Abstract
IMPORTANCE
Measures of neuronal loss are likely good surrogates for clinical and radiological disease progression in Alzheimer disease (AD). Cerebrospinal fluid (CSF) markers of neuronal injury or neurodegeneration may offer usefulness in predicting disease progression and guiding outcome assessments and prognostic decisions in clinical trials of disease-modifying therapies. Visinin-like protein 1 (VILIP-1) has demonstrated potential usefulness as a marker of neuronal injury in AD.
OBJECTIVE
To investigate the usefulness of CSF VILIP-1, tau, p-tau181, and Aβ42 levels in predicting rates of whole-brain and regional atrophy in early AD and cognitively normal control subjects over time.
DESIGN, SETTING, AND PARTICIPANTS
Longitudinal observational study of brain atrophy in participants with early AD and cognitively normal controls. Study participants had baseline CSF biomarker measurements and longitudinal magnetic resonance imaging assessments for a mean follow-up period of 2 to 3 years. Mixed linear models assessed the ability of standardized baseline CSF biomarker measures to predict rates of whole-brain and regional atrophy over the follow-up period. The setting was The Charles F. and Joanne Knight Alzheimer’s Disease Research Center, Washington University School of Medicine in St Louis. Participants (mean age, 72.6 years) were individuals with a clinical diagnosis of very mild AD (n = 23) and cognitively normal controls (n = 64) who were enrolled in longitudinal studies of healthy aging and dementia. The study dates were 2000 to 2010.
MAIN OUTCOMES AND MEASURES
Correlations between baseline CSF biomarker measures and rates of whole-brain or regional atrophy in the AD and control cohorts over the follow-up period.
RESULTS
Baseline CSF VILIP-1, tau, and p-tau181 levels (but not Aβ42 levels) predicted rates of whole-brain and regional atrophy in AD over the follow-up period. Baseline CSF VILIP-1 levels predicted whole-brain (P = .006), hippocampal (P = .01), and entorhinal (P = .001) atrophy rates at least as well as tau and p-tau181 in early AD. Cognitively normal controls whose CSF VILIP-1, tau, or p-tau181 levels were in the upper tercile had higher rates of whole-brain (P = .02, P = .003, and P = .02, respectively), hippocampal (P = .001, P = .01, and P = .02, respectively), and entorhinal (P = .007, P = .01, and P = .01, respectively) atrophy compared with those whose levels were in the lower 2 terciles.
CONCLUSIONS AND RELEVANCE
Cerebrospinal fluid VILIP-1 levels predict rates of whole-brain and regional atrophy similarly to tau and p-tau181 and may provide a useful CSF biomarker surrogate for neurodegeneration in early symptomatic and preclinical AD.
The aggregation and deposition of Aβ in the form of amyloid plaques and tau in the form of neurofibrillary tangles (NFTs) are estimated to begin approximately 10 to 15 years before the earliest signs of cognitive impairment, a stage referred to as preclinical Alzheimer disease (AD).1,2 However, substantial neuronal and synaptic loss in specific brain regions occurs before the first signs of cognitive impairment.3 Several lines of evidence suggest that neuronal and synaptic loss provides the best correlate for disease progression in AD.2,4 Structural magnetic resonance (MR) imaging measures of brain volume provide indirect estimates of neuronal, synaptic, and axonal loss5 and have been shown to be good surrogates for neurodegeneration in AD.4,5 Volumetric MR imaging measures reflect the cumulative outcome of different pathological substrates in AD, which may account for why they are good predictors of disease progression.5
Visinin-like protein 1 (VILIP-1) is a neuronal calcium-sensor protein6 that has demonstrated potential usefulness as a marker of neuronal injury in large-scale gene array analyses and animal models of brain injury.7 Our group has shown that cerebrospinal fluid (CSF) VILIP-1 and CSF VILIP-1/Aβ42 levels were prognostic of future cognitive decline in cognitively normal elderly over a mean follow-up period of 2 to 3 years,8 predicted rates of cognitive decline in early symptomatic AD similarly to p-tau181 and tau,9 and were elevated during the preclinical and clinical phases of dominantly inherited AD.10 Consistent with its potential usefulness as a marker of neurodegeneration, our group’s previous results also indicated that CSF VILIP-1 levels correlated with whole-brain and regional atrophy in cross-sectional studies of individuals with early symptomatic AD.8
Herein, we investigate the usefulness of CSF markers of neurodegeneration VILIP-1, tau, and p-tau181 as predictors of rates of brain atrophy in a longitudinal study of individuals with early symptomatic AD and cognitively normal control subjects who were followed up for 2 to 3 years. Our findings show that CSF VILIP-1 levels predicted rates of whole-brain and regional atrophy in early symptomatic AD at least as well as tau and p-tau181 over this follow-up period.
Methods
Participants
Participants (n = 87) were community-dwelling volunteers (mean [SE] age, 72.6 [0.8] years) enrolled in longitudinal studies of healthy aging and dementia through The Charles F. and Joanne Knight Alzheimer’s Disease Research Center, Washington University School of Medicine in St Louis. Study participants were in good general health, with no other medical illness that could contribute importantly to dementia and no contraindication to lumbar puncture or MR imaging. Apolipoprotein E (APOE) genotypes were obtained as previously described.11
The Clinical Dementia Rating (CDR) was used to denote the presence or absence of symptomatic AD and, when present, its severity.12,13 A CDR designation of 0 indicates cognitive normality, while a CDR designation of 0.5 denotes very mild symptomatic AD (encompassing mild cognitive impairment [MCI] due to AD), and CDR 1 and CDR 2 denote mild and moderate symptomatic AD, respectively.14 Annual clinical assessments included assignment of CDR, CDR sum of boxes,15 Mini-Mental State Examination,16 and a 1½-hour psychometric test battery.13 Baseline clinical assessments were the closest assessments before the time of the lumbar puncture (median interval, 3.4 months). All clinical diagnoses were made in accord with standard criteria.17,18 All individuals in the AD cohort (n = 23) had a clinical diagnosis of very mild symptomatic AD (CDR 0.5) at the baseline assessment. Our group has previously demonstrated that this CDR 0.5 cohort includes individuals who meet criteria for MCI, as well as those who have insufficient impairment to meet MCI criteria and might be designated as having pre-MCI.13 The rate of postmortem confirmation of a clinical diagnosis of AD in individuals who have been followed up longitudinally in our center is 92%, including the CDR 0.5 stage.13
Studies were approved by the Human Research Protection Office at Washington University School of Medicine in St Louis. Written informed consent was obtained from all participants.
CSF Collection and Processing
Cerebrospinal fluid samples (20–30 mL) were collected from all participants and analyzed for total tau, p-tau181, Aβ42 (Innotest; Innogenetics),19 and Aβ40 levels20 by enzyme-linked immunosorbent assays. The samples were analyzed for VILIP-1 by a microparticle-based immunoassay (Erenna; Singulex).
