Abstract
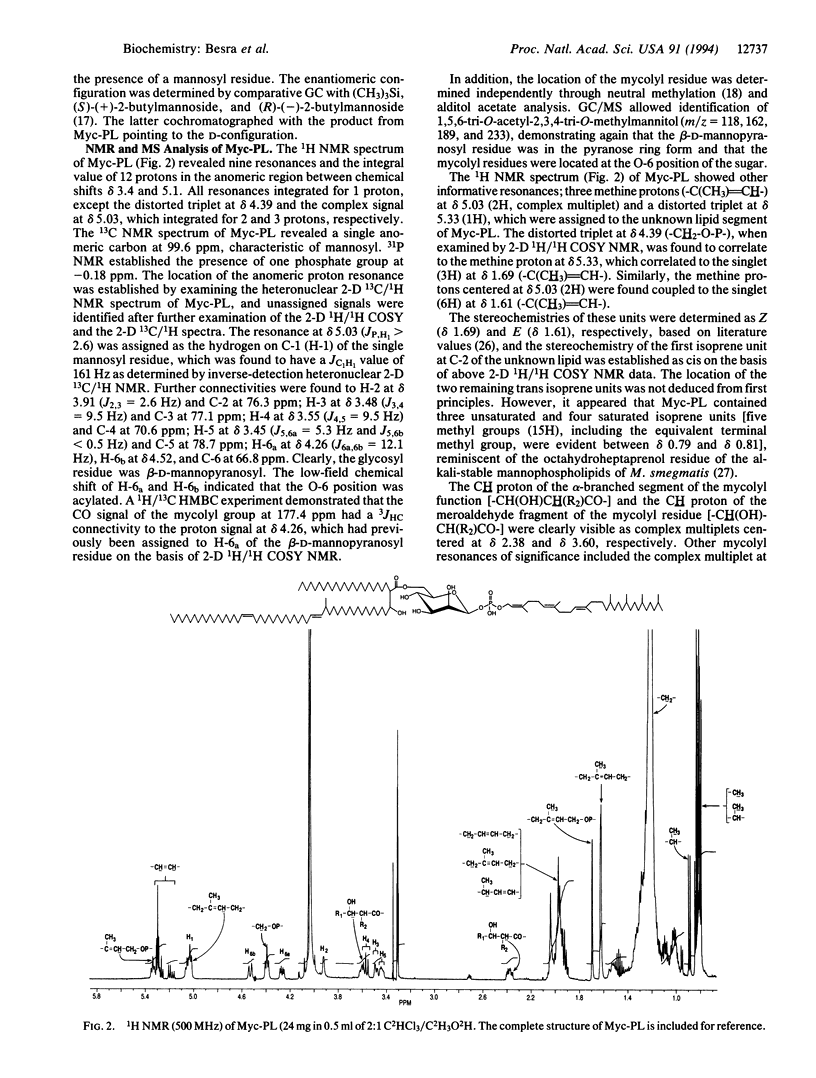
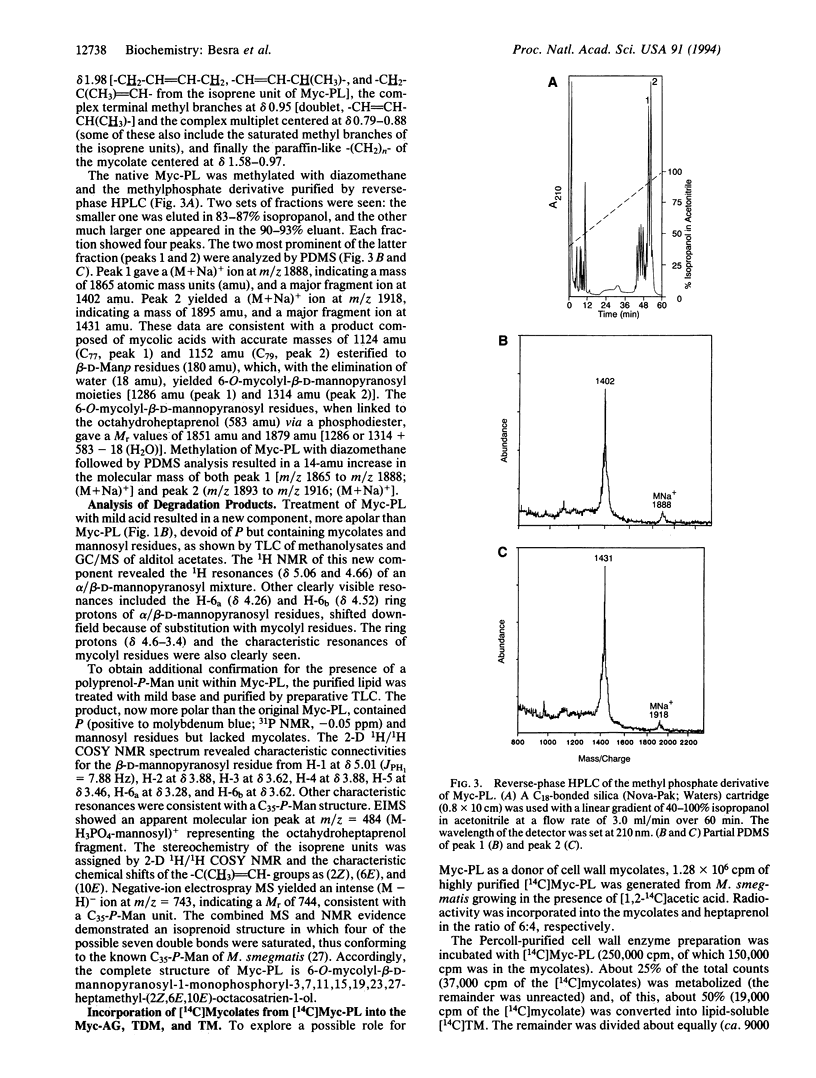
The mycolic acids are large (C70-90) alpha-alkyl, beta-hydroxy fatty acids and are the major determinants of the mycobacterial cell wall's impermeable barrier. The biosynthesis of mycolic acids is barely understood (they are probably the products of specialized elongation and Claisen-type condensation), and yet their synthesis is the site of action of several mainline antituberculosis drugs. We describe the isolation from Mycobacterium smegmatis and the full characterization of a 6-O-mycolyl-beta-D-mannopyranosyl-1-monophosphoryl-3,7,11,15,19,23 ,27- heptamethyl-(2Z,6E,10E)-octacosatrien-1-ol . The identification of a mycolyl-mannosylphosphopolyprenol supported by cell-free labeling experiments and earlier literature suggests unusual biochemical pathways in which mature mycolic acids are formed from beta-oxo precursors while attached to a mannosyl-P-polyprenol, in which form they are transported through the membrane prior to final deposition as arabinan-bound mycolates.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee A., Dubnau E., Quemard A., Balasubramanian V., Um K. S., Wilson T., Collins D., de Lisle G., Jacobs W. R., Jr inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science. 1994 Jan 14;263(5144):227–230. doi: 10.1126/science.8284673. [DOI] [PubMed] [Google Scholar]
- Besra G. S., McNeil M. R., Khoo K. H., Dell A., Morris H. R., Brennan P. J. Trehalose-containing lipooligosaccharides of Mycobacterium gordonae: presence of a mono-O-methyltetra-O-acyltrehalose "core" and branching in the oligosaccharide backbone. Biochemistry. 1993 Nov 30;32(47):12705–12714. doi: 10.1021/bi00210a020. [DOI] [PubMed] [Google Scholar]
- Bloch A. B., Cauthen G. M., Onorato I. M., Dansbury K. G., Kelly G. D., Driver C. R., Snider D. E., Jr Nationwide survey of drug-resistant tuberculosis in the United States. JAMA. 1994 Mar 2;271(9):665–671. [PubMed] [Google Scholar]
- Bloch K. Control mechanisms for fatty acid synthesis in Mycobacterium smegmatis. Adv Enzymol Relat Areas Mol Biol. 1977;45:1–84. doi: 10.1002/9780470122907.ch1. [DOI] [PubMed] [Google Scholar]
- Bloch K. Fatty acid synthases from Mycobacterium phlei. Methods Enzymol. 1975;35:84–90. doi: 10.1016/0076-6879(75)35141-0. [DOI] [PubMed] [Google Scholar]
