Abstract
Background
In 2004, the Mozambican Ministry of Health began a national scale-up of antiretroviral therapy (ART) using a vertical model of HIV clinics colocated within large urban hospitals. In 2006, the ministry expanded access by integrating ART into primary health care clinics.
Methods
We conducted a retrospective cohort study including adult ART-naive patients initiating ART between January 2006 and June 2008 in public sector clinics in Manica and Sofala provinces. Cox proportional hazards models with robust variances were used to estimate the association between clinic model (vertical/integrated), clinic location (urban/rural), and clinic experience (first 6 months/post first 6 months) and attrition occurring in early patient follow-up (≤6 months) and attrition occurring in late patient follow-up (>6 months), while controlling for age, sex, education, pre-ART CD4 count, World Health Organization stage and pharmacy staff burden.
Results
A total of 11,775 patients from 17 clinics were studied. The overall attrition rate was 37 per 100 person-years. Patients attending integrated clinics had a higher risk of attrition in late follow-up [hazard ratio (HR) = 1.75; 95% confidence interval (CI): 1.04 to 2.94], and patients attending urban clinics (HR = 0.57; 95% CI: 0.35 to 0.91) had a lower risk of attrition in late follow-up. Though not statistically significant, clinics open for longer than 6 months (HR = 0.71; 95% CI: 0.49 to 1.04) had a lower risk of attrition in early follow-up.
Conclusions
Patients attending vertical clinics had a lower risk of attrition. Utilizing primary health clinics to implement ART is necessary to reach higher levels of coverage; however, further implementation strategies should be developed to improve patient retention in these settings.
Keywords: Mozambique, antiretroviral therapy, health system, integration, attrition, implementation research
INTRODUCTION
In the past decade, antiretroviral therapy (ART) has dramatically scaled-up across low-income and middle-income countries. Between 2001 and 2010, a 22-fold increase in patients receiving ART occurred, with an estimated 6.6 million adults and children receiving ART.1 Countries in sub-Saharan Africa experienced some of the largest accelerations of ART coverage—in 2010 alone, the region reported a 31% increase in the number of people receiving ART, with more than 5,000,000 (49% coverage of estimated need) people receiving HIV treatment.1,2
Initially, ART programs in sub-Saharan Africa were implemented in large hospitals that offered targeted HIV care and treatment services.3–7 However, geographical distance and high transport costs were obstacles to both ART uptake and follow-up of patients.7–9 In an effort to reduce the burden placed on the hospital systems, to increase geographic coverage, and to reduce the time and costs of transport, many ART programs began integrating HIV care and treatment services into primary health care centers resulting in a dramatic expansion in the number of people initiating treatment.3–6,10,11 A few studies have demonstrated that these integrated programs resulted in faster enrollment and better retention.3,12–14 However, given the lack of conclusive evidence, experts have called for further assessments of the potential benefits and risks of integrated models of delivery.15,16
Similar to other high HIV prevalence countries in sub-Saharan Africa, Mozambique reported nearly 220,000 people on ART by the end of 2010, representing a more than 31-fold increase from the 7000 receiving ART in 2004.17 During the initial phase of ART scale-up in Mozambique, clinical services were delivered through a few newly developed clinics within established hospitals to facilitate high quality, targeted HIV services. Facing the common concerns of overwhelmed infrastructure, human resources, and poor geographic coverage as seen in other settings, the Ministry of Health (MOH) in 2006 began to integrate HIV care and treatment services into health centers offering primary health care services.3
To determine the effects of integration on quality of HIV care, we conducted a retrospective cohort study to examine the association of clinic type (ie, integrated versus vertical) with patient attrition from ART programs in Manica and Sofala provinces of Mozambique. Second, we examined the impact of clinic location and clinic experience with patient attrition from ART programs in this region.
METHODS
Study Setting
In response to the estimated 11.5% of the adult population infected with HIV,18 the Mozambican MOH initiated a national scale-up of antiretroviral therapy (ART), funded by the Global Fund for AIDS, Tuberculosis, and Malaria; the President's Emergency Plan for AIDS Relief (PEPFAR); and the Clinton Health Access Initiative. Using a centralized model of vertical, outpatient HIV clinics located within high-population urban centers, the MOH opened the first public sector ART program in 2003 within Manica and Sofala provinces in Beira, the capital city of Sofala; and in 2004, a second vertical ART delivery site was opened in Chimoio, the capital city of Manica. With the vertical model, HIV treatment clinics were stand-alone structures and were supported by parallel logistics, clinical, and pharmacy delivery systems. Designated health workers operated separate from the rest of the health care system. Voluntary testing and counseling, blood bank, and antenatal care sites identified HIV-positive patients who were then referred to the HIV clinic for care and treatment.3
As the MOH expanded ART access by integrating HIV care and treatment with other primary health care services in 2006, the number of clinics offering HIV care and treatment services in Manica and Sofala provinces increased to a total of 36 by 2008. The integrated clinics shared both physical space and human resources between ART and non-HIV outpatient services.3 Of the 36 clinics offering HIV care and treatment, 18 had electronic patient tracking systems.
