Abstract
Background
Triple receptor-negative breast cancers (TNBC) are higher grade and more likely to metastasize. Recurrences after 5 years are rare in TNBCs. Conversely, late recurrences are seen in estrogen receptor (ER)-positive (luminal) cancers. Disseminated tumor cells (DTCs) may be responsible for late recurrences. We compared rates of DTCs in basal and luminal subtypes.
Methods
We evaluated 205 stage I–III patients. DTCs were assessed from bone marrow aspirates using anti-cytokeratin (CK) antibody following cytospin, and the presence of ≥1 CK-positive cells was considered positive. Pathologic complete response (pCR) was defined as lack of invasive disease in primary tumor and regional lymph nodes after neoadjuvant chemotherapy (NAC). Statistical analyses used chi-square and Fischer's exact test.
Results
Median follow-up (f/u) was 27 months, and 40% of patients had NAC. Forty patients had TNBC, and 148 had luminal cancers. Seventeen percent of TNBC patients, and 27% of those with luminal subtype, had DTCs after NAC (P = NS). Following NAC, pCR occurred in 28% of TNBC and 23% of luminal patients. Luminal A subtypes were less likely to achieve pCR when compared with nonluminal A subtypes (16 versus 41%; P = 0.01). All TNBC patients who achieved pCR had complete eradication of DTCs, whereas 36% of luminal (A and B) subtypes had DTCs.
Conclusions
DTCs were found in 29% of stage I–III patients. TNBCs were more likely to have complete eradication of DTCs after pCR. Further study is needed to determine whether DTCs are responsible for late recurrences in patients with luminal cancers.
Breast cancer is a heterogeneous disease that is defined and classified using clinical and pathologic features to predict outcome and treatment response. Classically, these clinical features include age, tumor size, axillary node involvement, histological grade, estrogen (ER) and progesterone (PR) hormone receptor status, and ERBB2 (HER2/neu) amplification. Recent advances in molecular biology techniques have expanded the classical description of breast cancer tumors into distinct subtypes. Three of these subtypes represent ER-negative tumors (triple receptor negative, basal-like, and HER2/neu positive), and two are characterized by ER-positive tumors (luminal A and B).1 Tumors classified as luminal A express ER, with or without PR, and lack HER2 expression; luminal B subtypes express ER and HER2, with or without PR expression; HER2-positive subtypes express HER2 but lack both ER and PR expression.2 Basal-like (BL) and triple receptor-negative breast cancers (TNBC) are highly concordant; both are characterized by lack of expression of ER, PR, and HER2/neu.3 TNBCs represent 11–24% of all breast cancers; these patients typically present at younger age with larger average tumor size, higher grade, and higher rates of lymph node positivity than ER/PR-positive counterparts, and they appear more frequently in patients of African-American origin.4–7 Because of the aggressive nature and lack of receptors for targeted therapies, TNBC present a particular challenge in the clinic. Several large studies have demonstrated that TNBC patients are more likely to recur within the first 3 years following treatment.6,8 However, the rate of recurrence ≥5 years is 50% less than in ER/PR-positive patients.9 Increasing evidence suggests that TNBC patients may be more chemosensitive than ER/PR-positive patients in the neoadjuvant setting, since TNBC patients are more likely to achieve pathologic complete response (pCR) than ER/PR-positive patients.10 pCR predicts excellent survival regardless of receptor status, yet TNBC patients with residual disease following neoadjuvant chemotherapy (NAC) experience significantly shorter disease-free and overall survival than patients with ER/PR-positive tumors and residual disease. More extensive studies are warranted to identify TNBC patients who may not sufficiently benefit from NAC. Over the past decade, several investigators have reported on the prognostic significance of micrometastatic disease through detection of disseminated tumor cells (DTCs) within bone marrow of breast cancer patients. In several large, multi-center studies of stage I–III breast cancer patients, identification of CK-positive DTCs by immunocytochemistry (ICC) was an independent predictor of poor outcome on multivariate analysis.11–14 The largest study identified presence of DTCs in 31% of stage I–III breast cancer patients at time of diagnosis.12 DTC presence correlated with lymph node positivity and tumor size in the pooled analysis. However, several previous reports found that DTCs did not correlate with lymph node status, indicating that regional spread of disease is not required for cancer cells to spread hematogenously and compromise survival.11,13,14 While both pCR and absence of DTCs are excellent independent predictors of favorable prognosis in operable breast cancer, these two parameters have not been concurrently assessed. The work reported herein determined whether presence of DTC was associated with pCR and disease progression in luminal and TNBC subtypes.
