Abstract
Gustatory perception is inherently multimodal, since approximately the same time that intra-oral stimuli activate taste receptors, somatosensory information is concurrently sent to the CNS. We review evidence that gustatory perception is intrinsically linked to concurrent somatosensory processing. We will show that processing of multisensory information can occur at the level of the taste cells through to the gustatory cortex. We will also focus on the fact that the same chemical and physical stimuli that activate the taste system also activate the somatosensory system (SS), but they may provide different types of information to guide behavior.
Keywords: gustation, somatosensation, trigeminal system, sodium chloride, nicotine, multisensory integration, fixed ratio schedule, licking, generalized linear model
Introduction
The gustatory and oral somatosensory systems allow animals to differentiate between essential nutrients such as fats, proteins and carbohydrates and potentially harmful stimuli. Stimuli that must be rapidly rejected can range from a small piece of bone in the food that could cause one to choke, to a very hot liquid, and to potentially poisonous bitter tasting chemicals such as atropine or strychnine. Therefore, once a stimulus is inside the oral cavity the decision to ingest or reject it depends on its multisensory aspects including its taste, temperature and texture/viscosity. In this regard, it can be argued that gustatory perception is inherently multimodal, since approximately the same time that intra-oral stimuli activate taste receptors, somatosensory and thermal information is concurrently sent to the CNS1. Having multimodal inputs to the CNS typically means that the system will elicit faster, more robust and more reliable physiological responses than do their modality-specific component stimuli (Stanford and Stein, 2007).
In the following, we will review evidence that gustatory perception is intrinsically linked to concurrent somatosensory processing. We will show that processing of multisensory information can occur at the level of the taste cells through to the gustatory cortex. We will also focus on the fact that the same chemical and physical stimuli that activate the taste system also activate the somatosensory system (SS), but they may provide different types of information to guide behavior.
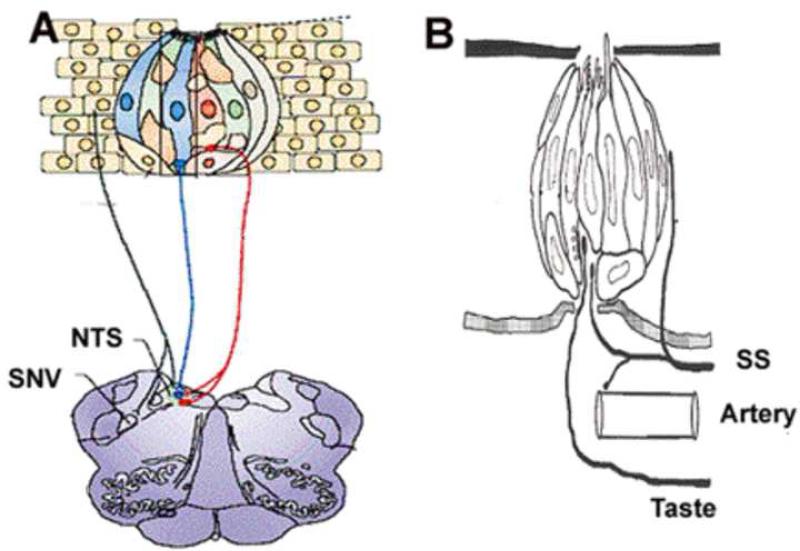
Figure 1 shows a schematic of stratified epithelium with an embedded taste bud together with its associated primary afferent neurons. Surrounding the taste bud is shown a somatosensory neuron. Taste information from the anterior tongue and palate is transmitted to the brain by special sensory branches of the facial (VII) nerve. Somatosensory information from these same areas is transmitted from the trigeminal (V) nerve. Other regions of the oral cavity that contain taste buds such as the posterior tongue, the pharynx, larynx and epiglottis are innervated either by the glossopharyngeal (IX) or vagal (X) nerves (Norgren, 1995). These two cranial nerves have both special and general sensory components. In addition to containing mechano- and thermosensors, the general sensory branches of cranial nerves V, IX, and X also contain nociceptors. One important point regarding the multisensory aspect of gustation is that branches from all three of these general sensory nerves project to the nucleus of the solitary tract (NST) where they co-mingle with projections from primary afferent neurons that innervate taste cells. In this regard it is not surprising that activation of these general sensory fibers can modulate taste responses (Boucher et al., 2003b; Boucher et al., 2003a). Somatosensory and gustatory pathways also converge in the thalamus (Ogawa et al., 1987) and cortex. Indeed, pathways of cranial nerves V, IX and X project to the insular cortex which has unimodal and multimodal neurons that respond to gustatory, visceral, mechanical and nociceptive stimuli (Hanamori et al., 1998; Hanamori et al., 1997; Kadohisa et al., 2004; Cerf-Ducastel et al., 2001; Scott and Plata-Salaman, 2003; Katz et al., 2001).
