Abstract
Background/Aims
Survival of patients with hepatocellular carcinoma (HCC) is determined by hepatic function and tumor extent. Recently, a new Model to Estimate Survival in Ambulatory HCC patients (MESIAH) was proposed to predict overall survival in ambulatory HCC patients. This study aimed to evaluate the prognostic performance of the MESIAH score in an independent cohort of HCC patients.
Methods
A cohort of 1,969 patients newly diagnosed with HCC at the National Cancer Center, Korea between January 2004 and December 2009 was used for validation of the MESIAH score. The model's performance was assessed using C-statistics, the likelihood ratio (LR) χ2 value, and Akaike information criterion (AIC).
Results
Patients in the cohort had a median age of 56 years and 83.2% were men. Hepatitis B virus infection was present in 74.6% and 81.6% had a Child-Pugh class A. The median overall survival was 21.4 months. The MESIAH score had a higher degree of discrimination, with a C-statistic of 0.792 (95% confidence interval [CI], 0.782–0.803), when compared with the Barcelona Clinic Liver Cancer (BCLC) staging system (0.665 [95% CI, 0.653–0.678], p<0.001). The LR χ2 value and the AIC of MESIAH were also better than those of BCLC, Cancer of the Liver Italian Program, Japan Integrated Scoring and Tokyo score. The observed survival in the cohort closely matched that predicted by the MESIAH score.
Conclusions
The new prognostication model MESIAH accurately estimated the overall survival of Korean HCC patients and may be useful in future research as well as individual patient care.
Keywords: hepatocellular carcinoma, prognosis, MELD, MESIAH, BCLC
Introduction
Accurate staging information is necessary to determine the prognosis of patients with cancer and to guide subsequent patient management.(1) In general, the curability of cancer is inversely proportional to the extent of the tumor; the most widely used staging method for cancer is the tumor, node, and metastasis (TNM) system. Hepatocellular carcinoma (HCC) is somewhat unique because the vast majority of HCC patients have underlying liver cirrhosis and the prognosis of HCC patients is determined not only by the tumor extent but also by hepatic function. Therefore, many staging systems developed for HCC include simultaneous measurement of the tumor extent and overall liver function. The Barcelona Clinic Liver Cancer (BCLC) staging system adopted by American and European liver societies as a guide in patient management employs variables representing liver function, such as the Child-Pugh score classification, performance status and cancer-related symptoms, and the tumor extent. (2, 3)
Recently, a new model to predict survival of patients with HCC has been proposed.(4) A strength of this multivariable Model to Estimate Survival in Ambulatory Patients with HCC (MESIAH) is that it consists of objective and reproducible variables such as age and morphologic characteristics such as tumor size and number, vascular invasion, and extrahepatic metastasis. In assessing liver function, the MESIAH score utilizes the Model for End-Stage Liver Disease (MELD) score, a widely validated measure to estimate short term mortality in patients with advanced liver disease from a wide variety of causes.(5-11) MELD was chosen over the Child-Pugh classification because MELD consists only of laboratory variables, consistent with the goal of maximizing reproducibility of the MESIAH score.
In addition to objectivity and reproducibility of its component variables, the MESIAH score is able to estimate survival probabilities over a wide span of prognosis. For example, in the original work that described the score, MESIAH was able to stratify patients by their risk of mortality even within the same BCLC categories. In this work, we further evaluate the predictive performance of the MESIAH score. Specifically, we assess discrimination of the MESIAH score in comparison to the BCLC system and other staging system (Cancer of the Liver Italian Program (CLIP) (12), Japan Integrated Scoring (JIS) (13), and Tokyo score(14)) and evaluate MESIAH's calibration in predicting survival.
