Abstract
Background
Mechanisms of acinar cell death in pancreatitis are poorly understood. Cytochrome c release is a central event in apoptosis in pancreatitis. Here, we assessed the regulation of pancreatic cytochrome c release by Ca2+, mitochondrial membrane potential (ΔΨm), and reactive oxygen species (ROS), the signals involved in acute pancreatitis. We used both isolated rat pancreatic mitochondria and intact acinar cells hyper-stimulated with cholecystokinin-8 (CCK-8; in vitro model of acute pancreatitis).
Results
Micromolar amounts of Ca2+ depolarised isolated pancreatic mitochondria through a mechanism different from the “classical” (ie, liver) mitochondrial permeability transition pore (mPTP). In contrast with liver, Ca2+-induced mPTP opening caused a dramatic decrease in ROS and was not associated with pancreatic mitochondria swelling. Importantly, we found that Ca2+-induced depolarisation inhibited cytochrome c release from pancreatic mitochondria, due to blockade of ROS production. As a result, Ca2+ exerted two opposite effects on cytochrome c release: Ca2+ per se stimulated the release, whereas Ca2+-induced depolarisation inhibited it. This dual effect caused a non-monotonous dose-dependence of cytochrome c release on Ca2+. In intact acinar cells, cytochrome c release, caspase activation and apoptosis were all stimulated by ROS and Ca2+, and inhibited by depolarisation, corroborating the findings on isolated pancreatic mitochondria.
Conclusions
These data implicate ROS as a key mediator of CCK-induced apoptotic responses. The results indicate a major role for mitochondria in the effects of Ca2+ and ROS on acinar cell death. They suggest that the extent of apoptosis in pancreatitis is regulated by the interplay between ROS, ΔΨm and Ca2+. Stabilising mitochondria against loss of ΔΨm may represent a strategy to mitigate the severity of pancreatitis.
Although acinar cell death is a key pathological response of pancreatitis, its mechanism remains poorly understood.1–8 In models of acute pancreatitis, acinar cells have been shown to die through both necrosis and apoptosis.1,3,4,7–9 The severity of experimental pancreatitis correlates directly with the extent of necrosis and, inversely, with apoptosis; furthermore, stimulating apoptosis decreases necrosis and the disease severity.1,3–5,7,8,10,11 Thus, shifting the pattern of death responses of pancreatitis towards apoptosis and away from necrosis could be of therapeutic value.1,8
Although morphological characteristics of apoptosis and necrosis are different, they are both mediated by mitochondrial membrane permeablisation,12–17 which causes release into the cytosol of cytochrome c, leading to activation of caspases, the proteases that execute apoptosis. On the other hand, mitochondrial permeabilisation causes a loss of mitochondrial membrane potential (ΔΨm), ultimately leading to depletion of intracellular ATP and necrosis. The balance between apoptosis and necrosis depends on the extent of cytochrome c release versus mitochondrial depolarisation. However, the factors that regulate cytochrome c release and dissipation of ΔΨm are not completely understood in general, and have not been investigated in pancreatitis.
The best-known mechanism of permeabilisation is through opening of the mitochondrial permeability transition pore (mPTP), a multiprotein complex involving cyclophilin D and adenine nucleotide translocase (ANT).16–20 “Classical” (eg, liver) mPTP opening is associated with swelling of the mitochondrial matrix and consequent rupture of the outer mitochondrial membrane (OMM),16,18–20 which allows the release of cytochrome c. Recent data on mice lacking cyclophilin D21,22 show, however, that cytochrome c can be released independently of mPTP, through OMM channel(s) comprised of the pro-apoptotic Bcl-2 proteins Bax and Bak.15,17,23,24
We have recently shown that both cytochrome c release and mitochondrial depolarisation occur and mediate cell death in pancreatitis.8,9 On the other hand, an abnormal, sustained increase in cytosolic Ca2+ ([Ca2+]i) and oxidative stress are key pathological signals associated with acute pancreatitis.25–28 However, very little is known of the regulation of death responses in pancreatitis by Ca2+ or reactive oxygen species (ROS). Moreover, pancreatic mitochondria are poorly characterised as compared with other organs.29–31
Here, we investigated the roles of Ca2+, ΔΨm and ROS in the regulation of cytochrome c release and apoptosis in both isolated pancreatic mitochondria and acinar cells. We found that properties of pancreatic mitochondria are different from liver mitochondria. In particular, in contrast to “classical” mPTP in liver mitochondria, Ca2+-induced mPTP opening in pancreatic mitochondria causes inhibition of ROS production. This, in turn, blocks cytochrome c release. Similarly, cytochrome c release in intact acinar cells (as well as caspase activation and apoptosis) is stimulated by Ca2+ and ROS, and inhibited by mitochondrial depolarisation. The results indicate that death responses of pancreatitis are regulated at the mitochondrial level by the interplay between Ca2+, ΔΨm and ROS.
MATERIALS AND METHODS
The experimental procedures are described in detail in the supplementary material.
Isolation of mitochondria
Mitochondria from rat pancreas and liver were isolated using the same procedure (modified from Wilson et al29,30 and Schild et al31), in a medium containing 250 mmol/l sucrose, 10 mmol/l Tris–HCl (pH 7.4), 0.5% bovine serum albumin (BSA), 0.25 µg/ml soybean trypsin inhibitor, and 1 mmol/l ethylene glycol tetraacetic acid (EGTA). For functional assays, the mitochondria were re-suspended in either medium A (250 mmol/l sucrose, 22 mmol/l KCl, 22 mmol/l triethanolamine (pH 7.4), 3 mmol/l MgCl2, 5 mmol/l KH2PO4, and 0.5% BSA); or medium B based on Ca2+/EGTA buffers (Molecular Probes, Eugene, Oregon, USA), which was used to maintain the free Ca2+ concentration, as indicated, from nominal “zero” to 39 µmol/l. The buffers contain varying ratios of 10 mmol/l K2EGTA and 10 mmol/l CaEGTA; 100 mmol/l KCl; and 30 mmol/l 3-morpholinopropanesulfonic acid (MOPS; pH 7.2). To the Ca2+/EGTA buffers we added 5 mmol/l KH2PO4 and 3 mmol/l MgCl2; and we determined (by using Fura-6F) that these additions did not change the free Ca2+ concentration. The medium also contained 10 mmol/l succinate (in most experiments) or 10 mmol/l glutamate plus 2.5 mmol/l malate as respiratory substrates. The osmolarity of medium B was between 319 mosm (at “zero” Ca2+) and 309 mosm (at 39 µmol/l free Ca2+). The measurements on isolated mitochondria were carried out at 22°C.
Isolation of acinar cells
Rat pancreatic acinar cells were isolated using a standard collagenase digestion procedure,8,9,32 and then incubated in medium 199 containing 0.1 µg/ml soybean trypsin inhibitor.
Mitochondrial respiration was measured using a Clark-type electrode. The value of the respiratory control ratio was >3 in all mitochondria preparations, and it did not change during the incubation of mitochondria at “zero” Ca2+ for 20 min of observation.
ΔΨm in mitochondria suspension was measured using a tetraphenyl phosphonium ion (TPP+)-sensitive electrode33 in the presence of 2µmol/l TPP+. An increase in ΔΨm causes TPP+ uptake by mitochondria and, correspondingly, a decrease of TPP+ in the medium. Alternatively, we used the ΔΨm-sensitive fluorescent probe tetramethylrhodamine methyl ester (TMRM; 0.5 µmol/l),34 either in a spectrofluorimeter cuvette (Shimadzu RF-1501; Shimadzu Scientific, Kyoto, Japan) or by using flow cytometry.
Intra-mitochondrial free Ca2+ was measured as described by Hotta et al35 in Fura-2-loaded mitochondria.
Mitochondrial swelling was assessed by measuring light scattering, using a Hitachi F4500 spectrofluorimeter (Hitachi High-Technologies, Tokyo, Japan), which reflects changes in the mitochondrial volume.36
ROS levels in the mitochondria suspension were measured using the Amplex Red (1µmol/l)/horseradish peroxidase (0.2 U/ml) fluorimetric method.37 ROS in pancreatic acinar cells were measured using either the rhodamine dye DHR123, which predominantly monitors mitochondrial ROS,38 or 2,7-dichlorofluorescein (DCF), which monitors both mitochondrial and non-mitochondrial ROS.39,40 Fluorescence images were obtained using a Leica TCS SP MP inverted confocal microscope (Leica Microsystems, Heidelberg, Germany).
Cytochrome c release was measured by western blot,8,9,32 caspase-3 activity was measured using a fluorogenic assay,8,9 and apoptosis in acinar cells was quantified using Hoechst 33258 staining.8,9
Statistical analysis of data
Statistical analysis was done by using the two-tailed Student t test. p Values <0.05 were considered statistically significant.
Reagents
Amplex Red, DHR123, DCF-DA, Fura-2/AM, Fura-6F, MitoTracker Red (CMXRos), TMRM, 1,2-bis(o-aminophenoxy) ethoxy-ethane-N′-tetraacetic acid (BAPTA-AM), thapsigargin, and Ca2+/EGTA buffer kits were from Molecular Probes. Bongkrekic acid, alamethicin, ruthenium red and Ru 360 were from Calbiochem (San Diego, California, USA). The caspase-3 substrate N-acetyl-Asp-Glu-Val-Asp-AMC (where AMC is 7-amino-4-methylcourmarin) (Ac-DEVD-AMC) and cyclosporin A were from Biomol (Plymouth Meeting, Philadelphia, USA). Antibodies against cytochrome c and complex IV cytochrome c oxidase (COX IV) were from BD Biosciences (San Diego, California, USA) and Molecular Probes, respectively. Cholecystokin-8 (CCK-8) was from Research Plus (Manasquan, New Jersey USA). All other reagents were from Sigma Chemical (St Louis, Missouri, USA).
RESULTS
Effect of Ca2+ on the pancreatic mitochondria membrane potential
The information on the properties of pancreatic mitochondria is very limited.29–31 We determined that rat pancreatic mitochondria isolated in the presence of 1 mmol/l EGTA displayed stable ΔΨm, as measured with both the TPP+ electrode (Supplementary fig 1A) and the fluorescent dye TMRM (Supplementary fig 1C). Addition of more EGTA did not affect ΔΨm; while the mitochondrial uncoupler CCCP completely dissipated ΔΨm (Supplementary fig 1A). Measurements of oxygen consumption (Supplementary fig 1B) demonstrated coupled respiration of pancreatic mitochondria isolated in the presence of 1 mmol/l EGTA then incubated in medium A. ADP produced state 3 respiration in a standard manner (Supplementary fig 1B). Mitochondria also developed uncoupled respiration after the addition of CCCP, comparable with a state 3 rate. The value of respiratory control ratio measured in the presence of succinate was greater than 3 in all mitochondria preparations (Supplementary fig 1B).
