Abstract
The complement anaphylatoxin C5a is a critical mediator of allergic contact dermatitis, bridging essential aspects of innate and adaptive immunity. This anaphylatoxin functions by interacting with two 7-transmembrane segment (TMS) receptors, the C5aR and C5L2. The C5aR is a classical G protein coupled receptor while C5L2 is deficient in coupling to G proteins due to variations in the sequence. Our previous work in human neutrophils revealed a unique role for C5L2 in negatively modulating anaphylatoxin receptor mediated cellular activation through interactions with β-arrestin. When C5L2 is deficient, C5aR mediated β-arrestin signaling is greatly enhanced. The work described here was undertaken first, to determine the impact of C5L2 deficiency in a murine model of contact sensitivity, and second, to determine whether the resultant exacerbation of inflammatory parameters reflects a negative modulatory function of C5L2 on the C5aR. First, we find dramatic increases in inflammation in C5L2−/− animals compared with wild type mice, and second, these increases are completely reversed following administration of monoclonal antibody against the C5aR. Thus, in allergic contact sensitivity, as in isolated human neutrophils, C5L2 functions to suppress C5a-C5aR mediated responses, further underscoring its role as a negative regulator of anaphylatoxin activity.
Keywords: complement, anaphylatoxins, C5a, C5L2, C5a receptor, contact sensitivity, oxazolone, murine DTH
Introduction
Allergic contact dermatitis is a common delayed type hypersensitivity reaction of the skin that results in more than 8 million outpatient visits per year in the United States (1, 2). The pathophysiology of this disease is examined primarily using animal models of contact sensitivity (CS) and involves induction of T-cell mediated skin inflammation resulting from exposure to a hapten in a sensitized animal (3). Current understanding describes at least two distinct phases of disease development: sensitization and elicitation. During the sensitization phase, the hapten painted on the animal’s body penetrates into the epidermis and is taken up by resident dendritic cells. The haptenated dendritic cells induce rapid (within 1 day) production of IgM by B-1 cells (4). Subsequent topical application of the same hapten to a remote site of the body initiates the elicitation phase, triggering an inflammatory cascade of cytokine and chemokine production, neutrophil infiltration, and mast cell activation, leading to T-cell recruitment. In mouse models, this process generally peaks at ~24 hours (5).
Initial paradigms supported the concept of CS as mediated solely by T cell activation. Work by Tsuji, et al (6–8), however, demonstrated a central role for complement activation by formation of IgM-antigen complexes. In mice deficient in C5 or the C5a receptor (C5aR) the inflammatory response fails to occur, indicating an important linkage of C5a in early activation of innate immunity that is required for subsequent elicitation of the acquired T cell response.
Studies have also invoked a role for C3 and/or C3a in CS although the mechanism is less clear. Purwar, et al (9), observed heightened contact sensitivity to multiple haptens in C3 deficient mice, as evidenced by increased swelling of challenged tissues, elevated tissue expression of IFN-γ, the chemokines CXCL-10, CCL-2, and CCL-17, as well as increased IFN-γ from splenocytes and draining lymph nodes. A subsequent study was undertaken to distinguish the individual roles of C3a and other C3 products using C3aR deficient mice (10). This work recapitulated the increased cytokine response in the sensitization phase, but no differences were observed in the elicitation phase in terms of both challenged tissue swelling and cytokine secretion. Thus the apparent protective role of C3 was thought to result from a downstream cascade involving C3b, and/or contributions of C5aR or C5L2.
The C5a anaphylatoxin is considered as one of the most potent pro-inflammatory components of the complement system, capable of activating neutrophils, monocytes, macrophages, and mast cells, among others, at nanomolar concentrations (11). It functions through two 7-TMS receptors, the C5aR and C5L2. The C5aR is a classical G protein coupled receptor (12), while C5L2 fails to couple to G proteins because of an amino acid replacement in the 2nd intracellular loop sequence (13, 14). C5L2 exhibits similar binding affinity for C5a and C5adesArg as the C5aR and was initially described as a decoy receptor (15). In human PMNs C5L2 has been shown to act as a negative modulator of C5a mediated responses (16). In the presence of a blocking antibody against C5L2, certain C5a mediated functions are markedly potentiated, including ERK1/2 activation and chemotaxis, resulting from increased C5aR-mediated β-arrestin signaling. Similar increases in inflammation and neutrophil activation have been observed in vivo in C5L2−/− mice (14, 17, 18). Inflammatory indices were markedly increased in C5L2−/− mice in a model of autoimmune glomerular nephritis induced by anti-neutrophil cytoplasmic antibodies (19), as well as a model of diabetes when animals were placed on a high-fat/high sucrose diet (20). In a mouse model of sepsis, blocking C5L2 resulted in increased IL-6 and TNF-α production (21). Similarly in human colonic epithelial cells C5a stimulation induced release of CXCL-8/IL-8, a response that was enhanced by blocking C5L2 (22).
The work presented here was undertaken to further elucidate the role of C5L2 as a modulator of C5a-C5aR mediated inflammation. We tested C5L2−/− mice in a model of oxalazone (OX) induced CS in comparison with wild type animals of the same genetic background. We demonstrate increased inflammation in the C5L2−/− animals in support of the negative modulatory role of C5L2 toward the C5aR. Further supporting this role of C5L2, we show that CS inflammation in C5L2−/− mice is reduced to the level of wild type animals by a blocking antibody against the C5aR.
Materials and Methods
Animals
The generation and initial characterization of C5L2 deficient mice have been previously described (17). Animals were backcrossed through at least 10 generations to the Balb/c background and maintained in the Children’s Hospital of Boston Animal Facility. All experiments utilized female wild type and C5L2−/− mice at 8–12 weeks of age. All studies were conducted in accordance with the Institutional Animal Care and Use Committee of Children’s Hospital.