Patients and Methods
A total of 2,509 HCC patients were seen at the Center for Liver Cancer, National Cancer Center (Goyang, South Korea) between January 2004 and December 2009. Of these, 1,969 patients were newly diagnosed with HCC and had not undergone prior anti-tumor therapy. These patients comprised the cohort for this study.(15). The diagnosis of HCC was based upon histology and/or clinico-radiologic evidence according to the Korean practice guidelines for HCC.(16, 17) The latter criteria consisted of the presence of 1 or more risk factors (hepatitis B virus [HBV], hepatitis C virus [HCV], or cirrhosis); a liver mass in a dynamic liver imaging (such as dynamic spiral computed tomography (CT), contrast-enhanced dynamic magnetic resonance imaging (MRI), or hepatic angiography) that demonstrates typical characteristics including enhancement in the arterial phase and washout in the delayed portal/venous phase; and/or elevated serum alpha-fetoprotein (AFP) levels. These patients were collected prospectively; relevant data on clinical and tumor characteristics were extracted retrospectively from the medical records. This study was approved by the Institutional Review Board of our institute (National Cancer Center, Goyang, South Korea).
The MELD score was calculated according to the original formula without rounding or lower and upper bounds in the variables and final score.(18) The MESIAH score was calculated according to the following formula: MESIAH score = 0.232 * (Age in Decades) + 0.099 * (MELD†) - 0.391 * (Albumin) + 0.290 * (Tumor size††) + 0.153 * (Tumor number†††) + 1.122 * (Vascular invasion) + 1.130 * (Metastasis) + 0.082 * (AFP††††) + 1 where † equals the MELD scores ≤ 13 set to 13, †† equals the number of nodules (1 = 1, 2 = 2, 3 = 3, 4 = 4, 5 = ≥5), ††† equals the size of the largest nodule (1 = ≤1 cm, 2 = 1–2 cm, 3 = 2–3 cm, 4 = 3–5 cm, 5 = 5–10 cm, 6 = 10–15 cm, 7 = 15–20, and 8 = >20), and †††† equals the ln(AFP) with AFP capped at 10,000 units.(4) CLIP, JIS and Tokyo score were also evaluated as previously described.(12-14)
Continuous variables were expressed as median values with interquartile ranges. Survival probabilities were estimated by the Kaplan-Meier method and difference in survival tested using the log-rank test as well as the Cox proportional hazard regression analysis. In assessing discrimination of the survival models, the concordance (C)-statistics, the likelihood ratio (LR) χ2 and the Akaike information criterion (AIC) were calculated. In evaluating model calibration, a χ2 statistic was calculated as a measure of agreement between predicted and observed event rates in deciles of predicted risk. We considered a value of 20 or higher to be indicative of poor calibration. All statistical analyses in this study were carried out using the STATA software version 12.0 (StataCorp LP, College Station, TX).
Results
Baseline characteristics
Characteristics of patients included in this study cohort are summarized in Table 1. There were 1,639 men (83.2%) and 330 women. The median age was 56 years (interquartile range [IQR], 49–64 years). HBV was the predominant cause of liver disease (74.6%), followed by HCV (9.3%). The median MELD score was 7.8 (IQR, 5.7–10.3) and most patients had a performance status of less than 2. Over 80% of the patients (n=1,969, 81.6%) had Child-Pugh class A and 328 (16.7%) had a Child-Pugh class B. According to the BCLC system, 93 (4.7%) were classified as stage 0, 390 (19.8%) as stage A, 184 (9.3%) as stage B, 1,266 (64.3%) as stage C, and 36 (1.8%) as stage D. Consequently, transarterial chemoembolization (TACE) was the most common choice of initial treatment, administered to 58.3% of patients. Curative treatment, including resection (18.6%), transplantation (1.5%), and local ablation therapy (3.6%) were performed in 467 patients.
Table 1. Characteristics of the validation cohort (n=1,969).