Figure 1. Schematic diagram of taste bud, taste receptor cell and associated neurons.
A. Illustration of a taste bud that is embedded in epithelium. The different types of taste receptor cells are indicated by different colors as they may contain different types of receptors and intracellular modulators. The gustatory neurons with their associated colors that match the associated TRCs indicate that they may respond best to those stimuli that activate the particular TRCs. These primary gustatory neurons project ipsilaterally to the rostral NTS. The black colored axon that is embedded in the epithelia that surrounds a taste bud is likely to be a nociceptor. These neurons project ipsilaterally to the spinal nucleus of V and have collaterals that project to the rostral NTS. Adapted from Simon et al. 2006. B. Schematic diagram of a taste bud with neurons (taste) and artery. Note that chemical stimuli in the mouth can directly activate receptors on taste cells but they must diffuse into the epithelium to activate somatosensory (SS) neurons. The activation of SS nociceptors can cause vasodilatation of the tongues arterial system to increase the tongue's temperature that, in turn, may affect taste responses (see text for additional details).
Taste-guided behavior is determined by input from both the gustatory and somatosensory system
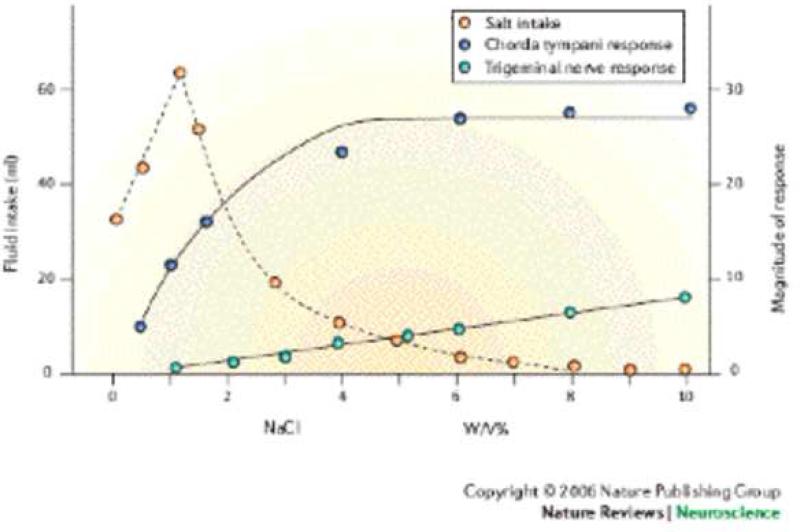
One outstanding example of how a combination of gustatory and somatosensory information guides ingestive behavior is found in a study by Kawamura and colleagues (Kawamura et al., 1968), where they measured how much NaCl a thirsty rat would ingest as a function of concentration (see Figure 2). To identify the taste component of this behavior they measured whole nerve responses from the chorda tympani (CT) nerve, and to measure the corresponding somatosensory component of this behavior they quantified whole nerve responses from the lingual branch of the trigeminal (TG) nerve. The responses of both nerves were measured as a function of NaCl concentration. They found that upon increasing the NaCl concentration, the subjects ingested a greater volume, since NaCl at concentrations up to 150 mM is hedonically positive. However, upon further increases in concentration the amount of fluid ingested reached a maximum and then decreased to a point where the animals refused to drink. Physiologically, the CT responses were observed to increase in a sigmoidal manner with increasing NaCl concentration, whereas the TG responses were initiated just at the concentration where the ingestion started to decrease. The TG responses then increased in a linear manner. The important point we wish to emphasize is that the behavioral responses followed the sensory input from both systems. In particular, taste information from the CT indicates that NaCl is a hedonically positive stimulus that should be ingested and this input dominated behavior at low to medium concentrations. At very high NaCl concentrations, the input from the TG dominates the behavior (even though the CT responses were large) as high NaCl concentrations activate TG nociceptors (Wang et al., 1993). At intermediate concentrations, where both systems contribute, how much an animal will drink will depend on many factors including a balance between hedonically positive and negative inputs as well as the subjects’ motivational state. This same type of behavioral response is also seen for sour (acidic) tastants, which at low concentrations are usually behaviorally acceptable but at high concentrations activate nociceptors (Carstens et al., 1998) and cause a painful sensation and thus will be avoided. In other words, behavioral responses to a particular tastant compound might change whenever somatosensory aspects of the stimulus are affected.
Figure 2. Salt intake reflects input from both gustatory and trigeminal nerves.
