A modest, 10% reduction in outpatient antibiotic prescribing among U.S. adults could result in a substantial 17% reduction in Clostridium difficile infections that originate in the community.
Keywords: antibacterial agents, Clostridium difficile, epidemiology, outpatients, public health surveillance
Abstract
Background. Antibiotic use predisposes patients to Clostridium difficile infections (CDI), and approximately 32% of these infections are community-associated (CA) CDI. The population-level impact of antibiotic use on adult CA-CDI rates is not well described.
Methods. We used 2011 active population- and laboratory-based surveillance data from 9 US geographic locations to identify adult CA-CDI cases, defined as C difficile-positive stool specimens (by toxin or molecular assay) collected from outpatients or from patients ≤3 days after hospital admission. All patients were surveillance area residents and aged ≥20 years with no positive test ≤8 weeks prior and no overnight stay in a healthcare facility ≤12 weeks prior. Outpatient oral antibiotic prescriptions dispensed in 2010 were obtained from the IMS Health Xponent database. Regression models examined the association between outpatient antibiotic prescribing and adult CA-CDI rates.
Methods. Healthcare providers prescribed 5.2 million courses of antibiotics among adults in the surveillance population in 2010, for an average of 0.73 per person. Across surveillance sites, antibiotic prescription rates (0.50–0.88 prescriptions per capita) and unadjusted CA-CDI rates (40.7–139.3 cases per 100 000 persons) varied. In regression modeling, reducing antibiotic prescribing rates by 10% among persons ≥20 years old was associated with a 17% (95% confidence interval, 6.0%–26.3%; P = .032) decrease in CA-CDI rates after adjusting for age, gender, race, and type of diagnostic assay. Reductions in prescribing penicillins and amoxicillin/clavulanic acid were associated with the greatest decreases in CA-CDI rates.
Conclusions and Relevance. Community-associated CDI prevention should include reducing unnecessary outpatient antibiotic use. A modest reduction of 10% in outpatient antibiotic prescribing can have a disproportionate impact on reducing CA-CDI rates.
Clostridium difficile infection (CDI) has become an increasingly common cause of healthcare-associated infectious diarrhea [1], and the national burden among patients admitted to a hospital setting is estimated to be 250 000 cases per year [2]. In recent years, CDI has been increasingly reported among mostly adult persons with no recent stays in hospitals or long-term care facilities [3–5]. Community-associated (CA) CDI represents as many as 32% of all CDI cases [5–7], and this statistic may be increasing [5, 8].
A recent meta-analysis using data from 8 studies across several regions in the world showed that exposure to several antibiotic categories, including clindamycin, fluoroquinolones, cephalosporins, penicillins, macrolides, and sulfonamides/trimethoprim, was associated with increased risk of CA CDI in adults [9]. Studies have demonstrated geographic variation and widespread overuse of antibiotics in US outpatient settings [10, 11]. Although previous studies have assessed patient-level risk for CDI in the community, an assessment of this risk on a population level is important to guide prevention strategies.
To determine the impact of outpatient antibiotic prescriptions on adult rates of CA CDI, we analyzed population-based CA-CDI rates from several US geographic locations and oral antibiotic prescribing practices in these regions using a national commercial pharmacy database. We then quantified the expected effect of lowering rates of antibiotic prescribing on CA-CDI rates.
METHODS
Emerging Infections Program
The Emerging Infections Program (EIP) C difficile surveillance is an active population- and laboratory-based surveillance that has tracked CDI in both inpatient and outpatient healthcare settings since 2009. The surveillance methods have been described elsewhere [12]. For this analysis, we used 2011 CDI surveillance data from 33 counties in 9 US states, including California (San Francisco County) Colorado (Adams, Arapahoe, Denver, Douglas, and Jefferson Counties), Connecticut (New Haven County), Georgia (Clayton, Cobb, DeKalb, Douglas, Fulton, Gwinnett, Newton, and Rockdale Counties), Maryland (Caroline, Cecil, Dorchester, Frederick, Kent, Somerset, Talbot, Queen Anne's, Washington, Wicomico, and Worcester Counties), Minnesota (Benton, Morrison, Stearns, and Todd Counties), New Mexico (Bernalillo County), New York (Monroe County), and Oregon (Klamath County), representing a total of 10.4 million persons under surveillance. We defined an adult CA-CDI case as a surveillance area resident ≥20 years of age with a positive C difficile toxin or molecular assay on a stool specimen who also met the following criteria: (1) the positive C difficile specimen was collected as an outpatient or ≤3 days after hospital admission (admission date = day 1); (2) the patient had no positive C difficile specimen within the previous 8 weeks; and (3) the patient had no documented overnight stay in a healthcare facility (hospital or long-term care facility) within the prior 12 weeks.
