Abstract
IMPORTANCE
Obesity is associated with chronic musculoskeletal pain and is a risk factor for disability and osteoarthritis.
OBJECTIVES
To describe the prevalence, sites, and intensity of musculoskeletal pain in adolescents with severe obesity; to evaluate associations between musculoskeletal pain and self-reported physical function as well as weight-related quality of life; and to evaluate the association between musculoskeletal pain and high-sensitivity C-reactive protein level.
DESIGN, SETTING, AND PARTICIPANTS
Teen–Longitudinal Assessment of Bariatric Surgery (Teen-LABS) is a prospective, observational study that collects standardized data on adolescents undergoing weight loss surgery at 5 US centers. We examined baseline data from this cohort between February 28, 2007, and December 30, 2011. We excluded adolescents with Blount disease and slipped capital femoral epiphyses. A total of 233 participants were included in these analyses.
MAIN OUTCOMES AND MEASURES
We assessed musculoskeletal pain and pain intensity of the lower back, hips, knees, and ankles/feet using the visual analog scale, categorizing musculoskeletal pain into lower back pain, lower extremity (hips, knees, and feet/ankles combined) pain, and no pain. We assessed self-reported physical function status with the Health Assessment Questionnaire Disability Index and assessed weight-related quality of life with the Impact of Weight on Quality of Life–Kids measure. We adjusted for sex, race, age at surgery, body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), and clinical depressive symptoms in regression analyses.
RESULTS
Among the 233 participants, the mean (SD) age at surgery was 17.1 (1.56) years and the median BMI was 50.4. Participants were predominantly female (77%), white (73%), and non-Hispanic (93%). Among the participants, 49% had poor functional status and 76% had musculoskeletal pain. Lower back pain was prevalent (63%), followed by ankle/foot (53%), knee (49%), and hip (31%) pain; 26% had pain at all 4 sites. In adjusted analyses, compared with pain-free participants, those reporting lower extremity pain had greater odds of having poor physical function according to scores on the Health Assessment Questionnaire Disability Index (odds ratio = 2.82; 95% CI, 1.35 to 5.88; P < .01). Compared with pain-free participants, those reporting lower extremity pain had significantly lower Impact of Weight on Quality of Life–Kids total scores (β = −9.42; 95% CI, −14.15 to −4.69; P < .01) and physical comfort scores (β = −17.29; 95% CI, −23.32 to −11.25; P < .01). After adjustment, no significant relationship was observed between musculoskeletal pain and high-sensitivity C-reactive protein level.
CONCLUSIONS AND RELEVANCE
Adolescents with severe obesity have musculoskeletal pain that limits their physical function and quality of life. Longitudinal follow-up will reveal whether weight loss surgery reverses pain and physical functional limitations and improves quality of life.
Childhood obesity is associated with comorbidities including hypertension, diabetes mellitus, sleep apnea, cardiovascular disease, and impaired quality of life.1–6 These comorbidites will worsen as more children and adolescents become severely obese (body mass index [BMI; calculated as weight in kilograms divided by height in meters squared] ≥120% of the 95th percentile or ≥40).7,8 Due to strong associations between obesity and joint pain as well as between obesity and the development of knee osteoarthritis (OA), childhood obesity negatively affects musculoskeletal (MSK) health.9–15
Obesity leads to the development and progression of knee OA through 2 mechanisms: (1) mechanical loading on the joint, causing wear and tear; and (2) adiposity-mediated joint inflammation.10,16–18 Thus, OA evolves from a stage of pre-OA (preradiographic and asymptomatic) to symptomatic disease causing pain and functional limitation, then radiologically evident disease, and ultimately joint death.19 In adults, weight loss by either dieting or weight loss surgery (WLS) leads to improvement in pain, quality of life, joint loads, and inflammation but does not reverse OA.20–23 Musculoskeletal pain in children and adolescents with obesity may herald a predisease state that may be reversible at early stages.
Joint pain is associated with childhood obesity in cross-sectional studies; the largest study showed that obese adolescents have increased odds of any pain (odds ratio [OR] = 1.33; P = .04) and knee pain (OR = 1.87; P = .001) compared with non-obese adolescents.11,24–26 However, there is a paucity of data examining the associations between MSK pain and physical functional status or between MSK pain and quality of life in adolescents with obesity and those with severe obesity.27 This lack of data makes it difficult to determine exercise ability or exercise adherence during weight management.
To address these knowledge gaps, baseline data from a unique multisite cohort of 242 teens undergoing WLS were used for the following: (1) to describe the prevalence, anatomical sites, and intensity of MSK pain in adolescents with severe obesity; (2) to evaluate associations between MSK pain and self-reported physical function as well as weight-related quality of life (WRQOL); and (3) to evaluate the association between MSK pain and level of high-sensitivity C-reactive protein (hs-CRP), a marker of inflammation. We hypothesized that adolescents with lower extremity (LE) pain would have poor self-reported physical functional status and lower WRQOL scores compared with those without LE pain and compared with those with low back pain. Secondarily, we hypothesized that joint pain would be associated with abnormal elevations of hs-CRP level.