Study Population
Study subjects were patients who initiated ART between January 1, 2006, and June 1, 2008, in 1 of 17 public-sector clinics with electronic clinic databases in Sofala and Manica provinces. Of the 18 clinics with electronic databases, one did not have information on a key confounder, pharmacy staff burden, leaving 17 clinics to be included in this analysis. We included only adult (≥15 years of age), ART-naïve, nonpregnant patients, as clinical pathways for these patients were distinct from other populations (ie, pregnant women and children), and therefore limited confounding in our results. For 2 study clinics, we excluded patients enrolling into treatment after December 31, 2007, and March 1, 2008, due to 2 distinct disruptive external events—regional flooding and temporary closure of the clinic, respectively.
Data Sources
This study utilized electronic clinic databases that contained patients’ sociodemographic characteristics, pharmacy and clinical data, and human resources information. These databases were primarily used by clinic management as tools for routine clinic monitoring and evaluation. Numerous cross-checks were incorporated within the databases to minimize data errors, and database managers performed queries to monitor data quality. In May 2009, the databases were collected for the purpose of this research, providing 11 months from the end of the study (June 30, 2008) for peer counselors to track patients who may have missed appointments. This approach made outcome ascertainment and transcription of data into the electronic database more comparable throughout the study period.
To evaluate the validity of data used in this study, we compared data from the clinic databases to a randomly selected sample of 520 paper-based patient medical records from the study clinics. Kappa scores and concordance correlation coefficients for sex, pre-ART CD4 value, and patient retention ranged from 0.91 to 0.97, indicating “almost perfect” agreement, and the dates of CD4 blood draw, ART initiation, and outcome agreed over 92% of the time.19
Measures
Clinic Characteristics
The clinic-level characteristics used in our study included the clinic model (vertical: HIV care and treatment services operated within a separate clinic and not integrated with regard to physical structure or human resources, versus integrated: HIV care and treatment services colocated with other services and human resources shared between services); the clinic location (rural or urban); and clinic experience (whether the clinic was in its first 6 months of offering HIV treatment or after its first 6 months of offering treatment).
Covariables
Patient characteristics considered as potential confounders in our analysis included age at enrollment at the ART clinic, sex, pre-ART CD4 cell count in cells per cubic millimeter (log transformed), pre-ART clinical World Health Organization (WHO) stage, education at ART clinic enrollment, and year of ART initiation. Clinic characteristics considered as potential confounders included pharmacy staff burden (number of pharmacy visits/number of pharmacy staff), treated as a time-varying covariate, because it has been shown to be an important predictor of patient attrition in other research.20 Consideration of which covariables to include was based on their theoretical plausibility to confound the relationship between our clinic-level exposures of interest and patient attrition.
Outcomes
Outcomes for patients who initiated HIV treatment were defined as (1) transferred—patients who transferred to receive treatment at another facility; (2) suspended—patients who suspended HIV treatment per clinicians’ recommendation but remained in care; (3) dead—patients who died due to any cause while receiving ART, as ascertained by reports from family members and active tracing of patients who missed a pharmacy visit; and (4) lost to follow-up—patients who failed to return for treatment for 2 months beyond their missed ART refill visit for reasons unrelated to patient death. If a patient failed to return for ART medication refill, “acti-vistas” (clinic-based peer counselors) were notified to begin actively tracing patients. The peer counselors visited the patient's residence and encouraged them to return for treatment at the clinic if the patient was still alive. If the patient had died, the date of death was documented. Patients were considered to be maintained on ART until they either experienced one of the aforementioned terminating events or reached the end of study period on June 30, 2008.
We defined our primary outcome measure, attrition from care, as patients classified as either dead or lost to follow-up. The date of attrition was defined as the date of death for those who died and the last pharmacy refill date for those who were lost to follow-up. The principal reason for combining the 2 outcomes was that differential ascertainment between those patients who were lost to follow-up and those who died could occur at clinics with larger patient volumes. Essentially, the workload for peer counselors could have compromised their ability to accurately determine whether or not patients who failed to return for pharmacy refills actually died, and therefore, these patients could be incorrectly classified as lost to follow-up.
