Abstract
Neurotransmitters, acting as chemical messengers, play an important role in neurotransmission, which governs many functional aspects of nervous system activity. Electrochemical probes have proven a very useful technique to study neurotransmission, especially to quantify and qualify neurotransmitters. With the emerging interests in probing neurotransmission at the level of single cells, single vesicles, as well as single synapses, probes that enable detection of neurotransmitters at the nanometer scale become vitally important. Electrochemical nanoprobes have been successfully employed in nanometer spatial resolution imaging of single nanopores of Si membrane and single Au nanoparticles, providing both topographical and chemical information, thus holding great promise for nanometer spatial study of neurotransmission. Here we present the current state of electrochemical nanoprobes for chemical detection of neurotransmitters, focusing on two types of nanoelectrodes, i.e. carbon nanoelectrode and nano-ITIES pipet electrode.
1. Introduction
Electroanalytical methods provide useful insight about neuro-transmitter exocytosis and its implications in cell communication.1–16 Typically, a micrometer sized carbon electrode can be used as a sensing platform for neurotransmitter detection via an electrochemical redox reaction, i.e. the loss or gain of electron(s) from the neurotransmitter molecule, where a current is generated. This signal can be used to provide quantitative information. Very often, electrode modifications are needed to detect neurotransmitters that are not redox active, such as acetylcholine. For instance, a carbon electrode modified with a combination of enzymes acetylcholine esterase and choline oxidase was used for the detection of acetylcholine, where the kinetics of the enzyme reaction can limit the electrode response.17–20 The use of pipet electrodes based on ionic transfer across an Interface between Two Immiscible Electrolyte Solutions (ITIES)21–36 is complementary in detecting neurotransmitters that are not redox active directly without electrode modification; ITIES based pipet electrodes can also detect electrochemically redox active neurotransmitters.37–40 Recent advances in nanoelectrode fabrication made it possible to downscale micrometer sized carbon electrodes and micro-pipette electrodes by 50 times or even 500 times.41–47 Thus, electroanalytical chemistry with nanoelectrode detection lays the ground to meet current challenges in electroanalytical-neurochemical studies, e.g. single vesicles and single synapses.
Here we will focus on electroanalytical detection of neuro-transmitters with electrodes on the order of hundreds or tens of nanometers. Electrochemical analysis with nanoelectrodes offers several advantages: (1) nanoelectrodes exhibit the same virtues as conventional ultramicroelectrodes, i.e. high diffusive mass transport; (2) low measurement background due to a decreased area contributing to electrode capacitance; and (3) nanoelectrodes offer superior spatial resolution for investigating biochemical and materials structures. Recent examples of breakthroughs in the use of nanoelectrodes include: topographical imaging as well as measurements of the ion flux of a single nanopore of Si nanoporous film using a 15 nm radius nanopipet electrode coupled with scanning electrochemical microscopy47 by Shen et al.,45 the imaging of the catalytic activity of single Au nanoparticle with a 14 nm radius Pt electrode by Sun et al.,49 and the study of single H2 nanobubble nucleation with Pt nanodisk electrode of radii less than 50 nm by Chen et al.50
Despite these advantages, nanoelectrodes have been slow to become routine probes in electroanalytical chemistry due to technical challenges in preparing electrodes with sizes that are two to three orders of magnitude smaller than traditionally used microelectrodes. Other limitations include the lack of availability for nanometer sized carbon fiber or metal wires. Great efforts have been made to prepare nanometer sized carbon electrodes, such as focused ion beam milling, pyrolytic deposition of carbon containing gas, and chemical vapor deposition.51–57
In this article, we begin with a brief review of the most commonly employed nanofabrication methods to prepare nano-electrodes for neurotransmitter detection, mainly carbon nano-electrode and nanopipet electrode. The following sections discuss the working principles of neurotransmitter detection with these two types of nanoprobes, their fabrication, as well as electrochemical application for the detection of neurotransmitters.
2. Working principles of carbon electrode and pipet electrode
The detection of neurotransmitters on a carbon electrode occurs via electron transfer, i.e. the loss or gain of electron(s), while the detection of neurotransmitters on an ITIES pipet electrode occurs via ionic transfer across the liquid–liquid interface. In both cases, a current signal providing quantitative information about neurotransmitter is generated. This results from the flow of electrons on a carbon electrode and flow of ions on an ITIES pipet electrode, respectively. The driving force for electron transfer or ionic transfer, i.e. potential of detection, is characteristic of each neurotransmitter and related to its molecular structure. The redox potential, in the case of carbon electrodes, or the transfer potential, in the case of ITIES probes can be used for identifying the neurotransmitters being detected. Sometimes, more than one neurotransmitter can have a similar detection potential. Fig. 1 shows the principle of detection of a neurotransmitter (NT) on both a carbon electrode (Fig. 1A) and an ITIES pipet electrode (Fig. 1B). In Fig. 1A, NTs need to be electrochemically redox active to be detected directly on a carbon electrode. For the detection of electrochemically non-redox active NTs, electrode modification (e.g. with enzymes) will be needed for their detection on a carbon electrode, where kinetics of enzymatic reactions could limit the response of carbon electrode.58–61 In Fig. 1B, any NTs that can be transferred across the interface of a pipet electrode can be identified and quantified. Thus ITIES pipet electrodes can detect both electrochemically active and non-active NTs directly. For instance, our lab has studied the detection of the electrochemically non-redox active NT acetylcholine, as well as redox active NTs tryptamine and serotonin on nanopipet electrodes.37 For the case of ITIES pipet electrodes, the transfer potential can be modulated via assisted ion transfer62 by adding an ionophore in the pipet. This can facilitate the transfer of ions and can be extremely useful when the ions cannot be transferred within the potential window. For both nano carbon electrode and nanopipet electrode, the steady-state limiting current, i, at the nanoelectrode can be calculated with the following expression:63
Fig. 1.
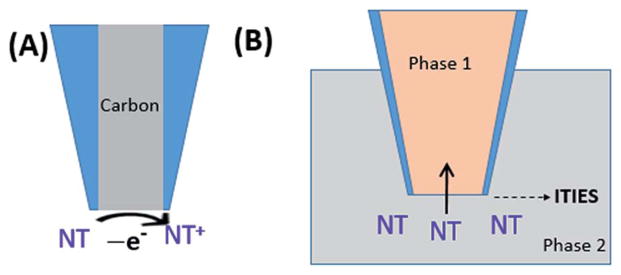
Principle of detection of neurotransmitters (NT) on a carbon electrode (A) and ITIES pipet electrode (B).
| (1) |
where x is a function of the quantity RG = rg/a (rg and a are outer and inner tip radii, respectively),64 n is the number of transferred charges in the tip reaction, F is Faraday’s constant, a is the radius of the pipet, D is the diffusion coefficient of the neurotransmitter measured, and c is the concentration of analyte in solution.
3. Carbon nanoelectrode for the detection of neurotransmitters
3.1 Carbon nanofiber electrodes
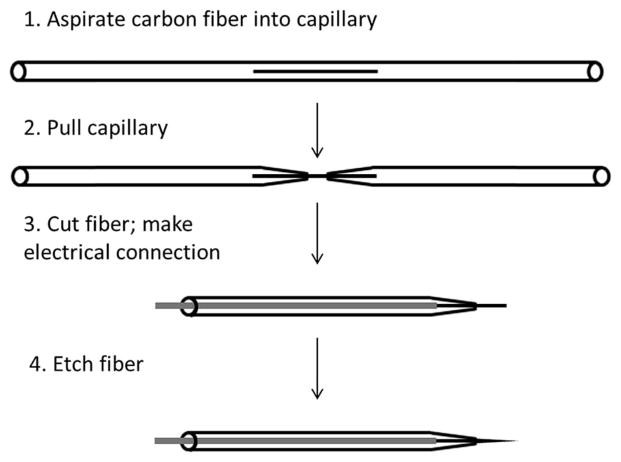
Reports of carbon electrodes with dimensions on the sub-micron scale can be traced back to the late 1980’s.65–68 Most are based on the typical fabrication process for carbon fiber microelectrodes (CFMEs),69–71 followed by further etching to achieve nm scale dimensions, as follows (Fig. 2): a carbon fiber with a diameter on the micron scale is aspirated into a glass capillary, which is then pulled to form two glass pipets with the fiber between the two nm sized openings. This fiber is then cut and electrical contact is made with a conductive liquid and a metal wire. From here, the carbon fiber can be etched down to nm dimensions in a variety of ways.
