Abstract
Objectives:
Two-thirds of adolescents with chronic musculoskeletal pain report a concurrent sleep problem. Both musculoskeletal pain and sleep problems can have deleterious effects on physiological and psychological well-being. We explored the prevalence of sleep problems and musculoskeletal pain, using data on 3568 adolescents from the Avon Longitudinal Study of Children.
Materials and Methods:
A comprehensive battery of questionnaires was administered to derive clinical phenotypes of musculoskeletal pain. Adolescents with single symptoms were compared with those reporting both musculoskeletal pain and sleep problems. Linear and logistic regression analyses were used to compare groups on pain-related variables and psychological complaints. The association between sociodemographic variables and comorbid musculoskeletal pain and sleep problems was assessed using logistic regression.
Results:
Over half the sample was female (n=2076, 58.2%) and the majority of European ancestry (n=3174, 97.7%). Only 5.5% (n=196) of participants were identified as having a pain condition, while 21.2% (n=749) reported a significant sleep problem, and 2.8% (n=99) reported comorbid musculoskeletal pain and sleep problems. Adolescents with comorbid problems experienced greater pain intensity and pain-related anxiety. Other psychological complaints were also higher in those who experienced concurrent problems, including depression, fatigue, concentration, and overall severity of psychological symptoms.
Discussion:
Comorbid sleep and pain problems were associated with a higher incidence of pain-related and psychological symptoms. Sleep problems may therefore be an important modifiable risk factor for alleviating distress in adolescents with musculoskeletal pain.
Key Words: sleep, chronic pain, adolescence, ALSPAC, musculoskeletal pain
Around two-thirds of adolescents with musculoskeletal pain report a concurrent sleep problem.1,2 The nature of these problems varies and can include: extended sleep onset latencies, difficulty with sleep maintenance, and daytime hypersomnolence. Both musculoskeletal pain and sleep problems can have deleterious effects on physiological and psychological well-being.3,4 Furthermore, sleep and pain problems may have interdependent effects on such processes, the mechanisms of which need to be better understood.
The prevalence of chronic musculoskeletal pain in adolescents ranges from 4% to 40%.5 Sleep problems are one of the most common comorbidities in pain populations.6 The presence of musculoskeletal pain and sleep problems can have a stark impact upon an adolescent’s physical, emotional, and social well-being.7 Both the physical and affective components of pain can interfere with sleep quality and quantity.8 In turn, the physiological and psychological consequences of sleep problems can hinder an individual’s ability to cope with the functional demands of chronic pain.9,10 This cyclic pattern, and the subsequent changes in both affective and attentional states, can impede long-term rehabilitation.11,12
Aviel et al13 found an association between comorbid problems and increased pain sensitivity in adolescents. Similarly, a large cross-sectional study of Chinese adolescents found that those with comorbid musculoskeletal pain and sleep problems reported more bodily pain sites, higher pain intensity and greater pain disability than those with pain alone.2
Comorbid sleep and pain problems can also have significant consequences for an adolescent’s functionality. Studies have found that children reporting comorbid symptoms have a reduced quality of life, are less active, and have greater functional impairments.13–16 Furthermore, those struggling with sleep problems, in addition to a chronic pain are seen to utilize medical services more frequently.15 Such factors can lead to reduced engagement with both education and social activities, meaning these adolescents may experience a significant deviation in their normal developmental trajectory.
The experience of musculoskeletal pain and concurrent sleep problems is also associated with an increase in psychological difficulties. Those with comorbid problems experience more depression and show impaired emotional and social functions.10,17 The development of a mental health disorder during adolescence may not only hinder coping strategies for dealing with the long-term sequelae of musculoskeletal pain, but can also predict difficulties in later life.18,19 Finally, pain is associated with impairments in concentration and increased fatigue in adult clinical samples.3 Both of these factors limit functionality in adolescents with chronic pain,20 but the associations between comorbid problems and such factors need to be further explored.
This large cross-sectional study of adolescents explored the prevalence of both sleep problems and musculoskeletal pain and their relationship with pain-related factors. To the best of our knowledge, this is the first cohort study to assess the relationship between these comorbid problems and both pain-related anxiety and psychological symptoms such as concentration difficulties. Such comorbidities have not yet been fully explored in epidemiological studies of adolescents. A number of detailed assessments were used to derive both clinical phenotypes of chronic musculoskeletal pain and a wide range of pain-related symptoms. Specifically, we investigated the associations between comorbid musculoskeletal pain and sleep problems, pain severity, pain-related disability, and pain. By comprehensively assessing pain-related symptoms in adolescents, it may be possible to identify modifiable therapeutic targets and/or risk factors that may alleviate distress at a later developmental stage.
On the basis of the cognitive-vulnerability models of pain,21 we hypothesized that rates of depression and anxiety would be higher in adolescents with comorbid musculoskeletal pain and sleep problems. Higher incidences of pain-related anxiety, intensity, and disability were also expected in those adolescents reporting comorbid sleep and pain problems. Similarly, we anticipated that having musculoskeletal pain with concurrent sleep problems would be associated with greater concentration difficulties and fatigue, the psychosocial and educational implications of which could be critical. Finally, we expected that sleep problems would be associated with a higher incidence of pain symptoms across musculoskeletal sites.
MATERIALS AND METHODS
Participants
The Avon Longitudinal Study of Parents and Children (ALSPAC)22 is a prospective population-based study investigating a wide range of genetic and environmental influences on the health and development of children. Between April 1, 1991 to December 31, 1992, 14,541 pregnant women were initially enrolled from the district formerly known as Avon (United Kingdom). A total of 14,062 children were born and 13,988 were alive at 12 months. The main focus of this study was the results obtained from research clinics held at age 17, which 5102 children attended. Ethical approval for the study was obtained from the ALSPAC Ethics and Law Committee and the Local Research Ethics Committees. Details for all of the data used are available on the study website using a fully searchable data dictionary.23,24
Measures
Musculoskeletal Pain
The pain questionnaire was administered to all participants attending a research clinic at the age of 17. The questionnaire used was assembled from domains and scales taken from questionnaires previously validated in UK populations, based on combinations of pain previously reported in the literature.25,26 The questionnaire covered 3 aspects of pain: the experience of localized pain, the overall experience of pain, and the impact of pain on aspects of life.