Methods
Study Design
Study methods for the Teen–Longitudinal Assessment of Bariatric Surgery (Teen-LABS) have been previously described in detail.27,28 Teen-LABS is a prospective, observational study designed to collect standardized clinical and laboratory data on adolescents undergoing WLS at 5 US centers. The study protocol, assent and consent forms, and data and safety monitoring plans were approved by the institutional review boards of the participating institutions (Nationwide Children’s Hospital, Cincinnati Children’s Hospital, Texas Children’s Hospital, University of Pittsburgh, and Children’s Hospital of Alabama) and by the independent data and safety monitoring board before study initiation. Written assent and consent were obtained from the participants. Consecutive adolescents (aged ≤19 years) at each Teen-LABS center between February 28, 2007, and December 30, 2011, were offered enrollment. The present analysis focuses on the baseline cohort of 242 participants. Adolescents with confounding conditions of Blount disease (n = 9) or slipped capital femoral epiphyses (n = 0) were excluded, resulting in a final sample of 233 participants. Research data were collected in a standardized fashion at all institutions within 30 days of surgery, during a study visit by trained staff.27
Pain Assessment
Pain was assessed by self-report on the Health Assessment Questionnaire Disability Index (HAQ-DI)29 using a 0- to 10-point visual analog scale (0 indicates no pain; 10, severe pain). Participants were asked, “How much pain have you had because of your weight in the past week?” Pain was rated at each anatomical site (lower back, hips, knees, ankles/feet). Presence of MSK pain was defined as pain with a score greater than 0 at any site. Site-specific pain was similarly defined (ie, scores >0). To assess pain intensity, site-specific pain was reported as a continuous variable, including and excluding a score of 0 (no pain).
Physical Functional Status Assessment
Physical functional status was assessed by self-report on the HAQ-DI, a well-validated tool that assesses the impact of chronic disease on functional ability.30,31 The HAQ-DI contains 20 items that measure physical disabilities during the past week in 8 activity categories: dressing and grooming, arising, eating, walking, hygiene, reach, grip, and activities. Items are scored on a 4-point scale, with 0 indicating no difficulty; 1, some difficulty; 2, much difficulty; and 3, cannot do. A standard score is computed using scores from the 8 categories, accounting for use of aids or devices.32 The standard score was treated as a binary variable where a score higher than 0 indicates poor self-reported physical function.
Weight-Related Quality of Life
The WRQOL was assessed using the Impact of Weight on Quality of Life–Kids measure, a self-report instrument for adolescents (aged 11–19 years) with 4 subscales (physical comfort, body esteem, social life, and family relations) and a summary total score. Raw scaled scores were transformed to a 0- to 100-point scale, with higher scores reflecting better WRQOL.33 The Impact of Weight on Quality of Life–Kids has demonstrated excellent psychometric properties, including discrimination among weight status groups and responsiveness to weight change.33–35 We restricted analyses to the summary total score and the physical comfort subscale score.
Marker of Inflammation
High-sensitivity CRP is a well-established nonspecific marker of systemic inflammation that has been correlated with pain and joint disease.36,37 It was collected and analyzed as part of a biospecimen panel and analyzed at a central core laboratory facility as previously described.27
Covariates
Sex, race (white vs nonwhite), age at surgery, and BMI were considered confounders in regression analyses. Clinical depressive symptoms assessed by the Beck Depression Inventory–II were also considered as confounders in the regression analyses because pain and WRQOL are highly correlated with depressive symptoms.38,39 The suggested total score of more than 17 was used as a conservative cut point for clinically elevated depressive symptoms.40
Statistical Analysis
Only 1.9% of values were missing among variables included in statistical models. Most participants (90%) had complete data. Missing data ranged from 1.7% (n = 4) for site-specific pain measures and hs-CRP to 6.0% (n = 14) for depressive symptoms. Missing data were assumed to be missing at random. Multivariate imputation by fully conditional specification was performed to address missing data. The SAS MI procedure (SAS version 9.4 statistical software; SAS Institute, Inc) was used to generate 25 imputed data sets for use in multivariable modeling analyses.
Descriptive statistics were calculated to summarize participant characteristics. Frequencies and percentages are reported for categorical measures. Means and standard deviations or medians and interquartile ranges were calculated for continuous variables. We ran χ2, Fisher exact, Wilcoxon rank sum, and t tests to evaluate participant characteristics by presence or absence of pain. In the statistical models, pain was evaluated as a categorical variable (no pain, back pain only, or LE pain [hip, knee, or ankle/foot pain]) and as a continuous variable. To assess the relationship between pain and presence of poor self-reported physical function, we calculated adjusted ORs by fitting a generalized linear mixed model (SAS Proc GLIMMIX). Linear mixed models (SAS Proc MIXED) were used to evaluate the association between pain and Impact of Weight on Quality of Life–Kids scores (total score and physical comfort score) as well as hs-CRP level. All models accounted for the correlation of participants by including a random intercept for clinical site and for potential confounders such as sex, race, age at surgery, BMI, and Beck Depression Inventory–II scores. Predictor terms were retained in the final models when P < .10. We used SAS Proc MiAnalyze to generate all estimates from the multiply imputed data sets. All reported P values were 2-sided and considered statistically significant at P ≤ .05.
Results
Participant Characteristics
Participants were predominantly female (77%), white (73%), and non-Hispanic (93%). The mean (SD) age was 17.1 (1.56) years and the median BMI was 50.4. Among the participants, 49% had poor physical functional status (ie, HAQ-DI score >0) and 76% had MSK pain. Compared with participants without pain, those reporting pain were older at surgery (mean [SD] age, 16.7 [1.46] vs 17.2 [1.58] years, respectively; P = .03), had a greater prevalence of clinically depressive symptoms (3 participants [6%] vs 29 [18%], respectively; P = .03) and HAQ-DI scores greater than 0 (17 participants [30%] vs 96 [56%], respectively; P < .01), had lower WRQOL scores (mean [SD] total score: 72.6 [16.77] vs 59.7 [17.33], respectively; P < .01; mean [SD] physical comfort score: 70.7 [22.64] vs 47.1 [22.72], respectively; P < .01), and had higher hs-CRP levels (median [interquartile range], 4.5 [2.5–8.8] vs 7.1 [3.4–13.3] mg/L; to convert to nanomoles per liter, multiply by 9.524; P = .03) (Table 1). No difference in BMI was detected for those with and without MSK pain (P = .17).
Table 1.