Statistical Analysis
Cox proportional hazards models were used to analyze time until attrition. Individuals who were categorized as transferred and suspended were treated as censored observations at the time of transfer or suspension. Follow-up time began at each patients’ initiation of HIV treatment and ended with the date of death, transfer, treatment suspension, loss to follow-up (date of last pharmacy visit), or June 30, 2008, whichever came first. Patients attending the 2 clinics with disruptive external events were censored on dates corresponding to the event—December 31, 2007, and March 1, 2008. Given that survival times were clustered at the clinic level, robust variances were used.21
Our primary analysis tested the association of clinic model and time-to-attrition, and in secondary analysis, we tested the association between clinic location and clinic experience with time-to-attrition. We evaluated the associations of these characteristics with attrition in both early patient follow-up, defined as a patient's first 6 months of follow-up, and late patient follow-up, defined as a patient's follow-up beginning 6 months after initiating treatment. Using backward stepwise regression with a criterion P value of <0.2 for inclusion in the final model, each of these analyses considered the following a priori–specified potential confounders as follows: pre-ART CD4 count, pre-ART WHO clinical stage, age at enrollment at the ART clinic, education, sex, year of ART initiation, and pharmacy staff burden. Using this procedure, year of ART initiation was dropped from the models for clinic model, clinic location, and clinic experience. We used residual plots to guide the choice of the best-fitting transformation for continuous variables. We allowed variables to have time-varying effects between early and late patient follow-up based on results from previous analyses.20
Data were missing for some patients on CD4 count (5%), education (7%) and WHO stage (9%). To address this, we utilized multiple imputation procedures to fill in missing values in the dataset for sensitivity analysis. Ten imputations were done, using fully conditional specifications including pre-ART CD4 count, pre-ART WHO clinical stage, age at enrollment, sex, education, attrition status, and survival time (log form).22 Results from the imputations were combined into a single set of parameter estimates for the “final” proportional hazards regression model that incorporated the uncertainty from the imputations.23
The study was approved by the institutional review boards of the Mozambique MOH and the University of Washington. All analyses were conducted in Stata version 11.1 (College Station, TX).
RESULTS
Patient Characteristics
Overall, 15,232 patients initiated HIV treatment during the study period. We excluded 810 patients who were transferred in from another facility, 1138 who were younger than 15 years, and 1491 who were pregnant at the time of enrollment, resulting in 11,775 patients being included in the study. Patients attending different clinic models (vertical versus integrated) were similar with regards to sex and age, but patients did have statistical differences for education, CD4 count, and WHO clinical stage (Table 1). A slightly higher percentage of patients attending integrated facilities received stavudine + lamivudine + efavirenz as their initial ART regimen. The proportion of patients retained in care at 24 months was 60.7% [95% confidence interval (CI): 59.0% to 62.3%] among those attending vertical facilities and 54.9% (95% CI: 52.3% to 57.4%) among those attending integrated facilities.
TABLE 1.
Characteristic of Patients Initiating ART by Clinic Model
| Vertical, n = 6190 | Integrated, n = 5585 | P | Total, N = 11,775 | |
|---|---|---|---|---|
| Female, n (%) | 3542 (57) | 3226 (58) | 0.60 | 6768 (57) |
| Age (yrs), n (%) | ||||
| ≤15 to <25 | 752 (12) | 721 (13) | 0.19 | 1473 (12) |
| ≤25 to <35 | 2412 (39) | 2234 (40) | 4646 (40) | |
| ≤35 to <45 | 1880 (30) | 1652 (30) | 3532 (30) | |
| ≤45 | 1133 (18) | 960 (17) | 2093 (18) | |
| Education (yrs), mean (SD) | 6 (4) | 5 (3) | <0.01 | 6 (3) |
| CD4 count (cells/μL), n (%) | ||||
| <100 | 1769 (30) | 1648 (31) | <0.01 | 3417 (31) |
| ≤100 to <200 | 2351 (40) | 1937 (36) | 4288 (38) | |
| ≤200 to <300 | 1126 (19) | 1053 (20) | 2179 (19) | |
| ≤300 | 622 (11) | 664 (12) | 1286 (11) | |
| WHO clinical stage, n (%) | ||||
| I/II | 1568 (27) | 1130 (24) | <0.01 | 2698 (26) |
| III | 3684 (64) | 3121 (66) | 6805 (65) | |
| IV | 492 (9) | 488 (10) | 980 (9) | |
| Initial ART regimen, n (%) | ||||
| D4T + 3TC + NVP | 5651 (91) | 4647 (84) | <0.01 | 10,298 (88) |
| D4T + 3TC + EFV | 462 (7) | 766 (14) | 1228 (10) | |
| AZT + 3TC + NVP | 70 (1) | 104 (2) | 174 (1) | |
| Follow-up time (person-years) | 5171 | 3945 | — | 9117 |
AZT, azidothymidine; 3TC, Lamivudine; D4T, stavudine; EFV, efavirenz; NVP, nevirapine.
