The number of Americans diagnosed with type 2 diabetes in 2012 was predicted by the American Diabetes Association (ADA) to be 23.3 million, with a projected cost of $306 billion: more than $1 for every $5 spent on healthcare [1]. While many type 2 diabetes therapies exist, non-adherence and ineffective daily blood glucose management promote associated complications. Most classic type 2 diabetes therapies aim to reduce peripheral insulin resistance, whereas more recently-developed therapeutics aim to improve beta-cell function by acting directly on the insulin-producing cells of the pancreatic islet. Furthermore, beta-cell therapies that work through G protein-coupled receptors modulate insulin secretion in response to stimulatory glucose, thus promoting a more physiological regulation of the insulin response. Examples of these therapeutics are the stable glucagon-like peptide 1 (GLP-1) agonists or dipeptidyl peptidase 4 (DPP-4) inhibitors, which both act through the GLP-1 receptor on the beta-cell to stimulate downstream signaling pathways (Figure 1). An emerging target, the E-prostanoid receptor 3 (EP3), plays a critical regulatory role in modulating beta-cell function and may be a key component in the development of beta-cell pathologies. It is well established that the endogenous ligand for EP3, prostaglandin E2 (PGE2), negatively regulates insulin secretion [2–4]. PGE2 is rapidly degraded in the bloodstream and is thought to act on its target tissues via autocrine or paracrine mechanisms [5]. Increased (1) plasma levels of PGE2 metabolites, (2) production of PGE2 from platelets and islet cells (3) expression of PGE2 synthetic enzymes, and (4) EP3 expression have all been linked with the pathophysiology of type 2 diabetes [6–10]. Furthermore, increased islet PGE2 production and EP3 expression have a negative impact on signaling through the GLP-1 receptor, which in beta-cells is a strong, endogenous potentiator of insulin secretion in response to food intake [6] (Figure 1). Increased EP3 signaling might explain the failure of type 2 diabetes drugs that act through the GLP-1 receptor in a subset of type 2 diabetic patients [6–11]. Although the EP3 receptor plays critical roles in islet biology, extrapancreatic function may be equally important in both type 2 diabetes pathophysiology. PGE2 signaling through EP3 appears to be a critical component of reducing lipolysis in adipose tissue. Mice lacking a critical phospholipase A2, an essential initiating component in PGE2 biosynthesis, have reduced PGE2 concentrations in adipose tissue and are resistant to weight gain [12]. Although these mice are protected from weight gain and present an increased catabolic phenotype, they are largely insulin resistant in the liver and have lowered peripheral glucose metabolism, most likely due in part to reduced fat mass [12]. In addition, EP3 signaling can mediate the migratory response of vascular smooth muscle cells (VSMCs). Pharmaceutical blockade of EP3 or its genetic deletion produces VSMCs that exhibit suppressed G protein-mediated signaling and altered polarity and directional migration, suggesting that blockade of EP3 might protect from dysfunctional vascular remodeling [13]. Furthermore, EP3-null mice exhibit reduced baseline mean arterial pressures, and pharmacological inhibition of EP3 blocks angiotensin 2-mediated vasoconstriction, suggesting the EP3 receptor as a target for antihypertensives [14]. Thus, it appears that PGE2 signaling through EP3 may play a significant role in the progression of obesity, diabetes, and co-morbidities such as cardiovascular disease.