Plots showing that as the NaCl concentration increases, the salt intake (open circles) initially increases until it reaches a maximum at 1% (wt/V) (0.17 M). The intake then monotonically decreases until the animals do not accept any NaCl after 7% (wt/V). With increasing NaCl concentration the chorda tympani or taste responses (closed circles) show a sigmoidal increase in activity. With increasing NaCl the activity obtained from the lingual branch of the trigeminal nerve increases linearly. Note that the maximum fluid intake occurs when the lingual nerve activity is essentially zero and the intake decreases as the lingual nerve activity increases. Thus, the hedonically positive aspects of NaCl are signaled by responses of the chorda tympani nerve whereas the hedonically negative aspects of NaCl are signaled by the trigeminal nerve. Therefore to explain the animals’ behavior, sensory information from both neuronal pathways needs to be taken into account. Figure taken from Simon et al with permission that was adopted from Kawamura et al (1968).
Compounds that are highly water-soluble and of medium and large molecular weight are unable to diffuse into the epithelium (at least in times relevant to gustatory processing) to directly activate thermoreceptors or nociceptors embedded in the epithelium or papillary layer. These compounds can of course activate taste receptors. Hydrophobic compounds, such the alkaloids that interact with bitter-responsive T2R receptors on taste cells (Chrandrashekar et al., 2000), have the potential to diffuse into the epithelium and interact with the somatosensory neurons. As a rule, if these hydrophobic stimuli produce aversive behavior when they interact with the taste system, then they will produce aversive behavior upon activating the somatosensory system. Nevertheless one must be aware that not all compounds that activate the somatosensory system will produce aversive behaviors. For example, consider menthol. At moderate concentrations, it activates TRPM8 receptors in cold fibers and induces a cooling sensation (Zanotto et al., 2007), whereas at higher concentrations it is irritating and has anesthetic effects (Dessirier et al., 2001; Green, 1992; Green and Schoen, 2007). In summary, behavioral responses to chemical stimuli are obtained from multisensory input.
The same stimuli activate gustatory and somatosensory neurons
In the preceding section we presented two examples of chemicals (NaCl, acid) that activate both the taste and somatosensory systems. In general, the same stimuli (chemical, thermal, and mechanical) that activate the taste system will, with notable exceptions, also activate the somatosensory system, although the consequences may be different.
It is established that the peripheral taste and SS systems are responsive to thermal and mechanical stimuli (Ogawa et al., 1968; Wang et al., 1993; Ogawa, 1994; Kadohisa et al., 2005; Yamamoto et al., 1981). We note that in the peripheral taste system (i.e., before the senses become integrated) changing temperature or mechanical stimuli will not necessarily be perceived as changes in these stimuli but rather to changes in taste perception (Diamond et al., 2005; Bartoshuk et al., 1982; Todrank and Bartoshuk, 1991). One example of how the taste and SS systems may interact is seen by stimulating lingual nerve, which on one hand will decrease chorda tympani responses to NaCl (Wang et al., 1995), and, on the other hand, increase the tongue's temperature (Wang et al., 1995). Increasing the tongue's temperature will also increase the intensity of sucrose solutions (Bartoshuk et al., 1982), perhaps via the activation of one the thermosensitive TRP channels in found taste cells (Talavera et al., 2005; Lyall et al., 2005). Despite the obvious importance of mechanical and thermal stimuli, in this section we will continue to focus on how selected chemical stimuli interact with both systems. In the following section we will elaborate on taste and SS interactions.
With regard to the interaction of chemicals there is one important difference in these two systems. Whereas chemical stimuli can directly interact with the apical surface of taste cells, they must diffuse into the epithelium for them to interact with SS neurons (Figure 1). This anatomical arrangement eliminates the direct interaction of large water-soluble compounds as well as most charged compounds with SS neurons since their permeability through stratified epithelia will be very slow relative to events associated with the processing of taste information. This does not mean that the SS system will not sense such compounds via mechanoreceptors or via heats of dilution, thermoreceptors, but it means that polymers such as cellulose, proteins such as thaumatin, and large organic salts are unlikely to directly activate SS neurons to produce a chemically gated sensation.
As noted above, hydrophobic compounds can activate taste cells and also diffuse into the epithelium to activate SS neurons. In this regard many, if not most, of these stimuli activate both systems. The rate that hydrophobic molecules will penetrate the epithelium depends on many factors that include the location of the nerve terminals, the concentration gradient and the membrane – water partition coefficient (Burnette, 1984). We now present two representative examples of the multisensory effects of the hydrophobic chemicals: capsaicin and nicotine.