Baseline Characteristics of Participants by MSK Pain Statusa
| Characteristic | Total (N = 233) |
MSK Pain (n = 173) |
No MSK Pain (n = 56) |
P Value |
|---|---|---|---|---|
| Age at surgery, mean (SD), y | 17.1 (1.56) | 17.2 (1.58) | 16.7 (1.46) | .03 |
| Female, No. (%) | 179 (77) | 131 (76) | 44 (79) | .66 |
| White, No. (%) | 171 (73) | 131 (76) | 36 (64) | .31 |
| Non-Hispanic, No. (%) | 216 (93) | 159 (92) | 53 (95) | .77 |
| BMI, median (IQR) | 50.4 (45.2–57.4) | 50.5 (45.5–57.4) | 48.8 (43.4–57.9) | .17 |
| HAQ-DI score, median (IQR)b,c | 0 (0–0.38) | 0.125 (0.0–0.500) | 0.0 (0.0–0.125) | <.01 |
| Impaired physical functional status, No. (%)c,d | 113 (49) | 96 (56) | 17 (30) | <.01 |
| BDI-II total score, median (IQR)e | 6.0 (2–12) | 7.0 (2–12) | 4.0 (1–8) | <.01 |
| Depressive symptoms, No. (%)e,f | 32 (15) | 29 (18) | 3 (6) | .03 |
| IWQOL score, mean (SD)g | ||||
| Total | 62.9 (17.93) | 59.7 (17.33) | 72.6 (16.77) | <.01 |
| Physical comfort | 53.1 (24.78) | 47.1 (22.72) | 70.7 (22.64) | <.01 |
| High-sensitivity C-reactive protein, median (IQR), mg/Lc | 6.3 (3.0–11.7) | 7.1 (3.4–13.3) | 4.5 (2.5–8.8) | .03 |
Abbreviations: BDI-II, Beck Depression Inventory–II; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HAQ-DI, Health Assessment Questionnaire Disability Index; IQR, interquartile range; IWQOL, Impact of Weight on Quality of Life–Kids; MSK, musculoskeletal.
SI conversion factor: To convert high-sensitivity C-reactive protein to nanomoles per liter, multiply by 9.524.
Musculoskeletal pain was defined as any reported level of lower back, hip, knee, or ankle/foot pain (data missing for 4 participants).
Score of 0 indicates normal physical functional status; score greater than 0, impaired physical functional status.
Data missing for 4 participants.
Score on HAQ-DI greater than 0.
Data missing for 14 participants.
Score on BDI-II of 17 or greater.
Data missing for 9 participants.
MSK Pain
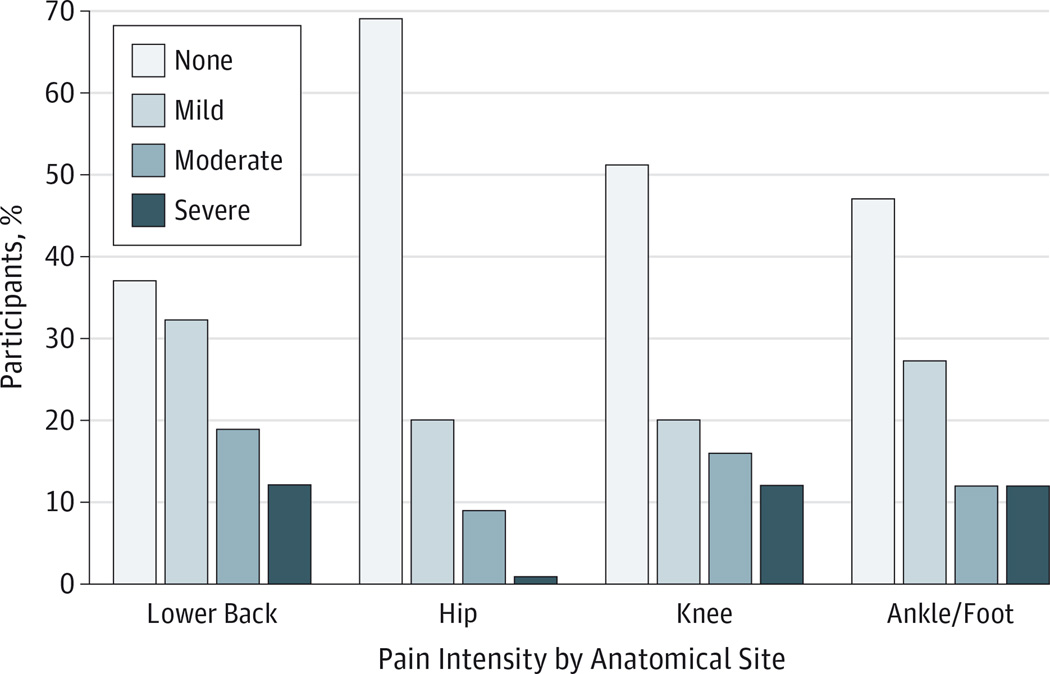
Lower back pain (63%) was most prevalent, followed by ankle/foot (53%), knee (49%), and hip (31%) pain. One-quarter (26%) of participants reported pain at all 4 sites (data not shown). Of those reporting pain, intensity was greatest at the knee (median score,4), followed by lower back (median score, 3), ankles/feet (median score, 3), and hips (median score, 2) (data not shown). Among those with a pain intensity score of 7 or greater, 12% had lower back pain, 12% had knee pain, 12% had ankle/foot pain, and 1% had hip pain (Figure).
Figure. Prevalence of Musculoskeletal Pain Intensity by Anatomical Site.
Musculoskeletal pain reported for anatomical sites on a 0- to 10-point visual analog scale and categorized by none (score of 0), mild (score of 1–3), moderate (score of 4–6), and severe (score of 7–10).
Physical Functional Status
Although half had an HAQ-DI score greater than 0, disability was more prevalent among those with MSK pain compared with those without (56% vs 30%, respectively; P < .01) (Table 1). The median HAQ-DI score was 0.125 (interquartile range, 0.0–0.500) for those reporting MSK pain. Participants scored higher than 0 (indicating some difficulty, much difficulty, or unable to perform specific tasks) in categories requiring gross motor tasks such as taking a tub bath, climbing stairs, and standing. Very few had scores higher than 0 on fine motor tasks such as lifting a glass to the mouth or opening jars (Table 2).