Initially, participants indicated whether they had any aches or pains that had lasted for longer than 1 day in the past month and, if so, whether this had started in the last 3 months, or before. Using a manikin they were asked to indicate the site of the pain. Participants were then provided with a list of regions (eg, shoulder, knee) and asked to indicate whether they had experienced no pain or pain ranging from not at all to slightly, moderately, very, or extremely troubling. Reports of any pain affecting the musculoskeletal sites listed were classed as regional pain symptoms. Choosing their most troublesome pain, they were asked to indicate whether, over the past 3 to 6 months, their pain had lasted for fewer than 7 days, 1 to 4 weeks, 1 to 3 months, or more than 3 months.
On the basis of the participants’ experience of chronic pain (pain lasting≥3 mo) variables were derived from pain patterns consistent with clinical phenotypes and, in particular, musculoskeletal pain.27 Two forms of musculoskeletal pain were identified: chronic regional pain (CRP) and chronic widespread pain (CWP). CRP comprised of moderately, very, or extremely troublesome pain lasting longer than 3 months affecting the knee, hip, shoulder, lower back, or a combination of these sites, as indicated by completion of the troublesome pain domain. CWP was based on diagnostic criteria for fibromyalgia (ie, pain on both sides of the body, above and below the waist, and in the axial skeleton that has been present for at least 3 months), based on completion of the pain manikin.28 These definitions for both the CRP and CWP phenotypes were consistent with guidelines outlined by the American College of Rheumatology and have previously been used to assess musculoskeletal pain in a large population study.27 Participants identified as having either CRP and/or CWP were grouped into one “musculoskeletal pain” group to ensure that a sufficient number of observation were available for regression analyses.
Pain-related Variables
In addition to the musculoskeletal conditions (CRP and CWP) derived, we also explored pain symptoms. Specifically, these were defined as any bothersome pain at selected musculoskeletal sites lasting for longer than 3 months. The location and number of symptoms were recorded for each participant.
Participants also completed a modified version of the Chronic Pain Grade Questionnaire (CPGQ).29 Participants were asked to rate their pain intensities over the past 6 months. Using a scale of 1 (no pain) to 10 (pain as bad as it could be) they were asked to rate their current pain, worst pain, and average pain intensity. These 3 items were compiled to create a pain intensity variable (Cronbach’s α=0.71). At the start of the pain battery, participants were asked to indicate whether they had aches or pains that had lasted for a day or longer in the past month? Those who responded “no” were instructed not to fill in the CPGQ, as such a reduced number of responses were available for this measure.
A third subscale, pain disability was also assessed. Participants were asked to rate how much impact pain had upon their social, recreational, and work-related activities in the past 6 months. Specifically, they are asked to indicate the number of days that pain has kept them from usual activities (0 to 6 d, 7 to 14 d, 15 to 30 d, 31 or more days) and how much pain had interfered with daily activities on a scale of 1 (no interference) and 10 (unable to carry on with activities). Finally, using a scale of 1 (no change) to 10 (extreme change), they then rated how much pain had changed their ability to take part in recreational, social, and family activities. Using scoring procedures recommended by Von Korff et al,29 the CPGQ was used to classify participants according to chronic pain grade: 0=no pain, I= low pain intensity and low levels of pain-related disability, II=high pain intensity and low levels of pain-related disability, III=moderate pain-related disability, and IV=severe pain-related disability. The sample was then further classified into those with low (grades 0, I, II) and high disability (grades III, IV). The scale has previously been used in both adolescent and young adult cohorts.30,31 Reliability for this subscale was tested and found to be acceptable (Cronbach’s α=0.72).
A final subscale, “pain-related anxiety” was taken from the Bath Adolescent Pain Questionnaire, which has previously been used and validated in adolescent samples.32 Using a scale of 1 (never) to 5 (always), participants were asked to rate the following statements: (1) I worry about my pain problem, (2) I avoid activities that cause pain, (3) When I think about my pain, it makes me upset, (4) Pain scares me, (5) I worry that I will do something that will make my pain worse, (6) When I have pain, I think something harmful is happening, (7) I am afraid to move due to pain. Reliability of the measure in the current sample was tested and found to be acceptable (Cronbach’s α=0.87).
Sleep and Psychological Symptoms
The Computerised Interview Schedule—Revised (CIS-R), a computerized interview schedule was used to establish the nature and severity of psychological symptoms.33,34 The interview is fully standardized and equally reliable whether conducted by a clinically trained interviewer or self-administered by a computer.33,35,36 The CIS-R is designed for, and has been widely used within adolescent birth cohorts including National Surveys of Psychiatric Morbidity and the 1958 birth cohort.36–38 The CIS-R has a maximum score of 57, with a score of >12 indicating the presence of a common mental disorder based on ICD-10 criteria. The interview begins with general questions to establish an overall picture of psychological and physical health. The main body of the CIS-R contains 14 sections: somatic symptoms, fatigue, concentration, sleep problems, irritability, depression, depressive ideas, worry, anxiety, phobia, obsessions, compulsions, and worry about physical health. Each section scores a particular type of psychological symptom, which, with the exception of “depressive ideas” ranges in severity between 0 and 4; “depressive ideas” scores between 0 and 5. Each subscale has mandatory questions to establish the existence of a particular symptom. If the participant affirms the existence of this symptom, a more detailed assessment follows. Specifically, participants are questioned about the frequency, duration, severity, and time since onset. A score of between 0 and 4 is generated based on these answers. Symptoms with scores of 2 or more should be considered as a significant psychological problem. Each of the 14 subscales provides a distinct measure of the proposed underlying construct, the reliability of which has been favorable.33 This criterion was used to establish those with a sleep problem and identifiable psychological problems including anxiety, concentration difficulties, etc.