Antibodies and reagents
Oxazolone, 4-Ethoxymethylene-2-phenyl-2oxazolin-5-one (OX) was purchased from Sigma-Aldrich. Anti-C5aR (Rat anti-mouse CD88 clone 20/70, low endotoxin) and rat isotype control IgG2b were obtained from AbD Serotec.
Contact sensitivity induction
On day 0, wild type and C5L2−/− mice were sensitized by topical application of 50µl of 3% OX dissolved in a mixture of acetone and olive oil (4:1v/v) to the shaved abdomen. On day 5, animals were challenged by topical application of 20µl of 0.5% OX to both sides of the experimental ear, while the contralateral ear received vehicle alone and served as the control. In experiments involving antibody blockade, anti-C5aR (35µg in 100µl PBS), isotype control IgG (35µg in 100µl PBS), or PBS (100µl) alone was injected retro-orbitally 1h prior to challenge. Twenty-four hours later, mice were sacrificed and analyzed for CS reactions. Ear thickness was measured using a thickness gage (Kafer J15, Long Island Indicator, Hauppauge, NY). Ears were harvested along the base of the lobes, and the wet weight difference between the control (vehicle only) and experimental (OX-challenged) ears was determined. In some experiments, the ears were subsequently dried under vacuum at 100°C overnight for determination of wet/dry weight ratios.
Myeloperoxidase assay
Ear samples (~30mg) were homogenized in HBSS, pH 7.5. Homogenates were suspended in 1% hexadecyltrimethylammonium bromide (HTAB, Sigma-Aldrich). Samples were subjected to multiple freeze/thaw cycles using liquid nitrogen, and insoluble material removed by centrifugation. Supernatants were assessed for myeloperoxidase using 3,3’,5,5’-tetramethylbenzidine (TMB, Sigma-Aldrich) by determining the absorbance at 450nm as previously described (23). Absorbance values obtained were converted to neutrophil equivalents based on a standard curve generated with purified cells, and results are expressed as MPO equivalent to the neutrophil numbers determined.
Cytokine and chemokine analyses
For determination of the tissue content of cytokines, control and OX-challenged ears were homogenized HBSS, pH7.5, and the supernatants used to assess levels of IFN-γ, IL-17A, IL-4, IL-5, IL-6, IL-10, and IL-12p40 using ELISA kits (OptEIA, BD Biosciences).
RNA extraction and quantitative real time PCR
For RNA analyses, excised ears were stored in RNA-Later (Qiagen) at 4°C for up to 1 week. RNA was extracted using a commercially available kit (RNAeasy Mini kit, Qiagen) and reverse transcribed (Superscript Vilo, LifeTechnologies) according to the manufacturer’s protocols. Quantitative PCR was performed using SYBR Green Supermix (BioRad). All primers were purchased from Integrated DNA Technologies and designed based on recommendations from LifeTechnologies Primer Design Tool. Amplified targets span at least one intron where possible and the amplification efficiency was determined at 92–100%. The primers utilized were as follows: IFN-γ (F5’-TGAACGCTACACACTGCATCTTGG-3’; R5’-CGACTCCTTTTCCGCTTCCTGAG-3’), CXCL-1 (F5-GCTGGGATTCACCTCAAGAA-3’; R5’-AGGTGCCATCAGAGCAGTCT-3’), CXCL-2 (F5’-TCCAGAGCTTGAGTGTGA-3’; R5’-AGGCACATCAGGTACGATCC-3’), CCL-2 (F5’-AGGTCCCTGTCATGCTTCTG-3’; R5’-TCTGGACCCATTCCTTCTTG-3’), IL-17A (F5’-TCCAGAAGGCCCTCAGACTA-3’; R5’-AGCATCTTCTCGACCCTGAA-3’), IL-6 (F5’-TCTGGGAAATCGTGGAAATGA-3’; R5’-TGGATGGTCTTGGTCCTTAGC-3’) IL-10 (F5’-CACTGCTATGCTGCCTGCTCTTAC-3’; R5’-AAAATCACTCTTCACCTGCTCCAC-3’), β-actin (F5’-TGTTACCAACTGGGACGACA-3’; R5’-ACCAGAGGCATACAGGGACA-3’), and β-2-microglobulin (B2M) (F5’-CTGACCGGCCTGTATGCTAT-3’; R5’-CAGTCTCAGTGGGGGTGAAT-3’). Results for cytokine and chemokine genes were determined by the ΔΔCT method, and normalized to both β-actin and B2M as reference housekeeping genes.
Lymph node cell cultures
For ex vivo stimulation experiments, bilateral inguinal and axillary lymph nodes were harvested from OX sensitized and challenged mice one day after challenge. Single-cell suspensions were prepared in complete Iscove’s medium supplemented with 10% FCS, 100U/ml penicillin and 10µg/ml streptomycin. Cells were cultured at 1×106/ml in the presence of 0 or 1µg/ml OX, and assessed for chemokine and cytokine production after 1 or 24h as described above.
Statistical Analysis
Data are presented as the mean ± SEM. The data were analyzed using Prism for Windows software (GraphPad) and statistical analyses were performed using Student’s t-test and two-way ANOVA. A P value ≤0.05 was considered statistically significant.