Fig. 2.
General procedure for producing carbon fiber nanoelectrodes, which can then be further modified according to specific detection needs. Order may be rearranged, as noted in the main text of this review.
In 1992, Ewing’s group reported a method for the fabrication of carbon fiber nanoelectrodes (CFNEs) whereby an oxygen/methane flame was used to etch the carbon fiber down to as small as 100 nm, followed by an extremely thin copolymer film coating of 2-allylphenol and phenol for insulation.72 This insulating layer was then removed from the very tip of the fiber, typically via scalpel, to create a disk-shaped electroactive area. Dopamine concentrations down to 99 μM were detected using a probe of electroactive diameter of about 200 nm. While they reported a 70% success rate for the fabrication of electrodes with tip diameters below 1.5 μm, the construction of those with tip diameters near 400 nm was near 10%.72
Besides the disk shaped carbon nanoelectrode as described above,72 the majority of literature employing carbon nano-electrode for neurotransmitter detection are conical shape, with a length most often in micrometer scale exposed. Since the exposed electroactive area is much larger compared to disk shaped nanoelectrode, typically much lower LODs are achieved on these conical carbon fiber nanoelectrode. In 1996, Zhang et al.73 reported a method employing argon ion beam etching to fabricate smooth cone shaped CFNEs with a success rate of 50–80% depending on tip diameter, using the following procedure: micron-sized carbon fibers were first etched with an argon ion beam to produce cone shaped tips with diameters ranging from about 50–500 nm. The thinned fibers were then mounted to the end of a copper wire and inserted into a pulled glass capillary, with ~10–100 μm of the fiber protruding from the glass tip, which was then sealed by a heating coil. Using differential pulse voltammetry (DPV), these CFNEs were able to detect down to 100 nM DA and 500 nM 5-HT in a phosphate buffer solution. While no selectivity study was reported on these conical CFNEs; a decrease in 5-HT steady-state oxidation current was observed with multiple scans due to an insulating film that forms on the surface of the electrode from the oxidation products of 5-HT, as reported in other works.74,75
While the use of ion beam73 etching allows for controllable and reproducible sizes by controlling the beam parameters, the process can be time consuming and costly. Another method of preparing carbon nanofiber electrode that does not require ion beam etching was employed by Cheng group of Wuhan University in 2001, in which the carbon fibers are flame fuse sealed to the tip of a pulled glass capillary (with inner diameter of ~20 μm) and flame etched and in order to reduce etching time while maintaining a smooth fiber/glass interface.76 In this method, carbon fibers connected to copper wire are first inserted into a pulled glass capillary, where 1 cm is allowed to protrude from the tip, which is then flame fused to the glass. Next, the bottom of the flame is used to etch the fiber down to a diameter of 100–300 nm and a length of approximately 200 μm. This flame sealing resulted in a smooth glass/fiber interface. These CFNEs were used to detect several catecholamines (dopamine, epinephrine, and norepinephrine) in a phosphate buffer solution using cyclic voltammetry as well as DPV, with detection limits for dopamine, epinephrine, and norepinephrine in the mid-nanomolar range (Table 1). The electrooxidation of serotonin formed an insulating film on the surface of the electrode,76 which can be overcome by using fast scan rates such as those used in fast scan cyclic voltammetry (FSCV).99
Table 1.
Summary of key features of nanoelectrodes used for the detection of neurotransmitters
| Ref. | Preparation method | Geometry/size | NTs | LOD | Fouling |
|---|---|---|---|---|---|
| 72 | Flame etched carbon fiber with insulating coating | Disk shape; diameter: 400–1500 nm | DA | N/A | N/A |
| 73 | Argon ion beam milling of micro carbon fibers | Conical shape; diameter: 50–500 nm, length: 10–100 μm | DA 5-HT |
100 nM (DPV) 500 nM (DPV) |
Decrease in iss with repeated 5-HT scans |
| 76 | Flame fusing and etching of carbon fiber | Conical shape; diameter: 100–300 nm, length: 200 μm | DA NE E 5-HT |
76 nM (CV); 40 nM (DPV) 70 nM (CV); 100 nM (DPV) 50 nM (CV); 220 nM (DPV) N/A (FSCV) |
5-HT insulating film forms |
| 77 | Flame fusing and etching of carbon fiber | Conical shape; diameter: ca. 100 nm | DA | N/A (FSCV) | N/A |
| 79 | Carbon microfiber flame etched, immersed in SWNTs | Conical shape; diameter: 100–300 nm, length: 100–1000 μm | DA E NE |
7.7 nM (CV) 38 nM (CV) 42 nM (CV) |
N/A |
| 89 | Plasma enhanced chemical vapor deposition | 4 mm diameter array of CNFs with ~5 μm length | DA 5-HT |
50 nM (DPV) 100 nM (DPV) |
Shoulder on 5-HT peak |
These nanoelectrodes were later used in combination with FSCV to amperometrically monitor dopamine release from live PC12 cells.77 The tip diameter of the probes, around 100 nm, nicely corresponds to the average size of a PC12 vesicle, around 99 nm. A nanoelectrode placed <500 nm above a cellular release site was able to detect amperometric spikes corresponding to the release of content from individual vesicles. The high spatial resolution obtained by these nanoelectrodes showed that most sites on a cell surface are actually inactive, supporting previous results that used a 2 μm diameter microelectrode to image chromaffin cell release sites.78 Additionally, this was the first report of direct electrochemical detection of sequential dopamine release from multiple vesicles at the same release site (Fig. 3).
Fig. 3.
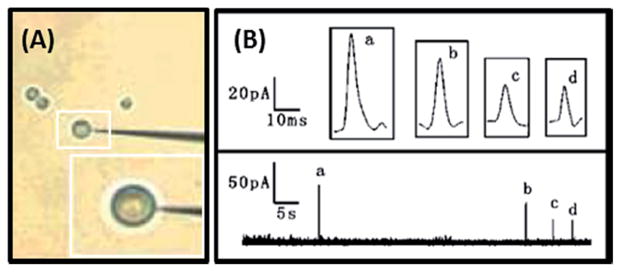
(A) Photograph of nanoelectrode-cell arrangement. (B) Spikes corresponding to sequential dopamine release from multiple vesicles with the electrode placed over the same position on the cell. Graphs a–d show magnified pictures of the individual current spikes. Modified from ref. 77.
The same group then took CFNEs prepared using this flame etching method and modified them with single-walled carbon nanotubes (SWNTs) by immersing the probes into a SWNT suspension and allowing the solution to evaporate under an infrared lamp.79 These SWNTs/CFNEs were able to detect dopamine, epinephrine, and norepinephrine in a Tris–HCL buffer down to low nanomolar levels (Table 1). This decrease in LOD compared with the bare CFNEs is attributed to the increased surface area of the modified probes. The increased surface area also affected the shape of the voltammogram at certain scan rates, where the CV is sigmoidal at rates less than 20 mV s−1, but peaked at higher rates. The stability of these modified CFNEs was also tested by taking up to 1000 cyclic voltammograms of a Tris–HCl buffer background solution with no analyte over a period of 15 days. The results of this test were good; though no stability tests were reported using a neuro-transmitter as the analyte.
