Abstract
Epidemiologic studies indicate that moderate alcohol consumption increases breast cancer risk in women. Understanding the mechanistic basis of this relationship has important implications for women’s health and breast cancer prevention. In this commentary, we focus on some recent epidemiologic studies linking moderate alcohol consumption to breast cancer risk, and place the results of those studies within the framework of our current understanding of the temporal and mechanistic basis of human carcinogenesis. This analysis supports the hypothesis that alcohol acts as a weak cumulative breast carcinogen, and may also be a tumor promoter. We discuss the implications of these mechanisms for the prevention and treatment of alcohol-related breast cancer, and present some considerations for future studies. Moderate alcohol consumption has been shown to benefit cardiovascular health, and recently been associated with healthy aging. Therefore, a better understanding of how moderate alcohol consumption impacts breast cancer risk will allow women to make better informed decisions about the risks and benefits of alcohol consumption in the context of their overall health and at different stages of their life. Such mechanistic information is also important for the development of rational clinical interventions to reduce ethanol-related breast cancer mortality.
Since the official classification of ethanol (alcohol) as carcinogenic to humans by the International Agency for Research on Cancer (IARC) 2007 (IARC, 2010), and subsequent epidemiologic studies, the relationship between alcohol drinking and breast cancer in women has attracted much attention. The issue is particularly salient in view of prevalence of breast cancer in Western societies, and reports linking surprisingly low amounts of alcohol consumption to an increased breast cancer incidence. Specifically, the results from a meta-analysis (Hamajima et al., 2002), as well as large epidemiologic studies (Allen et al., 2009; Chen et al., 2011; Li et al., 2010) indicate that each additional 10 g of alcohol/day increases a woman’s risk of breast cancer. In the US, a standard drink contains 14 grams of ethanol. Therefore, if these findings are correct, the implication is that a woman’s risk of breast cancer would be significantly increased by drinking slightly less than one drink per day. A commentary on one of these studies (Allen et al., 2009) took this relationship even further, stating that “There is no level of alcohol consumption that can be considered safe (Lauer and Sorlie, 2009)”.
In our view, drawing firm conclusions about safe levels of drinking based on epidemiologic studies alone is problematic for several reasons, including the lack of a detailed understanding of the temporal and mechanistic aspects of the relationship between alcohol and breast cancer. Towards this end, our goal here is to place the results of some of the recent epidemiologic studies within the framework of our current understanding of the temporal and mechanistic basis of human carcinogenesis, and the possible role of alcohol metabolism therein. We focus primarily on three large epidemiologic studies (Allen et al., 2009; Chen et al., 2011; Li et al., 2010) in part because they raised several important issues which impact the interpretation of other epidemiologic studies. Since these studies focused on middle-age and older women, the health implications need to be considered in the context of other data indicating beneficial effects of moderate alcohol drinking in this population (Sun et al., 2011). By identifying testable mechanistic interpretations of the epidemiologic data, we hope to stimulate additional research which will result in a better understanding of how alcohol drinking affects breast cancer risk in women, and ultimately lead to improvements in women’s health.
The Limits of Epidemiology
The science of epidemiology has made important contributions to human health by identifying the risks associated with cigarette smoke, environmental pollutants, and obesity. However, epidemiology is not intended to identify the mechanistic basis of disease, nor establish actual guidelines for safe levels of drinking. There are several reasons for this, as discussed below.
Self-Reported Alcohol Drinking
One problem with drawing firm conclusions about safe levels of drinking from epidemiologic studies is that such studies depend upon self-reporting of alcohol consumption, which is known to underestimates true consumption. Indeed, the authors of a large and influential meta-analysis (Hamajima et al., 2002) stated this point clearly, writing that “…self-reported information on alcohol consumption is known to underestimate true consumption…” and that “systematic under-reporting of consumption by both cases and controls would result in an overestimation of the relative risk of breast cancer for a given level of alcohol consumption.” Of course, it is precisely this issue that is the most relevant from a public health perspective, and for providing guidance for safe levels of drinking. In addition, as recently noted (Jimenez et al., 2012) underreporting alcohol drinking could potentially lead to spurious associations at lower levels of consumption.
Seven drinks per week or 1 drink per day?
In addition to underreporting actual alcohol consumption in epidemiologic studies, another limitation is the lack of information about drinking pattern. In some epidemiologic studies (Allen et al., 2009; Hamajima et al., 2002) women report consumption on a per week basis, which is converted into drinks / day by dividing by 7. While this is seemingly a simple and straightforward calculation, it raises a major interpretational issue. According to this approach, a woman who drinks 3–4 drinks on Friday and Saturday night and no alcohol during the rest of the week could accurately report drinking 7 drinks / week. After dividing by 7, she would be classified as drinking one drink / day, and her data combined with those of women who do in fact drink only one drink / day. Clearly, drinking multiple drinks in the same sitting will result in higher blood alcohol levels than from a single drink, which can result in qualitatively different metabolic consequences, such as the induction of CYP2E1 and formation of free radicals (see below). In support of this concept, a recent study (Chen et al., 2011) showed that binge drinking (defined as 4 or more drinks at one time for a woman) was modestly associated with breast cancer risk, after controlling for cumulative intake. It should also be noted here that the problem with underestimating actual consumption in epidemiologic studies is likely to be even more significant in analyses related to binge drinking. In contrast to moderate alcohol consumption, binge drinking is socially inappropriate, and therefore more likely to be intentionally underreported by participants (Giovannucci et al., 1993).
Temporal issues and their implication for mechanisms
Some epidemiologic studies relate lifetime breast cancer risk to alcohol consumption reported at a single point in time (Allen et al., 2009; Li et al., 2010). However, alcohol consumption is typically a lifelong habit. In addition, the development of breast cancer is a multi-step, time dependent process. Below we briefly discuss some temporal and mechanistic aspects of human carcinogenesis, followed by a consideration of the implications of this information for the interpretation of epidemiologic studies relating alcohol consumption to breast cancer risk.
Cancer is the result of multiple genetic and epigenetic changes (Vogelstein and Kinzler, 2004) which result in a set of phenotypes referred to as the “hallmarks of cancer” (Hanahan and Weinberg, 2000). Evidence from the number of mutations in cancer genomes also supports the concept that a mutator phenotype is an important early step in the carcinogenic process (Loeb, 2011). Early acquisition of a mutator phenotype increases the cellular mutation rate, thereby facilitating additional mutations necessary to produce an invasive cancer. However, in the epidemiologic literature, these fundamental aspects of cancer biology are generally not addressed. Furthermore, there is a lack of consideration of the length of time necessary for human cancer to develop, and the implications of this time course on the interpretation of epidemiologic studies.
