Abstract
Deficits in recognizing others' emotions are reported in many psychiatric and neurological disorders, including autism, schizophrenia, behavioral variant frontotemporal dementia (bvFTD) and Alzheimer's disease (AD). Most previous emotion recognition studies have required participants to identify emotional expressions in photographs. This type of assessment differs from real-world emotion recognition in important ways: Images are static rather than dynamic, include only 1 modality of emotional information (i.e., visual information), and are presented absent a social context. Additionally, existing emotion recognition batteries typically include multiple negative emotions, but only 1 positive emotion (i.e., happiness) and no self-conscious emotions (e.g., embarrassment). We present initial results using a new task for assessing emotion recognition that was developed to address these limitations. In this task, respondents view a series of short film clips and are asked to identify the main characters' emotions. The task assesses multiple negative, positive, and self-conscious emotions based on information that is multimodal, dynamic, and socially embedded. We evaluate this approach in a sample of patients with bvFTD, AD, and normal controls. Results indicate that patients with bvFTD have emotion recognition deficits in all 3 categories of emotion compared to the other groups. These deficits were especially pronounced for negative and self-conscious emotions. Emotion recognition in this sample of patients with AD was indistinguishable from controls. These findings underscore the utility of this approach to assessing emotion recognition and suggest that previous findings that recognition of positive emotion was preserved in dementia patients may have resulted from the limited sampling of positive emotion in traditional tests.
Keywords: empathy, emotion recognition, dementia, neurodegeneration, self-conscious emotion
The ability to identify others' emotions accurately (often referred to as empathic accuracy; Ickes, 1997) is critical for the formation and maintenance of interpersonal relationships. Being able to recognize the emotions of others provides invaluable clues as to their intentions and likely actions and provides a yardstick for evaluating the impact of one's own behavior on others. Across a broad spectrum of neurological and psychiatric functioning, emotion recognition ability has been found to be associated with successful interpersonal functioning and higher quality of life (Davis, 1983; Boice, 1983; Couture, Penn, & Roberts, 2006; Hooker & Park, 2002; Mueser, Doonan, Penn, Blanchard, Bellack, Nishith, et al., 1996; Mah, Arnold, & Grafman, 2004; Phillips, Scott, Henry, Mowat, & Bell, 2010). Emotion recognition relies on a complex neural network that includes circuitry related to sensation, perception, motor mimicry, interoception, and social judgment (Adolphs, 2002; Preston & de Waal, 2002). Not surprisingly, given this complexity, emotion recognition is vulnerable to a wide range of neurological and psychiatric disorders including behavioral variant frontotemporal dementia (bvFTD) (Keane, Calder, Hodges, & Young, 2002), AD (Cadieux & Greve, 1997), Hunting-ton's disease (S. A. Johnson et al., 2007), Parkinson's disease (Gray & Tickle-Degnen, 2010), traumatic brain injury (Radice-Neumann, Zupan, Babbage, & Willer, 2007), autism (Ashwin, Chapman, Colle, & Baron-Cohen, 2006), schizophrenia (Chan, Li, Cheung, & Gong, 2010), depression (Bediou, Saoud, Harmer, & Krolak-Salmon, 2009), and bipolar disorder (Rocca, Heuvel, Caetano, & Lafer, 2009).
Assessing Emotion Recognition Abilities
Investigations of emotion recognition have most often used photographs of emotional facial expressions and asked participants to identify the emotion expressed. These studies usually test recognition of a core set of “basic emotions” (Ekman, 1992), typically consisting of a group of negative emotions (e.g., anger, disgust, fear, sadness) and a single positive emotion (happiness). This approach has the advantage of being relatively easy to administer; however, it has several serious limitations in terms of ecological validity. First, real world emotions are dynamic, appearing and changing quickly (Ekman, 1992); in contrast, in the stimuli used for these studies, photographs are static and exposure times can be quite long (e.g., up to 30 s per image (Fernandez-Duque & Black, 2005). Second, judgments about emotion in the real world make use of multiple modes of information (visual, auditory, etc.) involving multiple bodily regions (e.g., face, posture, position); photographs used to assess emotion recognition only provide visual information and have typically been limited to the face. Third, real world emotion is typically embedded in richly interpersonal social contexts (Scherer, Matsumoto, Wallbott, & Kudoh, 1988); this contextual information is typically absent in photographs.
In addition to these threats to ecological validity, the typical photograph-based tasks have more subtle problems related to the breadth of emotions sampled that have not been widely discussed. These tasks typically include photographs of multiple negative emotions (e.g., fear, sadness, disgust, fear), but only a single positive emotion (happiness). Including multiple negative emotions but only one positive emotion makes recognition of specific negative emotions more difficult than positive emotions for purely statistical reasons. Simply stated, if participants recognize that a photograph is portraying a negative emotion, they still have to figure out which of several negative emotions is being expressed. In contrast, if they recognize that a photograph is depicting a positive emotion, then it must be happiness. For this reason, oft-reported findings that patients have more difficulty recognizing negative emotions than positive emotions (Fernandez-Duque & Black, 2005; Hargrave, Maddock, & Stone, 2002; Rosen, Perry, et al., 2002) may be an artifact of only having one positive emotion from which to choose. Finally, these tasks typically fail to sample the self-conscious emotions (e.g., embarrassment, pride, shame, guilt), which may be particularly important for effective social functioning (Keltner & Buswell, 1997).
Although the kinds of photograph recognition tasks described above are most common, other approaches have been utilized. For example, some studies have generated images of emotional facial expressions that are blends of two basic emotions (e.g., fear and surprise) or are low intensity versions of the full emotional expression (Blair & Cipolotti, 2000; Harmer, Perrett, Cowen, & Goodwin, 2001). Others have included photographs of self-conscious emotions along with basic emotions (e.g., Beer, Heerey, Keltner, Scabini, & Knight, 2003; Heerey, Keltner, & Capps, 2003). To assess modality effects, various studies have separately assessed recognition via emotional prosody and via emotional facial expressions (e.g., Drapeau, Gosselin, Gagnon, Peretz, & Lorrain, 2009; Perry et al., 2001). Recently, investigators have begun to use film stimuli in recognition studies, having participants watch brief films and identify the emotion depicted by the main character (Rankin et al., 2009; Werner et al., 2007). In several studies, participants tracked the fluctuating emotions of the main character in film clips (Goodkind et al., 2012; Zaki, Weber, Bolger, & Ochsner, 2009) or a spouse in a marital interaction (Levenson & Ruef, 1992; Sze, Goodkind, Gyurak, & Levenson, 2012) using a rating dial. Despite these encouraging developments, to our knowledge there have been no prior studies that utilized comprehensive assessment of multiple positive, negative, and self-conscious emotions using multimodal, dynamic, socially-embedded stimulus materials, such as those used in the present study.
Emotion Recognition in Neurodegenerative Disorders
As noted earlier, the assessment of emotion recognition is of great interest in a range of psychiatric and neurological disorders as well as in normal individuals. Our focus in the present study is to provide a preliminary evaluation of a new test of emotion recognition in a sample of dementia patients. Deficits in emotion recognition have been reported in diverse neurodegenerative conditions. One example is bvFTD, a subtype of frontotemporal dementia that leads to dramatic changes in emotional and interpersonal functioning. Although frontotemporal dementia has lower prevalence overall than Alzheimer's disease (AD), the two types of dementia have similar prevalence in early onset dementias that occur before the age of 65 (Ratnavalli, Brayne, Dawson, & Hodges, 2002). As bvFTD progresses, symptoms emerge that disrupt the person's ability to relate to others. These disruptions likely result in large part from patients with bvFTD showing deficits both in generating emotions (Sturm, Ascher, Miller, & Levenson, 2008; Sturm, Rosen, Allison, Miller, & Levenson, 2006) and in recognizing emotions in others (Goodkind et al., 2012; Kipps, Duggins, McCusker, & Calder, 2007).
In a recently proposed update to the diagnostic criteria for bvFTD, Raskovsky and colleagues note that loss of empathy is a core feature of the disease (Rascovsky et al., 2011). In contrast, the core features of AD are typically seen to involve deficits in cognitive function (e.g., memory, visuospatial processing, and executive functioning). Although deficits in emotional processing and interpersonal functioning in AD are certainly seen (Allender & Kaszniak, 1989; Bozeat, Gregory, Ralph, & Hodges, 2000; Cadieux & Greve, 1997), these functions are often spared early in the course of disease (Bozeat et al., 2000). Differences in the impact of these two diseases on emotion recognition would not be surprising, given that they target different large-scale neural networks (Seeley, Crawford, Zhou, Miller, & Greicius, 2009; Zhou et al., 2010).