Regional and Whole-Brain Volumetry
Of 309 participants who had baseline VILIP-1 measurements in our group’s previous study,8 a total of 192 participants underwent MR imaging within 1.1 years of their lumbar puncture (median interval, 1.7 months). Of these 192 participants, 87 individuals (64 with CDR 0 and 23 with AD) had at least 1 follow-up MR imaging session and were included in this study. Baseline MR imaging measures were based on the closest imaging session before the time of the lumbar puncture (median interval, 4.4 months).
Structural MR imaging was performed using a 3.0-Timaging system (Trio; Siemens) (n = 44) or a 1.5-T imaging system (Vision; Siemens) (n = 43) as previously described.21–26 Normalized whole-brain volumes were computed as the proportion of all voxels occupied by gray and white matter (equivalent to 100% minus the percentage of CSF) voxels, yielding a unit that represents the proportion of estimated total intracranial volume. Regional volume or cortical thickness estimates were obtained via an image analysis suite (Freesurfer 5.0; http://surfer.nmr.mgh.harvard.edu/) that implements an automated probabilistic labelling procedure.25,26 Regions of interest included the hippocampus, entorhinal cortex, parahippocampal gyrus, fusiform gyrus, posterior cingulate, and precuneus. The pericalcarine cortex was included as a control region because it is rarely affected in the early stages of AD27 (eMethods in the Supplement). In vivo amyloid imaging methods are also discussed in the eMethods in the Supplement.
Statistical Analysis
Mixed linear models (PROC MIXED, SAS version 9.2; SAS Institute Inc) assessed the ability of baseline CSF biomarkers, examined as continuous or categorical measures (dichotomized at the 33rd or 66th percentile value), to predict annual change in whole-brain or regional volume or thickness measures over time. Analyses were adjusted for age, sex, imaging system type, and APOE ε4 genotype.28,29
Baseline CSF biomarker measures were standardized to z scores before analyses. Estimated effects of CSF biomarkers on annual change in volume or thickness measures are reported as β estimates. t Tests or χ2 analyses were used to compare demographic, clinical, CSF biomarker, or MR imaging variables between the clinical groups using a software program (SPSS, version 15; SPSS Inc). Statistical significance was defined as P < .05 (eMethods in the Supplement).
Results
Baseline Characteristics of Study Participants
Table 1 summarizes baseline demographic, clinical, MR imaging, and CSF biomarker characteristics of the AD (n = 23) and control (n = 64) cohorts. The mean (SE) duration of follow-up was 2.7 (0.2) years (range, 0.9–7.9 years), with a mean of 2 MR imaging assessments for each participant. There were no significant differences in age, sex, education, or mean duration of follow-up between the AD and control cohorts. The AD cohort included a higher percentage of individuals with the APOE ε4 genotype and a higher percentage of individuals with amyloid binding on positron emission tomography with Pittsburgh Compound B compared with the control cohort.
Table 1.
Baseline Demographic, Clinical, Genotype, Magnetic Resonance Imaging, and Cerebrospinal Fluid (CSF) Biomarker Characteristics of Study Participantsa
| Variable | Clinical Dementia Rating 0 (n = 64) |
Alzheimer Disease (n = 23) |
P Value |
|---|---|---|---|
| Demographics | |||
| Age at lumbar puncture, mean (SE), y | 72.3 (0.8) | 73.6 (2.1) | .46 |
| Sex | .44 | ||
| Female-male ratio | 45:19 | 14:9 | |
| Female sex, No. (%) | 45 (70) | 14 (61) | |
| Education, mean (SE), y | 15.4 (0.4) | 14.0 (0.7) | .06 |
| APOE ε4 genotypeb | .04c | ||
| ε4+ to ε4− Ratio | 20:44 | 13:10 | |
| ε4+, No. (%) | 20 (31) | 13 (57) | |
| Duration of follow-up, mean (SE), y | 2.7 (0.2) | 2.5 (0.3) | .62 |
| Baseline Clinical Dementia Rating sum of boxes, mean (SE) | 0.02 (0.01) | 2.70 (0.50) | <.001c |
| Baseline Mini-Mental State Examination score, mean (SE) | 29.0 (0.2) | 26.0 (0.9) | <.001c |
| Positron emission tomography with PIBd | .005c | ||
| PIB+ to PIB− ratio | 13:37 | 8:3 | |
| PIB+, No./total number (%) | 13/50 (26) | 8/11 (73) | |
| Baseline Whole-Brain and Regional Volume or Cortical Thickness Measures, Mean (SE)e | |||
| Normalized whole-brain volume | 0.770 (0.004) | 0.740 (0.007) | .001c |
| Hippocampal volume, mm3 | 7438 (111) | 6310 (237) | <.001c |
| Entorhinal thickness, mm | 3.49 (0.05) | 3.22 (0.08) | .007c |
| Parahippocampal thickness, mm | 2.60 (0.04) | 2.45 (0.06) | .04c |
| Fusiform thickness, mm | 2.59 (0.03) | 2.46 (0.04) | .01c |
| Posterior cingulate thickness, mm | 2.48 (0.02) | 2.40 (0.03) | .04c |
| Precuneus thickness, mm | 2.26 (0.01) | 2.16 (0.03) | .003c |
| Pericalcarine thickness, mm | 1.52 (0.02) | 1.56 (0.04) | .28 |
| Baseline CSF Biomarker Measures, Mean (SE) | |||
| VILIP-1, pg/mL | 396 (16) | 549 (43) | <.001c |
| tau, pg/mL | 297 (21) | 543 (52) | <.001c |
| p-tau181, pg/mL | 53 (3) | 82 (6) | <.001c |
| Aβ42, pg/mL | 613 (30) | 436 (30) | .001c |
| tau to Aβ42 ratio | 0.62 (0.07) | 1.44 (0.18) | <.001c |
| p-tau181 to Aβ42 ratio | 0.11 (0.01) | 0.21 (0.02) | <.001c |
| VILIP-1 to Aβ42 ratio | 0.78 (0.06) | 1.39 (0.13) | <.001c |
Abbreviations: APOE, apolipoprotein E ε4; PIB, Pittsburgh Compound B.
t Tests or χ2 tests were used to compare demographic, clinical, genotype, CSF, and magnetic resonance imaging biomarker characteristics between the Alzheimer disease cohort (Clinical Dementia Rating 0.5) and control cohort (Clinical Dementia Rating 0).
The APOE ε4+ genotype was defined by the presence of at least 1 APOE ε4 allele.
P < .05.
Of 87 participants in the study, 61 underwent positron emission tomography with PIB, including 50 individuals in the control cohort (Clinical Dementia Rating 0) and 11 individuals in the Alzheimer disease cohort (Clinical Dementia Rating 0.5).
Values represent baseline normalized whole-brain volume (Clinical Dementia Rating 0, n = 64, and Clinical Dementia Rating 0.5, n = 23) and regional cortical volume or thickness measures in cubic millimeters or millimeters, respectively (Clinical Dementia Rating 0, n = 61, and Clinical Dementia Rating 0.5, n = 23).