METHODS
Data Source
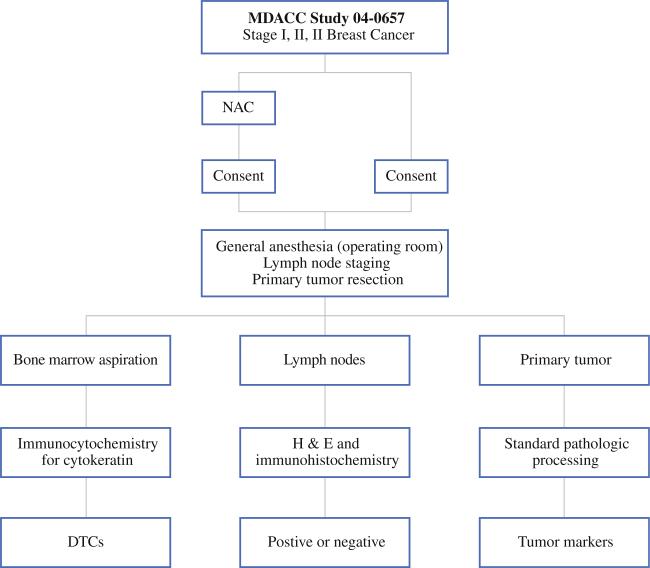
We reviewed data that had been collected from 205 patients with operable breast cancer (stages I – III) who were treated at The University of Texas M. D. Anderson Cancer Center between February 2005 and October 2007. These patients were enrolled as part of a research protocol, 04-0657, which studies DTCs in stage I–III breast cancer, and which is approved by our institutional review board (IRB). Patients with pathologically confirmed diagnosis of invasive breast cancer without clinical or radiographic evidence of metastases were eligible for this protocol. These patients consented to tissue, blood, and bone marrow collection. Irrespective of whether patients received NAC or not, samples (tissue, blood, and bone marrow) were collected at time of primary breast surgery (Fig. 1). Tumor resection was performed after these samples were collected under anesthesia.
FIG. 1.
Schema for DTC study
Information on prognostic markers (e.g., ER, PR, HER2, and Ki-67 proliferation index) and other clinical variables such as age at diagnosis, race, lymph node metastasis, menopausal status, lymphovascular invasion, and nuclear grade was abstracted and updated from the University of Texas M. D. Anderson Cancer Center's database (Clinic-Station®) in December 2009. Based on current literature, TNBC was defined by absence of ER, PR, and HER2 receptors. Luminal A was defined as those who showed absence of HER2 receptor, and expression of ER and/or PR receptors. Luminal B subtype was defined as those with HER2 and ER and/or PR receptors.15
Staging and Classification
Primary tumor–node–metastasis (TNM) staging and tumor grade were designated according to criteria from the American Joint Commission on Cancer (AJCC) and Black's nuclear grading system, respectively.16 Clinical tumor stage was defined by the clinician responsible for the patient after appropriate imaging and physical examination at the initial hospital visit; pathological stage was determined after primary tumor removal and lymph node staging. For patients undergoing NAC, clinical stage was used for analyses. For other patients, precedence was given to pathologic determination of tumor size.
Pathology and Staining for DTCs
Pathology
ER (Novocastra, clone GFII, dilution 1:35) and PR (Dako, clone PR 1294, dilution 1:200) status of primary tumor sections were immunostained using established procedures. HER2 gene amplification was assessed using fluorescence in situ hybridization (FISH, PathVision kit; Vysis, Downers Grove, IL). Response to a treatment regimen was deemed pCR only when there was no evidence of residual invasive component in the excised tumor and lymph nodes after completion of chemotherapy.