Among studies of bvFTD, there is general consensus that emotion recognition is impaired (Diehl-Schmid et al., 2007; Fernandez-Duque & Black, 2005; Keane et al., 2002; Lavenu, Pasquier, Lebert, Petit, & Van der Linden, 1999; Lough et al., 2006; Rosen et al., 2004; Rosen, Perry, et al., 2002; Snowden et al., 2008); however, there are inconsistencies with respect to which types of emotion (e.g., positive or negative) and which specific emotions are most impacted. For example, among negative emotions, some studies find diffuse impairment in the recognition of fear, anger, sadness, and disgust (Guaita et al., 2009; Lavenu et al., 1999; Rosen, Perry, et al., 2002) while others find selective preservation in recognition of one or more of these emotions (Keane et al., 2002; Kessels et al., 2007). The research is similarly mixed for recognition of surprise, with some findings of impairment (Guaita et al., 2009; Kessels et al., 2007) and some of no impairment (Keane et al., 2002; Lavenu et al., 1999; Lough et al., 2006). In terms of positive emotions, most studies have found that patients with bvFTD show no deficit in recognizing happiness (Fernandez-Duque & Black, 2005; Kessels et al., 2007; Lavenu et al., 1999; Lough et al., 2006), but again, there are exceptions (Guaita et al., 2009; Keane et al., 2002). In one of the few studies using dynamic stimuli (film clips), patients with FTD had deficits in the recognition of fear and sadness but were unimpaired in the recognition of happiness (Werner et al., 2007).
For AD, studies of emotion recognition deficits have also yielded mixed results, with evidence of both impaired (Albert, Cohen, & Koff, 1991; Allender & Kaszniak, 1989; Cadieux & Greve, 1997; Shimokawa et al., 2003) and intact (Burnham & Hogervorst, 2004; Lavenu et al., 1999) recognition overall. Several studies have found that disease severity or stimuli difficulty may account for differences between patients with AD and control participants (Spoletini et al., 2008). When considering specific emotions, the findings are also inconsistent. While some report deficits in recognizing disgust, anger, sadness, fear, and happiness (Cadieux & Greve, 1997; Drapeau et al., 2009; Hargrave et al., 2002), others report intact recognition for select emotions, such as disgust (Henry et al., 2008), anger (Drapeau et al., 2009; Weiss et al., 2008), surprise (Drapeau et al., 2009), and happiness (Burnham & Hogervorst, 2004; Lavenu et al., 1999; Weiss et al., 2008). It is important to consider the stage of the disease when comparing patients with different neurodegenerative disorders. Over time and in the severe stages, neurodegenerative disorders tend to converge and look more similar. The current study includes patients in the relatively early stages of bvFTD and AD.
The Current Study
We examined emotion recognition in patients with bvFTD and AD and in normal controls. We provided a preliminary test of a new assessment task that we designed that increases ecological validity and expands the scope of emotion recognition to include multiple positive and self-conscious emotions in addition to multiple negative emotions. In this task, participants were asked to identify the emotion experienced by target characters in brief (30 s) film clips. These stimuli were dynamic, multimodal (visual and auditory information), and the emotions occurred in socially embedded contexts. The set of 11 clips depicted four negative emotions (anger, fear, disgust, and sadness), four positive emotions (affection, amusement, calm, and enthusiasm), and three self-conscious emotions (embarrassment, pride, and shame). Based on previous research (Keane et al., 2002; Lavenu et al., 1999), we hypothesized patients with bvFTD would show impairment in ability to recognize negative emotions. Our inclusion of self-conscious emotions and a full range of positive emotions enabled us to test the hypothesis that this impairment would extend to these other types of emotions as well. Given the generally intact social behavior often reported for patients in the early stages of AD (Bozeat et al., 2000), we did not expect similarly profound deficits in emotion recognition for patients with AD.
Method
Participants
Patients diagnosed with bvFTD (N = 24) and AD (N = 23) were recruited by the Memory and Aging Center in the Department of Neurology at the University of California, San Francisco for participation in an ongoing program of collaborative research. Patients underwent neurological, neuropsychological, and neuro-imaging testing and were diagnosed using standard criteria for bvFTD (Neary et al., 1998) and AD (McKhann et al., 1984); all patients were in the relatively early stages of the disease. Diagnoses of bvFTD or AD were not given if symptoms were thought to be due to another cause (e.g., alcohol, schizophrenia). Neurologically normal controls (N = 24) were recruited through newspaper ads and confirmed to have no neurological or psychiatric conditions. All participants were given the Mini-Mental State Examination (MMSE; Folstein, Folstein, & McHugh, 1975) to assess their cognitive status.
Emotional Films
We have utilized emotional films extensively in prior studies of emotion generation and emotion recognition in both patient and normal populations (Gross & Levenson, 1995; Werner et al., 2007) and thus have a large library of these films. Drawing from this library and expanding it with a number of additional films, we embarked on the process of selecting a set of films to assess ability to recognize negative, positive, and self-conscious emotions. Starting with approximately 75 candidate film clips, we selected the final set of 11 films based on their emotional structure (the character experienced only one of the emotions of interest), complexity (thematically simple enough for use with dementia patients) and pilot data (ratings from undergraduates confirmed the target emotion). These films have not yet been compared with traditional measures of emotion recognition (i.e., static emotional facial expressions).
Below, we describe the content of the 11 film clips. Interested researchers may contact the corresponding author to obtain more detailed editing information necessary to create the individual clips.
Negative Emotions
Anger
The anger clip is a scene from the movie, “Best In Show,” depicting a woman (target character) frantically searching her hotel room for a lost item, yelling at the hotel manager and maid the entire time, blaming them for misplacing her item.
Disgust
The disgust clip is a scene from the movie, “Indiana Jones and the Temple of Doom,” depicting a dinner party. As one of the dishes, a plate of large beetlelike insects, is presented to a woman (target character), she watches others eating the insects with a disturbed expression, and asks the person next to her if she can borrow his hat so that she might “puke” in it.
Fear
The fear clip is a scene from the movie, “Pirates of the Caribbean,” depicting a woman (target character) running away from a pirate ghost, getting caught, and screaming several times during the chase.
Sadness
The sadness clip is a scene from the movie, “Playing by Heart,” depicting two women sitting on a bench at a cemetery. Both women are crying as one woman (target character) tells the other how much she will miss the man whose funeral they had just attended.
Positive Emotions
Affection
The affection clip is a scene from the movie, “High Fidelity,” depicting a man (target character) embracing a woman, while dancing with her to music, smiling, and kissing her on the cheek.
Amusement
The amusement clip is a scene from the movie, “Patch Adams,” depicting a doctor entertaining a child patient by wearing a clown nose and doing tricks. The child (target character) smiles and laughs in response to the doctor.
Calmness
The calmness clip is a scene from the movie, “The Graduate,” depicting a man (target character) lounging on a float in a swimming pool, wearing sunglasses, smiling, and drinking a beer as pleasant music plays in the background.
Enthusiasm
The enthusiasm clip is a scene from the movie, “My Best Friend's Wedding,” depicting a woman (target character) greeting two friends, hugging them, and saying how excited she is to meet them in a high, giggly voice.
Self-Conscious Emotions
Embarrassment
The embarrassment clip is a scene from the movie, “The Princess Diaries,” depicting a teenage girl (target character) seated in a classroom full of students. Another girl interrupts the lesson to point out that the target character is wearing a hat in class, violating the dress code. The teacher instructs the target character to take her hat off. As she does so, the other students laugh at her and tease her as she shrinks in her seat.
Shame
The shame clip is a scene from the movie, “Bend It Like Beckham,” depicting a girl (target character) wearing a soccer uniform sitting on a bench next to the soccer field talking to her coach, telling him how she got a scar on her leg as she tries to cover and hide the scar, averting his gaze.
Pride
The pride clip is a scene from the movie, “The Karate Kid,” depicting the end of a karate competition. The winner is being held up on his teammates' shoulders as they all cheer and a man (target character) watches and nods, with a slight smile, while uplifting music plays in the background.
Procedures
The assessment of emotion recognition was conducted at the Berkeley Psychophysiology Laboratory at the University of California, Berkeley (UCB) as part of a larger study of emotional functioning in dementia. Upon arrival, each participant signed a consent form (approved by the Committee for the Protection of Human Subjects at UCB) that described the experimental tasks and an experimenter also explained the procedure. During the emotion recognition task, participants were seated in a comfortable chair in a well-lit, 3 m × 6 m room. Films were presented on a 21-in. TV monitor placed directly in front of the participants. Participants were videotaped throughout the experimental tasks using a remote-controlled, high-resolution video camera that was partially concealed from view. Physiological measures (e.g., heart rate, skin conductance) were also obtained, but these were not used in the current study.
Participants viewed the 11 film clips in a fixed order (affection, amusement, anger, calmness, embarrassment, enthusiasm, disgust, fear, pride, sadness, shame). Each film clip was preceded by a 30 s baseline period, during which a black “X” was presented in the middle of a white screen and participants were instructed to relax and watch the X. Each clip began after the baseline fixation. Each stimulus clip was 37 s in length, for a total of 67 s of viewing time, including the baseline.