Individuals in the AD cohort exhibited the typical CSF biomarker phenotype of AD, with higher mean levels of tau and p-tau181 and lower mean levels of Aβ42. Baseline CSF VILIP-1 and VILIP-1/Aβ42 levels were higher in the AD cohort than in the control cohort. Compared with controls, individuals with AD had lower baseline whole-brain volumes, hippocampal volumes, and cortical thickness measures of the entorhinal, parahippocampal, fusiform, cingulate, and precuneus regions.
Correlations Between Baseline CSF Biomarker Measures and Annual Change in MR Imaging Measures in the AD Cohort
The mean (SE) adjusted rate of atrophy in the AD cohort was −0.007 (0.001) points per year for normalized whole-brain volume (−0.9% annual change from baseline), −271 (42) mm3 per year for hippocampal volume (−4.3% annual change from baseline), and −0.14 (0.03) mm per year for entorhinal thickness (−4.3% annual change from baseline), adjusting for age, sex, imaging system type, and APOE ε4 genotype. The mean (SE) adjusted rate of atrophy in the control cohort was −0.003 (0.001) points per year for normalized whole-brain volume (−0.4% annual change from baseline), −100 (25) mm3 per year for hippocampal volume (−1.3% annual change from baseline), and −0.05 (0.02) mm per year for entorhinal thickness (−1.3% annual change from baseline) (eTable 1 in the Supplement).
When examined as continuous variables, baseline CSF VILIP-1, tau, and p-tau181 levels (but not Aβ42 or Aβ40 levels) predicted annual change in normalized whole-brain volume in the AD cohort over the follow-up period (adjusting for age, sex, imaging system type, and APOE ε4 genotype). Similarly, baseline CSF VILIP-1, tau, and p-tau181 levels (but not Aβ42 or Aβ40 levels) were closely associated with rates of hippocampal, entorhinal, parahippocampal, fusiform, cingulate, and precuneus atrophy in the AD cohort. None of the CSF biomarker measures correlated with rates of pericalcarine atrophy in the AD cohort (Table 2).
Table 2.
Cerebrospinal Fluid (CSF) Biomarker Measures as Predictors of Annual Change in Whole-Brain and Regional Volume or Thickness Measures in Alzheimer Diseasea
| CSF Biomarker | P Value | β Estimate | P Value | β Estimate | P Value | β Estimate | P Value | β Estimate |
|---|---|---|---|---|---|---|---|---|
| Normalized Whole-Brain Volume | Hippocampal Volume | Entorhinal Thickness | Parahippocampal Thickness | |||||
| VILIP-1 | .006b | −0.004 | .01b | −95.0 | .001b | −0.093 | .006b | −0.087 |
| tau | .005b | −0.003 | .02b | −90.3 | .01b | −0.076 | .01b | −0.076 |
| p-tau181 | .006b | −0.003 | .04b | −76.1 | .008b | −0.076 | .03b | −0.064 |
| Aβ42 | .17 | 0.002 | .05 | 77.9 | .15 | 0.048 | .71 | 0.012 |
| Aβ40 | .19 | −0.001 | .17 | −57.4 | .24 | −0.039 | .79 | −0.009 |
| Fusiform Thickness | Posterior Cingulate Thickness | Precuneus Thickness | Pericalcarine Thickness | |||||
| VILIP-1 | .04b | −0.056 | .005b | −0.062 | .07 | −0.036 | .28 | 0.007 |
| tau | .04b | −0.057 | .04b | −0.049 | .08 | −0.033 | .52 | −0.005 |
| p-tau181 | .001b | −0.081 | .002b | −0.069 | .02b | −0.044 | .36 | −0.006 |
| Aβ42 | .58 | −0.017 | .38 | −0.023 | .90 | 0.003 | .44 | −0.006 |
| Aβ40 | .10 | −0.055 | .09 | −0.051 | .53 | −0.015 | .61 | −0.010 |
Abbreviation: VILIP-1, visinin-like protein 1.
In these analyses, CSF biomarker measures were standardized to z scores and examined as continuous variables. The given β estimate for a CSF biomarker measure reflects the difference in annual change in a volume or thickness measure per standardized biomarker unit. Analyses were adjusted for age, sex, imaging system type, and apolipoprotein E ε4 genotype. Cerebrospinal fluid Aβ40 levels were used as a control.
P < .05.
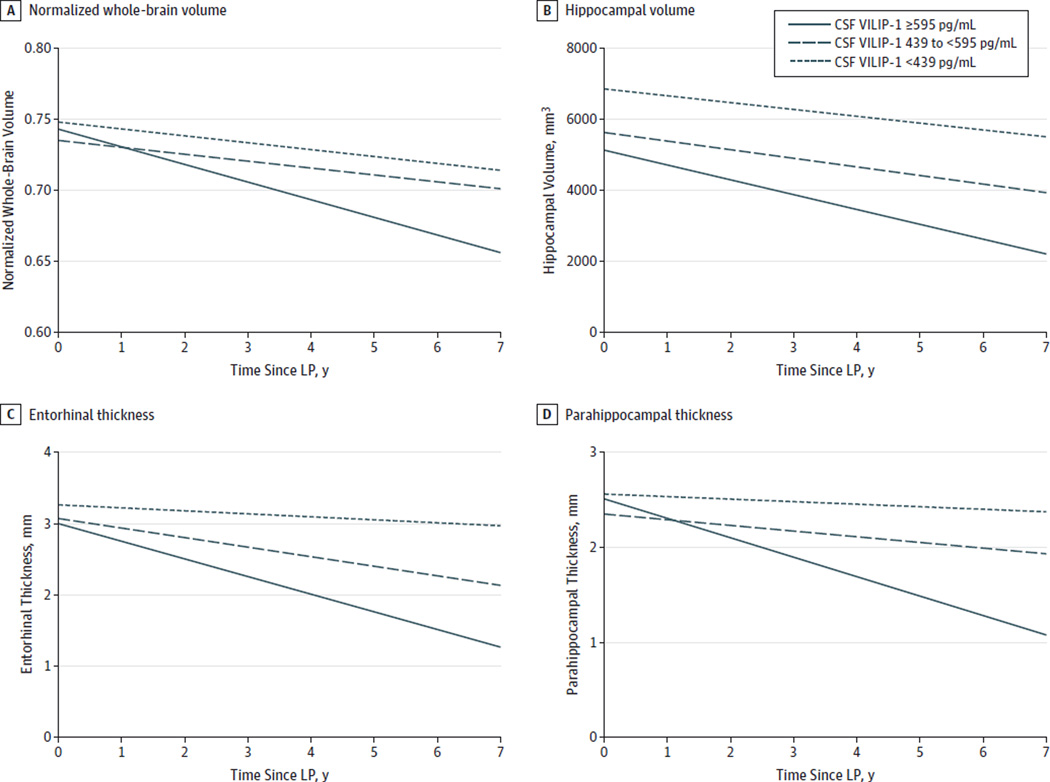
Analyses were then performed for CSF biomarker measures as categorical variables (adjusting for age, sex, imaging system type, and APOE ε4 genotype). Individuals in the AD cohort (n = 23) were divided into 3 terciles for each CSF biomarker measure (using the 33rd and 66th percentile values as cutoffs). Cerebrospinal fluid VILIP-1, tau, or p-tau181 levels in the upper tercile (corresponding to ≥595, ≥550, and ≥92 pg/mL, respectively) predicted more rapid rates of whole-brain atrophy than those in the lower 2 terciles over time (Table 3 and Figure, A). Similarly, compared with those in the lower 2 terciles, CSF VILIP-1, tau, and p-tau181 levels in the upper tercile predicted more rapid rates of hippocampal, entorhinal, and parahippocampal atrophy (Table 3 and Figure, B–D), as well as fusiform, cingulate, and precuneus atrophy (eTable 2 in the Supplement). There were no significant differences in rates of whole-brain or regional atrophy between individuals in the lower tercile of Aβ42 values (corresponding to <320 pg/mL) and those in the upper 2 terciles.