Results
C5L2−/− mice exhibit increased OX-induced contact sensitivity
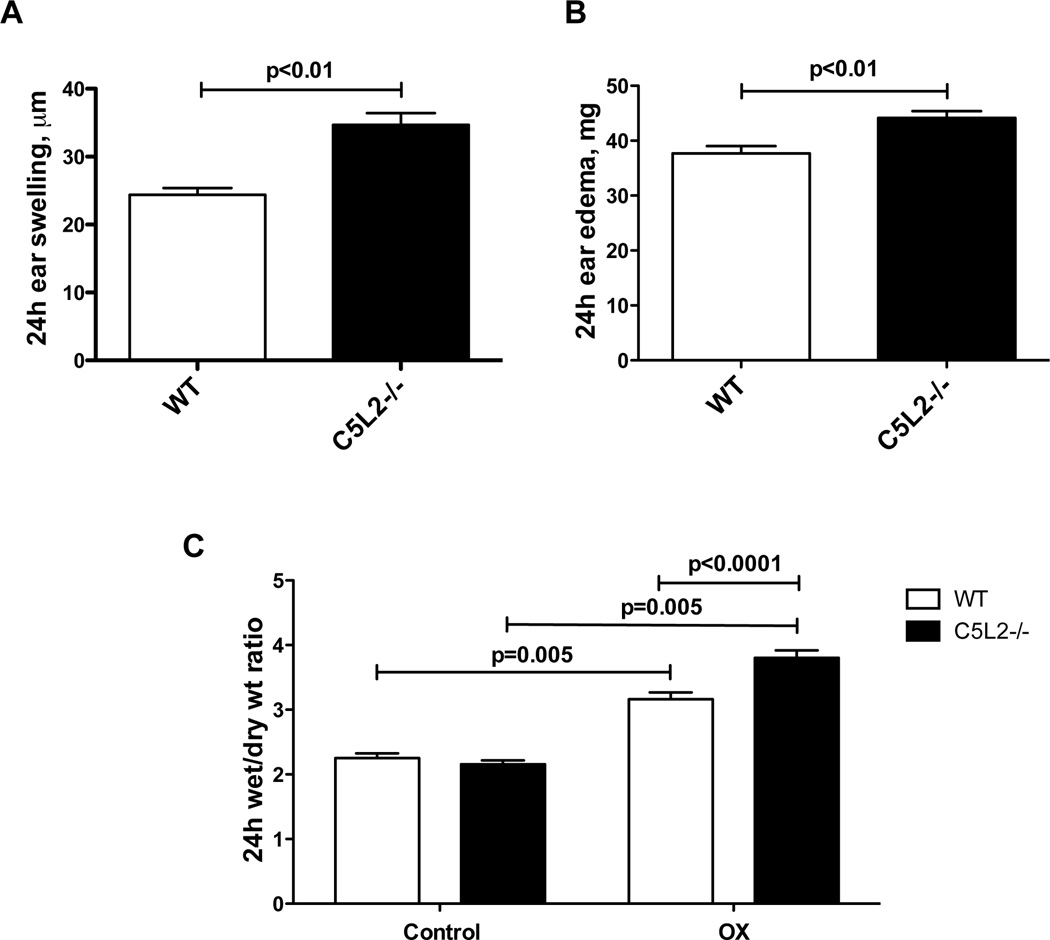
The inflammation associated with allergic contact sensitivity in the mouse has been shown to be critically dependent on early generation of C5a (4, 7, 24). In animals deficient in C5 or the C5aR, inflammation was not observed following hapten challenge. Our work in isolated human neutrophils has revealed a role for the second C5a receptor, C5L2, in attenuating C5a mediated responses (16). We therefore tested C5L2−/− mice in the model of OX induced contact sensitivity in comparison with wild type animals to test our hypothesis that C5L2 deficiency would exacerbate this response. Five days after initiating OX sensitization, mice were challenged by application of hapten to one ear, while the other received vehicle alone. Twenty-four hours later animals were sacrificed and evaluated for inflammatory responses. The challenged ears from both C5L2−/− and wild type mice exhibited significantly increased swelling following application of the hapten compared with the control ears, assessed as ear thickness, wet weight and the ratio of wet to dry weight (Figure 1). Importantly, the response in C5L2−/− mice was significantly greater than wild type animals for all three parameters. The increase in thickness was ~40% greater in C5L2−/− ears, and both wet weight and the wet/dry weight ratio were increased ~20%, compared with ears from wild type mice (P<0.01 for each measurement, n=9–10 mice/group).
Figure 1. C5L2 deficiency results in exacerbation of tissue swelling and edema associated with OX-induced contact sensitivity.
A. Ear swelling determined as the thickness of OX-challenged ears minus the thickness of vehicle treated control ears in C5L2−/− and wild type mice. B. Ear edema measured as the weight of OX-challenged ears minus vehicle-treated control ears in C5L2−/− and wild type mice. C. Wet/dry weight ratio for OX-challenged and vehicle treated control ears from C5L2−/− and wild type mice. Data are expressed as the mean ± SEM for each value, the significance of differences is indicated, n=9–10 mice/group.