Table 2.
Self-reported Physical Functional Status in the Participantsa
| Activity | Participants, % | |||
|---|---|---|---|---|
| Without Any Difficulty |
With Some Difficulty |
With Much Difficulty |
Unable to Do | |
| Take tub bath | 75 | 15 | 5 | 5 |
| Dress yourself | 77 | 20 | 3 | 0 |
| Vacuum/yardwork chores | 77 | 17 | 5 | 1 |
| Run errands/shop | 82 | 13 | 4 | 0 |
| Get in/out of bed | 83 | 14 | 3 | 0 |
| Climb 5 stairs | 83 | 13 | 3 | 1 |
| Pick up clothing on floor | 83 | 15 | 2 | 0 |
| Get in/out of car | 84 | 14 | 2 | 0 |
| Stand up | 85 | 11 | 2 | 2 |
| Walk on flat ground | 90 | 8 | 1 | 1 |
| Wash/dry body | 90 | 9 | 1 | 0 |
| Retrieve 5-lb object | 93 | 6 | 0 | 0 |
| Get on/off toilet | 96 | 3 | 0 | 1 |
| Shampoo hair | 97 | 3 | 0 | 0 |
| Cut meat | 98 | 1 | 0 | 0 |
| Lift glass to mouth | 99 | 0 | 0 | 0 |
| Open milk carton | 99 | 0 | 0 | 0 |
| Open jar | 99 | 0 | 0 | 0 |
| Open car door | 100 | 0 | 0 | 0 |
| Turn faucet on/off | 100 | 0 | 0 | 0 |
Self-reported physical functional status was assessed by the Health Assessment Questionnaire Disability Index, on which 20 items measure physical functional ability in 8 activity categories. Items are scored on a 4-point scale: 0 indicates without any difficulty; 1, with some difficulty; 2, with much difficulty; and 3, unable to do.
Association of MSK Pain and Physical Functional Status
After adjustment for covariates, compared with pain-free participants, those reporting LE pain had 282% greater odds of having poor physical function (OR = 2.82; 95% CI, 1.35–5.88) (Table 3). A significant sex-by-race interaction was noted (P = .03). Specifically, nonwhite boys were less likely to report poor physical function compared with nonwhite girls (OR = 0.06; 95% CI, 0.01–0.55). Higher levels of LE pain intensity were associated with poor self-reported physical function (OR = 1.41; 95% CI, 1.20–1.66). Low back pain intensity was not associated with poor self-reported physical function.
Table 3.
Final Adjusted Model Showing Assocation of Lower Extremity Pain With Impaired Self-reported Physical Functional Statusa
| Outcome | OR (95% CI) | P Value |
|---|---|---|
| Musculoskeletal pain | ||
| Lower extremity vs none | 2.82 (1.35–5.88) | <.01 |
| Lower back vs none | 1.33 (0.47–3.81) | .59 |
| Lower back vs lower extremity | 0.47 (0.19–1.16) | .10 |
| BMI | 1.06 (1.02–1.10) | <.01 |
| Depressive symptoms | 4.19 (1.54–11.36) | <.01 |
| Sex-by-race interaction | … | .03 |
| White race, male vs female | 0.86 (0.39–1.88) | .70 |
| Nonwhite race, male vs female | 0.06 (0.01–0.55) | .01 |
| Female, nonwhite vs white | 1.26 (0.59–2.68) | .55 |
| Male, nonwhite vs white | 0.09 (0.01–0.81) | .03 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); OR, odds ratio; ellipsis, not applicable.
Self-reported physical functional status was assessed by the Health Assessment Questionnaire Disability Index, where a score higher than 0 indicates impaired physical functional status. Analyses were adjusted for age at surgery, BMI, depressive symptoms (indicated by a score ≥17 on the Beck Depression Inventory–II), sex, and race. Age at surgery was not retained in the final model.
Association of MSK Pain and WRQOL
After adjustment for covariates, WRQOL total scores were almost 10 points lower among participants with LE pain compared with those without LE pain (β = −9.42; 95% CI, −14.15 to −4.69) (Table 4). Similarly, WRQOL total scores for participants with LE pain were significantly lower than those reporting only back pain (for lower back vs LE pain: β = 9.96; 95% CI, 3.82 to 16.11). Each 1-point increase in LE pain intensity was associated with a 2.5-point decrease in WRQOL total score (β = −2.52; 95% CI, −3.51 to −1.54). Notably, there was no association between WRQOL and those reporting only back pain.
Table 4.
Final Adjusted Model Showing Assocation of Lower Extremity Pain With Poor Weight-Related Quality of Lifea
| Outcome | IWQOL Total Score | IWQOL Physical Comfort Score | ||
|---|---|---|---|---|
| β (95% CI) | P Value | β (95% CI) | P Value | |
| Musculoskeletal pain | ||||
| Lower extremity vs none | −9.42 (−14.15 to −4.69) | <.01 | −17.29 (−23.32 to −11.25) | <.01 |
| Lower back vs none | 0.54 (−6.36 to 7.44) | .88 | −4.82 (−13.61 to 3.97) | .28 |
| Lower back vs lower extremity | 9.96 (3.82 to 16.11) | <.01 | 12.46 (4.64 to 20.28) | <.01 |
| Male vs female | 4.85 (0.25 to 9.45) | .04 | … | … |
| Nonwhite vs white | 4.05 (−0.52 to 8.62) | .08 | … | … |
| BMI | … | … | −0.73 (−1.03 to −0.43) | <.01 |
| Depressive symptoms | −15.37 (−21.19 to −9.55) | <.01 | −9.62 (−17.39 to −1.84) | .02 |
| Physical functional status | −10.84 (−14.91 to −6.78) | <.01 | −17.35 (−22.73 to −11.96) | <.01 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); IWQOL, Impact of Weight on Quality of Life–Kids; ellipses, not applicable.