Table 3.
Rates of Change in Normalized Whole-Brain Volume, Hippocampal Volume, and Entorhinal Thickness Measures as a Function of Cerebrospinal Fluid (CSF) Biomarker Measures in Alzheimer Diseasea
| CSF Biomarkerb | Mean (SE) | P Valuec | |
|---|---|---|---|
| Normalized Whole-Brain Volume, Points per Year | |||
| Lower 2 Terciles | Upper Tercile | ||
| VILIP-1 | −0.0048 (0.0017) | −0.0123 (0.0025) | .02d |
| tau | −0.0042 (0.0014) | −0.0092 (0.0018) | .03d |
| p-tau181 | −0.0044 (0.0018) | −0.0113 (0.0022) | .02d |
| Upper 2 Terciles | Lower Tercile | ||
| Aβ42 | −0.0061 (0.0011) | −0.0085 (0.0026) | .40 |
| Hippocampal Volume, mm3 per Year | |||
| Lower 2 Terciles | Upper Tercile | ||
| VILIP-1 | −220.2 (45.3) | −418.3 (76.6) | .03d |
| tau | −209.2 (44.3) | −423.3 (71.6) | .01d |
| p-tau181 | −202.4 (44.7) | −414.1 (67.9) | .02d |
| Upper 2 Terciles | Lower Tercile | ||
| Aβ42 | −236.7 (46.9) | −366.8 (85.1) | .18 |
| Entorhinal Cortical Thickness, mm per Year | |||
| Lower 2 Terciles | Upper Tercile | ||
| VILIP-1 | −0.083 (0.037) | −0.248 (0.051) | .01d |
| tau | −0.091 (0.039) | −0.220 (0.051) | .04d |
| p-tau181 | −0.088 (0.037) | −0.225 (0.051) | .03d |
| Upper 2 Terciles | Lower Tercile | ||
| Aβ42 | −0.124 (0.069) | −0.147 (0.039) | .77 |
Abbreviations: APOE, apolipoprotein E ε4; VILIP-1, visinin-like protein 1.
Mixed linear models were used to estimate rates of change in normalized whole-brain volume, hippocampal volume, and entorhinal thickness measures in Alzheimer disease (Clinical Dementia Rating 0.5) over time as a function of CSF biomarker measures (adjusting for age, sex, imaging system type, and APOE ε4 genotype). In these analyses, CSF biomarkers were examined as categorical variables (dichotomized at the 33rd or 66th percentile) to compare rates of brain atrophy between individuals in the upper tercile vs those in the lower 2 terciles for CSF biomarker measures (or the lower tercile vs the upper 2 terciles for Aβ42).
The 66th percentile cutoff values in the Alzheimer disease cohort were ≥595, ≥550, and ≥92 pg/mL for VILIP-1, tau, and p-tau181, respectively. Cerebrospinal fluid Aβ42 values were dichotomized at the 33rd percentile value of <320pg/mL.
P values reflect whether CSF biomarker measures (dichotomized at the 33rd or 66th percentile value) significantly predict rates of brain atrophy in Alzheimer disease (adjusting for age, sex, imaging system type, and APOE ε4 genotype).
P < .05.
Figure. Rates of Change in Normalized Whole-Brain Volume, Hippocampal Volume, and Entorhinal and Parahippocampal Thickness Measures as a Function of Cerebrospinal Fluid (CSF) Visinin-Like Protein 1 (VILIP-1) Terciles in Alzheimer Disease.
Mixed linear models were used to estimate rates of change in normalized whole-brain volume (A), hippocampal volume (B), and entorhinal (C) and parahippocampal (D) thickness measures in the Alzheimer disease cohort over time as a function of CSF VILIP-1 levels. Estimated slope and intercept for each of the 3 CSF VILIP-1 terciles are plotted, adjusting for age, sex, imaging system type, and apolipoprotein E ε4 genotype. Adjusted rates of brain atrophy in individuals in the Alzheimer disease cohort whose CSF VILIP-1 levels were in the upper, middle, and lower terciles were −0.012, −0.005, and −0.005 points per year, respectively, for normalized whole-brain volume; −418, −241, and −191 mm3 per year, respectively, for hippocampal volume; −0.25, −0.13, and −0.04 mm per year, respectively, for entorhinal thickness; and −0.21, −0.06, and −0.03 mm per year, respectively, for parahippocampal thickness. LP indicates lumbar puncture.
Correlations Between Baseline CSF Biomarker Measures and Annual Change in MR Imaging Measures in the Control Cohort
When examined as continuous variables, baseline CSF VILIP-1 levels (P = .03) and tau levels (P = .04) (but not p-tau181, Aβ42, or Aβ40 levels) predicted annual change in whole-brain volume in cognitively normal controls (adjusting for age, sex, imaging system type, and APOE ε4 genotype). None of the CSF biomarker measures (examined as continuous variables) correlated with annual change in regional volume or thickness measures in controls (eTable 3 in the Supplement). When examined as categorical variables, cognitively normal controls whose CSF VILIP-1, tau, or p-tau181 levels were in the upper tercile (corresponding to ≥427, ≥293, and ≥57 pg/mL, respectively) had more rapid rates of whole-brain, hippocampal, entorhinal, parahippocampal, and fusiform atrophy compared with those in the lower 2 terciles (Table 4, eTable 4 and eFigure in the Supplement). Cerebrospinal fluid Aβ42 terciles were not associated with rates of whole-brain or regional atrophy in controls. None of the CSF biomarker terciles correlated with rates of cingulate, precuneus, or pericalcarine atrophy in our control cohort.
Table 4.