Clinic Characteristics
Supplemental Digital Content 1 (see Figure, http://links.lww.com/QAI/A386) illustrates the distribution of vertical and integrated clinics throughout Manica and Sofala provinces, and characteristics of the 17 clinics included in this study are outlined in Table 2. Four clinics provided person-time for vertical clinics and 14 provided person-time for integrated facilities. One clinic shifted from a vertical to an integrated model, providing person-time for both categories. Among patients initiating treatment, the average number of days between enrolling at the facility and initiating treatment was 180 days for vertical clinics and 93 days for integrated clinics (P < 0.01). Within both clinic types, the average number of days between enrollment and treatment initiation increased over time.
TABLE 2.
Characteristic of Study Clinics by Clinic Model
| Vertical | Integrated | Total | |
|---|---|---|---|
| Number of clinics*†, n (%) | 4 (23) | 14 (82) | 17 (100)† |
| Clinic location* | |||
| Urban, n (%) | 2 (50) | 6 (43) | 8 (47) |
| Clinic experience* | |||
| ≤6 Months | 0 (0) | 13 (93) | 13 (76) |
| >6 Months† | 4 (100) | 14 (100) | 17 (100)† |
| No. days from enrollment into care until treatment initiation, mean (SD) | |||
| 2006 | 154 (188) | 86 (98) | 131 (167) |
| 2007 | 192 (264) | 91 (106) | 138 (202) |
| 2008 | 211 (304) | 105 (133) | 152 (231) |
| Combined | 180 (244) | 93 (111) | 139 (198) |
| Monthly no. ART-naive adults initiating ART per clinic, mean (SD) | |||
| 2006 | 60 (15) | 15 (6) | 39 (6) |
| 2007 | 66 (13) | 23 (3) | 33 (5) |
| 2008 | 52 (33) | 20 (1) | 26 (5) |
| Combined | 61 (18) | 19 (6) | 34 (7) |
Number of clinics providing person-time.
One clinic shifted from a vertical to an integrated facility and is included in the counts of both categories but is not double counted in the total.
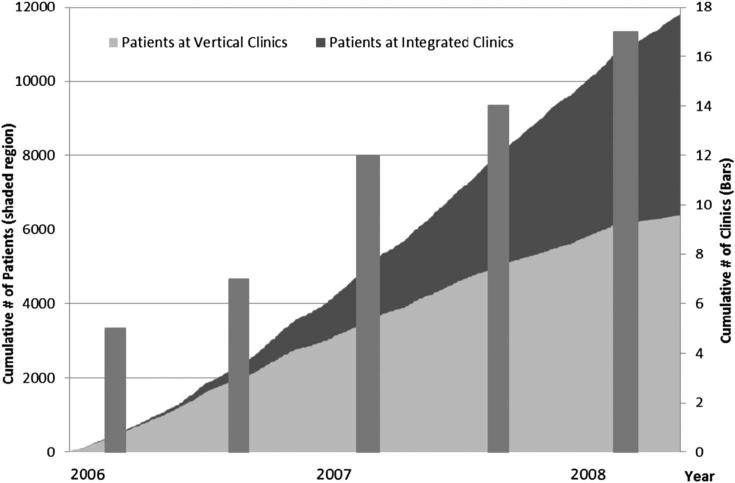
Figure 1 illustrates the increase in the number of clinics offering HIV treatment and the scale at which patients included in this study initiated ART over the study period, resulting in a total of 6190 patients initiating ART at vertical facilities and 5585 at integrated facilities. The average number of patients initiating ART per clinic month was 61 for vertical facilities and 19 for integrated facilities (P < 0.01). Eight (47%) clinics were located in an urban setting and 9 (53%) were in a rural environment. Regarding clinic experience, 13 clinics provide person-time to both the first 6 months and post 6 months timeframe, and 4 clinics provided person-time to only the post 6 months timeframe.
FIGURE 1.
The cumulative number of ART-naive adult patients initiating treatment by clinic type during the study period.