Based on studies of known chemical carcinogens in humans, it has been estimated that it takes at least 20 years from exposure to a carcinogen to a clinical cancer diagnosis (Bielas and Loeb, 2005; Pierce et al., 1992). This surprisingly long time frame has recently received additional support from whole genome analyses of human cancers. Deep sequencing analysis of pancreatic cancers, as well as mathematical modeling (Yachida et al., 2010), estimated that the time between a tumor-initiating mutation and the non-metastatic founder cell is ≈12 years. After that, additional years are necessary for that individual cancer cell to proliferate into a tumor large enough to be detected clinically. Based on measurements of the size of breast cancers in vivo, tumor volume doubling times have been estimated to be 100–200 days (Spratt et al., 1996; von Fournier et al., 1980). Assuming continuous exponential growth, with these doubling times, it would take roughly 8–16 years for a single cancer cell to grow into a clinically detectable tumor (Spratt et al., 1995; von Fournier et al., 1980). It should be also noted that breast cancers in women > 50 years old grow more slowly than those in younger women (Peer et al., 1993; Spratt et al., 1996).
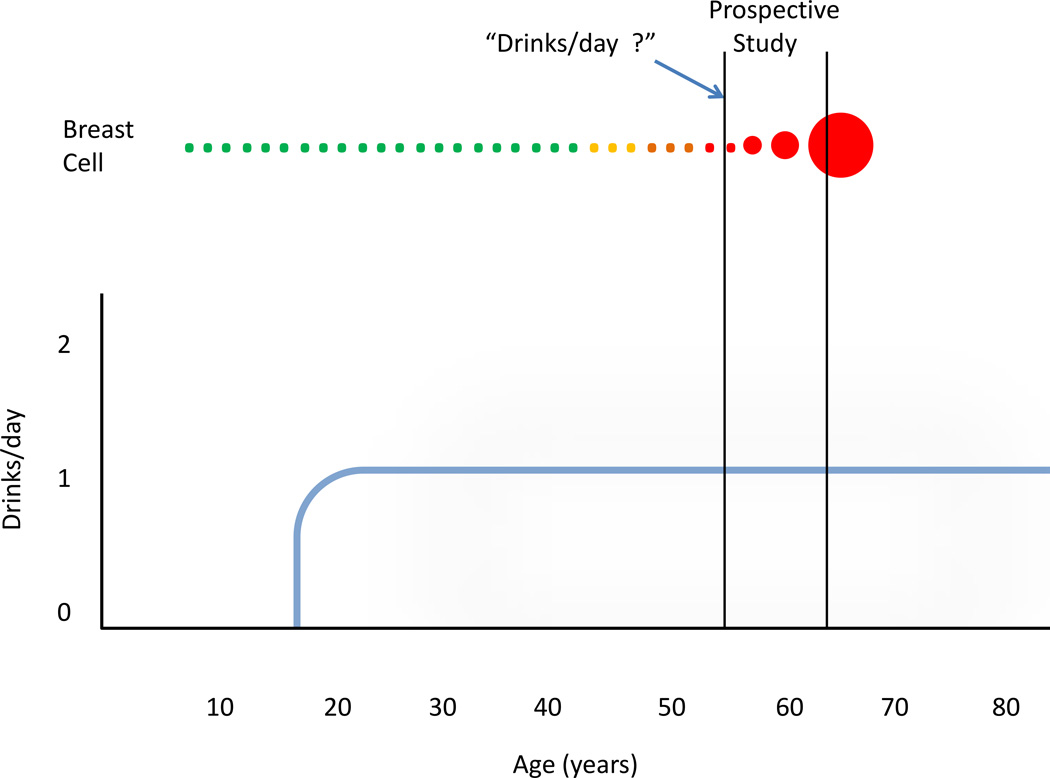
When the results of the recent epidemiologic studies are considered in view of the temporal considerations above, the results are illuminating. In the study by Allen et al (Allen et al., 2009), the average time between entry into the study and the end of the study was 7.1 years, while in the case of the Li et al, it was 7–12 years (Li et al., 2010). Therefore, for the women in both studies, the breast cancers they were diagnosed with during the study must have been initiated before they enrolled in the study. Consequently, the alcohol that these women reported drinking at the beginning of the study could not have been the initiating event in the breast cancers that they were diagnosed with. Figure 1 is a simplified graphic representation of these temporal relationships.
Figure 1.
Representation of the temporal relationship between lifetime alcohol consumption and breast cancer for a hypothetical woman who enters a prospective epidemiologic study (e.g. Allen et al., 2009). The top of the figure represents a normal breast cancer cell (green) which undergoes phenotypic changes (yellow, brown), becoming an immortalized cancer cell (red). The vertical black lines mark the beginning and end of the prospective study. The cancer cell grows over a period of years, ultimately becoming large enough to be detected by screening. The heavy blue line represents alcohol consumption remaining steady at 1 drink/day.
The above considerations have important implications for understanding how the relationship between moderate alcohol consumption by postmenopausal women and breast cancer incidence can be understood mechanistically. It seems that there are two quite different mechanistic possibilities, which are not mutually exclusive: The first possibility is that alcohol acts on a pre-existing tumor, either by increasing the growth rate of breast cancer cells, or by altering some other aspect of cancer cell biology which increases the likelihood of a clinical diagnosis within the time frame of a given study. We will refer to this as the tumor promoter hypothesis. Another possibility is that alcohol is a weak cumulative carcinogen, and that self-reported alcohol consumption at the time of study entry is a surrogate marker of lifetime exposure. We refer to this as the cumulative carcinogen hypothesis. Because these two explanations have potentially important but fundamentally different implications for disease prevention, we consider these possibilities separately below.
Possibility 1: Alcohol as a Breast Tumor Promoter
In the first possibility, alcohol consumption acts as a classical tumor promoter, decreasing the latency of breast cancer. Such a mechanism is consistent with the definition of a carcinogenic agent according to IARC guidelines (IARC, 2010). Possible tumor promoter mechanisms include increasing the growth rate of the tumor, and/or altering the biology of the tumor or its micro-environment in a manner that increases invasiveness.
