Summary
Ceritinib and other second-generation inhibitors have demonstrated promising anticancer activity in anaplastic lymphoma kinase (ALK)–positive non–small cell lung cancer (NSCLC). Specifically, they can overcome resistance due to certain gatekeeper mutations acquired following crizotinib exposure. These agents now provide new options for the management of ALK-positive NSCLC.
A mere 7 years ago, aberrant activation of the anaplastic lymphoma kinase (ALK) gene was identified as a potential therapeutic target for a subset of patients with non-small cell lung cancer (NSCLC; ref. 1). The ensuing development of therapeutic agents that target ALK and their application in the clinical setting can be described as no less than a “bolt out of the blue.” Approximately 5% of patients with NSCLC harbor an activating gene rearrangement involving ALKwhich is detected by using the fluorescent in situ hybridization (FISH) test. More recently, detection of ALK by immunohistochemistry and RT-PCR has been pursued for either screening or confirmation. ALK-positive disease has certain unique characteristics, including the presence of pleural and/or pericardial involvement, a greater propensity for metastasis, and lower likelihood of response to conventional systemic therapeutic approaches. Crizotinib, which was under development as an inhibitor of the c-MET pathway, was found to be associated with objective responses in patients with ALK-positive NSCLC in a phase I clinical trial, based on its ability to inhibit ALK. These initial observations formed the basis for the development of crizotinib specifically for patients with ALK-positive NSCLC that culminated in the approval of this agent by the FDA in 2011. Subsequent studies have demonstrated the superiority of crizotinib over standard chemotherapy regimens for patients with ALK-positive NSCLC (2). Crizotinib results in an objective response rate of approximately 60% and median progression-free survival of 10 months, and is associated with a good tolerability profile (3). For these reasons, crizotinib is now considered the “standard of care” for ALK-positive NSCLC (advanced-stage disease). This agent is presently being studied for patients with earlier stages of NSCLC, including as adjuvant therapy for patients with surgically resected, early-stage NSCLC (ALCHEMIST, EA 5412).
As is the case with other targeted therapies for oncogene-addicted tumors, resistance to crizotinib is inevitable and is observed in approximately 10 to 12 months. The molecular mechanisms of resistance include development of secondary gatekeeper mutations and the activation of alternate oncogenic pathways. The secondary mutations confer a structural change at the ATP-binding pocket that causes steric hindrance to binding by crizotinib (4). Unlike the situation with T790M mutation, observed in nearly 60% of NSCLC tumors bearing activating mutations in the EGFR gene, the secondary mutations associated with ALK inhibition are distributed over a number of locations on the gene (5). The functional consequences of some of these mutations, such as L1196M and G1269A, are known, but for certain other mutations, the exact mechanisms that contribute to resistance are not understood. The knowledge gained about resistance mechanisms has prompted the development of therapeutic approaches to treat patients with acquired resistance to crizotinib.
In this issue of Cancer Discovery, Friboulet and colleagues (6) report on the ability of ceritinib, a potent second-generation ALK inhibitor, to overcome resistance to crizotinib. Briefly, their article illustrates the following points: (i) ceritinib inhibited ALK signaling in two cell lines with L1196M and G1269A, the two most common resistance mutations; (ii) ceritinib was approximately 20-fold more potent in ALK-rearranged NSCLC cell lines and showed more sustained growth inhibition in xenograft models compared with crizotinib; (iii) the efficacy of ceritinib against crizotinib-resistant ALK likely derives from its Cl moiety of the pyrimidine hinge-binding core that is not hindered by the structural change in the binding pocket; and (iv) ceritinib was active in xenograft models that did not harbor a resistance mutation. These elegant experiments provide the mechanistic reasons underlying the robust anticancer activity of ceritinib in a phase I study for patients with ALK-positive NSCLC (7). The objective response rate was 58% for the 114 patients who were treated with ceritinib at doses above 400 mg/d. Notably, the objective response rate was similar for patients who had not been previously treated with an ALK inhibitor and those who had experienced disease progression on therapy with crizotinib. The responses were durable and lasted for a median of 8.2 months. Preliminary evidence suggested a longer progression-free survival among crizotinib-naïve patients compared with those who were resistant (10.4 and 6.9 months, respectively). Gastrointestinal adverse events, fatigue, and elevation of hepatic transaminases were the salient toxicities associated with ceritinib. These promising clinical data have resulted in ceritinib being accorded “accelerated approval” by the FDA in 2014.