After viewing each film clip, participants were shown an image of the target character displaying a neutral expression. This was intended to cue memory of the content of the film and the identity of the target character without cuing the target's emotion. A list of 11 emotions (affection, amusement, anger, calmness, embarrassment, enthusiasm, disgust, fear, pride, sadness, shame) was also presented and the participant was asked to indicate verbally the emotion the target character felt most strongly. Participants were given as much time as they needed to respond and their answers were recorded by the experimenter. Participants' memory of the film clip was then assessed using a single multiple-choice question about the film content (e.g., “What happened in the film? A) A woman sees her friends. B) A woman talks on the phone. C) A woman is in a store.”). This content question was included to assess for memory deficits that could negatively impact emotion recognition scores.
Data Reduction
A total emotion recognition score was calculated by summing correct answers across all films and dividing by the total number of films (11). Similar proportion correct scores were computed for the four negative (anger, disgust, fear, and sadness), four positive (affection, amusement, calmness, and enthusiasm), and three self-conscious emotion films (embarrassment, pride, and shame). A total memory score was calculated by summing the number of correct responses to the memory questions and dividing by the total number of films (11). The Cronbach's alpha reliability coefficients for the emotion recognition types were: positive emotions .63, negative emotions .72, and self-conscious emotions .65.
Results
Demographic Information
Table 1 shows demographic information and statistics. An analysis of variance revealed significant age differences between the three groups, F(2, 68) = 6.18, p = .003; post hoc analyses showed controls were older than patients with AD. A chi-square analysis revealed that the three groups differed in terms of distribution of sex (χ2(2, N = 74) = 14.33, p < .01); there was a larger proportion of males in the bvFTD group than in the AD or control groups. As expected, there were significant differences in MMSE scores between the three groups, F(2, 68) = 18.71, p < .001; post hoc analyses revealed that patients with AD and patients with bvFTD had lower scores than control participants (ps < .02), and that patients with AD had lower scores than patients with bvFTD (p < 01). Based on these findings, age and MMSE were included as covariates and sex was included as a between-subjects factor in all analyses.
Table 1. Participant Demographic Data.
| Normal control participants (n = 24) | AD (n = 23) | bvFTD (n = 24) | Test statistics | |
|---|---|---|---|---|
| Age (SD) | 67.51a (6.13) | 60.65b (8.69) | 64.36b (4.76) | F(2, 68) = 6.18, p < .01 |
| Males | 7a | 12a | 20b | χ2(2, N = 71) = 14.33, p < .01 |
| CDR (SD) | 0.00a (0.00) | .85b (.24) | 1.00b (.63) | F(2, 68) = 46.42, p < .01 |
| MMSE | 29.33a (1.13) | 22.04c (5.09) | 25.96b (4.82) | F(2, 68) = 18.71, p < .01 |
| Film memory | .98 (.04) | .94 (.14) | .94 (.12) | F(2, 68) = 1.23, p > .05 |
Note. Means (standard deviations) are reported. Groups with different subscripts differed from each other at p < .05. bvFTD = behavioral variant frontotemporal dementia; AD = Alzheimer's disease; MMSE = Mini-Mental State Examination (out of 30 points); CDR = clinical dementia rating (… range, with 0 indicating no impairment).
Memory Questions
An analysis of variance revealed no main effect of diagnosis on the memory questions, F(2, 68) = 1.23, p = .30. We interpreted this as indicating that we had been successful in choosing films that were comprehensible to both dementia patients and controls and providing some indirect indication that the groups did not differ greatly in hearing or vision.
Emotion Recognition
Emotion recognition was assessed using a 3 (diagnosis: bvFTD, AD, CTL) × 2 (sex: male, female) × 3 (film type: negative, positive, self-conscious) repeated measures analysis of covariance (ANCOVA). In this analysis, diagnosis and sex were between-subjects factors and film type was a within-subject factor; age and MMSE were the covariates. For emotion recognition, percent-correct scores for each film type were used because the number of positive, negative, and self-conscious films was not equal. Partial eta squared ( ) statistics representing the portion of explained variance in the dependent variable are reported for all ANCOVA effects, with .01–.05 representing a small effect, .06–.13 representing a medium effect, and .14 or greater representing a large effect (Cohen, 1992). Significant main effects were examined using least significant difference tests (adjusted for multiple comparisons); significant interactions were followed up using separate one-way general linear models (GLMs) for each type of film (Table 2).
Table 2. Emotion Recognition Scores Collapsed Over Types of Films Reported by Group.
| Normal control participants (n = 24) | AD (n = 23) | bvFTD (n = 24) | Test statistics | |
|---|---|---|---|---|
| Total emotion recognition | .91 (.06)a | .86 (.05)a | .54 (.05)b | F(2, 63) = 18.03, p < .001 |
| Positive emotion recognition | .93 (.06)a | .78 (.06) | .64 (.06)b | F(2, 63) = 5.65, p < .01 |
| Negative emotion recognition | .88 (.05)a | .95 (.05)a | .52 (.06)b | F(2, 63) = 20.34, p < .001 |
| Self-conscious emotion recognition | .92 (.07)a | .84 (.07)a | .45 (.07)b | F(2, 63) = 13.58, p < .001 |
Note. Means (standard errors) reported are estimated marginal means, corrected for participant age, sex, and MMSE. Groups with different subscripts differed from each other at p < .05. bvFTD = behavioral variant frontotemporal dementia; AD = Alzheimer's disease.
Preliminary analysis: Cognitive effects
In dementia samples, patients typically differ in cognitive capacity (both within and across diagnoses). Thus, in a preliminary analysis, we explored the relationship between cognitive capacity (as measured by the MMSE) and emotion recognition in the patients with bvFTD or AD. We found a significant relationship between MMSE and negative emotion recognition (N = 47, r = .33, p = .03), with greater cognitive capacity associated with greater emotion recognition. A similar relationship was observed for positive emotion, but it did not reach statistical significance (N = 47, r = .26, p = .08). The relationship between MMSE and self-conscious emotion recognition was not significant (N = 47, r = .09, p = .57). As noted earlier, MMSE was included as a covariate in all other analyses.
Diagnostic group effects
The main effect for diagnostic group was significant (F(2, 63) = 18.03, p < .001, ). Follow-up tests revealed that patients with bvFTD performed worse than patients with AD and control participants (p's<.01), but that patients with AD did not differ from control participants (p = .51).
Importantly, the Film Type × Diagnostic Group interaction was also significant (F(4, 126) = 3.70, p = .01, ). This interaction was decomposed using individual GLMs (controlling for age and MMSE) for positive, negative, and self-conscious films. For positive films, there was a significant effect of diagnostic group (F(2, 63) = 5.65, p < .01, ). Follow-up analyses revealed that patients with bvFTD performed significantly worse than control participants (p = .01) but not significantly worse than patients with AD (p = .09); control participants and patients with AD did not differ from each other (p = .12). For negative films, there was a main effect of diagnostic group (F(2, 63) = 20.34, p < .001, ). Patients with bvFTD were significantly worse than both control participants and patients with AD (ps < .001); control participants and patients with AD did not differ from one another. For self-conscious films, there was a main effect of diagnostic group (F(2, 63) = 13.58, p < .001, ). Patients with bvFTD were significantly worse than both control participants and patients with AD (ps < .001); control participants and patients with AD did not differ from one another (p = .47).
To summarize, patients with bvFTD had clear deficits in emotion recognition in positive, negative, and self-conscious films, with lower recognition scores than controls. In contrast, patients with AD showed no deficits in emotion recognition for any of the three kinds of films; their scores did not differ from controls. Comparing the two patient groups, patients with bvFTD had significantly lower recognition scores than patients with AD for negative and self-conscious emotions but not for positive emotions.
Sex differences
To explore possible sex differences in emotion recognition, we examined the main effect for sex, the Sex × Film interaction, and the Sex × Diagnosis × Film Type interaction. The main effect of sex was not statistically significant (F < 1) nor was the Sex × Diagnosis × Film Type (F(4, 126) = 2.28, p = .06, . The Sex × Film interaction was significant (F(2, 126) = 3.14, p = .047, ). When we decomposed this interaction using individual GLMs for positive, negative, and self-conscious films (controlling for age and MMSE), none of these follow-up analyses were significant. Thus within the limits of our small samples, and with the predominance of male bvFTD participants, we found no evidence for sex differences in emotion recognition for the positive, negative, or self-conscious films.
Film type effects
The main effect for film type did not reach significance, F(2, 126) = 2.92, p = .06, . However, because this effect approached significance, we conducted follow-up analyses examining differences between film types. Using pairwise comparisons, none of these differences between film types were significant (all ps > .10).