The Estrogen Hypothesis and its Complications
Perhaps the most common hypothesis in the epidemiologic literature is that alcohol increases breast cancer risk via alterations in circulating estrogen levels (Chen et al., 2011; Singletary and Gapstur, 2001). Consistent with this possibility, (Li et al., 2010) found a relationship between estrogen receptor (ER) positive tumors and alcohol drinking in postmenopausal women. Therefore, a possible explanation for the relationship between moderate alcohol consumption and breast cancer in postmenopausal women is that ongoing alcohol consumption could increase the growth rate, or affect other properties of ER positive cancers (Tyson et al., 2011), thereby increasing the probability that they would be diagnosed during the course of the study. A problem with this mechanism is that, as discussed below, moderate alcohol consumption does not increase serum estrogen levels in postmenopausal women.
The key study was that of Dorgan et al. (1994), in which 51 postmenopausal women underwent 8-week periods of drinking either 15 g of ethanol/day, 30 g of ethanol /day, or a placebo alcohol-free beverage. The order of the three drinking periods was chosen at random for each woman. The study participants agreed to consume only those foods and alcohol supplied to them by the study coordinators. In addition, body weights of all the participants were obtained weekly, and the caloric content of the diets adjusted to maintain body weight within a set range throughout the study. At the end of each 8 week exposure period, blood was collected for assay of steroid hormone levels.
The authors found that daily consumption of 15 g of ethanol/day did not significantly increase serum levels of any estrogens. The only hormone that was significantly elevated was dehydroepiandrosterone sulfate (DHEAS), which is an androgen primarily secreted by the adrenal gland. In women drinking 30 g of ethanol/day, DHEAS levels were elevated along with those of estrone sulfate. Notably, the most biologically potent estrogen, estradiol, was not elevated by either dose of alcohol.
The Dorgan study design left open the possibility that acute alcohol drinking could transiently increase estrogen levels. However, in another interventional study, Ginsburg et al found no effect of acute ethanol on serum estradiol levels in postmenopausal women not taking hormone replacement therapy (HRT) (Ginsburg et al., 1996).
Additional evidence against the ethanol – estrogen secretion mechanism comes from studies of postmenopausal women using HRT. While women in the Dorgan study were not using HRT, other studies have shown that alcohol does increase estrogen levels in postmenopausal women who are taking HRT(Ginsburg et al., 1996). Therefore, if the effect of moderate alcohol on breast cancer risk were due to increased estrogen levels, and alcohol only increases estrogen levels in postmenopausal women on HRT, it follows that the relationship between moderate alcohol consumption and breast cancer risk would only be observed in postmenopausal women on HRT. However, in the Allen study, the increased breast cancer risk per 10 g/day in current users of HRT was no different than in non-users. Similarly, Li et al. (2010) found that the multivariable adjusted risks of hormone receptor positive ductal cancers per drink per day among current HRT users were similar to those for never users.
In summary, daily consumption of 15 g of ethanol/day has no effect on serum estrogen levels in postmenopausal women, and the prediction based on the alcohol-estrogen mechanism is not confirmed by either the Allen et al or Li et al studies. Furthermore, a recent meta-analysis also found no effect of drinking less than 19 g of ethanol/day on estradiol levels (Key et al., 2011). Therefore, the observed relationship between daily consumption of 10 g of alcohol and breast cancer risk in postmenopausal women cannot be explained by a mechanism involving increased serum estrogen levels.
As noted above, daily consumption of 15 g of ethanol/day does increase DHEAS levels, and this finding is consistently observed in other studies (Key et al., 2011). However, relating increased DHEAS to breast cancer risk is complicated. On the one hand, DHEAS can be metabolized to estrogen in target tissues, including the breast, via a mechanism involving aromatization (Geisler et al., 2011). As such, DHEAS could be considered an estrogen precursor. On the other hand, DHEA is an androgen, and androgens inhibit breast cancer cell growth (Labrie et al., 2003). Thus, more research into the relationship between alcohol, DHEAS metabolism, and breast cancer risk is necessary.
Other Mechanisms by Which Ethanol can Promote Breast Cancer
While changes in sex hormones in relation to alcohol consumption have received the most attention in the epidemiology literature, moderate alcohol drinking may have other biological effects on an initiated tumor that could be of potential relevance to the observed breast cancer risk. The epithelial–mesenchymal transition (EMT) is emerging as an important mechanism in the development of invasive cancer (Kalluri and Weinberg, 2009). (Forsyth et al., 2010) recently found that ethanol stimulates the EMT in human breast cancer cells in vitro. Notably, the ethanol concentrations used in this work were consistent with those that could be attained during moderate drinking. If these in vitro results are applicable to human breast cancers in vivo, then ethanol stimulation of the EMT could provide a mechanistically and temporally plausible explanation for the observed relationship between moderate alcohol consumption on breast cancer risk. Similarly, a recent in vitro study showed that alcohol increased the invasiveness of human breast cancer cells (Wong et al., 2011b). Again, if the mechanisms described in this work are operative in vivo, they could provide clinical relevant targets for alcohol-related breast cancer.
Finally, a recent study reported a correlation between self-reported alcohol consumption, and global DNA methylation in breast tumors (Christensen et al., 2010). While this finding is consistent with ethanol acting as a tumor promoter by altering DNA methylation, it is somewhat difficult to interpret mechanistically, since no individual CpG loci showed statistically significant alcohol-related changes in methylation.
Summary
In summary, one explanation for the observed relationship between moderate alcohol consumption in postmenopausal women and breast cancer incidence is that alcohol consumption acts upon pre-existing breast cancer cells, or the tissue surrounding the tumor, in a manner that increases the likelihood of a diagnosis of invasive breast cancer. While studies do not support a mechanism involving increased estrogen levels in postmenopausal women, other plausible mechanisms include elevated DHEAS, which could be converted to estradiol in breast tissue, or ethanol stimulation of the EMT and tumor invasiveness. Further research into these possibilities could lead to clinical benefit in alcohol related breast cancer.
Possibility 2: Alcohol as a Weak Cumulative Breast Carcinogen
Statements about breast cancer risk from moderate alcohol consumed daily are based on relationship observed between self-reported daily alcohol consumption at the time of study entry (or 3 years after enrollment, in Allen et al) and the relative risk of breast cancer detected during the study. As noted above, temporal and mechanistic considerations demonstrate that drinking reported at the time of study enrollment could not have caused the cancers. Presumably, however, the women in these studies had been drinking alcohol for some period of time before entering the study. Previous studies (Longnecker et al., 1995b; Terry et al., 2006), support a relationship between lifetime alcohol consumption and breast cancer risk. Therefore, if alcohol acts as a cumulative carcinogen, and alcohol consumption by postmenopausal women is a surrogate measure of lifetime alcohol consumption, then assessing alcohol consumption in postmenopausal women monitors lifetime exposure to a presumably cumulative carcinogen.
