Abstract
Ovarian cancer (OVCA) is the most lethal gynecological malignancy. It is often diagnosed in advanced stages and despite therapy, 70% relapse within 2 years with incurable disease. Regimens with clinical benefit and minimal toxicity are urgently needed. More effective hormonal therapies would be appealing in this setting.
Estrogens (E2) are implicated in the etiology of OVCA. Estrogens drive proliferation and anti-estrogens inhibit ovarian cancer growth in vitro and in vivo. Despite estrogen receptor (ER) expression in 67% of OVCAs, small anti-estrogen therapy trials have been disappointing and the benefit of hormonal therapy has not been systematically studied in large well-designed trials. OVCAs often manifest de novo anti-estrogen resistance and those that initially respond invariably develop resistance. Estrogens stimulate ovarian cancer progression by transcriptional activation and cross talk between liganded ER and mitogenic pathways, both of which drive cell cycle progression. Estrogen deprivation and estrogen receptor (ER) blockade cause cell cycle arrest in susceptible OVCAs by increasing the cell cycle inhibitor, p27. This review summarizes and discusses scientific and epidemiological evidence supporting estrogen’s role in ovarian carcinogenesis, provides an overview of clinical trials of ER blockade and aromatase inhibitors in OVCA and reviews potential causes of antiestrogen resistance. Anti-estrogen resistance was recently shown to be reversed by dual ER and Src signaling blockade. Blocking cross-talk between ER and constitutively activated kinase pathways may improve anti-estrogen therapeutic efficacy in OVCA, as has been demonstrated in other cancers. Novel strategies to improve benefit from anti-estrogens by combining them with targeted therapies are reviewed.
Keywords: ovarian cancer, estrogen receptor, antiestrogen-resistance
Graphical abstract
Clinical impact of ovarian cancer
Ovarian cancer is the most lethal gynecological cancer in the United States and the fourth leading cause of cancer death in women. In 2011, approximately 21,990 women were diagnosed and over 15,000 women died from ovarian cancer (American Cancer Society, 2011). Above 70% of women are diagnosed with late stage III and IV disease [1]. Despite aggressive surgery to remove the bulk of the tumor at diagnosis and post-surgical treatment with platinum/ taxane-based chemotherapy, about 70% of ovarian cancers relapse within 2 years [2]. Recurrent disease has a poor prognosis, with a 5 year survival of 23% and 14% for stage III and IV disease, respectively [3]. Survival has minimally improved over the past decade for advanced stages and clearly new therapies are needed. The goals of salvage therapy are to palliate symptoms and to maximize quality of life. A less toxic regimen, such as hormonal therapy, is particularly appealing in this setting. Thus, convenient regimens that have clinical benefit with minimal toxicity are essential for this population.
Hormone replacement therapy and ovarian cancer risk
Although the etiologic origins of ovarian cancer are poorly understood, most tumors are thought to arise in the surface epithelium of the ovary itself or the fallopian tubes, influenced by changes in the hormonal environment of ovulation and pregnancy, as well as use of exogenous estrogen supplementation. Ovarian cancer increases dramatically in peri- and post-menopausal women. The age-specific incidence increases from 13/100,000 at age 45 to 43/100,000 at age 60. Compared to earlier reproductive years, circulating peri-menopausal estrogen levels are higher, coincident with the time period when ovarian cancer risk rises sharply [4]. Thus, adrenal pre-estrogenic hormones are likely important in ovarian carcinogenesis [5].
Increasing epidemiologic data implicate estrogen in the etiology of ovarian cancer. These data come from studies of hormone replacement therapy (HRT) in post-menopausal women. A meta-analysis of nine studies including the Million Women Study and the Women’s Health Initiative, showed the risk of ovarian cancer increased by 1.28 (1.20–1.36) in women who used hormonal replacement therapy compared to never users [6]. A recent meta-analysis of 14 studies revealed effects of formulation and duration of hormone replacement [estrogen alone (ET) or estrogen plus progesterone (EPP)] and risk of ovarian cancer [7]. Eight population-based case-control studies, five cohort studies, and 1 clinical trial, showed ovarian cancer risk was increased among ET users (RR per 5 years of use, RR=1.22 (95% CI, 1.18–1.27; P < .0001), and a lower but still statistically significant increased risk was seen with EPP use (RR= 1.10; 95% CI, 1.04–1.16; P = 0.001). The increased risk in ET users was statistically significantly greater than the risk in EPT users (P = 0.004) [7]. The duration and cumulative estrogen dose contributed most importantly to ovarian cancer risk. Twelve of thirteen studies showed an effect of ET duration on ovarian cancer risk [7]. Notably, estrogen use over 10 years increased ovarian cancer risk (RR= 1.45– 2.2) [8–10]. A cumulative estrogen dose of one gram increased ovarian cancer risk 1.056 fold, but the risk rose by 1.31 fold with 5 g cumulative estrogen intake (95% CI 1.003–1.112 and 1.01–1.7, respectively) [11]. These data support a moderate risk increase for ovarian cancer with hormone replacement therapy.
Expression and activity of estrogen receptors ERα and ERβ in ovarian cancer
There is increasing evidence that estrogens drive proliferation in a subset of ovarian cancer lines in cell culture and in xenograft models [12–15]. Moreover, anti-estrogens can inhibit proliferation of estrogen receptor positive OVCA cells in vitro and in vivo [13;15]. There are two types of estrogen receptor (ER): ERα and ERβ. The former is expressed in up to 60% of ovarian cancers (reviewed below); the latter is found in normal ovaries [16]. ERα activates expression of genes involved in cell survival and proliferation, thus promoting tumor growth and progression, while the function of ERβ has been found to be anti-proliferative and pro-apoptotic [17]. Growth response to estrogen in hormone responsive ovarian cancer cell lines was shown to be mediated by ERα and not by ERβ [15;18], since treatment with 17-β estradiol or an ERα specific agonist (PTT,4’, 4’, 4” –(4-Propyl-[1H]-pyrazole-1,2,5-tryl) trisphenol) induced cell proliferation [15]. This effect was not elicited by DPN (2,3-bis (4-hydroxyphenyl)-propionitrile), an ERβ selective agonist. When ERβ is coexpressed with ERα it may act as a brake on ERα-mediated effects, including cell proliferation [19].
Different roles in carcinogenesis have been proposed for ERβ. ERβ is encoded by ESR2 gene, which is expressed in different splice variants (ER β1–5). ERβ mRNA levels and protein levels are decreased in ovarian cancer samples compared to normal ovarian tissues [16;20–25], while ERα mRNA levels are similar or slightly higher in cancer compared to normal ovarian tissue. ERβ expression declines during tumorigenesis of breast, colon and prostate cancer [26–30]. In addition to its anti-proliferative role, exogenous expression of ERβ increased apoptosis in ovarian cancer cells [17]. In breast, prostate and ovarian cancer cell lines, transfection of ESR2 inhibited cell motility and invasion in a ligand independent fashion [17;31–33]. Antitumor effects of ERβ have been linked to its inhibition of cyclin A2 and cyclin D1 expression and upregulation of growth inhibitory p21 (WAF1) [33–37]. The ERβ expression has been inversely associated with stage of disease and positively associated with disease free (DFS) and overall survival (OS) in a recent RT-PCR based study of 161 malignant ovarian tissue samples [38]. In a study of 58 ovarian cancers and 12 normal ovaries, nuclear ERβ localization was seen in normal cells, while ERβ was shifted to the cytoplasm in tumor cells and cytoplasmic ERβ expression was associated with decreased DFS and OS [39].
There are several splice variants of ERβ (or ERβ1) that appear to have distinct levels and functions in cancers [23;40;41]. These ERβ splice variants are characterized by alternative 3’-exons (ERβ2, ERβ3, ERβ4, ERβ5) or by deletion of single or multiple exons (ie ERβΔ2, ERβΔ5/6). Promoter hypermethylation decreased ERβ1, ERβ2 and ERβ4 mRNAexpression in ovarian cancer lines and tissues compared to their normal counterparts. However, that of ERβ5 mRNA was significantly elevated in all ovarian cancers compared to normal ovary, and particularly so in clear cell adenocarcinoma [42]. ERβ5 has been shown to heterodimerize with ERβ1, and enhance its overall activity in a ligand-dependent manner [43]. However, little is known currently about the function of ERβ5 in ovarian cancer. All in all, this suggests that ERβ isoforms may be involved in the development and progression of ovarian cancer. ERβ1 may be more important as a tumor suppressor in ovarian cancer because ERβ1 is more comprehensively repressed in ovarian cancers compared to other ERβ isoforms. The identification of ERβ regulated specific genes involved in epithelial proliferation and apoptosis may advance our understanding of the progression of ovarian cancer and aid in the design of new targeted therapies. A similar decrease in ERβ1, ERβ2 and ERβ4 has also been reported in breast and prostate cancers [42;44;45].-. Given the current data available regarding the antitumor effects of ERβ, strategies to restore or increase its expression may have potential in cancer therapy.