By contrast, when isolated in the absence of EGTA, pancreatic mitochondria did not preserve membrane potential (Supplementary fig 1A). The mitochondria were able to restore ΔΨm after addition of EGTA (Supplementary fig 1A). In mitochondria isolated without EGTA respiration was inhibited; moreover, CCCP did not significantly stimulate oxygen consumption (Supplementary fig 1B). EGTA chelates both Ca2+ and Mg2+, as well as other divalent cations. Using different ion chelators (N, N, N′, N′-tetrakis (2-pyridylmethyl)ethylenediamine (TPEN), ethylenediaminetetraacetic acid (EDTA) and BAPTA) we showed that it is Ca2+ not Mg2+ (or other ions) that depolarises pancreatic mitochondria (data not shown).
We also measured (Supplementary fig 1C) that addition of Na+ into medium A (or substituting K+ for Na+) affected neither the basal ΔΨm nor Ca2+-induced depolarisation. These results provide evidence against a contribution of an Na+/Ca2+ exchanger, in accord with the fact that its activity is limited in mitochondria from non-excitable tissues.41
For comparison, we demonstrated that complete absence of Ca2+ is not as critical for the maintenance of the functional state of liver mitochondria, the most studied and best characterised type of mitochondria. The respiration rate and ΔΨm of isolated rat liver mitochondria were the same independent of whether they were isolated with or without EGTA (Supplementary fig 1D). These data show that pancreatic mitochondria are more sensitive to Ca2+-induced damage than liver mitochondria.
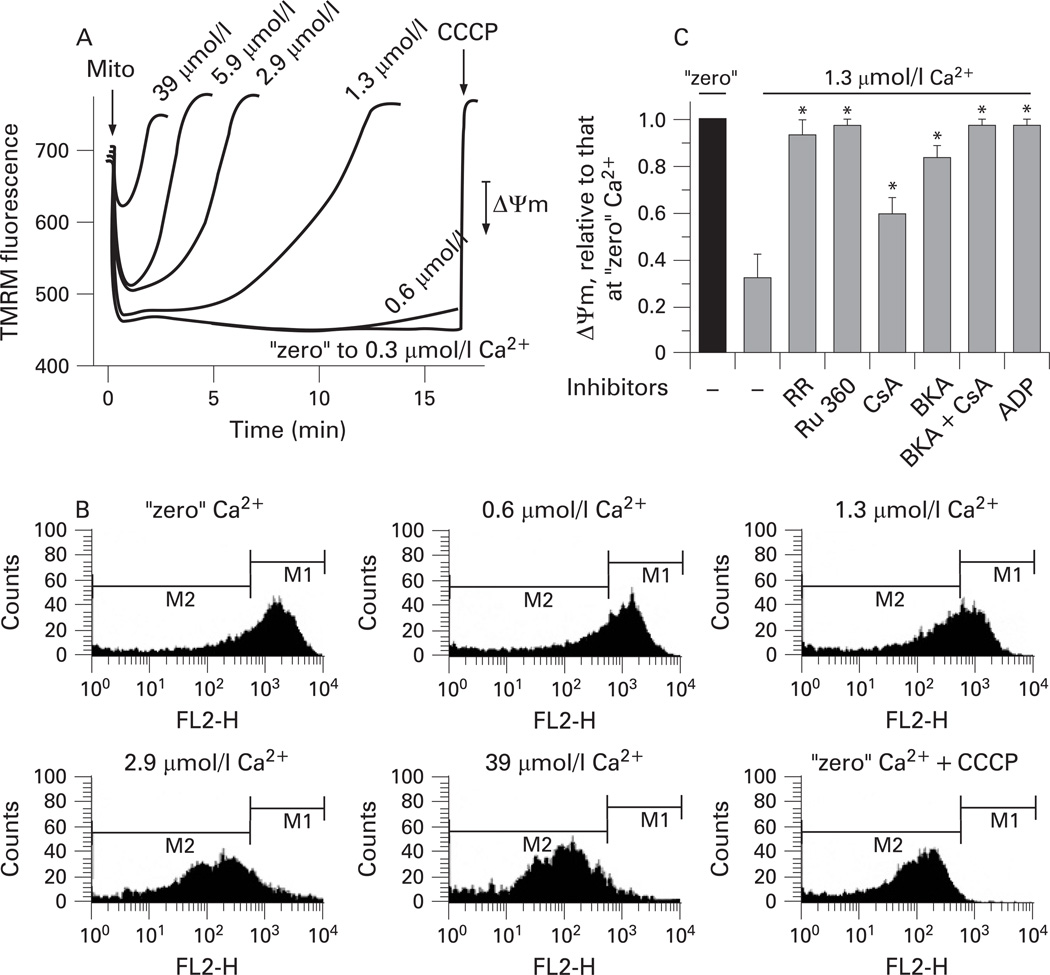
In subsequent experiments designed to characterise the regulation of ΔΨm by Ca2+, pancreatic mitochondria were isolated in the presence of 1 mmol/l EGTA and then incubated in medium B in which the concentration of free Ca2+ was clamped using Ca2+/EGTA buffers. Ca2+ in the range from “zero” to 39 µmol/l caused progressive dose-dependent depolarisation of pancreatic mitochondria (fig 1). The extent of depolarisation increased with Ca2+ concentration and duration of mitochondrial exposure to Ca2+ (fig 1A). For example, 10 min exposure to 1.3 µmol/l Ca2+ significantly depolarised the mitochondria whereas ΔΨm did not decrease during 1 min incubation at this Ca2+ concentration (fig 1A).
Figure 1.
Ca2+ dose- and time-dependently depolarises pancreatic mitochondria. Rat pancreatic mitochondria were isolated in the presence of 1 mmol/l ethylene glycol tetraacetic acid (EGTA) and incubated for the times indicated (A) or for 10 min (B,C) at different concentrations of free Ca2+ maintained with Ca2+/EGTA buffers (medium B). In this and other figures presenting the results on isolated mitochondria, 10 mmol/l succinate was used as the respiratory substrate if not stated otherwise. The mitochondrial membrane potential (ΔΨm) was measured by tetramethylrhodamine methyl ester (TMRM) fluorescence in the spectrofluorimeter cuvette (A,C) or by flow cytometry (B). Mitochondria (mito) and carbonyl cyanide m-chlorophenylhydrazone (CCCP; 5 µmol/l) were added as indicated. In (C), ΔΨm was measured in the presence and absence of the Ca2+ uniporter inhibitors, ruthenium red (RR; 1.5 µmol/l) and ruthenium red 360 (Ru 360; 2 µmol/l); or the mitochondrial permeability transition pore (mPTP) inhibitors cyclosporin A (CsA; 2 µmol/l), bongkrekic acid (BKA; 5 µmol/l) and ADP (100 µmol/l). ΔΨm values are means with the standard error (n=3) normalised to that for mitochondria incubated at “zero” Ca2+ without inhibitors. *p<0.05 vs mitochondria incubated without inhibitors at 1.3 µmol/l Ca2+. FL2H, TMRM fluorescence intensity; M1 and M2 are gating regions.
Using flow cytometry, we showed (fig 1B) that our preparations of pancreatic mitochondria did not contain distinct subpopulations that drastically differ in ΔΨm sensitivity to Ca2+. With increasing Ca2+, the ΔΨm histogram progressively shifted to the left, indicating mitochondrial depolarisation (fig 1B).
It is of note that Ca2+-induced mitochondrial depolarisation was prevented by the Ca2+ uniporter inhibitors ruthenium red and Ru 360 (fig 1C), indicating that it is due to mitochondrial Ca2+ overload.
Using an inhibitory approach, we tested whether mPTP opening mediates Ca2+-induced depolarisation of pancreatic mitochondria. Mitochondria were incubated with various mPTP inhibitors for 10 min at 1.3 µmol/l free Ca2+ (fig 1C). The exposure of mitochondria to this Ca2+ concentration induced a time-dependent decrease in ΔΨm, which reached 70% by 10 min (cf. fig 1A). The mPTP inhibitors applied, namely the ANT inhibitor, bongkrekic acid; the cyclophilin D inhibitor, cyclosporin A; and a broad-spectrum inhibitor of mitochondrial ion transport, ADP, all significantly restored ΔΨm (fig 1C). The potencies of the inhibitors were, however, different from those characteristic for “classical” (ie, liver) mPTP.18–20,42 In particular, whereas bongkrekic acid and ADP fully restored ΔΨm, the classical mPTP inhibitor cyclosporin A restored ΔΨm by less than 50%, at both 2 µmol/l (fig 1C) and 5 µmol/l (data not shown).
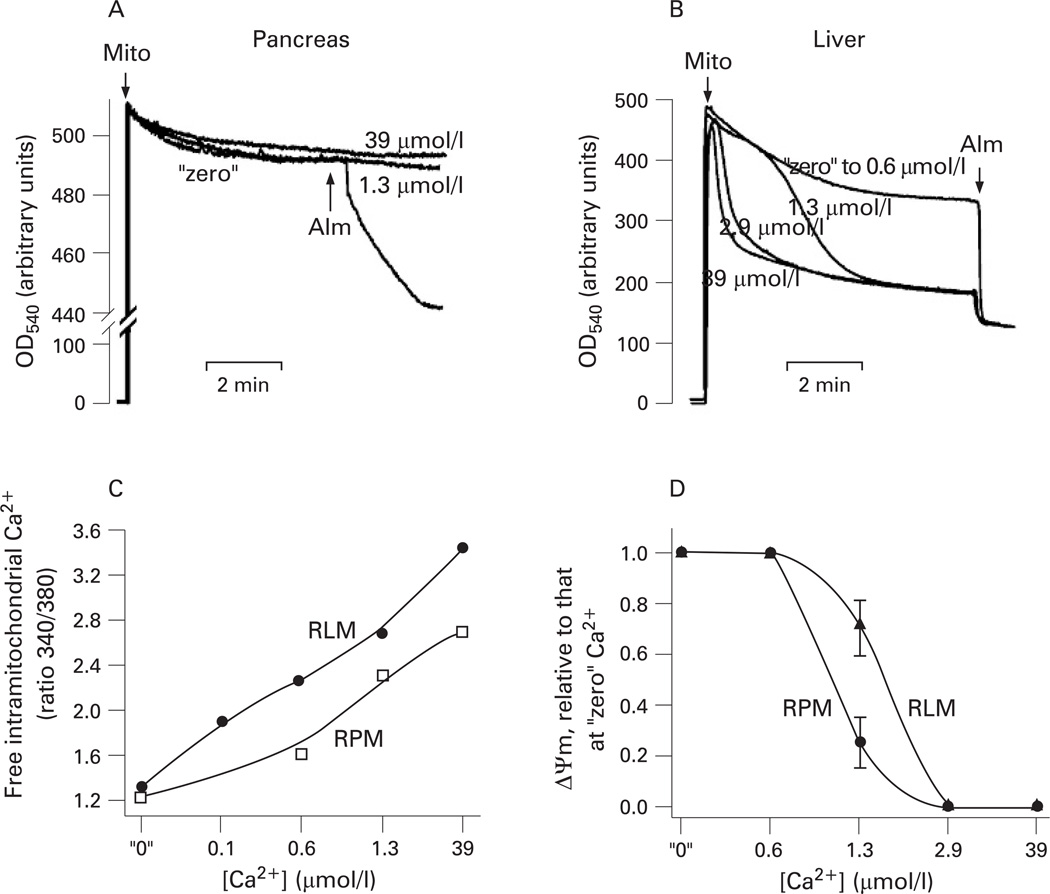
The mitochondrial depolarisation induced by mPTP opening (eg, in liver) is known to cause mitochondrial swelling, manifested by a decrease in light scattering. Mitochondrial swelling, in turn, leads to OMM rupture.17–20,42,43 Unexpectedly, we found that in pancreatic mitochondria Ca2+-induced dissipation of ΔΨm was not associated with a decrease in light scattering indicative of mitochondria swelling and OMM rupture (fig 2A). The pore-forming agent alamethicin (a positive control) caused a rapid decrease in light scattering (fig 2A), indicating swelling of pancreatic mitochondria. Alamethicin at the applied concentration completely depolarised pancreatic mitochondria (data not shown).