The recent report by Chen et al. (2011) also supports the lifetime carcinogen hypothesis. If we presume that the only effect of alcohol drinking was to increase the growth rate of pre-existing tumors, then the relationship between breast cancer risk and alcohol drinking would be limited to recent drinking. However, Chen et al found that when examined separately, alcohol consumption in early adulthood (18 to 40 years) and after 40 years, were both strongly associated with the risk of breast cancer (Chen et al., 2011). These findings are not consistent with a pure tumor-promoter type mechanism for ethanol and breast cancer, but are consistent with a cumulative carcinogen mechanism.
If we consider the significance of one additional self-reported drink / day in the context of lifetime exposure, the dose-response relationship looks quite different. Assuming for the purpose of illustration that each additional 10 grams/day reported by a woman upon entering a study corresponds to an additional 10 g/day from the age of beginning drinking (18 years old) to age 55, then this corresponds to ≈ 135,000 g, or 135 kg of ethanol over that time period. Even smaller differences in self-reported amounts at study entry would correspond to large additional amounts consumed over a lifetime. Of course, in reality the relationship between self-reported drinking by postmenopausal women and lifetime consumption is not so simple or linear. However, if alcohol consumption in postmenopausal women is proportional to lifetime consumption, the basic point still holds; small differences in self-reported drinks/day at study entry correspond to large differences in the amount of alcohol consumed over a lifetime, and it is these large differences in cumulative carcinogen exposure over the lifetime that are responsible for the small differences in breast cancer risk.
The hypothesis that chronic exposure to alcohol over many years could increase the risk of breast cancer in women is mechanistically plausible. Alcohol is metabolized by alcohol dehydrogenase (ADH) into acetaldehyde, a known genotoxin, and carcinogen which could increase breast cancer risk via multiple mechanisms (Seitz and Stickel, 2007). Both the ADHIB and ADH1C genes are expressed in the human breast, encoding alcohol dehydrogenases that are active at ethanol concentration that can be generated in the blood during social drinking. Consistent with these data, direct biochemical studies have shown that normal human breast tissue has the capacity to metabolize ethanol at low concentrations, and ADH immunoreactivity is also detectable in breast epithelial cells (Triano et al., 2003). Also, rodent mammary tissue can metabolize ethanol into acetaldehyde (Castro et al., 2006), providing a potential explanation for animal studies showing that chronic alcohol drinking causes mammary tumors in female mice (Watabiki et al., 2000).
An important question in this context is the expression of ALDH2 in the human breast, which would be protective against acetaldehyde generated from local alcohol metabolism. Publicly available datasets (http://www.nih.gov/geo) indicate low levels of ALDH2 mRNA expression in human mammary gland, but to our knowledge there is no published evidence of ALDH2 enzymatic activity in this tissue. If ALDH2 enzyme is active in the human breast epithelial cells, women expressing the inactive form of ALDH2 could be at elevated risk of breast cancer from alcohol drinking. One Japanese study (Kawase et al., 2009) did not observe this effect, but this study included only 11 such ALDH2-deficient individuals drinking more than 15 gm/day.
In addition to ADH, the ethanol-inducible CYP2E1 is also expressed in breast tissue. CYP2E1 can activate procarcinogens, and also generates oxygen radicals, which can cause lipid peroxidation, as an integral part of its catalytic activity (Caro and Cederbaum, 2004). Elevated levels of CYP2E1 were recently shown to increase levels of mutagenic DNA adducts in the esophagus, another target tissue for alcohol-related carcinogenesis (Millonig et al., 2011).
Drinking pattern, CYP2E1, and alcohol-related breast cancer
Aside from the publication of (Chen et al., 2011), little information is available in the literature on the topic of alcohol drinking pattern and breast cancer risk. One important reason for focusing on this issue is that drinking pattern strongly influences the blood alcohol concentration (BAC). The BAC in turn, determines the biochemical changes that take place in the human body after alcohol drinking. Specifically, the BAC produced in a woman after a single drink of alcohol is approximately 10 mM, which is above the ethanol Km for the alcohol dehydrogenases expressed in the breast (ADH1B and ADH1C). The ethanol-inducible CYP2E1 expressed in human breast becomes significant for ethanol metabolism at higher BAC > 20 mM (Salaspuro and Lieber, 1978). BACs in this range would require consuming multiple drinks in a given setting. As noted above, alcohol metabolism by CYP2E1 leads to the formation of mutagenic DNA adducts (Millonig et al., 2011). Based on these considerations, a plausible hypothesis is that alcohol-related breast cancer risk is correlated with the amount of time when a woman has BACs at a level sufficient to induce CYP2E1, which can be roughly related to binge drinking. Therefore, although not discussed by Chen et al. (2011) their observations linking binge drinking to breast cancer risk are in fact consistent with a mechanism involving genotoxicity from induction of CYP2E1 in breast tissue.