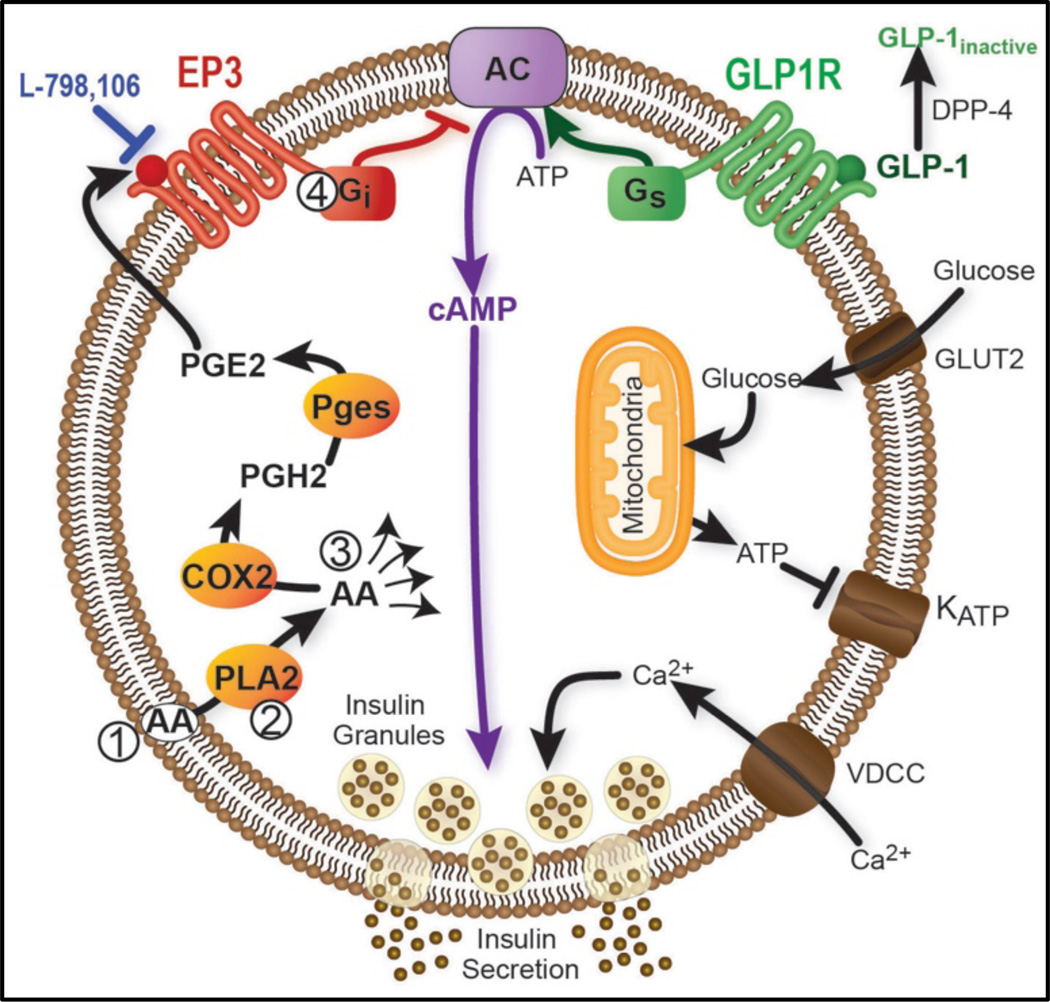
Figure 1. Arachidonic acid metabolism to PGE2 and how these signaling pathways interact with other beta-cell signaling pathways.
Arachidonic acid (AA) from dietary sources is incorporated into membrane phospholipids in the beta-cell. Next, AA is cleaved from the phospholipids by phospholipase A2 (PLA2). AA is also converted into numerous metabolites, some pro- and some anti-inflammatory. PGE2 is generated from AA by two sequential enzymatic steps: cyclooxygenase 2 (COX2)-mediated generation of PGH2, followed by prostaglandin E synthase (Pges)-mediated conversion to PGE2. PGE2 can activate the EP3 receptor on the beta-cell to block cAMP production, interfering with cAMP-mediated signaling through the GLP-1 receptor. The four steps to be explored in this grant are indicated by circled numerals.