Rates of Change in Normalized Whole-Brain Volume, Hippocampal Volume, and Entorhinal Thickness Measures as a Function of Cerebrospinal Fluid (CSF) Biomarker Measures in Control Subjectsa
| CSF Biomarkerb | Mean (SE) | P Valuec | |
|---|---|---|---|
| Normalized Whole-Brain Volume, Points per Year | |||
| Lower 2 Terciles | Upper Tercile | ||
| VILIP-1 | −0.0018 (0.0009) | −0.0051 (0.0011) | .02d |
| tau | −0.0016 (0.0008) | −0.0059 (0.0012) | .003d |
| p-tau181 | −0.0020 (0.0008) | −0.0056 (0.0014) | .02d |
| Upper 2 Terciles | Lower Tercile | ||
| Aβ42 | −0.0026 (0.0009) | −0.0035 (0.0011) | .51 |
| Hippocampal Volume, mm3 per Year | |||
| Lower 2 Terciles | Upper Tercile | ||
| VILIP-1 | −50.7 (27.8) | −199.4 (38.6) | .001d |
| tau | −57.7 (27.4) | −202.3 (44.3) | .01d |
| p-tau181 | −69.1 (27.2) | −192.5 (44.5) | .02d |
| Upper 2 Terciles | Lower Tercile | ||
| Aβ42 | −84.8 (30.9) | −123.9 (38.7) | .41 |
| Entorhinal Cortical Thickness, mm per Year | |||
| Lower 2 Terciles | Upper Tercile | ||
| VILIP-1 | −0.017 (0.019) | −0.104 (0.026) | .007d |
| tau | −0.027 (0.019) | −0.076 (0.029) | .01d |
| p-tau181 | −0.028 (0.019) | −0.078 (0.030) | .01d |
| Upper 2 Terciles | Lower Tercile | ||
| Aβ42 | −0.052 (0.021) | −0.036 (0.026) | .64 |
Abbreviations: APOE, apolipoprotein E ε4; VILIP-1, visinin-like protein 1.
Mixed linear models were used to estimate rates of change in normalized whole-brain volume, hippocampal volume, and entorhinal thickness measures in cognitively normal control subjects (Clinical Dementia Rating 0) over time as a function of CSF biomarker measures (adjusting for age, sex, imaging system type, and APOE ε4 genotype). In these analyses, CSF biomarkers were examined as categorical variables (dichotomized at the 33rd or 66th percentile) to compare rates of brain atrophy between individuals in the upper tercile vs those in the lower 2 terciles for CSF biomarker measures (or the lower tercile vs the upper 2 terciles for Aβ42).
The 66th percentile cutoff values in the control cohort were ≥427, ≥293, and ≥57 pg/mL for VILIP-1, tau, and p-tau181, respectively. Cerebrospinal fluid Aβ42 values were dichotomized at the 33rd percentile value of <502 pg/mL.
P values reflect whether CSF biomarker measures (dichotomized at the 33rd or 66th percentile value) significantly predict rates of brain atrophy in cognitively normal control subjects (adjusting for age, sex, imaging system type, and APOE ε4 genotype).
P < .05.
Discussion
VILIP-1 is a calcium-sensor protein6,30 that is abundantly expressed in neurons but not other cell types in the brain8,31 and has demonstrated potential usefulness as a marker of neuronal injury in large-scale gene arrays.7 The protein is found in close association with NFTs and amyloid plaques in AD brains but does not appear to be a component of NFTs.8,31 The results of previous studies30,31 suggest that VILIP-1 may be involved in calcium-mediated neurodegeneration in AD. Increased CSF VILIP-1 levels in AD but not in other types of dementia8,32,33 and altered expression patterns in AD are thought to reflect the selective vulnerability of VILIP-1-expressing neurons to altered calcium signaling pathways in the presence of AD pathology.8,31,34
Our group has previously shown that CSF VILIP-1 levels predict clinical disease progression similarly to p-tau181 and tau9 and correlate with cross-sectional measures of whole-brain and regional atrophy in early AD.8 Furthermore, CSF VILIP-1 levels are associated with amyloid load and future cognitive impairment in cognitively normal individuals.8 Together, these findings support the potential usefulness of CSF VILIP-1 as a biomarker surrogate for neurodegeneration in preclinical and early symptomatic AD.10
We herein investigate the usefulness of CSF VILIP-1, tau, and p-tau181 in predicting rates of brain atrophy in a well-characterized cohort of individuals with early symptomatic AD and cognitively normal controls who were followed up for 2 to 3 years. Our results suggest that CSF VILIP-1, tau, and p-tau181 levels predicted rates of whole-brain and regional atrophy of the hippocampus, entorhinal, parahippocampal, fusiform, cingulate, and precuneus regions in early AD over the follow-up period. In our cohort, individuals with baseline VILIP-1, tau, or p-tau181 values in the upper tercile had higher rates of whole-brain and regional atrophy compared with individuals in the lower 2 terciles over time. The ability of CSF VILIP-1, tau, and p-tau181 to predict rates of subsequent brain atrophy when examined as continuous measures, as well as the ordered gradation in rates of brain atrophy among these CSF biomarker terciles in AD, underscores their predictive ability independent of the cutoff values proposed in this study.
Our findings that baseline CSF tau and p-tau181 levels35–38 (but not Aβ42 levels37,39,40) predicted rates of whole-brain and regional atrophy in AD are consistent with previous reports and with our current understanding of the temporal sequence of pathological changes in early AD.2,41,42 Several lines of evidence suggest that NFT load43–45 and neuronal counts46,47 (but not cortical amyloid burden39,48) are associated with rates of brain atrophy in AD. Cerebrospinal fluid Aβ42 levels are thought to first decrease a decade or more before the onset of cognitive impairment.1,2,49 Once this low set point is reached,19 CSF Aβ42 levels do not change substantially over time in individuals with and without impairment.50,51 Conversely, following the first signs of cognitive impairment, progressive increase in NFT pathology and progressive neuronal and synaptic loss on a background of substantial Aβ accumulation are thought to continue into the more advanced stages of disease.9,42,49 While CSF Aβ42 levels are reflective of disease stage,37,52 it is likely that CSF tau and VILIP-1 levels (reflective of neurodegeneration) and p-tau181 levels (reflective of NFT burden) are more closely associated with disease intensity53 and with rates of subsequent brain atrophy in early symptomatic AD.9,52 Therefore, markers of amyloid pathology and markers of tangle pathology or neurodegeneration appear to have different prognostic roles in the different stages of disease.52
Volumetric MR imaging measures of whole-brain and regional atrophy provide indirect assessments of neuronal loss54 and have been proposed as useful radiological surrogates of neurodegeneration in AD.46,47,55 Data from several longitudinal MR imaging studies56–58 suggest a spatial pattern of regional volume loss that closely parallels the staging of neurofibrillary pathology by Braak et al.27 This AD signature of regional atrophy is characterized by predominantly early involvement of medial temporal structures (entorhinal cortex, hippocampus, and parahippocampal gyrus),54,57–59 as well as cingulate, fusiform, and precuneus in very mild AD or MCI,59,60 followed by spread of the pathology into the lateral temporal, inferior parietal, and orbitofrontal regions as the disease progresses.58,60 In very mild AD or MCI compared with controls, our group’s observations that baseline volume or thickness measures of these regions were lower and that rates of regional atrophy over the follow-up period were higher are consistent with the topographical distribution of neuronal loss in these very early stages.60,61
Medial temporal lobe structures are implicated as the sites of earliest neurodegeneration in AD, with an estimated volume loss of 10% to 25% in very mild AD or MCI compared with healthy controls.27,58,62 Our group has previously shown that CSF VILIP-1 levels are closely associated with baseline hippocampal, entorhinal, and parahippocampal volumes in early AD.8 Notably, we show herein that baseline CSF VILIP-1 levels predicted rates of whole-brain, hippocampal, entorhinal, and parahippocampal atrophy over the follow-up period at least as well as tau and p-tau181 in our longitudinal cohort. While CSF tau, p-tau181, and Aβ42 each reflect a specific pathological substrate of AD, brain atrophy (as a surrogate of neuronal loss) likely reflects the cumulative outcome of different and highly intricate pathological processes.55 Data from other cohorts suggest that CSF tau and Aβ42 explain only a fraction of regional volume changes in early AD.63 Together, these findings support the notion that markers that reflect neuronal loss or neurodegeneration (such as VILIP-1) may offer usefulness in predicting baseline or longitudinal brain volume loss that is at least comparable to that of CSF markers of tau or amyloid pathologies. Most important, such markers may be useful in monitoring clinical or radiological disease progression and in assessing response to potential disease-modifying therapies independent of changes to tau or Aβ42.