Additional Data Sources
Previous analysis of EIP data has shown that CA-CDI rates should be adjusted for race, sex, age, and use of nucleic acid amplification testing (NAAT) [13]. The NAAT usage was estimated based on a survey of laboratories serving the catchment population in 2011 [14].
Population denominator data for 2011 was obtained from US census to calculate incidence [15]. Data on oral antibiotic prescriptions dispensed during 2010 in the United States were extracted from the IMS Health Xponent database, which represents a 100% projection of prescription activity on the basis of a sample of greater than 70% of all US prescriptions. These data represent all outpatient antibiotic prescriptions, across all payers, including retail pharmacies and federal government and nongovernmental mail service pharmacies. Prescription counts were summarized by drug category, and patient age and sex, according to the county where the prescriber was located. Antibiotics were categorized according to the IMS Health Uniform System of Classification. Persons <20 years of age were excluded from the denominators because the case definition only includes persons ≥20 years of age.
The numbers of prescriptions and US census denominators were used to calculate prescribing rates [15].
Statistical Analysis
Generalized linear-mixed models with negative binomial distribution were built to examine the association between EIP site-specific antibiotic prescribing rates, demographic, and diagnostic factors with CA-CDI incidence. Because CDI incidence varied across surveillance sites, a random intercept was specified to account for the site variations. The candidate variables for the models included age, sex, race, percentage of urban population, NAAT usage, percentage of population between 18 and 64 years of age without insurance, and average of outpatient visits per hospital in each surveillance site. Final models were obtained using a backward selection with a stay criterion of P < .05. Separate models were created for each major class of oral antibiotic and for all oral antibiotics combined. The ratio of the generalized χ2 statistic and its degrees of freedom was calculated to evaluate the model fit. A ratio close to 1 indicates a good fit. Analyses were performed using SAS software, version 9.3 (SAS Institute Inc., Cary, NC).
Overall, 18% of CA-CDI cases did not have race information available in medical charts. When race was missing, data were imputed based on the known population distribution of race by age, sex, and surveillance site. Because the IMS Health Xponent database does not track the race of persons filling prescriptions and this information is also not available from other data sources, we assumed that antibiotics were prescribed in proportion to the prevalence of white and non-white persons in that EIP site, based on census data.
Human Subject Research Considerations
The EIP-CDI surveillance was approved by the Institutional Review Boards at the Centers for Disease Control and Prevention and participating EIP sites. Waivers of informed consent to review medical records were obtained in EIP sites where CDI was not reportable to the state health department.
RESULTS
Incidence of Community-Associated Clostridium difficile Infection
From January 1, 2011 through December 31, 2011, 4682 adult (≥20 years of age) CA-CDI cases were identified. Of these cases, 39% were ≥65 years of age, 37% were male, and 80% were of white race. Unadjusted CA-CDI incidence varied across the 9 sites (Figure 1), with the lowest incidence in California (40.7 cases per 100 000 population) and the highest incidence in Minnesota (139.3 cases per 100 000 population).
Figure 1.
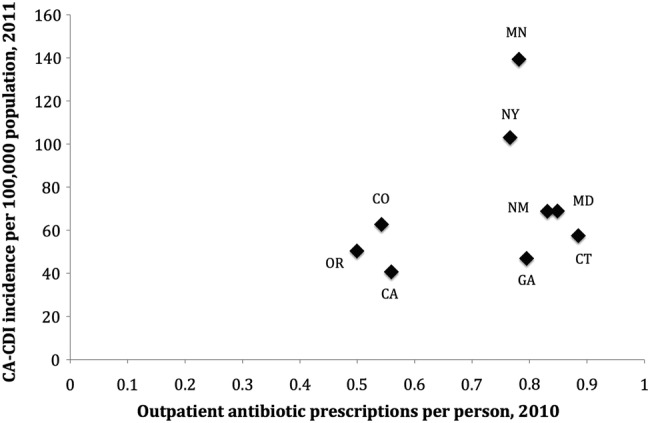
The 2011 community-associated Clostridium difficile (CA-CDI) unadjusted incidence and 2010 oral outpatient antibiotic prescription rates, by Emerging Infections Program site.