ERα Expression and prognostic importance of Erα protein in ovarian cancer
ERα (hereafter ER) is a nuclear hormone receptor superfamily member traditionally classified as a ligand activated transcription factor [46]. Upon ligand binding, ER undergoes conformational changes to form an “activated ER”, involving by dissociation of heat shock proteins (hsp) 90, and hsp70 [47] and other proteins so it can dimerize and bind to specific DNA sequences, estrogen response elements (EREs) and interact with a complex array of potential co-regulators to modulate the transcription of ER target genes. In addition, other mechanisms of estrogen-regulated transcription involve indirect non-genomic actions of ER via cross talk with signaling kinase pathways that ultimately lead to changes in estrogen-regulated genes. There is also evidence that the unliganded ER may become transcriptionally activated by selected posttranslational modifications [48]. Estrogen receptor expression has been reported in 36–77% of ovarian cancers in several small studies [49]. A review of 45 studies, including 2508 ovarian cancers, reported that 67% expressed ER and 47% PR, proportions similar to those reported for breast cancer [49]. Most retrospective studies have evaluated small tumor numbers, used archive specimens with prolonged storage and different immunohistochemical methods, giving rise to inconsistent reports. Most recently, we assayed 338 primary ovarian cancer samples from The Cancer Genome Atlas (TCGA) project by reverse phase protein array and found that ER was expressed in 67% of high grade serous ovarian cancers. We also found ESR1 mRNA and ER protein levels were highly correlated [15].
While reports of the prognostic importance of ER status have been inconsistent, the largest study to date (n=582 patients), using tissue array and immunohistochemistry (IHC), showed ER correlated with better outcome. Multivariate analysis showed that ER and progesterone receptor (PR) expression > 10% was of independent prognostic value for improved disease specific survival [ER: hazard ratio (HR) 0.80; 95% confidence interval (CI), 0.63–0.99; PR: HR 0.69; 95% CI, 0.51–0.94]. The prognostic value of ER and PR expression was additive, with a HR for recurrence of 0.48 (95% CI, 0.13–0.74) [50].
In breast and endometrial carcinoma, the expression of certain estrogen-regulated target genes has been shown to have prognostic importance. Detection of estrogen-regulated gene expression may also prove to have utility for ovarian cancer patients. Despite the correlation of ER expression with improved patient outcome in the studies reviewed above, a recent study in 83 advanced stage ovarian/primary peritoneal high-grade serous carcinomas evaluated expression of ERα by IHC and expression of six genes known to be induced by estrogen in the female reproductive tract by qRT-PCR. ERα expression correlated with poor prognosis [51]. High expression of ERα and estrogen-induced genes was associated with worse overall survival and it was a negative prognostic factor independent of other patient-dependent covariates such as age, race, and BMI. The conflicting findings with regard to ER as a prognostic factor may be a result of various factors, including differences in the method of receptor detection, the lack of standardization of the scoring system, and differences in patient stage and sizes of cohorts analyzed. Further studies are needed to identify the best method to evaluate ER expression (immunohistochemistry or gene expression analysis) as a biomarker for prognosis and to identify which patients may benefit from antiestrogen therapies.
Estrogen receptor driven gene expression in ovarian cancer
Several studies have identified ERα-target genes in ovarian cancer cells. These show some overlap with estrogen response genes in breast cancer cells, but also reveal unique targets. The biological effects of estrogens are classically mediated by ER which functions as a hormone inducible transcription factor that binds to the estrogen-responsive element (ERE) located often in regions far from the transcription start site of target genes thereby involving distal enhancer elements, that function to tether the ER complex to the target gene promoters [48]. In breast cancer cells, ERα is thought to mediate the mitogenic actions of estrogen by inducing expression of genes involved in cell proliferation. Estrogen-induced genes identified in ERα positive breast cancer cell lines include the progesterone receptor (PR), cathepsin D, c-fos, and pS2 [52;53] and studies in primary breast cancers have suggested these may have prognostic utility for predicting whether a tumor is estrogen sensitive and will respond to antiestrogen therapy [53;54]. In ovarian cancers, however, there is a notable lack of expression of many the classical estrogen-responsive genes (PR, c-fos, pS2) identified in breast models. Early studies have shown ER-responsive genes involved in ovarian cancer cell proliferation include: cathepsin D [55], c-fos [56], TGFα [57], stromal cell-derived factor 1 (SDF-1) [58], c-myc [59], and PR [56]. ER target genes in ovarian cancer associated with invasion include fibulin-1C [60;61], and cell cycle regulation include, cyclins D1, A and E [56]. The effects of E2 on gene expression in an ER+ ovarian cancer cell line, PEO1, were evaluated using DNA microarray containing 1200 cancer-related genes [18]. This study showed five transcripts had at least a 3-fold increase and 23 transcripts at least a 3-fold decrease in expression in E2 treated PEO1 compared to untreated cells. These ER targets were verified by real-time quantitative PCR. Gene up-regulated by E2, such as TNFDF1, FOSLI, TRAP1, CTSD (Cathepsin D) and TFAP4, are known to promote cell proliferation. Of particular note, however, was the number of down-regulated genes, especially those involved in maintenance of the cytoskeleton, suggesting a role for E2 signaling in ovarian cancer invasion and metastasis [18]. A limitation of this study is that expression changes were measured 24 hrs after E2 treatment, and thus genes affected may not be directed ER-targets. Further studies are warranted to better define direct ER targets in OVCA.
Only one study has described estrogen-mediated, promoter-specific and ER-α-dependent repression of target genes in ovarian cancer cells. This study showed the folate receptor (FR) α gene promoter is repressed in the presence of 17β-estradiol and derepressed by the antiestrogens tamoxifen and ICI 182,780. The ER corepressor, SMRT, enhanced the repression by 17β-estradiol/ER, but ER coactivators, including SRC family members, did not appreciably impact the ER ligand response [62].
Use of estrogen receptor blockers for ovarian cancer
While adjuvant hormonal therapy prevents disease recurrence and reduces mortality from ER positive breast cancer, the response to anti-estrogens in ovarian cancer is more limited and less encouraging. The potential impact of adjuvant hormonal therapy in ovarian cancer and the predictive value of hormonal receptors have not been studied in well-designed trials. Tamoxifen, an estrogen antagonist, and fulvestrant, a newer ER blocker,, have both been used primarily for recurrent ovarian cancer. Our review of twenty published trials of tamoxifen for recurrent ovarian cancer (total=695 pts, none of which required knowledge of ER status) showed an overall response rate (ORR) of only 13%. Interestingly, 35% of these patients showed stable disease (SD), defined as a lack of disease progression assessed by radiological imaging or CA-125 serum tumor marker level (Table 1). Among these studies, that of Hatch et al. [63] is the largest (n=105) and included patients that progressed after first line therapy and were thus not so heavily pretreated. Their ORR was 17% [9.5% complete response (CR); with 7.6% showing partial response (PR)] with a median complete response duration of 7.5 months (max 17 months). The remainder of the twenty trials in Table 1 included patients that were heavily pretreated, some of them resistant to chemotherapy. However, it is not possible to determine the platinum sensitivity status in these trials. If we select those trials in which at least 50% had not received more than 1 prior treatment (n=240) [63–67], OR rate doubles to 26% with a 9% CR rate. In addition, patients were not selected for tamoxifen therapy by their receptor status. Response rates for tamoxifen in breast cancer whenever receptor status was not routinely employed was around 30% which is lower than for patients with positive receptor status [68]. The majority, 13/19 trials, did not report if receptor status correlated to response. In Hatch et al, the patients with ER+ tumors had higher response rates to tamoxifen than ER- tumors [63]. In Swartz et al, all SD patients were ER+ [69]. In Shirey and Weiner et al., ER status did not correlate with response, but ER status was known in only 25–30% of patients enrolled [70;71]. No study was specifically designed to test if ER status correlated with response to tamoxifen. To conclude, tamoxifen has been studied primarily in retrospective studies, involving recurrent, heavily pretreated, platinum resistant ovarian cancers, treated irrespective of ER status. Tamoxifen activity in advanced ovarian cancer has not been systematically evaluated and its role may have been underestimated because the target responsive population has not been defined.
Table 1.