Specifically, the sleep interview uses 11 items to examine changes in sleep patterns, causes of sleep disturbance, and duration of sleep to assess the severity of the sleep problem. Participants are required to respond to the mandatory question: “Have you been having problems with trying to get to sleep or with getting back to sleep if you woke up or were woken up?” If the participant affirms “yes” to having sleep problems, they are then questioned regarding the frequency and severity of the problem. Examples include: “How many nights did you have problems with your sleep?,” where response options range from “none,” “between one and three nights,” and “four or more nights,” and “How long did you spend trying to get to sleep?” where response options range from “less than 15 minutes,” “between 15 minutes and an hour,” “between one and three hours,” and “three or more hours.” Using each item a weighted score is calculated for each subscore. Further information on the scoring of the CIS-R can be found in Golderg et al.34
Other Measures
Basic demographic information was also collected, including: sex, ethnicity, and parent’s marital status. Socioeconomic position indicators were obtained from self-administered questionnaires to the mothers. Social class was defined using the UK Registrar General’s occupational coding (SOC 90) and grouped into 6 categories: I (professional), II (managerial and technical), III (skilled manual or nonmanual), IV (semiskilled) manual, V (unskilled), VI (armed forces). The highest social class of either parent was used to define parental social class.
Statistical Analyses
On the basis of the outcomes from the pain battery and CIS-R, 4 groups were identified: (1) No condition, those adolescents with neither musculoskeletal pain or sleep problem; (2) Pain condition only, those identified as having either CRP and/or CWP from the pain battery but no identifiable sleep problem from the CIS-R; (3) Sleep condition only, those scoring>2 on the CIS-R sleep subscale but pain-free; (4) Pain and sleep condition, those identified as having both a musculoskeletal pain condition and a sleep problem from the aforementioned scales.
Proportions of those participants in each condition group reporting high pain disability and psychological symptoms (CIS sub score>2) were calculated. In particular, we explored the following CIS-R subscales: depression, anxiety, concentration, and fatigue. Previous studies have indicated an association between musculoskeletal pain and such psychological symptoms; however, the influence of sleep problems on such comorbidities remains poorly understood.20,39,40 The total CIS-R score was also assessed to examine whether any of our condition groups were more likely to report a common mental health disorder.
Pain-related variables and psychological symptoms were compared between the 4 conditions using multiple regression with both linear and logistic functions. Given that sex differences can influence pain response, we adjusted for sex in each of our regression analyses.41 Linear regression analysis was used to investigate the potential association between condition groups and continuous measures (pain intensity, pain-related anxiety, CIS-R score). For each linear model, the unstandardized regression coefficients (beta) are reported and can be interpreted as the difference in symptoms scores between each condition group and a comparison group. Multiple logistic regression was used to assess the associations between the 4 conditions and binary outcome measures (eg, CIS-R subscores and pain disability). Odds ratios (OR) are presented with 95% confidence intervals (CIs). Wald F tests (linear regressions) and χ2 tests (logistic regressions) and were used to evaluate the contrasts between condition groups. Specifically, for each group a Wald test was performed to test whether the pairwise difference between the coefficient/odds ratio of interest and the comparison group was different from zero.
Binary logistic regression was used to assess whether adolescents with sleep problems were more likely to report pain symptoms (yes/no) at certain musculoskeletal sites, compared with those without. A Mann-Whitney U test was used to examine whether a greater number of pain complaints were reported across the musculoskeletal system in adolescents with sleep problems.
Finally, Poisson regression was used to assess the association between sociodemographic variables and the development of comorbid musculoskeletal pain and sleep problems (relative risk ratios are presented with 95% CI). Analyses were conducted using STATA (version 13) with all available data points for each participant.
RESULTS
Characteristics of Participants
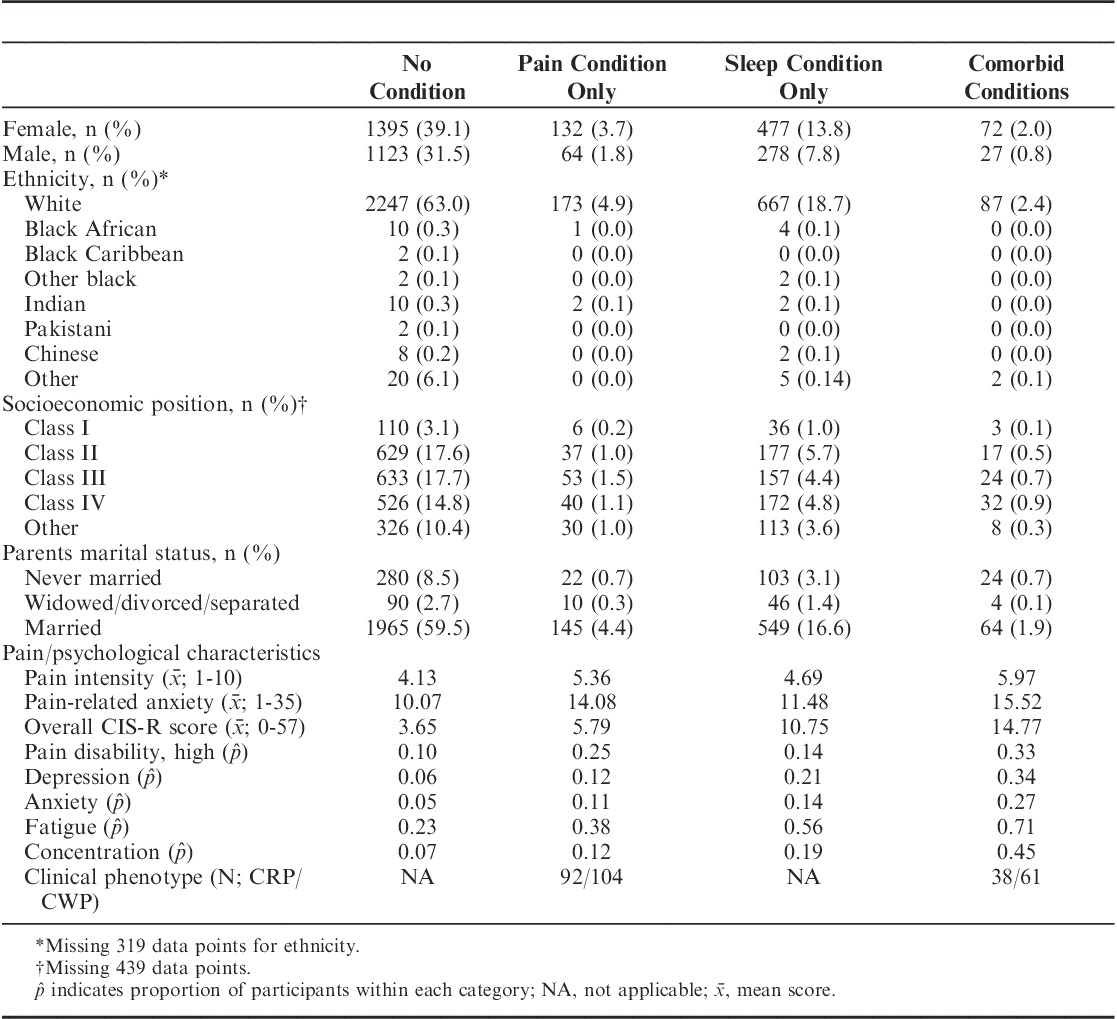
The pain battery and CIS-R were both completed by 3568 adolescents. Over half of the sample were female (n=2076, 58.2%) and the majority of European ancestry (n=3174, 97.7%). Only 5.5% (n=196) were identified as having a musculoskeletal pain condition, whereas 21.2% (n=749) reported a sleep problem, with 2.8% (n=99) reporting comorbid musculoskeletal pain and sleep problems. A total of 1658 participants responded “yes” to the question asking for an indication of pain in the last month and were required to complete the CPGQ. Out of these participants, only 1627 provided complete data. Full prevalence rates and demographic information are presented in Table 1.