Outpatient Antibiotic Prescribing Rates
In the EIP surveillance areas, 5 521 457 oral antibiotic prescriptions were filled among adults ≥20 years of age in 2010, corresponding to an oral antibiotic prescribing rate of 0.73 prescriptions per person. Antibiotic prescribing varied by EIP site, with the highest rate in Connecticut (0.88 prescriptions per capita) and the lowest rate in Oregon (0.50 prescriptions per capita) (Figure 1).
Antibiotic prescribing rates also varied by age and gender groups within EIP sites (Figure 2), and they were higher among males and females aged 65 years and older (0.91 and 1.02 prescriptions per capita, respectively). Antibiotic prescribing rates were lower among those aged 20–64, with a large discrepancy in antibiotic prescribing between males and females aged 20–64 (0.50 vs 0.85 prescriptions per capita, respectively). Among all adults, females were prescribed more antibiotics more frequently than males (0.88 vs 0.56 prescriptions per capita, respectively) in 2010.
Figure 2.
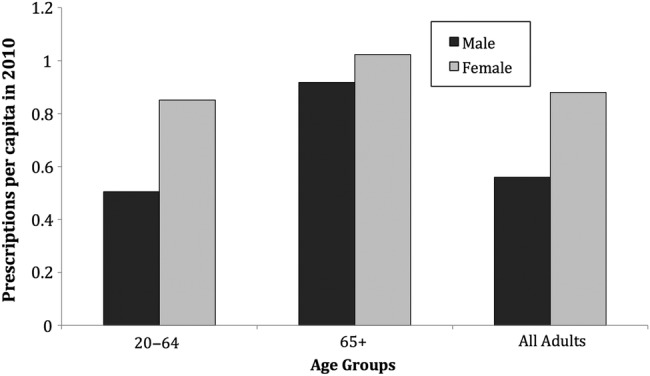
Oral outpatient antibiotic prescription rates among adults ≥20 years of age, by gender and age groups, in Emerging Infection Program surveillance sites, 2010. P < .001 comparing all gender pairs by chi-squared test.
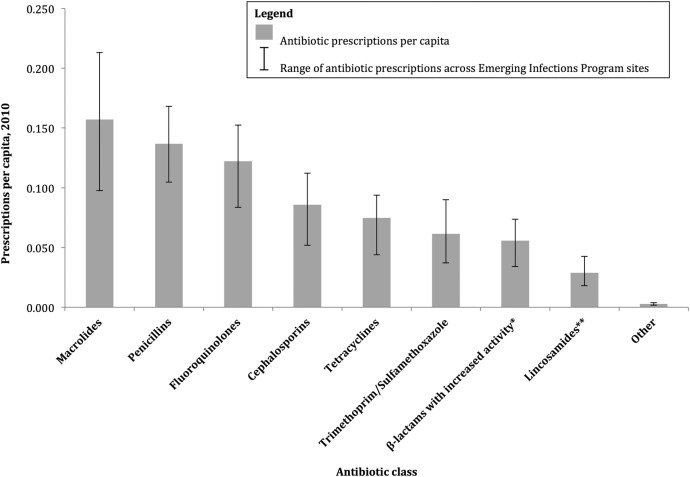
Across the EIP surveillance sites, the most common oral antibiotics prescribed were macrolides (0.157 prescriptions per capita), followed by penicillins (excluding β-lactam/β-lactamase inhibitor combinations) (0.137 prescriptions per capita), fluoroquinolones (0.122 prescriptions per capita), cephalosporins (0.086 prescriptions per capita), tetracyclines (0.075 prescriptions per capita), trimethoprim/sulfamethoxazole (0.061 prescriptions per capita), β-lactams with increased activity (amoxicillin/clavulanic acid) (0.056 prescriptions per capita), lincosamides (clindamycin) (0.029 prescriptions per capita), and others (0.003 prescriptions per capita) (Figure 3). There was variability across surveillance sites on the number of prescriptions per capita for each antibiotic category (Figure 3).
Figure 3.
Oral outpatient antibiotic prescription rates among adults ≥20 years of age, by drug class, in Emerging Infection Program sites, 2010. *Amoxicillin/clavulanic acid is the only oral antibiotic in this category. **Clindamycin is the only oral antibiotic in this category.
Estimated Reductions in Community-Associated Clostridium difficile Infection With Reductions in Outpatient Antibiotic Use
The final models included the following confounders: age group (20–64, ≥65), gender, race (white, non-white), and type of diagnostic assay (NAAT, other) (Table 1). The ratios of the generalized χ2 statistic to degrees of freedom ranged from 0.91 to 0.95, indicating that the variability in these data were properly modeled, without residual overdispersion.