Tamoxifen in treatment or recurrent or resistant EOC
| Study | N | Tamoxifen dose | CR | PR | SD | PD | Median survival (months) | Mean response duration (months) |
ER +status |
|---|---|---|---|---|---|---|---|---|---|
| Karagol et at (2007) [100] | 29 | 20 mg BID | 1 (3) | 2 (7) | 6 (21) | 20 (69) | 3 (SD +PD) vs 15 (CR + PR) | NS | NS |
| Hatch et al (2006) [63] | 105 | 20 mg BID | 10 (10) | 8 (8) | 40 (38) | 47 (45) | NS | 3 (for PR and SD) vs 7.5 (CR) | 62/105 |
| Rolski et al (1998) [101] | 47 | 40 mg daily | 1 (2) | 2 (4) | 22(47) | NA | NA | 6.9 | NS |
| Marth et al (1997) [102] | 65 | 30–40 mg daily | 2 (3) | 2 (3) | 50 (77) | 11 (17) | 5.5 (SD +PD) vs 6.2 (CR+ PR) | NS | NS |
| Gennatas et al (1996) [64] | 50 | 40 mg daily | 2 (4) | 26 (52) | NS | NS | NS | 18 | NS |
| Van Der Velden et al (1995) [66] | 30 | 20 mg BID | 2 (7) | 0 | 10 (33) | NS | NS | 11.5 | NS |
| Jager et al (1995) [103] | 33 | 30 mg daily | 0 | 0 | 2 (6) | NS | NS | NS | NS |
| Van der Vange et al (1995) [104] | 6 | 20 mg BID | 0 | 1 (17) | 1 (17) | NS | NS | NS | NS |
| Losa et al (1993) [105] | NA | 40 mg daily | 0 | 1 | 22 | 32 | NS | NS | NS |
| Ahlgren et al (1993) [67] | 29 | 80 mg qd × 30 days, f/u by 40 mg daily | 2 (7) | 7 (24) | 18 (62) | 6 (21) | 5 (NR) vs 36 (R) | 2 (NR) vs 24 (R) | NS |
| Osborne et al (1988) [106] | 51 | 100 mg/m2 in 24 hrs, f/u by 20 mg BID | 1 (2) | 0 | 0 | 50 (98) | NS | 2 | NS |
| Weiner et al (1987) [71] | 31 | 40 mg/m2 qd × 7, f/u by 10 mg bid | 1 (3) | 2 (6) | 6 (19) | 22 (71) | 7 (NR) vs 16 (R) | 14 | 4/11 |
| Quinn et al (1987) [65] | 40 | 20 mg BID | 5 (13) | 4 (10) | 12 (30) | NS | NS | NS | NS |
| Slevin et al (1986) [107] | 22 | 20 mg daily | 0 | 0 | 1(5) | 21 (95) | 3.5 (SD +PD) | 3 | NS |
| Landoni et al (1985) [108] | 55 | 40 mg daily | 0 | 0 | 19 (35) | NS | NS | NS | NS |
| Shirey et al (1985) [70] | 23 | 20–40 mg daily | 0 | 0 | 19 (83) | NS | NS | 2.5 | 6/23 |
| Hamerlynck et al (1985) [109] | 36 | 40 mg daily | 0 | 2 (6) | 7 (19) | NS | NS | NS | NS |
| Rowland et al (1985) [110] | 9 | 20 mg daily | 0 | 0 | NS | NS | NS | NS | 5/9 |
| Pagel et al (1983) [111] | 21 | NS | 1 (5) | 7 (3) | 12 (57) | NS | NS | NS | 10/12 |
| Schwartz et al (1982) [112] | 13 | 20 mg daily, increased to 40 mg for progression | 0 | 1 (8) | 4 (31) | 8 (62) | NS | NS | 4/13 |
| Total | 695 | 28 (4) | 65 (9) | 251 (35) | 217 (36) | 6.2–36 (R) | 2–24 | 91/173 |
CR: complete response, PR: partial response, SD stable disease, PD: progressive disease, R: responders, NR: non responder, NS: not stated, NA: not available
Tamoxifen has been studied in a limited manner when used as first line treatment after surgery for advanced disease or to augment effects of post-surgical chemotherapy (called “consolidation”) for advanced ovarian cancer. A randomized prospective trial (n=100) of stage III/IV patients treated with first line chemotherapy including cisplatin and doxorubicin with or without tamoxifen, found no difference between PFS and OS between both groups [69]. Limitations to this trial are that the majority of patients (54%) had residual disease (≥2cm) and tamoxifen was given only with chemotherapy for only 36 weeks which is much shorter than that recommended for breast cancer use in this setting. Tamoxifen was recently evaluated in the “consolidation” setting in a phase III randomized trial investigating thalidomide vs tamoxifen in patients with biochemical recurrence (where the tumor marker, CA-125, increased by two-fold but there was no radiographical evidence of disease) after first-line chemotherapy. Thalidomide effects were similar to the tamoxifen treated group in terms of progression free survival (PFS) but the thalidomide group had a worse median and overall survival with a higher risk of death (HR=1.76, 95% CI=1.16–2.68) and it was significantly more toxic [72]. Unfortunately, there was no control group without either drug, thus it was not clear if either drug gave benefit over no additional therapy. Markman et al retrospectively reviewed 56 women with asymptomatic recurrent ovarian cancer treated with tamoxifen prior to initiation of cytotoxic chemotherapy [73]. The median treatment duration on tamoxifen was 3 months, but 42% of patients were on tamoxifen for ≥ 6 months and 19% for ≥ 12 months. Reasons for discontinuation of tamoxifen were development of symptoms, radiographical evidence of disease, or continuing rise in CA-125 tumor marker. No standard of care exists for management of asymptomatic recurrent disease. Clinical trials investigating hormonal therapy in this setting are warranted. In summary, antiestrogens have not been thoroughly evaluated in the adjuvant or consolidation setting for early or advanced ovarian cancer with low volume disease.
The pure ER antagonist, Fulvestrant, has also shown activity in recurrent ovarian cancer. Recently, a small phase II trial for 31 heavily pretreated ER+ (preselected) recurrent ovarian cancer patients showed a 38% clinical benefit for fulvestrant (1 CR, 1 PR, 11 pt or 35% SD at 90 days) with no grade 2, 3 or 4 toxicities and quality of life scores improved [74]. Well designed prospective trials are needed to carefully evaluate the role of anti-estrogens and the predictive role of receptor status in ovarian cancer.
Aromatase expression & effects of aromatase inhibitors in ovarian cancer
Aromatase, expressed in fat, liver, muscle, brain, normal breast tissue, and breast and ovarian cancers [75], converts adrenal androstenedione to estrogen and is the predominate source of estrogen in postmenopausal women. There is increasing data that intra-tumoral estrogens, derived from in situ aromatization, function as autocrine growth factors that prompt cancer cell proliferation independently of circulating estrogen [76]. Aromatase inhibitors (AI) reduce estrogen production in post menopausal women by more than 90%. Aromatase expression, analyzed as either aromatase activity, mRNA expression or protein levels, has been found in 33–81% of ovarian cancers [77;78] This wide variability in aromatase detection results from small study size (average 20–40 specimens per study) and different methods used to measure activity (activity assays and mRNA). Aromatase is expressed in ovarian cancer epithelial cells, and in nearby stroma where there is frank ovarian cancer invasion [78;79].
AIs have shown some therapeutic activity against recurrent ovarian cancers, with modest response rates, and they may also stabilize disease. AIs have been shown to be superior to tamoxifen when used as adjuvant therapy for breast cancer [80]. In vitro studies demonstrated anti-tumor effects of AI on ovarian cancer cells which correlated with aromatase activity and ER expression [81]. In nine clinical trials (6 of letrozole, 2 of anastrozole and 1 of exemestane) totaling 300 patients, AIs for recurrent ovarian cancer have produced overall response rates of 8% (CR 1%, PR 7% and SD rates of 33%) (See Table 2). Few of these studies preselected patients based on hormone receptor or aromatase expression. One study by Smyth et al. recently showed that preselecting patients for study enrollment based on ER expression improved response to letrozole for recurrent ovarian cancer. In a study of 42 patients, overall response increased from 9% to 17% when patients were preselected for ER, and disease stabilization improved from 25 to 36%. These response rates approach that of salvage chemotherapy but with far less toxicity [82;83].
Table 2.
Aromatase inhibitors in persistent or recurrent EOC
| Study | N | Drug | CR | PR | SD | PD | Median Survival (months) |
Median time to progression(months) |
ER + status |
|---|---|---|---|---|---|---|---|---|---|
| Ramirez et al (2008) [113] | 33 | Letrozole | 0 | 1 (3) | 7 (21) | 23 (70) | 5.6 (NR) vs 10.9 (PR +SD) | 2 (SD) vs 4 (PR) | 33/33 (all platinum resistent) |
| Smyth et al (2007) [114] | 42 | Letrozole | 0 | 7 (17) | 11 (26) | NS | 11 (26) PFS >6mo, 2(5) PFS >2 yrs | NS | 42/42 |
| Kavanagh et al (2007) [84] | 13 | Letrozole | 2 (15) | 2 (15) | 5 (38) | 4 (31) | NS | NS | 13/13 |
| Gourley et al (2006) [115] | 33 | Letrozole | 0 | 3 (9) | 14 (42) | 16 (49) | NS | NS | NS |
| Papadimitriou et al (2004) [116] | 27 | Letrozole | 1 (4) | 3 (11) | 5 (18) | 18 (67) | 26.5 (NR) vs not reached for R +SD | 2.4 (NR) vs 17.6 (R+SD) | 20/27 |
| Bowman et al (2002) [82] | 54 | Letrozole | 0 | 5 (9) | 14 (26) | 30 (56) | 14 | NS | Mixed ER−/ER+ |
| Krasner et al (2005) [117] | 23 | Anastrozole | 1 (4) | 0 | 14 (61) | NS | NS | NS | 23/23 |
| Del Carmen et al (2003) [118] | 53 | Anastrozole | 0 | 1 (2) | 22 (42) | 30 (57) | NS | 2.8 | Mixed ER−/ER+ |
| Verma et al (2006) [119] | 22 | Exemestane | 0 | 0 | 8 (36) | NS | NS | 2.2 | 16/22 |
| Total | 300 | 4 (1) | 22 (7) | 100(33) | 121 (40) | 147/160 |
CR: complete response, PR: partial response, SD stable disease, PD: progressive disease, R: responders, NR: non responder, NS: not stated, ER: estrogen receptor
Biomarkers such as ER, PR, EGFR and HER-2 have been investigated as predictors of AI response for ovarian cancer. Results are encouraging but inconsistent. Three of nine trials using AIs for recurrent disease, showed biomarker levels in the diagnostic tumor correlated with response [82–84]. One study of 41 patients and another of 42 treated with letrozole showed elevated (pre-treatment) levels of ER, PR, EGFR and decreased HER-2 expression significantly correlated with better response. Another small study also showed ER/PR correlated with response. However, three studies (n=96 pts) showed no correlation between ER/PR or HER2 levels in the tumor at diagnosis and response. This discrepancy may be due to differences in ER assays, study size, or prior exposure to hormonal agents. Since not all patients will benefit from AIs it is important to develop molecular identifiers to predict response in ovarian cancer. In recurrent ovarian cancer, AIs appear to yield a similar clinical benefit (disease stabilization plus partial response) to tamoxifen (48% vs 41%). Given their favorable safety profiles, convenient use and moderate efficacy in the recurrent setting, AIs are a rational treatment option for ovarian cancer.