Clinic Characteristics and Attrition
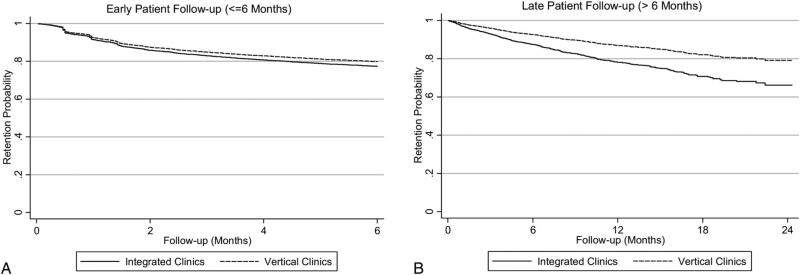
The relationship between clinic model, clinic location, and clinic experience are located in Table 3. The overall attrition rate was 58 per 100 person-years during early follow-up; 15 per 100 person-years during late follow-up for patients receiving treatment at vertical clinics compared with 55 per 100 person-years during early follow-up; and 22 per 100 person-years during late follow-up for patients receiving treatment at integrated clinics. Patients attending integrated clinics (HR = 1.75; 95% CI: 1.04 to 2.94) had a higher risk of attrition in late follow-up (P = 0.03) but did not have a significantly higher risk of attrition in early follow-up (HR = 0.97; 95% CI: 0.71 to 1.33; P = 0.87). Figure 2 illustrates the retention probability of patients receiving ART at integrated and vertical clinics during early and late patient follow-up.
TABLE 3.
Association Between Clinic Characteristics and Attrition
| Early Follow-Up (≤6 Months) |
Late Follow-Up (>6 Months) |
|||||||
|---|---|---|---|---|---|---|---|---|
| No. Events | Rate (Per 100 Person-Years) | Attrition, aHR* (95% CI) | P | No. Events | Rate (Per 100 Person-Years) | Attrition, aHR* (95% CI) | P | |
| Clinic model | ||||||||
| Vertical | 1488 | 57.70 | 1.00 (ref) | 0.87 | 431 | 15.13 | 1.00 (ref) | 0.03 |
| Integrated | 1226 | 55.37 | 0.97 (0.71 to 1.33) | 430 | 21.83 | 1.75 (1.04 to 2.94) | ||
| Clinic location | ||||||||
| Rural | 996 | 60.02 | 1.00 (ref) | 0.30 | 401 | 23.02 | 1.00 (ref) | 0.02 |
| Urban | 1721 | 58.83 | 0.84 (0.61 to 1.17) | 459 | 14.94 | 0.57 (0.35 to 0.91) | ||
| Clinic experience | ||||||||
| First 6 months | 150 | 76.00 | 1.00 (ref) | 0.08 | — | — | — | — |
| Post first 6 months | 2558 | 55.79 | 0.71 (0.49 to 1.04) | — | — | — | ||
Early attrition—fFirst 6 months of patient follow-up; late attrition—post first 6 months of patient follow-up.
Adjusted for pre-ART CD4 count, WHO stage, age, education, and pharmacy staff burden; CD4 count, education, and pharmacy staff burden allowed to have time time-varying effects in early/late follow-up.
FIGURE 2.
Retention probability of patients receiving HIV treatment at integrated and vertical clinics for attrition occurring during the first 6 months of patient follow-up (A) and attrition occurring after the first 6 months of patient follow-up (B).
Patients receiving treatment at urban clinics had an overall attrition rate of 59 per 100 person-years during early follow-up and 15 per 100 person-years during late follow-up compared with 60 per 100 person-years during early follow-up and 23 per 100 person-years during late follow-up for patients receiving treatment at rural clinics. Patients attending urban clinics [HR = 0.57 (95% CI: 0.35-0.91)] had a significantly lower risk of attrition in late follow-up compared with those attending rural clinics (P = 0.02), but did not have a significantly lower risk in early follow-up (HR = 0.84; 95% CI: 0.61 to 1.17; P = 0.30). For the first 6 months of patient follow-up, patients attending clinics after their first semester of offering treatment tend to have a lower risk of attrition (HR = 0.71; 95% CI: 0.49 to 1.04) than those attending clinics in their first semester of offering ART, but the results were not statistically significant (P = 0.08). In sensitivity analysis, findings from the complete case analysis were similar to results from multiple imputations.
DISCUSSION
In this retrospective cohort study of more than 11,700 patients, we evaluated the impact of clinic model, clinic location, and clinic experience on patient attrition in 17 public sector HIV care and treatment clinics in Central Mozambique. Patients attending urban clinics had a lower risk of late attrition, but patients attending clinics after their first semester of offering treatment did not have a significantly lower risk of attrition. Patients attending integrated clinics had a higher risk of late attrition compared with patients attending vertical clinics. This result has important implications as many HIV programs have integrated HIV care and treatment into primary health care settings, resulting in a dramatic increase in the number of clients receiving ART. Our findings should therefore stimulate the development and implementation of effective strategies to improve the retention of patients on ART in these settings.
