Abstract
Objective
We tested our hypothesis that abdominal obesity when associated with increased levels of systemic and CNS immuno-inflammatory mediators contributes to neurocognitive impairment (NCI).
Design
Cross-sectional
Setting
Six Academic Centers
Participants
152 patients with plasma HIV RNA <1,000copies/ml had clinical evaluations and cognitive function quantified by global deficit scores (GDS).
Outcome Measures
GDS, waist circumference (WC) and plasma IL-6, sCD163, and sCD14 and CSF sCD40L, sTNFrII, MCP-1, sICAM, and MMP-9.
Results
WC and plasma IL-6 levels positively correlated with GDS; the WC correlation was strongest in the high tertile of IL-6 (rho=0.39, p=0.005). IL-6 correlated with GDS only if WC was ≥99cm. In the high tertile of CSF sCD40L, a biomarker of macrophage and microglial activation, the correlation of IL-6 to GDS was strongest (rho=0.60, p<0.0001). Across 3-5 visits within ±1year of the index visit, GDS remained worse in patients with IL-6 levels in the high-versus-low tertile (p=0.02). Path analysis to explore potential mediators of NCI produced a strong, integrated model for patients in the high CSF sCD40L tertile. In this model, WC affected GDS both directly and via a second path that was mediated by IL-6. Inclusion of plasma sCD14 levels strengthened the model. NCI was more common in men and for individuals with components of the metabolic syndrome.
Conclusions
NC function was significantly linked to abdominal obesity, systemic inflammation (high IL-6), and immune activation in plasma (high sCD14) and CSF (high sCD40L). Abdominal obesity, inflammation, and CNS immune activation are potential therapeutic targets for NCI in HIV+ patients.
Keywords: Abdominal obesity, HIV Associated Neurocognitive Disorder, Inflammation and immune activation, Global deficit score, sCD40L, sCD14
INTRODUCTION
HIV-associated neurocognitive disorder (HAND) remains an important complication in the current antiretroviral therapy era, occurring in up to 50% of persons with HIV, although most cases are mild or asymptomatic 1,2. The prevalence, incidence and severity of HAND may increase as the HIV population ages 3. Even patients who have asymptomatic neurocognitive impairment (ANI), the mildest and most common form of HAND, progress to symptomatic HAND more rapidly than patients without HAND 4. The mechanisms for persistence and progression of HAND despite good suppression of HIV replication with anti-retroviral therapy (ART) remain uncertain 5.
We recently reported that the severity of neurocognitive impairment (NCI) in HIV infected (HIV+) patients was correlated with waist circumference 6 suggesting that abdominal obesity may be contributing to HAND. In HIV uninfected (HIV-) populations, a growing body of literature provides compelling evidence of the relationship of abdominal obesity and other components of the metabolic syndrome (MetS) to the increased risk of future NCI and severity of existing NCI 7-9. The impact of obesity and related cardiometabolic disorders on NCI in HIV+ and HIV- persons will become increasingly important since nearly 70% of Americans (~200 million) are obese or overweight 10 and the prevalence of pre-diabetes has nearly doubled in recent years 11. Already abdominal obesity and cardiometabolic risk factors, including components of MetS, are also highly prevalent in HIV+ patients 12-14, although the complete MetS is not as prevalent as expected in some HIV populations 14. We postulated that the contribution of abdominal obesity, a central component of MetS, to cognitive impairment may be substantive in HIV infection because of the high background of chronic inflammation in these patients. Indeed, in observational studies of HIV negative persons, the risk of NCI in obese patients is invariably associated with systemic inflammation 15-18.
We hypothesized that in HIV+ patients, systemic and central nervous system (CNS) inflammation and immune activation link abdominal obesity to NCI. In support of this hypothesis, when adipocytes accumulate excessive amounts of lipid, pro-inflammatory macrophages infiltrate adipose tissue and secrete pro-inflammatory cytokines (e.g. IL-6, TNFα), reactive oxygen species, and chemokines (MCP-1) 19-21. These mediators may enter the systemic circulation 21,22, increasing systemic inflammation and immune activation (e.g. elevations in sCD163) reflecting monocyte activation 23,24. Immune activation may also be due to intestinal translocation of microbial products including lipopolysaccharide 25,26 as reflected by increases in sCD14 26,27 that can further enhance adipose tissue inflammation via TLR signaling 21,28,29. We further postulate that these effects are super-imposed on chronic inflammation and immune activation from HIV that persists even when HIV infection is well-controlled 26,30.
We examined the relationship of measures of abdominal obesity to neurocognition and to immuno-inflammatory mediators obtained at a single study visit. Mediators included markers of systemic inflammation (IL-6) and immune activation (sCD163 and sCD14) and CSF biomarkers, including sTNFrII (inflammation), sICAM (vascular damage), MMP-9 (basement membrane remodeling), MCP-1 (monocyte chemotaxis) and sCD40L (CNS immune activation). We also explored the possibility that cardiometabolic variables associated with obesity such as blood pressure, plasma lipids, insulin resistance, and adiponectin levels enhance risks for NCI.
METHODS
Patient Selection and Characterization
Study subjects provided written informed consent approved by the local institutional review board of the participating institutions. Participants in the CNS HIV Antiretroviral Therapy Effects Research (CHARTER) study 1 were selected for this analysis (n=152) if they had all of the following: a) stored samples of plasma and CSF, b) plasma HIV RNA <1000copies/ml, c) comprehensive neuropsychological assessment, and d) no conditions such as developmental delay, head trauma, or prolonged substance abuse that would confound assessment of their cognitive performance. All participants in the CHARTER cohort were included if they met all four entry criteria. For persons with multiple qualifying visits, data from the first visit was used as the index visit for this substudy. This CHARTER sample overlapped that of our prior metabolic substudy (N=130) by 42% 6.
Mid waist circumference was measured in triplicate by a standardized anthropometric protocol used across the CHARTER study sites. Blood and CSF were collected at the index visit after an overnight fast and processed for clinical laboratory tests, T-lymphocyte subsets, and HIV RNA (Roche Amplicor; lower limit of quantitation 50 copies/mL) and additional aliquots were stored at -80C until assayed.