Exploratory analysis: Individual films
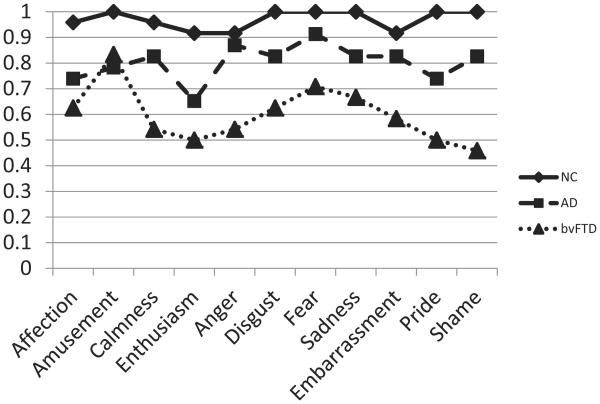
In the prior analyses, emotion recognition scores were aggregated into their a priori designations of positive, negative, and self-conscious film types. We also performed an exploratory 3 × 2 × 11 (Diagnosis × Sex × Film) ANCOVA with scores on each film entered individually and treated as a within-subject factor. In this analysis, there was a main effect of film (F(10, 630) = 2.03, p = .03, ), a significant interaction of diagnostic group by film (F(20, 630) = 1.92, p = .01, ) and a significant Diagnostic Group × Sex × Film interaction (F(20, 630) = 1.70, p = .03, ). We decomposed the significant film by diagnosis interaction by conducting individual GLM comparing the three diagnostic groups for each film. As indicated in Table 3, patients with bvFTD were worse than control participants on all four negative films (anger, disgust, fear, sadness), two positive films (calmness, enthusiasm, but not affection and amusement), and two self-conscious films (pride, shame, but not embarrassment). Patients with AD were impaired compared to control participants on just one film (enthusiasm) (see Figure 1).
Table 3. Exploratory Analysis (Repeated Measures) Examining Across the 11 Individual Films Without Consideration of Categories of Emotion Film Type.
| Film | Test statistic | Group differences | Effect size ( ) |
|---|---|---|---|
| Positive films | |||
| Affection | F(2, 63) = .97, p > .05 | .03 | |
| Amusement | F(2, 63) = .80, p > .05 | .03 | |
| Calmness | F(2, 63) = 4.22, p < .02 | bvFTD < AD, NC | .12 |
| Enthusiasm | F(2, 63) = 4.88, p < .02 | bvFTD, AD < NC | .13 |
| Negative films | |||
| Fear | F(2, 63) = 20.45, p < .001 | bvFTD < NC < AD | .39 |
| Anger | F(2, 63) = 7.10, p < .01 | bvFTD < AD, NC | .18 |
| Disgust | F(2, 63) = 4.27, p < .02 | bvFTD < AD, NC | .12 |
| Sadness | F(2, 63) = 9.65, p < .001 | bvFTD < AD, NC | .23 |
| Self-conscious films | |||
| Embarrassment | F(2, 63) = 1.35, p > .05 | .04 | |
| Pride | F(2, 63) = 12.06, p < .001 | bvFTD < AD, NC | .28 |
| Shame | F(2, 63) = 10.97, p < .001 | bvFTD < AD, NC | .26 |
Note. Effect sizes are reported as partial eta squared, with .01–.05 representing a small effect, .06–.13 representing a medium effect, and larger than .14 representing a large effect. bvFTD = behavioral variant frontotemporal dementia (n = 24); AD = Alzheimer's disease (n = 23); NC = normal control participants (n = 24).
Figure 1.
Uncorrected means for each of the 11 individual films by diagnostic group. NC = normal control participant; AD = Alzheimer's disease; bvFTD = behavioral variant frontotemporal dementia.
Relationship with traditional photo-based emotion recognition task
To evaluate the relationship between our new assessment task and more traditional tasks for assessing emotion recognition, a subset of participants in the current study (N = 48) completed an abbreviated version of the Comprehensive Affect Testing System (CATS; Froming, Weiner, Gregory, Levy, & Ekman, 2006) Affect Naming subtest. In this task, participants are shown a photograph of an emotionally expressive or neutral face and asked to identify which of seven basic emotions (happy, sad, anxious, angry, surprised, disgusted, or neutral) the person is feeling. The CATS total score correlated significantly (all ps < .05) with the total emotion recognition score (r = .78), positive emotion recognition score (r = .49), negative emotion recognition score (r = .72), and self-conscious emotion recognition score (r = .71).
Discussion
In this paper, we report results from a preliminary test of a new method for assessing emotion recognition in individuals with dementia. This method improves the ecological validity of stimuli typically used to assess emotion recognition and expands the scope of emotions assessed to include multiple positive and self-conscious emotions in addition to multiple negative emotions. Whereas prior tests had used static visual stimuli and were limited in the emotions tested (typically testing multiple negative emotions, but only one positive emotion [happiness], and no self-conscious emotions), our task used film stimuli in which the emotional information was dynamic, multimodal, and socially embedded and which assessed four negative, four positive, and three self-conscious emotions. We also compared the results from our new assessment task with those from a traditional measure of emotion recognition that uses static images.
Using our new assessment task with patients with two different types of dementia (bvFTD and AD) and normal controls, we found that patients with bvFTD were broadly impaired in emotion recognition, with lower recognition than controls for negative, positive, and self-conscious emotion regardless of whether emotions were examined by the three emotion types or by the 11 individual emotion films. In contrast, emotion recognition in patients with AD was relatively intact, with no deficits compared to controls in an analysis conducted by emotion type and only a deficit in recognizing enthusiasm in an analysis of individual films. Importantly, we found only limited support for prior findings (based on tests that sampled only a single positive emotion) that recognition of positive emotions was relatively preserved in these populations (e.g., Fernandez-Duque & Black, 2005).
Emotion Recognition Deficits in bvFTD
Negative emotions
Consistent with previous emotion recognition research using static images of emotional facial expressions (Fernandez-Duque & Black, 2005; Lavenu et al., 1999; Rosen et al., 2004) we found that patients with bvFTD had marked difficulties recognizing negative emotions. These difficulties were substantial and pervasive; effect sizes were large and deficits were found for all four negative emotions that were sampled. Because patients with AD did not show these deficits and all patient groups did well on our memory questions, it is not likely that this deficit in patients with bvFTD resulted from our stimuli being overly complex. Recognizing negative emotions in others is critical for successful social interactions. For example, assume a patient's behavior makes a family member angry. If this anger is recognized correctly, it might motivate the patient to change their behavior. If the anger is not recognized correctly, reparative action would be less likely. Poor recognition of emotions such as fear and sadness, which often signal distress and need to others, may be interpreted as a lack of empathy and concern in the patients. Consistent with this, caregivers often describe patients with bvFTD as showing a lack of emotional concern for others (Eslinger, Moore, Anderson, & Grossman, 2011) and as being cold-hearted (Rankin, Kramer, & Miller, 2005).
Positive emotions: Evidence against preserved recognition in bvFTD
Using film stimuli and sampling an expanded set of four positive emotions, we found that patients with bvFTD were also impaired in their ability to recognize positive emotions. Prior studies using static images and sampling a single positive emotion had typically come to a quite different conclusion, namely, that positive emotion recognition was preserved in bvFTD (Fernandez-Duque & Black, 2005). Why the differences in findings? As explained earlier, in the typical emotion recognition task, including only one positive emotional stimulus and multiple negative emotional stimuli can bias results toward not finding deficits in positive emotions because the identification task is easier when there are fewer choices. This bias is increased by the fact that positive emotions share a common signal, the smile. Thus, a participant need only detect this single expressive feature of a facial expression and connect it to positive emotion. Our task was not plagued by this problem because we sampled the same number of positive and negative emotions. Thus, even when a smile is detected, there was additional processing needed to recognize the associated positive emotion.
We believe that the deficits in recognizing positive emotions that patients with bvFTD showed using our task are quite consistent with caregiver reports and our own observations based on working with these patients. For example, partners' expressions of amusement and affection are often unreciprocated. As was the case with the negative emotions, failure to recognize positive emotions can have quite profound social consequences. Positive emotions expressed by others communicate information as to which behaviors are desired and appreciated. Failure to recognize these emotions can lead to more troublesome behaviors and fewer constructive behaviors. Many positive emotions serve to “build” social connections (Fredrickson, 1998). Failure to recognize and respond to positive emotions such as affection can contribute further to patients with bvFTD coming across as being cold and distant.
Positive emotions: A caveat
Although our findings challenge earlier findings that suggest that positive emotion recognition is relatively preserved in bvFTD, several aspects of our findings suggest that the deficits in positive emotion may not be quite as profound as those in negative and self-conscious emotions. First, although emotion recognition in patients with bvFTD was worse than controls for positive, negative, and self-conscious emotions, it was only worse for patients with bvFTD compared to patients with AD for negative and self-conscious emotions; moreover, the effect sizes for positive emotions were smaller than those for negative or self-conscious films. Second, when examining individual films, performance of patients with bvFTD was worse than controls for only two of the four positive films (for calmness and enthusiasm, but not for affection and amusement). Although each of these caveats could be explained in other ways (e.g., findings driven by positive emotion recognition abilities of patients with AD or differences in difficulty of particular films), together they suggest some caution in completely dismissing the notion that positive emotion recognition is preserved in bvFTD.