Figure 2.
Ca2+ induces swelling in liver but not pancreatic mitochondria. Mitochondria were isolated from rat pancreas and liver in the presence of 1 mmol/l ethylene glycol tetraacetic acid (EGTA) and incubated in medium B for the times indicated (A,B) or 10 min (C,D) at various concentrations of free Ca2+. (A,B) Changes in light scattering were measured at 540 nm in pancreatic (A) or liver (B) mitochondria (mito) suspension. The pore-forming agent alamethicin (Alm; 40 µmol/l) was added as indicated. (C) Changes in the intra-mitochondrial free Ca2+ were measured by the ratio of fluorescence intensities at 340 nm and 380 nm in rat pancreatic mitochondria (RPM) or rat liver mitochondria (RLM) loaded with Fura-2 and incubated at the indicated concentrations of external free Ca2+. The data are representative of two experiments with similar results. (D) The mitochondrial membrane potential (ΔΨm) was measured in RPM or RLM with a tetraphenyl phosphonium ion (TPP+) electrode, and its values normalised to those for mitochondria incubated at “zero” Ca2+. Values are means with the standard error from at least three different preparations of mitochondria.
We tested that our mitochondria preparations isolated from rat liver, indeed, underwent rapid Ca2+-induced swelling (at free Ca2+ concentrations >0.6 µmol/l) in the same experimental conditions (fig 2B). We further compared the intra-mitochondrial free Ca2+ levels in pancreatic and liver mitochondria (fig 2C). The results showed that in the whole range of external Ca2+ concentrations examined, the intra-mitochondrial free Ca2+ levels were ~3–4 times lower in pancreatic than in liver mitochondria. For example, at 0.6 µmol/l external Ca2+ the intra-mitochondrial free Ca2+ concentration was 2.8 µmol/l in pancreatic and 11.7 µmol/l in liver mitochondria. These data indicate that liver mitochondria retain more Ca2+ (ie, withstand higher [Ca2+]i) than pancreatic mitochondria. In accord with these data, we found that liver mitochondria were more resistant to Ca2+-induced loss of ΔΨm than pancreatic mitochondria (fig 2D).
The data in fig 2 indicate that the properties of mPTP operating in pancreatic mitochondria are different from “classical” (ie, liver) mPTP. To emphasise these differences, we termed the mPTP in pancreatic mitochondria “non-classical”.
Effect of Ca2+ on cytochrome c release from pancreatic mitochondria
Non-monotonous dependence of cytochrome c release on Ca2+ concentration
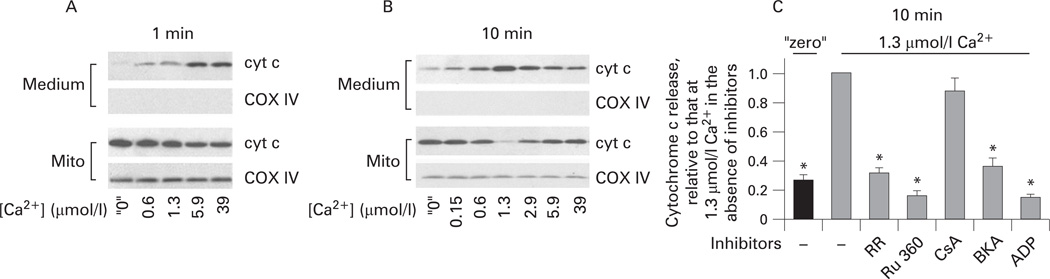
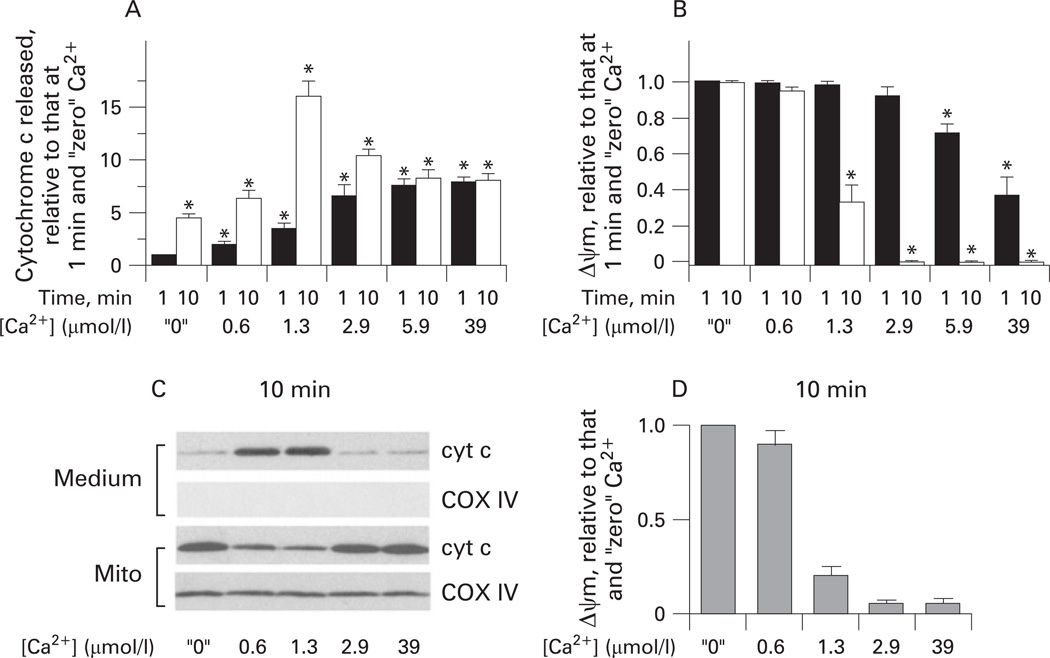
Figures 3–5 show the effect of Ca2+ on cytochrome c release from isolated pancreatic mitochondria into the incubation medium. These measurements were performed on the same mitochondrial preparations as those of ΔΨm (figs 1 and 2). At 1 min mitochondria exposure to Ca2+, cytochrome c release gradually increased with the increase in Ca2+ concentration from “zero” to 39 µmol/l (fig 3A). However, when cytochrome c release was measured after 10 min incubation, we found, surprisingly, a non-monotonous dependence of the extent of cytochrome c release on Ca2+ (fig 3B). That is, the amount of cytochrome c released into the medium was maximal at 1.3 µmol/l Ca2+ and decreased at greater Ca2+ concentrations (fig 3B).
Figure 3.
Dual effect of Ca2+ on cytochrome c (cyt c) release from pancreatic mitochondria. Rat pancreatic mitochondria were isolated in the presence of 1 mmol/l ethylene glycol tetraacetic acid (EGTA) and incubated for 1 min (A) or 10 min (B,C) in medium B at the indicated free Ca2+ concentrations. (A,B). Cytochrome c levels were measured by western blot analysis both in the incubation medium and the mitochondria pellet (mito). Blots were reprobed for the mitochondrial marker complex IV cytochrome C oxidase (COX IV) to assess the quality of mitochondrial separation and to confirm equal protein loading. (C) Cytochrome c release was measured in the presence and absence of the Ca2+ uniporter inhibitors, ruthenium red (RR; 1.5 µmol/l) and ruthenium red 360 (Ru 360; 2 µmol/l); or the mitochondrial permeability transition pore (mPTP) inhibitors cyclosporin A (CsA; 2 µmol/l), bongkrekic acid (BKA; 5 µmol/l) and ADP (100 µmol/l). The band intensity of cytochrome c released into the incubation medium was quantified by using densitometry and normalised to that at 1.3 µmol/l Ca2+ in the absence of the inhibitors. Values are means with the standard error (n=3). *p<0.05 vs mitochondria incubated at 1.3 µmol/l Ca2+ without inhibitors.
Figure 5.
Ca2+ exerts both mitochondrial membrane potential (ΔΨm)-dependent and -independent effects on cytochrome c (cyt c) release from pancreatic mitochondria. Rat pancreatic mitochondria were isolated in the presence of 1 mmol/l ethylene glycol tetraacetic acid (EGTA), and then incubated in medium B for 10 min at the indicated concentrations of free Ca2+, in the absence or presence of 1 µmol/l carbonyl cyanide m-chlorophenylhydrazone (CCCP). (A) Cytochrome c release was measured by western blot analysis. (B) The band intensity of cytochrome c released into the medium during 10 min incubation was quantified by using densitometry. Values are means with the standard error from at least three different preparations of mitochondria, normalised to those for mitochondria incubated at “zero” Ca2+ without CCCP. *p<0.05 vs mitochondria incubated without CCCP at the same concentration of Ca2+.
Importantly, cytochrome c release from pancreatic mitochondria appeared very sensitive to Ca2+. It was markedly stimulated by 1 min exposure to 0.6 µmol/l Ca2+, the concentration that is reached at the peak of the agonist-evoked [Ca2+]i signal;25,44 moreover, cytochrome c release was stimulated by 10 min exposure to as little as 0.15 µmol/l Ca2+ (fig 3A,B). It is of note that ruthenium red and Ru 360 (fig 3C) blocked Ca2+-induced cytochrome c release, indicating that it is due to mitochondrial Ca2+ overload.
The classical mPTP inhibitor cyclosporin A had no effect on cytochrome c release (fig 3C), in contrast with its 100% inhibitory effect on Ca2+-induced cytochrome c release from liver mitochondria.45 The ANT inhibitor bongrekic acid, as well as ADP, greatly inhibited cytochrome c release from pancreatic mitochondria (fig 3C).
Loss of ΔΨm inhibits Ca2+-induced cytochrome c release
We next examined the mechanisms underlying the inhibition of cytochrome c release that we observed (fig 3A,B) with prolonged (ie, 10 min) exposure of pancreatic mitochondria to Ca2+ concentrations >1.3 µmol/l. We noticed that the inhibition of cytochrome c release occurred under conditions (Ca2+ concentrations and incubation times) that resulted in dissipation of ΔΨm. Indeed, under conditions in which depolarisation was minimal, ie, at 1 min mitochondria exposure to Ca2+ (fig 1A), cytochrome c release monotonously increased with the increase in Ca2+ (fig 3A). It is of note that, at 39 µmol/l Ca2+, the amount of cytochrome c released during 1 min incubation was the same as at 5.9 µmol/l Ca2+ (fig 3A), which could be explained by the fact that mitochondria lost most of their ΔΨm within 1 min of exposure to 39 µmol/l Ca2+ (fig 1A).