C5L2−/− mice exhibit significantly increased inflammatory cell influx relative to wild type animals
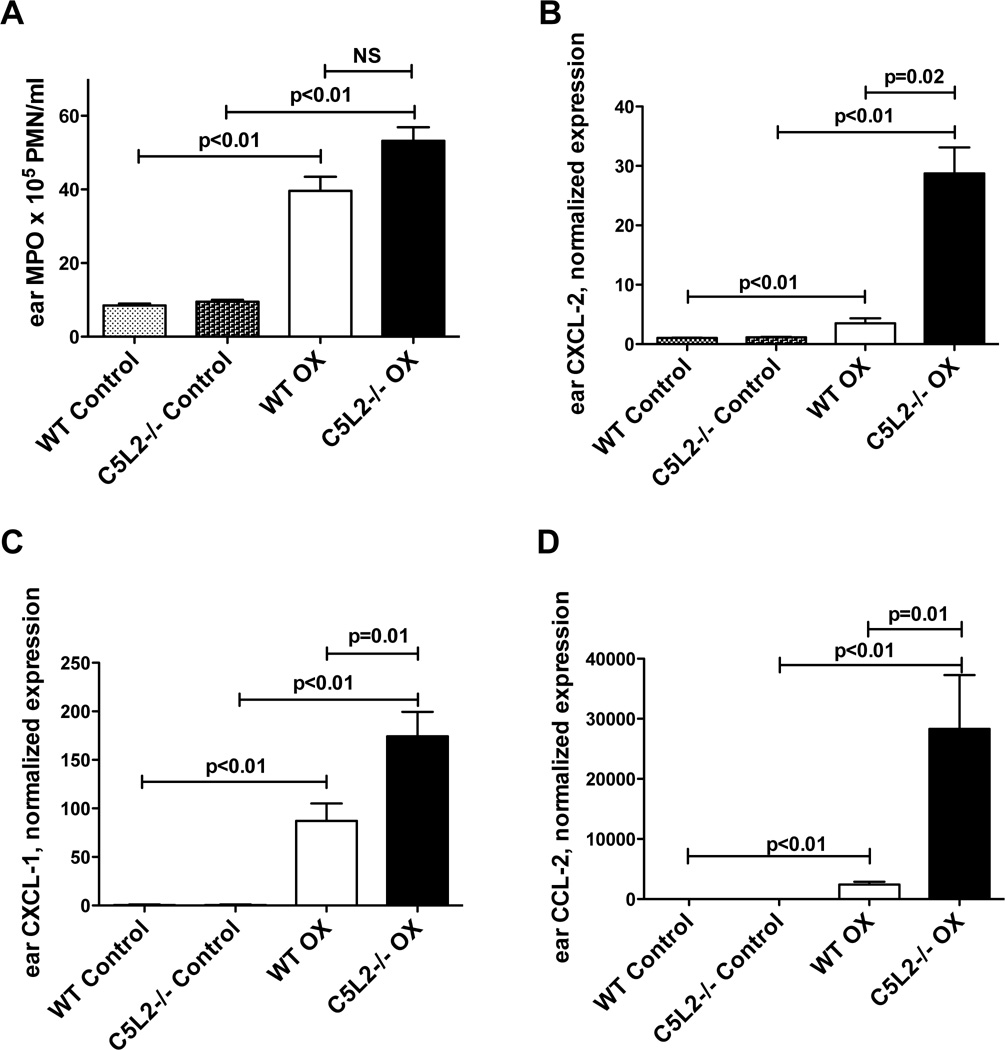
The CD8 T cell recruitment in contact sensitivity reactions has been shown to require neutrophil activation and infiltration resulting from generation of C5a (4, 7). We quantitated the tissue neutrophil content 24h after OX challenge by measuring myeloperoxidase (MPO) levels in tissue extracts from C5L2−/− and wild type mouse ears (Figure 2A). Consistent with the increased tissue swelling, hapten challenged ears from both C5L2−/− and wild type mice revealed significantly elevated levels of MPO at 24h relative to vehicle-treated control ears. Sensitized and challenged C5L2−/− animals exhibited ~35% greater MPO than wild type mice (equivalent to 5.5×106 PMN/ml compared with 4×106 PMN/ml for wild type mice, P<0.01, n=10–11 mice/group).
Figure 2. C5L2−/− mouse ears challenged with hapten exhibit increased inflammatory cell influx and pro-inflammatory chemokines relative to wild type animals.
A. MPO content of ears from C5L2−/− and wild type mice sensitized to OX, determined 24h after challenge with OX or vehicle (control). Data are expressed as the mean ± SEM MPO value, significance of differences is indicated, n=10–11 mice/group. B–D. Gene expression of CXCL-1 (B), CXCL-2 (C) and CCL-1 (D) in the ears of OX sensitized C5L2−/− and wild type mice 24h after challenge with OX or vehicle (control). Results are normalized to β-actin, mean ± SEM for 8–9 mice/group. Significance of differences is indicated.
Generation of chemokines including CXCL-1, CXCL-2 and CCL-2 has also been shown to contribute to the recruitment and activation of neutrophils and CD8 T cells and to further amplify cell-mediated inflammation in CS (7, 17, 25). We therefore assessed the gene expression for these chemokines by quantitative PCR in ears of C5L2−/− and wild type mice 24h after elicitation (Figure 2B–D). Consistent with previous studies, the challenged ears from both mouse strains exhibited dramatic increases in CXCL-1, CXCL-2 and CCL-2 mRNA compared with unchallenged control ears. For ears from C5L2−/− mice these values were further enhanced compared with wild type animals. CXCL-1 was increased ~2-fold compared with wild type (P<0.01), CXCL-2 was increased by >7-fold (P=0.02), and CCL-2 was increased by >10-fold (P=0.01) compared with ears from OX challenged wild type animals (n=8–9 mice/group). These increases in chemokine levels in C5L2−/− mice compared with wild type animals also supports a sequence in which complement activation and C5a generation precede chemokine production.