| Patient Characteristics (n, [IQR]) | Tumor Characteristics | ||
|---|---|---|---|
| Age (years) | 56 [49-64] | Size of the largest nodule | |
| Male | 1639 (83.2%) | ≤1cm | 2.5% |
| Etiology | 1-2cm | 12.7% | |
| HBV | 1469 (74.6%) | 2-3cm | 14.9% |
| HCV | 184 (9.3%) | 3-5cm | 19.7% |
| Alcoholic | 144 (7.4%) | 5-10cm | 26.9% |
| NBNCNA | 172 (8.7%) | 10-15cm | 15.7% |
| Bilirubin (mg/dl) | 0.9 [0.6-1.3] | 15-20cm | 6.4% |
| INR | 1.15 [1.06-1.27] | >20cm | 1.2% |
| Creatinine (mg/dl) | 1.0 [0.9-1.1] | Number of nodules | |
| Albumin (g/dl) | 3.8 [3.4-4.2] | 1 | 51.2% |
| C-P score | 5.0 [5.0-6.0] | 2 | 15.9% |
| C-P class (A/B/C) | 81.6%/16.7%/1.7% | 3 | 6.9% |
| MELD* | 7.8 [5.7-10.3] | 4 | 3.7% |
| MESIAH | 4.6 [3.7-5.8] | ≥5 | 22.4% |
| Performance status (%) | Vascular invasion | 674 (34.2%) | |
| 0/1/2 | 43.3/53.4/3.3 | Extrahepatic metastasis | 358 (18.2%) |
| Initial treatment | AFP (ng/ml) | 171.6 [14.0-3272.0] | |
| Transplantation | 30 (1.5%) | <20 | 29.0% |
| Resection | 367 (18.6%) | ≥20, <400 | 28.9% |
| Local ablation | 70 (3.6%) | ≥400 | 42.2% |
| TACE | 1,148 (58.3%) | ||
| Sorafenib | 32 (1.6%) | ||
| Cytotoxic chemotherapy | 78 (4.0%) | ||
| Radiation | 99 (5.0%) | ||
| Supportive care | 145 (7.4%) | ||
Number (proportion) or median [interquartile range] are shown.
HBV, hepatitis B virus; HCV, hepatitis C virus; NBNCNA, non-B/non-C/non-alcoholic; INR, internationalized normalized ratio; C-P, Child-Pugh; MELD, Model for End-Stage Liver Disease; MESIAH, Model to Estimate Survival in Ambulatory HCC; TACE, transarterial chemoembolization
The MELD score was calculated according to the original formula without rounding or lower and upper bounds in the variables and the final score.
Prognostic stratification according to the MESIAH score and BCLC staging system
The median overall survival of the cohort was 21.4 months (95% confidence interval [CI], 18.9–23.6). The 1-, 2-, 3-, 4-, and 5-year survival probability was 61.1%, 47.9%, 39.9%, 35.5%, and 31.5%, respectively.
The median MESIAH score was 4.6 (IQR, 3.7–5.8) and the distribution was skewed to the right with the mean score of 4.8 (Figure 1). Figure 2A describes survival of the cohort in quintiles of the MESIAH score; the 1-year survival was 95.7%, 91.4%, 69.5%, 34.8%, and 13.7%, respectively. The corresponding 3-year survival estimates were 84.9%, 65.6%, 34.0%, 12.7%, and 2.2%. A sensitivity analysis shown in Figure 2B in which liver transplantation was censored, the result did not change materially.
Figure 1.
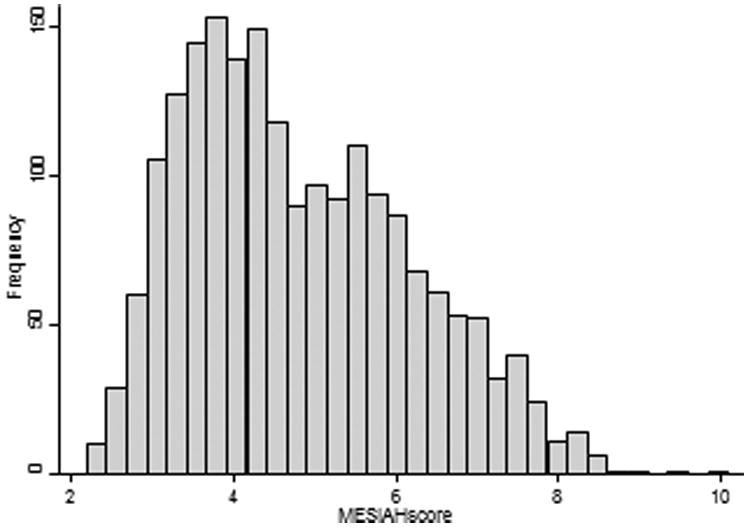
Distribution of the MESIAH score.
Figure 2.