DTC Immunostaining
Bone marrow aspirations of 10 ml each were collected from bilateral anterior superior iliac crests into ethylenediamine tetraacetic acid (EDTA)-containing tubes. Enrichment of tumor cells was performed by density gradient separation using Ficoll–Hypaque solution. Eight cytospin specimens were prepared and immunostained using a pancytokeratin (CK) cocktail of antibodies including AE1/AE3, CAM5.2, MNF116, CK8, and CK18. Distinct cytoplasmic and/or membranous staining of cells and clusters with cytomorphological features consistent with a malignant cell were identified as DTCs.
Statistical Analyses
Baseline tumor characteristics and patient demographics (including pCR) were tabulated and compared between patients receiving NAC and those receiving adjuvant chemotherapy or no chemotherapy using chi-square test and Fisher's exact test (Table 1). Odds ratios (and their 95% confidence intervals) of achieving pCR were calculated for biologic tumor subtypes. Statistical analyses were performed by statisticians using STATA-IC11 (StataCorp, College Station, TX), and P-values < 0.05 were considered statistically significant.
TABLE 1.
Comparison of neoadjuvant chemotherapy (NAC) patients versus non-NAC patients
| Variable | Adjuvant/no chemotherapy (N = 124) | NAC (N = 81) | P value |
|---|---|---|---|
| Age (years) | 54.3 | 50.6 | 0.02 |
| BMI (kg/m2) | 29.7 | 28.8 | 0.36 |
| Race | 0.78 | ||
| White | 78% (97) | 73% (59) | |
| Black | 10% (12) | 11% (9) | |
| Hispanic | 10% (13) | 15% (12) | |
| Other | 2% (3) | 1% (1) | |
| Tumor size (T)a | <0.01 | ||
| T1 | 57% (71) | 10% (8) | |
| T2 | 38% (47) | 35% (28) | |
| T3 | 3% (4) | 19% (15) | |
| T4 | 2% (2) | 37% (30) | |
| Stage (AJCC) | <0.01 | ||
| Stage 1 | 44% (54) | 1% (1) | |
| Stage 2 | 44% (55) | 37% (30) | |
| Stage 3 | 12% (15) | 62% (50) | |
| Premenopausal | 28% (35) | 39% (31) | 0.10 |
| LN statusb | 41% (51) | 60% (48) | 0.008 |
| ER | 79% (98) | 57% (46) | 0.001 |
| PR | 65% (80) | 42% (34) | 0.001 |
| HER2 gene amplification | 6% (8) | 33% (27) | <0.001 |
| Intrinsic subtypesc | |||
| TNBC subtype | 18% (22) | 22% (18) | 0.43 |
| Luminal A subtype | 76% (94) | 46% (37) | <0.001 |
| Luminal B subtype | 5% (6) | 14% (11) | 0.03 |
| HER2 subtype | 2% (2) | 19% (15) | <0.001 |
| LVI | 28% (34) | 30% (20) | 0.77 |
| High tumor grade | 35% (43) | 66% (53) | <0.001 |
| pCR | NA | 30% (24) | NA |
| DTCs | 32% (39) | 25% (20) | 0.29 |
AJCC AJCC Cancer Staging Manual,16 LVI presence of lymphovascular invasion, NAC neoadjuvant chemotherapy, DTCs disseminated tumor cells in bone marrow, pCR pathologic complete response,10 BMI body mass index
Tumor size: T1, ≤2 cm; T2, >2 cm and ≤5 cm; T3, >5 cm; T4, any tumor size extending to overlying skin or chest wall
LN status: presence of axillary lymph node metastases
TNBC: triple negative breast cancer (ER–, PR–, HER2–); luminal A subtype (ER+, and/or PR+, HER2–); luminal B subtype (ER+, and/or PR+, HER2+); HER2 subtype (ER–, PR–, HER2+)
RESULTS
Clinicopathologic Characteristics
Clinical characteristics of the 205 patients with stage I, II, or III operable breast cancer and the pathologic characteristics of their tissue samples are presented in Table 2. Mean age, body mass index (BMI), and follow-up time were 53 years (range 25–81 years), 29.3 kg/m2, and 27 months, respectively. The majority of the patients were White (76%, 156/205), and postmenopausal (68%, 139/205). Based on AJCC criteria, 39% (79/205) of patients had T1 and 37% (75/205) had T2 primary tumors. Sixty-eight percent (140/205) of patients in this cohort presented with stage I and II disease, while 32% (65/205) had stage III disease at presentation. High tumor grade was present in 47% (96/205) of patients. Axillary lymph node metastases were present in 48% (99/205). Estrogen receptors were positive in 70% (144/205) patients, PR in 56% (114/205) patients, and HER2 amplification was identified in 17% (35/205) patients. Due to a significant number of patients undergoing NAC (40%), reliable observation of lymphovascular invasion (LVI) in primary tumor specimen was observed in 29% (54/189) of patients.