Capsaicin is a pungent ingredient in chili pepper that produces a burning taste (Duner – Engstrom et al., 1986, Cliff and Green, 1996) sensation by activating TRPV1 receptors in nociceptors. Despite the fact that over 30 years ago it was found that capsaicin could activate CT neurons (Okuni, 1977), most researchers considered it to be a purely SS stimulant that selectively activates TRPV1 channels in nociceptors. Recently, Lyall and colleagues (Lyall et al., 2004) have shown that capsaicin also activates taste cells and their associated (CT) neurons via the activation of a TRPV1 splice variant. Moreover, they associated the activation of this receptor with amiloride–sensitive salt taste (Lyall et al., 2005). In this regard, capsaicin has both a taste (salty-bitter) and an irritating burning component. Perceptually, it is sometimes difficult to distinguish between bitter and mild burning taste sensations (Lim and Green, 2007), perhaps because both bitter and burning sensations indicate that the stimulus should not be ingested . There is another, albeit indirect, mechanism by which proinflammatory molecules like capsaicin can influence taste processing. This is by the activation of peptidergic perigemmal nociceptors that exhibit both afferent and efferent responses. The afferent responses are transmitted to the CNS where they are interpreted as pain. The efferent responses can cause the release of proinflammatory peptides, like Substance P (Yamasaki et al., 1984) or ATP which has receptors on taste cells (Chang et al., 1996) or primary afferent neurons (Finger et al., 2005) and thus upon their activation could modulate taste responses.
We next consider the alkaloid, nicotine, that at low concentrations produces a bitter taste sensation and at higher concentrations a burning sensation (Carstens et al., 2007). Its bitter taste sensation may arise from a still unidentified T2R receptor, nicotinic acetylcholine choline receptors (Simons et al., 2006), or perhaps its interaction of ENaC channels in taste cells (Lyall et al., 2007). In this regard, nicotine has been shown to activate chemosensory neurons from first order primary neurons (Dahl et al., 1997; Lyall et al., 2007) , to taste fibers in the NTS (Simons et al., 2006; Lemon and Smith, 2005; Scott and Mark, 1987), and finally neurons in the gustatory cortex (Katz et al., 2001; Soares et al.,2007). Nicotine's burning taste sensation arises from activation of nociceptors containing nicotinic acetylcholine receptors (Simons et al., 2003; Liu and Simon, 1996; Wang et al., 1993; Liu et al., 1998).
In the rat NTS, Simons and colleagues (Simons et al., 2006) have investigated the role of nicotine in modulating taste response. They found that nicotine, via the activation of nicotinic acetylcholine receptors, evoked NTS responses and attenuated NTS unit responses to the neurons’ preferred tastant. Upon removing trigeminal input they found nicotine still excited nearly all NTS units but no longer depressed tastant-evoked responses. The results indicate that excitation of trigeminal afferents could modulate taste responsive NTS units. This study is consistent with findings that showed that trigeminal deafferentation also modulated taste-responsive NTS neurons (Boucher et al., 2003a) and reduced ingestive behaviors elicited by preferred tastants (Szolcsanyi, 1993).
Responses in the gustatory cortex obtained from freely licking animals are fast and multisensory
Humans chew foods and animals lick for liquids. In regard to this review in these behaviors both the activation of SS and taste pathways often occurs simultaneously and in the same receptive field. To investigate the cortical gustatory responses of rats that are attending to taste stimuli by actively licking we recorded single neurons in rat gustatory cortex while they received tastants on an FR5 schedule. In this task we investigated the evoked responses where the subjects licked a dry sipper four times, and on the fifth lick received a variety of tastants (Stapleton et al., 2007; Stapleton et al., 2006). In this regard, we addressed whether we can discriminate, in a single lick (~ 150 ms), SS from gustatory responses. We also investigated whether there is sufficient information in the evoked spike trains in a single lick to discriminate dry licks from wet licks and to discriminate among the tastants. We note that trained rats can discriminate tastants in a single lick (~ 150 ms, (Halpern and Tapper, 1971)).