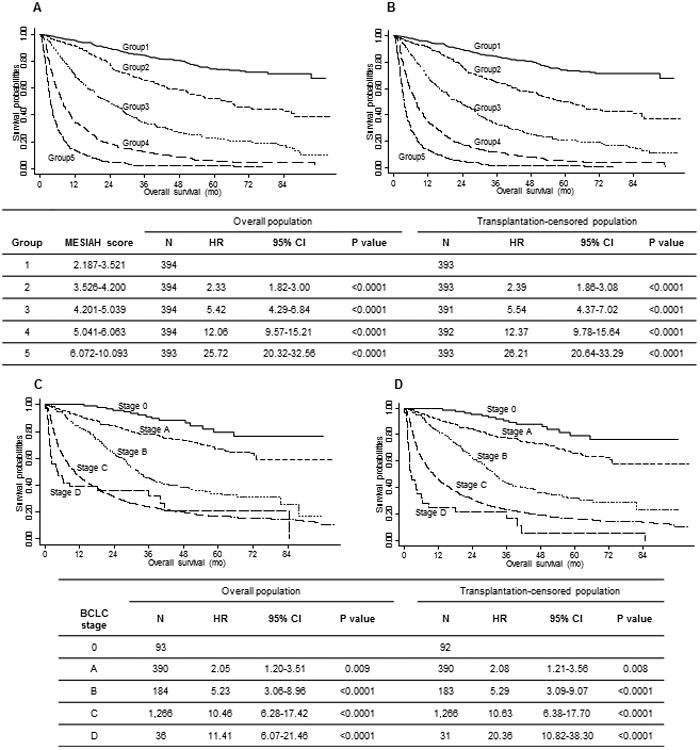
Cumulative overall survival curves and hazard ratio of patients with HCC stratified by the MESIAH score (A, B) and BCLC stage (C, D). A and C represent the overall population, while B and D represent the non-transplantation population.
The BCLC staging system was also able to stratify patients (Figure 2C). The 1-year survival was 100%, 91.6%, 83.2%, 46.2%, and 38.9% for BCLC stage 0, A, B, C, and D, respectively. The corresponding 3-year figures were 91.0%, 78.0%, 45.2%, 23.9%, and 32.1%, respectively. Again, censoring liver transplantation did not alter the results (Figure 2D).
Discrimination and Calibration of the MESIAH score
The overall C-statistic of the MESIAH model in this cohort was 0.792 (95% CI, 0.782–0.803) (Table 2). When compared with the BCLC system, the overall C-statistic of the MESIAH model was superior to that of the BCLC (0.665 [95% CI, 0.653–0.678], p < 0.001). Similarly, the LR χ2 values were higher and the AIC lower for MESIAH than those for the BCLC system. Table 2 also includes sensitivity analyses addressing subgroups of patients, in all of which MESIAH had a better discrimination than BCLC, CLIP, JIS and Tokyo score.
Table 2. Comparison of prognostic stratification of the MESIAH score and the BCLC system.
| Model | C-statistic | LR χ2 | AIC |
|---|---|---|---|
| All patients (n=1,969) | |||
| MESIAH | 0.792 (0.782-0.803) | 1263.0 | 17523.0 |
| BCLC | 0.665 (0.653-0.678) | 551.9 | 18240.1 |
| CLIP | 0.760 (0.748-0.771) | 1031,6 | 17764.4 |
| JIS | 0.753 (0.748-0.771) | 1025.3 | 17768.8 |
| Tokyo score | 0.716 (0.703-0.729) | 688.4 | 18111.7 |
| Without transplantation (n=1,870) | |||
| MESIAH | 0.789(0.778-0.800) | 1207.7 | 17123.6 |
| BCLC | 0.666(0.653-0.678) | 556.9 | 17780.4 |
| CLIP | 0.760 (0.748-0.772) | 1003.4 | 17337.9 |
| JIS | 0.752 (0.741-0.764) | 1016.1 | 17323.2 |
| Tokyo score | 0.724 (0.711-0.737) | 745.8 | 17599.5 |
| HBV (n=1,469) | |||
| MESIAH | 0.797 (0.785-0.810) | 1015.8 | 12624.1 |
| BCLC | 0.669 (0.655-0.684) | 444.4 | 13201.5 |
| CLIP | 0.767 (0.754-0.780) | 856.0 | 12793.9 |
| JIS | 0.761 (0.748-0.773) | 853.8 | 12794.1 |
| Tokyo score | 0.724 (0.709-0.738) | 570.8 | 13083.1 |
| HCV (n=184) | |||
| MESIAH | 0.755 (0.712-0.797) | 86.4 | 1051.4 |
| BCLC | 0.636 (0.592-0.679) | 31.3 | 1112.5 |
| CLIP | 0.724 (0.679-0.768) | 73.8 | 1074.0 |
| JIS | 0.694 (0.647-0.740) | 54.3 | 1091.5 |
| Tokyo score | 0.685 (0.639-0.732) | 47.2 | 1102.7 |
| NBNC (n=316) | |||
| MESIAH | 0.784 (0.755-0.813) | 156.2 | 1950.3 |
| BCLC | 0.658 (0.626-0.691) | 78.6 | 2033.9 |
| CLIP | 0.731 (0.697-0.764) | 113.6 | 2002.9 |