Further evidence for the role of ΔΨm in the effects of Ca2+ on cytochrome c release came from the comparison of the extent of cytochrome c release and depolarisation in mitochondria incubated for 1 min versus 10 min (fig 4A,B). At low Ca2+ concentrations (up to 1.3 µmol/l), at which mitochondria retained all or some ΔΨm, the cytochrome c release significantly increased between 1 and 10 min of incubation (fig 4A,B). By contrast, with greater Ca2+ concentrations (ie, at 2.9, 5.9 and 39 µmol/l) essentially all cytochrome c release occurred during the first minute; that is, in conditions in which mitochondria retained at least some ΔΨm. In other words, there was no additional cytochrome c release between 1 and 10 min of incubation of mitochondria under conditions in which ΔΨm dissipated to a non-measurable level (fig 4A,B). These results indicate that the inhibition of cytochrome c release with greater Ca2+ concentrations is due to Ca2+-induced loss of ΔΨm. The results also suggest that Ca2+-induced cytochrome c release depends on ΔΨm in a threshold manner. Partial depolarisation (such as that observed at 10 min mitochondria exposure to 1.3 µmol/l Ca2+ or 1 min exposure to 2.9 and 5.9 µmol/l Ca2+) did not block cytochrome c release; but dissipation of ΔΨm (eg, at 10 min exposure to 2.9, 5.9 or 39 µmol/l Ca2+) abolished it (fig 4A,B).
Figure 4.
Ca2+-induced loss of mitochondrial membrane potential (ΔΨm) correlates with inhibition of cytochrome c (cyt c) release from pancreatic mitochondria. Rat pancreatic mitochondria were isolated in the presence of 1 mmol/l ethylene glycol tetraacetic acid (EGTA), and then incubated in medium B for 1 or 10 min at the concentrations of free Ca2+ indicated, in the presence of 10 mmol/l succinate (A,B) or 10 mmol/l glutamate plus 2.5 mmol/l malate (C,D) as the respiratory substrates. (A,C) Cytochrome c levels were measured by western blot analysis both in the incubation medium and the mitochondria pellet (mito). Blots were re-probed for the mitochondrial marker complex IV cytochrome C oxidase (COX IV) to assess the quality of mitochondria separation and to confirm equal protein loading. The band intensity of cytochrome c released into the medium during 1 min or 10 min incubation was quantified by using densitometry. (B,D) ΔΨm was measured in the same mitochondrial preparations by using a tetraphenyl phosphonium ion (TPP+) electrode. In (A,B), the values are means with the standard error from at least three different preparations of mitochondria, normalised to those for mitochondria incubated for 1 min at “zero” Ca2+. *p<0.05 vs mitochondria incubated for 1 min at “zero” Ca2+. In (D), the values are means with the ranges from two preparations of mitochondria, normalised to those for mitochondria incubated for 10 min at “zero” Ca2+.
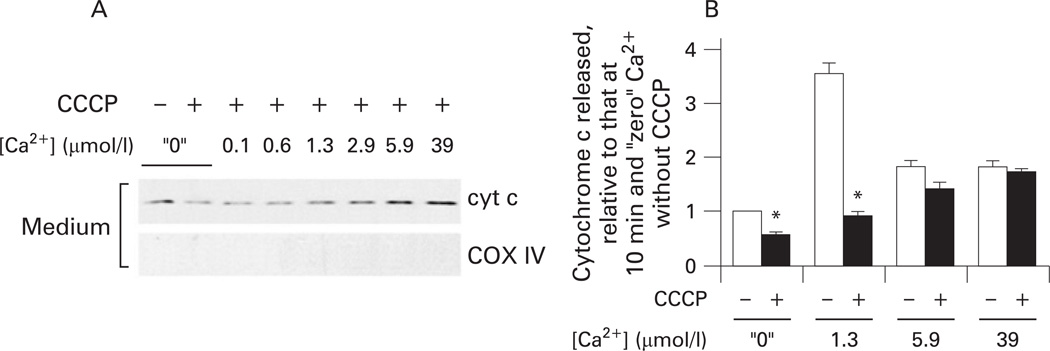
The data in figs 1–3 and 4A,B were obtained with succinate as the respiratory (complex II) substrate, as is customary in studies of the effects of Ca2+ on isolated mitochondria.45,46 Because, in intact cells, mitochondria mostly use complex I linked substrates, we also measured the effects of Ca2+ on ΔΨm and cytochrome c release using glutamate/malate as the respiratory substrate. In the presence of glutamate/malate, the effects of Ca2+ on cytochrome c release (fig 4C) and ΔΨm (fig 4D) in isolated pancreatic mitochondria were similar to those observed with succinate. In particular, with either substrate Ca2+ caused a gradual loss of ΔΨm but a non-monotonous release of cytochrome c.
To further prove that mitochondrial depolarisation inhibits the release of cytochrome c, we measured the dose dependence of the Ca2+ effect on cytochrome c release in the presence of CCCP; that is, under conditions where ΔΨm was completely dissipated (fig 5). In the presence of CCCP we observed a monotonous increase in the release of cytochrome c with Ca2+ (fig 5A). Comparison of the extent of cytochrome c release in the presence and absence of CCCP at the same Ca2+ concentration (fig 5B) showed that CCCP markedly decreased the extent of cytochrome c release from mitochondria exposed to low Ca2+ (≤ 1.3 µmol/l); ie, from mitochondria which retained all or some ΔΨm; but there was no effect of CCCP on the release of cytochrome c from mitochondria completely depolarised by Ca2+; eg, at 5.9 or 39 µmol/l Ca2+ (fig 5B).
The results in figs 3–5 indicate that Ca2+ has two opposite effects on the release of cytochrome c from pancreatic mitochondria: Ca2+ per se stimulates the release of cytochrome c, but depolarisation induced by Ca2+ inhibits it. As a result, such a dual effect causes the observed non-monotonous dependence of cytochrome c release on Ca2+.
ROS mediate cytochrome c release from pancreatic mitochondria
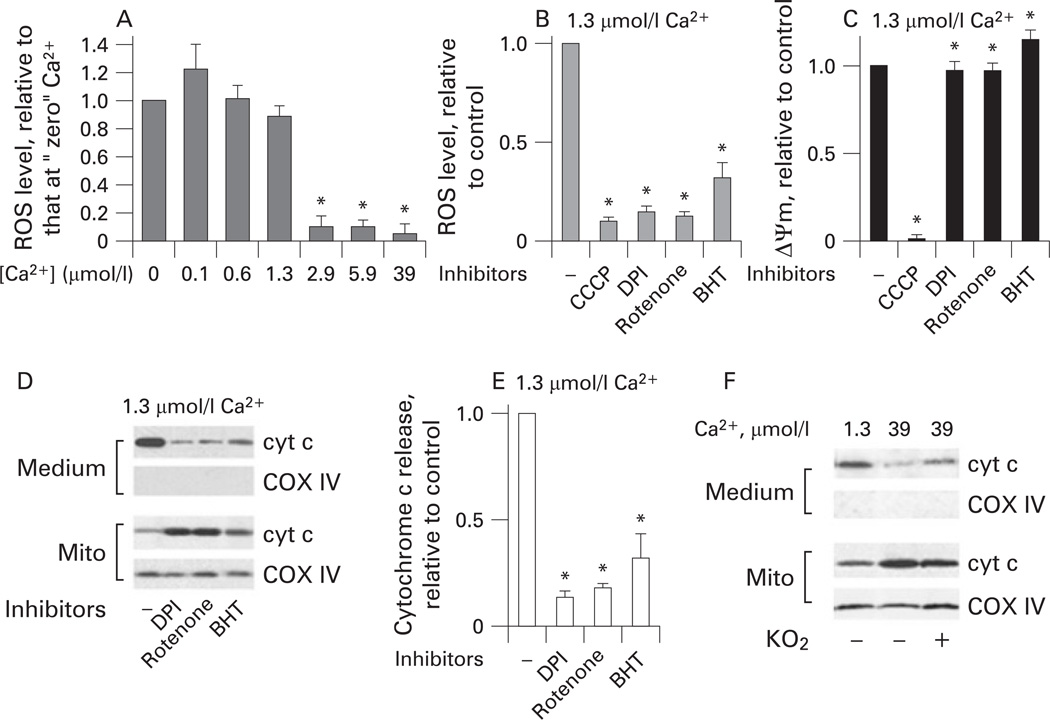
We hypothesised that the inhibitory effect of depolarisation on Ca2+-induced cytochrome c release could be due to inhibition of ROS production. Using the ROS-sensitive fluorescent dye Amplex Red, we measured the effect of Ca2+ on ROS levels in isolated pancreatic mitochondria. At low concentrations (≤ 1.3 µmol/l) Ca2+ had little effect on mitochondrial ROS, whereas greater Ca2+ concentrations completely blocked ROS production (fig 6A). Importantly, the blockade of ROS production occurred at Ca2+ concentrations that dissipated ΔΨm (figs 1A and 6A).
Figure 6.
Inhibition of mitochondrial reactive oxygen species (ROS) prevents Ca2+-induced cytochrome c (cyt c) release from pancreatic mitochondria. Rat pancreatic mitochondria were isolated in the presence of 1 mmol/l ethylene glycol tetraacetic acid (EGTA), and then incubated in medium B for 10 min at the indicated concentrations of free Ca2+, in the absence or presence of the inhibitors of mitochondrial ROS production: the mitochondrial uncoupler carbonyl cyanide m-chlorophenylhydrazone (CCCP; 5 µmol/l); the complex I inhibitors diphenyliodonium (DPI) (15 µmol/l) and rotenone (2 µmol/l); or the lipid peroxidation inhibitor butylated hydroxytoluene (BHT; 10 µmol/l). (A,B). Changes in ROS levels in the mitochondria suspension were measured with Amplex Red. (C) Mitochondrial membrane potential (ΔΨm) was measured in the same mitochondria preparations with a tetraphenyl phosphonium ion (TPP+) electrode. (D–F) Cytochrome c levels in the incubation medium and the mitochondria (mito) were measured by western blot analysis. Blots were re-probed for complex IV cytochrome c oxidase (COX IV) to assess the quality of mitochondria separation and to confirm equal protein loading. The band intensity of cytochrome c released into the incubation medium was quantified by using densitometry. (F) The effect of superoxide generation with KO2 (1 mmol/l) on the release of cytochrome c from mitochondria. Values are means with the standard error from at least three different preparations, normalised to those for mitochondria incubated at “zero” Ca2+ (A) or without inhibitors (B,C,E). *p<0.05 vs mitochondria incubated at “zero” Ca2+ (A) or without inhibitors (B,C,E).