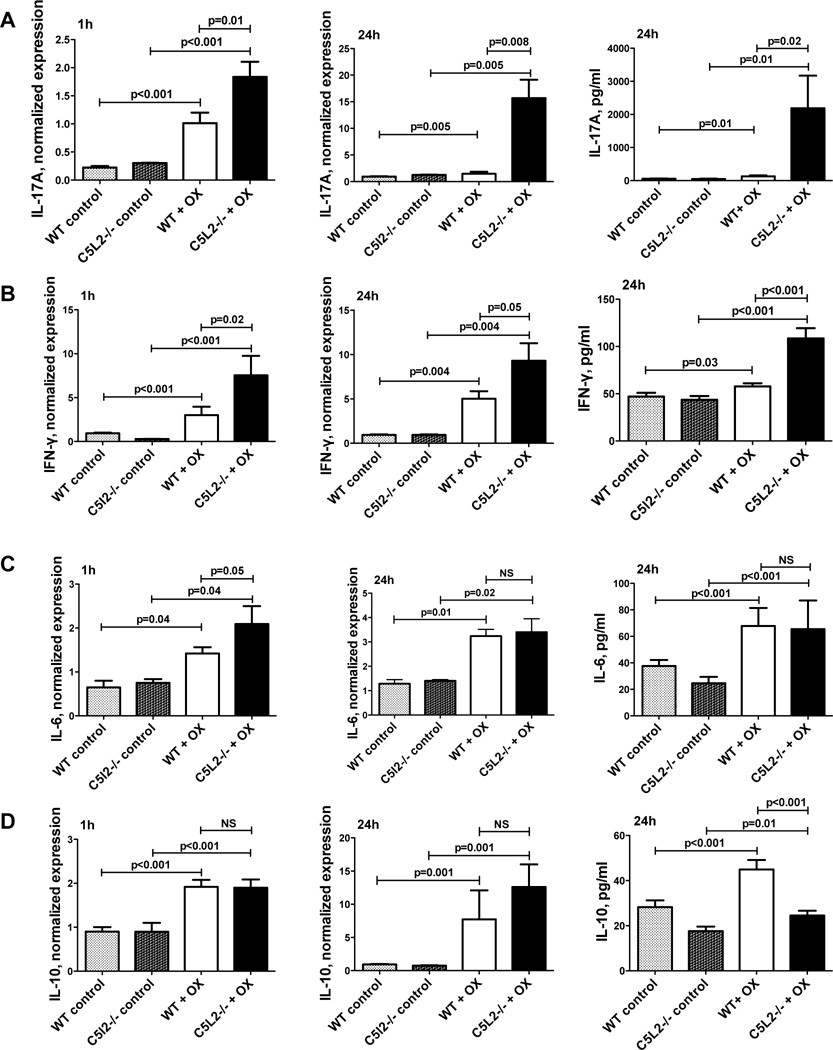
We also assessed the levels of a number of cytokines reported to participate in CS in the mouse ears 24h after OX challenge (Figure 3), and found increases in both C5L2−/− and wild type animals compared with unchallenged control tissues for IFN-γ, IL-5, IL-10, IL-12p40, and IL-17A. Importantly, C5L2−/− mouse ears exhibited significantly increased levels of all cytokines tested at this time point relative to wild type mice with the exception of the gene expression for IL-10. The IL-10 protein level was increased in C5L2−/− mice, and other cytokines for which we evaluated levels of protein as well as gene expression revealed good correlation between the two. These findings are consistent with the Th1/Th17 nature of contact dermatitis (10, 17, 26) and are also supportive of the negative modulatory role of C5L2 on C5a-C5aR mediated responses.
Figure 3. C5L2−/− mouse ears challenged with hapten exhibit increased Th1/Th17 cytokines relative to wild type animals.
A, C,&E. Expression analysis of IFN-γ, IL-17A, and IL-10 in C5L2−/− and wild type mouse ears following sensitization and challenge with OX or vehicle (control) by quantitative PCR. Results are normalized to β-actin, mean ± SEM for 6–9 mice/group. Significance of differences is indicated. B, D, F–H. Cytokine levels for IFN-γ, IL-17A, IL-10, IL-5 and IL-12 in C5L2−/− and wild type mouse ears following sensitization and challenge with OX or vehicle (control) determined by ELISA. Results are the mean ± SEM for 9–10 mice/group. Significance of differences is indicated.
C5aR blockade ameliorates the increases in inflammation associated with contact sensitivity in C5L2−/− mice
To further test our hypothesis that the enhanced contact sensitivity in C5L2−/− mice compared with wild type animals is the result of deficient negative regulation of C5a-C5aR signaling, we treated OX sensitized C5L2−/− and wild type mice with rat monoclonal anti-mouse C5aR or isotype control antibody 1 hour prior to OX challenge. We chose this antibody as it has been utilized effectively to block C5a-C5aR interactions by other investigators with no apparent untoward effects (27, 28). C5L2−/− mice treated with anti-C5aR mAb exhibited significant reductions in ear swelling associated with OX challenge assessed as increases in both thickness and weight (Figure 4). In the absence of mAb, the swelling of the challenged ears was increased by ~30% in C5L2−/− mice compared with wild type animals (P=0.03, n=6–8 mice/group) and this was completely reversed by treatment with the anti-C5aR mAb (P=0.03). Swelling was also reduced by anti-C5aR mAb in OX challenged ears from wild type animals (25% reduction, P=0.03). Ear edema was similarly increased 20% in C5L2−/− mice compared with wild type animals (p=0.006, n=7–8 mice/group) and treatment with the anti-C5aR mAb reduced this parameter ~45% in C5L2−/− mice (P=0.001) and 20% in wild type animals (P=0.01). These changes were mirrored by the changes in ear MPO content. In the absence of antibody blockade the MPO content of C5L2−/− ears was elevated by 60% compared with wild type mice (P=0.05, n=4–15 mice/group). Anti-C5aR mAb reduced this level ~20-fold for C5L2−/− ears and 8-fold for wild type ears (P<0.01 for both C5L2−/− and wild type ears). These data are consistent with the critical role of C5a in CS associated inflammation (7) and further support the role of C5L2 in the negative regulation of C5a-C5aR mediated responses (16).
Figure 4. Antibody blockade of the C5aR reverses the increases in inflammatory parameters in C5L2−/− mice.
OX sensitized C5L2−/− and wild type mice were treated with anti-C5aR mAb or isotype control 1h prior to OX challenge on one ear with vehicle alone on the other. After 24h animals were sacrificed and ears assessed for inflammatory changes as in Figures 1–3. Control in panels D–G represents the response of the vehicle treated ears from mice of either strain. Data are the mean ± SEM for n=3–8 mice /group. Significance of differences is indicated.
We then utilized quantitative PCR to evaluate expression of the chemoattractants CXCL-1, CXCL-2 and CCL-2 in the ears of C5L2−/− and wild type mice pre-treated with anti-C5aR mAb or isotype control prior to OX challenge. As we previously observed, ears from OX sensitized and challenged C5L2−/− mice exhibit ~2.5-fold more CXCL-1 and CXCL-2, and almost 4-fold more CCL-2 compared with wild type (P<0.01, n=3–7 mice/group). Pre-treatment with the anti-C5aR mAb resulted in reduction of 4-fold, 20-fold and ~3-fold for CXCL-1, CXCL-2 and CCL-2, respectively (P<0.01 for all three). The chemokine content of wild type ears was also reduced by blocking the C5aR but to a lesser extent (NS for CXCL-1, ~6-fold for CXCL-2 and 2.4-fold for CCL-2). Analysis of IFN-γ expression by both quantitative PCR and protein content yielded a similar result. Sensitized and challenged ears from C5L2−/− mice exhibited ~4-fold increases in both gene expression and protein compared with wild type animals (P<0.01, n=4–14 for both), and blocking the C5aR resulted in significant reduction in C5L2−/− mouse ears with minor reduction in ears from wild type mice.