Weight-related quality of life was assessed with IWQOL scores. Analyses were adjusted for age at surgery, BMI, depressive symptoms (indicated by a score ≥17 on the Beck Depression Inventory–II), sex, race, and physical functional status (a score of 0 on the Health Assessment Questionnaire Disability Index indicates normal physical functional status; a score >0 indicates impaired physical functional status). Age at surgery was not retained in the final model for IWQOL total score or physical comfort score; sex and race were not retained in the final model for IWQOL physical comfort score; and BMI was not retained in the final model for IWQOL total score.
The relationship between MSK pain and physical comfort was also evaluated. Compared with those without pain or with only back pain, physical comfort scores were lower for those with LE pain (for LE pain vs none: β = −17.29; 95% CI, −23.32 to −11.25; for lower back vs LE pain: β = 12.46; 95% CI, 4.64 to 20.28) (Table 4). As with total scores, each 1-point increase in LE pain severity was related to a 4-point decrease in physical comfort score (β = −3.96; 95% CI, −5.21 to −2.71), while no association was seen between physical comfort and back pain severity.
Association of MSK Pain and Inflammation
Crude analysis indicated that the group with MSK pain had higher hs-CRP values than the group with no MSK pain (Table 1), but after adjustment, no significant association was observed between MSK pain and hs-CRP level (data not shown). However, as expected, higher BMI values (β = 0.03; 95% CI, 0.01–0.05) and age (β = 0.14; 95% CI, 0.03–0.25) were associated with higher hs-CRP values.
Discussion
This is the first report, to our knowledge, describing relationships between baseline MSK pain, physical functioning, and WRQOL in a cohort of adolescents with severe obesity. This evidence shows that MSK pain is highly prevalent and occurs at multiple sites. Most important and, to our knowledge, novel in the literature is the analysis that highlights that LE pain is strongly associated with poor physical function and poor quality of life in adolescents with severe obesity.
Musculoskeletal pain is prevalent among children and adolescents with obesity, ranging from 12% to 44%, compared with nonobese counterparts.12,15,24,25,41 In a cohort of obese youths, the prevalence of LE pain was 70% and that of upper extremity or back pain was 27%.9 Our results are consistent with previous studies as most participants had MSK pain (76%), with pain commonly occurring in the lower back, followed by LE pain.11 Unlike previous studies, we found that a quarter of our participants had pain at all 4 sites.9
Comparison of MSK pain and its intensity for children and adolescents with obesity in the existing literature proves challenging owing to variable study design and pain assesment tools.25,26,41 For example, one study reported a pain intensity of overall pain with medians of 5 to 50 mm.26 Another documented that knee pain intensity ranged from means of 4 to 8 cm (40–80 mm) at all time points (present, worst, and average).25 Compared with other studies, our participants had prevalent lower back pain at a low intensity score of 2 of 10.26 However, when a score of 0 was excluded, pain intensity at the knee was highest at a level of 4 of 10. Thus, MSK pain is present and affects daily functioning among adolescents with severe obesity.
The effect of MSK pain on objective and reported physical function is poorly characterized in the literature. A few small studies have reported that adolescents with obesity have diminished strength, diminished physical activity levels (ultimately affecting performance and gait asymmetry), and loss of movement efficiency on functional performance tests.42,43 In children and adolescents with autoimmune arthritis, the median score on the Child Health Assessment Questionnaire (an instrument adapted from the HAQ) was 0.125.44 This level of poor physical function was similar to our adolescents with MSK pain (median HAQ-DI score, 0.125). This is concerning because pain-related functional impairment will worsen as these adolescents age if their weight trajectory is not altered.
Lower extremity pain had a profound association with physical function. The presence of LE pain increased the odds of poor physical functioning by nearly 3-fold, while lower back pain did not. This suggests that LE pain is the major contributor affecting physical function. Whether this can be reversed is a matter of speculation, but adult studies consistently show that most obese individuals have improved back and LE pain, improved self-reported physical function, and improved functional performance after WLS.23,45
Depression, race, and sex were covariates in these analyses, and we noted that they were associated with diminished physical function. These findings were expected as obesity has been associated with both depression and joint problems in children aged 10 to 17 years.46 We hypothesize that meaningful weight loss will have beneficial effects on pain and depression, and longitudinal analyses in this cohort will allow us to test this hypothesis.
Pain affects health-related quality of life, too, and compared with healthy-weight peers with comorbidities, children and adolescents with obesity consistently reported poorer health-related quality of life.3–7 In an obese cohort of medically managed children, LE pain was correlated with diminished quality of life on the Pediatric Quality of Life Inventory, especially in the physical domain.9 Our cohort reported low total and physical comfort scores on WRQOL compared with other obese cohorts.33,34 Those with MSK pain had total and physical comfort scores 10 and 20 points lower than those without MSK pain. Similarly, LE pain was strongly associated with poor physical comfort scores, suggesting there would likely be an important clinical impact by addressing LE pain symptoms.
We found no association between MSK pain and inflammation (hs-CRP level) after adjusting for BMI, which suggests that although cytokines affect pain through mechanisms of nociceptor activation, these pathways are complex and not specific to hs-CRP.47
This study has a number of limitations that should be high-lighted. First, as a cross-sectional observational study, causality for significant associations could not be addressed. Planned longitudinal studies should determine whether significant weight loss anticipated following WLS results in MSK pain reduction and improved physical functioning, and WRQOL out-comes may address causality. Second, our pain assessment tool was limited to describing pain during a week, which limits the understanding of pain chronicity and quality. Third, the physical examination data were not focused on the MSK system, which could suggest a cause for pain such as joint hypermobility or arthritis. Fourth, the HAQ-DI is not an ideal tool to assess function in obese adolescents since some of the questions may be less relevant to teens (running errands) and many questions pertain to upper extremity function rather than LE function. Moreover, there is a theoretical ceiling effect because the HAQ-DI does not discriminate well between people with low levels of self-reported physical functioning as is likely in this cohort.48 Fifth, although our data cannot be generalized to all adolescents with obesity, we can generalize our findings to those seeking bariatric surgery because our participants were older, white teens with a very high BMI. Additionally, our data suggest that these outcomes should be studied in a diverse group of teens who are obese.