Table 1.
Model Parameters of CA-CDI Association With 10% Increase in Oral Outpatient Antibiotic Prescribing, Adjusted for Gender, Race, and Use of Molecular Diagnostic Assays, Among Adults (≥20 Years Old), in Emerging Infections Program Surveillance Sites*
| Variable | Coefficient | Standard Error | Rate Ratio | 95% CI | P Value |
|---|---|---|---|---|---|
| Age 20–64 y | — | — | 1.00 (reference) | — | — |
| Age 65+ | 0.938 | 0.087 | 2.55 | 2.15–3.03 | <.0001 |
| Non-white race | — | — | 1.00 (reference) | — | — |
| White race | 0.339 | 0.071 | 1.4 | 1.22–1.61 | <.0001 |
| Male | 0.000 | — | 1.00 (reference) | — | — |
| Female | 0.194 | 0.088 | 1.21 | 1.02–1.44 | .028 |
| Nucleic acid amplification testing use increased by 10% | 0.065 | 0.028 | 1.07 | 01.01–1.13 | .019 |
| Antibiotic prescribing increased by 10% | 0.183 | 0.062 | 1.2 | 1.06–1.36 | .003 |
Abbreviations: CA-CDI, community-associated Clostridium difficile infections; CI, confidence interval.
*Generalized χ2/degree of freedom: 0.91.
These models were used to calculated the change in CA-CDI incidence among adults (≥20 years old) associated with a 10% change in antibiotic use, which has been shown to an achievable target in various quality improvement studies [16], adjusting for gender, race, and use of molecular diagnostic assays (Table 1). Overall, a 16.8% (6.0%–26.3%; P = .003) decrease in CA-CDI incidence was predicted for each 10% reduction in the use of all antibiotics (Table 2), corresponding to 885 (95% confidence interval [CI], 315–1390) fewer cases of CA CDI annually among all EIP sites combined. Separate models were also created to model the expected effects of reducing prescriptions of specific antibiotic classes by 10%. Among the drug classes, reductions in penicillin prescriptions were associated with the largest decrease in CA-CDI incidence (12.1%; 95% CI, 2.9%–20.5%; P = .012), followed by β-lactams with increased activity (9.4%; 95% CI, 1.6%–16.5%; P = .020), lincosamides (7.6%; 95% CI, 1.4%–13.4%; P = .017), cephalosporins (7.5%; 95% CI, .7%–13.8%; P = .031), trimethoprim/sulfamethoxazole (7.4%; 95% CI, .7%–13.7%; P = .030), tetracyclines (6.9%; 95% CI, 1.2%–12.3%; P = .019), and macrolides (6.8%; 95% CI, .03%–13.1%; P = .049). Of note, although a 10% reduction in fluoroquinolone prescribing was associated with a 4.8% (95% CI, −1.8%–11.1%; P = .149) decrease in CA CDI, these results were not statistically significant. Likewise, a separate model of a 10% reduction in fluoroquinolone prescribing among adults ≥65 years old was not associated with a statistically significant decrease in CA-CDI (5.4%; 95% CI, −1.0% to 11.4%; P = .095).
Table 2.