In contrast, no decrease in ROS levels was observed in isolated rat liver mitochondria in the whole range of Ca2+ concentrations, “zero” to 39 µmol/l (data not shown), in agreement with the literature data.47–50
We next measured the effect of inhibitors of mitochondrial ROS production47,48 on the Ca2+-induced cytochrome c release from pancreatic mitochondria. To inhibit mitochondrial ROS we applied the inhibitors of complex I, rotenone and diphenyliodonium (DPI); the anti-oxidant and lipid peroxidation inhibitor BHT; and CCCP. It is of note that DPI and rotenone inhibit ROS because they block the reverse electron transport from complex II to complex I, the main pathway of succinate-driven ROS generation.51 Rotenone and DPI markedly inhibited ROS production by mitochondria exposed to 1.3 µmol/l Ca2+ for 10 min (fig 6B). BHT also caused a significant (down to about 40% of control), but a lesser decrease in mitochondrial ROS (fig 6B). As expected, CCCP, which dissipates ΔΨm, completely blocked ROS production. Importantly, the ROS inhibitors (except CCCP) did not depolarise mitochondria (fig 6C).
All the ROS inhibitors drastically decreased cytochrome c release from mitochondria incubated at 1.3 µmol/l Ca2+ (figs 6D,E and 5B). Conversely, exogenous superoxide added in the form of KO252 significantly stimulated cytochrome c release in mitochondria incubated at 39 µmol/l Ca2+ (fig 6F); ie, in conditions in which ROS production was blocked (fig 6A). Of note, KO2 had no effect on ΔΨm (data not shown). The results in fig 6 indicate that ROS promote cytochrome c release from pancreatic mitochondria.
Taken together, the results in figs 3–6 elucidate the regulation of cytochrome c release from pancreatic mitochondria by Ca2+, ΔΨm and ROS. In particular, these results indicate that Ca2+-induced loss of ΔΨm inhibits cytochrome c release due to inhibition of ROS.
ROS and Ca2+ regulate cytochrome c release and apoptosis in pancreatic acinar cells
To corroborate the findings on isolated mitochondria that revealed key roles for ROS and Ca2+ in cytochrome c release, we performed experiments on rat pancreatic acinar cells stimulated with supramaximal CCK-8 (CCK). We have previously demonstrated8,9 cytochrome c release, downstream caspase-3 activation and apoptosis in this experimental system, which is considered an in vitro model of acute pancreatitis.1,2,6–9,25,53
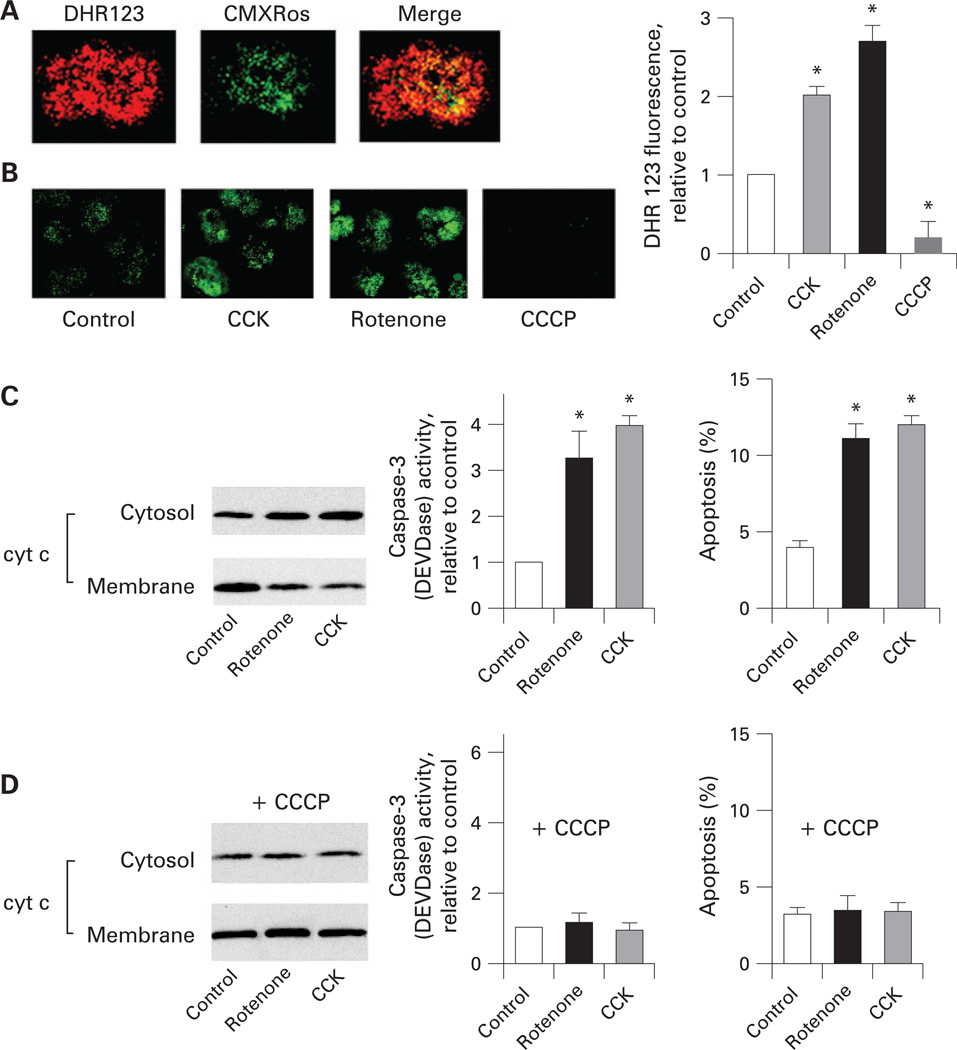
We first assessed the effect of CCK on mitochondrial ROS and the role of ROS in cytochrome c release, caspase activation and apoptosis in acinar cells (fig 7). To measure mitochondrial ROS in intact cells, we used the ROS-sensitive rhodamine dye DHR123.38 DHR123 fluorescence largely co-localised with that of the mitochondrial marker MitoTracker Red (CMXRos) (fig 7A), confirming mitochondrial localisation of DHR123 in acinar cells. The supramaximal CCK increased DHR123 fluorescence 2-fold (fig 7B), indicating an increase in ROS. These data are in accord with a previous report54 (using a different dye) that supramaximal CCK increases ROS in acinar cells.
Figure 7.
Reactive oxygen species (ROS) mediate cytochrome c (cyt c) release, caspase-3 activation, and apoptosis in pancreatic acinar cells. (A) Rat pancreatic acinar cells were labelled with the mitochondrial ROS-sensitive rhodamine dye DHR123 and the mitochondria marker MitoTracker Red (CMXRos), and analysed by confocal microscopy. (B) DHR123-labelled acinar cells were incubated for 15 min without (control) and with cholecystokinin-8 (CCK-8; 100 nmol/l), rotenone (5 µmol/l) or carbonyl cyanide m-chlorophenylhydrazone (CCCP; 5 µmol/l), and imaged using confocal microscopy. DHR123 fluorescence was quantified and normalised to the cell number in the field (at least 100 cells in three different cell preparations). Values are means with the standard error (n = 3) normalised to those in control (ie, untreated acinar cells). *p<0.05 vs control. (C,D). Pancreatic acinar cells were pre-incubated for 15 min with or without 5 µmol/l CCCP, and then incubated for 3 h with and without 5 µmol/l rotenone or 100 nmol/l CCK-8. In (C,D), cytochrome c levels were measured in cytosolic and membrane fractions by western blot analysis. Blots were re-probed for tubulin (cytosolic fractions) and for complex IV cytochrome c oxidase (COX IV, membrane fractions) to confirm equal protein loading (not shown). The band intensity of cytochrome c in the cytosolic fraction was quantified by using densitometry and normalised to that for tubulin in the same sample. Caspase-3 activity was measured with a fluorogenic assay, using Asp-Glu-Val-Asp-AMC (where AMC is 7-amino-4-methylcoumarin) (DEVD-AMC) as a substrate. Apoptosis was measured by the percentage of cells with apoptotic nuclear morphology using Hoechst 33258 staining. For each condition, at least 1000 cells were counted in three different acinar cell preparations. Values are means with the standard error (n=3) normalised to those in control (ie, acinar cells incubated without CCK and rotenone). *p<0.05 vs control cells.
To assess the role of mitochondrial ROS in apoptosis in acinar cells, we used rotenone, an inhibitor of complex I, which is routinely applied to stimulate mitochondrial ROS production in intact cells.47,55,56 Of note, in isolated mitochondria that use succinate as a substrate, rotenone produces the opposite effect of inhibiting ROS, which we utilised in the experiments in fig 6. The reason for these opposing effects of rotenone is that in isolated mitochondria using succinate as a substrate, rotenone blocks the reverse electron transport from complex II to complex I and thus inhibits ROS production.47,51 In contrast, cells mostly use complex I-linked substrates (eg, glutamate/malate), and, therefore, inhibiting electron transport through complex I with rotenone stimulates mitochondrial ROS in intact cells.56 We found that in pancreatic acinar cells rotenone, indeed, increased DHR123 fluorescence about 3-fold (fig 7B). We tested that rotenone did not cause a decrease in CMXRos fluorescence (ie, mitochondrial depolarisation) in acinar cells (data not shown).
Stimulating mitochondrial ROS production with rotenone greatly induced cytochrome c release, caspase-3 activity, and apoptosis in acinar cells (fig 7C). The magnitude of rotenone-induced apoptotic signals was similar to those induced by the supramaximal CCK, suggesting a major role for ROS in the CCK-induced apoptotic responses (fig 7C). Our findings are in accord with recent data57,58 showing that the pro-oxidant menadione increases ROS levels and stimulates apoptosis in rat pancreatic acinar cells.
CCCP, which blocked mitochondrial ROS production (fig 7B), completely abolished both CCK- and rotenone-induced cytochrome c release, caspase-3 activation, and apoptosis in acinar cells (fig 7D).