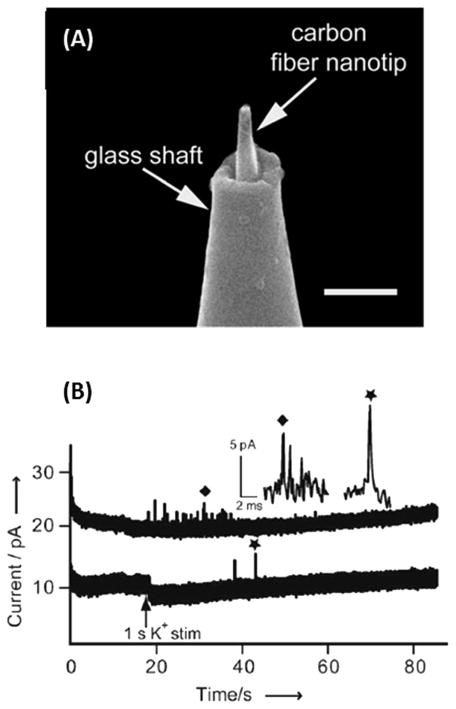
Recently, Li et al. reported on CFNEs that were able to fit inside an apparent synapse, specifically inside the space between a neuron’s soma and a neuronal varicosity.80 These CFNEs were prepared by flame etching a carbon fiber to a diameter of 50–200 nm, and sealing it inside a glass capillary with approximately 0.5–2 μm of the carbon nanotip protruding from the glass, in a similar manner to previous work.77 The carbon fibers were then further etched using a microforge, producing tips with a base radius near 100 nm and a length of less than 1 μm (Fig. 4A). This process produced nanoelectrodes with a success rate of over 75%. Inside the apparent synapse, complex sequences of events were detected, which were distinctly different from single spike events that are typically detected using CFMEs (Fig. 4B). Furthermore, using a CFNE inside the apparent synapse and a CFNE above the same varicosity (semi-artificial synapse), significantly more amperometric spikes corresponding to vesicle release were detected at the probe inside the synapse than at the one above (Fig. 4B), confirming non-uniform distribution of active hot spots at the synapse.81 While this study is insightful regarding vesicular release at the synapse, no information was obtained in terms of the chemical makeup of the release detected.
Fig. 4.
(a) Scanning electron micrograph of flame etched carbon fiber fused inside a sub-micropipette; scale bar is 1 μm. (b) Quantal amperometric spikes recorded inside the synapse (upper trace) and above the synapse (lower trace). Modified from ref. 80.
Most recently, Jill Venton’s group has reported on the use of carbon nanopipette electrodes (CNPEs), which are fabricated without the use of a carbon fiber, making them more robust for applications involving tissue implantation.82 Briefly, after laser pulling quartz capillaries, chemical vapor deposition was used to selectively deposit carbon inside the pipette. Next, 5 : 1 buffered hydrofluoric acid was used to etch away quartz at the tip of the electrode. Detailed descriptions of the fabrication process can be found elsewhere.83,84 This method involving chemical vapor deposition allows for batch fabrication of nanocarbon electrodes, resulting in an average tip diameter of 250 nm, with an exposed carbon length between 5 and 175 μm, controlled by the amount of time spent etching the quartz. Proper sealing of the CNPEs interface was examined by verifying that capacitance was stable as various pressures were applied inside the pipette. FSCV of 1 μM dopamine was used to compare CNPEs to CFMEs. While the ratio of background current to peak oxidative current was higher at CNPEs, the average difference between the oxidative and reductive peak potentials was significantly lower for CNPEs. Octopamine and serotonin were also detected using FSCV at the CNPEs, each with a waveform specialized for the respective analyte that reduces electrode fouling. Octopamine showed a larger secondary peak at the CNPE compared to the CFME, indicating possible oxidation product adsorption at the surface of the CNPE. Based on the ratio of background current to peak oxidative current, the CNPEs show a higher sensitivity to serotonin and the same sensitivity to octopamine relative to the CFMEs. Finally, the CNPEs were used for in vivo FSCV measurements of endogeneous extracellular dopamine in the Drosophila melanogaster.
3.2 Carbon nanofiber-based arrays
Arrays of carbon nanofibers are an attractive option for the simultaneous detection of NTs at numerous cell sites, as well as statistical reliability. The Koehne lab has developed such arrays85–89 (Fig. 5a and b) using plasma enhanced chemical vapor deposition to grow vertical carbon nanofibers on silicon substrates. These arrays of nanoelectrodes can differentiate certain catecholamines and their interferents, due to their high conductivity and COOH functionality at defect sites.85 Ascorbic acid, a negatively charged molecule present in the brain up to 1000× higher concentrations than dopamine and serotonin, is typically oxidized at similar potentials, making it a common interference in electrochemical detection of catecholamines.90 These nanofiber arrays however, were capable of distinguishing DA, 5-HT, and AA simultaneously in a ternary mixture, with LODs of 50 nM for dopamine and 100 nM for serotonin using DPV (Fig. 5c).85,86 Again, the oxidation products of 5-HT show adverse effects, in this case creating a shoulder on the 5-HT peak after multiple scans.85
Fig. 5.
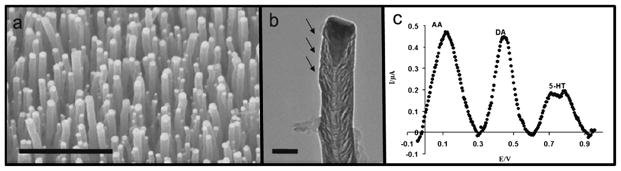
(a) SEM image of an array of vertically aligned CNFs. Scale bar is 1 μm. (b) TEM image of an individual nanofiber. Arrows highlight active sites. Scale bar is 0.1 μm. (c) Baseline-corrected differential pulse voltammogram of a mixture of 1 mM ascorbic acid, 10 μM dopamine, and 10 μM serotonin measured using CNF array. Modified from ref. 85 and 86.
A similar carbon nanofiber based array was used in a series of FSCV experiments89 to compare its behavior with carbon fiber microelectrodes (CFME).91,92 Similar to a CFME, it was found that positively charged dopamine adsorbs to the carbon surface of the CNF array, while negatively charged ascorbic acid does not. Additionally, a two minute pretreatment with isopropyl alcohol significantly enhanced the signal-to-noise ratio, as was seen in previous studies using CFMEs. Altogether, the results of these studies show that the behavior of DA detection at CNF based electrodes is very similar to that at CFMEs, and due to their smaller size, response time is faster for the CNF based electrodes.89 While there have been several reports of carbon microelectrode arrays for the detection of release from cell cultures93 or in vivo,94,95 no reports were found in which a carbon nanoelectrode based array was used for the study of cells.
3.3 Selectivity and stability of carbon electrodes
Selectivity of carbon electrodes to neurotransmitters of interest is of great importance, since dopamine, norepinephrine, serotonin, ascorbic acid, and certain neurotransmitter metabolites all oxidize within a narrow potential window. Furthermore, carbon fiber microelectrodes (CFMEs) are known to foul quickly when exposed to brain tissue, meaning that significant decreases in faradaic current occur quickly after implantation for an in vivo experiment.96–99 In order to improve the selectivity and reduce fouling at the electrode surface, a number of selectively permeable materials have been used as a coating for the micrometer sized carbon fiber electrode.