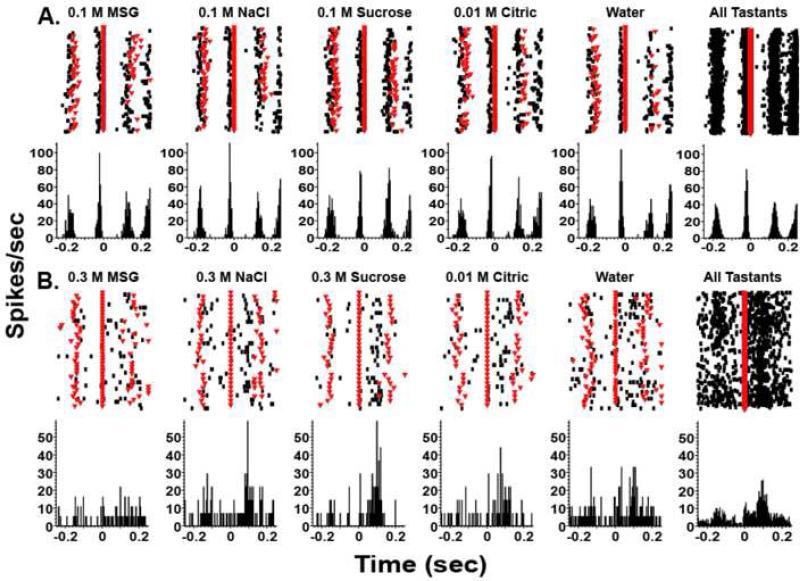
Here we present two distinct types of neurons, both of which were active at some time in the lick cycle. One type was activated in a temporally precise manner before the lick cycle (Figure 3A) and thus is obviously not chemosensitive. Neurons of this type could simply reflect oromotor responses. A second type of neuron was activated both by licking a dry sipper and by the delivery of tastants (Figure 3B). Typically, the firing rates are low when tastants are delivered to anesthetized or passively stimulated subjects (Yamamoto et al., 1984; Yaxley, Rolls, and Sienkiewicz, 1990), but when the subjects receive tastants by licking the firing rates are much higher. Licking alone elicits a small response (Figure 4), but the response for licking plus the concurrent tastant delivery was much larger for such chemosensory neurons. This suggests that the somatosensory information elicited by licking combines in a supralinear manner with the chemosensory input, as would be expected for multimodal processing (Laurienti et al., 2005; Stanford and Stein, 2007). Although the PSTHs depicted in Figure 3B are clearly different for licking alone versus licking plus the tastant delivery, such examples of supra-additivity are often not as clear (Laurienti et al., 2005). Consequently, to separate the chemosensory and somatosensory components we have analyzed the neural responses with a generalized linear model (GLM). In this approach, the spike train from each neuron is modeled as a log-linear Poisson regression (Stapleton et al., 2007). Briefly, the logarithm of the Poisson rate is modeled as a linear combination of explanatory variables, such as indicators of the various tastant/concentration combinations and of the time lapse since the lick onset. With the GLM we have successfully distinguished between reinforced and unreinforced licks, separated tastants and their concentrations, and measured the evolution of the chemosensory response over the course of a single lick. These results suggest that tastant-evoked responses in the rat gustatory cortex process multimodal information on a rapid timescale and provide the physiological basis by which animals might discriminate between tastants during a single lick. In summary, we have shown that at all levels of the gustatory axis processing is inherently multisensory.
Figure 3. Gustatory processing is multimodal and rapid.
Single unit recordings from the primary gustatory cortex of rats licking on a FR5 (fixed ratio) schedule in which they licked a dry sipper four times and every fifth lick they received a tastant (at time 0 seconds as indicated by a solid red line). The tastants were delivered in blocks of eight. The dry licks before and after tastant delivery are indicated by inverted triangles. The upper parts of each figure are raster plots and each dot indicates an action potential. Below are peri-stimulus time histograms (PSTHs). A. This trace is an example of a non-chemosensory response whose activity correlated with licking and preceded the licking of the sipper. It is important to note the temporal precision of the spikes and that the responses were the same for all tastants. Adapted from Stapleton et al. 2006. B. An example of a chemosensitive neuron is presented. It is seen that the neuron is unresponsive to 0.3 M MSG but clearly responsive to the other taste stimuli including water. The panel on the right hand side, which represents the responses to all tastants, clearly shows that there is activity generated in the dry lick preceding the tastant delivery, indicating that this neuron responds to somatosensory stimulation.
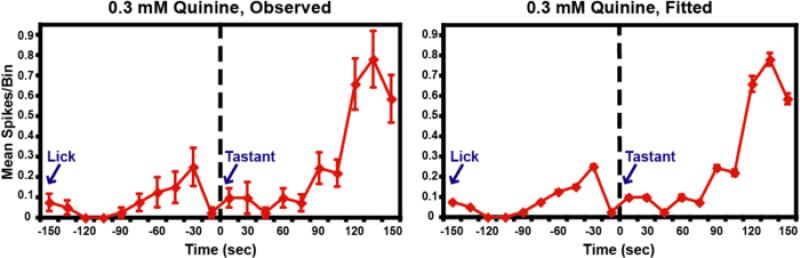
Figure 4. Chemosensory and somatosensory information is summed supra-linearly.
The panel on the left depicts the mean number of spikes in each 15 ms bin following the unreinforced lick and the tastant delivery (blue arrows). The elapsed time is presented on the abscissa, and the mean ± S.E.M. spike count per bin is depicted on the ordinate. Zero on the abscissa indicates the time of tastant delivery. Note the lick-related activity that occurred at about -30 ms. When 0.3 mM quinine HCl was delivered, the magnitude of the multimodal response was much larger than the lick response alone. The corresponding activity displayed a peak at 135 ms. The panel on the right depicts the best-fit curve as returned by the GLM. It is seen that the GLM accurately reconstructs both the magnitude and temporal profile of the response to quinine.