Self-conscious emotions
The ability to recognize self-conscious emotions such as embarrassment, shame, and pride has not been studied previously in dementia patients. Using our new task, we found patients with bvFTD had clear deficits in recognizing these emotions. In prior studies we also found dramatic impairment in these patients in the ability to generate self-conscious emotions (Sturm et al., 2008, 2006). Thus, self-conscious emotions may be an area of emotional functioning that is profoundly altered in bvFTD. We believe that deficits in self-conscious emotion processing are particularly important for understanding some of the problems patients with bvFTD display in the social realm. Self-conscious emotions are inherently social (i.e., they involve comparisons of one's own behavior against social norms) and regulatory (i.e., they signal the need to engage in corrective actions to adjust behavior so that it complies with social norms; Keltner & Buswell, 1997). Deficits in recognizing (and producing) these emotions can have dramatic consequences. For example, if our behavior causes other people to be embarrassed, their emotional response should be a powerful signal for us to stop behaving in that way. Conversely, another person's pride in response to something we do suggests that this behavior is socially valued and should be continued. Difficulties in recognizing self-conscious emotions can result in behaviors that are mismatched to the needs of others and that can create serious problems for maintaining important social relationships.
Intact Emotion Recognition in AD
Across all three kinds of emotions that we measured, emotion recognition in patients with AD was not significantly impaired. Thus, at least at the relatively early disease stages that we are studying, it appears that the ability to recognize emotions in others is relatively spared. This finding is consistent with clinical observations that suggest that emotional functioning remains relatively intact in the early stages of AD (Bozeat et al., 2000). These patients often maintain the ability to manage successful social interactions even in the face of memory and other cognitive problems. Other research from our laboratory also suggests relative preservation of emotional functioning in AD, including findings of greater emotional impairment in bvFTD than in AD (Ascher et al., 2010; Goodkind, Gyurak, McCarthy, Miller, & Levenson, 2010). Finally, we should note that these results are consistent with the different patterns of neurodegeneration typically found in bvFTD and AD, with bvFTD targeting circuits that are critical for social and emotional functioning (e.g., insular, frontopolar, and anterior cingulate regions) that are often spared in the early stages of AD (Rosen, Gorno-Tempini, et al., 2002; Seeley et al., 2009).
Sex Differences in Emotion Recognition
In the current study, the distribution of males and females varied as a function of diagnostic group. The predominance of males in the bvFTD group is consistent with other studies reporting demographic characteristics of this disease and may reflect a greater vulnerability in males (Johnson et al., 2005; Ratnavalli et al., 2002). Because of these differences, sex was included as a factor in all analyses. We did not find an overall difference in emotion recognition between males and females. There was a significant Sex × Film Type interaction; however, the sex differences did not reach significance in the positive, negative, or self-conscious films.
Sex differences in overall emotional expressivity have been widely reported (e.g., Hall, 1990). In realms related to emotion recognition, however, sex differences appear to be more nuanced. For example, women generally self-report higher levels of empathy (Eisenberg & Lennon, 1983) and show greater amygdalar, subcortical, and medial prefrontal reactivity to negative emotional stimuli (Stevens & Hamann, 2012). However, in studies assessing emotion recognition using objective criteria (e.g., the criteria used in the present study), we and others have generally not found differences between men and women (Eisenberg & Lennon, 1983; Levenson & Ruef, 1992; Russell, Tchanturia, Rahman, & Schmidt, 2007; Soto & Levenson, 2009). In such studies, superior performance by women may require priming participants to feel sympathy for the target (Klein & Hodges, 2001) or emphasizing performance speed (Hampson, van Anders, & Mullin, 2006). Examining in greater detail the effect of sex for emotion recognition in neurodegenerative disease is an important avenue for future studies.
Cognitive Functioning and Emotion Recognition
Successfully identifying others' emotions under real-world conditions draws on a rich set of processes, including many that would typically be termed “cognitive” (e.g., maintaining and shifting attention, scanning and detecting details, and updating information concerning the other person's current state). This is reflected in prior findings of associations between deficits in emotion recognition and deficits in cognitive performance in normal, psychiatric, and neurological populations (Bryson, Bell, & Lysaker, 1997; Grattan, Bloomer, Archambault, & Eslinger, 1994; Mah, Arnold, & Grafman, 2004; Mathersul et al., 2008; Rankin et al., 2005; Shamay-Tsoory, Tomer, Berger, & Aharon-Peretz, 2003). In our exploratory analyses of the relationship between emotion recognition and overall cognitive functioning (measured via the MMSE), we found that lower MMSE scores were related to a lower ability to recognize negative emotions but not to recognition of positive or self-conscious emotions. The design of our study did not allow for additional exploration of this finding; however, it does suggest that future research should consider whether there are differences in the cognitive processing needed to identify different emotions.
Implications for Studies of Emotion Recognition in Other Patient Groups
In addition to expanding our understanding of emotion recognition abilities and deficits in two neurodegenerative diseases, we believe this study has implications for expanding assessment of emotion recognition in other psychiatric and neurological populations. A pattern similar to that previously found in bvFTD of impaired negative and intact positive emotion recognition has been reported in several other patient groups. For example, patients with schizophrenia and unaffected family members showed deficits in recognizing negative emotions but were unimpaired in recognition of positive emotion (Leppänen et al., 2008; Namiki et al., 2007). Similar findings in autism spectrum disorders have also been presented (Ashwin et al., 2006). In a meta-analysis of emotion recognition studies in Parkinson's disease, effect sizes were much larger for impairments in recognizing anger, disgust, fear, and sadness than for happiness (Gray & Tickle-Degnen, 2010). Importantly, all of these conclusions were based on emotion recognition tasks that used static images and that sampled many more negative than positive emotions. Before accepting that this pattern of deficits in negative emotion recognition and preservation of positive emotion recognition truly characterizes these disorders, it will be important to repeat these studies using the kind of emotion recognition assessment utilized in the present study (i.e., using more ecologically valid stimulus materials and assessing recognition for similar numbers of positive and negative emotions).
In addition, studies of emotion recognition in other populations could benefit from assessment of ability to recognize self-conscious emotions. Although rarely studied, prior attempts have been promising. Children with autism were found to perform more poorly than comparison children at identifying self-conscious emotions (embarrassment, shame) but were unimpaired at recognizing other emotions such as anger, contempt, disgust, fear, happiness, sadness, and surprise (Heerey et al., 2003). Similarly, patients with orbitofrontal cortex damage were worse than control participants at identifying self-conscious but not other emotions (Beer et al., 2003). Given the important role that recognition of self-conscious emotions plays in supporting successful social behavior (see earlier discussion), this is clearly an area that would benefit from future study.
Strengths and Limitations
In this study we evaluated a new test for assessing emotion recognition based on film stimuli that have greater ecological validity (dynamic, multimodal, socially embedded stimuli) and allows for broader sampling of emotions than has been the case with traditional approaches using images of facial expressions (static, single-modality, removed from social context). Using this test, we found deficits in emotion recognition in patients with bvFTD that go beyond the problems with negative emotion reported previously to include problems with both positive and self-conscious emotions. These findings suggest that deficits in emotion recognition in bvFTD may be even more pervasive than previously thought and shed additional light on some of the oft-observed problems these patients have in navigating their social worlds.
This study can be viewed as an initial “proof of concept” for assessing emotion recognition across positive, negative, and self-conscious emotions using film stimuli. A subset of participants in the present study were also assessed with a more traditional test of emotion recognition using static photographs of emotional faces. The correlations for overall scores between the two tests were .78, or approximately 60% shared variance. This indicates a reasonable level of concurrent validity for the new test, but also indicates that there is ample unshared variance for differences between the tests to emerge. Importantly, the correlations for positive emotion scores (an area where our new test assesses an expanded array of positive emotions) were lower (correlations of .49, or approximately 25% shared variance), which would be consistent with there being differences in how the two kinds of tests assess positive emotion recognition.
Further development and testing of our new task using larger and more diverse samples is clearly warranted. This should include: (a) expanding the test set with multiple stimuli for the different emotions, (b) carrying out more extensive psychometric testing, (c) evaluating order of presentation effects, and (d) evaluating the effects of prior experience with the particular films used. In addition, the intriguing findings using the MMSE support the value of exploring the influence of more specific cognitive abilities on emotion recognition, similar to our previous work studying specific cognitive correlates of emotion regulation ability (Gyurak, Goodkind, Kramer, Miller, & Levenson, 2012; Gyurak et al., 2009;).
Summary
The current study provides a preliminary evaluation of a new test for assessing emotion recognition using film stimuli that has greater ecological validity and samples a wider range of emotions than previous tests using static photographic images. Using this test with a sample of patients with two kinds of dementia and with normal controls, we found clear impairment in recognition of negative, positive, and self-conscious emotions in patients with bvFTD compared with patients with AD and controls. BvFTD is a disease that negatively impacts socioemotional functioning, creating profound social and interpersonal difficulties for families and caregivers (Ascher et al., 2010; de Vugt et al., 2006). The ability to recognize the emotions of others is critical for successful interpersonal interactions and for maintaining intimate relationships. We believe that these findings underscore the contribution that deficits in ability to recognize negative, positive, and self-conscious emotions play in bvFTD. Patients who cannot recognize others' emotions will be unable to use these rich sources of social information as a guide for helping monitor, regulate, and correct their own behavior. In contrast, relative preservation of these abilities in the early stages of AD suggests an area of preserved functioning that can be leveraged in ways that can improve interactions with loved ones and preserve general quality of life as long as possible in these patients and their families. We expect that this approach will be useful with a broad range of neurological and psychiatric patient groups to help characterize areas of impaired and preserved functioning in the realm of emotion recognition.