Finally, it is possible that participants’ self-reports of physical function and pain were exaggerated in the hope of under-going WLS. However, this is unlikely to bias our results as the data collection was done after the decision for surgery was taken.
Conclusions
Musculoskeletal pain was highly prevalent in our cohort of adolescents with severe obesity. Lower extremity pain predicted physical functional limitations and impaired physical comfort related to weight. These problems likely lead to a cycle of more severe obesity and greater functional limitations, putting these adolescents at greater risk for diseases or conditions associated with sedentary behavior as well as greater risk for early and progressive degenerative joint disease.14 The possibility of reversing these limitations and risks with significant weight loss is compelling and of urgent public health importance.49 Health care practitioners working with severely obese adolescents should be cognizant of LE pain in these patients and how it may affect exercise ability at entrance, adherence to exercise, and other physical activity recommendations; these issues can be addressed early in caring for their patients. Emphasis should be given to recommendations for activities that do not exacerbate LE pain. Future studies will need to focus on the longitudinal outcomes in this cohort as well as prospective studies on the impact of weight loss on pain, objective function, LE joint loads, and LE joint integrity.
At a Glance.
Musculoskeletal and lower extremity pain occurs in three-quarters of the adolescents in the Teen-LABS cohort preparing for bariatric surgery.
Lower extremity pain is associated with self-reported physical functional limitations.
Lower extremity pain is associated with impaired physical health-related quality of life.
Clinicians caring for severely obese children should be cognizant of and address musculoskeletal and lower extremity pain and associated limitations early in treatment.
Longitudinal studies will demonstrate whether weight loss can mitigate musculoskeletal pain and physical functional limitations.
Acknowledgments
Dr Courcoulas reported receiving grants from the National Institute of Diabetes and Digestive and Kidney Diseases and from EndoGastric Solutions as well as personal fees from Ethicon Scientific outside this work. Dr Inge reported receiving grants from Ethicon Endosurgery outside this work.
Funding/Support: The Teen-LABS consortium was funded by cooperative agreements with the National Institute of Diabetes and Digestive and Kidney Diseases through grants U01DK072493, UM1DK072493, and UM1DK095710 (University of Cincinnati). The study was also supported by grants UL1 TR000077-04 (Cincinnati Children’s Hospital Medical Center), UL1RR025755 (Nationwide Children’s Hospital), M01-RR00188 (Texas Children’s Hospital/Baylor College of Medicine), UL1 RR024153 and UL1TR000005 (University of Pittsburgh), and UL1 TR000165 (University of Alabama at Birmingham) from the National Institutes of Health.
Role of the Funder/Sponsor: The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional Contributions: We gratefully acknowledge the dedication and expertise of the coinvestigators and research coordinators at each site and the administrative, data management, data quality/integrity, and analyst staff at the Data Coordinating Center, Cincinnati, Ohio.
Footnotes
Author Contributions: Dr Jenkins had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Bout-Tabaku, Michalsky, Baughcum, Brandt, Helmrath, Inge.
Acquisition, analysis, or interpretation of data: Bout-Tabaku, Michalsky, Jenkins, Baughcum, Zeller, Courcoulas, Buncher, Harmon, Chen, Inge.
Drafting of the manuscript: Bout-Tabaku, Michalsky, Jenkins, Baughcum, Harmon.
Critical revision of the manuscript for important intellectual content: Bout-Tabaku, Michalsky, Jenkins, Baughcum, Zeller, Brandt, Courcoulas, Buncher, Helmrath, Chen, Inge.
Statistical analysis: Jenkins, Buncher.
Obtained funding: Michalsky, Zeller, Courcoulas, Harmon, Inge.
Administrative, technical, or material support: Michalsky, Zeller, Courcoulas, Helmrath.
Study supervision: Bout-Tabaku, Michalsky.
Conflict of Interest Disclosures: No other disclosures were reported.