An ideal electrode coating would offer increased selectivity toward analytes of interest as well as resistance to biofouling. For instance, Naflon, a perfluorinated cation exchange polymer, is a coating that is often used due to its exclusion of most anions, including ascorbic acid which is a common interferent present in the brain at high concentrations.99–103 Chitosan, a chemically inert biopolymer has also been shown to be selective toward serotonin against ascorbic acid.104,105 Furthermore, coating of micro carbon fibers with fibronectin, a glycoprotein primarily used in cell culture, showed higher resistance to biofouling compared to uncoated electrodes.106 Similar results have been shown by coating micro carbon fiber electrodes with base-hydrolyzed cellulose acetate (BCA), which forms a steric barrier preventing large molecules such as proteins from reaching the electrode surface, resulting in increased resistance to biofouling.107 Recently, Michael Heien’s group reported CFMEs modified with a combination of poly(3,4-ethyl-enedioxythiophene) (PEDOT) and Naflon.108 Unlike Naflon alone, which does not adhere well to carbon fibers,109 this PEDOT:Naflon copolymer creates a uniform, ≈100 nm thick coating, giving the advantages of increased sensitivity to dopamine plus reduced biofouling in vivo.108 Singh et al. compared the performance of CFMEs that were coated with Naflon, BCA, and fibronectin.110 The selectivity and stability of electrodes with these coatings were compared with uncoated carbon fibers after being exposed to brain tissue. This study found that Naflon is well suited for improving the sensitivity and selectivity of CFMEs, but has a similar decrease in oxidative current as a bare carbon fiber due to fouling. On the other hand, BCA and fibronectin showed much smaller reductions in current after fouling compared with Naflon and bare fibers, yet these coatings actually lead to decreased sensitivity toward dopamine compared with bare CFMEs.
Overall, the current carbon fiber nanoelectrode (CFNE) literature does not have in depth discussions regarding selectivity. It would be interesting to see how these selective coatings used for CFMEs affect the performance of CFNEs.
4. Fabrication and characterization of nanopipet electrode for ITIES measurements
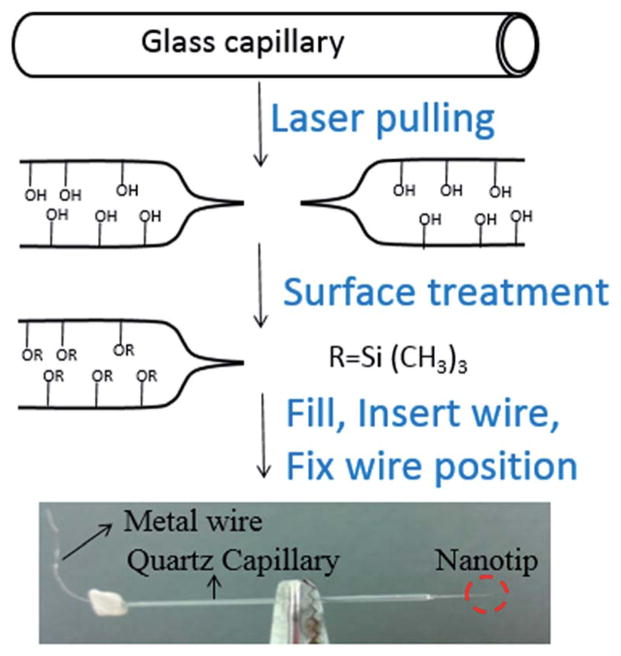
We now turn to the detection of neurotransmitters via ITIES pipettes. The process of nanopipet fabrication relies on three basic steps: (1) creation of nanometer orifice at the end of pipet, (2) surface treatment of the generated nanopipet and (3) assembly the nanopipet electrode. This process is summarized in Fig. 6 and described in more detail below.
Fig. 6.
Fabrication process of nanopipet electrode. In the surface treatment process, listed here is trimethylsilyl (–Si(CH3)3) as an example, however other types of silanes can be used as well.
Fabrication starts from a quartz capillary, from which the nano-orifice can be generated via laser heating and pulling. We have found that the capillaries made from quartz glass with outer diameter of 1 mm and inner diameter of 0.7 mm result in reproducible pipets. Sometimes borosilicate glass is used as starting material to fabricate the nanopipet electrode. When borosilicate glass is used, less heat will be needed due to the lower melting point of borosilicate glass. Laser heating and pulling can be done on a commercial laser puller such as the P-2000 (Sutter Instrument Co), with a set of pulling parameters. Often, pulling parameters need to be adjusted due to variations in the condition of the puller.
The size of the nanometer orifice at the end of the nanopipet electrode can be inspected with scanning electron microscopy (SEM). Surface coating with conducting materials (e.g. Au–Pd) is necessary for high resolution SEM image of the nano-orifice, because charging occurs on glass surface under the electron beam. This charging phenomenon blurs the electron image of nano-orifice, which makes it difficult or even impossible to check the accurate size of the nanopipet.
The surface treatment of the nanopipet is very critical for the formation of stable ITIES. During surface treatment, a silane, such as chlorotrimethylsilane, reacts with –OH group on the surface of the glass, and the glass surface changes from hydrophilic to hydrophobic, eventually yielding –O–Si(CH3)3 groups. One method for surface treatment is by immersing the pipet into a silane solution.111 Another method is to expose pipet to vapor of silane, which was first introduced by the group of Amemiya to prepare micro- and nanopipets. Later Mirkin’s group112 and Shen’s group37 adopted this procedure to prepare nanopipet electrodes. For the vapor reaction, a plastic desiccator can be used as a reactor where the pulled pipets can be placed, and a vacuum can be created in the desiccator to then expose the pipets to the vapors of the silane.
Once a nanopipet has been prepared, the next step is to fill the nanopipet with an electrolyte solution of 1,2-dichloroethane or nitrobenzene. Sometimes, an ionophore will be used to facilitate the transfer of certain ions. This filling process can be done by creating a gentle vibration such as tapping with fingers or small objects. The last step of nanopipet preparation is insertion of an inner reference electrode, typically a Pt wire. The location of Pt wire needs to be fixed to prevent possible dislocation during usage, which can break the nanopipet.
5. Application of nanopipet electrode to neurotransmitter detection
Our group has successfully detected both electrochemically redox active (e.g. tryptamine and serotonin) and non-redox active (e.g. acetylcholine (ACh)) neurotransmitters quantitatively on nanopipet electrodes with radii of 7–35 nm (Fig. 7). The multifunctional detection is based on ion transfer across a nano-interface of water and 1,2-DCE. An electrolyte of ionic liquid, TDDATFAB, was used in the 1,2-DCE phase.37 No electrode modification was needed for the detection of acetylcholine on these nanopipet electrodes. Though the transfer of ACh across macro-ITIES was observed dating back to 1981 by Vanysek et al.113 and across micro-ITIES was reported by Campbell et al. in 1989,114 our work37 is the first time when ACh transfer at nanometer scale-ITIES was observed and its transfer was quantitatively measured with cyclic voltammetry and amperometry i–t, as shown below.
Fig. 7.
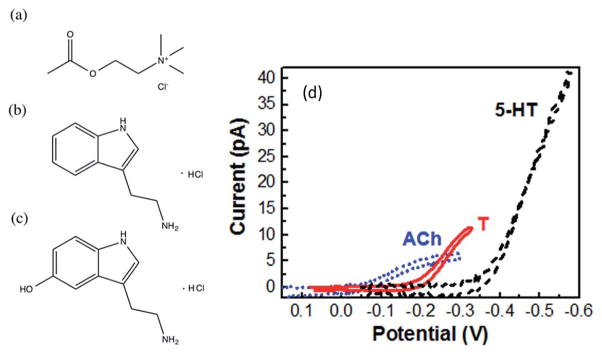
Cyclic voltammograms (d) of acetylcholine (ACh), tryptamine (T), and serotonin (5-HT) on nanopipet electrodes, with molecular structures shown in (a), (b), (c) respectively. Electrode radii are the following: ≈7 nm for ACh, ≈35 nm for 5-HT, ≈19 nm for T. Modified from ref. 37.