TABLE 2.
Clinicopathological characteristics (N = 205)
| Variable | Percentage/mean | Number |
|---|---|---|
| Age (years) | 52.8 | — |
| BMI (kg/m2) | 29.3 | — |
| Race | ||
| White | 76 | 156 |
| Black | 10 | 21 |
| Hispanic | 12 | 25 |
| Other | 2 | 3 |
| Tumor size (T)a | ||
| T1 | 39 | 79 |
| T2 | 36 | 75 |
| T3 | 9 | 19 |
| T4 | 16 | 32 |
| Stage (AJCC) | ||
| 1 | 27 | 55 |
| 2 | 42 | 85 |
| 3 | 32 | 65 |
| Premenopausal | 33 | 66 |
| LN statusb | 48 | 99 |
| ER | 70 | 144 |
| PR | 56 | 114 |
| HER2 gene amplification | 17 | 35 |
| Intrinsic subtypesc | ||
| TNBC subtype | 20 | 40 |
| Luminal A subtype | 64 | 131 |
| Luminal B subtype | 8 | 17 |
| HER2 subtype | 8 | 17 |
| LVI | 29 | 54 |
| High tumor grade | 47 | 96 |
| NAC | 40 | 81 |
| DTCs | 29 | 59 |
AJCC AJCC Cancer Staging Manual,16 LVI presence of lymphovascular invasion, NAC neoadjuvant chemotherapy, DTCs disseminated tumor cells in bone marrow, pCR pathologic complete response,10 BMI body mass index
Tumor size: T1, ≤2 cm; T2, >2 cm and ≤5 cm; T3, >5 cm; T4, any tumor size extending to overlying skin or chest wall
LN status: presence of axillary lymph node metastases
TNBC: triple negative breast cancer (ER–, PR–, HER2–); luminal A subtype (ER+, and/or PR+, HER2–); luminal B subtype (ER+, and/or PR+, HER2+); HER2 subtype (ER–, PR–, HER2+)
DTCs and Biologic Subtypes
DTCs were present in 29% (59/205) of all patients included in this cohort. Presence of DTCs did not correlate with lymph node status. Information from the three tumor markers (ER, PR, and HER2) was used to classify patients into four biological subtypes: TNBC, luminal A, luminal B, and HER2. Based on this classification, 20% (40/205) of patients had TNBC subtype, 64% (131/205) had luminal A subtype, 8% (17/205) had luminal B subtype, and 8% (17/205) had HER2 subtype. Prevalence of DTCs was 30% (12/40) in TNBC, 28% (36/131) in luminal A, 35% (6/17) in luminal B, and 29% (5/17) in HER2 subtype patients. Crude odds ratios (OR) for presence of DTCs (primary outcome) were calculated for tumor markers (ER, PR, and HER2) and biological tumor subtypes and were found to be nonsignificant (Table 3).
TABLE 3.