| JIS | 0.730 (0.699-0.762) | 133.5 | 1981.0 |
| Tokyo score | 0.686 (0.651-0.722) | 84.3 | 2036.2 |
LR, likelihood ratio; AIC, Akaike information criterion; MESIAH, Model to Estimate Survival in Ambulatory HCC; BCLC, Barcelona Clinic Liver Cancer; HBV, hepatitis B virus; HCV, hepatitis C virus; NBNC, non-B non-C
Figure 3 compares actual survival for 36 months with the predicted survival by the MESIAH model. While the MESIAH model tended to overestimate survival, there was overall agreement between the predicted and actual survival in the whole cohort (Figure 3A) and the subset of non-transplant patients (Figure 3B). Because of the large sample size, the difference between the predicted and actual survival did reach statistical significance in both comparisons (χ2 statistics of 5303.7 [p < 0.01] and 8328.7 [p < 0.01], respectively).
Figure 3.
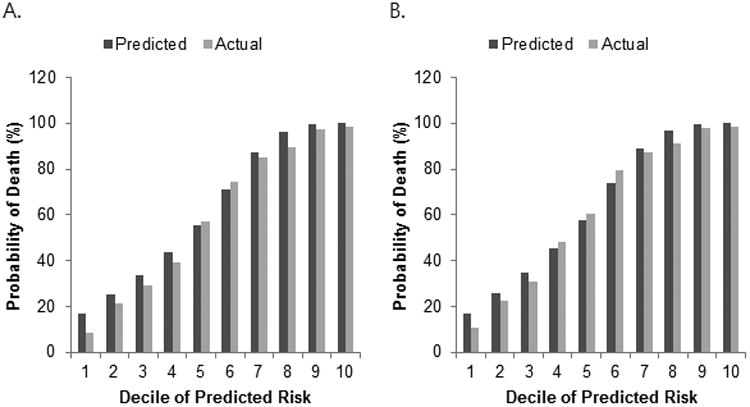
Three-year predictions and actual values for survival in all patients (A) and non-transplantation patients (B) with HCC. X-axes refer to the deciles of predicted risk based on the MESIAH score.
Discussion
This is a validation study of the new MESIAH scoring system to predict survival in patients with HCC.
An earlier cohort (2000-2003) at our institution was used for external validation of this model in the original description of the model, so it may limit our findings. However, the current cohort (2004–2009) did not overlap with the first one and is larger and more recent, making it appropriate for validation of the MESIAH system. For example, living donor liver transplantation became available at this center in 2005 and the cohort included a small proportion of patients (5%, n=99) that underwent liver transplantation with 30 patients accepting transplantation as the initial treatment. Further, beginning in 2007, sorafenib was also administered to a small number of patients as the initial treatment of choice; however, sorafenib use may be hampered by its high cost and the reimbursement policies of the Korean health insurance system. Satisfactory performance of the MESIAH model in this new group of patients further advocates the robustness of the model.
Many staging systems for HCC have included a measure of liver function such as Child-Pugh classification. Although the Child-Pugh classification or the MELD score is originally designed for patients with cirrhosis, both are useful parameters to predict prognosis of patients with liver diseases across the presence or absence of cirrhosis.(8, 19) The MELD score has the advantage of being composed of objective and easily obtainable variables(7), and the MESIAH utilizes the MELD score.