Mechanisms of growth arrest by aromatase inhibitors and anti-estrogens
Estrogen deprivation and ER blockade cause breast and ovarian cancer cells to arrest in the G1 phase of the cell cycle [15;85]. G1 cell cycle progression is governed by a family of cyclin-dependent kinases (cdks) that are activated by binding of different cyclins and inhibited by cdk inhibitors, such as p21 and p27. ER blockade by tamoxifen, fulvestrant or estrogen withdrawal arrests cells in G1 by stabilizing p27 [15;85]. In normal rhesus ovarian surface epithelium, low dose estrogen, tamoxifen or fulvestrant all caused G1 arrest by induction of p53 and p21 [86]. Our work and that of others has shown that cell cycle regulation and in particular the mechanisms of G1 arrest mediated by anti-estrogens in ovarian cancer are similar to that in breast cancer [12;13;15;85;87].
Mechanism of resistance to anti-estrogens in ovarian cancer
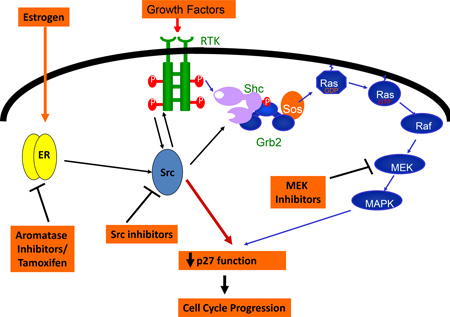
Although hormonal therapies as single agents are well tolerated and show modest activity in recurrent ovarian cancer, the benefit is of short duration with an average of 3–4 months. In both breast and ovarian cancer, many patients manifest initial de novo resistance and those that do initially respond, often develop resistance to hormonal agents. In breast cancer, resistance can arise through cross-talk between ER and constitutively activated receptor tyrosine kinase (RTK) pathways [88]. Until recently, ligand activated ER transcriptional activity was thought to be the major mechanism whereby ER regulates cell behavior. Cross talk between estrogen bound ER and signal transduction may account for more ER-mediated functions than previously recognized. These effects do not initially affect estrogen dependent transcription and have been termed non-genomic actions of the ER. In breast cancer, it has been shown that estrogen stimulates breast cancer proliferation by rapid estrogen-stimulated ER-dependent activation of cSrc and of MEK/MAPK pathways, leading to p27 degradation and G1 progression in breast cancer cells [87;89–91].
The Src, Ras/Raf/MAPK and PI3K/AKT pathways have all been implicated in ovarian cancer proliferation, invasion, metastasis and survival [92;93]. cSrc can activate both Ras/Raf/MAPK and PI3K/AKT pathways downstream of RTKs (EGFR/HER2, PDGFR), and is over-expressed and activated in ovarian cancer cell lines and tumors compared to normal ovarian epithelium [94]. Src directly associates with EGFR and HER2, both of which are involved in ovarian tumorigenesis. Src inhibition reverses resistance to platinums and taxanes in ovarian cancer cells [95]. There is evidence for cross-talk between HER2/MAPK and estrogen signaling in ovarian cancer [96]. Recent pre-clinical data showed that a MEK inhibitor combined with fulvestrant additively decreased ovarian cancer cell growth via increased p21 and p53 in vitro and decreased endometrial cancer in vivo [86]. We recently found that Src inhibition with saracatinib reverses fulvestrant resistance in ER+ ovarian cancer cell lines and in an ovarian cancer xenograft model. Estrogen activated Src promoted Src binding to the ER in the cytoplasm and ER translocation to the nucleus. Src inhibition together with ER blockade more effectively arrested cell cycle progression and inhibited transcription of ER target genes such as Myc and FOSL1 [15]. A better understanding of ER signaling in ovarian cancer will permit refinement of combinations of targeted therapy with standard hormonal agents to improve treatment.
There is also evidence that ERα may be activated by kinase pathways in a ligand independent manner in ovarian cancer. One study showed ERK2 mediated phosphorylation of ERα in response to CD44’s [a major hyaluronan (HA)1 receptor] interaction with hyaluronan and IQGAP1, independent of estrogen in ovarian cancer cells [97]. In addition, there is evidence for crosstalk between ERα and leptin signaling [98]. Leptin promotes ovarian cancer cell proliferation, at least in part, by ER transcriptional activation via the STAT-3 signaling pathway mediated by ERK and PI3K pathways independent of estrogen [98]. Thus targeting crosstalk between liganded and/or un-liganded ER and kinase signaling pathways may have potential to overcome anti-estrogen resistance.
CONCLUSION
Ovarian cancer arises from surface epithelium which expresses ERα receptors. The observation that more than 60% of primary ovarian and breast tumors express epithelial ERα suggests there could be parallels between estrogen action in ovarian and breast cancer cells [15;49;54]. The ERs (ERα and ERβ) belong to the nuclear receptor family and function as hormone-inducible transcription factors in target cells [99]. Growth response to estrogens promote proliferation in ovarian cancer cell lines by ERα and not by ERβ [15;18]. Despite the majority of ovarian cancers expressing the ER, antiestrogens have shown disappointing therapeutic efficacy in part due to lack of verification of ER target expression and also due to drug resistance. It is unclear whether ER expression is a predictor of response to antiestrogens since most trials failed to preselect patients based on ER and did not correlate response with ER status. Molecular analysis of gene expression or pharmacogenomics may help identify new markers that are more predictive of response. There may prove to be a role for anti-estrogens in the post-operative (adjuvant) setting in OVCA to prevent or reduce cancer recurrence as is the case for breast cancer. Unfortunately, trials evaluating anti-estrogens in ovarian cancer patients in a true adjuvant setting have never been initiated. The trials that have compared chemotherapy with and without hormonal therapy for recurrent disease are inconclusive due to small sample size, inadequate treatment design and lack of ER ascertainment. Patients most likely to benefit would likely be those with optimally debulked ER positive tumors after post-operative chemotherapy. Such an adjuvant therapy study would require a cooperative group effort to permit randomization of sufficient patients to placebo vs anti-estrogen in this context.
Preclinical models demonstrate that targeting estrogen activated kinase pathways in combination with ER blockade reverses anti-estrogen resistance in ovarian cancer [15]. Given the preclinical and early clinical data, combinations of hormonal and molecular targeted therapies warrant further investigation in clinical trials for ovarian cancer. Although significant advances have been made in our understanding of ER action in breast cancer, the molecular pathways involved in hormone-stimulated proliferation in the ovary remain less clear. Defining ER-stimulated pathways that mediate proliferation in response to hormone will clarify the role of the receptor and its target genes in the onset and progression of ER+ ovarian cancers. This type of research may yield much needed new diagnostic or prognostic markers for clinical use. Such investigations may also reveal whether the mitogenic actions of estrogen in the ovary and breast occur through common mechanisms and ultimately provide avenues for development of improved ER-targeted therapeutics.
Highlights.