Neurocognitive Assessment
A senior neuropsychologist (RH) used guidelines from a consensus panel along with all available historical and testing data to rate cognitive co-morbidity in participants from all six CHARTER sites 1. A standardized battery of 12 neuropsychological tests that assess cognitive domains commonly affected by HIV was administered to all participants (average time = 2-2.5 hours) and scored by centrally-certified research psychometrists. The component tests in this battery have been described previously 1. To adjust for age, education, and race/ethnicity, raw test scores were converted to demographically-corrected deficit scores using published methods and best available normative data 31.
Global deficit scores (GDS), ranging from 0-5, are calculated by averaging the individual deficit scores from each test. Higher scores indicate poorer cognitive functioning. A GDS-based diagnosis of NCI requires an average score for all tests of ≥0.50, which indicates mildly impaired performance on at least half of the tests 32. Neurocognitive impairment was also evaluated by the Clinical Rating (CR) score 31 in this cohort. The correlations of the independent variables to GDS and the CR scores were similar but generally stronger with GDS. We elected to report our results as GDS, the more objective measure of cognitive impairment.
Biomarker Measurements
Plasma and CSF specimens stored at -80C were thawed once and tested using commercially available immunoassays according to manufacturers’ protocols. Four biomarkers (high sensitivity IL-6, soluble CD14, soluble CD163, soluble CD40L) were measured by traditional, plate-based enzyme-linked immunosorbent assays (Quantikine; R&D Systems, Minneapolis, MN USA). The other four biomarkers (MCP-1, sTNFr-II, sICAM-1, MMP-9) were assayed using individual single-plex bead-based immunoassays (EMD Millipore, Billerica, MA). Plasma samples were tested for hs-IL-6, sCD14 and sCD163; CSF samples were tested for sCD40L, MCP-1, sTNFr-II, sICAM-1, and MMP-9. Precision was ensured by assaying specimens in duplicate and repeating the assays for samples with coefficients of variation greater than 20% or outliers that were more than three standard deviations from the mean levels of each biomarker.
Fasting glucose, insulin and adiponectin levels in plasma were assayed using an automated chemiluminescent analyzer (Immulite 1000, Siemens Healthcare Diagnostics, Deerfield, IL) 33. Plasma for lipopolysaccharide (LPS) was quantified using the Endpoint Chromogenic LAL assay (Lonza, Basel, Switzerland) 34.
Statistical Analysis
Characteristics of the study cohort at the index visit are presented as median with first and third quartile for continuous variables. Frequencies and percentages were reported for binary or categorical variables. The primary cross-sectional analyses used biomarker measures at the index visit. A correlation matrix was used to assess associations among waist circumference , cognition (GDS) and index visit biomarkers levels. To examine the possible non-linear effects of biomarkers on the relationships between cognition (GDS) with abdominal obesity and inflammation (IL-6), the cohort was then divided into sub-groups defined by high or low tertile of plasma and CSF biomarkers. Pearson's correlation and linear regression were used to analyze the relationships of waist circumference and biomarkers to GDS. Estimated correlation coefficients and regression slopes were compared between high versus low tertile subgroup for each biomarker.
Because GDS had been measured every 6 months in CHARTER participants, we investigated effects of high versus low IL-6 from the index visit relative to changes in GDS at 6 and 12 months before and after the index visits. To account for each participant having multiple GDS scores, repeated measure analysis was adopted to examine trajectories of GDS over multiple visits between high versus low IL-6 tertile subgroups. Within each gender, participants were also divided into abdominally obese and non-obese subgroups using metabolic syndrome criteria (waist circumference of ≥88cm for women and ≥102cm for men) 35. Prevalence of NCI, components of metabolic syndrome, and plasma adiponectin were compared between obese versus non-obese subgroup within each gender by Wilcoxon signed rank test or T-test if data was normally distributed. The differences between female obese and male obese participants were evaluated using the Wilcoxon test.
Path analysis was used to identify statistically acceptable models describing the data 36. Assuming linear relationships, several linear path models were fitted by analyzing the covariance matrices. The goodness of fit (GOF) of the overall model was assessed using the chi-square test. Two other GOF indices, the non-normed fit index 37 and the comparative fit index (CFI), were also examined (Bentler, PM in BMDP Software [1989], Los Angeles, California). Models were further investigated if the GOF p-value was >0.1, and both NNFI and CFI were >0.90, suggesting that the model fits the data well since higher p values indicate better performance in path analysis.
RESULTS
Patient Characteristics
The 152 participants averaged almost 50 years of age, were 85% male and about half were White. They had been receiving ART for an average of nearly 15 years (Table 1). Although their self-reported nadir CD4+ T-cells was 117cells/mm3, the majority had achieved excellent immune recovery with current CD4+ T-cell counts of over 500cells/mm3. Eighty two percent had undetectable plasma HIV RNA and 93% of patients had no detectable HIV in their CSF. Their cardiometabolic characteristics including systolic blood pressure, fasting plasma lipids and glycemic control were generally good and none had prior myocardial infarction or stroke (data not shown). Their neurocognitive function varied from normal to moderately impaired. Twenty eight percent had NCI (GDS ≥0.5).
Table 1.