Acknowledgments
This work was supported by grants from the National Institute on Aging Grants AG017766 and AG019724; National Institute of Mental Health Grant MH020006; the State of California Alzheimer's disease Research Center of California Grant 03-75271; and the Hellman Family Center. These sponsors had no involvement in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
The authors report no conflict of interest.
Contributor Information
Madeleine S. Goodkind, Mental Illness Research, Education, and Clinical Center, Veterans Affairs Palo Alto Health Care System, Palo Alto, California and Department of Psychiatry and Behavioral Science, Stanford University School of Medicine
Virginia E. Sturm, Memory and Aging Center, University of California, San Francisco
Elizabeth A. Ascher, Department of Psychology, University of California, Berkeley
Suzanne M. Shdo, Memory and Aging Center, University of California, San Francisco
Bruce L. Miller, Memory and Aging Center, University of California, San Francisco
Katherine P. Rankin, Memory and Aging Center, University of California, San Francisco
Robert W. Levenson, Department of Psychology, University of California, Berkeley
References
- Adolphs R. Neural systems for recognizing emotion. Current Opinion in Neurobiology. 2002;12:169–177. doi: 10.1016/s0959-4388(02)00301-x. http://dx.doi.org/10.1016/S0959-4388(02)00301-X. [DOI] [PubMed] [Google Scholar]
- Albert MS, Cohen C, Koff E. Perception of affect in patients with dementia of the Alzheimer type. Archives of Neurology. 1991;48:791–795. doi: 10.1001/archneur.1991.00530200027013. http://dx.doi.org/10.1001/archneur.1991.00530200027013. [DOI] [PubMed] [Google Scholar]
- Allender J, Kaszniak AW. Processing of emotional cues in patients with dementia of the Alzheimer's type. The International Journal of Neuroscience. 1989;46:147–155. doi: 10.3109/00207458908986252. http://dx.doi.org/10.3109/00207458908986252. [DOI] [PubMed] [Google Scholar]
- Ascher EA, Sturm VE, Seider BH, Holley SR, Miller BL, Levenson RW. Relationship satisfaction and emotional language in frontotemporal dementia and Alzheimer disease patients and spousal caregivers. Alzheimer Disease and Associated Disorders. 2010;24:49–55. doi: 10.1097/WAD.0b013e3181bd66a3. http://dx.doi.org/10.1097/WAD.0b013e3181bd66a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwin C, Chapman E, Colle L, Baron-Cohen S. Impaired recognition of negative basic emotions in autism: A test of the amygdala theory. Social Neuroscience. 2006;1:349–363. doi: 10.1080/17470910601040772. http://dx.doi.org/10.1080/17470910601040772. [DOI] [PubMed] [Google Scholar]
- Bediou B, Saoud M, Harmer C, Krolak-Salmon P. L'analyse des visages dans la dépression [Analyzing facial expressions in depression] L'Évolution Psychiatrique. 2009;74:79–91. http://dx.doi.org/10.1016/j.evopsy.2008.12.015. [Google Scholar]
- Beer JS, Heerey EA, Keltner D, Scabini D, Knight RT. The regulatory function of self-conscious emotion: Insights from patients with orbitofrontal damage. Journal of Personality and Social Psychology. 2003;85:594–604. doi: 10.1037/0022-3514.85.4.594. http://dx.doi.org/10.1037/0022-3514.85.4.594. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Cipolotti L. Impaired social response reversal. A case of ‘acquired sociopathy’. Brain: A Journal of Neurology. 2000;123:1122–1141. doi: 10.1093/brain/123.6.1122. http://dx.doi.org/10.1093/brain/123.6.1122. [DOI] [PubMed] [Google Scholar]
- Boice R. Observational skills. Psychological Bulletin. 1983;93:3–29. [PubMed] [Google Scholar]
- Bozeat S, Gregory CA, Ralph MA, Hodges JR. Which neuropsychiatric and behavioural features distinguish frontal and temporal variants of frontotemporal dementia from Alzheimer's disease? Journal of Neurology, Neurosurgery & Psychiatry. 2000;69:178–186. doi: 10.1136/jnnp.69.2.178. http://dx.doi.org/10.1136/jnnp.69.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryson G, Bell M, Lysaker P. Affect recognition in schizophrenia: A function of global impairment or a specific cognitive deficit. Psychiatry Research. 1997;71:105–113. doi: 10.1016/s0165-1781(97)00050-4. http://dx.doi.org/10.1016/S0165-1781(97)00050-4. [DOI] [PubMed] [Google Scholar]
- Burnham H, Hogervorst E. Recognition of facial expressions of emotion by patients with dementia of the Alzheimer type. Dementia and Geriatric Cognitive Disorders. 2004;18:75–79. doi: 10.1159/000077813. http://dx.doi.org/10.1159/000077813. [DOI] [PubMed] [Google Scholar]
- Cadieux NL, Greve KW. Emotion processing in Alzheimer's disease. Journal of the International Neuropsychological Society: JINS. 1997;3:411–419. [PubMed] [Google Scholar]
- Chan RCK, Li H, Cheung EFC, Gong QY. Impaired facial emotion perception in schizophrenia: A meta-analysis. Psychiatry Research. 2010;178:381–390. doi: 10.1016/j.psychres.2009.03.035. http://dx.doi.org/10.1016/j.psychres.2009.03.035. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. http://dx.doi.org/10.1037/0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: A review. Schizophrenia Bulletin. 2006;32(Suppl 1):S44–63. doi: 10.1093/schbul/sbl029. http://dx.doi.org/10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MH. Measuring individual differences in empathy: Evidence for a multidimensional approach. Journal of Personality and Social Psychology. 1983;44:113–126. [Google Scholar]
- de Vugt ME, Riedijk SR, Aalten P, Tibben A, van Swieten JC, Verhey FRJ. Impact of behavioural problems on spousal caregivers: A comparison between Alzheimer's disease and frontotemporal dementia. Dementia and Geriatric Cognitive Disorders. 2006;22:35–41. doi: 10.1159/000093102. http://dx.doi.org/10.1159/000093102. [DOI] [PubMed] [Google Scholar]
- Diehl-Schmid J, Pohl C, Ruprecht C, Wagenpfeil S, Foerstl H, Kurz A. The Ekman 60 Faces Test as a diagnostic instrument in frontotemporal dementia. Archives of Clinical Neuropsychology. 2007;22:459–464. doi: 10.1016/j.acn.2007.01.024. http://dx.doi.org/10.1016/j.acn.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Drapeau J, Gosselin N, Gagnon L, Peretz I, Lorrain D. Emotional recognition from face, voice, and music in dementia of the Alzheimer type. Annals of the New York Academy of Sciences. 2009;1169:342–345. doi: 10.1111/j.1749-6632.2009.04768.x. http://dx.doi.org/10.1111/j.1749-6632.2009.04768.x. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Lennon R. Sex differences in empathy and related capacities. Psychological Bulletin. 1983;94:100–131. http://dx.doi.org/10.1037/0033-2909.94.1.100. [Google Scholar]
- Ekman P. Are there basic emotions? Psychological Review. 1992;99:550–553. doi: 10.1037/0033-295x.99.3.550. http://dx.doi.org/10.1037/0033-295X.99.3.550. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Moore P, Anderson C, Grossman M. Social cognition, executive functioning, and neuroimaging correlates of empathic deficits in frontotemporal dementia. The Journal of Neuropsychiatry and Clinical Neurosciences. 2011;23:74–82. doi: 10.1176/appi.neuropsych.23.1.74. http://dx.doi.org/10.1176/appi.neuropsych.23.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Duque D, Black SE. Impaired recognition of negative facial emotions in patients with frontotemporal dementia. Neuropsychologia. 2005;43:1673–1687. doi: 10.1016/j.neuropsychologia.2005.01.005. http://dx.doi.org/10.1016/j.neuropsychologia.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. http://dx.doi.org/10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL. What Good Are Positive Emotions? Review of General Psychology: Journal of Division 1, of the American Psychological Association. 1998;2:300–319. doi: 10.1037/1089-2680.2.3.300. http://dx.doi.org/10.1037/1089-2680.2.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froming KB, Weiner SG, Gregory AL, Levy CM, Ekman P. The Comprehensive Affect Testing System: An emotion processing system-Clinical version. Gainesville, Florida: Psychology Software Inc; 2006. [Google Scholar]