OX-stimulated C5L2−/− lymph node cells exhibit increased chemokine and cytokine production compared with wild type cells
To corroborate our in vivo findings, we also examined the ex vivo response of combined inguinal and axillary lymph node cells harvested 24h following OX challenge from C5L2−/− and wild type mice. As the conditions of these cultures do not include a significant source of complement, the results are consistent with previously described evidence for local synthesis and secretion of alternative pathway complement proteins by T cells and dendritic cells (1, 29–31). These cells have demonstrated capability for producing anaphylatoxins. Cells were incubated with 0 or 1µg/ml OX and evaluated for production of the same chemokines and cytokines assessed in vivo (Figure 5). Within 1h of initiating the cultures, OX stimulated cells from sensitized and challenged wild type mice exhibited a tendency toward increased CXCL-1 gene expression relative to unstimulated cells and significant increases in CXCL-2 and CCL-2 (P≤0.01, n=4–5 mice/group). For OX stimulated cells from C5L2−/− mice gene expression of all three chemokines was significant at 1h (P≤0.01, n=4–5 mice/group), and these cells also exhibited greater chemokine production compared with cells from wild type animals, ~55% for CXCL-1 (P=0.02), ~35%, for CXCL-1 (NS), and CXCL-2 (P=0.002, n=4–9 mice/group). After 24h in culture these changes were much more dramatic. The increases were significantly different resulting from OX stimulation of both mouse strains for all three chemokines compared with unstimulated cells. In addition OX stimulated cells from C5L2−/− animals exhibited elevated gene expression for all three chemokines compared with identically stimulated cells from wild type mice. CXCL-1 was increased >3.5-fold in C5L2−/− cells compared with wild type (P=0.03), CXCL-2 was increased ~25% (P=0.01), and CCL-2 was increased >5-fold (P=0.002, n=5–9 mice/group).
Figure 5. Lymph node cells from sensitized C5L2−/− mice incubated with OX exhibit increased gene expression for pro-inflammatory chemokines compared with wild type mice.
Cells were incubated with 0 or 1µg/ml OX for 1 or 24h as indicated and expression of CXCL-1 (A&B), CXCL-2 (C&D) and CCL-1 (E&F) was analyzed by quantitative PCR. Results are normalized to β-actin, mean ± SEM for 4–9 mice/group. Significance of differences is indicated.
Within 1h of initiating lymph node cultures, expression of IFN-γ, IL-17A, IL-6, and IL-10 was elevated in OX stimulated cells from both C5L2−/− and wild type mice (Figure 6). As for the chemokines, the changes in cytokine gene expression and protein levels were generally more pronounced after 24h. IL-6 gene expression and protein were elevated for OX stimulated cells compared to controls, but at 24h there was no difference between C5L2−/− and wild type cells. Similarly, IL-10 gene expression was increased with OX stimulation in cells from both mouse strains, equally for C5L2−/− and wild type cells. C5L2−/− cells exhibited reduced protein relative to cells from wild type mice. In contrast, IL-10 protein in the ears of OX sensitized and challenged C5L2−/− mice was elevated relative to wild type animals (see Figure 3F), possibly reflecting the role of distinct cell types in lymph nodes and intact dermal tissues.
Figure 6. Lymph node cells from sensitized C5L2−/− mice incubated with OX exhibit increased gene expression for pro-inflammatory cytokines compared with wild type mice.
Cells were incubated with 0 or 1µg/ml OX for 1 or 24h as indicated and expression of IFN-γ (A), IL-17A (B), IL-6 (C) and IL-10 (D) determined by quantitative PCR or ELISA. Data are the mean ± SEM for n=3–8 mice/group. Results are normalized to β-actin for qPCR, mean ± SEM for 4–9 mice/group. Significance of differences is indicated.
Discussion
Previous investigations have demonstrated a critical role for early complement activation and generation of the C5a anaphylatoxin in allergic contact sensitivity (6–8, 24). C5a exhibits high affinity interactions with the C5aR, a classical G protein coupled 7-TMS receptor, as well as the atypical receptor, C5L2, which is deficient in G protein coupling. The work described here was initiated to determine the impact of C5L2 in a mouse model of allergic CS induced by oxazolone. Our work in human neutrophils revealed enhanced C5a-C5aR mediated responses in the presence of a blocking antibody against C5L2 (16), and we therefore expected C5L2 deficient mice to reveal exacerbated CS reactions. Examination of the mechanism of action of C5L2 in neutrophils revealed a molecular association between C5L2 and β-arestin that normally restricts the interaction between β-arrestin and the C5aR following ligand stimulation. When the C5L2-β-arrestin interaction is blocked, C5aR-β-arrestin interactions are potentiated and enhanced signaling through this pathway is observed. In OX-induced CS we find that C5a-C5aR mediated responses are similarly potentiated in C5L2−/− mice. Indeed all aspects of CS are exacerbated in C5L2−/− mice including tissue swelling, neutrophil influx, chemokine and cytokine production, supporting the essential role of early complement activation and generation of C5a. Additional evidence for the specificity of the enhanced CS associated with C5L2 deficiency is provided by treating animals with a monoclonal antibody against the mouse C5aR. This not only reverses the increased responses associated with C5L2 deficiency, but in most instances, ameliorates the responses observed for wild type animals as well. In particular, we observed significant reductions following anti-C5aR pre-treatment in the ear swelling, edema, neutrophil influx (as tissue MPO) and increased gene expression of CXCL-2 and CCL-2 associated with OX-induced CS. In contrast, this treatment did not alter CXCL-1 or IFN-γ in wild type mice. While early work showed that C5a production contributes a majority of the inflammatory activity associated with CS, involvement of additional pathways is also evident, particularly at later time points following hapten challenge (7).