Even though PGE2 signaling through EP3 may be important in metabolic disease progression, EP3 has other functions that lend caution to EP3 as a therapeutic target. First, signaling through EP3 reduces cancer cell proliferation and tumorigenesis through G12-mediated Rho activation [15]. Blockade of EP3 also prevents apoptosis, which protects from stroke injury [16], but could potentially interfere with cancer cell killing. Extending from these findings, caution should be taken to ensure that pharmaceutical blockade of EP3 does not promote tumorigenesis in vivo. In addition, whole-body EP3-null mice develop obesity, insulin resistance, glucose intolerance, and eat considerably more than wild-type controls [17]. Part of this phenotype can be explained by increased night eating, most likely due to unstable sleep patterns, as PGE2 (acting through hypothalamic EP3) may act as a somnogen [18]. Furthermore, high levels of PGE2 and increased signaling through EP3 augment nitric oxide synthase expression, an enzyme critical in the brain development of newborns [19]. This signaling cascade is suggested to play a significant role in connecting brain circulation and synaptic activity in perinatal development. A drug that did not cross the blood/brain barrier might bypass these negative consequences of inactivation of brain EP3 signaling. Another way to target a GPCR is to interfere with its downstream signaling mechanisms. EP3 is one of four known E-prostanoid receptors (EP1-4) and is widely expressed across tissues, with highest expression in the kidney, uterus, and pancreas [20]. When bound by PGE2, EP3 contributes to a reduction in intracellular cyclic AMP (cAMP) concentrations by inhibiting adenylyl cyclase activity [21, 22] (Figure 1). A unique aspect of EP3 is that multiple splice variants exist in every species tested, differing only in their C-terminus (Table 1). The identity of the C-terminal tail appears to determine which G protein-mediated signaling cascades will be activated, as well as whether the receptor exhibits constitutive activity or the ability to be desensitized by agonist. Of note, in the pancreatic beta-cell, the EP3 receptor can couple to a unique Gi subfamily member, Gz [21, 23]. Gz is the most biochemically-unique and tissue-restricted Gi -subfamily G protein [24, 25]. Determining which splice variant is responsible for Gz -coupling in the beta-cell may lead to new ways to specifically target this interaction. In sum, we have learned much about the role of PGE2-mediated EP3 signaling in metabolic diseases such as type 2 diabetes, yet we still have a long way to go before confirming that EP3 is a suitable target for new type 2 diabetes therapeutics. Overall, though, the results seem promising.
Table 1. Human, Mouse, and Rat EP3 splice variants, organized based on unique C-terminal sequence and arranged with their species homologs.
The C-terminus of these splice variants appears to impact on G protein coupling, constitutive activity, and desensitization to agonist. The G proteins with the most evidence for coupling are shown in Bold. Within each set of homologs (shown in grey or white shading), receptor properties are consistent, with the exception of the constitutive activity of Hs Var 4 and Hs Var 5.
| Species | Variant | Unique C-terminal Sequence | G-prot. coupling |
Ref. | Constitutive activity |
Ref. | De- sensitized? |
Ref. |
|---|---|---|---|---|---|---|---|---|
| Hs | Var 4 |
IRYHTNNYASSSTSLPCQCSSTLMW SDHLER |
Gi, Gq, Gβγ |
(3, 25– 27) |
None (Gi) | (28) | ||
| Mm | alpha |
IRDHTNYASSSTS_LPCPGSSALMWS DQLER |
Gi, G12, Gβγ |
(29–31) | Partial (Gi) Full (G12) |
(29, 32) |
Yes-Slow & persistent |