Promising anticancer activity has also been demonstrated with other second-generation ALK inhibitors recently. Alectinib, another ALK inhibitor with several-fold higher potency over crizotinib, was associated with a response rate of approximately 55% in a phase I study for patients with resistance to crizotinib. On a more exciting note, a phase I study conducted in Japan with alectinib for crizotinib-naïve patients reported a response rate of >90% (8). AP26113, another potent inhibitor of ALK, demonstrated a response rate of approximately 70% in a cohort of crizotinib-refractory patients. A number of other highly potent ALK inhibitors are currently in clinical evaluation as well, increasing the number of agents that are being studied for this relatively small molecular subset of NSCLC. Unlike crizotinib, the second-generation agents were specifically developed as inhibitors of ALK. They are capable of achieving target inhibition at lower doses and can therefore overcome resistance that might result from subtherapeutic exposure to crizotinib within the tumor. This effect could explain the efficacy of the newer agents even in patients without the gatekeeper mutations following crizotinib therapy. This could also explain the lower incidence of gatekeeper mutations with acquired resistance to crizotinib, compared with the nearly 60% prevalence of T790M following EGFR inhibition. As logical extensions of these observations, randomized studies are already under way to compare the efficacy of the second-generation agents with crizotinib in first-line therapy for ALK-positive NSCLC. It is hoped that higher efficacy and delay in the onset of resistance can be achieved with this strategy.
Although the availability of a plethora of new agents against ALK is an exciting prospect for patients, one cannot overlook the obvious challenges this raises. With ceritinib, objective responses have been observed at doses >400 mg/d. However, the dose used for the clinical studies is 750 mg/d, which is the maximum-tolerated dose (MTD) of ceritinib. The other second-generation ALK inhibitors are also being given at the MTD, though activity has been observed at lower doses. Does a higher dose result in greater efficacy for patients? Are the incremental toxicities noted with the higher doses offset by the greater efficacy? With the intense competition between these agents in the development cycle, it is unlikely that the dose-response issue will be addressed in clinical studies. This issue is also particularly relevant as the doses studied may not be conducive to the development of second-generation combination regimens. It is widely held that the use of rational combination approaches is most likely to take us closer to the goal of cure for patients with driver mutations.
Friboulet and colleagues (6) also provide information about resistance mechanisms to ceritinib. In a cohort of 11 patients who underwent biopsy upon emergence of resistance to ceritinib, none of the patients harbored the sensitive gatekeeper mutations. These early observations require further study in larger cohorts of patients, though it is highly likely that the spectrum of resistance mechanisms will be different from that with crizotinib. Inhibition of HSP90 could prove to be a useful strategy to manage acquired resistance to the second-generation ALK inhibitors (9). ALK is a sensitive client protein to HSP90, and therefore objective responses have been noted with various inhibitors, including ganetespib, AUY922, and retispamycin (10). Because the target is the chaperone, as opposed to the oncoprotein itself, HSP90 inhibitors are likely to be active regardless of the specific secondary ALK mutation. Combination approaches of ALK inhibitors with HSP90 inhibitors could be a potential therapeutic strategy to delay or overcome resistance. The exciting early data with agents targeting immune checkpoints in NSCLC also provide the potential for new combination regimens with ALK inhibitors.