Association of DTCs with ER, PR, HER2, TNBC, luminal and high grade (grade III) for 205 patients
| Variable | OR | 95% CI | P value |
|---|---|---|---|
| ER (+) versus ER (–) | 0.95 | 0.49–1.84 | 0.88 |
| PR (+) versus PR (–) | 0.93 | 0.50–1.70 | 0.80 |
| HER2 (+) versus HER2 (–) | 1.16 | 0.53–2.56 | 0.70 |
| TNBCs versus non-TNBCs | 1.08 | 0.51–2.29 | 0.85 |
| Luminals (A and B) versus nonluminals (A and B) | 0.92 | 0.50–1.68 | 0.78 |
| Grade III versus Grade I and II combined | 0.90 | 0.49–1.66 | 0.75 |
DTCs After Neoadjuvant Chemotherapy
Forty percent (81/205) of patients received NAC, of whom 30% (24/81) achieved pCR. When compared with patients who either received adjuvant chemotherapy or no systemic chemotherapy, patients who received NAC were more likely to be younger (mean 50.6 years versus 54.3 years), were significantly different with respect to tumor size (P < 0.01), AJCC stage (P < 0.01), axillary lymph node metastases (P = 0.008), ER status (P = 0.001), PR status (P = 0.001), and HER2 amplification (P < 0.001), and had higher grade tumors (P < 0.001) (Table 1). Surprisingly, DTCs were identified in 25% (20/81) of patients after receiving NAC. In this subset of 81 patients, 22% (18/81) were TNBC, 46% (37/81) were luminal A, 14% (11/81) were luminal B, and 19% (15/81) were HER2 subtypes. Following NAC, prevalence of DTCs was 17% (3/18) in TNBC patients, 24% (9/37) in luminal A, 36% (4/11) in luminal B, and 27% (4/15) in HER2 subtype patients. In this subgroup, presence of DTCs did not correlate with biologic tumor subtypes, axillary lymph node metastases, or pCR achievement (Table 4a).
TABLE 4.
Association of DTCs and pCR with biologic subtypes and LN status in neoadjuvant patients (n = 81)
| Biologic subtypes | Odds ratio (OR) | 95% Confidence interval | P value |
|---|---|---|---|
| a. DTCs | |||
| Luminal Aa | 0.96 | 0.30–2.98 | 0.94 |
| Luminal Ba | 1.93 | 0.36–8.70 | 0.33c |
| Luminal A and Ba | 1.38 | 0.43–1.67 | 0.54 |
| Basal/TNBCa | 0.54 | 0.09–2.29 | 0.37c |
| HER2a | 1.13 | 0.23–1.55 | 0.84c |
| LN statusb | 0.94 | 0.49–1.80 | 0.84 |
| pCR | 1.39 | 0.39–4.55 | 0.54 |
| b. pCR | |||
| Luminal Aa | 0.28 | 0.08–0.88 | 0.01 |
| Luminal Ba | 2.23 | 0.48–9.89 | 0.22 |
| Luminal A and Ba | 0.46 | 0.15–1.34 | 0.11 |
| Basal/TNBCa | 0.89 | 0.22–3.16 | 0.84 |
| HER2a | 3.57 | 0.95–13.41 | 0.002 |
| LN statusb | 0.11 | 0.03–0.37 | <0.001 |
TNBC: triple negative breast cancer (ER–, PR–, HER2–); luminal A subtype (ER+, and/or PR+, HER2–); luminal B subtype (ER+, and/or PR+, HER2+); HER2 subtype (ER–, PR–, HER2+)
LN status: presence of axillary lymph node metastases
P value determined by Fischer's exact test when any single cell frequency was less than 5
Pathologic Complete Response, Biologic Subtypes, and Relapse Following Neoadjuvant Chemotherapy
Pathologic complete response occurred in 30% (24/81) of patients (Table 1). Prevalence of pCR in biologic tumor subtypes is enumerated in Table 5. Odds ratios for achieving pCR in biologic tumor subtypes following NAC are calculated in Table 4b. In this subgroup of 81 NAC patients, luminal A cancers had lesser odds of achieving pCR (16%) (6/37) as compared with non-luminal A sub-types (41%) (18/44) (OR = 0.28; 95% CI = 0.08–0.88; P = 0.01). Patients with luminal B subtype were more likely to achieve pCR [46% (5/11) versus 27% (19/70)] as compared with non-luminal B patients, but this finding did not reach statistical significance (OR = 2.23, 95% CI = 0.48–9.89; P = 0.22). TNBC patients had higher odds of achieving pCR, but this was not statistically different from those who had one or more than one receptor positive (OR = 0.89; 95% CI = 0.22–3.16; P = 0.84). Patients with HER2-positive tumors (53%, 8/15) had significantly greater chance of achieving pCR as compared with HER2-negative (24%, 16/66) patients (OR = 3.57; 95% CI = 0.95–3.41; P = 0.002). Patients with axillary lymph node metastases (12%, 6/48) were significantly less likely to achieve pCR as compared with those who did not have axillary lymph nodes metastases (56%, 18/32) at time of presentation (OR = 0.11; 95% CI = 0.03–0.37; P < 0.001).