Patient Characteristics at the Index Visit
| Total Cohort (N=152) | Non-obese (N=101) | Obese** (N=51) | P value | |
|---|---|---|---|---|
| Age, years | 49 (22, 69) * | 50 (22, 68) | 49 (31, 69) | 0.82 |
| Gender, Male (%) | 129 (85%) | 94 (94%) | 35 (69%) | <0.0001 |
| Education, years | 13 (7, 20) | 13 (7, 18) | 13 (9, 20) | 0.43 |
| Ethnicity/Race | ||||
| White (%) | 79 (52%) | 53 (52%) | 26 (51%) | 0.86 |
| Hispanic (%) | 22 (14%) | 12 (12%) | 10 (20%) | 0.20 |
| African American (%) | 48 (32%) | 35 (35%) | 13 (25%) | 0.25 |
| Comorbid Conditions | ||||
| Tobacco exposure ever (%) | 120 (79%) * | 78 (77%) | 42 (82%) | 0.47 |
| IV drug use ever (%) | 38 (25%) | 39 (29%) | 9 (18%) | 0.14 |
| Alcohol exposure | 4.0 (2.7, 7.6) | 4.0 (2.5, 7.3) | 4.2 (2.9, 7.6) | 0.67 |
| HCV antibody positive | 42 (26%) | 28 (26%) | 14 (25%) | 0.92 |
| Body mass index kg/m2 | 25.4 (17.3, 41.1) | 23.6 (17.3, 31.1) | 30.9 (22.0, 41.1) | N/A |
| WC for men, cm | 94 (71, 140) | 90 (71, 101) | 108 (102, 140) | N/A |
| WC for women, cm | 94 (75, 133) | 78 (75, 85) | 96 (88, 133) | N/A |
| Diagnosis of diabetes (%) | 15 (10%) | 9 (9%) | 6 (12%) | 0.58 |
| HIV Disease Status | ||||
| AIDS CDC diagnosis | 114 (75%) | 78 (77%) | 36 (71%) | 0.37 |
| Duration of HIV, years | 14.9 (1.5, 29.5) | 15.5 (2.7, 28.6) | 14.5 (1.5, 29.5) | 0.43 |
| Current CD43, cells/mm3 | 549 (65, 3199) | 504 (65, 1869) | 612 (75, 3199) | 0.04 |
| Nadir CD43, cells/mm3 | 117 (0, 816) | 101 (0, 816) | 155 (0, 369) | 0.57 |
| Plasma VL, c/mL (log10) | 1.7 (1.6, 3.0) | 1.7 (1.6, 3.0) | 1.6 (1.6, 2.6) | 0.03 |
| Plasma VL, undetectable | 124 (82%) | 80 (79%) | 44 (86%) | 0.29 |
| CSF VL, c/mL (log10) | 1.7 (1.6, 3.5) | 1.7 (1.6, 3.5) | 1.6 (1.6, 2.5) | 0.04 |
| CSF VL detectable (%) | 10 (7%) | 8 (8%) | 2 (4%) | 0.35 |
| Currently on ARV (%) | 151 (99%) | 100 (99%) | 51 (100%) | 0.66 |
| Duration of current ARV mo. | 26 (13-42) | 26 (15, 42) | 26 (12, 42) | 0.79 |
| Neurocognitive Impairment | ||||
| Global deficit score (GDS) | 0.21 (0.00, 2.36) | 0.14 (0.00, 2.36) | 0.29 (0.00, 1.93) | 0.04 |
| No. with HAND (%) | 43 (28%) | 21 (21%) | 22 (43%) | 0.004 |
Median (first and third quartiles) or frequency (percentage)
Waist circumference ≥88cm for women and ≥102cm for men
Abdominal Obesity and Neurocognitive Function
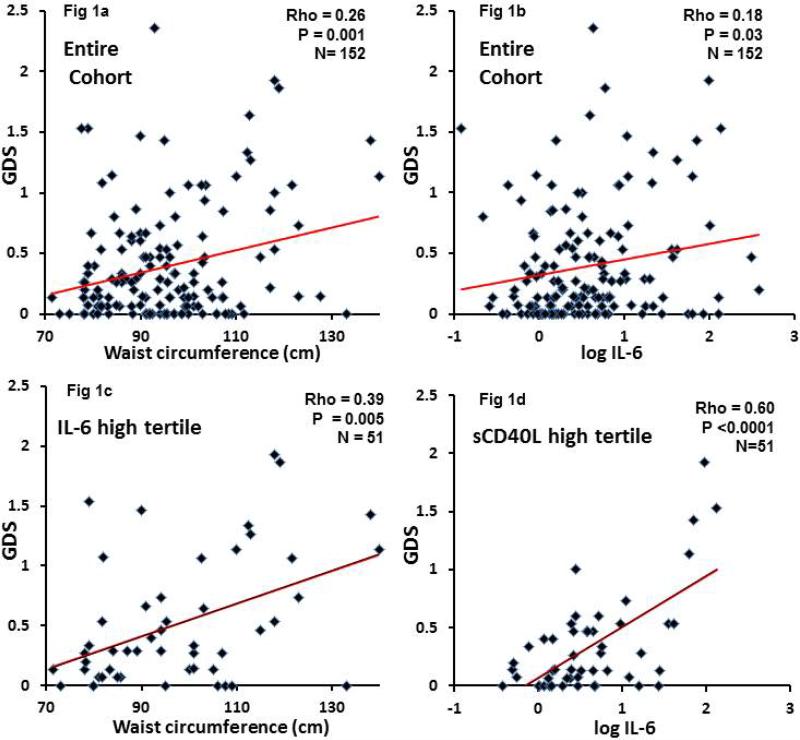
Waist circumference varied widely (70-136 cm) and correlated with GDS across the entire cohort (rho=0.26, p=0.001; Figure 1a). Moreover, GDS was significantly greater for patients defined as abdominally obese (n=51) according to criteria for metabolic syndrome, than for non-obese patients (0.29 compared to 0.14, p=0.04; Table 1). Further, 43% of these abdominally obese patients versus only 21% of non-obese patients met criteria for global NCI (odds ratio=2.89, p=0.004).
Figure 1.
Figure shows relationship of global deficit scores (GDS) to waist circumference (left panels) and log10 IL-6 levels (right panels). Data is for the entire study population (Fig 1a and 1b) and for the highest tertiles of IL-6 (Fig 1c) and of sCD40L (Fig 1d). Correlations of GDS with either WC or IL-6 were stronger in those patients with the highest tertiles of IL-6 and of sCD40L.
Effects of Immuno-inflammatory Markers on GDS
Appendix Table 1 summarizes the correlations of between biomarkers, waist circumference and GDS. For the entire group, only plasma IL-6 correlated significantly with GDS (rho=0.18, p=0.003, Figure 1b); however, this correlation was limited to patients with waist circumference ≥99cm (rho=0.33, p=0.02). To understand how the biomarkers of immune activation and inflammation might affect the relationship of GDS to waist circumference or IL-6, each biomarker was divided into tertiles. Pearson correlations of GDS to waist circumference or IL-6 were compared between the high and low tertiles for each biomarker (Appendix Table 2). For waist circumference, the subgroups with the high tertile of each biomarker had larger correlation coefficients than the low subgroups for all of the biomarkers except for MCP-1. However, the difference between the high and low tertiles was statistically significant only for IL-6 (p=0.03). In the high tertile of IL-6, waist circumference correlated with GDS (rho=0.39, p=0.005), which is a stronger correlation than for the entire cohort of 152 patients (rho=0.26; Figure 1c compared to Figure 1a); if the 28 patients with detectable plasma viral loads were excluded (remaining n=124), the respective correlation coefficients were rho=0.42 (p=0.006) and rho-0.36 (p=0.02)—data not shown.