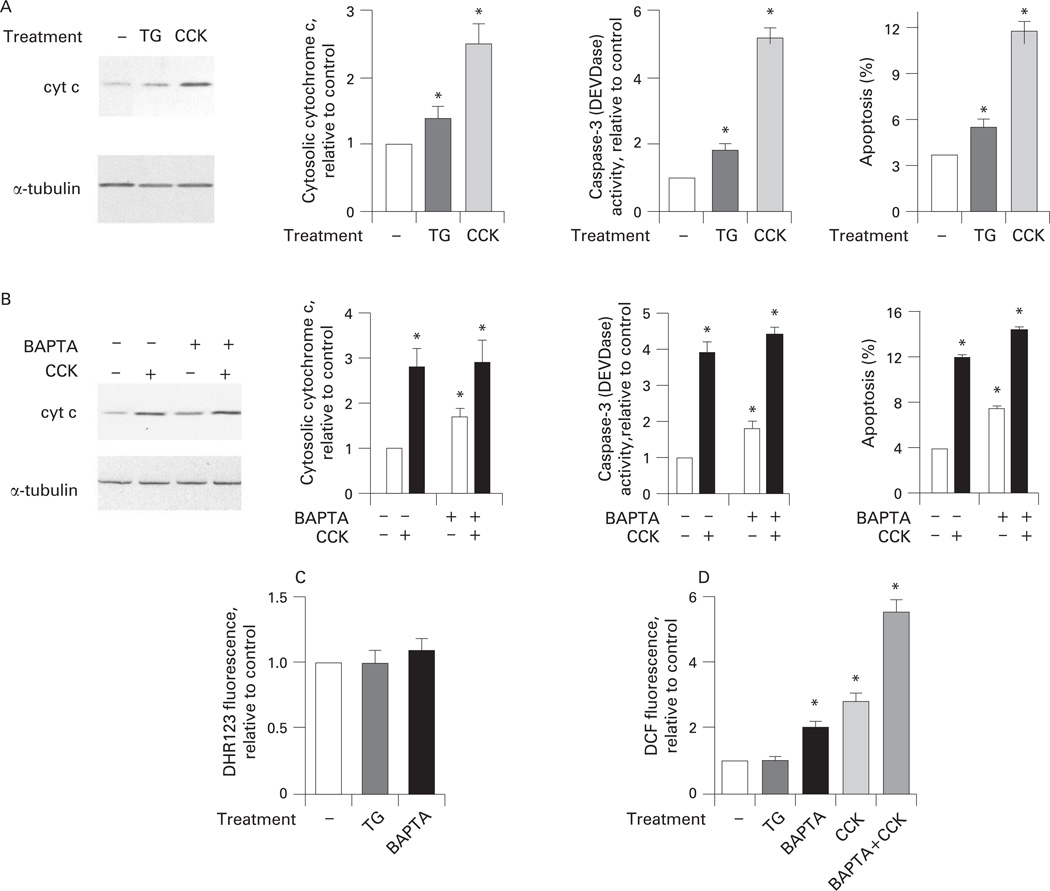
Next, to assess the role of Ca2+ we applied thapsigargin, an inhibitor of the endoplasmic reticulum Ca2+–ATPase, which induces a sustained increase in [Ca2+]i in acinar cells (to approximately 300 nmol/l, compared with the basal of about 100 nmol/l) and thus mimics the effect of supramaximal CCK.44,59 We also used buffering of [Ca2+]i with the intracellular Ca2+ chelator BAPTA. Incubation of acinar cells with thapsigargin significantly stimulated cytochrome c release, caspase-3 activity and apoptosis (fig 8A). The effects of thapsigargin were, however, less than those elicited by supramaximal CCK (fig 8A). BAPTA, which prevents CCK-induced increase in [Ca2+]i,44 had two effects on apoptotic responses in acinar cells (fig 8B).
Figure 8.
Cholecystokinin (CCK)-induced apoptosis in pancreatic acinar cells is mediated through both Ca2+-dependent and -independent mechanisms. (A) Isolated rat pancreatic acinar cells were incubated for 3 h with and without CCK-8 (100 nmol/l) or thapsigargin (TG; 1 µmol/l). (B) Prior to the addition of CCK, cells were pre-incubated for 40 min with or without 50 µmol/l 1,2-bis(o-aminophenoxy)ethoxy-ethane-N′-tetraacetic acid (BAPTA-AM), washed, and re-suspended in the same buffer containing no BAPTA. The incubation continued for 3 more hours with and without CCK. In (A,B), cytochrome c (cyt c) levels were measured in cytosolic fractions by western blot analysis. Blots were re-probed for tubilin to confirm equal protein loading. Cytochrome c band intensity was quantified by using densitometry and normalised to that for tubulin in the same sample. Caspase-3 activity was measured with a fluorogenic assay, using Asp-Glu-Val-Asp-AMC (where AMC is 7-amino-4-methylcoumarin) (DEVD-AMC) as a substrate. Apoptosis was measured by the percentage of cells with apoptotic nuclear morphology using Hoechst 33258 staining. For each condition, at least 1000 cells were counted in three different acinar cell preparations. Values are means with the standard error (n = 3) normalised to those in controls (ie, acinar cells incubated without CCK, thapsigargin or BAPTA). *p<0.05 vs control cells. (C, D) Cells were labelled with (C) the rhodamine dye DHR123 sensitive to mitochondrial reactive oxygen species (ROS) or (D) 2,7-dichlorofluorescein (DCF) sensitive to both mitochondrial and non-mitochondrial ROS. Cells labelled with either DHR123 or DCF were incubated for 15 min without (control) and with thapsigargin (TG; 1 µmol/l) or CCK-8 (100 nmol/l), and imaged using confocal microscopy. DHR123 or DCF fluorescence was quantified and normalised to the cell number in the field. In experiments with BAPTA, cells were pre-incubated for 40 min with or without 50 µmol/l BAPTA-AM, washed, and re-suspended in the same buffer containing no BAPTA. Values are means with the standard error from three different cell preparations, normalised to those in controls (ie, untreated acinar cells). *p<0.05 vs control.
BAPTA loading increased basal levels (ie, in untreated cells) of cytochrome c release, caspase-3 activity and apoptosis. As a result, in the presence of BAPTA the CCK-induced cytochrome c release, caspase-3 activation and apoptosis were all less than in cells without BAPTA (fig 8B). For example, CCK stimulated apoptosis 3-fold in control cells and 2.1-fold in BAPTA-loaded cells.
We tested whether the increases in the basal levels of cytochrome c release and downstream apoptosis caused by BAPTA could be mediated through ROS. There are data on other cell types60,61 showing that BAPTA stimulates ROS-dependent cell death; for example, BAPTA induces death of cortical cells through stimulating lipoxygenase-mediated ROS production.61 Using DHR123, we did not find any significant increase in mitochondrial ROS in BAPTA-loaded acinar cells (fig 8C). However, BAPTA caused an approximately 2-fold increase in intracellular ROS measured with DCF (fig 8D), a fluorescent dye sensitive to both mitochondrial and non-mitochondrial ROS.39,40 Thus, the pro-apoptotic effect of BAPTA can be explained by its stimulation of ROS. Indeed, the anti-oxidant N-acetylcysteine abrogated BAPTA-induced caspase-3 activation in acinar cells (data not shown).
In contrast, thapsigargin had no effect on either DHR123 or DCF fluorescence in acinar cells (fig 8C,D), indicating the lack of its effect on either mitochondrial or non-mitochondrial ROS. This indicates that, by itself, an increase in [Ca2+]i elicited with thapsigargin does not increase ROS in acinar cells. Therefore, the pro-apoptotic effect of thapsigargin (fig 8A) is mediated through an increase in [Ca2+]i.
Comparison between the two sets of conditions, CCK versus control and BAPTA+CCK versus BAPTA, allows the contributions of Ca2+ and ROS to the effect of CCK on the apoptotic responses in acinar cells to be assessed. The fold increase in DCF fluorescence induced by the supramaximal CCK was the same (approximately three times) in the absence of BAPTA and in BAPTA-loaded acinar cells (fig 8D), indicating that CCK-induced increases in ROS were similar in controls and BAPTA-loaded cells. At the same time, the effects of CCK on apoptotic responses were less in BAPTA-loaded than in control cells (fig 8B), reflecting the contribution of the CCK-induced [Ca2+]i signal, which is abolished by BAPTA.
The results in figs 7 and 8, corroborating our findings on isolated mitochondria, indicate that cytochrome c release, caspase-3 activation and apoptosis in acinar cells are mediated by increases in both [Ca2+]i and ROS, and are inhibited by loss of ΔΨm. In particular, sustained increase in [Ca2+]i stimulates apoptotic responses in acinar cells. The results also indicate that Ca2+ is not the only signal mediating the CCK-induced apoptotic responses; that is, these responses are mediated by both Ca2+-dependent and -independent mechanisms.
DISCUSSION
Mechanisms regulating acinar cell death in pancreatitis remain poorly understood. We have recently shown8,9 that cytochrome c release is a common event in models of acute pancreatitis. Experimental pancreatitis is also associated with sustained elevations of [Ca2+]i, which can locally reach low micromolar concentrations26 and are believed to mediate trypsinogen activation and other pathological responses of pancreatitis.53,62 Oxidative stress is implicated in acute pancreatitis; however, the roles of ROS and the targets of ROS in acinar cells are not known.27,28,63
Here, we investigated the roles of Ca2+, ΔΨm and ROS in the regulation of cytochrome c release in pancreatic mitochondria. We used both isolated pancreatic mitochondria and acinar cells hyperstimulated with CCK.
The properties of pancreatic mitochondria are poorly characterised.29–31 We found that pancreatic mitochondria are more sensitive to Ca2+ than liver mitochondria and maintain their functional state only if isolated in the presence of EGTA. Ca2+ at low micromolar concentrations depolarised isolated pancreatic mitochondria, which was prevented by inhibition of the Ca2+ uniporter, indicating mPTP opening. Further, we found that the mPTP in pancreatic mitochondria has unusual properties, different from the “classical” (eg, liver) mPTP. A key characteristic of “classical” mPTP opening is mitochondria swelling, leading to OMM rupture.18–20 By contrast, Ca2+-induced depolarisation of isolated pancreatic mitochondria was not associated with swelling. Further, in pancreatic mitochondria Ca2+-induced depolarisation caused a dramatic decrease in ROS, in contrast with ROS burst induced by mPTP opening in mitochondria from liver and other organs.47–51,64,65 Of note, endogenous ROS production in mitochondria is mediated by electrons that have escaped from the electron transport chain; thus, dissipation of ΔΨm (eg, with CCCP) blocks ROS production.47 The reasons why the “classical” mPTP-mediated ROS burst occurs in spite of depolarisation are not quite clear, but it is likely due to inhibition of mitochondrial anti-oxidant capacity resulting from swelling.47–51,64,65
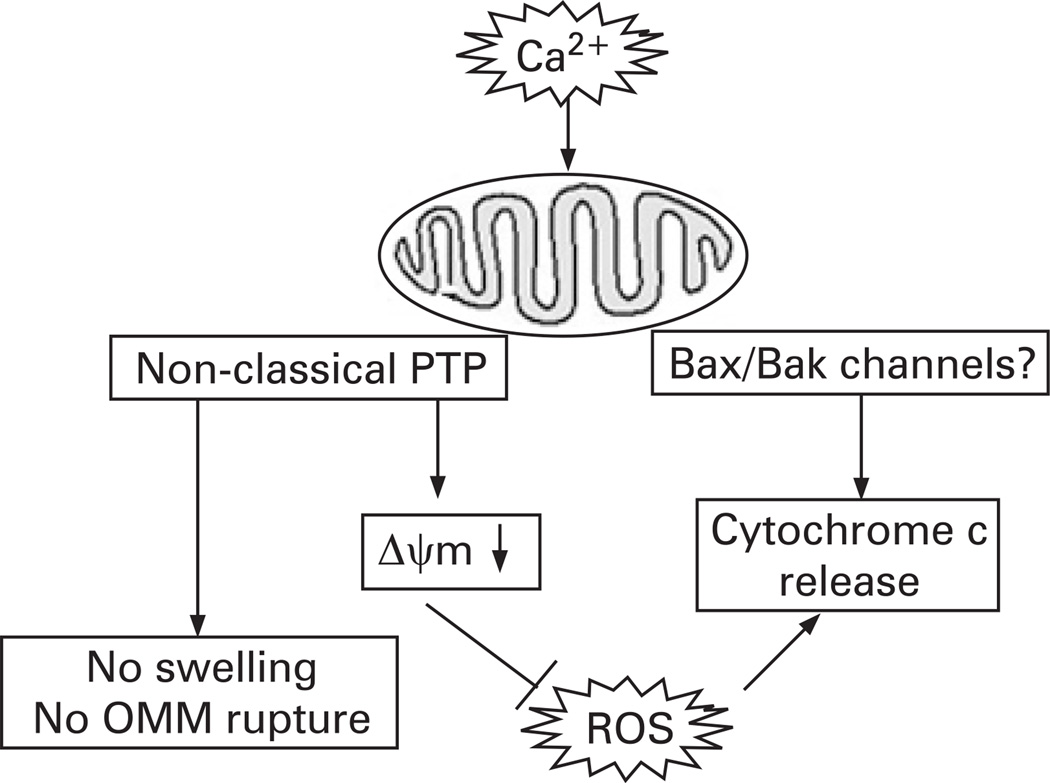
The central finding in our study is that in pancreatic mitochondria, Ca2+-induced cytochrome c release occurs in the absence of OMM rupture. Moreover, we found that the Ca2+-induced, mPTP-mediated depolarisation negatively regulates cytochrome c release, most likely through inhibition of ROS. Thus, although Ca2+ per se stimulates cytochrome c release, Ca2+-induced depolarisation inhibits it. Such a dual effect results in the observed non-monotonous dependence of cytochrome c release on Ca2+. The regulations of cytochrome c release by Ca2+, ΔΨm and ROS in pancreatic mitochondria are depicted in fig 9.