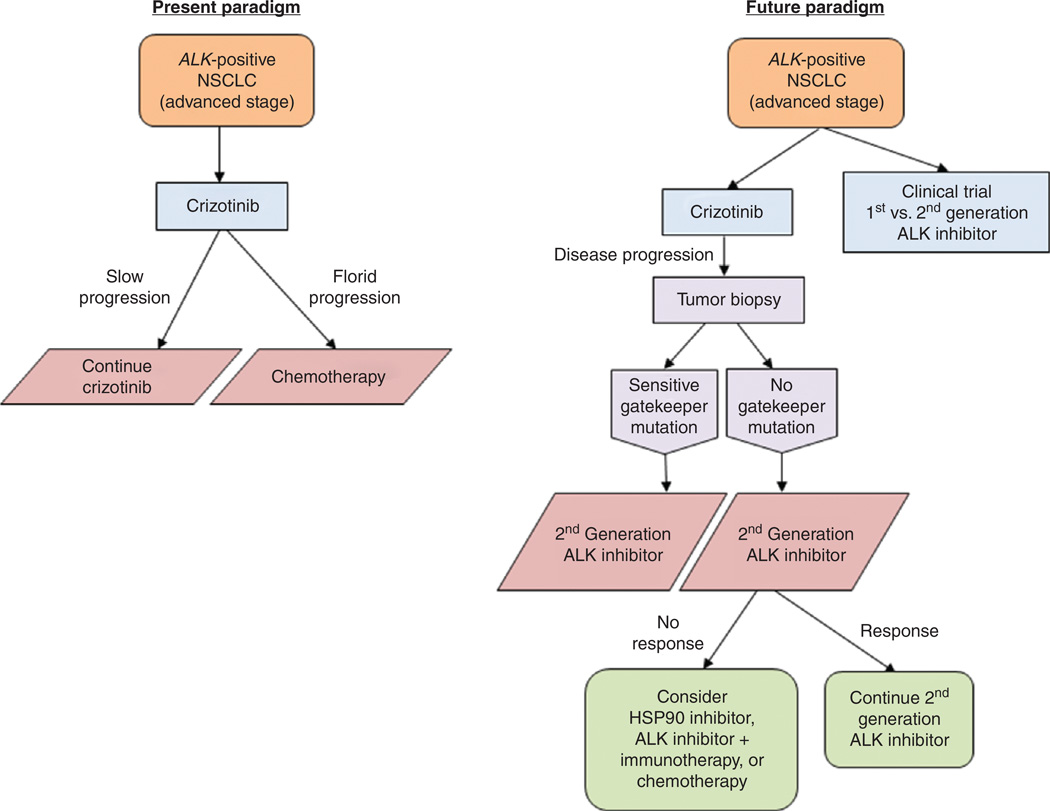
In summary, these recent developments raise a number of exciting new options for patients with ALK-positive NSCLC. The treatment paradigms are poised to change in short order, with the emergence of the second-generation ALK inhibitors (Fig. 1). Whether they will be used as first-line therapy or sequenced after crizotinib to achieve maximal benefits from both is the critical question. With the exciting results shown in this issue of Cancer Discovery by Friboulet and colleagues (6), the case for obtaining tumor biopsy at the time of resistance to guide therapy is getting stronger for patients with ALK-positive NSCLC. All of these data strengthen the impetus to conduct molecular testing in patients with NSCLC, so that every patient with ALK-positive disease is detected and provided access to the specific targeted therapies.
Figure 1.
Treatment algorithm for patients with advanced-stage lung cancer with ALK gene rearrangement.
Footnotes
Disclosure of Potential Conflicts of Interest
S.S. Ramalingam is a consultant/advisory board member of Novartis and Genentech. No potential conflicts of interest were disclosed by the other author.
REFERENCES
- 1.Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 2.Shaw AT, Kim DW, Nakagawa K, Seto T, Crinó L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–2394. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 3.Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi YL, Soda M, Yamashita Y, Ueno T, Takashima J, Nakajima T, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med. 2010;363:1734–1739. doi: 10.1056/NEJMoa1007478. [DOI] [PubMed] [Google Scholar]
- 5.Doebele RC, Pilling AB, Aisner DL, Kutateladze TG, Le AT, Weickhardt AJ, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res. 2012;18:1472–1482. doi: 10.1158/1078-0432.CCR-11-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friboulet L, Li N, Katayama R, Lee CC, Gainor JF, Crystal AS, et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non- small cell lung cancer. Cancer Discov. 2014;4:662–673. doi: 10.1158/2159-8290.CD-13-0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaw AT, Kim DW, Mehra R, Tan DS, Felip E, Chow LQ, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med. 2014;370:1189–1197. doi: 10.1056/NEJMoa1311107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seto T, Kiura K, Nishio M, Nakagawa K, Maemondo M, Inoue A, et al. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): a single-arm, open-label, phase 1–2 study. Lancet Oncol. 2013;14:590–598. doi: 10.1016/S1470-2045(13)70142-6. [DOI] [PubMed] [Google Scholar]
- 9.Normant E, Paez G, West KA, Lim AR, Slocum KL, Tunkey C, et al. The Hsp90 inhibitor IPI-504 rapidly lowers EML4-ALK levels and induces tumor regression in ALK-driven NSCLC models. Oncogene. 2011;30:2581–2586. doi: 10.1038/onc.2010.625. [DOI] [PubMed] [Google Scholar]
- 10.Socinski MA, Goldman J, El-Hariry I, Koczywas M, Vukovic V, Horn L, et al. A multicenter phase II study of ganetespib monotherapy in patients with genotypically defined advanced non-small cell lung cancer. Clin Cancer Res. 2013;19:3068–3077. doi: 10.1158/1078-0432.CCR-12-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]