Examination of the role of C3a in allergic CS revealed enhanced cytokine production in the sensitization phase in C3aR deficient mice but no change in ear swelling in the elicitation phase (32). We find that mice deficient in the C3aR also exhibit a reduction in expression of C5L2 protein resulting from formation of a heterodimer between the two (NP Gerard, unpublished observations). Since the impact of C5L2 deficiency is enhanced responses to C5a, we expect a similar phenotype in C3aR deficient animals as well. Thus, the result in this case is not a reflection of a protective role for C3a but rather an indirect effect due to alteration in expression of C5L2. The absence of observed changes in the elicitation phase may be explained by the transient nature of the reaction, as the contribution of C5a was reduced as a function of time, while the apparent role of chemokines became more pronounced (8).
The results of the lymph node cultures closely mirror those of the mouse ears in that the inflammatory parameters associated with allergic contact sensitivity are elevated in OX-stimulated cells from C5L2−/− mice compared with wild type animals. The absence of a significant serum source for complement supports the previously identified ability of dendritic cells and T cells to locally synthesize and secrete alternative complement pathway components which may then result in generation of anaphylatoxins (1, 30, 31). Importantly, this observation, coupled with evidence based on antibody blockade of the C5aR, lends further support to the role of C5L2 as a negative regulator of C5a-C5aR mediated reactions.
Footnotes
This work was supported in part by NIH grants HL051366 and AI039759
References
- 1.Lalli PN, Strainic MG, Yang M, Lin F, Medof ME, Heeger PS. Locally produced C5a binds to T cell-expressed C5aR to enhance effector T-cell expansion by limiting antigen-induced apoptosis. Blood. 2008;112:1759–1766. doi: 10.1182/blood-2008-04-151068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen DE. Contact dermatitis: a quarter century perspective. J Am Acad Dermatol. 2004;51:S60–S63. doi: 10.1016/j.jaad.2003.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Askenase PW, Szczepanik M, Itakura A, Kiener C, Campos RA. Extravascular T-cell recruitment requires initiation begun by Valpha14+ NKT cells and B-1 B cells. Trends Immunol. 2004;25:441–449. doi: 10.1016/j.it.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Tsuji RF, Szczepanik M, Kawikova I, Paliwal V, Campos RA, Itakura A, Akahira-Azuma M, Baumgarth N, Herzenberg LA, Askenase PW. B cell-dependent T cell responses: IgM antibodies are required to elicit contact sensitivity. J Exp Med. 2002;196:1277–1290. doi: 10.1084/jem.20020649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krasteva M, Kehren J, Ducluzeau MT, Sayag M, Cacciapuoti M, Akiba H, Descotes J, Nicolas JF. Contact dermatitis I. Pathophysiology of contact sensitivity. Eur J Dermatol. 1999;9:65–77. [PubMed] [Google Scholar]
- 6.Tsuji RF, Geba GP, Wang Y, Kawamoto K, Matis LA, Askenase PW. Required early complement activation in contact sensitivity with generation of local C5-dependent chemotactic activity, and late T cell interferon gamma: a possible initiating role of B cells. J Exp Med. 1997;186:1015–1026. doi: 10.1084/jem.186.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsuji RF, Kawikova I, Ramabhadran R, Akahira-Azuma M, Taub D, Hugli TE, Gerard C, Askenase PW. Early local generation of C5a initiates the elicitation of contact sensitivity by leading to early T cell recruitment. J Immunol. 2000;165:1588–1598. doi: 10.4049/jimmunol.165.3.1588. [DOI] [PubMed] [Google Scholar]
- 8.Tsuji RF, Kikuchi M, Askenase PW. Possible involvement of C5/C5a in the efferent and elicitation phases of contact sensitivity. J Immunol. 1996;156:4444–4450. [PubMed] [Google Scholar]
- 9.Purwar R, Baumer W, Niebuhr M, Tschernig T, Kietzmann M, Werfel T. A protective role of complement component 3 in T cell-mediated skin inflammation. Exp Dermatol. 2011;20:709–714. doi: 10.1111/j.1600-0625.2011.01295.x. [DOI] [PubMed] [Google Scholar]
- 10.Campos RA, Szczepanik M, Lisbonne M, Itakura A, Leite-de-Moraes M, Askenase PW. Invariant NKT cells rapidly activated via immunization with diverse contact antigens collaborate in vitro with B-1 cells to initiate contact sensitivity. J Immunol. 2006;177:3686–3694. doi: 10.4049/jimmunol.177.6.3686. [DOI] [PubMed] [Google Scholar]
- 11.Klos A, Wende E, Wareham KJ, Monk PN. International Union of Pharmacology. LXXXVII. Complement peptide C5a, C4a, and C3a receptors. Pharmacol Rev. 2013;65:500–543. doi: 10.1124/pr.111.005223. [DOI] [PubMed] [Google Scholar]
- 12.Gerard NP, Lu B, Liu P, Craig S, Fujiwara Y, Okinaga S, Gerard C. An anti-inflammatory function for the complement anaphylatoxin C5a-binding protein, C5L2. J Biol Chem. 2005;280:39677–39680. doi: 10.1074/jbc.C500287200. [DOI] [PubMed] [Google Scholar]
- 13.Ohno M, Hirata T, Enomoto M, Araki T, Ishimaru H, Takahashi TA. A putative chemoattractant receptor, C5L2, is expressed in granulocyte and immature dendritic cells, but not in mature dendritic cells. Mol Immunol. 2000;37:407–412. doi: 10.1016/s0161-5890(00)00067-5. [DOI] [PubMed] [Google Scholar]