Figure 9.
Schematic illustrating the regulation of cytochrome c release in pancreatic mitochondria. ΔΨm, mitochondrial membrane potential; OMM, outer mitochondrial membrane; PTP, permeability transition pore; ROS, reactive oxygen species.
Differently, in liver mitochondria, Ca2+ only stimulates cytochrome c release.45 These differences could be due to the “non-classical” properties of pancreatic mitochondria mPTP. In liver, Ca2+-induced mPTP opening causes ROS burst, which promotes cytochrome c release, whereas mPTP opening in pancreatic mitochondria blocks ROS production, thus inhibiting cytochrome c release.
One mechanism whereby ROS may promote cytochrome c release is peroxidation of cardiolipin, a major phospholipid that anchors cytochrome c to the inner mitochondrial membrane.45,46,66 Its oxidation by ROS facilitates cytochrome c detachment, thus increasing cytochrome c availability for release.
The permeability system mediating cytochrome c release from pancreatic mitochondria in the absence of OMM rupture likely involves the pro-apoptotic proteins Bax and Bak17,23,24 Interestingly, our results with bongrekic acid indicate that ANT may be involved in both Ca2+-induced cytochrome c release and depolarisation of pancreatic mitochondria.
Importantly, the results on intact acinar cells hyperstimulated with CCK (the in vitro model of acute pancreatitis) corroborate those on isolated mitochondria. We found that (1) [Ca2+]i increase mediates, in part, the CCK-induced cytochrome c release, caspase-3 activation and apoptosis in acinar cells; (2) increasing mitochondrial ROS stimulates cytochrome c release and apoptosis; and (3) dissipation of ΔΨm prevents CCK-induced apoptotic responses. Thus, in acinar cells Ca2+ and ROS stimulate whereas mitochondrial depolarisation inhibits cytochrome c release. The results indicate that apoptotic responses in acinar cells are mediated by both Ca2+-dependent and Ca2+-independent mechanisms. On the other hand, the results suggest a major role for ROS in CCK-induced apoptotic responses.
In conclusion, our study presents several novel findings. First, it reveals unusual, “non-classical” properties of the mPTP in pancreatic mitochondria; in particular, that the Ca2+-induced depolarisation occurs without mitochondria swelling and results in ROS decrease. Second, the results indicate a critical role for ROS in cytochrome c release and apoptosis in pancreatic acinar cells. Third, we found that in pancreatic mitochondria Ca2+-induced loss of ΔΨm does not stimulate but, on the contrary, inhibits cytochrome c release, most likely through blocking ROS production. To our knowledge, this is the first demonstration of a negative feedback between mitochondrial depolarisation and cytochrome c release. The implication of this negative regulatory mechanism for cell death responses, in general, is that loss of ΔΨm, while facilitating necrosis, may at the same time limit apoptosis. Fourth, our results demonstrate regulation of the pattern of acinar cell death at the mitochondrial level, by the interplay between Ca2+, ΔΨm and ROS. Specifically, the inhibition of cytochrome c release by Ca2+-induced mitochondrial depolarisation suggests a molecular mechanism underlying the inverse correlation between necrosis and apoptosis observed in experimental pancreatitis. Therefore, stabilising mitochondria against ROS decrease and loss of ΔΨm may represent a therapeutic strategy to shift the death response of pancreatitis away from necrosis and thus mitigate the disease severity.
Acknowledgements
We thank Dr AV Panov for help with the equipment to measure mitochondria characteristics, and Dr SJ Pandol for stimulating discussions.
Funding: This study was supported by NIH grant DK059936 (to ASG), an AGA Foundation Designated Research Scholar Award in Pancreatitis (to OAM) and by the Hirshberg Foundation.
Footnotes
Supplementary methods and a figure are published online only at http://gut.bmj.com/content/vol58/issue3
Competing interests: None.
REFERENCES
- 1.Gukovskaya AS, Pandol SJ. Cell death pathways in pancreatitis and pancreatic cancer. Pancreatology. 2004;4:567–586. doi: 10.1159/000082182. [DOI] [PubMed] [Google Scholar]
- 2.Halangk W, Lerch MM. Early events in acute pancreatitis. Clin Lab Med. 2005;25:1–15. doi: 10.1016/j.cll.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Kaiser AM, Saluja AK, Sengupta A, et al. Relationship between severity, necrosis, and apoptosis in five models of experimental acute pancreatitis. Am J Physiol Cell Physiol. 1995;269:C1295–C1304. doi: 10.1152/ajpcell.1995.269.5.C1295. [DOI] [PubMed] [Google Scholar]
- 4.Gukovskaya AS, Perkins P, Zaninovic V, et al. Mechanisms of cell death after pancreatic duct obstruction in the opossum and the rat. Gastroenterology. 1996;110:875–884. doi: 10.1053/gast.1996.v110.pm8608898. [DOI] [PubMed] [Google Scholar]
- 5.Sandoval D, Gukovskaya A, Reavey P, et al. The role of neutrophils and platelet-activating factor in mediating experimental pancreatis. Gastroenterology. 1996;111:1081–1091. doi: 10.1016/s0016-5085(96)70077-x. [DOI] [PubMed] [Google Scholar]
- 6.Pandol SJ, Saluja AK, Imrie CW, et al. Acute pancreatitis: bench to the bedside. Gastroenterology. 2007;132:1127–1151. doi: 10.1053/j.gastro.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 7.Bhatia M. Apoptosis versus necrosis in acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2004;286:G189–G196. doi: 10.1152/ajpgi.00304.2003. [DOI] [PubMed] [Google Scholar]
- 8.Mareninova OA, Sung KF, Hong P, et al. Cell death in pancreatitis: caspases protect from necrotizing pancreatitis. J Biol Chem. 2006;281:3370–3381. doi: 10.1074/jbc.M511276200. [DOI] [PubMed] [Google Scholar]
- 9.Gukovskaya AS, Gukovsky I, Jung Y, et al. Cholecystokinin induces caspase activation and mitochondrial dysfunction in pancreatic acinar cells. Roles in cell injury processes of pancreatitis. J Biol Chem. 2002;277:22595–22604. doi: 10.1074/jbc.M202929200. [DOI] [PubMed] [Google Scholar]
- 10.Saluja A, Hofbauer B, Yamaguchi Y, et al. Induction of apoptosis reduces the severity of caerulein-induced pancreatitis in mice. Biochem Biophys Res Commun. 1996;220:875–878. doi: 10.1006/bbrc.1996.0498. [DOI] [PubMed] [Google Scholar]
- 11.Bhatia M, Wallig MA, Hofbauer B, et al. Induction of apoptosis in pancreatic acinar cells reduces the severity of acute pancreatitis. Biochem Biophys Res Commun. 1998;246:476–483. doi: 10.1006/bbrc.1998.8519. [DOI] [PubMed] [Google Scholar]
- 12.Fiers W, Beyaert R, Declercq W, et al. More than one way to die: apoptosis, necrosis and reactive oxygen damage. Oncogene. 1999;18:7719–7730. doi: 10.1038/sj.onc.1203249. [DOI] [PubMed] [Google Scholar]
- 13.Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 2004;16:663–669. doi: 10.1016/j.ceb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Golstein P, Kroemer G. Cell death by necrosis: towards a molecular definition. Trends Biochem Sci. 2007;32:37–43. doi: 10.1016/j.tibs.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong JS. Mitochondrial membrane permeabilization: the sine qua non for cell death. Bioessays. 2006;28:253–260. doi: 10.1002/bies.20370. [DOI] [PubMed] [Google Scholar]
- 16.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 17.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 18.Bernardi P. The permeability transition pore. Control points of a cyclosporin Asensitive mitochondrial channel involved in cell death. Biochim Biophys Acta. 1996;1275:5–9. doi: 10.1016/0005-2728(96)00041-2. [DOI] [PubMed] [Google Scholar]
- 19.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341:233–249. [PMC free article] [PubMed] [Google Scholar]
- 20.Halestrap AP, Kerr PM, Javadov S, et al. Elucidating the molecular mechanism of the permeability transition pore and its role in reperfusion injury of the heart. Biochim Biophys Acta. 1998;1366:79–94. doi: 10.1016/s0005-2728(98)00122-4. [DOI] [PubMed] [Google Scholar]
- 21.Baines CP, Kaiser RA, Purcell NH, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa T, Shimizu S, Watanabe T, et al. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 23.Zong WX, Lindsten T, Ross AJ, et al. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 2001;15:1481–1486. doi: 10.1101/gad.897601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei MC, Zong WX, Cheng EH, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sutton R, Criddle D, Raraty MG, et al. Signal transduction, calcium and acute pancreatitis. Pancreatology. 2003;3:497–505. doi: 10.1159/000075581. [DOI] [PubMed] [Google Scholar]
- 26.Raraty MG, Connor S, Criddle DN, et al. Acute pancreatitis and organ failure: pathophysiology, natural history, and management strategies. Curr Gastroenterol Rep. 2004;6:99–103. doi: 10.1007/s11894-004-0035-0. [DOI] [PubMed] [Google Scholar]
- 27.Dabrowski A, Konturek SJ, Konturek JW, et al. Role of oxidative stress in the pathogenesis of caerulein-induced acute pancreatitis. Eur J Pharmacol. 1999;377:1–11. doi: 10.1016/s0014-2999(99)00421-5. [DOI] [PubMed] [Google Scholar]
