 3485 (58%) | 2394 (59%) | 1091 (57%) | 1077 (57%) |
| Never Drinker | 2479 (42%) | 1641 (41%) | 838 (43%) | 802 (43%) |
| Former Drinker | 5474 (94%) | 3704 (93%) | 1770 (94%) | 1733 (94%) |
| Current Drinker | 378 (6%) | 262 (7%) | 116 (6%) | 103 (6%) |
Appendix Table 2. Incidence of Stroke Associated with Expanded Lesion Size Categories and Ordered by Prevalence.
Though sample sizes were small in individual categories, Hazard Ratios appeared elevated whenever very small lesions (<3mm) were present.
| Group | <3 | 3–7 | 8–20 | >20 | Lesion Group |
N (Prevalence) | Stroke Hazard Ratios |
|---|---|---|---|---|---|---|---|
| 1 | Absent | Absent | Absent | Absent | No Lesions | 1611 (86%) | -ref- |
| 2 | Absent | Present | Absent | Absent | ≥3 only | 84 (4.5%) | 1.02 (0.46, 2.23) p=0.963 |
| 3 | Present | Absent | Absent | Absent | <3 only | 50 (2.7%) | 3.50 (1.87, 6.55) p<0.001 |
| 4 | Absent | Absent | Present | Absent | ≥3 only | 47 (2.5%) | 3.00 (1.43, 6.33) p=0.004 |
| 5 | Absent | Present | Present | Absent | ≥3 only | 27 (1.4%) | 4.56 (2.04, 10.19) p<0.001 |
| 6 | Absent | Absent | Absent | Present | ≥3 only | 16 (0.9%) | 0.86 (0.12, 6.24) p=0.878 |
| 7 | Present | Present | Absent | Absent | Both | 14 (0.7%) | 14.76 (6.29, 34.60) p<0.001 |
| 8 | Present | Absent | Present | Absent | Both | 9 (0.5%) | 8.85 (3.46, 22.61) p<0.001 |
| 9 | Absent | Present | Absent | Present | ≥3 only | 7 (0.4%) | 3.22 (0.73, 14.13) p=0.121 |
| 10 | Present | Present | Present | Absent | Both | 6 (0.3%) | 2.93 (0.40, 21.63) p=0.293 |
| 11 | Absent | Absent | Present | Present | ≥3 only | 3 (0.2%) | - |
| 12 | Present | Absent | Absent | Present | Both | 2 (0.1%) | - |
| 13 | Present | Present | Absent | Present | Both | 2 (0.1%) | - |
| 14 | Present | Present | Present | Present | Both | 2 (0.1%) | - |
| 15 | Absent | Present | Present | Present | ≥3 only | 1 (0.1%) | - |
| 16 | Present | Absent | Present | Present | Both | 0 (0.0%) | - |
Unable to estimate stroke hazard ratios for the sample sizes <6.
Footnotes
“This is the prepublication, author-produced version of a manuscript accepted for publication in Annals of Internal Medicine. This version does not include post-acceptance editing and formatting. The American College of Physicians, the publisher of Annals of Internal Medicine, is not responsible for the content or presentation of the author-produced accepted version of the manuscript or any version that a third party derives from it. Readers who wish to access the definitive published version of this manuscript and any ancillary material related to this manuscript (e.g., correspondence, corrections, editorials, linked articles) should go to Annals.org or to the print issue in which the article appears. Those who cite this manuscript should cite the published version, as it is the official version of record.”
Conflict of interest Disclosures: None.
B. Gwen Windham MD MHS, Bradley Deere, MD, Michael E. Griswold, PhD, Wanmei Wang, MS, Thomas Mosley, PhD, Kenneth Butler, PhD, University of Mississippi Medical Center, 2500 N. State Street, Jackson, MS 39216
Dave Knopman, MD, Mayo Clinic, 200 First Street SW, Rochester, MN 55905
Dean Shibata, MD MHS, University of Washington, 1959 NE Pacific St., Seattle, WA 98195
Daniel Bezerra, MD PhD, Visconde Silva 52 / 804, Rio de Janeiro – RJ, ZIP 22271-092, Phone + 55-21-25279063
Rebecca Gottesman, MD PhD, Johns Hopkins Medicine, Phipps 446D, 600 N. Wolfe Street, Baltimore, MD
Gerardo Heiss, MD PhD, University of North Carolina at Chapel Hill, 137 E. Franklin Street, Suite 306, Chapel Hill, NC 27514-4145
References
- 1.Fisher CM. Lacunar strokes and infarcts: a review. Neurology. 1982;32(8):871–876. doi: 10.1212/wnl.32.8.871. [DOI] [PubMed] [Google Scholar]
- 2.Longstreth WT, Bernick C, Manolio TA, Bryan N, Jungreis CA, Price TR. Lacunar infarcts defined by magnetic resonance imaging of 3660 elderly people: the Cardiovascular Health Study. Arch Neurol. 1998;55(9):1217–1225. doi: 10.1001/archneur.55.9.1217. [DOI] [PubMed] [Google Scholar]
- 3.Bernick C, Kuller L, Dulberg C, Longstreth WT, Jr, Manolio T, Beauchamp N, et al. Silent MRI infarcts and the risk of future stroke: the cardiovascular health study. Neurology. 2001;57(7):1222–1229. doi: 10.1212/wnl.57.7.1222. [DOI] [PubMed] [Google Scholar]