We assessed the relationship of IL-6 to GDS in the subgroups composed of patients in the high and low tertiles of other markers (Appendix Table 2). Patients in the high tertile of CSF sCD40L, had the strongest correlation of IL-6 to GDS (rho=0.60, p<0.0001; Figure 1b compared to Figure 1d). This was a substantially greater correlation than in the entire cohort (rho=0.18). In contrast, in patients in the lowest tertile of sCD40L, IL-6 levels were unrelated to GDS (rho=0.01, p=0.93). The correlations of IL-6 to GDS in the high versus low tertile for other biomarkers did not differ (Appendix Table 2).
Path Analysis and Models
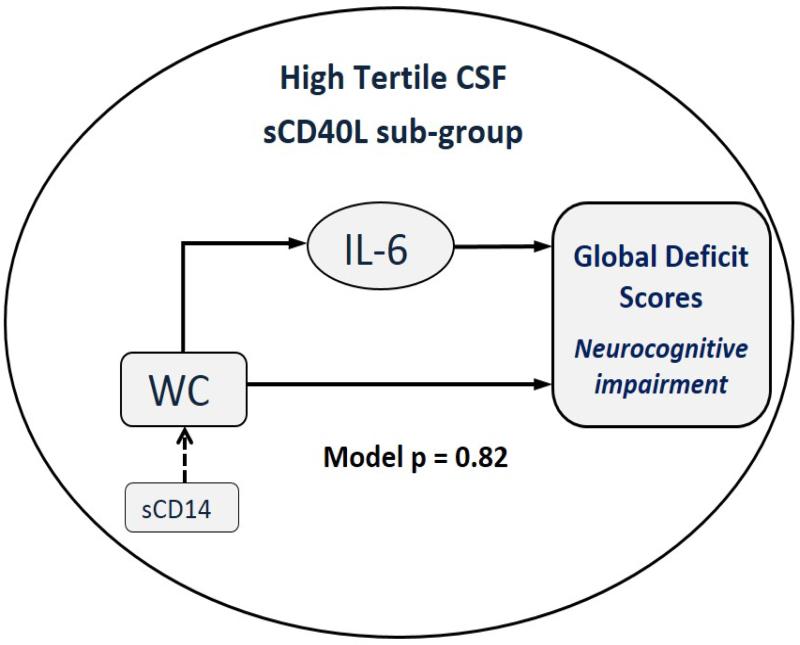
We conducted path analysis to further investigate the relationships of waist circumference and IL-6 to GDS and the interaction of these relationships with levels of other biomarkers. The model that best fit the data was for patients who had CSF sCD40L in the high tertile (goodness of fit p=0.82, Chi-square of 0.41 with two degrees of freedom, Figure 2). In path analysis, larger p values indicate a better fit of the model to the data. In this model, waist circumference was a direct predictor of GDS, and also involved a second pathway in which IL-6 mediated the effects of waist circumference on GDS. Inclusion of sCD14 as an interaction term with waist circumference increased the model's goodness of fit from p=0.55 to p=0.82. Inclusion of other biomarkers or cardiometabolic variables (e.g. blood pressure, lipids, insulin sensitivity, and adiponectin) as predictors, mediators or interaction terms did not further improve the goodness of fit.
Figure 2.
Figure depicts the model derived from path analysis and regression coefficients linking waist circumference (WC) to global deficit scores (GDS) in patients with high CSF levels of sCD40L. Solid lines represent regression coefficients each with p<0.05 and arrows show the direction of the relationship. For sCD14, p=0.07 and is an interaction term with waist circumference (WC). The Path analysis generated the following relationship:
1. Log (IL-6)=0.011*WC: 10cm increase in WC => 12% increase in IL-6 (exp[0.011*10]=1.116=111.6%)
2. WC = 0.006*sCD14 (in 1000): 100,000 increase in sCD14 => 0.6cm increase in WC
3. Sqrt (GDS) = 0.005*WC + 0.28*log(IL-6).
In the top pathway, WC is a predictor of GDS and its effects are mediated by IL-6. In the lower pathway, WC is direct predictor of GDS. In Path analysis, higher P values reflect better fit of data to the model and, thus, this is a strong model since p=0.82.
Effects of Gender and Metabolic Syndrome Components on GDS
We investigated the effects of metabolic syndrome components and gender on the relationship of waist circumference to GDS. In the 23 women in our cohort, GDS was higher in abdominally obese than in non-obese women (p=0.05 by t-test; Table 2). Likewise, GDS was higher in obese than non-obese men (p=0.01). Almost half (49%) of obese men had NCI compared to 22% of non-obese men (p=0.04). GDS in obese men was two-fold higher than in obese women, but the number of obese women was relatively small (N=16) and the difference between genders did not reach statistical significance (p=0.08). For the obese men, components of metabolic syndrome (systolic blood pressure, HDL-C, fasting triglycerides, insulin resistance by HOMA-IR) and plasma adiponectin were significantly worse than for obese women (Table 2).
Table 2.
Difference in Neurocognitive Function and Metabolic Syndrome Components by Gender and Obesity
| Variables from N=152 Age* | WOMEN (WC<88) N=7 50 (39, 55) | WOMEN (WC>88) N=16 49 (41, 55) | P value** | Men (WC <102) N=94 50 (44, 54) | MEN (WC≥102) N=35 49 (45, 57) | P value | P value Obese W vs M |
|---|---|---|---|---|---|---|---|
| Global Deficit Score | 0.07 (0.00, 0.20)* | 0.23 (0.00, 0.54) | 0.21 (0.05)† | 0.20 (0.07, 0.47) | 0.47 (0.13, 1.07) | 0.01 | 0.08 |
| No. with NCI†† (%) | 0 | 5 (31) | 0.27 | 21(22) | 17(49) | 0.004 | 0.25 |
| Systolic pressure mm Hg | 119 (116, 123) | 106 (100, 120) | 0.62 | 126 (116, 135) | 130 (120, 138) | 0.39 | 0.0008 |
| Total Cholesterol, mg/dL | 164 (131, 200) | 204 (192, 229) | 0.03 | 180 (157, 204) | 183 (163, 209) | 0.53 | 0.04 |
| LDL-Cholesterol, mg/dL | 85 (50, 114) | 118 (101, 139) | 0.03 | 98 (78, 120) | 104 (82, 129) | 0.38 | 0.10 |
| HDL-cholesterol, mg/dL | 55 (47, 77) | 58 (54, 66) | 0.44 | 47 (37, 59) | 38 (29, 50) | 0.006 | <0.0001 |
| Triglycerides, mg/dL | 131 (60, 171) | 113 (88, 147) | 0.66 | 143 (89, 210) | 201 (131, 308) | 0.01 | 0.0007 |
| HOMA-IR | 1.19 (1.04, 1.27) | 2.00 (1.37, 3.24) | 0.05 | 1.92 (1.04, 3.81) | 4.16 (2.70, 6.81) | 0.0003 | 0.01 |
| Plasma adiponectin, ng/ml × 103 | 5.3 (3.9, 9.4) | 4.5 (3.5, 6.8) | 0.49 | 4.3 (2.4, 6.0) | 3.2 (2.5, 3.9) | 0.04 | 0.009 |
Medians (first and third quartiles)
Wilcoxon nonparametric p-values were used for all other comparisons
0.05 by T test
neurocognitive impairment
Potential Confounding by Other Inflammatory Conditions
We examined whether other inflammatory conditions affected GDS. In this subgroup of the CHARTER cohort, Hepatitis C virus (HCV) seropositivity, lifetime intravenous drug use, lifetime cigarette smoking, and alcohol use 38 were similar in persons with and without NCI (data not shown). Inclusion of these parameters into the path analysis did not strengthen and actually weakened the model (data not shown).