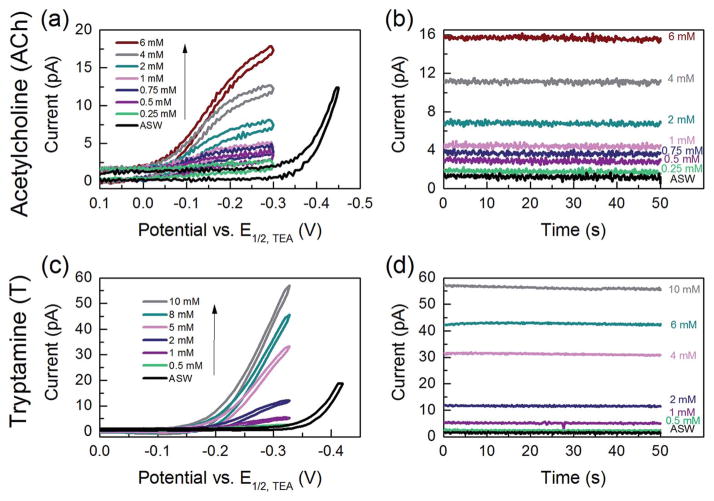
Both cyclic voltammetry and amperometry were used to detect various concentrations of ACh and T (Fig. 8). The detection was linear in the range of 0.25–6 mM for acetylcholine and of 0.5–10 mM for tryptamine in artificial seawater. Detection of serotonin was linear in the range of 0.15–8 mM in LiCl solution. The limit of detection for serotonin in LiCl on a radius ≈35 nm nanopipet electrode was 90 μM, and for acetylcholine on a radius ≈7 nm nanopipet electrode was 205 μM, and for tryptamine on a radius ≈19 nm nanopipet electrode was 86 μM. In comparison to macro ITIES, our nanoITIES electrode, though ~million times smaller in size, the LODs achieved is in the same order of magnitude, only slightly higher than the LODs achieved on an electrode with radius of millimeter. For instance, a detection limit of 50 μM was achieved for the detection of dopamine with an ITIES electrode area of 0.126 cm2 (radius = 2 mm).38 Most importantly, nanoITIES pipet electrode can be used to image nanometer scale structures, e.g. single cell, single vesicles. For instance, topography as well as ion transfer properties across single nanopore have been imaged with nanometer scale ITIES pipet electrode and scanning electrochemical microscope as demonstrated by Shen et al.47 For interrogating individual neurotransmission sites, computer simulations have shown local acetylcholine and serotonin concentrations to be well above the LOD for these nanoITIES pipet electrode.115,116
Fig. 8.
(a) Cyclic voltammograms and (b) amperometric i–t curves for 0.25–6 mM acetylcholine using a nanopipet probe with a radius of 7 nm in Cell 1; applied potential E = −0.25 V vs. E1/2,TEA for i–t curves. (c) Cyclic voltammograms and (d) amperometric i–t curves for 0.5–10 mM tryptamine using a nanopipet probe with a radius of 19 nm in Cell 1; applied potential E = −0.32 V vs. E1/2,TEA for i–t curves. Modified from ref. 37.
The matrix in which NT detection is being performed can have a strong influence on the potential window in which the ions can transfer. The matrix can be tailored to the purpose of the experiment, for example artificial seawater can be useful for studies of common invertebrate neuronal models such as Aplysia californica.37 Should vertebrate studies be of interest, one could also use artificial cerebrospinal fluid (ACSF) as the detection medium. The current ITIES literature lacks information regarding ACSF as a matrix, though understanding how this matrix behaves with ITIES could be of importance to certain applications.
The selectivity of ITIES probes can be tailored to certain neurotransmitters by selecting the appropriate filling solution for the pipet. For example, certain neurotransmitters such as dopamine and γ-aminobutyric acid (GABA) do not undergo unfacilitated transfer even within a large potential window such as LiCl,37 meaning that DA and GABA cannot be detected using an ionophore-free pipet. However, if DA detection is desired, several studies at macro- and micro- have shown that an ionophore such as dibenzo-18-crown-6 can facilitate the transfer of DA to occur within relevant potential windows.117–120 The assisted ion transfer of DA at nanopipet hasn’t been reported yet and work is currently in progress in our lab to detect DA at nanopipet electrode via assisted ion transfer. Ascorbic acid (AA) is a common interferent in biological fluids as well, particularly for the detection of neurotransmitters, where AA is present at much higher concentrations. However, AA does not transfer within the potential window of common nitrobenzene/water and 1,2-DCE/water interfaces.118–121 Thus, the nano-ITIES pipet electrode is insensitive to this common interferent, without any electrode modification necessary.
6. Conclusions and outlook
We have reviewed the most recent application of nano-electrodes, including nano-ITIES pipet electrode and nano carbon fiber electrode, for the detection of neurotransmitters (NTs). We believe the electrochemical detection of neurotransmitters with nanoelectrodes will become an active research area in the following years for two reasons. First, nanoelectrodes have very low background current from double layer charging, thus can provide increased signal to noise ratio. Second, nanoelectrodes can provide nanometer spatial resolution imaging and chemical measurement as demonstrated in the work by Shen et al.,47 Sun et al.49 and Chen et al.50
The work in our lab by Colombo et al.37 demonstrate for the first time that nano-ITIES pipet electrodes show great potential to detect a broad range of NTs, including both electrochemically redox active (e.g. serotonin and tryptamine) and non-redox active (e.g. acetylcholine) ones. The nano-ITIES sensor probe is selective towards detection of Ach, T and 5-HT against dopamine and ascorbic acid. The detection is explored in matrix of artificial sea water (ASW), biological media for the animal model in our research. For the study of different media, the similar methodology can be extended to test applicability of our nano-ITIES probes in different biological media. The detection on a nano-ITIES probe presented is very sensitive, providing similar LOD as macro ITIES probes, size millions times of nanoprobes. This can be attributed to very low background provided by nanoelectrode. The assisted ion transfer for the detection of NTs at nanopipet electrode is still yet to be explored.
While current nanocarbon electrode literature lacks in depth discussion about selectivity, it would be interesting to see how current electrode modification regimes reported on micro-carbon electrodes as we presented here can be used to improve selectivity at nanocarbon electrodes. Majority carbon nano-electrodes for the detection of NTs mainly consists of protruded carbon fiber electrode with micrometer long electroactive carbon fiber exposed; work related with detection of NTs on nanometer carbon disk electrode is very few.72 Carbon fiber nanoelectrodes have much lower LODs compared to disk shaped carbon nanoelectrode (with polymer insulation to expose only the very end of tip), this is because carbon fiber nano-electrodes typically have micrometer long electroactive carbon fiber exposed and thus have significantly higher electroactive area.
Overall, we envision that with the development of nano-electrode fabrication technologies, electroanalytical methods with nanoelectrodes can find broad application for neuro-transmitter detection at the levels of single authentic synapses, vesicles and single cells in vivo.
Acknowledgments
We gratefully appreciate the support by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award number R21NS085665.
Biographies

Dr Mei Shen is an Assistant Research Professor of Chemistry at the University of Illinois at Urbana-Champaign. She completed her PhD work at The University of Texas at Austin with Prof. Allen J. Bard and postdoctoral work at University of Pittsburgh with Prof. Shigeru Amemiya. Since arriving at University of Illinois at 2012, her group’s research has been interdisciplinary across nano-electrochemistry and neuroscience. Her group is developing novel nanosensor probes to detect a broad range of neurotransmitters. Another research focus in her lab is nanometer spatial resolution imaging of neurotransmission at single neuronal structure with scanning electrochemical microscopy and nanoelectrode.

Michelle Colombo is a second year Analytical Chemistry graduate student in Mei Shen and Jonathan Sweedler’s research groups at University of Illinois at Urbana-Champaign. She spent her undergraduate career at Wayne State University in Detroit, MI, where she earned a B.S. in Chemistry. Her current research focuses on the development of nano-scale electrochemical probes for the imaging of neurochemical processes using scanning electrochemical microscopy.