Our study has several strengths. The included clinics had standardized protocols with regards to tracing patients, delivering HIV services, and recording data. The databases used for this study had high levels of completeness and agreement compared with patients’ paper charts. Our study utilized a large population of patients among a diverse set of clinics within the Mozambican health system. In addition, our results relaxed the proportional hazards assumption by looking at attrition in early and late patient follow-up. Disaggregating attrition occurring in early and late patient follow-up allowed us to look at associations with clinic-level exposures at periods of time when different factors may be involved in attrition. For example, early attrition may reflect that severely ill patients are more likely to die or be lost to follow-up during early follow-up, whereas later attrition may better reflect long-term adherence structures and be more sensitive to clinic characteristics. We also adjusted for a key confounder, pharmacy staff burden, an important predictor of attrition in previous research.20 Furthermore, our findings were robust to sensitivity analyses.
The principal limitation of our study is the nonrandom assignment of clinics and patients to our clinic-level characteristics of interest. Our study was observational in nature; therefore, the potential for mismeasured or unmeasured characteristics to confound our results existed. In addition, other factors correlated with clinic-level exposures could have driven our associations. We attempted to address these concerns by adjusting for available patient and clinic characteristics known to influence attrition from HIV treatment, but the potential for bias still existed. Vertical clinics were the largest facilities in the provinces and are different from other facilities with regard to overall resource allocation, management, clinical skills, staffing levels, training opportunities, and patient experiences. Due to the number of clinics in our study population, we were not able to adjust for a number of clinic-level factors simultaneously.
Generalizability to other settings requires careful consideration. Clinics were included in the study based on whether they had an operational electronic database and were not selected through a random process. Therefore, the relatively small number of clinics may not be representative of all clinics in Mozambique. Another limitation of the study was combining loss to follow-up and mortality into one measure of attrition due to our concern over differential outcome misclassification between clinic types. A consequence of this approach was that the inclusion of mortality made our outcome measure of attrition less sensitive to our clinic exposures of interest. Our retention estimates, however, were similar to a recent study from Mozambique,24 but were lower than retention estimates from a recent systematic review of HIV treatment cohorts in sub-Saharan Africa.25
With these analyses, we continue to build upon our studies looking at the quality of HIV care in Mozambique.3,19,20,26–28 Integration of HIV care and treatment services into the existing primary health care system dramatically improved access to care and potentially reduced the time from enrolling into care to ART initiation among those initiating treatment. Even though parallel systems were built, vertical clinics were originally tailored to address the specific needs of HIV care and treatment delivery that could explain, in part, why there was a lower risk of attrition among patients attending these clinics. As HIV services moved into the broader public health system, the delivery of HIV services could have met some complexities as services were being delivered within facilities that do not focus entirely on HIV. We adjusted for pharmacy staff burden in our analyses, but over-burdened logistics systems, laboratory facilities, and management staff are potential reasons that higher risk of attrition among patients attending integrated sites was observed.
However, vertical clinics are not a functional model to reach scale in Mozambique, and integration of HIV care and treatment into primary health care clinics is an important strategy to increase ART coverage. Even in urban areas, there are insufficient resources to develop large separate HIV care and treatment services to meet demand and reduce lost to follow-up between testing and entry into care and treatment. Offering HIV care and treatment through the integrated model in rural settings dramatically increased geographic coverage of ART. However, patients attending these sites also had higher rates of attrition, possibly due to more travel burden among a dispersed population with less public transportation options, or less flexible schedules related to employment or farming activities.
Patient experiences within the facility also need to be considered when interpreting our results. Results from a qualitative study conducted in some of the same clinics included in this study indicated that clients were unhappy with pharmacies in integrated sites, as they did not provide adequate privacy for patients picking up their medications among other people from their communities.28 For example, one client mentioned, “Many feel scared to be in the pharmacy in the middle of a lot of people, to pick-up the medication and sign for it. People begin to question, ‘what type of medication is that?’ Many do not arrive at the pharmacy for this reason. They feel scared.”28 These findings highlight the need to understand patient's experiences in the integrated setting to ensure an environment for clients that is conducive for continued engagement in services.
CONCLUSIONS
In our study of more than 11,700 people initiating ART from December 1, 2006, to June 1, 2008, among 17 public sector HIV care and treatment clinics, we found a significantly higher risk of late attrition for patients attending integrated clinics and for clients attending clinics in rural settings. It is important to remember the benefits that integrating ART into primary health care services has afforded HIV-positive clients. This integration brought essential HIV care and treatment services closer to communities in need and resulted in a dramatic increase in the number of patients initiating ART. However, understanding the areas where improvements can be made and developing strategies to address the challenges observed in the integrated environment is vital to retaining patients on ART. Further research should seek to understand the challenges faced by clients, where ART is offered alongside primary health care services and in rural settings, and continue to identify potential interventions that address these issues.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the patients and providers at the clinics included in the study.