TABLE 1.
Sociodemographic Information and Characteristics of Participants With Musculoskeletal Pain and Sleep Conditions, Alone and in Combination (N=3568)
Pain Characteristics and Psychological Symptoms
The pain characteristics and psychological symptoms reported by the 4 comparison groups are presented in Table 2. Results are presented for comparisons between the musculoskeletal only and comorbid problems groups using a Wald test, unless stated. Results from the linear regression analyses indicate pain intensity was higher in the 3 condition groups compared with the comparison group. Pairwise comparisons indicated that those with comorbid problems reported a higher average pain intensity compared with adolescents with musculoskeletal pain alone (F1,1622=8.51, P=0.004). Those with both musculoskeletal pain and sleep problems also reported greater pain-related anxiety (F1,3354=6.29, P=0.01). Finally, maximum CIS-R scores were also higher in those reporting comorbid problems (F1,3563=174.75, P<0.001), indicating that these adolescents had a greater likelihood of reporting a common mental health problem.
TABLE 2.
Associations Between Pain and Psychological Neurotic Symptoms in Those With Musculoskeletal Pain and Sleep Conditions, Alone and in Combination
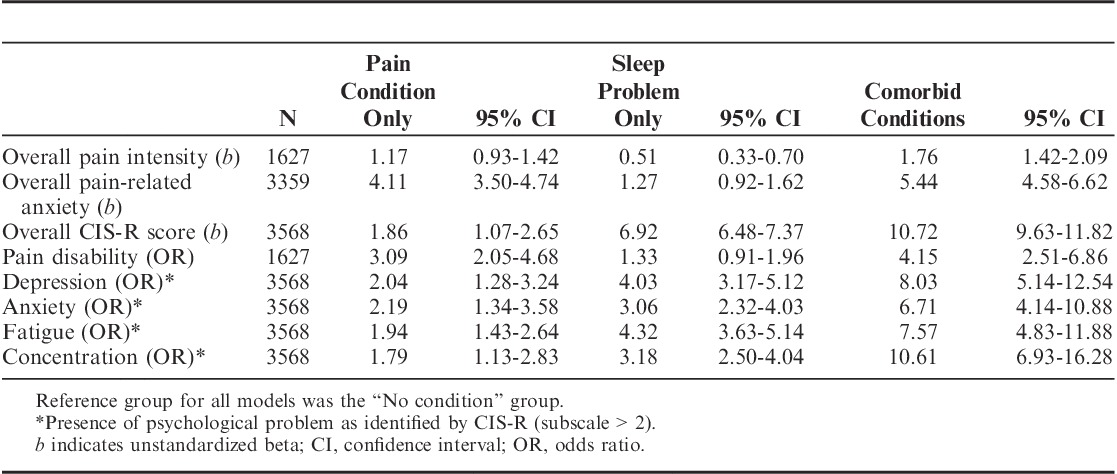
The binary logistic regression analyses presented in Table 2 indicate the association between pain groups and the occurrence of both pain disability (low/high) and psychological symptoms (no/yes). Associations were found between pain group and the psychological symptoms assessed, between which group differences were apparent upon assessment of the Wald statistic. Specifically, each of the psychological symptoms assessed were found to be greater in the comorbidity groups compared with the musculoskeletal pain-only group; depression (χ21=19.29, P<0.001), anxiety (χ21=11.80, P<0.001), fatigue (χ21=25.50, P<0.001), concentration (χ21=35.52, P<0.001). Finally, there is strong evidence for an association between those in the comorbid group and high pain disability (OR=4.07; 95% CI, 2.49-6.63). Despite disability being high in the comorbid group, comparison tests found little evidence to indicate that this was greater than the pain-alone group (χ21=1.04, P=0.3).