TABLE 5.
Presence of DTCs after pCR and relapses
| Biologic subtypesa | Achieved pCRb | Relapses | DTCs after pCR |
|---|---|---|---|
| Luminal A | 16% (6/37) | 0% (0/7) | 29% (2/7) |
| Luminal B | 45% (5/11) | 0% (0/5) | 40% (2/5) |
| Luminal A and B | 23% (11/48) | 0% (0/11) | 36% (4/11) |
| Basal/TNBC | 28% (5/18) | 0% (0/5) | 0% (0/5) |
| HER2 | 53% (8/15) | 13% (1/8) | 38% (3/8) |
| No pCRb | Relapses | DTCs | |
|---|---|---|---|
| Luminal A | 54% (31/57) | 16% (5/31) | 23% (7/31) |
| Luminal B | 11% (6/57) | 0% (0/6) | 33% (2/6) |
| Luminal A and B | 65% (37/57) | 14% (5/37) | 24% (9/37) |
| Basal/TNBC | 23% (13/57) | 39% (5/13) | 23% (3/13) |
| HER2 | 12% (7/57) | 29% (2/7) | 14% (1/7) |
TNBC: triple negative breast cancer (ER–, PR–, HER2–); luminal A subtype (ER+, and/or PR+, HER2–); luminal B subtype (ER+, and/or PR+, HER2+); HER2 subtype (ER–, PR–, HER2+)
pCR is numerator; biologic subtype is denominator
pCR and DTCs in Biologic Subtypes
Twenty-eight percent (5/18) of TNBC patients achieved pCR after NAC, and none (0/5) of these patients had DTCs (Table 5). Twenty-three percent (11/48) of luminal (A and B) patients achieved pCR after NAC, and more than one-third (36%, 4/11) of the luminal (A and B) patients who achieved pCR had DTCs (Table 5).
DISCUSSION
This study reports on 205 stage I–III breast cancer patients, most of whom had early-stage disease (75% of the patients had T1 or T2 tumors, and only 49% had positive lymph nodes). However, DTCs were identified in almost one-third of these patients, in agreement with previously published reports.12–14 Also, lymph node status did not predict DTC presence, suggesting independent routes of spread and possibly independent biology for lymphatic and hematogenous metastases. Finally, neither tumor size nor any other primary tumor characteristic (including ER, PR, or HER2 expression) reliably predicted the presence of DTCs. These findings support the idea that DTCs add prognostic information that may be complementary to, yet independent of, lymph node status. Several large prospective studies have demonstrated that DTC presence independently predicts both short- and long-term outcome from operable breast cancer.11–14 Published studies suggest that disseminated cells are often dormant and possess a predominately cancer stem-cell phenotype, which may explain their resistance to chemotherapy.17–20 Several smaller studies have shown persistence of DTCs in a significant number of patients after completion of systemic chemotherapy, and persistence of these cells correlated with worse survival.11,21 In our study we found that 25% of patients had DTCs after completion of NAC. Given the discrepancies in chemotherapeutic response rates and time to recurrence between TNBC and ER-positive patients, we hypothesized that DTC might be identified at different rates depending on biologic subtype. The data did not support this hypothesis, as there was no significant difference in rates of DTC between different subtypes. However, we found that none of the TNBC patients who had pCR had DTCs, yet one-third of luminal patients retained DTCs even after pCR. While there were not enough events to determine with statistical significance the prognostic value of these findings, the data support a role for DTCs in late recurrences in luminal subtype patients. Larger studies with longer follow-up periods are needed to document significance, especially in ER-positive patients who may not recur until several years post diagnosis. In any case, a significant number of patients had DTCs after NAC, suggesting that NAC is not particularly efficient at eradicating DTCs. Several recent studies have assessed pCR and outcome utilizing an expanded tumor subtyping system (luminal A, luminal B, TNBC, and HER2). Increasing evidence suggests that TNBC patients may