Effects of Systemic Inflammation on GDS over Time
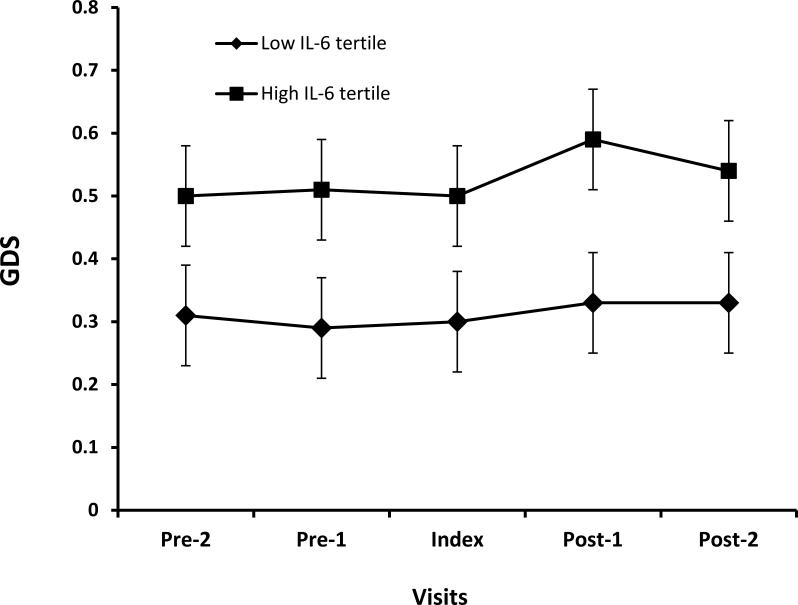
Of the 152 participants, 144 had at least three visits 6 months apart within ± 12 months of the index visit. The average difference in GDS was 0.22 in the high versus low tertiles of IL-6 (p=0.02) and the difference was stable over time (Figure 3).
Figure 3.
Figure shows change in Global Deficit Score (GDS) Over Time in High and Low IL-6 tertiles. Pre-2 and Pre-1 are CHARTER visits at -12 and -6 months before the index visit, respectively. Post-1 and Post-2 are visits 6 and 12 months, respectively, after the index visit when IL-6 was measured. The lines with boxes are GDS values for subjects with IL-6 in high tertile and line with diamonds are for subjects in low IL-6 tertile. The average difference in GDS over time between the two groups is 0.22 (p=0.02).
DISCUSSION
In this subgroup of the CHARTER cohort, we confirmed our earlier finding that waist circumference correlated with neurocognitive function as measured by GDS 6. In the current study, waist circumference was correlated with GDS only in abdominally obese patients. Indeed, the odds ratio for NCI in obese subjects was nearly three times greater than in non-obese patients. We found that the correlation of waist circumference to GDS was substantially greater in patients with the highest plasma levels of IL-6 (rho=0.39) compared to the entire cohort of 152 patients (rho=0.26). In addition, systemic IL-6 was correlated strongly with GDS in the high tertile of CSF sCD40L (rho=0.60). A model generated by path analysis confirmed these findings and indicated that increasing waist circumference was directly predictive of abnormalities in GDS and by a separate path in which IL-6 mediated the effects of waist circumference on GDS. The model was strongest in the group with highest levels of CSF sCD40L, a marker of microglial and CNS macrophage activation, and when plasma sCD14, was included as an interaction term with waist circumference. In addition to these cross-sectional findings, patients in the highest IL-6 tertile at their index visit had higher GDS than those in the lowest IL-6 tertile and the difference was stable over one-to-two years.
Waist circumference and IL-6 were most strongly linked to NCI for patients with the highest levels of CSF sCD40L, the soluble form of the transmembrane glycoprotein ligand that binds CD40. The severity of NCI in HAND is related to the number of activated microglia, which express the CD40 transmembrane protein. Similarly, microglia bearing primarily the CD40 epitope occur with other neurodegenerative disorders including Alzheimer's, multiple sclerosis and Parkinson's disease 39. In HIV, CD40 signaling in microglia synergize with the effects of HIV Tat further enhancing CNS inflammation and contributing to dementia 40. Blood sCD40L or CD40L bearing T and B lymphocytes entering the CNS may bind to and activate CD40 bearing microglia to secrete pro-inflammatory mediators (TNFα, IFNγ, nitric oxide, and other neurotoxins) important in neurodegeneration. Further, endothelial cells in the brain avidly bind sCD40L 41,42, which could contribute to inflammation and leakage of sCD40L from the neurovascular unit into CSF 43-45. Finally, plasma and CSF levels of sCD40L have been correlated with NCI in patients with HIV and may remain elevated and are often unaffected by cART 40. However, we did not measure sCD40L in plasma. It is thus possible that sCD40L in the CSF originated in part from CD40 bearing inflammatory macrophages in abdominal adipose tissue 46,47 or platelet aggregation (a rich source of the ligand) elsewhere 48. Defining the source of sCD40L will be important in unraveling the mechanism linking obesity and NCI in HIV+ and possibly in HIV- populations.