Previous studies have evaluated accuracy of different staging systems in predicting survival of patients with HCC; however, the preferred treatment strategy varies by the region or country, according to availability of different therapeutic options, reimbursement schemes, and prevailing consensus within the medical community. These disparities may potentially affect patient prognosis. For example, in Asian countries, TACE tends to be widely used as an initial treatment modality, even in an advanced stage of disease.(20) On the other hand, Western countries have utilized liver transplantation as the preferred option that provides the best chance for cure. The fact that the MESIAH score, developed in a Western setting, repeatedly validates well in Asian patient cohorts attests to its applicability in a wide range of patients.
Traditionally, scholars from Asian countries favored the JIS (21-23), while those from Western countries favored the BCLC system.(2, 24) More recently, its ability to guide management has led to increasing acceptance of the BCLC system worldwide. However, it remains uncertain whether the BCLC's prognostic utility decreases when selection of treatment deviates from what is recommended, which may occur as a result of availability or affordability of treatment as well as other cultural or healthcare system related reasons. The satisfactory performance of the MESIAH model independent of the initial choice of treatment modality may represent another advantage.
Discrimination refers to the ability to rank patients correctly according to their risk of death. In all measures of discrimination, MESIAH performed better than BCLC in this cohort. Calibration, on the other hand, describes how closely the predicted probabilities agree numerically with the actual outcomes. Since MESIAH is unique in its ability to calculate estimated survival, there are no other staging systems to compare against it. As seen in Figure 3, there was overall agreement between the actual and predicted survival. There was, however, statistically significantly difference, which was mostly in patients in the deciles with the lowest mortality. One potential explanation is improvement of surgical skills since about third fourths of resection was performed in patients with the lower 4 deciles of predicted risk based on the MESIAH score. Another plausible reason is control of chronic hepatitis B with antiviral therapy. There is an increasing body of literature that suggest that antiviral therapy in HCC patients may prolong survival. (25)
In summary, the MESIAH score performed well in estimating survival in this cohort of Korean HCC patients enriched with HBV-related liver disease. In its ability to discriminate patients with a wide range of survival probability, MESIAH outperformed the commonly used BCLC staging system. We conclude that these data further validate the MESIAH score in a setting disparate from its original derivation with regard to the ethnicity of the patient, etiology of underlying liver disease, and cross-cultural differences in therapeutic choices.
Acknowledgments
Grant Support: This study was supported by the National Cancer Center, Korea (Grant #1110050).
Abbreviations
- AFP
alpha-fetoprotein
- AIC
Akaike information criterion
- BCLC
Barcelona Clinic Liver Cancer
- CI
confidence interval
- CLIP
Cancer of the Liver Italian Program
- CT
computed tomography
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HCC
hepatocellular carcinoma
- IQR
interquartile range
- LR
likelihood ratio
- JIS
Japan Integrated Scoring
- MELD
model for end-stage liver disease
- MESIAH
Model to Estimate Survival in Ambulatory HCC
- MRI
magnetic resonance imaging
- TACE
transarterial chemoembolization
Footnotes
Disclosures: The authors state that they have no potential conflicts of interest.