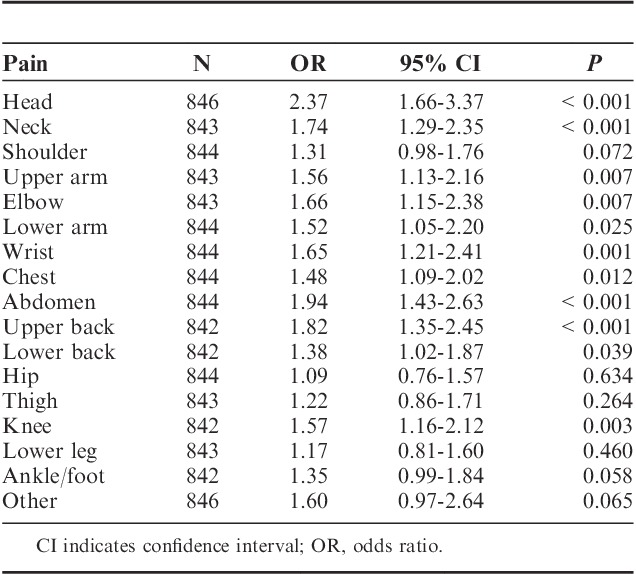
Nearly a quarter of our sample (23.8%, N=849) reported having a pain problem (>3 mo) and indicated that it had affected a particular musculoskeletal site. Those adolescents also reporting a sleep problem were found to have a greater number of pain symptoms across a number of musculoskeletal sites. In particular, sleep problems were associated with a higher likelihood of pain in the head (OR=2.37; 95% CI, 1.66-3.37; P<0.001) and abdomen (OR=1.94; 95% CI, 1.43-2.63; P<0.001). However, there was weak evidence to suggest that poor sleep was associated with hip (OR=1.09; 95% CI, 0.76-1.57; P=0.63) or leg pain (OR=1.17; 95% CI, 0.81-1.60; P=0.46). A full list of musculoskeletal sites is presented in Table 3. A Mann-Whitney U test indicated that participants reporting sleep problems reported a greater number of pain symptoms across musculoskeletal sites, compared with normal sleepers (M=6.92 vs. 5.49, z=−50.8, P<0.001).
TABLE 3.
Odds Ratios for Reporting Pain Complaints (>3 mo) in Adolescents With Sleep Problem Associations Between Sleep Problems and Regional Pain Symptoms (>3 mo)
Sociodemographic Factors, Musculoskeletal Pain, and Sleep Problems
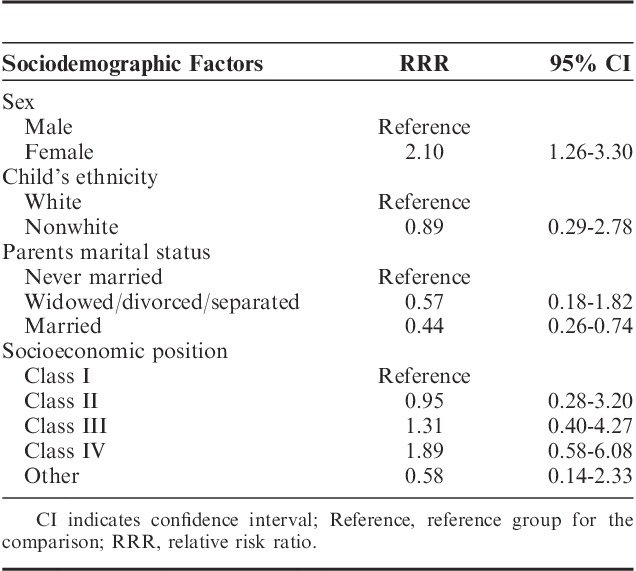
Results from the logistic regression analyses indicated that girls had a greater likelihood of reporting comorbid musculoskeletal pain and sleep problems (relative risk ratio=2.10; 95% CI, 1.26-3.30; P=0.003), whereas those with married parents were less likely to report comorbid problems (relative risk ratio=0.44; 95% CI, 0.26-0.74; P=0.002). No associations were found between either ethnicity or socioeconomic position and the presence of comorbid problems. Results from these analyses are presented in Table 4.
TABLE 4.
Sociodemographic Factors Associated With Comorbid Musculoskeletal Pain and Sleep Problems (N=2268)
DISCUSSION
Our results indicate that adolescents with comorbid musculoskeletal pain and sleep problems experience greater pain intensity and psychological problems (eg, depression and anxiety) compared with those with pain alone. Such findings are consistent with the previous literature in suggesting that relationship shared by sleep and pain might affect both physiological and psychological processes.2,42,43 Importantly this is the first cohort study to find an association between heightened levels of pain-related anxiety and comorbid sleep and pain problems. Furthermore, our findings indicate that functionality might be reduced in adolescents with comorbid sleep and pain problems as both concentration difficulties and fatigue were more frequently reported.
Pain intensity was greater in those adolescents reporting concurrent musculoskeletal pain and sleep problems, thereby complimenting previous studies.2 We may speculate that sleep problems enhance pain sensation by impaired endogenous pain inhibition,44 activation of systems involved in neurogenic inflammation,45 and/or alterations in both opioidergic and/or sertoninergic neurotransmission.46 Further studies are required to clarify the reciprocal relationship between pain, sleep, and their correlates.
Reports of any pain affecting musculoskeletal sites were also assessed. Interestingly, adolescents with sleep problems were more likely to report pain across a significant number of musculoskeletal regions (eg, head, neck, lower back). The number of pain symptoms across multiple sites was also seen to be higher in this group. Such findings indicate that we need to further explore the influence of sleep on bothersome pain, in addition to its relationship with clinical phenotypes such as CRP and CWP.
Adolescents with concurrent musculoskeletal pain and sleep problems reported greater pain-related anxiety. Specifically, these adolescents worried more about pain and would prefer to avoid activities that they perceived might cause pain. Previous studies have found that pain-related anxiety is associated with disability, pain intensity, and behavioral performance.47 These suggest that pain-related anxiety and hypervigilance to pain symptoms is actually more disabling than pain intensity.48,49 Similar work is required in adolescent populations to help explore the relationship between affective pain states and sleep.
Over half (53.5%) of those experiencing both musculoskeletal pain and sleep problems reached the threshold for significant mental health difficulties, as defined by the ICD-10. This could have important consequences for their psychological well-being as they progress through to adulthood. Upon analysis of the individual psychological symptoms, depression was found to be higher in those adolescents with comorbid musculoskeletal pain and sleep problems. Studies have found that both adults and adolescents with depression are less able to cope with the sensory and affective elements of pain.50–52 Comorbid depression also reduces the likelihood of a return to musculoskeletal health.53 Indeed our results are suggestive of a relationship between comorbid sleep problems and higher rates of depression, which may mean these adolescents are less able to cope with their on-going pain status.