- 28.Reinheckel T, Prause J, Nedelev B, et al. Oxidative stress affects pancreatic proteins during the early pathogenesis of rat caerulein pancreatitis. Digestion. 1999;60:56–62. doi: 10.1159/000007589. [DOI] [PubMed] [Google Scholar]
- 29.Wilson JS, Korsten MA, Lieber CS. The isolation and properties of mitochondria from rat pancreas. Biochem Biophys Res Commun. 1984;121:545–551. doi: 10.1016/0006-291x(84)90216-x. [DOI] [PubMed] [Google Scholar]
- 30.Wilson JS, Korsten MA, Leo MA, et al. New technique for the isolation of functional rat pancreatic mitochondria and its application to models of pancreatic injury. J Lab Clin Med. 1986;107:51–58. [PubMed] [Google Scholar]
- 31.Schild L, Matthias R, Stanarius A, et al. Induction of permeability transition in pancreatic mitochondria by cerulein in rats. Mol Cell Biochem. 1999;195:191–197. doi: 10.1023/a:1006988625831. [DOI] [PubMed] [Google Scholar]
- 32.Gukovskaya AS, Gukovsky I, Zaninovic V, et al. Pancreatic acinar cells produce, release, and respond to tumor necrosis factor-alpha. Role in regulating cell death and pancreatitis. J Clin Invest. 1997;100:1853–1862. doi: 10.1172/JCI119714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamo N, Muratsugu M, Hongoh R, et al. Membrane potential of mitochondria measured with an electrode sensitive to tetraphenyl phosphonium and relationship between proton electrochemical potential and phosphorylation potential in steady state. J Membr Biol. 1979;49:105–121. doi: 10.1007/BF01868720. [DOI] [PubMed] [Google Scholar]
- 34.Scaduto RCJ, Grotyohann LW. Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys J. 1999;76:469–477. doi: 10.1016/S0006-3495(99)77214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hotta Y, Ishikawa N, Ohashi N, et al. Effects of SM-20550, a selective Na+–H+ exchange inhibitor, on the ion transport of myocardial mitochondria. Mol Cell Biochem. 2001;219:83–90. doi: 10.1023/a:1011019010140. [DOI] [PubMed] [Google Scholar]
- 36.Beavis AD, Brannan RD, Garlid KD. Swelling and contraction of the mitochondrial matrix. I. A structural interpretation of the relationship between light scattering and matrix volume. J Biol Chem. 1985;260:13424–13433. [PubMed] [Google Scholar]
- 37.Zhou M, Diwu Z, Panchuk-Voloshina N, et al. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal Biochem. 1997;253:162–168. doi: 10.1006/abio.1997.2391. [DOI] [PubMed] [Google Scholar]
- 38.Bassoe CF, Li N, Ragheb K, et al. Investigations of phagosomes, mitochondria, and acidic granules in human neutrophils using fluorescent probes. Cytometry B Clin Cytom. 2003;51:21–29. doi: 10.1002/cyto.b.10003. [DOI] [PubMed] [Google Scholar]
- 39.Tarpey MM, Wink DA, Grisham MB. Methods for detection of reactive metabolites of oxygen and nitrogen: in vitro and in vivo considerations. Am J Physiol Regul Integr Comp Physiol. 2004;286:R431–R444. doi: 10.1152/ajpregu.00361.2003. [DOI] [PubMed] [Google Scholar]
- 40.Vaquero EC, Edderkaoui M, Pandol SJ, et al. Reactive oxygen species produced by NAD(P)H oxidase inhibit apoptosis in pancreatic cancer cells. J Biol Chem. 2004;279:34643–34654. doi: 10.1074/jbc.M400078200. [DOI] [PubMed] [Google Scholar]
- 41.Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- 42.Zoratti M, Szabo I, De Marchi U. Mitochondrial permeability transitions: how many doors to the house? Biochim Biophys Acta. 2005;1706:40–52. doi: 10.1016/j.bbabio.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 43.Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- 44.Gukovskaya AS, Hosseini S, Satoh A, et al. Ethanol differentially regulates NF-kappaB activation in pancreatic acinar cells through calcium and protein kinase C pathways. Am J Physiol Gastrointest Liver Physiol. 2004;286:G204–G213. doi: 10.1152/ajpgi.00088.2003. [DOI] [PubMed] [Google Scholar]
- 45.Petrosillo G, Ruggiero FM, Pistolese M, et al. Ca2+-induced reactive oxygen species production promotes cytochrome c release from rat liver mitochondria via mitochondrial permeability transition (MPT)-dependent and MPT-independent mechanisms: role of cardiolipin. J Biol Chem. 2004;279:53103–53108. doi: 10.1074/jbc.M407500200. [DOI] [PubMed] [Google Scholar]
- 46.Ott M, Robertson JD, Gogvadze V, et al. Cytochrome c release from mitochondria proceeds by a two-step process. Proc Natl Acad Sci U S A. 2002;99:1259–1263. doi: 10.1073/pnas.241655498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andreyev AY, Kushnareva YE, Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc) 2005;70:200–214. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- 48.Brookes PS, Yoon Y, Robotham JL, et al. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 49.Petrosillo G, Ruggiero FM, Paradies G. Role of reactive oxygen species and cardiolipin in the release of cytochrome c from mitochondria. FASEB J. 2003;17:2202–2208. doi: 10.1096/fj.03-0012com. [DOI] [PubMed] [Google Scholar]
- 50.Zoccarato F, Cavallini L, Alexandre A. Respiration-dependent removal of exogenous H2O2 in brain mitochondria: inhibition by Ca2+ J Biol Chem. 2004;279:4166–4174. doi: 10.1074/jbc.M308143200. [DOI] [PubMed] [Google Scholar]
- 51.Votyakova TV, Reynolds IJ. DeltaPsi(m)-Dependent and -independent production of reactive oxygen species by rat brain mitochondria. J Neurochem. 2001;79:266–277. doi: 10.1046/j.1471-4159.2001.00548.x. [DOI] [PubMed] [Google Scholar]
- 52.Kishioka SY, Takekawa T, Yamada A. Electrochemical determination of the superoxide ion concentration from KO2 dissolved in dimethyl sulfoxide. Anal Sci. 2004;20:1465–1466. doi: 10.2116/analsci.20.1465. [DOI] [PubMed] [Google Scholar]
- 53.Saluja AK, Bhagat L, Lee HS, et al. Secretagogue-induced digestive enzyme activation and cell injury in rat pancreatic acini. Am J Physiol Gastrointest Liver Physiol. 1999;276:G835–G842. doi: 10.1152/ajpgi.1999.276.4.G835. [DOI] [PubMed] [Google Scholar]
- 54.Granados MP, Salido GM, Pariente JA, et al. Generation of ROS in response to CCK-8 stimulation in mouse pancreatic acinar cells. Mitochondrion. 2004;3:285–296. doi: 10.1016/j.mito.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 55.Fiskum G, Starkov A, Polster BM, et al. Mitochondrial mechanisms of neural cell death and neuroprotective interventions in Parkinson’s disease. Ann N Y Acad Sci. 2003;991:111–119. doi: 10.1111/j.1749-6632.2003.tb07469.x. [DOI] [PubMed] [Google Scholar]
- 56.Li N, Ragheb K, Lawler G, et al. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J Biol Chem. 2003;278:8516–8525. doi: 10.1074/jbc.M210432200. [DOI] [PubMed] [Google Scholar]
- 57.Gerasimenko JV, Gerasimenko OV, Palejwala A, et al. Menadione-induced apoptosis: roles of cytosolic Ca(2+) elevations and the mitochondrial permeability transition pore. J Cell Sci. 2002;115:485–497. doi: 10.1242/jcs.115.3.485. [DOI] [PubMed] [Google Scholar]
- 58.Criddle DN, Gillies S, Baumgartner-Wilson HK, et al. Menadione-induced reactive oxygen species generation via redox cycling promotes apoptosis of murine pancreatic acinar cells. J Biol Chem. 2006;281:40485–40492. doi: 10.1074/jbc.M607704200. [DOI] [PubMed] [Google Scholar]
- 59.Voronina SG, Barrow SL, Gerasimenko OV, et al. Effects of secretagogues and bile acids on mitochondrial membrane potential of pancreatic acinar cells: comparison of different modes of evaluating DeltaPsim. J Biol Chem. 2004;279:27327–27338. doi: 10.1074/jbc.M311698200. [DOI] [PubMed] [Google Scholar]
- 60.Han KS, Kang HJ, Kim EY, et al. 1,2-bis(2-Aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid induces caspase-mediated apoptosis and reactive oxygen species-mediated necrosis in cultured cortical neurons. J Neurochem. 2001;78:230–239. doi: 10.1046/j.1471-4159.2001.00394.x. [DOI] [PubMed] [Google Scholar]
- 61.Wie MB, Koh JY, Won MH, et al. BAPTA/AM, an intracellular calcium chelator, induces delayed necrosis by lipoxygenase-mediated free radicals in mouse cortical cultures. Prog Neuropsychopharm Biol Psychiatry. 2001;25:1641–1659. doi: 10.1016/s0278-5846(01)00202-0. [DOI] [PubMed] [Google Scholar]
- 62.Raraty M, Ward J, Erdemli G, et al. Calcium-dependent enzyme activation and vacuole formation in the apical granular region of pancreatic acinar cells. Proc Natl Acad Sci U S A. 2000;97:13126–13131. doi: 10.1073/pnas.97.24.13126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chvanov M, Petersen OH, Tepikin A. Free radicals and the pancreatic acinar cells: role in physiology and pathology. Phil Trans R Soc Lond B Biol Sci. 2005;360:2273–2284. doi: 10.1098/rstb.2005.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Starkov AA, Polster BM, Fiskum G. Regulation of hydrogen peroxide production by brain mitochondria by calcium and Bax. J Neurochem. 2002;83:220–228. doi: 10.1046/j.1471-4159.2002.01153.x. [DOI] [PubMed] [Google Scholar]
- 65.Vercesi AE, Kowaltowski AJ, Oliveira HC, et al. Mitochondrial Ca2+ transport, permeability transition and oxidative stress in cell death: implications in cardiotoxicity, neurodegeneration and dyslipidemias. Front Biosci. 2006;11:2554–2564. doi: 10.2741/1990. [DOI] [PubMed] [Google Scholar]
- 66.Gogvadze V, Orrenius S, Zhivotovsky B. Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochim Biophys Acta. 2006;1757:639–647. doi: 10.1016/j.bbabio.2006.03.016. [DOI] [PubMed] [Google Scholar]