- 14.Okinaga S, Slattery D, Humbles A, Zsengeller Z, Morteau O, Kinrade MB, Brodbeck RM, Krause JE, Choe HR, Gerard NP, Gerard C. C5L2, a nonsignaling C5A binding protein. Biochemistry. 2003;42:9406–9415. doi: 10.1021/bi034489v. [DOI] [PubMed] [Google Scholar]
- 15.Scola AM, Johswich KO, Morgan BP, Klos A, Monk PN. The human complement fragment receptor, C5L2, is a recycling decoy receptor. Mol Immunol. 2009;46:1149–1162. doi: 10.1016/j.molimm.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bamberg CE, Mackay CR, Lee H, Zahra D, Jackson J, Lim YS, Whitfeld PL, Craig S, Corsini E, Lu B, Gerard C, Gerard NP. The C5a receptor (C5aR) C5L2 is a modulator of C5aR-mediated signal transduction. J Biol Chem. 2010;285:7633–7644. doi: 10.1074/jbc.M109.092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kish DD, Li X, Fairchild RL. CD8 T cells producing IL-17 and IFN-gamma initiate the innate immune response required for responses to antigen skin challenge. J Immunol. 2009;182:5949–5959. doi: 10.4049/jimmunol.0802830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li R, Coulthard LG, Wu MC, Taylor SM, Woodruff TM. C5L2: a controversial receptor of complement anaphylatoxin, C5a. Faseb J. 2013;27:855–864. doi: 10.1096/fj.12-220509. [DOI] [PubMed] [Google Scholar]
- 19.Xiao H, Schreiber A, Heeringa P, Falk RJ, Jennette JC. Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am J Pathol. 2007;170:52–64. doi: 10.2353/ajpath.2007.060573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisette A, Munkonda MN, Oikonomopoulou K, Paglialunga S, Lambris JD, Cianflone K. C5L2 receptor disruption enhances the development of diet-induced insulin resistance in mice. Immunobiology. 2013;218:127–133. doi: 10.1016/j.imbio.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Gao H, Neff TA, Guo RF, Speyer CL, Sarma JV, Tomlins S, Man Y, Riedemann NC, Hoesel LM, Younkin E, Zetoune FS, Ward PA. Evidence for a functional role of the second C5a receptor C5L2. Faseb J. 2005;19:1003–1005. doi: 10.1096/fj.04-3424fje. [DOI] [PubMed] [Google Scholar]
- 22.Cao Q, McIsaac SM, Stadnyk AW. Human colonic epithelial cells detect and respond to C5a via apically expressed C5aR through the ERK pathway. Am J Physiol Cell Physiol. 2012;302:C1731–C1740. doi: 10.1152/ajpcell.00213.2011. [DOI] [PubMed] [Google Scholar]
- 23.Bozic CR, Lu B, Hopken UE, Gerard C, Gerard NP. Neurogenic amplification of immune complex inflammation. Science. 1996;273:1722–1725. doi: 10.1126/science.273.5282.1722. [DOI] [PubMed] [Google Scholar]
- 24.Norman MU, Hulliger S, Colarusso P, Kubes P. Multichannel fluorescence spinning disk microscopy reveals early endogenous CD4 T cell recruitment in contact sensitivity via complement. J Immunol. 2008;180:510–521. doi: 10.4049/jimmunol.180.1.510. [DOI] [PubMed] [Google Scholar]
- 25.Engeman T, Gorbachev AV, Kish DD, Fairchild RL. The intensity of neutrophil infiltration controls the number of antigen-primed CD8 T cells recruited into cutaneous antigen challenge sites. J Leukoc Biol. 2004;76:941–949. doi: 10.1189/jlb.0304193. [DOI] [PubMed] [Google Scholar]
- 26.Pennino D, Eyerich K, Scarponi C, Carbone T, Eyerich S, Nasorri F, Garcovich S, Traidl-Hoffmann C, Albanesi C, Cavani A. IL-17 amplifies human contact hypersensitivity by licensing hapten nonspecific Th1 cells to kill autologous keratinocytes. J Immunol. 2010;184:4880–4888. doi: 10.4049/jimmunol.0901767. [DOI] [PubMed] [Google Scholar]
- 27.Lajoie S, Lewkowich IP, Suzuki Y, Clark JR, Sproles AA, Dienger K, Budelsky AL, Wills-Karp M. Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nat Immunol. 2010;11:928–935. doi: 10.1038/ni.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rittirsch D, Flierl MA, Nadeau BA, Day DE, Huber-Lang M, Mackay CR, Zetoune FS, Gerard NP, Cianflone K, Kohl J, Gerard C, Sarma JV, Ward PA. Functional roles for C5a receptors in sepsis. Nat Med. 2008;14:551–557. doi: 10.1038/nm1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heeger PS, Lalli PN, Lin F, Valujskikh A, Liu J, Muqim N, Xu Y, Medof ME. Decay-accelerating factor modulates induction of T cell immunity. J Exp Med. 2005;201:1523–1530. doi: 10.1084/jem.20041967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strainic MG, Liu J, Huang D, An F, Lalli PN, Muqim N, Shapiro VS, Dubyak GR, Heeger PS, Medof ME. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 2008;28:425–435. doi: 10.1016/j.immuni.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwan WH, Hashimoto D, Paz-Artal E, Ostrow K, Greter M, Raedler H, Medof ME, Merad M, Heeger PS. Antigen-presenting cell-derived complement modulates graft-versus-host disease. J Clin Invest. 2012;122:2234–2238. doi: 10.1172/JCI61019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niebuhr M, Baumer W, Kietzmann M, Wichmann K, Heratizadeh A, Werfel T. Participation of complement 3a receptor (C3aR) in the sensitization phase of Th2 mediated allergic contact dermatitis. Exp Dermatol. 2012;21:52–56. doi: 10.1111/j.1600-0625.2011.01403.x. [DOI] [PubMed] [Google Scholar]