References
- 1.Wightman RM, May LJ, Michael AC. Anal Chem. 1988;60:769A–779A. doi: 10.1021/ac00164a001. [DOI] [PubMed] [Google Scholar]
- 2.Wightman RM, Haynes CL. Nat Neurosci. 2004;7:321–322. doi: 10.1038/nn0404-321. [DOI] [PubMed] [Google Scholar]
- 3.Amatore C, Arbault S, Bonifas I, Lemaitre F, Verchier Y. Chem Phys Chem. 2007;8:578–585. doi: 10.1002/cphc.200600607. [DOI] [PubMed] [Google Scholar]
- 4.Amatore C, Arbault S, Bouret Y, Guille M, Lemaitre F, Verchier Y. Chem Bio Chem. 2006;7:1998–2003. doi: 10.1002/cbic.200600194. [DOI] [PubMed] [Google Scholar]
- 5.Anderson BB, Chen G, Gutman DA, Ewing AG. J Neurosci Methods. 1999;88:153–161. doi: 10.1016/s0165-0270(99)00024-2. [DOI] [PubMed] [Google Scholar]
- 6.Chen G, Gavin PF, Luo G, Ewing AG. J Neurosci. 1995;15:7747–7755. doi: 10.1523/JNEUROSCI.15-11-07747.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow RH, von Ruden L, Neher E. Nature. 1992;356:60–63. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- 8.Michael Adrian C, Borland Laura M., editors. Electrochemical methods for neuroscience. CRC Press/Taylor & Francis; Boca Raton: 2007. [PubMed] [Google Scholar]
- 9.Leszczyszyn DJ, Jankowski JA, Viveros OH, Diliberto EJ, Jr, Near JA, Wightman RM. J Biol Chem. 1990;265:14736–14737. [PubMed] [Google Scholar]
- 10.Alvarez de Toledo G, Fernandez-Chacon R, Fernandez JM. Nature. 1993;363:554–558. doi: 10.1038/363554a0. [DOI] [PubMed] [Google Scholar]
- 11.Uchiyama Y, Maxson MM, Sawada T, Nakano A, Ewing AG. Brain Res. 2007;1151:46–54. doi: 10.1016/j.brainres.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haynes CL, Buhler LA, Wightman RM. Biophys Chem. 2006;123:20–24. doi: 10.1016/j.bpc.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wightman RM, Schroeder TJ, Finnegan JM, Ciolkowski EL, Pihel K. Biophys J. 1995;68:383–390. doi: 10.1016/S0006-3495(95)80199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venton BJ, Wightman RM. Anal Chem. 2003;75:414A–421A. [Google Scholar]
- 15.Adams RA. Anal Chem. 1976;48:1126–1138. [Google Scholar]
- 16.Bruns D, Jahn R. Nature. 1995;377:62–65. doi: 10.1038/377062a0. [DOI] [PubMed] [Google Scholar]
- 17.Hale PD, Wightman RM. Mol Cryst Liq Cryst. 1988;160:269–279. [Google Scholar]
- 18.Kawagoe JL, Nieaus DE, Wightman RM. Anal Chem. 1991;63:2961–2965. doi: 10.1021/ac00024a029. [DOI] [PubMed] [Google Scholar]
- 19.Tamiya E, Sugiura Y, Navera EN, Mizoshita S, Nakajima K, Akiyama A, Karube I. Anal Chim Acta. 1991;251:129–134. [Google Scholar]
- 20.Barkhimer TV, Kirchhoff JR, Hudson RA, Messer WS, Jr, Tillekeratne LMV. Anal Bioanal Chem. 2008;392:651–662. doi: 10.1007/s00216-008-2307-2. [DOI] [PubMed] [Google Scholar]
- 21.Amemiya S, Kim J, Izadyar A, Kabagambe B, Shen M, Ishimatsu R. Electrochim Acta. 2013;110:836–845. doi: 10.1016/j.electacta.2013.03.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amemiya S. Heterogeneous Electron Transfer Reactions. In: Bard AJ, Mirkin MV, editors. Scanning Electrochemical Microscopy. 2. ch 6. Taylor and Francis; 2012. pp. 127–156. [Google Scholar]
- 23.Samec Z, Samcová E, Girault HH. Talanta. 2004;63:21–32. doi: 10.1016/j.talanta.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 24.Samec Z. Pure Appl Chem. 2004;76:2147–2180. [Google Scholar]
- 25.Senda M, Kakiuchi T, Osakais T. Electrochim Acta. 1991;36:253–262. [Google Scholar]
- 26.Wang Y, Velmurugan J, Mirkin MV, Rodgers PJ, Kim J, Amemiya S. Anal Chem. 2010;82:77–83. doi: 10.1021/ac902244s. [DOI] [PubMed] [Google Scholar]
- 27.Reymond F, Fermín D, Lee HJ, Girault HH. Electrochim Acta. 2000;45:2647–2662. [Google Scholar]
- 28.Rodgers PJ, Amemiya S. Anal Chem. 2007;79:9276–9285. doi: 10.1021/ac0711642. [DOI] [PubMed] [Google Scholar]
- 29.Amemiya S, Bard AJ. Anal Chem. 2000;72:4940–4948. doi: 10.1021/ac0004207. [DOI] [PubMed] [Google Scholar]
- 30.Vanỳsek P. Anal Chem. 1990;62:827–835. [Google Scholar]
- 31.Mirkin MV, Liu B. Electroanalysis. 2000;12:1433–1446. [Google Scholar]
- 32.Mirkin MV, Liu B. Anal Chem. 2001;73:670A–677A. doi: 10.1021/ac012648f. [DOI] [PubMed] [Google Scholar]
- 33.Stockmann T, Montgomery JAM, Ding Z. J Electroanal Chem. 2012;684:6–12. [Google Scholar]
- 34.Stockmann TJ, Zhang J, Montgomery AM, Ding Z. Anal Chim Acta. 2014;821:41–47. doi: 10.1016/j.aca.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 35.Liu S, Li Q, Shao Y. Chem Soc Rev. 2011;40:2236–2253. doi: 10.1039/c0cs00168f. [DOI] [PubMed] [Google Scholar]
- 36.Arrigan DWM, Herzog G, Scanlon MD, Strutwolf J. Bioanalytical Applications of Electroanalytical Chemistry at Liquid–Liquid Microinterfaces. In: Bard Allen J, Zoski Cynthia G., editors. A Series of Advances. Vol. 25. CRC Press; 2013. pp. 105–178. ch. 3. [Google Scholar]
- 37.Colombo ML, Sweedler JV, Shen M. Anal Chem. 2015;87:5095–5100. doi: 10.1021/ac504151e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arrigan DWM, Ghita M, Beni V. Chem Commun. 2004:732–733. doi: 10.1039/b316493d. [DOI] [PubMed] [Google Scholar]
- 39.Herzog G, McMahon B, Lefoix M, Mullins ND, Collins CJ, Moynihan HA, Arrigan DWM. J Electroanal Chem. 2008;622:109–114. [Google Scholar]
- 40.Tatsumi AH, Ueda TJ. Electroanal Chem. 2011;655:180–183. [Google Scholar]
- 41.Wei C, Bard AJ, Feldberg SW. Anal Chem. 1997;69:4627–4633. [Google Scholar]
- 42.Cai C, Mirkin MV. J Am Chem Soc. 2006;128:171–179. doi: 10.1021/ja055091k. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Velmurugan J, Mirkin MV. Isr J Chem. 2010;50:291–305. [Google Scholar]