Summary
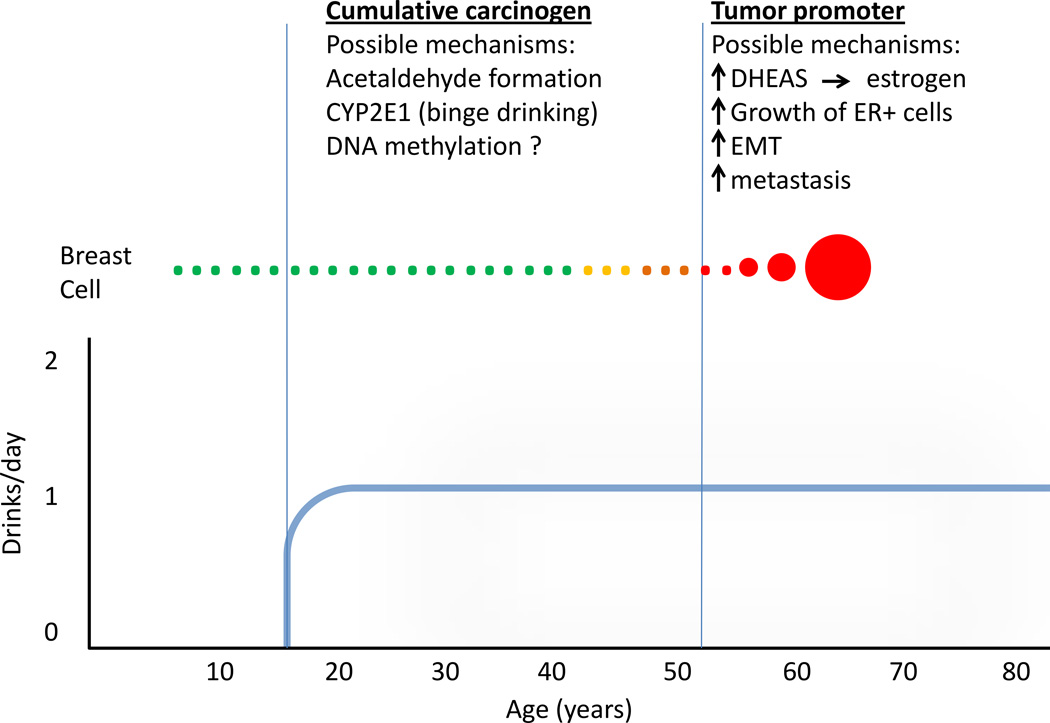
In summary, epidemiologic studies are consistent with alcohol acting as a cumulative carcinogen, and possibly a tumor promoter as well. Figure 2 illustrates these alternatives, as well as some of the most plausible mechanisms discussed above.
Figure 2.
Simplified model to illustrate how alcohol could act as both a cumulative carcinogen and a tumor promoter, depending upon when the drinking occurs. The possible mechanisms discussed in the text are shows above each phase.
Implications for Breast Cancer Prevention and Treatment
The different mechanistic interpretations of the epidemiologic data discussed above have significantly different implications for women’s health and disease prevention. To paraphrase Bradford Hill (1965), the most important question from a public health standpoint is whether the frequency of an undesirable event (in this case breast cancer), will be influenced by a change in the environmental feature (in this case alcohol drinking). As discussed below, the answer to this question very much depends upon the mechanistic basis for the observed relationship.
If the cumulative carcinogen model is correct, breast cancers that women were diagnosed with were caused by alcohol drinking earlier in life. Therefore, the cessation of drinking by postmenopausal women would have no effect on their breast cancer risk. Rather, the greatest overall benefit to women’s health would come from educating younger women about the long-term risks of breast cancer from alcohol drinking. In view of the work of (Chen et al., 2011) indicating that binge drinking is associated with alcohol-related breast cancer risk, that information should be disseminated as well. In this context, recent data indicating that binge drinking by women appears to be increasing (Keyes et al., 2011) is particular cause for concern.
The question of whether postmenopausal women should stop drinking is not without consequence. According to epidemiologic studies, moderate alcohol consumption decreases the risk of cardiovascular disease in women by 17% (Hvidtfeldt et al., 2010) and stroke by 21% (Jimenez et al., 2012). Notably, the magnitude of these health benefits of moderate alcohol drinking are comparable to the 8–27% increased breast cancer risk of moderate alcohol drinking (Allen et al., 2009; Chen et al., 2011; Li et al., 2010). Therefore, suggesting that postmenopausal women stop drinking due to concern about breast cancer risk may in fact be counterproductive to their overall health and mortality (see also Fuchs et al., 1995). This is especially true if, as proposed above, alcohol acts as a cumulative carcinogen, and most if not all of the effects of alcohol on breast cancer risk were the result of drinking (or binge drinking) decades earlier in life. In contrast, the mechanistic basis for the cardiovascular benefits of alcohol are well documented, including relatively acute effects on lipid profile, platelets, and the atherogenic process (Zakhari, 1999). The need to consider alcohol-related breast cancer risk in the context of the overall effect of alcohol on women’s health risk was recognized many years ago (Longnecker et al., 1995a).
In contrast, if the tumor promoter model is correct, and alcohol acts on a latent tumor, then women without tumors are not at elevated risk of breast cancer from alcohol drinking. If so, then the cessation of drinking by these women would have no benefit in terms of breast cancer risk. For women with latent tumors, cessation of drinking could delay the diagnosis of breast cancer. As discussed above, the epidemiologic results do not support a pure tumor promoter mechanism. However, it is possible that ethanol acts as both a weak cumulative carcinogen and tumor promoter. Therefore understanding the mechanistic basis of the tumor promoter effect may be important clinically.
It is clear that even one drink/day significantly increases serum DHEAS levels in postmenopausal women (Dorgan et al., 1994). DHEAS can be metabolized to estrogen in situ, by pathways involving steroid sulfatase and aromatase activities (Geisler et al., 2011). The resulting estrogen could then increase the growth rate or other characteristics of ER+ breast cancers. If this hypothesis is correct, then aromatase inhibitors (Goss et al., 2011), and/or steroid sulfatase inhibitors (Geisler et al., 2011) would be protective against alcohol related breast cancer.
In a similar manner, the elucidation of other tumor promoter mechanisms may have implications for clinical intervention in alcohol-related breast cancer. If the tumor promoting effects of ethanol involve stimulation of the EMT or tumor invasiveness, then drugs to counteract these effects could also be beneficial (Creighton et al., 2010).
Future Directions
Multiple large epidemiologic studies have shown that a relationship exists between self-reported alcohol drinking and breast cancer in women (Allen et al., 2009; Chen et al., 2011; Hamajima et al., 2002; IARC, 2010; Li et al., 2010). Therefore, additional epidemiologic studies further documenting this relationship are unlikely to increase our understanding. To assess mechanisms, and therefore develop mechanism based interventions, more information about drinking patterns over the lifetime in relation to breast cancer risk is needed. The study of Chen et al. (2011) represents an important first step in this direction, but more data are needed. Application of the lifetime exposure classification metrics which capture binge-type drinking at different age periods (e.g. Kerr and Ye, 2010) could be useful.
From the mechanistic standpoint, some of the issues could be addressed by animal models. Regarding the tumor promoter mechanism, a recent report (Wong et al., 2011a) showed that ethanol promotion of mammary tumors in transgenic mice depended upon ovarian hormones, clearly consistent with an estrogen mediated tumor promoter mechanism. However, the extent to which mammary tumors in this and other rodent models are related to breast cancer in humans is an important unanswered question.