Inclusion of sCD14 improved our path model, suggesting that it may contribute to neurocognitive impairment. sCD14 reflects systemic immune activation resulting from intestinal translocation of bacterial products including lipopolysaccharide (LPS). We were unable to correlate levels of LPS with sCD14 or GDS, but that may be due to widely acknowledged technical difficulties with the LPS assay. Regardless, adipocytes and microglia have toll-like receptors and in conjunction with CD14 avidly bind the TLR-4 ligand LPS, which leads to their activation and outpouring of pro-inflammatory mediators leading to adipose tissue inflammation 28,29 and neuro- inflammation 49-52. Moreover, elevated plasma sCD14 has been associated with HIV dementia 53. Our model is consistent with the postulate that intestinal translocation of microbial products promotes inflammation and immune activation and may be important in the pathogenesis of neurocognitive impairment.
When abdominal obesity was analyzed by sex-specific waist circumference criteria that define obesity for the metabolic syndrome (MetS) 35, the association of waist circumference on cognitive function was greater in men than women. Specifically, abdominally obese men (waist circumference ≥102cm) had double the GDS and 50% more of them met criteria for NCI than abdominally obese women (waist circumference ≥88). However, the number of women was relatively small and the prevalence of abdominal obesity was very high in women (70%), raising concern about the validity of these gender differences.
Components of the MetS (systolic blood pressure, HDL-C, fasting triglycerides and insulin resistance) and low adiponectin were significantly worse in obese than non-obese men. Thus, in obese HIV+ patients, components of MetS could increase risks for NCI 54 as demonstrated in non-infected populations 15-18,55. They may also mediate the effects of waist circumference on NCI, despite the fact that their individual inclusion in the path analysis did not improve the goodness of fit of the data to the model.
This study had several limitations. First, its design was primarily cross-sectional except for the measurement of change in cognitive impairment (GDS) over time. Thus, we can only speculate that there might be a causal relationship of obesity, inflammation, and immune activation to NCI, but the observed correlations support our a priori hypotheses. Based on our entry criteria of HIV viral load of <1000 copies/ml, availability of waist circumference, and stored samples of both plasma and CSF, too few subjects satisfied all of the requirements over time to evaluate whether our observed immunologic correlations with NCI were truly causative. Second, without abdominal adipose tissue biopsies, we cannot be certain that systemic IL-6 levels were primarily from inflamed abdominal fat. Other unidentified sources of inflammation (e.g. toxins, subclinical infections, or from mediators emanating from gut associated lymphoid tissue) may have contributed to elevations in plasma IL-6. These other sources of inflammation should be evaluated in larger, prospective, longitudinal studies to assess their contribution and relationship to NCI. Third, we did not measure sCD40L in plasma and thus its origin in CSF is uncertain. Fourth, the lack of relationship of GDS to other CSF inflammatory biomarkers was surprising but consistent with differences reported in prior studies 56. We suspect that this variability may relate to differences in study populations, concurrent cardiometabolic variables, neurocognitive test instruments, or laboratory methods (multiplex versus single platform assays).
Currently, cART is the only established treatment for HAND and appears to leave many patients with persisting NCI. Our study suggests several possible therapeutic strategies that should be evaluated to prevent or treat HAND, namely by reducing abdominal obesity and/or systemic and CNS inflammation/immune activation. For example, life style interventions of diet and exercise can reduce obesity. If those are unsuccessful, tesamorelin, a growth hormone releasing hormone (GHRH) analogue that selectively reduces abdominal VAT, has improved neurocognition in HIV-uninfected participants 57. Another option includes anti-inflammatory drugs that are being studied in HIV+ patients and could be evaluated for their effects on NC performance. Finally, because of the association of insulin resistance with NCI in obese HIV+ patients as suggested by our data and other studies 54,58-60, insulin-sensitizing therapies offer another option to improve cognition. Thus, in HIV+ patients with abdominal obesity, a number of strategies for treatment and prevention of NCI could be evaluated along with studies to define the underlying mechanisms of their effects.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the study participants who volunteered for CHARTER and contributions of the research staff and other faculty including Drs. Ann Collier (University Washington), Benjamin Gelman (UTMB Galveston), Justin McArthur (Johns Hopkins University, David Simpson (Johns Hopkins University)
Study Design: The CHARTER Steering Committee and manuscript authors contributed to the study design.
Authors: FRS, JAM, and JH were the primary authors; all authors listed in the Masthead contributed to data collection, and reviewed and contributed to the writing of the Abstract presented at CROI (2014) and penultimate version of the manuscript.
Study Assays: Assays were conducted in the laboratories of SL at UCSD and USC Diabetes and Obesity Research Institute Metabolic Core.
Data Management and Analyses: CS was responsible for collection and collation of clinical and neurocognitive data from the CHARTER data base and JH conducted the statistical analyses.
Grant Support: Grant support was provided by N01 MH22005, HHSN271201000036C and R01 AG18169.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare
Meeting Presentation: Data from this study was presented in part at Conference on Retroviruses and Opportunistic Infection, Boston MA, March 3-6, 2014.