Supported by a grant from the United States Agency for International Development as part of the President's Emergency Plan for AIDS Relief.
Footnotes
The authors B.L. and J.L. performed the literature search. B.H.L., M.A.M., J.P., K.S., and S.S.G. contributed to the study design. B.H.L., J.L., and M.K. assisted with data collection. B.H.L. and M.A.M. informed the data analysis. All authors contributed to the interpretation of study results and article preparation.
Portions of these data have been previously presented as an abstract at the International AIDS Conference, 2011, Rome, Italy.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
REFERENCES
- 1.UNAIDS [January 31, 2012];AIDS at 30: nations at the crossroads. 2011 Available at: http://www.unaids.org/unaids_resources/aidsat30/aids-at-30.pdf.
- 2.WHO/UNAIDS Global HIV/AIDS response—epidemic update and health sector progress towards universal access: progress report 2011. 2011:1–223. Available at: http://whqlibdoc.who.int/publications/2011/9789241502986_eng.pdf. February 2, 2012.
- 3.Pfeiffer J, Montoya P, Baptista AJ, et al. Integration of HIV/AIDS services into African primary health care: lessons learned for health system strengthening in Mozambique—a case study. [January 31, 2012];J Int AIDS Soc. 2010 13:3. doi: 10.1186/1758-2652-13-3. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgiartid=2828398&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stringer JSA, Zulu I, Levy J, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. [August 18, 2011];JAMA. 2006 296:782–793. doi: 10.1001/jama.296.7.782. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16905784. [DOI] [PubMed] [Google Scholar]
- 5.Chan AK, Mateyu G, Jahn A, et al. Outcome assessment of decentralization of antiretroviral therapy provision in a rural district of Malawi using an integrated primary care model. [September 25, 2011];Trop Med Int Health. 2010 15(suppl 1):90–97. doi: 10.1111/j.1365-3156.2010.02503.x. Available at: http://www.ncbi.nlm.nih.gov/pubmed/20586966. [DOI] [PubMed] [Google Scholar]
- 6.Topp SM, Chipukuma JM, Giganti M, et al. Strengthening health systems at facility-level: feasibility of integrating antiretroviral therapy into primary health care services in lusaka, zambia. [October 2, 2011];PloS one. 2010 5:e11522. doi: 10.1371/journal.pone.0011522. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2903482&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deribe K, Hailekiros F, Biadgilign S, et al. Defaulters from antiretroviral treatment in Jimma University specialized hospital, Southwest Ethiopia. [December 15, 2011];Trop Med Int Health. 2008 13:328–333. doi: 10.1111/j.1365-3156.2008.02006.x. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18298607. [DOI] [PubMed] [Google Scholar]
- 8.Zachariah R, Harries AD, Manzi M, et al. Acceptance of anti-retroviral therapy among patients infected with HIV and tuberculosis in rural Malawi is low and associated with cost of transport. [September 30, 2011];PloS one. 2006 1:e121. doi: 10.1371/journal.pone.0000121. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1762339&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu JKL, Chen SC-C, Wang K-Y, et al. True outcomes for patients on anti-retroviral therapy who are “lost to follow-up” in Malawi. [February 8, 2012];Bull World Health Organ. 2007 85:550–554. doi: 10.2471/BLT.06.037739. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2636367&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massaquoi M, Zachariah R, Manzi M, et al. Patient retention and attrition on antiretroviral treatment at district level in rural Malawi. [September 19, 2011];Trans R Soc Trop Med Hyg. 2009 103:594–600. doi: 10.1016/j.trstmh.2009.02.012. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19298993. [DOI] [PubMed] [Google Scholar]
- 11.Spira TJ, Ellerbrock TV. Commentary on Greig et al, “Similar mortality and reduced loss to follow-up in integrated compared with vertical programs providing antiretroviral treatment in Sub-Saharan Africa”. [November 2, 2011];J Acquir Immune Defic Syndr. 2012 59:82–84. doi: 10.1097/QAI.0b013e31824c1985. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22330609. [DOI] [PubMed] [Google Scholar]
- 12.Fredlund VG, Nash J. How far should they walk? Increasing antiretroviral therapy access in a rural community in northern KwaZulu-Natal, South Africa. [February 8, 2012];J Infect Dis. 2007 196(suppl 3):S469–S473. doi: 10.1086/521115. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18181696. [DOI] [PubMed] [Google Scholar]