REFERENCES
- 1.Nadeau KJ, Maahs DM, Daniels SR, Eckel RH. Childhood obesity and cardiovascular disease: links and prevention strategies. Nat Rev Cardiol. 2011;8(9):513–525. doi: 10.1038/nrcardio.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russell M, Trevisan M, Stranges S. Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med. 2010;362(19):1840. doi: 10.1056/NEJMc1002801. [DOI] [PubMed] [Google Scholar]
- 3.Daniels SR, Greer FR Committee on Nutrition. Lipid screening and cardiovascular health in childhood. Pediatrics. 2008;122(1):198–208. doi: 10.1542/peds.2008-1349. [DOI] [PubMed] [Google Scholar]
- 4.Manco M, Morandi A, Marigliano M, Rigotti F, Manfredi R, Maffeis C. Epicardial fat, abdominal adiposity and insulin resistance in obese pre-pubertal and early pubertal children. Atherosclerosis. 2013;226(2):490–495. doi: 10.1016/j.atherosclerosis.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 5.Michelson PH, Williams LW, Benjamin DK, Barnato AE. Obesity, inflammation, and asthma severity in childhood: data from the National Health and Nutrition Examination Survey 2001–2004. Ann Allergy Asthma Immunol. 2009;103(5):381–385. doi: 10.1016/S1081-1206(10)60356-0. [DOI] [PubMed] [Google Scholar]
- 6.Schwimmer JB, Burwinkle TM, Varni JW. Health-related quality of life of severely obese children and adolescents. JAMA. 2003;289(14):1813–1819. doi: 10.1001/jama.289.14.1813. [DOI] [PubMed] [Google Scholar]
- 7.Skelton JA, Cook SR, Auinger P, Klein JD, Barlow SE. Prevalence and trends of severe obesity among US children and adolescents. Acad Pediatr. 2009;9(5):322–329. doi: 10.1016/j.acap.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skinner AC, Skelton JA. Prevalence and trends in obesity and severe obesity among children in the United States, 1999–2012. JAMA Pediatr. 2014;168(6):561–566. doi: 10.1001/jamapediatrics.2014.21. [DOI] [PubMed] [Google Scholar]
- 9.Bout-Tabaku S, Briggs MS, Schmitt LC. Lower extremity pain is associated with reduced function and psychosocial health in obese children. Clin Orthop Relat Res. 2013;471(4):1236–1244. doi: 10.1007/s11999-012-2620-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sowers MR, Karvonen-Gutierrez CA. The evolving role of obesity in knee osteoarthritis. Curr Opin Rheumatol. 2010;22(5):533–537. doi: 10.1097/BOR.0b013e32833b4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stovitz SD, Pardee PE, Vazquez G, Duval S, Schwimmer JB. Musculoskeletal pain in obese children and adolescents. Acta Paediatr. 2008;97(4):489–493. doi: 10.1111/j.1651-2227.2008.00724.x. [DOI] [PubMed] [Google Scholar]
- 12.Taylor ED, Theim KR, Mirch MC, et al. Orthopedic complications of overweight in children and adolescents. Pediatrics. 2006;117(6):2167–2174. doi: 10.1542/peds.2005-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wills AK, Black S, Cooper R, et al. Life course body mass index and risk of knee osteoarthritis at the age of 53 years: evidence from the 1946 British birth cohort study. Ann Rheum Dis. 2012;71(5):655–660. doi: 10.1136/ard.2011.154021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Widhalm HK, Seemann R, Hamboeck M, et al. Osteoarthritis in morbidly obese children and adolescents, an age-matched controlled study. Knee Surg Sports Traumatol Arthrosc. doi: 10.1007/s00167-014-3068-4. [published online May 20, 2014]. [DOI] [PubMed] [Google Scholar]
- 15.Krul M, van der Wouden JC, Schellevis FG, van Suijlekom-Smit LW, Koes BW. Musculoskeletal problems in overweight and obese children. Ann Fam Med. 2009;7(4):352–356. doi: 10.1370/afm.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Felson DT, Anderson JJ, Naimark A, Walker AM, Meenan RF. Obesity and knee osteoarthritis: the Framingham Study. Ann Intern Med. 1988;109(1):18–24. doi: 10.7326/0003-4819-109-1-18. [DOI] [PubMed] [Google Scholar]
- 17.Karvonen-Gutierrez CA, Harlow SD, Mancuso P, Jacobson J, Mendes de Leon CF, Nan B. Leptin levels are associated with radiographic knee osteoarthritis among a cohort of mid-life women. Arthritis Care Res (Hoboken) doi: 10.1002/acr.21922. [published online December 19, 2012]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teichtahl AJ, Wang Y, Wluka AE, Cicuttini FM. Obesity and knee osteoarthritis: new insights provided by body composition studies. Obesity (Silver Spring) 2008;16(2):232–240. doi: 10.1038/oby.2007.30. [DOI] [PubMed] [Google Scholar]
- 19.Felson DT. Developments in the clinical understanding of osteoarthritis. Arthritis Res Ther. 2009;11(1):203. doi: 10.1186/ar2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Messier SP, Gutekunst DJ, Davis C, DeVita P. Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis Rheum. 2005;52(7):2026–2032. doi: 10.1002/art.21139. [DOI] [PubMed] [Google Scholar]
- 21.Messier SP, Mihalko SL, Legault C, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310(12):1263–1273. doi: 10.1001/jama.2013.277669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richette P, Poitou C, Garnero P, et al. Benefits of massive weight loss on symptoms, systemic inflammation and cartilage turnover in obese patients with knee osteoarthritis. Ann Rheum Dis. 2011;70(1):139–144. doi: 10.1136/ard.2010.134015. [DOI] [PubMed] [Google Scholar]
- 23.Vincent HK, Ben-David K, Conrad BP, Lamb KM, Seay AN, Vincent KR. Rapid changes in gait, musculoskeletal pain, and quality of life after bariatric surgery. Surg Obes Relat Dis. 2012;8(3):346–354. doi: 10.1016/j.soard.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 24.de Sá Pinto AL, de Barros Holanda PM, Radu AS, Villares SM, Lima FR. Musculoskeletal findings in obese children. J Paediatr Child Health. 2006;42(6):341–344. doi: 10.1111/j.1440-1754.2006.00869.x. [DOI] [PubMed] [Google Scholar]
- 25.Deere KC, Clinch J, Holliday K, et al. Obesity is a risk factor for musculoskeletal pain in adolescents: findings from a population-based cohort. Pain. 2012;153(9):1932–1938. doi: 10.1016/j.pain.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Tsiros MD, Buckley JD, Howe PR, Walkley J, Hills AP, Coates AM. Musculoskeletal pain in obese compared with healthy-weight children. Clin J Pain. 2014;30(7):583–588. doi: 10.1097/AJP.0000000000000017. [DOI] [PubMed] [Google Scholar]