- Goodkind MS, Gyurak A, McCarthy M, Miller BL, Levenson RW. Emotion regulation deficits in frontotemporal lobar degeneration and Alzheimer's disease. Psychology and Aging. 2010;25:30–37. doi: 10.1037/a0018519. http://dx.doi.org/10.1037/a0018519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkind MS, Sollberger M, Gyurak A, Rosen HJ, Rankin KP, Miller B, Levenson R. Tracking emotional valence: The role of the orbitofrontal cortex. Human Brain Mapping. 2012;33:753–762. doi: 10.1002/hbm.21251. http://dx.doi.org/10.1002/hbm.21251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grattan LM, Bloomer RH, Archambault FX, Eslinger PJ. Cognitive flexibility and empathy after frontal lobe lesion. Neuropsychiatry, Neuropsychology, & Behavioral Neurology. 1994;7:251–259. [Google Scholar]
- Gray HM, Tickle-Degnen L. A meta-analysis of performance on emotion recognition tasks in Parkinson's disease. Neuropsychology. 2010;24:176–191. doi: 10.1037/a0018104. http://dx.doi.org/10.1037/a0018104. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. Emotion elicitation using films. Cognition and Emotion. 1995;9:87–108. http://dx.doi.org/10.1080/02699939508408966. [Google Scholar]
- Guaita A, Malnati M, Vaccaro R, Pezzati R, Marcionetti J, Vitali SF, Colombo M. Impaired facial emotion recognition and preserved reactivity to facial expressions in people with severe dementia. Archives of Gerontology and Geriatrics. 2009;49(Suppl 1):135–146. doi: 10.1016/j.archger.2009.09.023. http://dx.doi.org/10.1016/j.archger.2009.09.023. [DOI] [PubMed] [Google Scholar]
- Gyurak A, Goodkind MS, Kramer JH, Miller BL, Levenson RW. Executive functions and the down-regulation and up-regulation of emotion. Cognition and Emotion. 2012;26:103–118. doi: 10.1080/02699931.2011.557291. http://dx.doi.org/10.1080/02699931.2011.557291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyurak A, Goodkind MS, Madan A, Kramer JH, Miller BL, Levenson RW. Do tests of executive functioning predict ability to downregulate emotions spontaneously and when instructed to suppress? Cognitive, Affective & Behavioral Neuroscience. 2009;9:144–152. doi: 10.3758/CABN.9.2.144. http://dx.doi.org/10.3758/CABN.9.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JA. Nonverbal sex differences: Accuracy of communication and expressive style. XII. Baltimore, MD: Johns Hopkins University Press; 1990. [Google Scholar]
- Hampson E, van Anders S, Mullin LI. A female advantage in the recognition of emotional facial expressions: Test of an evolutionary hypothesis. Evolution and Human Behavior. 2006;27:401–416. http://dx.doi.org/10.1016/j.evolhumbehav.2006.05.002. [Google Scholar]
- Hargrave R, Maddock RJ, Stone V. Impaired recognition of facial expressions of emotion in Alzheimer's disease. The Journal of Neuropsychiatry and Clinical Neurosciences. 2002;14:64–71. doi: 10.1176/jnp.14.1.64. http://dx.doi.org/10.1176/jnp.14.1.64. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Perrett DI, Cowen PJ, Goodwin GM. Administration of the beta-adrenoceptor blocker propranolol impairs the processing of facial expressions of sadness. Psychopharmacology. 2001;154:383–389. doi: 10.1007/s002130000654. http://dx.doi.org/10.1007/s002130000654. [DOI] [PubMed] [Google Scholar]
- Heerey EA, Keltner D, Capps LM. Making sense of self-conscious emotion: Linking theory of mind and emotion in children with autism. Emotion. 2003;3:394–400. doi: 10.1037/1528-3542.3.4.394. http://dx.doi.org/10.1037/1528-3542.3.4.394. [DOI] [PubMed] [Google Scholar]
- Henry JD, Ruffman T, McDonald S, O'Leary MAP, Phillips LH, Brodaty H, Rendell PG. Recognition of disgust is selectively preserved in Alzheimer's disease. Neuropsychologia. 2008;46:1363–1370. doi: 10.1016/j.neuropsychologia.2007.12.012. http://dx.doi.org/10.1016/j.neuropsychologia.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Hooker C, Park S. Emotion processing and its relationship to social functioning in schizophrenia patients. Psychiatry Research. 2002;112:41–50. doi: 10.1016/s0165-1781(02)00177-4. [DOI] [PubMed] [Google Scholar]
- Ickes WJ. Empathic accuracy. New York, NY: Guilford Press; 1997. [Google Scholar]
- Johnson JK, Diehl J, Mendez MF, Neuhaus J, Shapira JS, Forman M, et al. Miller BL. Frontotemporal lobar degeneration: Demographic characteristics of 353 patients. Archives of Neurology. 2005;62:925–930. doi: 10.1001/archneur.62.6.925. http://dx.doi.org/10.1001/archneur.62.6.925. [DOI] [PubMed] [Google Scholar]
- Johnson SA, Stout JC, Solomon AC, Langbehn DR, Aylward EH, Cruce CB, et al. Paulsen JS the Predict-HD Investigators of the Huntington Study Group. Beyond disgust: Impaired recognition of negative emotions prior to diagnosis in Huntington's disease. Brain: A Journal of Neurology. 2007;130:1732–1744. doi: 10.1093/brain/awm107. http://dx.doi.org/10.1093/brain/awm107. [DOI] [PubMed] [Google Scholar]
- Keane J, Calder AJ, Hodges JR, Young AW. Face and emotion processing in frontal variant frontotemporal dementia. Neuropsychologia. 2002;40:655–665. doi: 10.1016/s0028-3932(01)00156-7. http://dx.doi.org/10.1016/S0028-3932(01)00156-7. [DOI] [PubMed] [Google Scholar]
- Keltner D, Buswell BN. Embarrassment: Its distinct form and appeasement functions. Psychological Bulletin. 1997;122:250–270. doi: 10.1037/0033-2909.122.3.250. http://dx.doi.org/10.1037/0033-2909.122.3.250. [DOI] [PubMed] [Google Scholar]
- Kessels RPC, Gerritsen L, Montagne B, Ackl N, Diehl J, Danek A. Recognition of facial expressions of different emotional intensities in patients with frontotemporal lobar degeneration. Behavioural Neurology. 2007;18:31–36. doi: 10.1155/2007/868431. http://dx.doi.org/10.1155/2007/868431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipps CM, Duggins AJ, McCusker EA, Calder AJ. Disgust and happiness recognition correlate with anteroventral insula and amygdala volume respectively in preclinical Huntington's disease. Journal of Cognitive Neuroscience. 2007;19:1206–1217. doi: 10.1162/jocn.2007.19.7.1206. http://dx.doi.org/10.1162/jocn.2007.19.7.1206. [DOI] [PubMed] [Google Scholar]
- Klein KJK, Hodges SD. Gender differences, motivation, and empathic accuracy: When it pays to understand. Personality and Social Psychology Bulletin. 2001;27:720–730. http://dx.doi.org/10.1177/0146167201276007. [Google Scholar]
- Lavenu I, Pasquier F, Lebert F, Petit H, Van der Linden M. Perception of emotion in frontotemporal dementia and Alzheimer disease. Alzheimer Disease and Associated Disorders. 1999;13:96–101. doi: 10.1097/00002093-199904000-00007. http://dx.doi.org/10.1097/00002093-199904000-00007. [DOI] [PubMed] [Google Scholar]
- Leppänen JM, Niehaus DJH, Koen L, Du Toit E, Schoeman R, Emsley R. Deficits in facial affect recognition in unaffected siblings of Xhosa schizophrenia patients: Evidence for a neurocognitive endophenotype. Schizophrenia Research. 2008;99:270–273. doi: 10.1016/j.schres.2007.11.003. http://dx.doi.org/10.1016/j.schres.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Ruef AM. Empathy: A physiological substrate. Journal of Personality and Social Psychology. 1992;63:234–246. http://dx.doi.org/10.1037/0022-3514.63.2.234. [PubMed] [Google Scholar]
- Lough S, Kipps CM, Treise C, Watson P, Blair JR, Hodges JR. Social reasoning, emotion and empathy in frontotemporal dementia. Neuropsychologia. 2006;44:950–958. doi: 10.1016/j.neuropsychologia.2005.08.009. http://dx.doi.org/10.1016/j.neuropsychologia.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Mah L, Arnold MC, Grafman J. Impairment of social perception associated with lesions of the prefrontal cortex. The American Journal of Psychiatry. 2004;161:1247–1255. doi: 10.1176/appi.ajp.161.7.1247. http://dx.doi.org/10.1176/appi.ajp.161.7.1247. [DOI] [PubMed] [Google Scholar]
- Mathersul D, Palmer DM, Gur RC, Gur RE, Cooper N, Gordon E, Williams LM. Explicit identification and implicit recognition of facial emotions: II. Core domains and relationships with general cognition. Journal of Clinical and Experimental Neuropsychology. 2008;31:278–291. doi: 10.1080/13803390802043619. http://dx.doi.org/10.1080/13803390802043619. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. http://dx.doi.org/10.1212/WNL.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mueser KT, Doonan R, Penn DL, Blanchard JJ, Bellack AS, Nishith P, DeLeon J. Emotion recognition and social competence in chronic schizophrenia. Journal of Abnormal Psychology. 1996;105:271–275. doi: 10.1037//0021-843x.105.2.271. [DOI] [PubMed] [Google Scholar]