An important question is whether ethanol is a complete carcinogen in mammary tissue. The most compelling animal data is that of (Watabiki et al., 2000) who showed that 45% of female mice given 10–15 % ethanol in drinking water for 23 months developed mammary tumors, compared to 0% in control mice drinking water alone. While this is an impressive result, it is worth noting that the study design did not control for total caloric intake, and blood alcohol concentrations were not measured. Furthermore, the study used ICR mice, which are an outbred strain. In view of these issues, and the importance of this study, replication of this finding in a standard inbred mouse strain with control for total calories would be extremely valuable. Such a result would provide a foundation for future studies using genetically modified mice to rigorously address some of the clinically relevant mechanistic questions raised above, such as the potential role of CYP2E1.
Conclusion
Multiple epidemiologic studies have identified a relationship between moderate alcohol consumption and breast cancer risk in women. However, important questions remain about the mechanistic and temporal basis of this relationship, which impact the interpretation of the epidemiologic results, and their implications for disease prevention and women’s health. With refined epidemiological study designs, as well as mechanism-based research, it should be possible to provide better answers to these questions, so that women, in consultation with their health care providers, can make informed decisions about the health consequences of alcohol consumption at different stages of their lives. Such information should also allow the development of rational clinical interventions to reduce ethanol-related breast cancer mortality.
References
- Allen NE, Beral V, Casabonne D, Kan SW, Reeves GK, Brown A, Green J. Moderate alcohol intake and cancer incidence in women. J Natl Cancer Inst. 2009;101:296–305. doi: 10.1093/jnci/djn514. [DOI] [PubMed] [Google Scholar]
- Bielas JH, Loeb LA. Mutator phenotype in cancer: timing and perspectives. Environ Mol Mutagen. 2005;45:206–213. doi: 10.1002/em.20111. [DOI] [PubMed] [Google Scholar]
- Caro AA, Cederbaum AI. Oxidative stress, toxicology, and pharmacology of CYP2E1. Annu Rev Pharmacol Toxicol. 2004;44:27–42. doi: 10.1146/annurev.pharmtox.44.101802.121704. [DOI] [PubMed] [Google Scholar]
- Castro GD, De Castro CR, Maciel ME, Fanelli SL, De Ferreyra EC, Gomez MI, Castro JA. Ethanol-induced oxidative stress and acetaldehyde formation in rat mammary tissue: potential factors involved in alcohol drinking promotion of breast cancer. Toxicology. 2006;219:208–219. doi: 10.1016/j.tox.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Chen WY, Rosner B, Hankinson SE, Colditz GA, Willett WC. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA. 2011;306:1884–1890. doi: 10.1001/jama.2011.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen BC, Kelsey KT, Zheng S, Houseman EA, Marsit CJ, Wrensch MR, Wiemels JL, Nelson HH, Karagas MR, Kushi LH, Kwan ML, Wiencke JK. Breast cancer DNA methylation profiles are associated with tumor size and alcohol and folate intake. PLoS Genet. 2010;6:e1001043. doi: 10.1371/journal.pgen.1001043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton CJ, Chang JC, Rosen JM. Epithelial-mesenchymal transition (EMT) in tumor-initiating cells and its clinical implications in breast cancer. J Mammary Gland Biol Neoplasia. 2010;15:253–260. doi: 10.1007/s10911-010-9173-1. [DOI] [PubMed] [Google Scholar]
- Dorgan JF, Reichman ME, Judd JT, Brown C, Longcope C, Schatzkin A, Campbell WS, Franz C, Kahle L, Taylor PR. The relation of reported alcohol ingestion to plasma levels of estrogens and androgens in premenopausal women (Maryland, United States) Cancer Causes Control. 1994;5:53–60. doi: 10.1007/BF01830726. [DOI] [PubMed] [Google Scholar]
- Forsyth CB, Tang Y, Shaikh M, Zhang L, Keshavarzian A. Alcohol stimulates activation of Snail, epidermal growth factor receptor signaling, and biomarkers of epithelial-mesenchymal transition in colon and breast cancer cells. Alcohol Clin Exp Res. 2010;34:19–31. doi: 10.1111/j.1530-0277.2009.01061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs CS, Stampfer MJ, Colditz GA, Giovannucci EL, Manson JE, Kawachi I, Hunter DJ, Hankinson SE, Hennekens CH, Rosner B. Alcohol consumption and mortality among women. N Engl J Med. 1995;332:1245–1250. doi: 10.1056/NEJM199505113321901. [DOI] [PubMed] [Google Scholar]
- Geisler J, Sasano H, Chen S, Purohit A. Steroid sulfatase inhibitors: promising new tools for breast cancer therapy? J Steroid Biochem Mol Biol. 2011;125:39–45. doi: 10.1016/j.jsbmb.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Ginsburg ES, Mello NK, Mendelson JH, Barbieri RL, Teoh SK, Rothman M, Gao X, Sholar JW. Effects of alcohol ingestion on estrogens in postmenopausal women. JAMA. 1996;276:1747–1751. doi: 10.1001/jama.1996.03540210055034. [DOI] [PubMed] [Google Scholar]
- Giovannucci E, Stampfer MJ, Colditz GA, Manson JE, Rosner BA, Longnecker MP, Speizer FE, Willett WC. Recall and selection bias in reporting past alcohol consumption among breast cancer cases. Cancer Causes Control. 1993;4:441–448. doi: 10.1007/BF00050863. [DOI] [PubMed] [Google Scholar]