Greater levels of fatigue were also associated with adolescents experiencing both musculoskeletal pain and sleep problems compared with those reporting pain alone, consistent with previous findings.43,54 Patients with musculoskeletal pain commonly report fatigue, which may stem either from side effects of analgesic medications or from comorbid mental health difficulties. Fatigue may also be a direct result of central sensitization within the spinal cord causing a neuroendocrinological imbalance,55 or of constant hypervigilance to the threat of pain symptoms. Increased reports of concentration difficulties were also associated with our comorbid group. Such difficulties—in addition to persistent fatigue—may have significant consequences for learning outcomes, school performance, and even attendance. Fatigue and concentration difficulties are also associated with reduced functionality15 and might lead to reduced engagement with recreational and social activities.
Pain-related disability should also be considered as an important risk factor, which itself can further impair behavioral and emotional well-being. In combination with the aforementioned functional difficulties, the high levels of pain-related disability reported in our comorbid group can have significant developmental consequences during adolescence. Such factors can influence an adolescent’s physical and psychosocial well-being, which may lead to further functional impairment and mental health difficulties. Health care professionals, in addition to parents and teachers should be made aware of such risks and promote rehabilitation where possible.
Overall, both the sensory and affective elements of pain were heightened in those with comorbid pain and sleep problems. This is in keeping with Lewin and Dahl’s model11 that suggests that those in musculoskeletal pain become hypervigilant to the perceived threat of their pain symptoms. This preoccupation can lead to heightened states of arousal, particularly before bed. Studies in both adults and adolescents have found that presleep arousal can disrupt both sleep quality and quantity, acting to prolong insomnia symptoms.56,57 Over time, such disturbances can not only affect physical elements of pain but also contribute to maladaptive sleep-related cognitions. As this vicious cycle develops, both musculoskeletal pain and sleep problems are likely to be maintained.
Extended sleep problems can affect both behavioral and emotional regulation. As discussed, adolescents with comorbid symptoms pay greater attention to pain but also experience lower mood and enhanced rumination over pain-related worries. Results from this study are consistent with this position. Such factors can impede coping strategies and reduce motivation to achieve long-term goals.12 Following the principles of a cognitive-vulnerability model of stress and pain,21 such vicious cycles might be more problematic in those children attempting to cope with both musculoskeletal pain and sleep disorders. Concurrent sleep problems may impair coping strategies, and thus adolescents are psychologically ill-equipped to deal with the short and long-term consequences of musculoskeletal pain.
When one considers the complex set of associations shared by these parameters, it is possible to see how targeting one domain for therapeutic manipulation, might alleviate problems elsewhere in the cycle. Sleep itself undergoes significant changes during adolescence. The increasing prevalence of electronic devices and additional psychosocial pressures are delaying sleep onset times in adolescents, meaning sleep-wake cycles are becoming increasingly more disturbed and overall sleep quality is reduced.58 Such factors can aggravate and enhance any existing sleep problems in those dealing with musculoskeletal pain. Therefore, greater consideration of sleep patterns in adolescent pain samples might provide a simple therapeutic target. Equally, following cognitive-vulnerability models, other comorbidities identified in this study can also be targeted, such as treatment of depression and anxiety. Finally, it is possible to suggest that psychological therapies targeting pain-related anxiety might assist in alleviating the musculoskeletal pain. Studies assessing the efficacy of such treatments will provide a better understanding of the relationship shared by sleep, pain, and their correlates.
Although this study has revealed some interesting associations, there are limitations to consider. First, the data are cross-sectional meaning we cannot make any inferences regarding causality between the parameters investigated. Our findings therefore need to be integrated with both experimental and longitudinal studies to fully delineate the proposed mechanisms. Second, we must take care in over interpreting the results given that subjective self-report measures of sleep were used. For example; the metric assessing “sleep problems” does not provide us with a quantitative index of sleep disturbance and quality. Objective and longitudinal measures are required to examine which elements of sleep are associated with pain-related outcomes. Third, prevalence estimates may vary somewhat due to the use of different criteria, scales, and definitions for both musculoskeletal pain and sleep problems across studies. Prevalence estimates have been wide ranging for adolescent musculoskeletal pain in previous studies, for example Auvinen et al59 report high prevalence estimates of musculoskeletal pain (42% to 52%) based on a dichotomous response to a single-item measure. This may speak of the different measures and scales used to assess musculoskeletal pain. The criterion used for CWP in the present study is consistent with the diagnostic criteria for fibromyalgia and therefore may be considered as a more stringent and representative assessment. Such comprehensive assessments may have contributed to the low numbers of participants within both the pain-alone and comorbid groups. The low numbers observed are another limitation of the present study and gave rise to CRP and CWP groups being combined. Herein, it must be noted that the proportion of CWP cases in both the pain-only group and comorbid group was very similar (∼40%). As such, we believe that the group differences identified are not the result of variations in subgroup symptomology. We must also acknowledge the homogeneity of our sample given that all participants were tested at age 17. In addition to the sample being nonclinical, this limits the generalizations we can make from the sample. Finally, it would have been useful to include information on what medication each participant was taking for their pain (if any). This information was not collected, however.
In conclusion, our results suggest that sleep problems are associated with greater pain intensity, disability, and psychological symptoms in adolescents with musculoskeletal pain. More work is required to establish the etiology of sleep problems and their correlates in adolescents with musculoskeletal pain. Once we develop a better understanding of these associations, novel clinical targets can be established, which can be manipulated for therapeutic gain.
ACKNOWLEDGMENTS
The authors are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses.
Footnotes
The UK Medical Research Council (London, UK) and the Wellcome Trust, London, UK (Grant ref: 102215/2/13/2) and the University of Bristol (Bristol, UK) provide core support for ALSPAC. Funding from British Heart Foundation (Birmingham, UK), Cancer Research UK (London, UK), Economic and Social Research Council (Swindon, UK), Medical Research Council (London, UK), and the National Institute for Health Research (London, UK), under the auspices of the UK Clinical Research Collaboration (London, UK), is gratefully acknowledged. This publication is the work of the authors and L.H. will serve as guarantor for the contents of this paper. M.R.M. is a member of the United Kingdom Centre for Tobacco and Alcohol Studies, a UKCRC Public Health Research: Centre of Excellence (London, UK).