- Namiki C, Hirao K, Yamada M, Hanakawa T, Fukuyama H, Hayashi T, Murai T. Impaired facial emotion recognition and reduced amygdalar volume in schizophrenia. Psychiatry Research: Neuroimaging. 2007;156:23–32. doi: 10.1016/j.pscychresns.2007.03.004. http://dx.doi.org/10.1016/j.pscychresns.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Benson DF. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Rosen HR, Kramer JH, Beer JS, Levenson RL, Miller BL. Hemispheric dominance for emotions, empathy and social behaviour: Evidence from right and left handers with frontotemporal dementia. Neurocase. 2001;7:145–160. doi: 10.1093/neucas/7.2.145. http://dx.doi.org/10.1093/neucas/7.2.145. [DOI] [PubMed] [Google Scholar]
- Phillips LH, Scott C, Henry JD, Mowat D, Bell JS. Emotion perception in Alzheimer's disease and mood disorder in old age. Psychology and Aging. 2010;25:38–47. doi: 10.1037/a0017369. http://dx.doi.org/10.1037/a0017369. [DOI] [PubMed] [Google Scholar]
- Preston SD, de Waal FBM. Empathy: Its ultimate and proximate bases. Behavioral and Brain Sciences. 2002;25:49–58. doi: 10.1017/s0140525x02000018. [DOI] [PubMed] [Google Scholar]
- Radice-Neumann D, Zupan B, Babbage DR, Willer B. Overview of impaired facial affect recognition in persons with traumatic brain injury. Brain Injury. 2007;21:807–816. doi: 10.1080/02699050701504281. http://dx.doi.org/10.1080/02699050701504281. [DOI] [PubMed] [Google Scholar]
- Rankin KP, Kramer JH, Miller BL. Patterns of cognitive and emotional empathy in frontotemporal lobar degeneration. Cognitive and Behavioral Neurology. 2005;18:28–36. doi: 10.1097/01.wnn.0000152225.05377.ab. http://dx.doi.org/10.1097/01.wnn.0000152225.05377.ab. [DOI] [PubMed] [Google Scholar]
- Rankin KP, Salazar A, Gorno-Tempini ML, Sollberger M, Wilson SM, Pavlic D, et al. Miller BL. Detecting sarcasm from paralinguistic cues: Anatomic and cognitive correlates in neurodegenerative disease. NeuroImage. 2009;47:2005–2015. doi: 10.1016/j.neuroimage.2009.05.077. http://dx.doi.org/10.1016/j.neuroimage.2009.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Miller BL. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain: A Journal of Neurology. 2011;134:2456–2477. doi: 10.1093/brain/awr179. http://dx.doi.org/10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnavalli E, Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology. 2002;58:1615–1621. doi: 10.1212/wnl.58.11.1615. http://dx.doi.org/10.1212/WNL.58.11.1615. [DOI] [PubMed] [Google Scholar]
- Rocca CC, Heuvel E, Caetano SC, Lafer B. Facial emotion recognition in bipolar disorder: A critical review. Revista Brasileira de Psiquiatria (São Paulo) 2009;31:171–180. doi: 10.1590/s1516-44462009000200015. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Gorno-Tempini ML, Goldman WP, Perry RJ, Schuff N, Weiner M, et al. Miller BL. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58:198–208. doi: 10.1212/wnl.58.2.198. http://dx.doi.org/10.1212/WNL.58.2.198. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Pace-Savitsky K, Perry RJ, Kramer JH, Miller BL, Levenson RW. Recognition of emotion in the frontal and temporal variants of frontotemporal dementia. Dementia and Geriatric Cognitive Disorders. 2004;17:277–281. doi: 10.1159/000077154. http://dx.doi.org/10.1159/000077154. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Perry RJ, Murphy J, Kramer JH, Mychack P, Schuff N, et al. Miller BL. Emotion comprehension in the temporal variant of frontotemporal dementia. Brain: A Journal of Neurology. 2002;125:2286–2295. doi: 10.1093/brain/awf225. http://dx.doi.org/10.1093/brain/awf225. [DOI] [PubMed] [Google Scholar]
- Russell TA, Tchanturia K, Rahman Q, Schmidt U. Sex differences in theory of mind: A male advantage on Happé's “cartoon” task. Cognition and Emotion. 2007;21:1554–1564. http://dx.doi.org/10.1080/02699930601117096. [Google Scholar]
- Scherer K, Matsumoto D, Wallbott H, Kudoh T. Emotional experience in cultural context: A comparison between Europe, Japan, and the USA. In: Scherer K, editor. Facets of emotion: Recent research. Hillsdale, NJ: Erlbaum; 1988. pp. 5–30. [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. http://dx.doi.org/10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Tomer R, Berger BD, Aharon-Peretz J. Characterization of empathy deficits following prefrontal brain damage: The role of the right ventromedial prefrontal cortex. Journal of Cognitive Neuroscience. 2003;15:324–337. doi: 10.1162/089892903321593063. http://dx.doi.org/10.1162/089892903321593063. [DOI] [PubMed] [Google Scholar]
- Shimokawa A, Yatomi N, Anamizu S, Torii S, Isono H, Sugai Y. Recognition of facial expressions and emotional situations in patients with dementia of the Alzheimer and vascular types. Dementia and Geriatric Cognitive Disorders. 2003;15:163–168. doi: 10.1159/000068479. http://dx.doi.org/10.1159/000068479. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Austin NA, Sembi S, Thompson JC, Craufurd D, Neary D. Emotion recognition in Huntington's disease and frontotemporal dementia. Neuropsychologia. 2008;46:2638–2649. doi: 10.1016/j.neuropsychologia.2008.04.018. http://dx.doi.org/10.1016/j.neuropsychologia.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Soto JA, Levenson RW. Emotion recognition across cultures: The influence of ethnicity on empathic accuracy and physiological linkage. Emotion. 2009;9:874–884. doi: 10.1037/a0017399. http://dx.doi.org/10.1037/a0017399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoletini I, Marra C, Di Iulio F, Gianni W, Sancesario G, Giubilei F, et al. Spalletta G. Facial emotion recognition deficit in amnestic mild cognitive impairment and Alzheimer disease. The American Journal of Geriatric Psychiatry. 2008;16:389–398. doi: 10.1097/JGP.0b013e318165dbce. http://dx.doi.org/10.1097/JGP.0b013e318165dbce. [DOI] [PubMed] [Google Scholar]
- Stevens JS, Hamann S. Sex differences in brain activation to emotional stimuli: A meta-analysis of neuroimaging studies. Neuropsychologia. 2012;50:1578–1593. doi: 10.1016/j.neuropsychologia.2012.03.011. http://dx.doi.org/10.1016/j.neuropsychologia.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Sturm VE, Ascher EA, Miller BL, Levenson RW. Diminished self-conscious emotional responding in frontotemporal lobar degeneration patients. Emotion. 2008;8:861–869. doi: 10.1037/a0013765. http://dx.doi.org/10.1037/a0013765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm VE, Rosen HJ, Allison S, Miller BL, Levenson RW. Self-conscious emotion deficits in frontotemporal lobar degeneration. Brain: A Journal of Neurology. 2006;129:2508–2516. doi: 10.1093/brain/awl145. http://dx.doi.org/10.1093/brain/awl145. [DOI] [PubMed] [Google Scholar]
- Sze JA, Goodkind MS, Gyurak A, Levenson RW. Aging and emotion recognition: Not just a losing matter. Psychology and Aging. 2012;27:940–950. doi: 10.1037/a0029367. Advance online publication. http://dx.doi.org/10.1037/a0029367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss EM, Kohler CG, Vonbank J, Stadelmann E, Kemmler G, Hinterhuber H, Marksteiner J. Impairment in emotion recognition abilities in patients with mild cognitive impairment, early and moderate Alzheimer disease compared with healthy comparison subjects. The American Journal of Geriatric Psychiatry. 2008;16:974–980. doi: 10.1097/JGP.0b013e318186bd53. http://dx.doi.org/10.1097/JGP.0b013e318186bd53. [DOI] [PubMed] [Google Scholar]
- Werner KH, Roberts NA, Rosen HJ, Dean DL, Kramer JH, Weiner MW, et al. Levenson RW. Emotional reactivity and emotion recognition in frontotemporal lobar degeneration. Neurology. 2007;69:148–155. doi: 10.1212/01.wnl.0000265589.32060.d3. http://dx.doi.org/10.1212/01.wnl.0000265589.32060.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, Weber J, Bolger N, Ochsner K. The neural bases of empathic accuracy. PNAS Proceedings of the National Academy of Sciences of the United States of America. 2009;106:11382–11387. doi: 10.1073/pnas.0902666106. http://dx.doi.org/10.1073/pnas.0902666106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Greicius MD, Gennatas ED, Growdon ME, Jang JY, Rabinovici GD, et al. Seeley WW. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer's disease. Brain: A Journal of Neurology. 2010;133:1352–1367. doi: 10.1093/brain/awq075. http://dx.doi.org/10.1093/brain/awq075. [DOI] [PMC free article] [PubMed] [Google Scholar]