(33) |
| Rn | A |
IRDHTNYASSSTS_LPCPGSSVLMWS DQLER |
Gi | (34) | Full (Gi) | (34) | Yes | (35) |
| Hs | Var 5 |
VANAVSSCSNDGQKGQPISLSNEIIQ TEA |
Gi, Gs, Gq, Gβγ |
(3, 25, 26, 36) |
None (Gi) | (28) | Yes-Slow & persistent |
(36) |
| Mm | gamma |
VANAVSSCSSDGQKGQAISLSNEVVQ PGP |
Gi, Gs, Gβγ |
(30, 33, 37) |
Full (Gi), None (Gs) |
(38) | ||
| Rn | B |
VANAVSSCSSDQQKGQIASLSNEVVH PGP |
||||||
| Hs | Var 6 | MRKRRLREQEEFWGN |
Gi, Gq, Gs |
(3, 25, 26, 36) |
Partial (Gi) | (28) | Yes-Rapid & transient |
(36) |
| Hs | Var 7 | EEFWGN |
Gi, Gq, Gβγ |
(3, 25, 26, 36) |
Full (Gi) | (28) | Yes-Rapid & transient |
(36) |
| Hs | Var 8 |
MRKRRLREQLICSLQNSQIQRATAHC GQVQTYRVLNREEMEVLVSSINVYTR ISTVKTE |
Gi | (3, 26) | ||||
| Mm | Beta | MMNNLKWTFIAVPVSLGLRISSPREG |
Gi, Gβγ, G12 |
(29–31) | None (Gi), None (G12) |
(29, 32) | None | (33) |
| Rn | Beta | MMNNLKRSFIAIPASLSMRISSPREG | None | (35) | ||||
| Rn | D | FSLCFNR |
REFERENCES
- 1.Herman WH. The economic costs of diabetes: is it time for a new treatment paradigm? Diabetes Care. 2013;36:775–776. doi: 10.2337/dc13-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robertson RP, Chen M. A role for prostaglandin E in defective insulin secretion and carbohydrate intolerance in diabetes mellitus. J Clin Invest. 1977;60:747–753. doi: 10.1172/JCI108827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robertson RP, Tsai P, Little SA, Zhang HJ, Walseth TF. Receptor mediated adenylate cyclase-coupled mechanism for PGE2 inhibition of insulin secretion in HIT cells. Diabetes. 1987;36:1047–1053. doi: 10.2337/diab.36.9.1047. [DOI] [PubMed] [Google Scholar]
- 4.Sharp GW. Mechanisms of inhibition of insulin release. Am J Physiol. 1996;271:1781–1799. doi: 10.1152/ajpcell.1996.271.6.C1781. [DOI] [PubMed] [Google Scholar]
- 5.Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–2282. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 6.Kimple ME, Keller MP, Rabaglia MR, Pasker RL, Neuman JC, Truchan NA, et al. Prostaglandin E2 receptor, EP3, is induced in diabetic islets and negatively regulates glucose- and hormone-stimulated insulin secretion. Diabetes. 2013;62:1904–1912. doi: 10.2337/db12-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Persaud SJ, Muller D, Belin VD, Kitsou-Mylona I, Asare-Anane H, Papadimitriou A, et al. The role of arachidonic acid and its metabolites in insulin secretion from human islets of langerhans. Diabetes. 2007;56:197–203. doi: 10.2337/db06-0490. [DOI] [PubMed] [Google Scholar]
- 8.Robertson RP. Prostaglandins, glucose homeostasis, and diabetes mellitus. Annu Rev Med. 1983;34:1–12. doi: 10.1146/annurev.me.34.020183.000245. [DOI] [PubMed] [Google Scholar]
- 9.Robertson RP. Dominance of cyclooxygenase-2 in the regulation of pancreatic islet prostaglandin synthesis. Diabetes. 1998;47:1379–1383. doi: 10.2337/diabetes.47.9.1379. [DOI] [PubMed] [Google Scholar]
- 10.Robertson RP, Chen M, McRae JR, Metz SA. Improvement of insulin secretion in diabetics by a prostaglandin synthesis inhibitor. Adv Exp Med Biol. 1979;119:227–231. doi: 10.1007/978-1-4615-9110-8_32. [DOI] [PubMed] [Google Scholar]
- 11.Blonde L, Montanya E. Comparison of liraglutide versus other incretin related anti hyperglycaemic agents. Diabetes Obes Metab. 2012;2:20–32. doi: 10.1111/j.1463-1326.2012.01575.x. [DOI] [PubMed] [Google Scholar]