Modeled Reduction of Adult (≥20 Years Old) Community-Associated Clostridium difficile Infections (CA-CDI) Associated With 10% Reductions in Oral Outpatient Antibiotic Prescribing, Adjusted for Gender, Race, and Use of Molecular Diagnostic Assays, in Emerging Infections Program Surveillance Sites
| Antibiotic Class | Prescriptions Per Capita, 2010 | CA-CDI Cases, 2011 | Absolute CA-CDI Reduction for Each 10% Reduction in Drug Use | 95% Confidence Interval | CA-CDI Rate Reduction for Each 10% Reduction in Drug Use | 95% Confidence Interval | P Value |
|---|---|---|---|---|---|---|---|
| All classes | 0.731 | 5284 | 885 | (315–1390) | 16.8% | (6.0%–26.3%) | .003 |
| Penicillins | 0.137 | 5284 | 639 | (151–1081) | 12.1% | (2.9%–20.5%) | .012 |
| β-lactam, increased activitya | 0.056 | 5284 | 495 | (84–873) | 9.4% | (1.6%–16.5%) | .020 |
| Lincosamidesb | 0.029 | 5284 | 401 | (75–707) | 7.6% | (1.4%–13.4%) | .017 |
| Cephalosporins | 0.086 | 5284 | 396 | (39–730) | 7.5% | (.7%–13.8%) | .031 |
| Trimethoprim/sulfamethoxazole | 0.061 | 5284 | 392 | (39–722) | 7.4% | (.7%–13.7%) | .030 |
| Tetracyclines | 0.075 | 5284 | 365 | (62–651) | 6.9% | (1.2%–12.3%) | .019 |
| Macrolides | 0.157 | 5284 | 360 | (1–694) | 6.8% | (.03%–13.1%) | .049 |
| Fluoroquinolones (all adults) | 0.122 | 5284 | 255 | (−94–581) | 4.8% | (−1.8%–11.0%) | .149 |
| Fluoroquinolones (age 65+) | 0.238 | 1880 | 102 | (−18 to 215) | 5.4% | (−1.0%–11.4%) | .095 |
| Other antibiotics | 0.003 | 5284 | 104 | (−375 to 542) | 2.0% | (−7.1%–10.3%) | .658 |
aAmoxicillin/clavulanic acid is the only oral antibiotic in this category.
bClindamycin is the only oral antibiotic in this category.
DISCUSSION
Based on these data collected in 9 diverse US geographic areas, we expect that reducing all oral outpatient antibiotic prescribing among adults by 10% should result in a substantial reduction (17%) in CA-CDI rates. Our finding is particularly important in the context of how frequently antibiotics are prescribed unnecessarily in the outpatient setting. A study from Chitnis et al [12], using the same study sites as our study, reported 64% of CA-CDI cases were recently exposed to antibiotics, and the most frequent indication was an upper respiratory infection—a well recognized source of inappropriate antibiotic use in outpatient settings [17–19]. A recent US study based on the National Hospital Ambulatory Medical Care surveys suggested that up to 25% of ambulatory antibiotic prescriptions for adults were for conditions in which antibiotics are rarely indicated [11], suggesting persistent overuse of antibiotics in the outpatient setting and many opportunities for improving antibiotic use. Based on our data, CA-CDI reduction should be added to the list of benefits of improving outpatient antibiotic prescribing, which already includes reducing drug-resistant respiratory infections and preventing adverse drug events. Furthermore, interventions aimed at reducing unnecessary antibiotic use in the outpatient setting should be included in CA-CDI prevention efforts.
In adults, numerous studies have highlighted the limited efficacy of antibiotics for treating acute upper respiratory tract infections, including acute cough/bronchitis and acute pharyngitis [19, 20]. Despite these studies, antibiotics continue to be frequently prescribed to treat these infections in the United States, including 60% of cases of acute pharyngitis [21] (antibiotics are only indicated for streptococcal pharyngitis, comprising approximately 10% of acute pharyngitis [22]) and 71% for cases of ambulatory visits for bronchitis [23]. Treatment guidelines were developed for both adult and pediatric patients, in addition to education materials for primary care providers and patients [24–26]. In 2003, the Centers for Disease Control and Prevention launched the “Get Smart: Know When Antibiotics Work” campaign with a focus on reducing unnecessary prescription of antibiotics for acute upper respiratory infections in the community. A 4-month, regional “Get Smart” media campaign was associated with a 3.8%–8.8% reduction in regional antibiotic dispensing [25], demonstrating that reducing unnecessary outpatient antibiotic use is feasible. However, further efforts may be needed to encourage widespread adoptions of effective interventions [27–29].
The dental setting is another common source of antibiotic exposure. In a previous study using the same EIP surveillance system, 15% of CA-CDI cases with antibiotic exposure in the prior 12 weeks had been prescribed antibiotics by their dentist [12]. Recent guidelines have recommended more restrictive use of antibiotics for prevention of infective endocarditis [30] and orthopedic implant infections [31] during dental procedures. Additional work is needed to evaluate and improve adherence to these guidelines to reduce unnecessary antibiotic use in dental settings.
Finally, our analysis showed that females were prescribed more antibiotics than males; an association also seen with CA-CDI rates where females have higher CDI rates compared to males [13]. Increased use of antibiotics in females may explain the gender disparities observed in CDI rates.