- Goss PE, Ingle JN, Ales-Martinez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, Mctiernan A, Robbins J, Johnson KC, Martin LW, Winquist E, Sarto GE, Garber JE, Fabian CJ, Pujol P, Maunsell E, Farmer P, Gelmon KA, Tu D, Richardson H. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381–2391. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- Hamajima N, Hirose K, Tajima K, Rohan T, Calle EE, Heath CW, Jr, Coates RJ, Liff JM, Talamini R, Chantarakul N, Koetsawang S, Rachawat D, Morabia A, Schuman L, Stewart W, Szklo M, Bain C, Schofield F, Siskind V, Band P, Coldman AJ, Gallagher RP, Hislop TG, Yang P, Kolonel LM, Nomura AM, Hu J, Johnson KC, Mao Y, De Sanjose S, Lee N, Marchbanks P, Ory HW, Peterson HB, Wilson HG, Wingo PA, Ebeling K, Kunde D, Nishan P, Hopper JL, Colditz G, Gajalanski V, Martin N, Pardthaisong T, Silpisornkosol S, Theetranont C, Boosiri B, Chutivongse S, Jimakorn P, Virutamasen P, Wongsrichanalai C, Ewertz M, Adami HO, Bergkvist L, Magnusson C, Persson I, Chang-Claude J, Paul C, Skegg DC, Spears GF, Boyle P, Evstifeeva T, Daling JR, Hutchinson WB, Malone K, Noonan EA, Stanford JL, Thomas DB, Weiss NS, White E, Andrieu N, Bremond A, Clavel F, Gairard B, Lansac J, Piana L, Renaud R, Izquierdo A, Viladiu P, Cuevas HR, Ontiveros P, Palet A, Salazar SB, Aristizabel N, Cuadros A, Tryggvadottir L, Tulinius H, Bachelot A, Le MG, Peto J, Franceschi S, Lubin F, Modan B, Ron E, Wax Y, Friedman GD, Hiatt RA, Levi F, Bishop T, Kosmelj K, Primic-Zakelj M, Ravnihar B, Stare J, Beeson WL, Fraser G, Bullbrook RD, Cuzick J, Duffy SW, Fentiman IS, Hayward JL, Wang DY, McMichael AJ, McPherson K, Hanson RL, Leske MC, Mahoney MC, Nasca PC, Varma AO, Weinstein AL, Moller TR, Olsson H, Ranstam J, Goldbohm RA, van den Brandt PA, Apelo RA, Baens J, de la Cruz JR, Javier B, Lacaya LB, Ngelangel CA, La Vecchia C, Negri E, Marubini E, Ferraroni M, Gerber M, Richardson S, Segala C, Gatei D, Kenya P, Kungu A, Mati JG, Brinton LA, Hoover R, Schairer C, Spirtas R, Lee HP, Rookus MA, van Leeuwen FE, Schoenberg JA, McCredie M, Gammon MD, Clarke EA, Jones L, Neil A, Vessey M, Yeates D, Appleby P, Banks E, Beral V, Bull D, Crossley B, Goodill A, Green J, Hermon C, Key T, Langston N, Lewis C, Reeves G, Collins R, Doll R, Peto R, Mabuchi K, Preston D, Hannaford P, Kay C, Rosero-Bixby L, Gao YT, Jin F, Yuan JM, Wei HY, Yun T, Zhiheng C, Berry G, Cooper Booth J, Jelihovsky T, MacLennan R, Shearman R, Wang QS, Baines CJ, Miller AB, Wall C, Lund E, Stalsberg H, Shu XO, Zheng W, Katsouyanni K, Trichopoulou A, Trichopoulos D, Dabancens A, Martinez L, Molina R, Salas O, Alexander FE, Anderson K, Folsom AR, Hulka BS, Bernstein L, Enger S, Haile RW, Paganini-Hill A, Pike MC, Ross RK, Ursin G, Yu MC, Longnecker MP, Newcomb P, Bergkvist L, Kalache A, Farley TM, Holck S, Meirik O Collaborative Group on Hormonal Factors in Breast Cancer. Alcohol, tobacco and breast cancer--collaborative reanalysis of individual data from 53 epidemiological studies, including 58,515 women with breast cancer and 95,067 women without the disease. Br J Cancer. 2002;87:1234–1245. doi: 10.1038/sj.bjc.6600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hill AB. The Environment and Disease: Association or Causation? Proc R Soc Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvidtfeldt UA, Tolstrup JS, Jakobsen MU, Heitmann BL, Gronbaek M, O'Reilly E, Balter K, Goldbourt U, Hallmans G, Knekt P, Liu S, Pereira M, Pietinen P, Spiegelman D, Stevens J, Virtamo J, Willett WC, Rimm EB, Ascherio A. Alcohol intake and risk of coronary heart disease in younger, middle-aged, and older adults. Circulation. 2010;121:1589–1597. doi: 10.1161/CIRCULATIONAHA.109.887513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Alcohol Consumption and Ethyl Carbamate Lyon. 2010 [PMC free article] [PubMed] [Google Scholar]
- Jimenez M, Chiuve SE, Glynn RJ, Stampfer MJ, Camargo CA, Jr, Willett WC, Manson JE, Rexrode KM. Alcohol consumption and risk of stroke in women. Stroke. 2012;43:939–945. doi: 10.1161/STROKEAHA.111.639435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawase T, Matsuo K, Hiraki A, Suzuki T, Watanabe M, Iwata H, Tanaka H, Tajima K. Interaction of the effects of alcohol drinking and polymorphisms in alcohol-metabolizing enzymes on the risk of female breast cancer in Japan. J Epidemiol. 2009;19:244–250. doi: 10.2188/jea.JE20081035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr WC, Ye Y. Relationship of life-course drinking patterns to diabetes, heart problems, and hypertension among those 40 and older in the 2005 U.S. National Alcohol Survey. J Stud Alcohol Drugs. 2010;71:515–525. doi: 10.15288/jsad.2010.71.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key TJ, Appleby PN, Reeves GK, Roddam AW, Helzlsouer KJ, Alberg AJ, Rollison DE, Dorgan JF, Brinton LA, Overvad K, Kaaks R, Trichopoulou A, Clavel-Chapelon F, Panico S, Duell EJ, Peeters PH, Rinaldi S, Fentiman IS, Dowsett M, Manjer J, Lenner P, Hallmans G, Baglietto L, English DR, Giles GG, Hopper JL, Severi G, Morris HA, Hankinson SE, Tworoger SS, Koenig K, Zeleniuch-Jacquotte A, Arslan AA, Toniolo P, Shore RE, Krogh V, Micheli A, Berrino F, Barrett-Connor E, Laughlin GA, Kabuto M, Akiba S, Stevens RG, Neriishi K, Land CE, Cauley JA, Lui LY, Cummings SR, Gunter MJ, Rohan TE, Strickler HD. Circulating sex hormones and breast cancer risk factors in postmenopausal women: reanalysis of 13 studies. Br J Cancer. 2011;105:709–722. doi: 10.1038/bjc.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Li G, Hasin DS. Birth Cohort Effects and Gender Differences in Alcohol Epidemiology: A Review and Synthesis. Alcohol Clin Exp Res. 2011;35:2101–2112. doi: 10.1111/j.1530-0277.2011.01562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrie F, Luu-The V, Labrie C, Belanger A, Simard J, Lin SX, Pelletier G. Endocrine and intracrine sources of androgens in women: inhibition of breast cancer and other roles of androgens and their precursor dehydroepiandrosterone. Endocr Rev. 2003;24:152–182. doi: 10.1210/er.2001-0031. [DOI] [PubMed] [Google Scholar]