- 44.Amemiya S, Kim J, Izadyar A, Kabagambe B, Shen M, Ishimatsu R. Electrochim Acta. 2013;110:836–845. doi: 10.1016/j.electacta.2013.03.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McKelvey K, Kinnear SL, Perry D, Momotenko D, Unwin PR. J Am Chem Soc. 2014;136:13735–13744. doi: 10.1021/ja506139u. [DOI] [PubMed] [Google Scholar]
- 46.Shi W, Sa N, Thakar R, Baker LA. Analyst. 2015 doi: 10.1039/c4an02073f. ahead of print. [DOI] [PubMed] [Google Scholar]
- 47.Shen M, Ishimatsu R, Kim J, Amemiya S. J Am Chem Soc. 2012;134:9856–9859. doi: 10.1021/ja3023785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bard AJ, Mirkin MV, editors. Scanning Electrochemical Microscopy. Marcel Dekker; New York: 2001 pp. [Google Scholar]
- 49.Sun T, Yu Y, Zacher BJ, Mirkin MV. Angew Chem, Int Ed. 2014;53:1–6. doi: 10.1002/anie.201408408. [DOI] [PubMed] [Google Scholar]
- 50.Chen Q, Luo L, Faraji H, Feldberg SW, White HS. J Phys Chem Lett. 2014;5:3539–3544. doi: 10.1021/jz501898r. [DOI] [PubMed] [Google Scholar]
- 51.Thakar R, Weber Anna E, Morris Celeste A, Baker Lane A. Analyst. 2013;138:5973–5982. doi: 10.1039/c3an01216f. [DOI] [PubMed] [Google Scholar]
- 52.Clausmeyer J, Actis P, Lopez Cordoba A, Korchev Y, Schuhmann W. Electrochem Commun. 2014;40:28–30. [Google Scholar]
- 53.Hu K, Gao Y, Wang Y, Yu Y, Zhao X, Rotenberg SA, Gokmese E, Mirkin MV, Friedman G, Gogotsi Y. J Solid State Electrochem. 2013;17:2971–2977. [Google Scholar]
- 54.Singhal R, Bhattacharyya S, Orynbayeva Z, Vitol E, Friedman G, Gogotsi Y. Nanotechnology. 2010;21:1–9. doi: 10.1088/0957-4484/21/1/015304. [DOI] [PubMed] [Google Scholar]
- 55.Kim BM, Murray T, Bau HH. Nanotechnology. 2005;16:1317–1320. [Google Scholar]
- 56.Yu Y, Gao Y, Hu KK, Blanchard PY, Noel JM, Nareshkumar T, Phani KL, Friedman G, Gogotsi Y, Mirkin MV. Chem Electro Chem. 2015;2:58–63. [Google Scholar]
- 57.Actis P, Tokar S, Clausmeyer J, Babakinejad B, Mikhaleva S, Cornut R, Takahashi Y, Cordoba AL, Novak P, Shevchuck AI, Dougan JA, Kazarian SG, Gorelkin PV, Erofeev AS, Yaminsky IV, Unwin PR, Schuhmann W, Klenerman D, Rusakov DA, Sviderskaya EV, Korchev YE. ACS Nano. 2014;8:875–884. doi: 10.1021/nn405612q. [DOI] [PubMed] [Google Scholar]
- 58.Hale PD, Wightman RM. Mol Cryst Liq Cryst. 1988;160:269–279. [Google Scholar]
- 59.Kawagoe JL, Nieaus DE, Wightman RM. Anal Chem. 1991;63:2961–2965. doi: 10.1021/ac00024a029. [DOI] [PubMed] [Google Scholar]
- 60.Tamiya E, Sugiura Y, Navera EN, Mizoshita S, Nakajima K, Akiyama A, Karube I. Anal Chim Acta. 1991;251:129–134. [Google Scholar]
- 61.Barkhimer TV, Kirchhoff JR, Hudson RA, Messer WS, Jr, Tillekeratne LMV. Anal Bioanal Chem. 2008;392:651–662. doi: 10.1007/s00216-008-2307-2. [DOI] [PubMed] [Google Scholar]
- 62.Reymond F, Carrupt PA, Girault HH. J Electroanal Chem. 1998;449:49–65. [Google Scholar]
- 63.Lefrou C. J Electroanal Chem. 2006;592:103–112. [Google Scholar]
- 64.Kim J, Shen M, Nioradze N, Amemiya S. Anal Chem. 2012;84:3489–3492. doi: 10.1021/ac300564g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abe T, Itaya K, Uchida I. Chem Lett. 1988:399–402. [Google Scholar]; Kawagoe KT, Jankowski JA, Wightman RM. Anal Chem. 1991;63:1589–1594. doi: 10.1021/ac00015a017. [DOI] [PubMed] [Google Scholar]
- 66.Schulte A, Chow RH. Anal Chem. 1998;70:985–990. doi: 10.1021/ac970934e. [DOI] [PubMed] [Google Scholar]; Stamford JA. Anal Chem. 1986;58:1033–1036. doi: 10.1021/ac00297a011. [DOI] [PubMed] [Google Scholar]
- 67.Armstrongjames M, Fox K, Millar J. J Neurosci Methods. 1980;2:431–432. doi: 10.1016/0165-0270(80)90009-6. [DOI] [PubMed] [Google Scholar]
- 68.Meulemans A, Poulain B, Baux G, Tauc L, Henzel D. Anal Chem. 1986;58:2088–2091. [Google Scholar]
- 69.Dayton MA, Brown JC, Stutts KJ, Wightman RM. Anal Chem. 1980;52:946–950. [Google Scholar]
- 70.Ponchon JL, Cespuglio R, Gonon F, Jouvet M, Pujol JF. Anal Chem. 1979;51:1483–1486. doi: 10.1021/ac50045a030. [DOI] [PubMed] [Google Scholar]
- 71.Ewing AG, Dayton MA, Wightman RM. Anal Chem. 1981;53:1842–1847. [Google Scholar]
- 72.Strein TG, Ewing AG. Anal Chem. 1992;64:1368–1373. [Google Scholar]
- 73.Zhang XJ, Zhang WM, Zhou XY, Ogorevc B. Anal Chem. 1996;68:3338–3343. doi: 10.1021/ac9600969. [DOI] [PubMed] [Google Scholar]
- 74.Lau YY, Wong DKY, Luo G, Ewing AG. Electroanalysis. 1992;4:865–869. [Google Scholar]
- 75.Henstridge MC, Wildgoose GG, Compton RG. J Phys Chem C. 2009;113:14285–14289. [Google Scholar]
- 76.Huang WH, Pang DW, Tong H, Wang ZL, Cheng JK. Anal Chem. 2001;73:1048–1052. doi: 10.1021/ac0008183. [DOI] [PubMed] [Google Scholar]
- 77.Wu WZ, Huang WH, Wang W, Wang ZL, Cheng JK, Xu T, Zhang RY, Chen Y, Liu J. J Am Chem Soc. 2005;127:8914–8915. doi: 10.1021/ja050385r. [DOI] [PubMed] [Google Scholar]
- 78.Robinson IM, Finnegan JM, Monck JR, Wightman RM, Fernandez JM. Proc Natl Acad Sci U S A. 1995;92:2474–2478. doi: 10.1073/pnas.92.7.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen RS, Huang WH, Tong H, Wang ZL, Cheng JK. Anal Chem. 2003;75:6341–6345. doi: 10.1021/ac0340556. [DOI] [PubMed] [Google Scholar]
- 80.Li YT, Zhang SH, Wang L, Xiao RR, Liu W, Zhang XW, Zhou Z, Amatore C, Huang WH. Angew Chem, Int Ed. 2014;53:12456–12460. doi: 10.1002/anie.201404744. [DOI] [PubMed] [Google Scholar]
- 81.Sudhof TC. Neuron. 2012;75:11–25. doi: 10.1016/j.neuron.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rees HR, Anderson SE, Privman E, Bau HH, Venton BJ. Anal Chem. 2015;87:3849–3855. doi: 10.1021/ac504596y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Singhal R, Bhattacharyya S, Orynbayeva Z, Vitol E, Friedman G, Gogotsi Y. Nanotechnology. 2010;21:1–9. doi: 10.1088/0957-4484/21/1/015304. [DOI] [PubMed] [Google Scholar]