- 4.Bokura H, Kobayashi S, Yamaguchi S, Iijima K, Nagai A, Toyoda G, et al. Silent Brain Infarction and Subcortical White Matter Lesions Increase the Risk of Stroke and Mortality: A Prospective Cohort Study. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2006;15(2):57–63. doi: 10.1016/j.jstrokecerebrovasdis.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Buyck JF, Dufouil C, Mazoyer B, Maillard P, Ducimetiere P, Alperovitch A, et al. Cerebral white matter lesions are associated with the risk of stroke but not with other vascular events: the 3-City Dijon Study. Stroke. 2009;40(7):2327–2331. doi: 10.1161/STROKEAHA.109.548222. [DOI] [PubMed] [Google Scholar]
- 6.Debette S, Beiser A, DeCarli C, Au R, Himali JJ, Kelly-Hayes M, et al. Association of MRI Markers of Vascular Brain Injury With Incident Stroke, Mild Cognitive Impairment, Dementia, and Mortality. Stroke. 2010;41(4):600–606. doi: 10.1161/STROKEAHA.109.570044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Folsom AR, Yatsuya H, Mosley TH, Psaty BM, Longstreth WT. Risk of intraparenchymal hemorrhage with magnetic resonance imaging-defined leukoaraiosis and brain infarcts. Annals of Neurology. 2012;71(4):552–559. doi: 10.1002/ana.22690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerdes VEA, Kwa VIH, ten Cate H, Brandjes DPM, Büller HR, Stam J. Cerebral white matter lesions predict both ischemic strokes and myocardial infarctions in patients with established atherosclerotic disease. Atherosclerosis. 2006;186(1):166–172. doi: 10.1016/j.atherosclerosis.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi S, Okada K, Koide H, Bokura H, Yamaguchi S. Subcortical silent brain infarction as a risk factor for clinical stroke. Stroke. 1997;28(10):1932–1939. doi: 10.1161/01.str.28.10.1932. [DOI] [PubMed] [Google Scholar]
- 10.Kuller LH, Longstreth WT, Arnold AM, Bernick C, Bryan RN, Beauchamp NJ, et al. White Matter Hyperintensity on Cranial Magnetic Resonance Imaging. Stroke. 2004;35(8):1821–1825. doi: 10.1161/01.STR.0000132193.35955.69. [DOI] [PubMed] [Google Scholar]
- 11.Longstreth WT, Jr, Diehr P, Beauchamp NJ, Manolio TA. Patterns on cranial magnetic resonance imaging in elderly people and vascular disease outcomes. Arch Neurol. 2001;58(12):2074. doi: 10.1001/archneur.58.12.2074. [DOI] [PubMed] [Google Scholar]
- 12.Manolio TA, Kronmal RA, Burke GL, Poirier V, O'Leary DH, Gardin JM, et al. Magnetic resonance abnormalities and cardiovascular disease in older adults. The Cardiovascular Health Study. Stroke. 1994;25(2):318–327. doi: 10.1161/01.str.25.2.318. [DOI] [PubMed] [Google Scholar]
- 13.Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MMB. Silent Brain Infarcts and White Matter Lesions Increase Stroke Risk in the General Population. Stroke. 2003;34(5):1126–1129. doi: 10.1161/01.STR.0000068408.82115.D2. [DOI] [PubMed] [Google Scholar]
- 14.Wong TY, Klein R, Sharrett AR, Couper DJ, Klein BE, Liao DP, et al. Cerebral white matter lesions, retinopathy, and incident clinical stroke. JAMA. 2002;288(1):67–74. doi: 10.1001/jama.288.1.67. [DOI] [PubMed] [Google Scholar]
- 15.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9(7):689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 16.Potter GM, Doubal FN, Jackson CA, Chappell FM, Sudlow CL, Dennis MS, et al. Enlarged perivascular spaces and cerebral small vessel disease. Int J Stroke. 2013 doi: 10.1111/ijs.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rouhl RP, van Oostenbrugge RJ, Knottnerus IL, Staals JE, Lodder J. Virchow-Robin spaces relate to cerebral small vessel disease severity. J Neurol. 2008;255(5):692–696. doi: 10.1007/s00415-008-0777-y. [DOI] [PubMed] [Google Scholar]
- 18.Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. The Lancet Neurology. 2013;12(5):483–497. doi: 10.1016/S1474-4422(13)70060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart Disease and Stroke Statistics—2012 Update. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohira T, Shahar E, Chambless LE, Rosamond WD, Mosley TH, Jr, Folsom AR. Risk factors for ischemic stroke subtypes: the Atherosclerosis Risk in Communities study. Stroke. 2006;37(10):2493–2498. doi: 10.1161/01.STR.0000239694.19359.88. [DOI] [PubMed] [Google Scholar]