Acknowledgements
This study was supported in part by NIH grant DC-01065 and grants from Philip Morris Inc. USA and Philip Morris International.
Footnotes
We do not consider olfactory stimuli in this report.
Reference List
- Bartoshuk LM, Rennet K, Rodin BE, Stevens JC. Effect of temperature on the perceived sweetness of sucrose. Physiol & Behav. 1982;28:905–910. doi: 10.1016/0031-9384(82)90212-8. [DOI] [PubMed] [Google Scholar]
- Boucher Y, Simons CT, Faurion A, Azerad J, Carstens E. Trigeminal modulation of gustatory neurons in the nucleus of the solitary tract. Brain Research. 2003a;973:265–274. doi: 10.1016/s0006-8993(03)02526-5. [DOI] [PubMed] [Google Scholar]
- Boucher Y, Simons CT, Faurion A, Azerad J, Carstens E. Trigeminal modulation of gustatory neurons in the nucleus of the solitary tract. Brain Research. 2003b;973:265–274. doi: 10.1016/s0006-8993(03)02526-5. [DOI] [PubMed] [Google Scholar]
- Burnette RR. A monte-carlo model for the passive diffusion of drugs across the stratum corneum. Int. J. Pharmaceutics. 1984;22:89–97. [Google Scholar]
- Carstens E, Albin KC, Simons CT, Carstens MI. Time Course of Self-Desensitization of Oral Irritation by Nicotine and Capsaicin. Chem. Senses bjm048. 2007 doi: 10.1093/chemse/bjm048. [DOI] [PubMed] [Google Scholar]
- Carstens E, Kuenzler N, Handwerker KO. Activation of neurons in rat trigeminal subnucleus caudalis by different irritant chemicals applied to the oral or ocular mucosa. J. Neurophysiol. 1998;80:465–492. doi: 10.1152/jn.1998.80.2.465. [DOI] [PubMed] [Google Scholar]
- Cerf-Ducastel B, van de Moortele PF, MacLeod P, Le Bihan D, Faurion A. Interaction of gustatory and lingual somatosensory perceptions at the cortical level in the human: a functional magnetic image study. Chem Sens. 2001;26:371–383. doi: 10.1093/chemse/26.4.371. [DOI] [PubMed] [Google Scholar]
- Chang G-Q, Vigna SR, Simon SA. Localization of substance P NK-1 receptors in rat tongue. Regulatory Peptides. 1996;63:85–89. doi: 10.1016/0167-0115(96)00021-3. [DOI] [PubMed] [Google Scholar]
- Chrandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zucker CS, Ryba NJP. T2Rs function as bitter taste receptors. Cell. 2000;100:703–711. doi: 10.1016/s0092-8674(00)80706-0. [DOI] [PubMed] [Google Scholar]
- Dahl M, Erickson RP, Simon SA. Neural responses to bitter compounds in the rat. Brain Res. 1997;756:22–34. doi: 10.1016/s0006-8993(97)00131-5. [DOI] [PubMed] [Google Scholar]
- Dessirier J-M, O'Mahoney M, Carstens E. Oral irritant properties of menthol sensitizing and desentsitizing effects of repeated application and cross-desensitization to nicotine. Physiol & Behav. 2001;73:25–36. doi: 10.1016/s0031-9384(01)00431-0. [DOI] [PubMed] [Google Scholar]
- Diamond J, Breslin PAS, Doolittle N, Nagata H, Dalton P. Flavor Processing: Perceptual and Cognitive Factors in Multi-modal Integration. Chem. Senses. 2005;30:i232–i233. doi: 10.1093/chemse/bjh199. [DOI] [PubMed] [Google Scholar]
- Green BG. The sensory effects of l-menthol on human skin. Somatosens. Mot. Res. 1992;9:235–244. doi: 10.3109/08990229209144774. [DOI] [PubMed] [Google Scholar]
- Green BG, Schoen KL. Thermal and nociceptive sensations from menthol and their suppression by dynamic contact. Behavioural Brain Research. 2007;176:284–291. doi: 10.1016/j.bbr.2006.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger TE, Danilova V, Barrows J, Dianna L, Bartel DL, Alison J, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP Signaling Is Crucial for Communication from Taste Buds to Gustatory Nerves. Science. 2005;310:1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- Halpern BP, Tapper DN. Taste stimuli: quality coding time. Science. 1971;171:1256–1258. doi: 10.1126/science.171.3977.1256. [DOI] [PubMed] [Google Scholar]