- 84.Kim BM, Murray T, Bau HH. Nanotechnology. 2005;16:1317–1320. [Google Scholar]
- 85.Rand E, Periyakaruppan A, Tanaka Z, Zhang DA, Marsh MP, Andrews RJ, Lee KH, Chen B, Meyyappan M, Koehne JE. Biosens Bioelectron. 2013;42:434–438. doi: 10.1016/j.bios.2012.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang DA, Rand E, Marsh M, Andrews RJ, Lee KH, Meyyappan M, Koehne JE. Mol Neurobiol. 2013;48:380–385. doi: 10.1007/s12035-013-8531-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Koehne JE, Marsh M, Boakye A, Douglas B, Kim IY, Chang SY, Jang DP, Bennet KE, Kimble C, Andrews R, Meyyappan M, Lee KH. Analyst. 2011;136:1802–1805. doi: 10.1039/c1an15025a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cassell AM, Li J, Nguyen-Vu TB, Koehne JE, Chen H, Andrews R, Meyyappan M. J Nanosci Nanotechnol. 2009;9:5038–5046. doi: 10.1166/jnn.2009.gr06. [DOI] [PubMed] [Google Scholar]
- 89.Koehne JE, Marsh M, Boakye A, Douglas B, Kimble CJ, Bennet KE, Meyyappan M, Lee KH. Amino Acids. 2011;41:S41. [Google Scholar]
- 90.Stamford JA, Justice JB. Anal Chem. 1996;68:A359–A363. [PubMed] [Google Scholar]; Zen JM, Chen PJ. Anal Chem. 1997;69:5087–5093. [Google Scholar]
- 91.Bath BD, Michael DJ, Trafton BJ, Joseph JD, Runnels PL, Wightman RM. Anal Chem. 2000;72:5994–6002. doi: 10.1021/ac000849y. [DOI] [PubMed] [Google Scholar]
- 92.Ranganathan S, Kuo TC, McCreery RL. Anal Chem. 1999;71:3574–3580. [Google Scholar]
- 93.Zhang B, Adams KL, Luber SJ, Eves DJ, Heien ML, Ewing AG. Anal Chem. 2008;80:1394–1400. doi: 10.1021/ac702409s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guitchounts G, Markowitz JE, Liberti WA, Gardner TJ. J Neural Eng. 2013;10:1–13. doi: 10.1088/1741-2560/10/4/046016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen YC, Hsu HL, Lee YT, Su HC, Yen SJ, Chen CH, Hsu WL, Yew TR, Yeh SR, Yao DJ, Chang YC, Chen H. J Neural Eng. 2011;8:1–7. doi: 10.1088/1741-2560/8/3/034001. [DOI] [PubMed] [Google Scholar]
- 96.Cahill PS, Walker QD, Finnegan JM, Mickelson GE, Travis ER, Wightman RM. Anal Chem. 1996;68:3180–3186. doi: 10.1021/ac960347d. [DOI] [PubMed] [Google Scholar]
- 97.Capella P, Ghasemzadeh B, Mitchell K, Adams RN. Electroanalysis. 1990;2:175–182. [Google Scholar]
- 98.Luthman J, Friedemann MN, Hoffer BJ, Gerhardt GA. J Neural Transm. 1997;104:379–397. doi: 10.1007/BF01277658. [DOI] [PubMed] [Google Scholar]
- 99.Jackson BP, Dietz SM, Wightman RM. Anal Chem. 1995;67:1115–1120. doi: 10.1021/ac00102a015. [DOI] [PubMed] [Google Scholar]
- 100.Rice ME, Nicholson C. Anal Chem. 1989;61:1805–1810. doi: 10.1021/ac00192a005. [DOI] [PubMed] [Google Scholar]
- 101.Gerhardt GA, Oke AF, Nagy G, Moghaddam B, Adams RN. Brain Res. 1984;290:390–395. doi: 10.1016/0006-8993(84)90963-6. [DOI] [PubMed] [Google Scholar]
- 102.Brazell MP, Kasser RJ, Renner KJ, Feng J, Moghaddam B, Adams RN. J Neurosci Methods. 1987;22:167–172. doi: 10.1016/0165-0270(87)90011-2. [DOI] [PubMed] [Google Scholar]
- 103.Hashemi P, Dankoski EC, Petrovic J, Keithley RB, Wightman RM. Anal Chem. 2009;81:9462–9471. doi: 10.1021/ac9018846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Özel RE, Wallace KN, Andreescu S. Anal Chim Acta. 2011;695:89–95. doi: 10.1016/j.aca.2011.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cruz J, Kawasaki M, Gorski W. Anal Chem. 2000;72:680–686. doi: 10.1021/ac990954b. [DOI] [PubMed] [Google Scholar]
- 106.Trouillon R, Cheung C, Patel BA, O’Hare D. Analyst. 2009;134:784–793. doi: 10.1039/b811958a. [DOI] [PubMed] [Google Scholar]
- 107.Marinesco S, Carew TJ. J Neurosci Methods. 2002;117:87–97. doi: 10.1016/s0165-0270(02)00093-6. [DOI] [PubMed] [Google Scholar]
- 108.Vreeland RF, Atcherley CW, Russell WS, Xie JY, Lu D, Laude ND, Porreca F, Heien ML. Anal Chem. 2015;87:2600–2607. doi: 10.1021/ac502165f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ross AE, Venton JB. Analyst. 2012;137:3045–3051. doi: 10.1039/c2an35297d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Singh YS, Sawarynski LE, Dabiri PD, Choi WR, Andrews AM. Anal Chem. 2011;83:6658–6666. doi: 10.1021/ac2011729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cai C, Mirkin MV. J Am Chem Soc. 2006;128:171–179. doi: 10.1021/ja055091k. [DOI] [PubMed] [Google Scholar]
- 112.Wang Y, Kececi K, Velmurugan J, Mirkin MV. Chem Sci. 2013;4:3606–3616. doi: 10.1039/C2SC21502K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vanysek P, Behrendt M. J Electroanal Chem. 1981;130:287–292. [Google Scholar]
- 114.Campbell JA, Girault HH. J Electroanal Chem. 1989;266:465–469. [Google Scholar]
- 115.Aidoo AY, Ward K. Math Comput Model. 2006;44:952–962. [Google Scholar]
- 116.Bunin MA, Wightman RM. J Neurosci. 1998;18:4854–4860. doi: 10.1523/JNEUROSCI.18-13-04854.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhan DP, Mao SN, Zhao Q, Chen Z, Hu H, Jing P, Zhang MQ, Zhu ZW, Shao YH. Anal Chem. 2004;76:4128–4136. doi: 10.1021/ac035339t. [DOI] [PubMed] [Google Scholar]
- 118.Arrigan DWM, Ghita M, Beni V. Chem Commun. 2004:732–733. doi: 10.1039/b316493d. [DOI] [PubMed] [Google Scholar]
- 119.Beni V, Ghita M, Arrigan DWM. Biosens Bioelectron. 2005;20:2097–2103. doi: 10.1016/j.bios.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 120.Ribeiro JA, Miranda IM, Silva F, Pereira CM. Phys Chem Chem Phys. 2010;12:15190–15194. doi: 10.1039/c0cp00751j. [DOI] [PubMed] [Google Scholar]
- 121.Berduque A, Zazpe R, Arrigan DWM. Anal Chim Acta. 2008;611:156–162. doi: 10.1016/j.aca.2008.01.077. [DOI] [PubMed] [Google Scholar]