To our knowledge, this is the first study to use variation in CA-CDI rates and antibiotic prescribing across various settings to quantify the potential effect of reducing outpatient antibiotic prescribing on CDI rates. If outpatient healthcare providers wish to target efforts to reduce unnecessary antibiotic use to maximize impact on CA-CDI in their community, then, based on our study, reductions in prescriptions for oral penicillins and β-lactams with increased activity (amoxicillin/clavulanic acid) will potentially have the greatest impact on associated reductions in CA CDI. These antibiotic classes are among those most often prescribed for upper respiratory infections in the United States [18].
Exposure to fluoroquinolone antibiotics has been well described as a risk factor for Clostridium difficile infection in the inpatient setting [32, 33], and a recent meta-analysis concluded that exposure to fluoroquinolones was associated with a more than 5-fold increased risk for developing CA CDI [9]. Given the well described association between fluoroquinolone exposure and individual risk for CDI, it was surprising that our study did not find a significant association between reducing fluoroquinolone prescriptions and CA-CDI rates. This may be explained by geographic variability in CA-CDI strains. The North American Pulsed Field Gel Electrophoresis type 1 (NAP1) strain is known to have high resistance to fluoroquinolones [34], and reductions in fluoroquinolone use have been associated with decreasing NAP1 prevalence [35]. Our analysis was limited to 9 US geographic locations, where the NAP1 strain was present in approximately 21% of CA-CDI cases [36], compared with approximately 36% during the same time period in England [35] and approximately 30% in Canada [37]. Therefore, the lower prevalence of NAP1 strain among CA-CDI cases in our surveillance system may explain the lack of association between fluoroquinolone use and predicted changes in CA-CDI incidence. Further research is needed to determine whether varying C difficile strain prevalence affects the success of specific interventions to reduce inappropriate antibiotic use.
There are several limitations to this study. First, this is a large-scale ecological study and, as such, no inferences of individual-level association between an antibiotic exposure and development of CA-CDI can be made. In addition, the EIP program operates in several geographic areas with diverse populations; these specific areas were not randomly selected to be representative, and, therefore, our findings may not be generalizable to the entire United States. Furthermore, it is not known whether clinicians have equal sensitivity for ordering CDI diagnostic testing across geographic areas. There are also limitations to using the IMS Health Xponent antibiotic prescribing data. This database only tracks the number of prescriptions filled at a pharmacy and does not assess whether the patient used the medication as prescribed. Location data is also recorded by the county of the prescribing physician and not by the county of residence for the patient. Although this could potentially introduce inflated prescribing results in urban counties with higher concentrations of physicians, we chose to analyze aggregate adjacent counties within metropolitan areas to minimize this bias. The IMS Xponent also does not capture patient race data; as a result, we had to assume equal antibiotic prescribing for white and non-white persons in our mixed model. We could not identify any US national data that has determined whether racial disparities exist for prescriptions of all antibiotics, and the literature regarding specific indications is mixed, with some evidence suggesting that non-whites are more likely to be prescribed antibiotics for upper respiratory tract infections in outpatient settings [38], although African-Americans may be less likely to receive inappropriate antibiotics for acute asthma exacerbations and otitis media [39]. Further research regarding racial disparities in antibiotic prescribing is needed to improve the accuracy of our models. Finally, 2011 antibiotic prescribing data were not available at the time of this study to directly match the period of CA-CDI surveillance. However, very little year-to-year variation in antibiotic prescribing has been observed comparing 2010 to datasets from previous years, and, thus, we do not expect this to significantly affect our findings. The IMS Health Xponent data captures the largest proportion of US outpatient antibiotic prescribing and is the most robust data source available.
CONCLUSIONS
In conclusion, implementation of outpatient interventions to reduce inappropriate antibiotic use could substantially decrease CA-CDI rates. Oral penicillins and β-lactams with increased activity (amoxicillin/clavulanic acid), which are commonly used to treat acute respiratory infections, were the 2 antibiotic classes for which reduction in use was associated with the greatest declines in CA-CDI rates. Further research is necessary to understand whether the prevalence of particular C difficile strains influences the targeting of programs to reduce inappropriate antibiotic use. Our study suggests that reductions in CA-CDI rates would be an additional benefit of interventions to improve antibiotic use in US outpatient settings.