REFERENCES
- 1.Roth-Isigkeit A, Thyen U, Stoven H, et al. Pain among children and adolescents: restrictions in daily living and triggering factors. Pediatrics. 2005;115:e152–e162. [DOI] [PubMed] [Google Scholar]
- 2.Siu YF, Chan S, Wong KM, et al. The comorbidity of chronic pain and sleep disturbances in a community adolescent sample: prevalence and association with sociodemographic and psychosocial factors. Pain Med. 2012;13:1292–1303. [DOI] [PubMed] [Google Scholar]
- 3.Hart RP, Martelli MF, Zasler ND. Chronic pain and neuropsychological functioning. Neuropsychol Rev. 2000;10:131–149. [DOI] [PubMed] [Google Scholar]
- 4.Louca M, Short MA. The effect of one night’s sleep deprivation on adolescent neurobehavioral performance. Sleep. 2014;37:1799–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King S, Chambers CT, Huguet A, et al. The epidemiology of chronic pain in children and adolescents revisited: a systematic review. Pain. 2011;152:2729–2738. [DOI] [PubMed] [Google Scholar]
- 6.Valrie CR, Bromberg MH, Palermo T, et al. A systematic review of sleep in pediatric pain populations. J Dev Behav Pediatr. 2013;34:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eccleston C, Clinch J. Adolescent chronic pain and disability: a review of the current evidence in assessment and treatment. Paediatr Child Health. 2007;12:117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004;8:119–132. [DOI] [PubMed] [Google Scholar]
- 9.Long AC, Krishnamurthy V, Palermo TM. Sleep disturbances in school-age children with chronic pain. J Pediatr Psychol. 2008;33:258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palermo TM, Kiska R. Subjective sleep disturbances in adolescents with chronic pain: relationship to daily functioning and quality of life. J Pain. 2005;6:201–207. [DOI] [PubMed] [Google Scholar]
- 11.Lewin DS, Dahl RE. Importance of sleep in the management of pediatric pain. J Dev Behav Pediatr. 1999;20:244–252. [DOI] [PubMed] [Google Scholar]
- 12.Dahl RE, Lewin DS. Pathways to adolescent health sleep regulation and behavior. J Adolesc Health. 2002;31:175–184. [DOI] [PubMed] [Google Scholar]
- 13.Aviel YB, Stremler R, Benseler SM, et al. Sleep and fatigue and the relationship to pain, disease activity and quality of life in juvenile idiopathic arthritis and juvenile dermatomyositis. Rheumatology (Oxford). 2011;50:2051–2060. [DOI] [PubMed] [Google Scholar]
- 14.Palermo TM, Fonareva I, Janosy NR. Sleep quality and efficiency in adolescents with chronic pain: relationship with activity limitations and health-related quality of life. Behav Sleep Med. 2008;6:234–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palermo TM, Law E, Churchill SS, et al. Longitudinal course and impact of insomnia symptoms in adolescents with and without chronic pain. J Pain. 2012;13:1099–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LaPlant MM, Adams BS, Haftel HM, et al. Insomnia and quality of life in children referred for limb pain. J Rheumatol. 2007;34:2486–2490. [PubMed] [Google Scholar]
- 17.Luyster FS, Chasens ER, Wasko MC, et al. Sleep quality and functional disability in patients with rheumatoid arthritis. J Clin Sleep Med. 2011;7:49–55. [PMC free article] [PubMed] [Google Scholar]
- 18.Fombonne E, Wostear G, Cooper V, et al. The Maudsley long-term follow-up of child and adolescent depression. 2. Suicidality, criminality and social dysfunction in adulthood. Br J Psychiatry. 2001;179:218–223. [DOI] [PubMed] [Google Scholar]
- 19.Kashikar-Zuck S, Goldschneider KR, Powers SW, et al. Depression and functional disability in chronic pediatric pain. Clin J Pain. 2001;17:341–349. [DOI] [PubMed] [Google Scholar]
- 20.Gold JI, Mahrer NE, Yee J, et al. Pain, fatigue, and health-related quality of life in children and adolescents with chronic pain. Clin J Pain. 2009;25:407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riskind JH, Alloy LB. Cognitive vulnerability to psychological disorders: overview of theory, design, and methods. J Clin Social Clin Psychol. 2006;25:705–725. [Google Scholar]
- 22.Boyd A, Golding J, Macleod J, et al. Cohort profile: the ‘Children of the 90s’—the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol. 2012;42:111–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Golding J, Pembrey M, Jones R, et al. ALSPAC—the Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatr Perinat Epidemiol. 2001;15:74–87. [DOI] [PubMed] [Google Scholar]
- 24.University of Bristol. Avon Longitudinal Study of Parents and Children: data dictionary, 2015. Available at: http://www.bris.ac.uk/alspac/researchers/data-access/data-dictionary/. Accessed April 2015.