- 22.Price TR, Manolio TA, Kronmal RA, Kittner SJ, Yue NC, Robbins J, et al. Silent brain infarction on magnetic resonance imaging and neurological abnormalities in community-dwelling older adults. The Cardiovascular Health Study. CHS Collaborative Research Group. Stroke. 1997;28(6):1158–1164. doi: 10.1161/01.str.28.6.1158. [DOI] [PubMed] [Google Scholar]
- 23.van der Flier WM, van Straaten EC, Barkhof F, Verdelho A, Madureira S, Pantoni L, et al. Small vessel disease and general cognitive function in nondisabled elderly: the LADIS study. Stroke. 2005;36(10):2116–2120. doi: 10.1161/01.STR.0000179092.59909.42. [DOI] [PubMed] [Google Scholar]
- 24.de Laat KF, Tuladhar AM, van Norden AG, Norris DG, Zwiers MP, de Leeuw FE. Loss of white matter integrity is associated with gait disorders in cerebral small vessel disease. Brain. 2011;134(Pt 1):73–83. doi: 10.1093/brain/awq343. [DOI] [PubMed] [Google Scholar]
- 25.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42(9):2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 28.Bryan RN, Cai J, Burke G, Hutchinson RG, Liao D, Toole JF, et al. Prevalence and anatomic characteristics of infarct-like lesions on MR images of middle-aged adults: the atherosclerosis risk in communities study. AJNR Am J Neuroradiol. 1999;20(7):1273–1280. [PMC free article] [PubMed] [Google Scholar]
- 29.Liao D, Cooper L, Cai J, Toole J, Bryan N, Burke G, et al. The prevalence and severity of white matter lesions, their relationship with age, ethnicity, gender, and cardiovascular disease risk factors: the ARIC Study. Neuroepidemiology. 1997;16(3):149–162. doi: 10.1159/000368814. [DOI] [PubMed] [Google Scholar]
- 30.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12(8):822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bezerra DC, Sharrett AR, Matsushita K, Gottesman RF, Shibata D, Mosley TH, Jr, et al. Risk factors for lacune subtypes in the Atherosclerosis Risk in Communities (ARIC) Study. Neurology. 2012;78(2):102–108. doi: 10.1212/WNL.0b013e31823efc42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liao D, Cooper L, Cai J, Toole JF, Bryan NR, Hutchinson RG, et al. Presence and severity of cerebral white matter lesions and hypertension, its treatment, and its control. The ARIC Study. Atherosclerosis Risk in Communities Study. Stroke. 1996;27(12):2262–2270. doi: 10.1161/01.str.27.12.2262. [DOI] [PubMed] [Google Scholar]
- 33.Rosamond WD, Folsom AR, Chambless LE, Wang CH, McGovern PG, Howard G, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30(4):736–743. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- 34.Robins M, Baum HM. The National Survey of Stroke. Incidence. Stroke. 1981;12(2 Pt 2 Suppl 1):I45–I57. [PubMed] [Google Scholar]
- 35.Deramecourt V, Slade JY, Oakley AE, Perry RH, Ince PG, Maurage CA, et al. Staging and natural history of cerebrovascular pathology in dementia. Neurology. 2012;78(14):1043–1050. doi: 10.1212/WNL.0b013e31824e8e7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doubal FN, MacLullich AM, Ferguson KJ, Dennis MS, Wardlaw JM. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke. 2010;41(3):450–454. doi: 10.1161/STROKEAHA.109.564914. [DOI] [PubMed] [Google Scholar]
- 37.Zhu YC, Tzourio C, Soumare A, Mazoyer B, Dufouil C, Chabriat H. Severity of dilated Virchow-Robin spaces is associated with age, blood pressure, and MRI markers of small vessel disease: a population-based study. Stroke. 2010;41(11):2483–2490. doi: 10.1161/STROKEAHA.110.591586. [DOI] [PubMed] [Google Scholar]
- 38.Kalantarian S, Ay H, Gollub RL, Lee H, Retzepi K, Mansour M, et al. Association Between Atrial Fibrillation and Silent Cerebral InfarctionsA Systematic Review and Meta-analysisAtrial Fibrillation and Silent Cerebral Infarctions. Annals of Internal Medicine. 2014;161(9):650–658. doi: 10.7326/M14-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vermeer SE, Longstreth WT, Jr, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol. 2007;6(7):611–619. doi: 10.1016/S1474-4422(07)70170-9. [DOI] [PubMed] [Google Scholar]
- 40.Fu JH, Mok V, Lam W, Wong A, Chu W, Xiong Y, et al. Effects of statins on progression of subclinical brain infarct. Cerebrovasc Dis. 2010;30(1):51–56. doi: 10.1159/000313614. [DOI] [PubMed] [Google Scholar]
- 41.Knopman DS, Penman AD, Catellier DJ, Coker LH, Shibata DK, Sharrett AR, et al. Vascular risk factors and longitudinal changes on brain MRI: the ARIC study. Neurology. 2011;76(22):1879–1885. doi: 10.1212/WNL.0b013e31821d753f. [DOI] [PMC free article] [PubMed] [Google Scholar]