Acknowledgments
We acknowledge the following individuals for their contributions with implementation of surveillance and collection of data: Joelle Nadle, MPH, Erin Garcia, MPH, and Erin Parker, MPH (California Emerging Infections Program); Wendy Bamberg, MD and Helen Johnston, MPH (Colorado Emerging Infections Program); Carol Lyons, MPH (Connecticut Emerging Infections Program); Olivia Almendares, MPH, Leigh Ann Clark, MPH, Andrew Revis, MPH, and Zirka Thompson, MPH (Georgia Emerging Infections Program); Rebecca Perlmutter, MPH (Maryland Emerging Infections Program); Ruth Lynfield, MD (Minnesota Emerging Infections Program); Nathan Blacker (New Mexico Emerging Infections Program); Rebecca Tsay, MPH, Deborah Nelson, RN (New York Emerging Infections Program); Valerie Ocampo, RN, IMPH (Oregon Emerging Infections Program); Rebecca Roberts, MS (Respiratory Diseases Branch, Centers for Disease Control and Prevention [CDC], Atlanta, GA); and Robert Hunkler (IMS Health).
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of the US government.
Financial support. This work was supported by the Centers for Disease Control and Prevention Emerging Infections Program and the National Center for National Center for Emerging and Zoonotic Infectious Diseases.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Lucado J, Gould C, Elixhauser A. Clostridium Difficile Infections (CDI) in Hospital Stays, 2009: Statistical Brief #124. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD, 2011. [Google Scholar]
- 2.Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013. 2013; Available at: http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed 12 December 2014.
- 3.Centers for Disease Control and Prevention (CDC). Severe Clostridium difficile-associated disease in populations previously at low risk--four states, 2005. MMWR Morb Mortal Wkly Rep 2005; 54:1201–5. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC). Surveillance for community-associated Clostridium difficile--Connecticut, 2006. MMWR Morb Mortal Wkly Rep 2008; 57:340–3. [PubMed] [Google Scholar]
- 5.Kutty PK, Woods CW, Sena AC et al. Risk factors for and estimated incidence of community-associated Clostridium difficile infection, North Carolina, USA. Emerg Infect Dis 2010; 16:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karlstrom O, Fryklund B, Tullus K, Burman LG. A prospective nationwide study of Clostridium difficile-associated diarrhea in Sweden. The Swedish C. difficile Study Group. Clin Infect Dis 1998; 26:141–5. [DOI] [PubMed] [Google Scholar]
- 7.Lessa FC. Community-associated Clostridium difficile infection: how real is it? Anaerobe 2013; 24:121–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dumyati G, Stevens V, Hannett GE et al. Community-associated Clostridium difficile infections, Monroe County, New York, USA. Emerg Infect Dis 2012; 18:392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deshpande A, Pasupuleti V, Thota P et al. Community-associated Clostridium difficile infection and antibiotics: a meta-analysis. J Antimicrob Chemother 2013; 68:1951–61. [DOI] [PubMed] [Google Scholar]
- 10.Hicks LA, Taylor TH Jr, Hunkler RJ. U.S. outpatient antibiotic prescribing, 2010. N Engl J Med 2013; 368:1461–2. [DOI] [PubMed] [Google Scholar]
- 11.Shapiro DJ, Hicks LA, Pavia AT, Hersh AL. Antibiotic prescribing for adults in ambulatory care in the USA, 2007–09. J Antimicrob Chemother 2014; 69:234–40. [DOI] [PubMed] [Google Scholar]
- 12.Chitnis AS, Holzbauer SM, Belflower RM et al. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Intern Med 2013; 173:1359–67. [DOI] [PubMed] [Google Scholar]
- 13.Lessa FC, Mu Y, Winston LG et al. Determinants of Clostridium difficile infection incidence across diverse United States geographic locations. Open Forum Infect Dis 2014; 1:ofu048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen J, Limbago B, Dumyati G et al. Impact of changes in Clostridium difficile testing practices on stool rejection policies and C. difficile positivity rates across multiple laboratories in the United States. J Clin Microbiol 2014; 52:632–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Center for Health Statistics. Vintage 2011 Bridged-race postcensal population estimates for July 1, 2010 - July 1, 2011, by year, county, single-year of age (0 to 85+ years), bridged-race, Hispanic origin, and sex. Available at: http://www.cdc.gov/nchs/nvss/bridged_race.htm Accessed 23 August 2013.