- DAWSON R. M. A hydrolytic procedure for the identification and estimation of individual phospholipids in biological samples. Biochem J. 1960 Apr;75:45–53. doi: 10.1042/bj0750045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffe M., Brennan P. J., McNeil M. Predominant structural features of the cell wall arabinogalactan of Mycobacterium tuberculosis as revealed through characterization of oligoglycosyl alditol fragments by gas chromatography/mass spectrometry and by 1H and 13C NMR analyses. J Biol Chem. 1990 Apr 25;265(12):6734–6743. [PubMed] [Google Scholar]
- Danielson S. J., Gray G. R. Structures of the two homologous series of dialkene mycolic acids from Mycobacterium smegmatis. J Biol Chem. 1982 Oct 25;257(20):12196–12203. [PubMed] [Google Scholar]
- Datta A. K., Takayama K. Biosynthesis of a novel 3-oxo-2-tetradecyloctadecanoate-containing phospholipid by a cell-free extract of Corynebacterium diphtheriae. Biochim Biophys Acta. 1993 Aug 11;1169(2):135–145. doi: 10.1016/0005-2760(93)90198-i. [DOI] [PubMed] [Google Scholar]
- Jardine I., Scanlan G., McNeil M., Brennan P. J. Plasma desorption mass spectrometric analysis of mycobacterial glycolipids. Anal Chem. 1989 Mar 1;61(5):416–422. doi: 10.1021/ac00180a008. [DOI] [PubMed] [Google Scholar]
- Lacave C., Lanéelle M. A., Lanéelle G. Mycolic acid synthesis by Mycobacterium aurum cell-free extracts. Biochim Biophys Acta. 1990 Feb 23;1042(3):315–323. doi: 10.1016/0005-2760(90)90159-u. [DOI] [PubMed] [Google Scholar]
- Lee Y. C., Ballou C. E. Complete structures of the glycophospholipids of mycobacteria. Biochemistry. 1965 Jul;4(7):1395–1404. doi: 10.1021/bi00883a026. [DOI] [PubMed] [Google Scholar]
- McNeil M., Daffe M., Brennan P. J. Location of the mycolyl ester substituents in the cell walls of mycobacteria. J Biol Chem. 1991 Jul 15;266(20):13217–13223. [PubMed] [Google Scholar]
- Nikaido H. Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science. 1994 Apr 15;264(5157):382–388. doi: 10.1126/science.8153625. [DOI] [PubMed] [Google Scholar]
- Qureshi N., Sathyamoorthy N., Takayama K. Biosynthesis of C30 to C56 fatty acids by an extract of Mycobacterium tuberculosis H37Ra. J Bacteriol. 1984 Jan;157(1):46–52. doi: 10.1128/jb.157.1.46-52.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimakata T., Iwaki M., Kusaka T. In vitro synthesis of mycolic acids by the fluffy layer fraction of Bacterionema matruchotii. Arch Biochem Biophys. 1984 Feb 15;229(1):329–339. doi: 10.1016/0003-9861(84)90159-0. [DOI] [PubMed] [Google Scholar]