References
- 1.Heaton RK, Clifford DB, Franklin DR, Jr., et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010 Dec 7;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tozzi V, Balestra P, Bellagamba R, et al. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr. 2007 Jun 1;45(2):174–182. doi: 10.1097/QAI.0b013e318042e1ee. [DOI] [PubMed] [Google Scholar]
- 3.McCombe JA, Vivithanaporn P, Gill MJ, Power C. Predictors of symptomatic HIV-associated neurocognitive disorders in universal health care. HIV Med. 2013 Feb;14(2):99–107. doi: 10.1111/j.1468-1293.2012.01043.x. [DOI] [PubMed] [Google Scholar]
- 4.Grant I, Franklin DR, Jr., Deutsch R, et al. Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology. 2014 Jun 10;82(23):2055–2062. doi: 10.1212/WNL.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valcour V, Sithinamsuwan P, Letendre S, Ances B. Pathogenesis of HIV in the central nervous system. Curr HIV/AIDS Rep. 2011 Mar;8(1):54–61. doi: 10.1007/s11904-010-0070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCutchan JA, Marquie-Beck JA, Fitzsimons CA, et al. Role of obesity, metabolic variables, and diabetes in HIV-associated neurocognitive disorder. Neurology. 2012 Feb 14;78(7):485–492. doi: 10.1212/WNL.0b013e3182478d64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yaffe K, Haan M, Blackwell T, Cherkasova E, Whitmer RA, West N. Metabolic syndrome and cognitive decline in elderly Latinos: findings from the Sacramento Area Latino Study of Aging study. J Am Geriatr Soc. 2007 May;55(5):758–762. doi: 10.1111/j.1532-5415.2007.01139.x. [DOI] [PubMed] [Google Scholar]
- 8.Panza F, Solfrizzi V, Logroscino G, et al. Current epidemiological approaches to the metabolic-cognitive syndrome. J Alzheimers Dis. 2012;30(Suppl 2):S31–75. doi: 10.3233/JAD-2012-111496. [DOI] [PubMed] [Google Scholar]
- 9.Solfrizzi V, Scafato E, Capurso C, et al. Metabolic syndrome, mild cognitive impairment, and progression to dementia. The Italian Longitudinal Study on Aging. Neurobiol Aging. 2011 Nov;32(11):1932–1941. doi: 10.1016/j.neurobiolaging.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. Jama. 2012 Feb 1;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 11.Selvin E, Parrinello CM, Sacks DB, Coresh J. Trends in prevalence and control of diabetes in the United States, 1988-1994 and 1999-2010. Ann Intern Med. 2014 Apr 15;160(8):517–525. doi: 10.7326/M13-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sattler FR, Qian D, Louie S, et al. Elevated blood pressure in subjects with lipodystrophy. Aids. 2001 Oct 19;15(15):2001–2010. doi: 10.1097/00002030-200110190-00013. [DOI] [PubMed] [Google Scholar]
- 13.Sattler F. Body habitus changes related to lipodystrophy. Clin Infect Dis. 2003 Apr 1;36(Suppl 2):S84–90. doi: 10.1086/367563. [DOI] [PubMed] [Google Scholar]
- 14.Samaras K, Wand H, Law M, Emery S, Cooper D, Carr A. Prevalence of metabolic syndrome in HIV-infected patients receiving highly active antiretroviral therapy using International Diabetes Foundation and Adult Treatment Panel III criteria: associations with insulin resistance, disturbed body fat compartmentalization, elevated C-reactive protein, and [corrected] hypoadiponectinemia. Diabetes Care. 2007 Jan;30(1):113–119. doi: 10.2337/dc06-1075. [DOI] [PubMed] [Google Scholar]
- 15.Yaffe K, Kanaya A, Lindquist K, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. Jama. 2004 Nov 10;292(18):2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- 16.Yaffe K. Metabolic syndrome and cognitive disorders: is the sum greater than its parts? Alzheimer Dis Assoc Disord. 2007 Apr-Jun;21(2):167–171. doi: 10.1097/WAD.0b013e318065bfd6. [DOI] [PubMed] [Google Scholar]
- 17.Dik MG, Jonker C, Comijs HC, et al. Contribution of metabolic syndrome components to cognition in older individuals. Diabetes Care. 2007 Oct;30(10):2655–2660. doi: 10.2337/dc06-1190. [DOI] [PubMed] [Google Scholar]
- 18.Roberts RO, Geda YE, Knopman DS, et al. Metabolic syndrome, inflammation, and nonamnestic mild cognitive impairment in older persons: a population-based study. Alzheimer Dis Assoc Disord. 2010 Jan-Mar;24(1):11–18. doi: 10.1097/WAD.0b013e3181a4485c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998 Mar;83(3):847–850. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 20.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011 Jun;121(6):2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006 Jul;116(7):1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Apovian CM, Bigornia S, Mott M, et al. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arterioscler Thromb Vasc Biol. 2008 Sep;28(9):1654–1659. doi: 10.1161/ATVBAHA.108.170316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zanni MV, Burdo TH, Makimura H, Williams KC, Grinspoon SK. Relationship between monocyte/macrophage activation marker soluble CD163 and insulin resistance in obese and normal-weight subjects. Clin Endocrinol (Oxf) 2012 Sep;77(3):385–390. doi: 10.1111/j.1365-2265.2011.04284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subramanian S, Tawakol A, Burdo TH, et al. Arterial inflammation in patients with HIV. Jama. 2012 Jul 25;308(4):379–386. doi: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006 Dec;12(12):1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 26.Deeks SG, Tracy R, Douek DC. Systemic Effects of Inflammation on Health during Chronic HIV Infection. Immunity. 2013 Oct 17;39(4):633–645. doi: 10.1016/j.immuni.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nockher WA, Bergmann L, Scherberich JE. Increased soluble CD14 serum levels and altered CD14 expression of peripheral blood monocytes in HIV-infected patients. Clin Exp Immunol. 1994 Dec;98(3):369–374. doi: 10.1111/j.1365-2249.1994.tb05499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Creely SJ, McTernan PG, Kusminski CM, et al. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab. 2007 Mar;292(3):E740–747. doi: 10.1152/ajpendo.00302.2006. [DOI] [PubMed] [Google Scholar]