be more chemosensitive than luminal subtype tumors in the neoadjuvant setting. Leidtke et al. conducted a large retrospective study involving more than 1,100 stage I–III breast cancer patients, including 255 TNBC patients. Given the aggressive nature (high nuclear grade) exhibited by TNBC tumors, these patients were more likely to achieve pCR after NAC than were those with luminal A tumors (28 versus 16%, respectively). Indeed, pCR was associated with high-grade tumors (P ≤ 0.0001), and with excellent survival regardless of receptor status. As expected, TNBC patients with residual disease following NAC experienced significantly shorter disease-free and overall survival than patients with ER/PR-positive tumors and residual disease.10 In concordance with the Liedtke report, patients in our study with luminal A subtype tumors were least likely to achieve pCR (OR = 0.28, P = 0.01). In contrast to that study, we found no correlation between pCR and TNBC patients (OR = 0.89, P = 0.84). The Liedtke report contained 24% HER2-positive patients (including HER2 and luminal B subtypes), which is significantly higher than the 17% (combined HER2 positive and luminal B) we observed in our study population. Another possible explanation for the different findings between these studies is that our study utilized fluorescence in situ hybridization (FISH) to assess HER2 status, while the Liedtke study employed both FISH and immunocytochemistry. In addition, all of the patients in the aforementioned study received NAC, compared with 40% of the patients included in this study. In our study HER2 positivity significantly predicted pCR (OR = 3.57, P < 0.002), which is in agreement with previously published reports.22 Given the aggressive nature of TNBC, it is not surprising that TNBC patients with residual disease following NAC are more likely to recur within the first 3 years following treatment.6,8 However, long-term follow-up studies indicate that the rate of recurrence for TNBC patients ≥5 years is 50% less than in ER-positive patients.6,9 In fact, risk of recurrence and death in non-TNBC patients was steady and continued for 17 years after diagnosis. In addition, only 44% of patients, regardless of receptor status, experience local recurrence prior to distant recurrence.6 These observations support the hypothesis that cells unaffected by NAC may survive at distant sites (such as bone marrow) for many years, resulting in recurrence several years following diagnosis.
In conclusion, we found that a significant number of patients had DTCs after NAC. To our knowledge this is the first report demonstrating absence of DTCs in TNBC patients achieving pCR. Though the numbers of patients in the TNBC and luminal subgroups were relatively small, these findings support further investigation into the role of DTCs in late recurrences in ER-positive patients. These preliminary findings also suggest that DTCs may be useful in stratifying recurrence risk, as a significant number of patients harboring DTCs without pCR in this study exhibited early disease progression. In addition, none of the ER-positive patients who were DTC negative and achieved pCR have relapsed thus far. These observations warrant larger trials with longer follow-up periods to fully assess the significance of DTCs in predicting disease recurrence in patients with different biologic subtypes of breast cancer.
ACKNOWLEDGMENT
This work was supported by DoD Breast Cancer Research program (Award #DAMD 17-03-01-0669), Society of Surgical Oncology Clinical Investigator Award (A. Lucci), The Morgan Welch Inflammatory Breast Cancer Program at U.T. M.D. Anderson Cancer Center, and the State of Texas Rare and Aggressive Breast Cancer Research Program.
Footnotes
FINANCIAL DISCLOSURE STATEMENT No financial conflict of interests to report for any authors.