- 12.Jaworski K, Ahmadian M, Duncan RE, Sarkadi-Nagy E, Varady KA, Hellerstein MK, et al. AdPLA ablation increases lipolysis and prevents obesity induced by high-fat feeding or leptin deficiency. Nat Med. 2009;15:159–168. doi: 10.1038/nm.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J, Zou F, Tang J, Zhang Q, Gong Y, Wang Q, et al. Cyclooxygenase-2-Derived Prostaglandin E2 Promotes Injury-Induced Vascular Neointimal Hyperplasia Through the E-prostanoid 3 Receptor. Circ Res. 2013;113:104–114. doi: 10.1161/CIRCRESAHA.113.301033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen L, Miao Y, Zhang Y, Dou D, Liu L, Tian X, et al. Inactivation of the E-prostanoid 3 receptor attenuates the angiotensin II pressor response via decreasing arterial contractility. Arterioscler Thromb Vasc Biol. 2012;32:3042–3132. doi: 10.1161/ATVBAHA.112.254052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macias-Perez IM, Zent R, Carmosino M, Breyer MD, Breyer RM, Pozzi A, et al. Mouse EP3 alpha, beta, and gamma receptor variants reduce tumor cell proliferation and tumorigenesis in vivo. J Biol Chem. 2008;283:12538–12545. doi: 10.1074/jbc.M800105200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikeda-Matsuo Y, Tanji H, Narumiya S, Sasaki Y. Inhibition of prostaglandin E2 EP3 receptors improves stroke injury via anti-inflammatory and anti-apoptotic mechanisms. J Neuroimmunol. 2011;238:34–43. doi: 10.1016/j.jneuroim.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez-Alavez M, Klein I, Brownell SE, Tabarean IV, Davis CN, Conti B, et al. Night eating and obesity in the EP3R-deficient mouse. Proc Natl Acad Sci U S A. 2007;104:3009–3014. doi: 10.1073/pnas.0611209104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ram A, Pandey HP, Matsumura H, Kasahara-Orita K, Nakajima T, Takahata R, et al. CSF levels of prostaglandins, especially the level of prostaglandin D2, are correlated with increasing propensity towards sleep in rats. Brain Res. 1997;751:81–89. doi: 10.1016/s0006-8993(96)01401-1. [DOI] [PubMed] [Google Scholar]
- 19.Dumont I, Peri KG, Hardy P, Hou X, Martinez-Bermudez AK, Molotchnikoff S, et al. PGE2, via EP3 receptors, regulates brain nitric oxide synthase in the perinatal period. Am J Physiol. 1998;275:1812–1821. doi: 10.1152/ajpregu.1998.275.6.R1812. [DOI] [PubMed] [Google Scholar]
- 20.Kotani M, Tanaka I, Ogawa Y, Usui T, Mori K, Ichikawa A, et al. Molecular cloning and expression of multiple isoforms of human prostaglandin E receptor EP3 subtype generated by alternative messenger RNA splicing: multiple second messenger systems and tissue-specific distributions. Mol Pharmacol. 1995;48:869–879. [PubMed] [Google Scholar]
- 21.Kimple ME, Moss JB, Brar HK, Rosa TC, Truchan NA, Pasker RL, et al. Deletion of GαZ protein protects against diet-induced glucose intolerance via expansion of β-cell mass. J Biol Chem. 2012;287:20344–20355. doi: 10.1074/jbc.M112.359745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Negishi M, Sugimoto Y, Ichikawa A. Molecular mechanisms of diverse actions of prostanoid receptors. Biochim Biophys Acta. 1995;1259:109–119. doi: 10.1016/0005-2760(95)00146-4. [DOI] [PubMed] [Google Scholar]
- 23.Kimple ME, Nixon AB, Kelly P, Bailey CL, Young KH, Fields TA, et al. A role for G(z) in pancreatic islet beta-cell biology. J Biol Chem. 2005;280:31708–31713. doi: 10.1074/jbc.M506700200. [DOI] [PubMed] [Google Scholar]
- 24.Casey PJ, Fong HK, Simon MI, Gilman AG. Gz, a guanine nucleotide binding protein with unique biochemical properties. J Biol Chem. 1990;265:2383–2390. [PubMed] [Google Scholar]
- 25.Fields TA, Casey PJ. Signalling functions and biochemical properties of pertussis toxin resistant G-proteins. Biochem J. 1997;321:561–571. doi: 10.1042/bj3210561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Regan JW, Bailey TJ, Donello JE, Pierce KL, Pepperl DJ, Zhang D, et al. Molecular cloning and expression of human EP3 receptors: evidence of three variants with differing carboxyl termini. Br J Pharmacol. 1994;112:377–385. doi: 10.1111/j.1476-5381.1994.tb13082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmid A, Thierauch KH, Schleuning WD, Dinter H, et al. Splice variants of the human EP3 receptor for prostaglandin E2. Eur J Biochem. 1995;228:23–30. doi: 10.1111/j.1432-1033.1995.tb20223.x. [DOI] [PubMed] [Google Scholar]