- 29.Roncon-Albuquerque R, Jr., Moreira-Rodrigues M, Faria B, et al. Attenuation of the cardiovascular and metabolic complications of obesity in CD14 knockout mice. Life Sci. 2008 Sep 26;83(13-14):502–510. doi: 10.1016/j.lfs.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 30.Tenorio AR, Zheng Y, Bosch RJ, et al. Soluble Markers of Inflammation and Coagulation but Not T-Cell Activation Predict Non-AIDS-Defining Morbid Events During Suppressive Antiretroviral Treatment. J Infect Dis. 2014 May 1; doi: 10.1093/infdis/jiu254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blackstone K, Moore DJ, Franklin DR, et al. Defining neurocognitive impairment in HIV: deficit scores versus clinical ratings. Clin Neuropsychol. 2012;26(6):894–908. doi: 10.1080/13854046.2012.694479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carey CL, Woods SP, Gonzalez R, et al. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol. 2004 May;26(3):307–319. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- 33.Sattler F, Bhasin S, He J, et al. Testosterone threshold levels and lean tissue mass targets needed to enhance skeletal muscle strength and function: the HORMA trial. J Gerontol A Biol Sci Med Sci. 2011 Jan;66(1):122–129. doi: 10.1093/gerona/glq183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blodget E, Shen C, Aldrovandi G, et al. Relationship between microbial translocation and endothelial function in HIV infected patients. PLoS One. 2012;7(8):e42624. doi: 10.1371/journal.pone.0042624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005 Oct 25;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 36.Wright S. Correlation and causation. J Agricultural Res. 1921;20:557–585. [Google Scholar]
- 37.Bentler PM, Bonnett DG. Significance tests of goodness-of-fit in the analysis of covariance structures. Psychological Bulletin. 1980;88:586–606. [Google Scholar]
- 38.Byrd DA, Fellows RP, Morgello S, et al. Neurocognitive impact of substance use in HIV infection. J Acquir Immune Defic Syndr. 2011 Oct 1;58(2):154–162. doi: 10.1097/QAI.0b013e318229ba41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salemi J, Obregon DF, Cobb A, et al. Flipping the switches: CD40 and CD45 modulation of microglial activation states in HIV associated dementia (HAD). Mol Neurodegener. 2011;6(1):3. doi: 10.1186/1750-1326-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sui Z, Sniderhan LF, Schifitto G, et al. Functional synergy between CD40 ligand and HIV-1 Tat contributes to inflammation: implications in HIV type 1 dementia. J Immunol. 2007 Mar 1;178(5):3226–3236. doi: 10.4049/jimmunol.178.5.3226. [DOI] [PubMed] [Google Scholar]
- 41.Omari KM, Dorovini-Zis K. CD40 expressed by human brain endothelial cells regulates CD4+ T cell adhesion to endothelium. J Neuroimmunol. 2003 Jan;134(1-2):166–178. doi: 10.1016/s0165-5728(02)00423-x. [DOI] [PubMed] [Google Scholar]
- 42.Omari KM, Chui R, Dorovini-Zis K. Induction of beta-chemokine secretion by human brain microvessel endothelial cells via CD40/CD40L interactions. J Neuroimmunol. 2004 Jan;146(1-2):203–208. doi: 10.1016/j.jneuroim.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 43.Ramirez SH, Fan S, Dykstra H, et al. Dyad of CD40/CD40 ligand fosters neuroinflammation at the blood-brain barrier and is regulated via JNK signaling: implications for HIV-1 encephalitis. J Neurosci. 2010 Jul 14;30(28):9454–9464. doi: 10.1523/JNEUROSCI.5796-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Avison MJ, Nath A, Greene-Avison R, et al. Inflammatory changes and breakdown of microvascular integrity in early human immunodeficiency virus dementia. J Neurovirol. 2004 Aug;10(4):223–232. doi: 10.1080/13550280490463532. [DOI] [PubMed] [Google Scholar]
- 45.Sitati E, McCandless EE, Klein RS, Diamond MS. CD40-CD40 ligand interactions promote trafficking of CD8+ T cells into the brain and protection against West Nile virus encephalitis. J Virol. 2007 Sep;81(18):9801–9811. doi: 10.1128/JVI.00941-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poggi M, Engel D, Christ A, et al. CD40L deficiency ameliorates adipose tissue inflammation and metabolic manifestations of obesity in mice. Arterioscler Thromb Vasc Biol. 2011 Oct;31(10):2251–2260. doi: 10.1161/ATVBAHA.111.231357. [DOI] [PubMed] [Google Scholar]
- 47.Goldfine AB, Silver R, Aldhahi W, et al. Use of salsalate to target inflammation in the treatment of insulin resistance and type 2 diabetes. Clin Transl Sci. 2008 May;1(1):36–43. doi: 10.1111/j.1752-8062.2008.00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishikawa M, Vowinkel T, Stokes KY, et al. CD40/CD40 ligand signaling in mouse cerebral microvasculature after focal ischemia/reperfusion. Circulation. 2005 Apr 5;111(13):1690–1696. doi: 10.1161/01.CIR.0000160349.42665.0C. [DOI] [PubMed] [Google Scholar]
- 49.Lehnardt S, Massillon L, Follett P, et al. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci U S A. 2003 Jul 8;100(14):8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004 Sep 15;173(6):3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- 51.Chakravarty S, Herkenham M. Toll-like receptor 4 on nonhematopoietic cells sustains CNS inflammation during endotoxemia, independent of systemic cytokines. J Neurosci. 2005 Feb 16;25(7):1788–1796. doi: 10.1523/JNEUROSCI.4268-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suh HS, Zhao ML, Choi N, Belbin TJ, Brosnan CF, Lee SC. TLR3 and TLR4 are innate antiviral immune receptors in human microglia: role of IRF3 in modulating antiviral and inflammatory response in the CNS. Virology. 2009 Sep 30;392(2):246–259. doi: 10.1016/j.virol.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ancuta P, Kamat A, Kunstman KJ, et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One. 2008;3(6):e2516. doi: 10.1371/journal.pone.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valcour VG, Sacktor NC, Paul RH, et al. Insulin resistance is associated with cognition among HIV-1-infected patients: the Hawaii Aging With HIV cohort. J Acquir Immune Defic Syndr. 2006 Dec 1;43(4):405–410. doi: 10.1097/01.qai.0000243119.67529.f5. [DOI] [PubMed] [Google Scholar]
- 55.Panza F, Frisardi V, Seripa D, et al. Metabolic syndrome, mild cognitive impairment, and dementia. Curr Alzheimer Res. 2011 Aug;8(5):492–509. doi: 10.2174/156720511796391818. [DOI] [PubMed] [Google Scholar]
- 56.Cassol E, Misra V, Morgello S, Gabuzda D. Applications and limitations of inflammatory biomarkers for studies on neurocognitive impairment in HIV infection. J Neuroimmune Pharmacol. 2013 Dec;8(5):1087–1097. doi: 10.1007/s11481-013-9512-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baker LD, Barsness SM, Borson S, et al. Effects of Growth Hormone-Releasing Hormone on Cognitive Function in Adults With Mild Cognitive Impairment and Healthy Older Adults: Results of a Controlled Trial. Arch Neurol. 2012 Aug 6;:1–10. doi: 10.1001/archneurol.2012.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Craft S, Watson GS. Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol. 2004 Mar;3(3):169–178. doi: 10.1016/S1474-4422(04)00681-7. [DOI] [PubMed] [Google Scholar]
- 59.Zhao WQ, Alkon DL. Role of insulin and insulin receptor in learning and memory. Mol Cell Endocrinol. 2001 May 25;177(1-2):125–134. doi: 10.1016/s0303-7207(01)00455-5. [DOI] [PubMed] [Google Scholar]
- 60.Luchsinger JA, Tang MX, Shea S, Mayeux R. Hyperinsulinemia and risk of Alzheimer disease. Neurology. 2004 Oct 12;63(7):1187–1192. doi: 10.1212/01.wnl.0000140292.04932.87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.