- Siakotos A. N. Analytical separation of nonlipid water soluble substances and gangliosides from other lipids by dextran gel column chromatography. J Am Oil Chem Soc. 1965 Nov;42(11):913–919. doi: 10.1007/BF02632444. [DOI] [PubMed] [Google Scholar]
- Takayama K., Armstrong E. L. Isolation, characterization, and function of 6-mycolyl-6'-acetyltrehalose in the H37Ra strain of Myocobacterium tuberculosis. Biochemistry. 1976 Jan 27;15(2):441–447. doi: 10.1021/bi00647a032. [DOI] [PubMed] [Google Scholar]
- Takayama K., Goldman D. S. Enzymatic synthesis of mannosyl-1-phosphoryl-decaprenol by a cell-free system of Mycobacterium tuberculosis. J Biol Chem. 1970 Dec 10;245(23):6251–6257. [PubMed] [Google Scholar]
- Takayama K., Schnoes H. K., Armstrong E. L., Boyle R. W. Site of inhibitory action of isoniazid in the synthesis of mycolic acids in Mycobacterium tuberculosis. J Lipid Res. 1975 Jul;16(4):308–317. [PubMed] [Google Scholar]
- Takayama K., Schnoes H. K., Semmler E. J. Characterization of the alkali-stable mannophospholipids of Mycobacterium smegmatis. Biochim Biophys Acta. 1973 Aug 23;316(2):212–221. doi: 10.1016/0005-2760(73)90011-8. [DOI] [PubMed] [Google Scholar]
- Walker R. W., Prome J. C., Lacave C. S. Biosynthesis of mycolic acids. Formation of a C32 beta-keto ester from palmitic acid in a cell-free system of Corynebacterium diphtheriae. Biochim Biophys Acta. 1973 Oct 17;326(1):52–62. doi: 10.1016/0005-2760(73)90027-1. [DOI] [PubMed] [Google Scholar]
- Wheeler P. R., Besra G. S., Minnikin D. E., Ratledge C. Stimulation of mycolic acid biosynthesis by incorporation of cis-tetracos-5-enoic acid in a cell-wall preparation from Mycobacterium smegmatis. Biochim Biophys Acta. 1993 Apr 7;1167(2):182–188. doi: 10.1016/0005-2760(93)90160-b. [DOI] [PubMed] [Google Scholar]
- Wong M. Y., Steck P. A., Gray G. R. The major mycolic acids of Mycobacterium smegmatis. Characterization of their homologous series. J Biol Chem. 1979 Jul 10;254(13):5734–5740. [PubMed] [Google Scholar]
- Yokoyama K., Ballou C. E. Synthesis of alpha 1----6-mannooligosaccharides in Mycobacterium smegmatis. Function of beta-mannosylphosphoryldecaprenol as the mannosyl donor. J Biol Chem. 1989 Dec 25;264(36):21621–21628. [PubMed] [Google Scholar]