- 28.Yang J, Xia M, Goetzl EJ, An S. Cloning and expression of the EP3-subtype of human receptors for prostaglandin E2. Biochem Biophys Res Commun. 1994;198:999–1006. doi: 10.1006/bbrc.1994.1142. [DOI] [PubMed] [Google Scholar]
- 29.Jin J, Mao GF, Ashby B. Constitutive activity of human prostaglandin E receptor EP3 isoforms. Br J Pharmacol. 1997;121:317–323. doi: 10.1038/sj.bjp.0701121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hasegawa H, Negishi M, Katoh H, Ichikawa A. Two isoforms of prostaglandin EP3 receptor exhibiting constitutive activity and agonist-dependent activity in Rho-mediated stress fiber formation. Biochem Biophys Res Commun. 1997;234:631–636. doi: 10.1006/bbrc.1997.6655. [DOI] [PubMed] [Google Scholar]
- 31.Irie A, Segi E, Sugimoto Y, Ichikawa A, Negishi M. Mouse prostaglandin E receptor EP3 subtype mediates calcium signals via Gi in cDNA transfected Chinese hamster ovary cells. Biochem Biophys Res Commun. 1994;204:303–309. doi: 10.1006/bbrc.1994.2460. [DOI] [PubMed] [Google Scholar]
- 32.Sugimoto Y, Negishi M, Hayashi Y, Namba T, Honda A, Watabe A, et al. Two isoforms of the EP3 receptor with different carboxyl-terminal domains. Identical ligand binding properties and different coupling properties with Gi proteins. J Biol Chem. 1993;268:2712–2718. [PubMed] [Google Scholar]
- 33.Hasegawa H, Negishi M, Ichikawa A. Two isoforms of the prostaglandin E receptor EP3 subtype different in agonist-independent constitutive activity. J Biol Chem. 1996;271:1857–1860. doi: 10.1074/jbc.271.4.1857. [DOI] [PubMed] [Google Scholar]
- 34.Negishi M, Sugimoto Y, Irie A, Narumiya S, Ichikawa A. Two isoforms of prostaglandin E receptor EP3 subtype. Different COOH-terminal domains determine sensitivity to agonist-induced desensitization. J Biol Chem. 1993;268:9517–9521. [PubMed] [Google Scholar]
- 35.Takeuchi K, Takahashi N, Abe T, Ito O, Tsutsumi E, Taniyama Y, et al. Functional difference between two isoforms of rat kidney prostaglandin receptor EP3 subtype. Biochem Biophys Res Commun. 1994;203:1897–1903. doi: 10.1006/bbrc.1994.2409. [DOI] [PubMed] [Google Scholar]
- 36.Neuschäfer-Rube F, Hermosilla R, Kuna M, Pathe-Neuschäfer-Rube A, Schülein R, Püschel GP, et al. A Ser/Thr cluster within the C-terminal domain of the rat prostaglandin receptor EP3alpha is essential for agonist-induced phosphorylation, desensitization and internalization. Br J Pharmacol. 2005;145:1132–1142. doi: 10.1038/sj.bjp.0706282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.An S, Yang J, So SW, Zeng L, Goetzl EJ. Isoforms of the EP3 subtype of human prostaglandin E2 receptor transduce both intracellular calcium and cAMP signals. Biochemistry. 1994;33:14496–14502. doi: 10.1021/bi00252a016. [DOI] [PubMed] [Google Scholar]
- 38.Irie A, Sugimoto Y, Namba T, Harazono A, Honda A, Watabe A, et al. Third isoform of the prostaglandin-E-receptor EP3 subtype with different C-terminal tail coupling to both stimulation and inhibition of adenylate cyclase. Eur J Biochem. 1993;217:313–318. doi: 10.1111/j.1432-1033.1993.tb18248.x. [DOI] [PubMed] [Google Scholar]
- 39.Negishi M, Hasegawa H, Ichikawa A. Prostaglandin E receptor EP3 gamma isoform, with mostly full constitutive Gi activity and agonist-dependent Gs activity. FEBS Lett. 1996;386:165–168. doi: 10.1016/0014-5793(96)00354-7. [DOI] [PubMed] [Google Scholar]