- 13.Bedelu M, Ford N, Hilderbrand K, et al. Implementing antiretroviral therapy in rural communities: the Lusikisiki model of decentralized HIV/AIDS care. [December 3, 2011];J Infect Dis. 2007 196(suppl 3):S464–S468. doi: 10.1086/521114. Available at: http://jid.oxfordjournals.org/content/196/Supplement_3/S464.full. [DOI] [PubMed] [Google Scholar]
- 14.Greig J, O'brien D, Ford N, et al. Similar mortality and reduced loss to follow-up in integrated compared with vertical programs providing anti-retroviral treatment in sub-Saharan Africa. [January 9, 2012];J Acquir Immune Defic Syndr. 2012 59:e92–e98. doi: 10.1097/QAI.0b013e31824206c7. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22134144. [DOI] [PubMed] [Google Scholar]
- 15.Grépin KA. Leveraging HIV programs to deliver an integrated package of health services: some words of caution. [January 12, 2012];J Acquir Immune Defic Syndr. 2011 57(suppl 2):S77–S79. doi: 10.1097/QAI.0b013e31821f6afa. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21857301. [DOI] [PubMed] [Google Scholar]
- 16.Atun R, de Jongh T, Secci F, et al. A systematic review of the evidence on integration of targeted health interventions into health systems. [November 2, 2011];Health policy Plan. 2010 25:1–14. doi: 10.1093/heapol/czp053. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19959485. [DOI] [PubMed] [Google Scholar]
- 17.Ministério da Saúde [January 31, 2012];Programa de tratemento antiretroviral (TARV)–dados TARV nacionais. 2010 Available at: http://www.misau.gov.mz/pt/hiv_sida/dados_tarv_nacionais.
- 18.United Nations Development Programme . Mozambique National Human Development Report 2007. Vol. 2007. United Nations Development Programme; Maputo, Mozambique: p. 59. [Google Scholar]
- 19.Lambdin B, Micek M, Koepsell T, et al. An assessment of the accuracy and availability of data in electronic patient tracking systems for patients receiving HIV treatment in central Mozambique. [November 2, 2011];BMC Health Serv Res. 2012 12:30. doi: 10.1186/1472-6963-12-30. Available at: http://www.biomedcentral.com/1472-6963/12/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lambdin BH, Micek MA, Koepsell TD, et al. Patient volume, human resource levels, and attrition from HIV treatment programs in central Mozambique. [January 31, 2012];J Acquir Immune Defic Syndr. 2011 57:e33–e39. doi: 10.1097/QAI.0b013e3182167e90. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21372723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo Y, Manatunga AK. Nonparametric estimation of the concordance correlation coefficient under univariate censoring. [January 31, 2012];Biometrics. 2007 63:164–172. doi: 10.1111/j.1541-0420.2006.00664.x. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17447941. [DOI] [PubMed] [Google Scholar]
- 22.van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. [January 31, 2012];Stat Med. 1999 18:681–694. doi: 10.1002/(sici)1097-0258(19990330)18:6<681::aid-sim71>3.0.co;2-r. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10204197. [DOI] [PubMed] [Google Scholar]
- 23.Rubin DB. Multiple Imputation for Non-Response in Surveys. John Wiley & Sons; New York, NY: 1987. [Google Scholar]
- 24.Lahuerta M, Lima J, Elul B, et al. Patients enrolled in HIV care in Mozambique: baseline characteristics and follow-up outcomes. [October 17, 2012];J Acquir Immune Defic Syndr. 2011 58:e75–e86. doi: 10.1097/QAI.0b013e31822ac0a9. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3422887&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fox MP, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007–2009: systematic review. [October 17, 2012];Trop Med Int Health. 2010 15(suppl 1):1–15. doi: 10.1111/j.1365-3156.2010.02508.x. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2948795&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Micek MA, Gimbel-Sherr K, Baptista AJ, et al. Loss to follow-up of adults in public HIV care systems in central Mozambique: identifying obstacles to treatment. [August 9, 2011];J Acquir Immune Defic Syndr. 2009 52:397–405. doi: 10.1097/QAI.0b013e3181ab73e2. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2784145&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sherr KH, Micek MA, Gimbel SO, et al. Quality of HIV care provided by non-physician clinicians and physicians in Mozambique: a retrospective cohort study. [November 18, 2011];AIDS. 2010 24(suppl 1):S59–S66. doi: 10.1097/01.aids.0000366083.75945.07. Available at: http://www.ncbi.nlm.nih.gov/pubmed/20023441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lara J, Lambdin BH. Exploratory analysis of the facility-level factors associated with HAART abandonment in central Mozambique.. Presented at: APHA; Philadelphia, PA. November 11, 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.