- 27.Inge TH, Zeller MH, Jenkins TM, et al. Teen-LABS Consortium. Perioperative outcomes of adolescents undergoing bariatric surgery: the Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study. JAMA Pediatr. 2014;168(1):47–53. doi: 10.1001/jamapediatrics.2013.4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inge TH, Zeller M, Harmon C, et al. Teen-Longitudinal Assessment of Bariatric Surgery: methodological features of the first prospective multicenter study of adolescent bariatric surgery. J Pediatr Surg. 2007;42(11):1969–1971. doi: 10.1016/j.jpedsurg.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruce B, Fries JF. The Stanford Health Assessment Questionnaire: dimensions and practical applications. Health Qual Life Outcomes. 2003;1:20. doi: 10.1186/1477-7525-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krishnan E, Sokka T, Häkkinen A, Hubert H, Hannonen P. Normative values for the Health Assessment Questionnaire disability index: benchmarking disability in the general population. Arthritis Rheum. 2004;50(3):953–960. doi: 10.1002/art.20048. [DOI] [PubMed] [Google Scholar]
- 31.Golightly YM, Hannan MT, Shi XA, Helmick CG, Renner JB, Jordan JM. Association of foot symptoms with self-reported and performance-based measures of physical function: the Johnston County osteoarthritis project. Arthritis Care Res (Hoboken) 2011;63(5):654–659. doi: 10.1002/acr.20432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fries JF. Arthritis, Rheumatism, and Aging Medical Information System (ARAMIS) [Accessed January 20, 2014]; http://aramis.stanford.edu. [PubMed] [Google Scholar]
- 33.Kolotkin RL, Zeller M, Modi AC, et al. Assessing weight-related quality of life in adolescents. Obesity (Silver Spring) 2006;14(3):448–457. doi: 10.1038/oby.2006.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Modi AC, Loux TJ, Bell SK, Harmon CM, Inge TH, Zeller MH. Weight-specific health-related quality of life in adolescents with extreme obesity. Obesity (Silver Spring) 2008;16(10):2266–2271. doi: 10.1038/oby.2008.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeller MH, Reiter-Purtill J, Ratcliff MB, Inge TH, Noll JG. Two-year trends in psychosocial functioning after adolescent Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2011;7(6):727–732. doi: 10.1016/j.soard.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stannus OP, Jones G, Blizzard L, Cicuttini FM, Ding C. Associations between serum levels of inflammatory markers and change in knee pain over 5 years in older adults: a prospective cohort study. Ann Rheum Dis. 2013;72(4):535–540. doi: 10.1136/annrheumdis-2011-201047. [DOI] [PubMed] [Google Scholar]
- 37.Shimura Y, Kurosawa H, Sugawara Y, et al. The factors associated with pain severity in patients with knee osteoarthritis vary according to the radiographic disease severity: a cross-sectional study. Osteoarthritis Cartilage. 2013;21(9):1179–1184. doi: 10.1016/j.joca.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 38.Kashikar-Zuck S, Zafar M, Barnett KA, et al. Quality of life and emotional functioning in youth with chronic migraine and juvenile fibromyalgia. Clin J Pain. 2013;29(12):1066–1072. doi: 10.1097/AJP.0b013e3182850544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stevanovic D, Susic G. Health-related quality of life and emotional problems in juvenile idiopathic arthritis. Qual Life Res. 2013;22(3):607–612. doi: 10.1007/s11136-012-0172-0. [DOI] [PubMed] [Google Scholar]
- 40.Beck AT, Sr, Brown GK. Manual for Beck Depression Inventory–II. San Antonio, TX: Psychological Corp; 1996. [Google Scholar]
- 41.Hainsworth KR, Davies WH, Khan KA, Weisman SJ. Co-occurring chronic pain and obesity in children and adolescents: the impact on health-related quality of life. Clin J Pain. 2009;25(8):715–721. doi: 10.1097/AJP.0b013e3181a3b689. [DOI] [PubMed] [Google Scholar]
- 42.Tsiros MD, Buckley JD, Howe PR, et al. Day-to-day physical functioning and disability in obese 10- to 13-year-olds. Pediatr Obes. 2013;8(1):31–41. doi: 10.1111/j.2047-6310.2012.00083.x. [DOI] [PubMed] [Google Scholar]
- 43.Gushue DL, Houck J, Lerner AL. Effects of childhood obesity on three-dimensional knee joint biomechanics during walking. J Pediatr Orthop. 2005;25(6):763–768. doi: 10.1097/01.bpo.0000176163.17098.f4. [DOI] [PubMed] [Google Scholar]
- 44.Weiss PF, Beukelman T, Schanberg LE, Kimura Y, Colbert RA CARRA Registry Investigators. Enthesitis-related arthritis is associated with higher pain intensity and poorer health status in comparison with other categories of juvenile idiopathic arthritis: the Childhood Arthritis and Rheumatology Research Alliance Registry. J Rheumatol. 2012;39(12):2341–2351. doi: 10.3899/jrheum.120642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hooper MM, Stellato TA, Hallowell PT, Seitz BA, Moskowitz RW. Musculoskeletal findings in obese subjects before and after weight loss following bariatric surgery. Int J Obes (Lond) 2007;31(1):114–120. doi: 10.1038/sj.ijo.0803349. [DOI] [PubMed] [Google Scholar]
- 46.Halfon N, Larson K, Slusser W. Associations between obesity and comorbid mental health, developmental, and physical health conditions in a nationally representative sample of US children aged 10 to 17. Acad Pediatr. 2013;13(1):6–13. doi: 10.1016/j.acap.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 47.Kidd BL, Urban LA. Mechanisms of inflammatory pain. Br J Anaesth. 2001;87(1):3–11. doi: 10.1093/bja/87.1.3. [DOI] [PubMed] [Google Scholar]
- 48.White DK, Wilson JC, Keysor JJ. Measures of adult general functional status: SF-36 Physical Functioning Subscale (PF-10), Health Assessment Questionnaire (HAQ), Modified Health Assessment Questionnaire (MHAQ), Katz Index of Independence in Activities of Daily Living, Functional Independence Measure (FIM), and Osteoarthritis-Function-Computer Adaptive Test (OA-Function-CAT) Arthritis Care Res (Hoboken) 2011;63(suppl 11):S297–S307. doi: 10.1002/acr.20638. [DOI] [PubMed] [Google Scholar]
- 49.US Bone and Joint Initiative. The Burden of Musculoskeletal Disease in the United States. 2nd ed. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2011. [Google Scholar]