Most OVCAs express estrogen receptor and estrogens drive ovarian carcinogenesis
Anti-estrogen therapies are well tolerated in recurrent OVCA with minimal toxicity
Efficacy of Anti-estrogen therapies is limited by resistance
Targeting kinase pathways with ER blockade is a mechanism to reverse anti-estrogen resistance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Hensley ML. Epithelial ovarian cancer. Curr Treat Options Oncol. 2002 Apr;3(2):131–141. doi: 10.1007/s11864-002-0059-3. [DOI] [PubMed] [Google Scholar]
- 2.Tummala MK, McGuire WP. Recurrent ovarian cancer. Clin Adv Hematol Oncol. 2005 Sep;3(9):723–736. [PubMed] [Google Scholar]
- 3.Makar AP. Hormone therapy in epithelial ovarian cancer. Endocr Relat Cancer. 2000 Jun;7(2):85–93. doi: 10.1677/erc.0.0070085. [DOI] [PubMed] [Google Scholar]
- 4.Prior JC. Ovarian aging and the perimenopausal transition: the paradox of endogenous ovarian hyperstimulation. Endocrine. 2005 Apr;26(3):297–300. doi: 10.1385/ENDO:26:3:297. [DOI] [PubMed] [Google Scholar]
- 5.Quirk JT, Natarajan N, Mettlin CJ. Age-specific ovarian cancer incidence rate patterns in the United States. Gynecol Oncol. 2005 Oct;99(1):248–250. doi: 10.1016/j.ygyno.2005.06.052. [DOI] [PubMed] [Google Scholar]
- 6.Beral V. Ovarian cancer and hormone replacement therapy in the Million Women Study. The Lancet. 2007;369(9574):1703–1710. doi: 10.1016/S0140-6736(07)60534-0. [DOI] [PubMed] [Google Scholar]
- 7.Pearce CL, Chung K, Pike MC, Wu AH. Increased ovarian cancer risk associated with menopausal estrogen therapy is reduced by adding a progestin. Cancer. 2009;115(3):531–539. doi: 10.1002/cncr.23956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez C, Patel AV, Calle EE, Jacob EJ, Thun MJ. Estrogen replacement therapy and ovarian cancer mortality in a large prospective study of US women. J Am Med Assoc. 2001 Mar 21;285(11):1460–1465. doi: 10.1001/jama.285.11.1460. [DOI] [PubMed] [Google Scholar]
- 9.Riman T, Dickman PW, Nilsson S, Correia N, Nordlinder H, Magnusson CM, et al. Hormone replacement therapy and the risk of invasive epithelial ovarian cancer in Swedish women. J Natl Cancer Inst. 2002 Apr 3;94(7):497–504. doi: 10.1093/jnci/94.7.497. [DOI] [PubMed] [Google Scholar]
- 10.Lacey JV, Jr, Mink PJ, Lubin JH, Sherman ME, Troisi R, Hartge P, et al. Menopausal hormone replacement therapy and risk of ovarian cancer. J Am Med Assoc. 2002 Jul 17;288(3):334–341. doi: 10.1001/jama.288.3.334. [DOI] [PubMed] [Google Scholar]
- 11.Glud E, Kjaer SK, Thomsen BL, Hogdall C, Christensen L, Hogdall E, et al. Hormone therapy and the impact of estrogen intake on the risk of ovarian cancer. Arch Intern Med. 2004 Nov 8;164(20):2253–2259. doi: 10.1001/archinte.164.20.2253. [DOI] [PubMed] [Google Scholar]
- 12.Langdon SP, Hirst GL, Miller EP, Hawkins RA, Tesdale AL, Smyth JF, et al. The regulation of growth and protein expression by estrogen in vitro: a study of 8 human ovarian carcinoma cell lines. J Steroid Biochem Mol Biol. 1994 Aug;50(3–4):131–135. doi: 10.1016/0960-0760(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 13.Langdon SP, Crew AJ, Ritchie AA, Muir M, Wakeling A, Smyth JF, et al. Growth inhibition of oestrogen receptor-positive human ovarian carcinoma by anti-oestrogens in vitro and in a xenograft model. Eur J Cancer. 1994;30A(5):682–686. doi: 10.1016/0959-8049(94)90545-2. [DOI] [PubMed] [Google Scholar]
- 14.Armaiz-Pena GN, Mangala LS, Spannuth WA, Lin YG, Jennings NB, Nick AM, et al. Estrous cycle modulates ovarian carcinoma growth. Clin Cancer Res. 2009 May 1;15(9):2971–2978. doi: 10.1158/1078-0432.CCR-08-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simpkins F, Hevia-Paez P, Sun J, Ullmer W, Gilbert CA, da Silva T, et al. Src Inhibition with Saracatinib Reverses Fulvestrant Resistance in ER-Positive Ovarian Cancer Models In Vitro and In Vivo. Clinical Cancer Research. 2012 Aug 15; doi: 10.1158/1078-0432.CCR-12-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pujol P, Rey JM, Nirde P, Roger P, Gastaldi M, Laffargue F, et al. Differential expression of estrogen receptor-alpha and -beta messenger RNAs as a potential marker of ovarian carcinogenesis. Cancer Res. 1998 Dec 1;58(23):5367–5373. [PubMed] [Google Scholar]
- 17.Bardin A, Hoffmann P, Boulle N, Katsaros D, Vignon F, Pujol P, et al. Involvement of Estrogen Receptor Beta in Ovarian Carcinogenesis. Cancer Research. 2004 Aug 15;64(16):5861–5869. doi: 10.1158/0008-5472.CAN-04-0552. [DOI] [PubMed] [Google Scholar]
- 18.O'Donnell AJ, Macleod KG, Burns DJ, Smyth JF, Langdon SP. Estrogen receptor-alpha mediates gene expression changes and growth response in ovarian cancer cells exposed to estrogen. Endocr Relat Cancer. 2005 Dec;12(4):851–866. doi: 10.1677/erc.1.01039. [DOI] [PubMed] [Google Scholar]
- 19.Matthews J, Gustafsson JA. Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol Interv. 2003 Aug 1;3(5):281–292. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- 20.Brandenberger AW, Tee MK, Jaffe RB. Estrogen Receptor Alpha (ER-alpha) and Beta (ER-beta) mRNAs in Normal Ovary, Ovarian Serous Cystadenocarcinoma and Ovarian Cancer Cell Lines: Down-Regulation of ER-beta in Neoplastic Tissues. Journal of Clinical Endocrinology & Metabolism. 1998 Mar 1;83(3):1025–1028. doi: 10.1210/jcem.83.3.4788. [DOI] [PubMed] [Google Scholar]
- 21.Hillier SG, Anderson RA, Williams AR, Tetsuka M. Expression of oestrogen receptor alpha and beta in cultured human ovarian surface epithelial cells. Molecular Human Reproduction. 1998 Aug 1;4(8):811–815. doi: 10.1093/molehr/4.8.811. [DOI] [PubMed] [Google Scholar]
- 22.Rutherford T, Brown WD, Sapi E, Aschkenazi S, Munoz A, Mor G. Absence of estrogen receptor-beta expression in metastatic ovarian cancer. Obstet Gynecol. 2000 Sep 1;96(3):417–421. doi: 10.1016/s0029-7844(00)00917-0. [DOI] [PubMed] [Google Scholar]
- 23.Fujimura M, Hidaka T, Kataoka K, Yamakawa Y, Akada S, Teranishi A, et al. Absence of Estrogen Receptor-[alpha] Expression in Human Ovarian Clear Cell Adenocarcinoma Compared With Ovarian Serous, Endometrioid, and Mucinous Adenocarcinoma. Am J Surg Pathol. 2001;25(5) doi: 10.1097/00000478-200105000-00016. [DOI] [PubMed] [Google Scholar]
- 24.Li AJ, Baldwin RL, Karlan BY. Estrogen and progesterone receptor subtype expression in normal and malignant ovarian epithelial cell cultures. American Journal of Obstetrics and Gynecology. 2003 Jul;189(1):22–27. doi: 10.1067/mob.2003.328. [DOI] [PubMed] [Google Scholar]
- 25.Lindgren PR, Cajander S, Backstrom T, Gustafsson J-A, Makela S, Olofsson JI. Estrogen and progesterone receptors in ovarian epithelial tumors. Molecular and Cellular Endocrinology. 2004 Jun 30;221(1–2):97–104. doi: 10.1016/j.mce.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 26.Fixemer T, Remberger K, Bonkhoff H. Differential expression of the estrogen receptor beta (ER-beta) in human prostate tissue, premalignant changes, and in primary, metastatic, and recurrent prostatic adenocarcinoma. The Prostate. 2003;54(2):79–87. doi: 10.1002/pros.10171. [DOI] [PubMed] [Google Scholar]
- 27.Foley EF, Jazaeri AA, Shupnik MA, Jazaeri O, Rice LW. Selective Loss of Estrogen Receptor Beta in Malignant Human Colon. Cancer Research. 2000 Jan 1;60(2):245–248. [PubMed] [Google Scholar]
- 28.Park BW, Kim KS, Heo MK, Ko SS, Hong SW, Yang WI, et al. Expression of estrogen receptor-beta in normal mammary and tumor tissues: is it protective in breast carcinogenesis? Breast Cancer Res Treat. 2003 Jul 1;80(1):79–85. doi: 10.1023/A:1024406223619. [DOI] [PubMed] [Google Scholar]
- 29.Roger P, Sahla ME, Makela S, Gustafsson J-A, Baldet P, Rochefort H. Decreased Expression of Estrogen Receptor Beta Protein in Proliferative Preinvasive Mammary Tumors. Cancer Research. 2001 Mar 3;61(6):2537–2541. [PubMed] [Google Scholar]