Similarly to previous cohorts,8 our control cohort in the present study included individuals whose CSF biomarker values are comparable to those in AD, many of whom had evidence of amyloid retention on positron emission tomography with Pittsburgh Compound B and therefore likely represent a subset of cognitively normal individuals with preclinical AD. In the cohort studied herein, cognitively normal controls whose baseline CSF VILIP-1, tau, or p-tau181 values were in the upper tercile had more rapid rates of whole-brain and regional atrophy compared with those in the lower 2 terciles. Notably, CSF biomarker levels and rates of whole-brain and regional atrophy in this subset of controls were similar to those of individuals in the AD cohort. These results are in line with previously reported associations between NFT load and neuronal loss in preclinical AD10,64 and with proposed models of disease progression suggesting that AD pathology is associated with ongoing neurodegeneration even before symptom onset.2,65–67 In the presence of AD pathology, substantial volume loss in vulnerable brain regions, particularly higher rates of medial temporal lobe atrophy, can be detected on structural MR imaging years before the first signs of cognitive impairment.68,69
Conclusions
Our findings highlight the potential usefulness of CSF VILIP-1 as a secondary or tertiary outcome measure in clinical trials of disease-modifying therapies in both preclinical and symptomatic AD.70 By providing a CSF surrogate of neurodegeneration, CSF VILIP-1 and tau may assist in guiding trial design and contribute to diagnostic and outcome assessments. The incorporation of CSF biomarkers in these trials may assist with participant selection by enriching study populations with individuals who are likely to progress within the study period. While MR imaging measures of volume loss provide insight into regional patterns of neuronal loss or subtypes of AD pathology,60 CSF markers of neurodegeneration (such as VILIP-1 and tau) may provide univariate indicators to monitor disease progression and response to therapies over shorter follow-up periods and complement information provided by MR imaging.
Our study is limited by the small sample size of our AD and control cohorts and the short duration of follow-up. It will be of interest in the future to validate these findings in larger cohorts with longer durations of follow-up from different centers. Efforts to standardize the VILIP-1 immunoassay used in this study are underway. Studies with individuals in the more advanced stages of disease and those with longitudinal CSF biomarker measurements will be needed to provide further insight into the usefulness of CSF VILIP-1 as a biomarker surrogate of neurodegeneration in AD.
Supplementary Material
Acknowledgments
Dr Fagan reported serving on the scientific advisory boards of IBL International and Roche and reported being a consultant for AbbVie. Dr Holtzman reported being a cofounder of and on the scientific advisory board for C2N Diagnostics and reported serving as a consultant for Genentech, AstraZeneca, and NeuroPhage.
Funding/Support: This work was supported by grants P50-AG05681, P01-AG03991, and P01-AG026276 from the National Institutes of Health (Dr Morris); by Eli Lilly and Company (Dr Holtzman); by The JPB Foundation (Dr Holtzman); by Siemens Healthcare Diagnostics (Dr Ladenson); and by The Charles F. and Joanne Knight Alzheimer’s Disease Research Center.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional Contributions: We acknowledge the contributions of Kim Lipsey and the Clinical, Psychometrics, Biomarker, Imaging, and Biostatistics cores of The Charles F. and Joanne Knight Alzheimer’s Disease Research Center.
Footnotes
Author Contributions: Dr Tarawneh had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Tarawneh, Head, Buckles, Fagan, Ladenson, Morris, Holtzman.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Tarawneh, Holtzman.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Tarawneh.
Obtained funding: Fagan, Ladenson, Morris, Holtzman.
Administrative, technical, or material support: Ladenson, Morris, Holtzman.
Conflict of Interest Disclosures: No other disclosures were reported.
REFERENCES
- 1.Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999;45(3):358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 2.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price JL, Ko AI, Wade MJ, Tsou SK, McKeel DW, Morris JC. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Arch Neurol. 2001;58(9):1395–1402. doi: 10.1001/archneur.58.9.1395. [DOI] [PubMed] [Google Scholar]
- 4.Jack CR, Jr, Shiung MM, Weigand SD, et al. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology. 2005;65(8):1227–1231. doi: 10.1212/01.wnl.0000180958.22678.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vemuri P, Wiste HJ, Weigand SD, et al. Alzheimer’s Disease Neuroimaging Initiative. MRI and CSF biomarkers in normal, MCI, and AD subjects: predicting future clinical change. Neurology. 2009;73(4):294–301. doi: 10.1212/WNL.0b013e3181af79fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braunewell KH, Klein-Szanto AJ. Visinin-like proteins (VSNLs): interaction partners and emerging functions in signal transduction of a subfamily of neuronal Ca2+-sensor proteins. Cell Tissue Res. 2009;335(2):301–316. doi: 10.1007/s00441-008-0716-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laterza OF, Modur VR, Crimmins DL, et al. Identification of novel brain biomarkers. Clin Chem. 2006;52(9):1713–1721. doi: 10.1373/clinchem.2006.070912. [DOI] [PubMed] [Google Scholar]
- 8.Tarawneh R, D’Angelo G, Macy E, et al. Visinin-like protein-1: diagnostic and prognostic biomarker in Alzheimer disease. Ann Neurol. 2011;70(2):274–285. doi: 10.1002/ana.22448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tarawneh R, Lee JM, Ladenson JH, Morris JC, Holtzman DM. CSF VILIP-1 predicts rates of cognitive decline in early Alzheimer disease. Neurology. 2012;78(10):709–719. doi: 10.1212/WNL.0b013e318248e568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fagan AM, Xiong C, Jasielec MS, et al. Dominantly Inherited Alzheimer Network. Longitudinal change in CSF biomarkers in autosomal-dominant Alzheimer’s disease. Sci Transl Med. 2014;6(226):226ra30. doi: 10.1126/scitranslmed.3007901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talbot C, Lendon C, Craddock N, Shears S, Morris JC, Goate A. Protection against Alzheimer’s disease with apoE epsilon 2. Lancet. 1994;343(8910):1432–1433. doi: 10.1016/s0140-6736(94)92557-7. [DOI] [PubMed] [Google Scholar]