- Hanamori T, Kunitake T, Kato K, Kannan H. Convergence of afferent inputs from the chorda tympani, lingual-tonsillar and pharyngeal branches of the glossopharyngeal nerve, and superior laryngeal nerve on the neurons in the insular cortex in rats. Brain Research. 1997;763:267–270. doi: 10.1016/s0006-8993(97)00483-6. [DOI] [PubMed] [Google Scholar]
- Hanamori T, Kunitake T, Kato K, Kannan H. Responses of Neurons in the Insular Cortex to Gustatory, Visceral, and Nociceptive Stimuli in Rats. J Neurophysiol. 1998;79:2535–2545. doi: 10.1152/jn.1998.79.5.2535. [DOI] [PubMed] [Google Scholar]
- Kadohisa M, Rolls ET, Verhagen JV. Orbitofrontal cortex: neuronal representation of oral temperature and capsaicin in addition to taste and texture. Neuroscience. 2004;127:207–221. doi: 10.1016/j.neuroscience.2004.04.037. [DOI] [PubMed] [Google Scholar]
- Kadohisa M, Verhagen JV, Rolls ET. The primate amygdala: Neuronal representations of the viscosity, fat texture, temperature, grittiness and taste of foods. Neuroscience. 2005;132:33–48. doi: 10.1016/j.neuroscience.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Katz DB, Simon SA, Nicolelis MAL. Dynamic and multimodal response of gustatory cortical neurons. J. Neurosci. 2001;21:4478–4489. doi: 10.1523/JNEUROSCI.21-12-04478.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura Y, Okamoto J, Funakoshi M. A role of oral afferents in aversion to taste solutions. Physiol. Behav. 1968;3:537–542. [Google Scholar]
- Laurienti PJ, Perrault TJ, Stanford TR, Wallace MT, Stein BE. On the use of superadditivity as a metric for characterizing multisensory integration in functional neuroimaging studies. Eperimental Brain Research. 2005;166:289–297. doi: 10.1007/s00221-005-2370-2. [DOI] [PubMed] [Google Scholar]
- Lemon CH, Smith DV. Neural Representation of Bitter Taste in the Nucleus of the Solitary Tract. J Neurophysiol. 2005;94:3719–3729. doi: 10.1152/jn.00700.2005. [DOI] [PubMed] [Google Scholar]
- Lim J, Green BG. The Psychophysical Relationship between Bitter Taste and Burning Sensation: Evidence of Qualitative Similarity. Chem. Senses. 2007;32:31–39. doi: 10.1093/chemse/bjl033. [DOI] [PubMed] [Google Scholar]
- Liu L, Chang G-Q, Jiao Y, Simon SA. Neuronal nicotinic acetylcholine receptors in rat trigeminal ganglia. Brain Res. 1998;809:238–245. doi: 10.1016/s0006-8993(98)00862-2. [DOI] [PubMed] [Google Scholar]
- Liu L, Simon SA. Capsaicin and nicotine both activate a subset of rat trigeminal ganglion neurons. Am. J. Physiol. 1996;270:C1807–C1814. doi: 10.1152/ajpcell.1996.270.6.C1807. [DOI] [PubMed] [Google Scholar]
- Lyall V, Heck GL, Phan TH, Mummalaneni S, Malik SA, Vinnikova AK, DeSimone JA. Ethanol modulates the VR-1 variant amiloride-insensitive salt taste receptor. II. Effect on chorda tympani salt responses. J. Gen. Physiol. 2005;125:587–600. doi: 10.1085/jgp.200509264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall V, Heck GL, Vinnikova AK, Ghosh S, Phan TH, Alam RI, Russell OF, Malik SA, Bigbee JW, DeSimone JA. The mammalian amiloride-insensitive non-specific salt taste receptor is a vanilloid receptor-1 variant. J Physiol (Lond) 2004;558:147–159. doi: 10.1113/jphysiol.2004.065656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall V, Phan TH, Mummalaneni S, Mansouri M, Heck GL, Kobal G, DeSimone JA. Effect of nicotine on chorda tympani responses to salty and sour stimuli. J Neurophysiol. 2007 doi: 10.1152/jn.00366.2007. in press. [DOI] [PubMed] [Google Scholar]
- Norgren R. Gustatory system. In: Paxinos G, editor. The Rat Nervous System. Academic Press; San Diego: 1995. pp. 751–771. [Google Scholar]
- Ogawa H. Gustatory cortex in primates. Anatomy and physiology. Neurosci. Res. 1994;20:1–13. doi: 10.1016/0168-0102(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Hyama T, Ito S. Response properties of the parabrachio-thalamic taste and mechanoreceptive neurons in rats. Exp Brain Res. 1987;68:449–457. doi: 10.1007/BF00249789. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Sato M, Yamashita S. Multiple sensitivity of chorda tympani fibres of the rat and hamster to gustatory and thermal stimuli. J. Physiol. 1968;199:223–240. doi: 10.1113/jphysiol.1968.sp008650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuni Y. Response of chorda tympani fibers of the rat to pungent spices and irritants in pungent spices. Shikwa. Gakuho. 1977;77:1323–1349. [PubMed] [Google Scholar]