- 30.Skliris GP, Munot K, Bell SM, Carder PJ, Lane S, Horgan K, et al. Reduced expression of oestrogen receptor beta in invasive breast cancer and its re-expression using DNA methyl transferase inhibitors in a cell line model. J Pathol. 2003;201(2):213–220. doi: 10.1002/path.1436. [DOI] [PubMed] [Google Scholar]
- 31.Lazennec G, Bresson D, Lucas A, Chauveau C, Vignon F. ER-beta Inhibits Proliferation and Invasion of Breast Cancer Cells. Endocrinology. 2001 Sep 1;142(9):4120–4130. doi: 10.1210/endo.142.9.8395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng J, Lee EJ, Madison LD, Lazennec G. Expression of estrogen receptor-beta in prostate carcinoma cells inhibits invasion and proliferation and triggers apoptosis. FEBS Letters. 2004 May 21;566(1–3):169–172. doi: 10.1016/j.febslet.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 33.Treeck O, Pfeiler G, Mitter D, Lattrich C, Piendl G, Ortmann O. Estrogen receptor - beta1 exerts antitumoral effects on SK-OV-3 ovarian cancer cells. Journal of Endocrinology. 2007 Jun 1;193(3):421–433. doi: 10.1677/JOE-07-0087. [DOI] [PubMed] [Google Scholar]
- 34.Lazennec G. Estrogen receptor beta, a possible tumor suppressor involved in ovarian carcinogenesis. Cancer Letters. 2006 Jan 18;231(2):151–157. doi: 10.1016/j.canlet.2005.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu MM, Albanese C, Anderson CM, Hilty K, Webb P, Uht RM, et al. Opposing Action of Estrogen Receptors alpha and beta on Cyclin D1 Gene Expression. Journal of Biological Chemistry. 2002 Jul 5;277(27):24353–24360. doi: 10.1074/jbc.M201829200. [DOI] [PubMed] [Google Scholar]
- 36.Planas-Silva MD, Weinberg RA. Estrogen-dependent cyclin E-cdk2 activation through p21 redistribution. Mol Cell Biol. 1997;17(7):4059–4069. doi: 10.1128/mcb.17.7.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Worsley SD, Ponder BA, Davies BR. Overexpression of cyclin D1 in epithelial ovarian cancers. Gynecol Oncol. 1997 Feb;64(2):189–195. doi: 10.1006/gyno.1996.4569. [DOI] [PubMed] [Google Scholar]
- 38.Chan KK, Wei N, Liu SS, Xiao-Yun L, Cheung AN, Ngan HY. Estrogen receptor subtypes in ovarian cancer: a clinical correlation. Obstet Gynecol. 2008 Jan;111(1):144–151. doi: 10.1097/01.AOG.0000296715.07705.e9. [DOI] [PubMed] [Google Scholar]
- 39.De Stefano I, Zannoni GF, Prisco MG, Fagotti A, Tortorella L, Vizzielli G, et al. Cytoplasmic expression of estrogen receptor beta (ER-beta) predicts poor clinical outcome in advanced serous ovarian cancer. Gynecologic Oncology. 2011 Sep;122(3):573–579. doi: 10.1016/j.ygyno.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 40.Esslimani-Sahla M, Simony-Lafontaine J, Kramar A, Lavaill R, Mollevi C, Warner M, et al. Estrogen Receptor Beta (ER-beta) Level but Not Its ER-beta cx Variant Helps to Predict Tamoxifen Resistance in Breast Cancer. Clinical Cancer Research. 2004 Sep 1;10(17):5769–5776. doi: 10.1158/1078-0432.CCR-04-0389. [DOI] [PubMed] [Google Scholar]
- 41.Park BW, Kim KS, Heo MK, Yang WI, Kim SI, Kim JH, et al. The changes of estrogen receptor-beta variants expression in breast carcinogenesis: Decrease of estrogen receptor-beta2 expression is the key event in breast cancer development. J Surg Oncol. 2006;93(6):504–510. doi: 10.1002/jso.20336. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki F, Akahira Ji, Miura I, Suzuki T, Ito K, Hayashi Si, et al. Loss of estrogen receptor beta isoform expression and its correlation with aberrant DNA methylation of the 5'-untranslated region in human epithelial ovarian carcinoma. Cancer Science. 2008;99(12):2365–2372. doi: 10.1111/j.1349-7006.2008.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leung Yk, Mak P, Hassan S, Ho SM. Estrogen receptor (ER)-beta isoforms: A key to understanding ER-beta signaling. Proceedings of the National Academy of Sciences. 2006 Aug 29;103(35):13162–13167. doi: 10.1073/pnas.0605676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao C, Lam EWF, Sunters A, Enmark E, De Bella MT, Coombes RC, et al. Expression of estrogen receptor [beta] isoforms in normal breast epithelial cells and breast cancer: regulation by methylation. Oncogene. 2003 Oct 23;22(48):7600–7606. doi: 10.1038/sj.onc.1207100. [DOI] [PubMed] [Google Scholar]
- 45.Zhu X, Leav I, Leung Yk, Wu M, Liu Q, Gao Y, et al. Dynamic Regulation of Estrogen Receptor-beta Expression by DNA Methylation During Prostate Cancer Development and Metastasis. The American Journal of Pathology. 2004 Jun;164(6):2003–2012. doi: 10.1016/s0002-9440(10)63760-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsai MJ, O'Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994 Jan 1;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 47.Klinge CM. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res. 2001 Jul 15;29(14):2905–2919. doi: 10.1093/nar/29.14.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carroll JS, Brown M. Estrogen receptor target gene: an evolving concept. Mol Endocrinol. 2006 Aug;20(8):1707–1714. doi: 10.1210/me.2005-0334. [DOI] [PubMed] [Google Scholar]
- 49.Rao BR, Slotman BJ. Endocrine role in ovarian cancer. Endocr Relat Cancer. 1996;3(4):309–326. [Google Scholar]
- 50.Hogdall EV, Christensen L, Hogdall CK, Blaakaer J, Gayther S, Jacobs IJ, et al. Prognostic value of estrogen receptor and progesterone receptor tumor expression in Danish ovarian cancer patients: from the 'MALOVA' ovarian cancer study. Oncol Rep. 2007 Nov;18(5):1051–1059. [PubMed] [Google Scholar]
- 51.Schlumbrecht MP, Xie SS, Shipley GL, Urbauer DL, Broaddus RR. Molecular clustering based on ER[alpha] and EIG121 predicts survival in high-grade serous carcinoma of the ovary/peritoneum. Mod Pathol. 2011 Mar;24(3):453–462. doi: 10.1038/modpathol.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rio MC, Bellocq JP, Gairard B, Rasmussen UB, Krust A, Koehl C, et al. Specific expression of the pS2 gene in subclasses of breast cancers in comparison with expression of the estrogen and progesterone receptors and the oncogene ERBB2. Proceedings of the National Academy of Sciences. 1987 Dec 1;84(24):9243–9247. doi: 10.1073/pnas.84.24.9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rochefort H. Estrogen-induced genes in breast cancer, and their medical importance. Bull Acad Natl Med. 1999 Jan 1;183(5):955–968. [PubMed] [Google Scholar]
- 54.Jordan VC, Morrow M. Tamoxifen, Raloxifene, and the Prevention of Breast Cancer. Endocrine Reviews. 1999 Jun 1;20(3):253–278. doi: 10.1210/edrv.20.3.0368. [DOI] [PubMed] [Google Scholar]
- 55.Rochefort H, Chalbos D, Cunat S, Lucas A, Platet N, Garcia M. Estrogen regulated proteases and antiproteases in ovarian and breast cancer cells. The Journal of Steroid Biochemistry and Molecular Biology. 2001 Mar;76(1–5):119–124. doi: 10.1016/s0960-0760(00)00142-4. [DOI] [PubMed] [Google Scholar]
- 56.Albanito L, Madeo A, Lappano R, Vivacqua A, Rago V, Carpino A, et al. G Protein-Coupled Receptor 30 (GPR30) Mediates Gene Expression Changes and Growth Response to 17beta-Estradiol and Selective GPR30 Ligand G-1 in Ovarian Cancer Cells. Cancer Research. 2007 Feb 15;67(4):1859–1866. doi: 10.1158/0008-5472.CAN-06-2909. [DOI] [PubMed] [Google Scholar]
- 57.Simpson BJB, Langdon SP, Rabiasz GJ, Macleod KG, Hirst GL, Bartlett JMS, et al. Estrogen regulation of transforming growth factor-alpha in ovarian cancer. The Journal of Steroid Biochemistry and Molecular Biology. 1998 Feb 1;64(3–4):137–145. doi: 10.1016/s0960-0760(97)00159-3. [DOI] [PubMed] [Google Scholar]
- 58.Hall JM, Korach KS. Stromal Cell-Derived Factor 1, a Novel Target of Estrogen Receptor Action, Mediates the Mitogenic Effects of Estradiol in Ovarian and Breast Cancer Cells. Molecular Endocrinology. 2003 May 1;17(5):792–803. doi: 10.1210/me.2002-0438. [DOI] [PubMed] [Google Scholar]