- 12.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 13.Storandt M, Grant EA, Miller JP, Morris JC. Longitudinal course and neuropathologic outcomes in original vs revised MCI and in pre-MCI. Neurology. 2006;67(3):467–473. doi: 10.1212/01.wnl.0000228231.26111.6e. [DOI] [PubMed] [Google Scholar]
- 14.Morris JC, Blennow K, Froelich L, et al. Harmonized diagnostic criteria for Alzheimer’s disease: recommendations. J Intern Med. 2014;275(3):204–213. doi: 10.1111/joim.12199. [DOI] [PubMed] [Google Scholar]
- 15.Berg L, Miller JP, Baty J, Rubin EH, Morris JC, Figiel G. Mild senile dementia of the Alzheimer type, 4: evaluation of intervention. Ann Neurol. 1992;31(3):242–249. doi: 10.1002/ana.410310303. [DOI] [PubMed] [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 17.Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20(4):210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 18.Berg L, McKeel DW, Jr, Miller JP, et al. Clinicopathologic studies in cognitively healthy aging and Alzheimer’s disease: relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch Neurol. 1998;55(3):326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- 19.Fagan AM, Mintun MA, Mach RH, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Aβ42 in humans. Ann Neurol. 2006;59(3):512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 20.Cirrito JR, May PC, O’Dell MA, et al. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-β metabolism and half-life. J Neurosci. 2003;23(26):8844–8853. doi: 10.1523/JNEUROSCI.23-26-08844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buckner RL, Head D, Parker J, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23(2):724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 22.Head D, Snyder AZ, Girton LE, Morris JC, Buckner RL. Frontal-hippocampal double dissociation between normal aging and Alzheimer’s disease. Cereb Cortex. 2005;15(6):732–739. doi: 10.1093/cercor/bhh174. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20(1):45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 24.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 26.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 27.Braak H, Braak E, Bohl J. Staging of Alzheimer-related cortical destruction. Eur Neurol. 1993;33(6):403–408. doi: 10.1159/000116984. [DOI] [PubMed] [Google Scholar]
- 28.Tosun D, Schuff N, Truran-Sacrey D, et al. Alzheimer’s Disease Neuroimaging Initiative. Relations between brain tissue loss, CSF biomarkers, and the ApoE genetic profile: a longitudinal MRI study. Neurobiol Aging. 2010;31(8):1340–1354. doi: 10.1016/j.neurobiolaging.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hostage CA, Choudhury KR, Murali Doraiswamy P, Petrella JR Alzheimer’s Disease Neuroimaging Initiative. Mapping the effect of the apolipoprotein E genotype on 4-year atrophy rates in an Alzheimer disease–related brain network. Radiology. 2014;271(1):211–219. doi: 10.1148/radiol.13131041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braunewell K, Riederer P, Spilker C, Gundelfinger ED, Bogerts B, Bernstein HG. Abnormal localization of two neuronal calcium sensor proteins, visinin-like proteins (VILIPs)-1 and-3, in neocortical brain areas of Alzheimer disease patients. Dement Geriatr Cogn Disord. 2001;12(2):110–116. doi: 10.1159/000051244. [DOI] [PubMed] [Google Scholar]
- 31.Schnurra I, Bernstein HG, Riederer P, Braunewell KH. The neuronal calcium sensor protein VILIP-1 is associated with amyloid plaques and extracellular tangles in Alzheimer’s disease and promotes cell death and tau phosphorylation in vitro: a link between calcium sensors and Alzheimer’s disease? Neurobiol Dis. 2001;8(5):900–909. doi: 10.1006/nbdi.2001.0432. [DOI] [PubMed] [Google Scholar]
- 32.Lee JM, Blennow K, Andreasen N, et al. The brain injury biomarker VLP-1 is increased in the cerebrospinal fluid of Alzheimer disease patients. Clin Chem. 2008;54(10):1617–1623. doi: 10.1373/clinchem.2008.104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo X, Hou L, Shi H, et al. CSF levels of the neuronal injury biomarker visinin-like protein-1 in Alzheimer’s disease and dementia with Lewy bodies. J Neurochem. 2013;127(5):681–690. doi: 10.1111/jnc.12331. [DOI] [PubMed] [Google Scholar]
- 34.Braunewell KH. The visinin-like proteins VILIP-1 and VILIP-3 in Alzheimer’s disease: old wine in new bottles. Front Mol Neurosci. 2012;5:20. doi: 10.3389/fnmol.2012.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henneman WJ, Vrenken H, Barnes J, et al. Baseline CSF p-tau levels independently predict progression of hippocampal atrophy in Alzheimer disease. Neurology. 2009;73(12):935–940. doi: 10.1212/WNL.0b013e3181b879ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hampel H, Bürger K, Pruessner JC, et al. Correlation of cerebrospinal fluid levels of tau protein phosphorylated at threonine 231 with rates of hippocampal atrophy in Alzheimer disease. Arch Neurol. 2005;62(5):770–773. doi: 10.1001/archneur.62.5.770. [DOI] [PubMed] [Google Scholar]
- 37.Wahlund LO, Blennow K. Cerebrospinal fluid biomarkers for disease stage and intensity in cognitively impaired patients. Neurosci Lett. 2003;339(2):99–102. doi: 10.1016/s0304-3940(02)01483-0. [DOI] [PubMed] [Google Scholar]
- 38.Tosun D, Schuff N, Shaw LM, Trojanowski JQ, Weiner MW Alzheimer’s Disease NeuroImaging Initiative. Relationship between CSF biomarkers of Alzheimer’s disease and rates of regional cortical thinning in ADNI data. J Alzheimers Dis. 2011;26(suppl 3):77–90. doi: 10.3233/JAD-2011-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Josephs KA, Whitwell JL, Ahmed Z, et al. Beta-amyloid burden is not associated with rates of brain atrophy. Ann Neurol. 2008;63(2):204–212. doi: 10.1002/ana.21223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schuff N, Woerner N, Boreta L, et al. Alzheimer’s Disease Neuroimaging Initiative. MRI of hippocampal volume loss in early Alzheimer’s disease in relation to ApoE genotype and biomarkers. Brain. 2009;132(pt 4):1067–1077. doi: 10.1093/brain/awp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tarawneh R, Holtzman DM. Biomarkers in translational research of Alzheimer’s disease. Neuropharmacology. 2010;59(4–5):310–322. doi: 10.1016/j.neuropharm.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tarawneh R, Holtzman DM. Critical issues for successful immunotherapy in Alzheimer’s disease: development of biomarkers and methods for early detection and intervention. CNS Neurol Disord Drug Targets. 2009;8(2):144–159. doi: 10.2174/187152709787847324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Csernansky JG, Hamstra J, Wang L, et al. Correlations between antemortem hippocampal volume and postmortem neuropathology in AD subjects. Alzheimer Dis Assoc Disord. 2004;18(4):190–195. [PubMed] [Google Scholar]