- 25.Mallen CD, Peat G, Thomas E, et al. Is chronic musculoskeletal pain in adulthood related to factors at birth? A population-based case-control study of young adults. Eur J Epidemiol. 2006;21:237–243. [DOI] [PubMed] [Google Scholar]
- 26.Parsons S, Carnes D, Pincus T, et al. Measuring troublesomeness of chronic pain by location. BMC Musculoskelet Disord. 2006;7:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deere KC, Clinch J, Holliday K, et al. Obesity is a risk factor for musculoskeletal pain in adolescents: findings from a population-based cohort. Pain. 2012;153:1932–1938. [DOI] [PubMed] [Google Scholar]
- 28.Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Arthritis Rheum. 1990;33:160–172. [DOI] [PubMed] [Google Scholar]
- 29.Von Korff M, Ormel J, Keefe FJ, et al. Grading the severity of chronic pain. Pain. 1992;50:133–149. [DOI] [PubMed] [Google Scholar]
- 30.Plesh O, Gansky SA, Curtis DA. Chronic pain in a biracial cohort of young women. Open Pain J. 2012;5:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Gessel H, Gassmann J, Kroner-Herwig B. Children in pain: recurrent back pain, abdominal pain, and headache in children and adolescents in a four-year-period. J Pediatr. 2011;158:977–983. e1-2. [DOI] [PubMed] [Google Scholar]
- 32.Eccleston C, Jordan A, McCracken LM, et al. The Bath Adolescent Pain Questionnaire (BAPQ): development and preliminary psychometric evaluation of an instrument to assess the impact of chronic pain on adolescents. Pain. 2005;118:263–270. [DOI] [PubMed] [Google Scholar]
- 33.Lewis G, Pelosi AJ, Araya R, et al. Measuring psychiatric disorder in the community: a standardized assessment for use by lay interviewers. Psychol Med. 1992;22:465–486. [DOI] [PubMed] [Google Scholar]
- 34.Goldberg DP, Cooper B, Eastwood MR, et al. A standardized psychiatric interview for use in community surveys. Br J Prev Soc Med. 1970;24:18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patton GC, Coffey C, Posterino M, et al. A computerised screening instrument for adolescent depression: population-based validation and application to a two-phase case-control study. Soc Psychiatry Psychiatr Epidemiol. 1999;34:166–172. [DOI] [PubMed] [Google Scholar]
- 36.Bell T, Watson M, Sharp D, et al. Factors associated with being a false positive on the General Health Questionnaire. Soc Psychiatry Psychiatr Epidemiol. 2005;40:402–407. [DOI] [PubMed] [Google Scholar]
- 37.Brugha TS, Meltzer H, Jenkins R, et al. Comparison of the CIS-R and CIDI lay diagnostic interviews for anxiety and depressive disorders. Psychol Med. 2005;35:1089–1091. [DOI] [PubMed] [Google Scholar]
- 38.Brugha TS, Morgan Z, Bebbington P, et al. Social support networks and type of neurotic symptom among adults in British households. Psychol Med. 2003;33:307–318. [DOI] [PubMed] [Google Scholar]
- 39.Ando S, Yamasaki S, Shimodera S, et al. A greater number of somatic pain sites is associated with poor mental health in adolescents: a cross-sectional study. BMC Psychiatry. 2013;13:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Compas BE, Boyer MC, Stanger C, et al. Latent variable analysis of coping, anxiety/depression, and somatic symptoms in adolescents with chronic pain. J Consult Clin Psychol. 2006;74:1132–1142. [DOI] [PubMed] [Google Scholar]
- 41.Fillingim RB, King CD, Ribeiro-Dasilva MC, et al. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10:447–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang NK, Wright KJ, Salkovskis PM. Prevalence and correlates of clinical insomnia co-occurring with chronic back pain. J Sleep Res. 2007;16:85–95. [DOI] [PubMed] [Google Scholar]
- 43.Wong WS, Fielding R. The co-morbidity of chronic pain, insomnia, and fatigue in the general adult population of Hong Kong: prevalence and associated factors. J Psychosom Res. 2012;73:28–34. [DOI] [PubMed] [Google Scholar]
- 44.Smith MT, Edwards RR, McCann UD, et al. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30:494–505. [DOI] [PubMed] [Google Scholar]
- 45.Lautenbacher S, Kundermann B, Krieg J-C. Sleep deprivation and pain perception. Sleep Med Rev. 2006;10:357–369. [DOI] [PubMed] [Google Scholar]
- 46.Foo H, Mason P. Brainstem modulation of pain during sleep and waking. Sleep Med Rev. 2003;7:145–154. [DOI] [PubMed] [Google Scholar]
- 47.Simons LE, Kaczynski KJ. The Fear Avoidance model of chronic pain: examination for pediatric application. J Pain. 2012;13:827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swinkels-Meewisse IE, Roelofs J, Oostendorp RA, et al. Acute low back pain: pain-related fear and pain catastrophizing influence physical performance and perceived disability. Pain. 2006;120:36–43. [DOI] [PubMed] [Google Scholar]
- 49.Crombez G, Vlaeyen JW, Heuts PH, et al. Pain-related fear is more disabling than pain itself: evidence on the role of pain-related fear in chronic back pain disability. Pain. 1999;80:329–339. [DOI] [PubMed] [Google Scholar]
- 50.Edwards RR, Smith MT, Kudel I, et al. Pain-related catastrophizing as a risk factor for suicidal ideation in chronic pain. Pain. 2006;126:272–279. [DOI] [PubMed] [Google Scholar]
- 51.van Tilburg MA, Spence NJ, Whitehead WE, et al. Chronic pain in adolescents is associated with suicidal thoughts and behaviors. J Pain. 2011;12:1032–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bair MJ, Robinson RL, Katon W, et al. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433–2445. [DOI] [PubMed] [Google Scholar]
- 53.Davies KA, Macfarlane GJ, Nicholl BI, et al. Restorative sleep predicts the resolution of chronic widespread pain: results from the EPIFUND study. Rheumatology. 2008;47:1809–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Creavin ST, Dunn KM, Mallen CD, et al. Co-occurrence and associations of pain and fatigue in a community sample of Dutch adults. Eur J Pain. 2010;14:327–334. [DOI] [PubMed] [Google Scholar]
- 55.McBeth J, Silman AJ, Gupta A, et al. Moderation of psychosocial risk factors through dysfunction of the hypothalamic–pituitary–adrenal stress axis in the onset of chronic widespread musculoskeletal pain: findings of a population-based prospective cohort study. Arthritis Rheum. 2007;56:360–371. [DOI] [PubMed] [Google Scholar]
- 56.Smith MT, Perlis ML, Smith MS, et al. Sleep quality and presleep arousal in chronic pain. J Behav Med. 2000;23:1–13. [DOI] [PubMed] [Google Scholar]
- 57.Lewandowski AS, Palermo TM, De la Motte S, et al. Temporal daily associations between pain and sleep in adolescents with chronic pain versus healthy adolescents. Pain. 2010;151:220–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh GK, Kenney MK. Rising prevalence and neighborhood, social, and behavioral determinants of sleep problems in US children and adolescents, 2003–2012. Sleep Disorders. 2013;2013:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Auvinen JP, Tammelin TH, Taimela SP, et al. Is insufficient quantity and quality of sleep a risk factor for neck, shoulder and low back pain? A longitudinal study among adolescents. Eur Spine J. 2010;19:641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]