- 59.Chien CH, Wang FF, Hamilton TC. Transcriptional activation of c-myc proto-oncogene by estrogen in human ovarian cancer cells. Mol Cell Endocrinol. 1994 Feb 1;99(1):11–19. doi: 10.1016/0303-7207(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 60.Moll F, Katsaros D, Lazennec G, Hellio N, Roger P, Giacalone PL, et al. Estrogen induction and overexpression of fibulin-1C mRNA in ovarian cancer cells. Oncogene. 2002 Feb 7;21(7):1097–1107. doi: 10.1038/sj.onc.1205171. [DOI] [PubMed] [Google Scholar]
- 61.Clinton GM, Rougeot C, Derancourt J, Roger P, Defrenne A, Godyna S, et al. Estrogens increase the expression of fibulin-1, an extracellular matrix protein secreted by human ovarian cancer cells. Proceedings of the National Academy of Sciences. 1996 Jan 9;93(1):316–320. doi: 10.1073/pnas.93.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kelley KMM, Rowan BG, Ratnam M. Modulation of the Folate Receptor Alpha Gene by the Estrogen Receptor: Mechanism and Implications in Tumor Targeting. Cancer Research. 2003 Jun 1;63(11):2820–2828. [PubMed] [Google Scholar]
- 63.Hatch KD, Beecham JB, Blessing JA, Creasman WT. Responsiveness of patients with advanced ovarian carcinoma to tamoxifen. A Gynecologic Oncology Group study of second-line therapy in 105 patients. Cancer. 1991 Jul 15;68(2):269–271. doi: 10.1002/1097-0142(19910715)68:2<269::aid-cncr2820680209>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 64.Gennatas C, Dardoufas C, Karvouni H, Kairi E, Zourlas P. Phase II trial of tamoxifen in patients with advanced epithelial ovarian cancer. Proc Am Soc Clin Oncol. 1996;15 [Google Scholar]
- 65.Quinn MA. Hormonal therapy of ovarian cancer. In: Sharp F, Scoute WP, editors. Ovarian cancer: the way ahead. London: Royal College of Obstetricians and Gynecologi; 1987. pp. 383–393. [Google Scholar]
- 66.Van Der Velden J, Gitsch G, Wain GV, Friedlander ML, Hacker NF. Tamoxifen in patients with advanced epithelial ovarian cancer. Int J Gynecol Cancer. 1995 Jul 1;5(4):301–305. doi: 10.1046/j.1525-1438.1995.05040301.x. [DOI] [PubMed] [Google Scholar]
- 67.Ahlgren JD, Ellison NM, Gottlieb RJ, Laluna F, Lokich JJ, Sinclair PR, et al. Hormonal palliation of chemoresistant ovarian cancer: three consecutive phase II trials of the Mid-Atlantic Oncology Program. Journal of Clinical Oncology. 1993 Oct 1;11(10):1957–1968. doi: 10.1200/JCO.1993.11.10.1957. [DOI] [PubMed] [Google Scholar]
- 68.Jordan VC. Studies on the estrogen receptor in breast cancer - 20 years as a target for the treatment and prevention of cancer. Breast Cancer Res Treat. 1995 Jan 1;36:267–285. doi: 10.1007/BF00713399. [DOI] [PubMed] [Google Scholar]
- 69.Schwartz PE, Chambers JT, Kohorn EI, Chambers SK, Weitzman H, Voynick IM, et al. Tamoxifen in combination with cytotoxic chemotherapy in advanced epithelial ovarian cancer. A prospective randomized trial. Cancer. 1989 Mar 15;63(6):1074–1078. doi: 10.1002/1097-0142(19890315)63:6<1074::aid-cncr2820630606>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 70.Shirey DR, Kavanagh JJ, Gershenson DM, Freedman RS, Copeland LJ, Jones LA. Tamoxifen therapy of epithelial ovarian cancer. Obstet Gynecol. 1985 Oct 1;66(4):575–578. [PubMed] [Google Scholar]
- 71.Weiner SA, Alberts DS, Surwit EA, Davis J, Grosso D. Tamoxifen therapy in recurrent epithelial ovarian carcinoma. Gynecol Oncol. 1987 Jun 1;27(2):208–213. doi: 10.1016/0090-8258(87)90294-0. [DOI] [PubMed] [Google Scholar]
- 72.Hurteau JA, Brady MF, Darcy KM, McGuire WP, Edmonds P, Pearl ML, et al. Randomized phase III trial of tamoxifen versus thalidomide in women with biochemical-recurrent- only epithelial ovarian, fallopian tube or primary peritoneal carcinoma after a complete response to first-line platinum/taxane chemotherapy with an evaluation of serum vascular endothelial growth factor (VEGF): A Gynecologic Oncology Group Study. Gynecologic Oncology. 2010 Dec;119(3):444–450. doi: 10.1016/j.ygyno.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 73.Markman M, Webster K, Zanotti K, Rohl J, Belinson J. Use of tamoxifen in asymptomatic patients with recurrent small-volume ovarian cancer. Gynecologic Oncology. 2004 May;93(2):390–393. doi: 10.1016/j.ygyno.2004.01.035. [DOI] [PubMed] [Google Scholar]
- 74.Argenta PA, Thomas SG, Judson PL, Downs LS, Jr, Geller MA, Carson LF, et al. A phase II study of fulvestrant in the treatment of multiply-recurrent epithelial ovarian cancer. Gynecol Oncol. 2009 Feb 22;113(2):205–209. doi: 10.1016/j.ygyno.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 75.Cunat S, Rabenoelina F, Daures JP, Katsaros D, Sasano H, Miller WR, et al. Aromatase expression in ovarian epithelial cancers. J Steroid Biochem Mol Biol. 2005 Jan;93(1):15–24. doi: 10.1016/j.jsbmb.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 76.Labrie F, Belanger A, Simard J, Van L, Labrie C. DHEA and peripheral androgen and estrogen formation: intracinology. Ann N Y Acad Sci. 1995 Dec 29;774:16–28. doi: 10.1111/j.1749-6632.1995.tb17369.x. [DOI] [PubMed] [Google Scholar]
- 77.Slotman BJ, Kuhnel R, Rao BR, Dijkhuizen GH, de GJ, Stolk JG. Importance of steroid receptors and aromatase activity in the prognosis of ovarian cancer: high tumor progesterone receptor levels correlate with longer survival. Gynecol Oncol. 1989 Apr;33(1):76–81. doi: 10.1016/0090-8258(89)90607-0. [DOI] [PubMed] [Google Scholar]
- 78.Kitawaki J, Noguchi T, Yamamoto T, Yokota K, Maeda K, Urabe M, et al. Immunohistochemical localisation of aromatase and its correlation with progesterone receptors in ovarian epithelial tumours. Anticancer Res. 1996 Jan;16(1):91–97. [PubMed] [Google Scholar]
- 79.Thompson MA, Adelson MD, Kaufman LM, Marshall LD, Coble DA. Aromatization of testosterone by epithelial tumor cells cultured from patients with ovarian carcinoma. Cancer Res. 1988 Nov 15;48(22):6491–6497. [PubMed] [Google Scholar]
- 80.Wong ZW, Ellis MJ. First-line endocrine treatment of breast cancer: aromatase inhibitor or antioestrogen? Br J Cancer. 2004 Jan 12;90(1):20–25. doi: 10.1038/sj.bjc.6601508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sasano H, Sato S, Ito K, Yajima A, Nakamura J, Yoshihama M, et al. Effects of aromatase inhibitors on the pathobiology of the human breast, endometrial and ovarian carcinoma. Endocr Relat Cancer. 1999 Jun;6(2):197–204. doi: 10.1677/erc.0.0060197. [DOI] [PubMed] [Google Scholar]
- 82.Bowman A, Gabra H, Langdon SP, Lessells A, Stewart M, Young A, et al. CA125 response is associated with estrogen receptor expression in a phase II trial of letrozole in ovarian cancer: identification of an endocrine-sensitive subgroup. Clin Cancer Res. 2002 Jul;8(7):2233–2239. [PubMed] [Google Scholar]
- 83.Smyth JF, Gourley C, Walker G, MacKean MJ, Stevenson A, Williams AR, et al. Antiestrogen therapy is active in selected ovarian cancer cases: the use of letrozole in estrogen receptor-positive patients. Clin Cancer Res. 2007 Jun 15;13(12):3617–3622. doi: 10.1158/1078-0432.CCR-06-2878. [DOI] [PubMed] [Google Scholar]
- 84.Kavanagh JJ, et al. Anti-tumor activity of letrozole in patients with recurrent advanced low malignant potential or low-grade serous ovarian tumors. J Clin Oncol. 2007;25 [June 20 Supplement]. [Google Scholar]
- 85.Cariou S, Donovan JC, Flanagan WM, Milic A, Bhattacharya N, Slingerland JM. Down-regulation of p21WAF1/CIP1 or p27Kip1 abrogates antiestrogen-mediated cell cycle arrest in human breast cancer cells. Proc Natl Acad Sci USA. 2000 Aug 1;97(16):9042–9046. doi: 10.1073/pnas.160016897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wright JW, Stouffer RL, Rodland KD. High-dose estrogen and clinical selective estrogen receptor modulators induce growth arrest, p21, and p53 in primate ovarian surface epithelial cells. J Clin Endocrinol Metab. 2005 Jun;90(6):3688–3695. doi: 10.1210/jc.2004-2456. [DOI] [PubMed] [Google Scholar]