- 16.Ranji SR, Steinman MA, Shojania KG, Gonzales R. Interventions to reduce unnecessary antibiotic prescribing: a systematic review and quantitative analysis. Medical Care 2008; 46:847–62. [DOI] [PubMed] [Google Scholar]
- 17.Gill JM, Fleischut P, Haas S et al. Use of antibiotics for adult upper respiratory infections in outpatient settings: a national ambulatory network study. Fam Med 2006; 38:349–54. [PubMed] [Google Scholar]
- 18.Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA 2009; 302:758–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith SM, Fahey T, Smucny J, Becker LA. Antibiotics for acute bronchitis. Cochrane Database Syst Rev 2014; 3:CD000245. [DOI] [PubMed] [Google Scholar]
- 20.Little P, Stuart B, Moore M et al. Amoxicillin for acute lower-respiratory-tract infection in primary care when pneumonia is not suspected: a 12-country, randomised, placebo-controlled trial. Lancet Infect Dis 2013; 13:123–9. [DOI] [PubMed] [Google Scholar]
- 21.Barnett ML, Linder JA. Antibiotic prescribing to adults with sore throat in the United States, 1997–2010. JAMA Intern Med 2014; 174:138–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wessels MR. Clinical practice. Streptococcal pharyngitis. N Engl J Med 2011; 364:648–55. [DOI] [PubMed] [Google Scholar]
- 23.Barnett ML, Linder JA. Antibiotic prescribing for adults with acute bronchitis in the United States, 1996–2010. JAMA 2014; 311:2020–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnold SR, Straus SE. Interventions to improve antibiotic prescribing practices in ambulatory care. Cochrane Database Syst Rev 2005; CD003539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzales R, Corbett KK, Wong S et al. “Get smart Colorado”: impact of a mass media campaign to improve community antibiotic use. Medical Care 2008; 46:597–605. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. Get Smart: Know When Antibiotics Work. Available at: http://www.cdc.gov/getsmart/ Accessed 22 October 2013.
- 27.Taur Y, Smith MA. Adherence to the Infectious Diseases Society of America guidelines in the treatment of uncomplicated urinary tract infection. Clin Infect Dis 2007; 44:769–74. [DOI] [PubMed] [Google Scholar]
- 28.Vernacchio L, Vezina RM, Mitchell AA. Management of acute otitis media by primary care physicians: trends since the release of the 2004 American Academy of Pediatrics/American Academy of Family Physicians clinical practice guideline. Pediatrics 2007; 120:281–7. [DOI] [PubMed] [Google Scholar]
- 29.Linder JA, Schnipper JL, Tsurikova R et al. Self-reported familiarity with acute respiratory infection guidelines and antibiotic prescribing in primary care. Int J Qual Health Care 2010; 22:469–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson W, Taubert KA, Gewitz M et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. J Am Dent Assoc 2007; 138:739–45, 47–60. [DOI] [PubMed] [Google Scholar]
- 31.Rethman MP, Watters W III, Abt E et al. The American Academy of Orthopaedic Surgeons and the American Dental Association clinical practice guideline on the prevention of orthopaedic implant infection in patients undergoing dental procedures. J Bone Joint Surg Am 2013; 95:745–7. [DOI] [PubMed] [Google Scholar]
- 32.Kallen AJ, Thompson A, Ristaino P et al. Complete restriction of fluoroquinolone use to control an outbreak of Clostridium difficile infection at a community hospital. Infect Control Hosp Epidemiol 2009; 30:264–72. [DOI] [PubMed] [Google Scholar]
- 33.Pepin J, Saheb N, Coulombe MA et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis 2005; 41:1254–60. [DOI] [PubMed] [Google Scholar]
- 34.Loo VG, Poirier L, Miller MA et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med 2005; 353:2442–9. [DOI] [PubMed] [Google Scholar]
- 35.Wilcox MH, Shetty N, Fawley WN et al. Changing epidemiology of Clostridium difficile infection following the introduction of a national ribotyping-based surveillance scheme in England. Clin Infect Dis 2012; 55:1056–63. [DOI] [PubMed] [Google Scholar]
- 36.Limbago BM, Long CM, Thompson AD et al. Clostridium difficile strains from community-associated infections. J Clin Microbiol 2009; 47:3004–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller M, Gravel D, Mulvey M et al. Health care-associated Clostridium difficile infection in Canada: patient age and infecting strain type are highly predictive of severe outcome and mortality. Clin Infect Dis 2010; 50:194–201. [DOI] [PubMed] [Google Scholar]
- 38.Ma J, Stafford RS. Quality of US outpatient care: temporal changes and racial/ethnic disparities. Arch Intern Med 2005; 165:1354–61. [DOI] [PubMed] [Google Scholar]
- 39.Vanderweil SG, Tsai CL, Pelletier AJ et al. Inappropriate use of antibiotics for acute asthma in United States emergency departments. Acad Emerg Med 2008; 15:736–43. [DOI] [PubMed] [Google Scholar]