- Scott TR, Mark GP. The taste system encodes toxicity. Brain Res. 1987;414:197–203. doi: 10.1016/0006-8993(87)91347-3. [DOI] [PubMed] [Google Scholar]
- Scott TR, Plata-Salaman CR. Taste in the monkey cortex. Physiol & Behav. 2003;67:489–511. doi: 10.1016/s0031-9384(99)00115-8. [DOI] [PubMed] [Google Scholar]
- Simons CT, Boucher Y, Carstens MI, Carstens E. Nicotine suppression of gustatory responses of neurons in the nucleus of the solitary tract. J Neurophysiol. 2006;96:1877–1886. doi: 10.1152/jn.00345.2006. [DOI] [PubMed] [Google Scholar]
- Simons CT, Sudo S, Sudo M, Carstens E. Mecamylamine reduces nicotine cross-desensitization of trigeminal caudalis neuronal responses to oral chemical irritation. Brain Research. 2003;991:249–253. doi: 10.1016/s0006-8993(03)03539-x. [DOI] [PubMed] [Google Scholar]
- Soares ES, Stapleton JR, Rodriguez A, Fitzsimmons N, Oliveira L, Nicolelis MAL, Simon SA. Behavioral and neural responses to gustatory stimuli delivered non-contingently through intra-oral cannulas. Physiology & Behavior. doi: 10.1016/j.physbeh.2007.05.038. In Press, Corrected Proof. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford TR, Stein BE. Superadditivity in multisensory integration: putting the computation in context. Neuroreport. 2007;18:787–792. doi: 10.1097/WNR.0b013e3280c1e315. [DOI] [PubMed] [Google Scholar]
- Stapleton JA, Lavine M, Nicolelis MAL, Simon SA. Ensembles of gustatory cortical neurons anticipate and discriminate between tastants in a single lick. Frontiers in Neuroscience. 2007 doi: 10.3389/neuro.01.1.1.012.2007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton JA, Lavine M, Wolpert R, Nicolelis MAL, Simon SA. Rapid taste responses in the gustatory cortex during licking. J. Neurosci. 2006;26:4126–4138. doi: 10.1523/JNEUROSCI.0092-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szolcsanyi J. Actions of capsaicin on sensory receptors. In: Wood J, editor. Capsaicin in the study of pain. Acadmic Press; New York: 1993. pp. 1–26. [Google Scholar]
- Talavera K, Yasumatsu K, Voets T, Droogmans G, Shigemura N, Ninomiya Y, Margolskee RF, Nilius B. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature. 2005;438:1022–1025. doi: 10.1038/nature04248. [DOI] [PubMed] [Google Scholar]
- Todrank J, Bartoshuk LM. A taste illusion: Taste sensation localized by touch. Physiology & Behavior. 1991;50:1027–1031. doi: 10.1016/0031-9384(91)90432-n. [DOI] [PubMed] [Google Scholar]
- Wang Y, Erickson RE, Simon SA. Chemical selectivity of lingual nerve fibers to chemical stimuli. J. Gen. Physiol. 1993;101:1–24. doi: 10.1085/jgp.101.6.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Erickson RE, Simon SA. Modulation of chorda tympani nerve activity by lingual nerve stimulation. J. Neurophysiol. 1995;73:1468–1483. doi: 10.1152/jn.1995.73.4.1468. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Yuyama N, Kawamura Y. Cortical neurons responding to tactile, thermal and taste stimulations of the rat tongue. Br. Res. 1981;221:411–415. doi: 10.1016/0006-8993(81)91075-1. [DOI] [PubMed] [Google Scholar]
- Yamasaki H, Kubota Y, Takagi H, Tohyama M. Immunoelectron-microscopic study on the fine structure of substance -P containing fibers in taste buds of the rat. J. Comp. Neurol. 1984;227:380–392. doi: 10.1002/cne.902270308. [DOI] [PubMed] [Google Scholar]
- Zanotto KL, Merrill AW, Carstens MI, Carstens E. Neurons in superficial trigeminal subnucleus caudalis responsive to oral cooling, menthol, and other irritant stimuli. J Neurophysiol. 2007;97:966–978. doi: 10.1152/jn.00996.2006. [DOI] [PubMed] [Google Scholar]