- 87.Chen Y, Guggisberg N, Jorda M, Gonzalez-Angulo A, Hennessy B, Mills GB, et al. Combined Src and Aromatase Inhibition Impairs Human Breast Cancer Growth In vivo and Bypass Pathways Are Activated in AZD0530-Resistant Tumors. Clin Cancer Res. 2009 May 15;15(10):3396–3405. doi: 10.1158/1078-0432.CCR-08-3127. [DOI] [PubMed] [Google Scholar]
- 88.Osborne CK, Shou J, Massarweh S, Schiff R. Crosstalk between estrogen receptor and growth factor receptor pathways as a cause for endocrine therapy resistance in breast cancer. Clin Cancer Res. 2005 Jan 15;11(2 Pt 2):865s–870s. [PubMed] [Google Scholar]
- 89.Chu I, Sun J, Arnaout A, Kahn H, Hanna W, Narod S, et al. p27 phosphorylation by Src regulates inhibition of cyclin E-Cdk2. Cell. 2007 Jan 26;128(2):281–294. doi: 10.1016/j.cell.2006.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen Y, Alvarez EA, Azzam D, Wander SA, Guggisberg N, Jorda M, et al. Combined Src and ER blockade impairs human breast cancer proliferation in vitro and in vivo. Breast Cancer Res Treat. 2010 Jul 29; doi: 10.1007/s10549-010-1024-7. [DOI] [PubMed] [Google Scholar]
- 91.Donovan JC, Milic A, Slingerland JM. Constitutive MEK/MAPK activation leads to p27(Kip1) deregulation and antiestrogen resistance in human breast cancer cells. J Biol Chem. 2001 Nov 2;276(44):40888–40895. doi: 10.1074/jbc.M106448200. [DOI] [PubMed] [Google Scholar]
- 92.Talapatra S, Thompson CB. Growth factor signaling in cell survival: implications for cancer treatment. J Pharmacol Exp Ther. 2001 Sep;298(3):873–878. [PubMed] [Google Scholar]
- 93.Ishizawar R, Parsons SJ. c-Src and cooperating partners in human cancer. Cancer Cell. 2004 Sep;6(3):209–214. doi: 10.1016/j.ccr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 94.Wiener JR, Windham TC, Estrella VC, Parikh NU, Thall PF, Deavers MT, et al. Activated SRC protein tyrosine kinase is overexpressed in late-stage human ovarian cancers. Gynecol Oncol. 2003 Jan;88(1):73–79. doi: 10.1006/gyno.2002.6851. [DOI] [PubMed] [Google Scholar]
- 95.George JA, Chen T, Taylor CC. SRC tyrosine kinase and multidrug resistance protein-1 inhibitions act independently but cooperatively to restore paclitaxel sensitivity to paclitaxel-resistant ovarian cancer cells. Cancer Res. 2005 Nov 15;65(22):10381–10388. doi: 10.1158/0008-5472.CAN-05-1822. [DOI] [PubMed] [Google Scholar]
- 96.Mullen P, Cameron DA, Hasmann M, Smyth JF, Langdon SP. Sensitivity to pertuzumab (2C4) in ovarian cancer models: cross-talk with estrogen receptor signaling. Mol Cancer Ther. 2007 Jan;6(1):93–100. doi: 10.1158/1535-7163.MCT-06-0401. [DOI] [PubMed] [Google Scholar]
- 97.Bourguignon LYW, Gilad E, Rothman K, Peyrollier K. Hyaluronan-CD44 Interaction with IQGAP1 Promotes Cdc42 and ERK Signaling, Leading to Actin Binding, Elk-1/Estrogen Receptor Transcriptional Activation, and Ovarian Cancer Progression. Journal of Biological Chemistry. 2005 Mar 25;280(12):11961–11972. doi: 10.1074/jbc.M411985200. [DOI] [PubMed] [Google Scholar]
- 98.Choi JH, Lee KT, Leung PCK. Estrogen receptor alpha pathway is involved in leptin-induced ovarian cancer cell growth. Carcinogenesis. 2011 Apr 1;32(4):589–596. doi: 10.1093/carcin/bgq276. [DOI] [PubMed] [Google Scholar]
- 99.Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem. 2001 Oct 5;276(40):36869–36872. doi: 10.1074/jbc.R100029200. [DOI] [PubMed] [Google Scholar]
- 100.Karagol H, Saip P, Uygun K, Caloglu M, Eralp Y, Tas F, et al. The efficacy of tamoxifen in patients with advanced epithelial ovarian cancer. Med Oncol. 2007 Jan 1;24(1):39–43. doi: 10.1007/BF02685901. [DOI] [PubMed] [Google Scholar]
- 101.Rolski J, Pawlicki M. Evaluation of efficacy and toxicity of tamoxifen in patients with advanced chemotherapy resistant ovarian cancer. Ginekol Pol. 1998 Jul 1;69(7):586–589. [PubMed] [Google Scholar]
- 102.Marth C, Sorheim N, Kaern J, Trope C. Tamoxifen in the treatment of recurrent ovarian carcinoma. Int J Gynecol Cancer. 1997;7:256–261. doi: 10.1016/s0959-8049(00)00228-8. [DOI] [PubMed] [Google Scholar]
- 103.Jager W, Sauerbrei W, Beck E, Maasen V, Stumpfe M, Meier W, et al. A randomized comparison of triptorelin and tamoxifen as treatment of progressive ovarian cancer. Anticancer Res. 1995;15:2639–2642. [PubMed] [Google Scholar]
- 104.van der Vange N, Greggi S, Burger CW, Kenemans P, Vermorken JB. Experience with hormonal therapy in advanced epithelial ovarian cancer. Acta Oncol. 1995 Jan 1;34(6):813–820. doi: 10.3109/02841869509127191. [DOI] [PubMed] [Google Scholar]
- 105.Losa G, et al. Treatment of advanced ovarian cancer by hormone. Int J Gynecol Cancer. 1993;3(Suppl 1):66. [Google Scholar]
- 106.Osborne RJ, Malik ST, Slevin ML, Harvey VJ, Spona J, Salzer H, et al. Tamoxifen in refractory ovarian cancer: the use of a loading dose schedule. Br J Cancer. 1988 Jan 1;57(1):115–116. doi: 10.1038/bjc.1988.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Slevin ML, Harvey VJ, Osborne RJ, Shepherd JH, Williams CJ, Mead GM. A phase II study of tamoxifen in ovarian cancer. Eur J Cancer Clin Oncol. 1986 Mar 1;22(3):309–312. doi: 10.1016/0277-5379(86)90396-2. [DOI] [PubMed] [Google Scholar]
- 108.Landoni F, Bonazzi C, Regallo M, Scalambrino S, Vassena L, Mangioni C. Antiestrogen as last-line treatment in epithelial ovarian cancer. Chemi-oterapia. 1985;4(suppl to No. 2):1059–1060. [Google Scholar]
- 109.Hemerlynck JV, Vermorken JB, Van der Burgh ME, et al. Tamoxifen therapy in advanced ovarian cancer: a phase II study. Proc Am Soc Clin Oncol. 1985;4 [Google Scholar]
- 110.Rowland K, Bonomi P, Wilbanks G, Yordan E, Graham J, Dunne C. Hormone receptors in ovarian carcinoma. Proc Am Soc Clin Oncol. 1985;4:117. [Google Scholar]
- 111.Pagel J, Rose CTS, Hald I. Treatment of advanced ovarian carcinoma with tamoxifen: a phase II trial; Proc 2nd Eur Conf Clin Oncol; 1983. [Google Scholar]
- 112.Schwartz PE, Keating G, MacLusky N, Naftolin F, Eisenfeld A. Tamoxifen therapy for advanced ovarian cancer. Obstet Gynecol. 1982 May 1;59(5):583–588. [PubMed] [Google Scholar]
- 113.Ramirez PT, Schmeler KM, Milam MR, Slomovitz BM, Smith JA, Kavanagh JJ, et al. Efficacy of letrozole in the treatment of recurrent platinum- and taxane-resistant high-grade cancer of the ovary or peritoneum. Gynecologic Oncology. 2008 Jul;110(1):56–59. doi: 10.1016/j.ygyno.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 114.Smyth JF, Gourley C, Walker G, MacKean MJ, Stevenson A, Williams ARW, et al. Antiestrogen Therapy Is Active in Selected Ovarian Cancer Cases: The Use of Letrozole in Estrogen ReceptorGÇôPositive Patients. Clinical Cancer Research. 2007 Jun 15;13(12):3617–3622. doi: 10.1158/1078-0432.CCR-06-2878. [DOI] [PubMed] [Google Scholar]
- 115.Gourley C, Mackean M, Stevenson A, et al. Phase II study of letrozole in estrogen receptor (ER) positive relapsed epithelial ovarian cancer (EOC) J Clin Oncol. 2006;24 [Google Scholar]
- 116.Papadimitriou CA, Markaki S, Siapkaras J, Vlachos G, Efstathiou E, Grimani I, et al. Hormonal therapy with letrozole for relapsed epithelial ovarian cancer. Long-term results of a phase II study. Oncology. 2004;66(2):112–117. doi: 10.1159/000077436. [DOI] [PubMed] [Google Scholar]
- 117.Krasner C, Debernardo R, Findley M, et al. Phase II trial of anastrazole in combination with gefitinib in women with asymptomatic mullerian cancer. J Clin Oncol; ASCO Annual Meeting Proceedings; 2005. p. 5063. [Google Scholar]
- 118.del Carmen MG, Fuller AF, Matulonis U, Horick NK, Goodman A, Duska LR, et al. Phase II trial of anastrozole in women with asymptomatic mullerian cancer. Gynecol Oncol. 2003 Dec;91(3):596–602. doi: 10.1016/j.ygyno.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 119.Verma S, Le T, Baines K, Rabout L, Hopkins L, Fung K, et al. Phase II study of exemestane (E) in refractory ovarian cancer (ROC) J Clin Oncol. 2006;24 [Google Scholar]