Author Contributions: Study concept and design: JWP and WRK; analysis and interpretation of data: BHK and JWP; drafting of the manuscript: BHK and BHN; critical revision of the manuscript for important intellectual content: JWP and WRK; statistical analysis: BHN; study supervision: JWP
References
- 1.Pons F, Varela M, Llovet JM. Staging systems in hepatocellular carcinoma. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2005;7:35–41. doi: 10.1080/13651820410024058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–43. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Yang JD, Kim WR, Park KW, et al. Model to estimate survival in ambulatory patients with hepatocellular carcinoma. Hepatology. 2012;56:614–21. doi: 10.1002/hep.25680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, Ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864–71. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 6.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–70. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 7.Kamath PS, Kim WR. The model for end-stage liver disease (MELD) Hepatology. 2007;45:797–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- 8.Dunn W, Jamil LH, Brown LS, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology. 2005;41:353–8. doi: 10.1002/hep.20503. [DOI] [PubMed] [Google Scholar]
- 9.Srikureja W, Kyulo NL, Runyon BA, Hu KQ. MELD score is a better prognostic model than Child-Turcotte-Pugh score or Discriminant Function score in patients with alcoholic hepatitis. J Hepatol. 2005;42:700–6. doi: 10.1016/j.jhep.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 10.Amitrano L, Guardascione MA, Bennato R, Manguso F, Balzano A. MELD score and hepatocellular carcinoma identify patients at different risk of short-term mortality among cirrhotics bleeding from esophageal varices. J Hepatol. 2005;42:820–5. doi: 10.1016/j.jhep.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 11.Teh SH, Nagorney DM, Stevens SR, et al. Risk factors for mortality after surgery in patients with cirrhosis. Gastroenterology. 2007;132:1261–9. doi: 10.1053/j.gastro.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 12.A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751–5. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 13.Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score) J Gastroenterol. 2003;38:207–15. doi: 10.1007/s005350300038. [DOI] [PubMed] [Google Scholar]
- 14.Tateishi R, Yoshida H, Shiina S, et al. Proposal of a new prognostic model for hepatocellular carcinoma: an analysis of 403 patients. Gut. 2005;54:419–25. doi: 10.1136/gut.2003.035055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwak HW, Park JW, Nam BH, et al. Clinical outcomes of a cohort series of patients with hepatocellular carcinoma in a hepatitis B virus-endemic area. Journal of gastroenterology and hepatology. 2013 doi: 10.1111/jgh.12470. [DOI] [PubMed] [Google Scholar]
- 16.Park JW. Practice Guideline for Diagnosis and Treatment of Hepatocellular Carcinoma. Clin Mol Hepatol. 2004;10:88–98. [PubMed] [Google Scholar]
- 17.Korean Liver Cancer Study Group, National Cancer Center Korea. Special Contribution : Practice guidelines for management of hepatocellular carcinoma 2009. Clin Mol Hepatol. 2009;15:391–423. doi: 10.3350/kjhep.2009.15.3.391. [DOI] [PubMed] [Google Scholar]
- 18.Wiesner R, Edwards E, Freeman R, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91–6. doi: 10.1053/gast.2003.50016. [DOI] [PubMed] [Google Scholar]
- 19.Schroeder RA, Marroquin CE, Bute BP, Khuri S, Henderson WG, Kuo PC. Predictive indices of morbidity and mortality after liver resection. Ann Surg. 2006;243:373–9. doi: 10.1097/01.sla.0000201483.95911.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park JW, Sherman M, Colombo M, et al. Observations of hepatocellular carcinoma (HCC) management patterns from the global HCC BRIDGE study: first characterization of the full study population. J Clin Oncol. 2012;30 suppl abstr 4033. [Google Scholar]
- 21.Kudo M, Chung H, Haji S, et al. Validation of a new prognostic staging system for hepatocellular carcinoma: the JIS score compared with the CLIP score. Hepatology. 2004;40:1396–405. doi: 10.1002/hep.20486. [DOI] [PubMed] [Google Scholar]
- 22.Toyoda H, Kumada T, Kiriyama S, et al. Comparison of the usefulness of three staging systems for hepatocellular carcinoma (CLIP, BCLC, and JIS) in Japan. The American journal of gastroenterology. 2005;100:1764–71. doi: 10.1111/j.1572-0241.2005.41943.x. [DOI] [PubMed] [Google Scholar]
- 23.Huo TI, Lin HC, Hsia CY, et al. The model for end-stage liver disease based cancer staging systems are better prognostic models for hepatocellular carcinoma: a prospective sequential survey. The American journal of gastroenterology. 2007;102:1920–30. doi: 10.1111/j.1572-0241.2007.01370.x. [DOI] [PubMed] [Google Scholar]
- 24.Cillo U. Prospective validation of the Barcelona Clinic Liver Cancer staging system. J Hepatol. 2006;44:723–31. doi: 10.1016/j.jhep.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 25.Wu CY, Chen YJ, Ho HJ, et al. Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection. JAMA. 2012;308:1906–14. doi: 10.1001/2012.jama.11975. [DOI] [PubMed] [Google Scholar]