- 44.Whitwell JL, Josephs KA, Murray ME, et al. MRI correlates of neurofibrillary tangle pathology at autopsy: a voxel-based morphometry study. Neurology. 2008;71(10):743–749. doi: 10.1212/01.wnl.0000324924.91351.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silbert LC, Quinn JF, Moore MM, et al. Changes in premorbid brain volume predict Alzheimer’s disease pathology. Neurology. 2003;61(4):487–492. doi: 10.1212/01.wnl.0000079053.77227.14. [DOI] [PubMed] [Google Scholar]
- 46.Bobinski M, de Leon MJ, Wegiel J, et al. The histological validation of post mortem magnetic resonance imaging–determined hippocampal volume in Alzheimer’s disease. Neuroscience. 2000;95(3):721–725. doi: 10.1016/s0306-4522(99)00476-5. [DOI] [PubMed] [Google Scholar]
- 47.Zarow C, Vinters HV, Ellis WG, et al. Correlates of hippocampal neuron number in Alzheimer’s disease and ischemic vascular dementia. Ann Neurol. 2005;57(6):896–903. doi: 10.1002/ana.20503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chételat G, Villemagne VL, Bourgeat P, et al. Australian Imaging Biomarkers and Lifestyle Research Group. Relationship between atrophy and β-amyloid deposition in Alzheimer disease. Ann Neurol. 2010;67(3):317–324. doi: 10.1002/ana.21955. [DOI] [PubMed] [Google Scholar]
- 49.Ingelsson M, Fukumoto H, Newell KL, et al. Early Aβ accumulation and progressive synaptic loss, gliosis, and tangle formation in AD brain. Neurology. 2004;62(6):925–931. doi: 10.1212/01.wnl.0000115115.98960.37. [DOI] [PubMed] [Google Scholar]
- 50.Andreasen N, Hesse C, Davidsson P, et al. Cerebrospinal fluid β-amyloid(1–42) in Alzheimer disease: differences between early- and late-onset Alzheimer disease and stability during the course of disease. Arch Neurol. 1999;56(6):673–680. doi: 10.1001/archneur.56.6.673. [DOI] [PubMed] [Google Scholar]
- 51.Blennow K, Zetterberg H, Minthon L, et al. Longitudinal stability of CSF biomarkers in Alzheimer’s disease. Neurosci Lett. 2007;419(1):18–22. doi: 10.1016/j.neulet.2007.03.064. [DOI] [PubMed] [Google Scholar]
- 52.van Rossum IA, Visser PJ, Knol DL, et al. Injury markers but not amyloid markers are associated with rapid progression from mild cognitive impairment to dementia in Alzheimer’s disease. J Alzheimers Dis. 2012;29(2):319–327. doi: 10.3233/JAD-2011-111694. [DOI] [PubMed] [Google Scholar]
- 53.Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol. 2004;61(3):378–384. doi: 10.1001/archneur.61.3.378. [DOI] [PubMed] [Google Scholar]
- 54.de Leon MJ, George AE, Stylopoulos LA, Smith G, Miller DC. Early marker for Alzheimer’s disease: the atrophic hippocampus. Lancet. 1989;2(8664):672–673. doi: 10.1016/s0140-6736(89)90911-2. [DOI] [PubMed] [Google Scholar]
- 55.Risacher SL, Saykin AJ. Neuroimaging and other biomarkers for Alzheimer’s disease: the changing landscape of early detection. Annu Rev Clin Psychol. 2013;9:621–648. doi: 10.1146/annurev-clinpsy-050212-185535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baron JC, Chételat G, Desgranges B, et al. In vivo mapping of gray matter loss with voxel-based morphometry in mild Alzheimer’s disease. Neuroimage. 2001;14(2):298–309. doi: 10.1006/nimg.2001.0848. [DOI] [PubMed] [Google Scholar]
- 57.Chételat G, Desgranges B, De La Sayette V, Viader F, Eustache F, Baron JC. Mapping gray matter loss with voxel-based morphometry in mild cognitive impairment. Neuroreport. 2002;13(15):1939–1943. doi: 10.1097/00001756-200210280-00022. [DOI] [PubMed] [Google Scholar]
- 58.Braak H, Braak E, Bohl J, Bratzke H. Evolution of Alzheimer’s disease related cortical lesions. J Neural Transm Suppl. 1998;54:97–106. doi: 10.1007/978-3-7091-7508-8_9. [DOI] [PubMed] [Google Scholar]
- 59.Whitwell JL, Shiung MM, Przybelski SA, et al. MRI patterns of atrophy associated with progression to AD in amnestic mild cognitive impairment. Neurology. 2008;70(7):512–520. doi: 10.1212/01.wnl.0000280575.77437.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fan Y, Resnick SM, Wu X, Davatzikos C. Structural and functional biomarkers of prodromal Alzheimer’s disease: a high-dimensional pattern classification study. Neuroimage. 2008;41(2):277–285. doi: 10.1016/j.neuroimage.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Misra C, Fan Y, Davatzikos C. Baseline and longitudinal patterns of brain atrophy in MCI patients, and their use in prediction of short-term conversion to AD: results from ADNI. Neuroimage. 2009;44(4):1415–1422. doi: 10.1016/j.neuroimage.2008.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bobinski M, de Leon MJ, Tarnawski M, et al. Neuronal and volume loss in CA1 of the hippocampal formation uniquely predicts duration and severity of Alzheimer disease. Brain Res. 1998;805(1–2):267–269. doi: 10.1016/s0006-8993(98)00759-8. [DOI] [PubMed] [Google Scholar]
- 63.Fjell AM, Walhovd KB, Fennema-Notestine C, et al. Alzheimer’s Disease Neuroimaging Initiative. CSF biomarkers in prediction of cerebral and clinical change in mild cognitive impairment and Alzheimer’s disease. J Neurosci. 2010;30(6):2088–2101. doi: 10.1523/JNEUROSCI.3785-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jack CR, Jr, Dickson DW, Parisi JE, et al. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology. 2002;58(5):750–757. doi: 10.1212/wnl.58.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jack CR, Jr, Holtzman DM. Biomarker modeling of Alzheimer’s disease. Neuron. 2013;80(6):1347–1358. doi: 10.1016/j.neuron.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Desikan RS, McEvoy LK, Thompson WK, et al. Alzheimer’s Disease Neuroimaging Initiative. Amyloid-β–associated clinical decline occurs only in the presence of elevated p-tau. Arch Neurol. 2012;69(6):709–713. doi: 10.1001/archneurol.2011.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ridha BH, Barnes J, Bartlett JW, et al. Tracking atrophy progression in familial Alzheimer’s disease: a serial MRI study. Lancet Neurol. 2006;5(10):828–834. doi: 10.1016/S1474-4422(06)70550-6. [DOI] [PubMed] [Google Scholar]
- 69.Schott JM, Fox NC, Frost C, et al. Assessing the onset of structural change in familial Alzheimer’s disease. Ann Neurol. 2003;53(2):181–188. doi: 10.1002/ana.10424. [DOI] [PubMed] [Google Scholar]
- 70.Holtzman DM, Goate A, Kelly J, Sperling R. Mapping the road forward in Alzheimer’s disease. Sci Transl Med. 2011;3(114):114ps48. doi: 10.1126/scitranslmed.3003529. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.