- Lauer MS, Sorlie P. Alcohol, cardiovascular disease, and cancer: treat with caution. J Natl Cancer Inst. 2009;101:282–283. doi: 10.1093/jnci/djp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CI, Chlebowski RT, Freiberg M, Johnson KC, Kuller L, Lane D, Lessin L, O'Sullivan MJ, Wactawski-Wende J, Yasmeen S, Prentice R. Alcohol consumption and risk of postmenopausal breast cancer by subtype: the women's health initiative observational study. J Natl Cancer Inst. 2010;102:1422–1431. doi: 10.1093/jnci/djq316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb LA. Human cancers express mutator phenotypes: origin, consequences and targeting. Nat Rev Cancer. 2011;11:450–457. doi: 10.1038/nrc3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker MP, Newcomb PA, Mittendorf R, Greenberg ER, Clapp RW, Bogdan GF, Baron J, Macmahon B, Willett WC. Risk of breast cancer in relation to lifetime alcohol consumption. J Natl Cancer Inst. 1995a;87:923–929. doi: 10.1093/jnci/87.12.923. [DOI] [PubMed] [Google Scholar]
- Longnecker MP, Paganini-Hill A, Ross RK. Lifetime alcohol consumption and breast cancer risk among postmenopausal women in Los Angeles. Cancer Epidemiol Biomarkers Prev. 1995b;4:721–725. [PubMed] [Google Scholar]
- Millonig G, Wang Y, Homann N, Bernhardt F, Qin H, Mueller S, Bartsch H, Seitz HK. Ethanol-mediated carcinogenesis in the human esophagus implicates CYP2E1 induction and the generation of carcinogenic DNA-lesions. Int J Cancer. 2011;128:533–540. doi: 10.1002/ijc.25604. [DOI] [PubMed] [Google Scholar]
- Peer PG, Van Dijck JA, Hendriks JH, Holland R, Verbeek AL. Age-dependent growth rate of primary breast cancer. Cancer. 1993;71:3547–3551. doi: 10.1002/1097-0142(19930601)71:11<3547::aid-cncr2820711114>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Thurmond L, Rosbrook B. Projecting international lung cancer mortality rates: first approximations with tobacco-consumption data. J Natl Cancer Inst Monogr. 1992:45–49. [PubMed] [Google Scholar]
- Salaspuro MP, Lieber CS. Non-uniformity of blood ethanol elimination: its exaggeration after chronic consumption. Ann Clin Res. 1978;10:294–297. [PubMed] [Google Scholar]
- Seitz HK, Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer. 2007;7:599–612. doi: 10.1038/nrc2191. [DOI] [PubMed] [Google Scholar]
- Singletary KW, Gapstur SM. Alcohol and breast cancer: review of epidemiologic and experimental evidence and potential mechanisms. JAMA. 2001;286:2143–2151. doi: 10.1001/jama.286.17.2143. [DOI] [PubMed] [Google Scholar]
- Spratt JS, Meyer JS, Spratt JA. Rates of growth of human solid neoplasms: Part I. J Surg Oncol. 1995;60:137–146. doi: 10.1002/jso.2930600216. [DOI] [PubMed] [Google Scholar]
- Spratt JS, Meyer JS, Spratt JA. Rates of growth of human neoplasms: Part II. J Surg Oncol. 1996;61:68–83. doi: 10.1002/1096-9098(199601)61:1<68::aid-jso2930610102>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Sun Q, Townsend MK, Okereke OI, Rimm EB, Hu FB, Stampfer MJ, Grodstein F. Alcohol Consumption at Midlife and Successful Ageing in Women: A Prospective Cohort Analysis in the Nurses' Health Study. PLoS Med. 2011;8:e1001090. doi: 10.1371/journal.pmed.1001090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry MB, Zhang FF, Kabat G, Britton JA, Teitelbaum SL, Neugut AI, Gammon MD. Lifetime alcohol intake and breast cancer risk. Ann Epidemiol. 2006;16:230–240. doi: 10.1016/j.annepidem.2005.06.048. [DOI] [PubMed] [Google Scholar]
- Triano EA, Slusher LB, Atkins TA, Beneski JT, Gestl SA, Zolfaghari R, Polavarapu R, Frauenhoffer E, Weisz J. Class I alcohol dehydrogenase is highly expressed in normal human mammary epithelium but not in invasive breast cancer: implications for breast carcinogenesis. Cancer Res. 2003;63:3092–3100. [PubMed] [Google Scholar]
- Tyson JJ, Baumann WT, Chen C, Verdugo A, Tavassoly I, Wang Y, Weiner LM, Clarke R. Dynamic modelling of oestrogen signalling and cell fate in breast cancer cells. Nat Rev Cancer. 2011;11:523–532. doi: 10.1038/nrc3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- Von Fournier D, Weber E, Hoeffken W, Bauer M, Kubli F, Barth V. Growth rate of 147 mammary carcinomas. Cancer. 1980;45:2198–2207. doi: 10.1002/1097-0142(19800415)45:8<2198::aid-cncr2820450832>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Watabiki T, Okii Y, Tokiyasu T, Yoshimura S, Yoshida M, Akane A, Shikata N, Tsubura A. Long-term ethanol consumption in ICR mice causes mammary tumor in females and liver fibrosis in males. Alcohol Clin Exp Res. 2000;24:117S–122S. [PubMed] [Google Scholar]
- Wong AW, Dunlap SM, Holcomb VB, Nunez NP. Alcohol Promotes Mammary Tumor Development via the Estrogen Pathway in Estrogen Receptor Alpha-Negative HER2/neu Mice. Alcohol Clin Exp Res Oct. 2011a;7 doi: 10.1111/j.1530-0277.2011.01654.x. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Wong AW, Paulson QX, Hong J, Stubbins RE, Poh K, Schrader E, Nunez NP. Alcohol promotes breast cancer cell invasion by regulating the Nm23-ITGA5 pathway. J Exp Clin Cancer Res. 2011b;30:75. doi: 10.1186/1756-9966-30-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, Kamiyama M, Hruban RH, Eshleman JR, Nowak MA, Velculescu VE, Kinzler KW, Vogelstein B, Iacobuzio-Donahue CA. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–1117. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakhari S. Molecular mechanisms underlying alcohol-induced cardioprotection: contribution of hemostatic components. Introduction to the symposium. Alcohol Clin Exp Res. 1999;23:1108–1110. doi: 10.1111/j.1530-0277.1999.tb04232.x. [DOI] [PubMed] [Google Scholar]