REFERENCES
- 1.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 2.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertucci F, Finetti P, Cervera N, et al. How basal are triple-negative breast cancers? Int J Cancer. 2008;123:236–40. doi: 10.1002/ijc.23518. [DOI] [PubMed] [Google Scholar]
- 4.Millikan RC, Newman B, Tse CK, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109:123–39. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheang MC, Voduc D, Bajdik C, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008;14:1368–76. doi: 10.1158/1078-0432.CCR-07-1658. [DOI] [PubMed] [Google Scholar]
- 6.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–34. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 7.Dolle JM, Daling JR, White E, et al. Risk factors for triple-negative breast cancer in women under the age of 45 years. Cancer Epidemiol Biomarkers Prev. 2009;18:1157–66. doi: 10.1158/1055-9965.EPI-08-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tischkowitz M, Brunet JS, Begin LR, et al. Use of immunohistochemical markers can refine prognosis in triple negative breast cancer. BMC Cancer. 2007;7:134. doi: 10.1186/1471-2407-7-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nofech-Mozes S, Trudeau M, Kahn HK, et al. Patterns of recurrence in the basal and non-basal subtypes of triple-negative breast cancers. Breast Cancer Res Treat. 2009;118:131–7. doi: 10.1007/s10549-008-0295-8. [DOI] [PubMed] [Google Scholar]
- 10.Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–81. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 11.Braun S, Pantel K, Muller P, et al. Cytokeratin-positive cells in the bone marrow and survival of patients with stage I, II, or III breast cancer. N Engl J Med. 2000;342:525–33. doi: 10.1056/NEJM200002243420801. [DOI] [PubMed] [Google Scholar]
- 12.Braun S, Vogl FD, Naume B, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med. 2005;353:793–802. doi: 10.1056/NEJMoa050434. [DOI] [PubMed] [Google Scholar]
- 13.Cote RJ, Rosen PP, Lesser ML, et al. Prediction of early relapse in patients with operable breast cancer by detection of occult bone marrow micrometastases. J Clin Oncol. 1991;9:1749–56. doi: 10.1200/JCO.1991.9.10.1749. [DOI] [PubMed] [Google Scholar]
- 14.Gebauer G, Fehm T, Merkle E, et al. Epithelial cells in bone marrow of breast cancer patients at time of primary surgery: clinical outcome during long-term follow-up. J Clin Oncol. 2001;19:3669–74. doi: 10.1200/JCO.2001.19.16.3669. [DOI] [PubMed] [Google Scholar]
- 15.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 16.Edge SB. AJCC cancer staging manual. Springer; Berlin: 2010. [Google Scholar]
- 17.Muller V, Stahmann N, Riethdorf S, et al. Circulating tumor cells in breast cancer: correlation to bone marrow micrometastases, heterogeneous response to systemic therapy and low proliferative activity. Clin Cancer Res. 2005;11:3678–85. doi: 10.1158/1078-0432.CCR-04-2469. [DOI] [PubMed] [Google Scholar]
- 18.Balic M, Lin H, Young L, et al. Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin Cancer Res. 2006;12:5615–21. doi: 10.1158/1078-0432.CCR-06-0169. [DOI] [PubMed] [Google Scholar]
- 19.Becker S, Solomayer E, Becker-Pergola G, et al. Primary systemic therapy does not eradicate disseminated tumor cells in breast cancer patients. Breast Cancer Res Treat. 2007;106:239–43. doi: 10.1007/s10549-006-9484-5. [DOI] [PubMed] [Google Scholar]
- 20.Slade MJ, Singh A, Smith BM, et al. Persistence of bone marrow micrometastases in patients receiving adjuvant therapy for breast cancer: results at 4 years. Int J Cancer. 2005;114:94–100. doi: 10.1002/ijc.20655. [DOI] [PubMed] [Google Scholar]
- 21.Janni W, Rack B, Schindlbeck C, et al. The persistence of isolated tumor cells in bone marrow from patients with breast carcinoma predicts an increased risk for recurrence. Cancer. 2005;103:884–91. doi: 10.1002/cncr.20834. [DOI] [PubMed] [Google Scholar]
- 22.Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–34. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]