Abstract
Marijuana use by adolescents has been on the rise since the early 1990’s. With recent legalization and decriminalization acts passed, cannabinoid exposure in adolescents will undoubtedly increase. Human studies are limited in their ability to examine underlying changes in brain biochemistry making rodent models valuable. Studies in adult and adolescent animals show region and sex specific downregulation of the cannabinoid 1 (CB1) receptor following chronic cannabinoid treatment. However, although sex-dependent changes in behavior have been observed during the drug abstinence period following adolescent cannabinoid exposure, little is known about CB1 receptor expression during this critical time. In order to characterize CB1 receptor expression following chronic adolescent Δ-9-tetrahydrocannabinol (THC) exposure, we used [3H]CP55,940 binding to assess CB1 receptor expression in the dentate gyrus and areas CA1, CA2, and CA3 of the hippocampus in both male and female adolescent rats at both 24 hours and 2 weeks post chronic THC treatment. Consistent with other reported findings, we found downregulation of the CB1 receptor in the hippocampal formation at 24 hours post treatment. While this downregulation persisted in both sexes following two weeks of abstinence in the CA2 region, in females, this downregulation also persisted in areas CA1 and CA3. Expression in the dentate gyrus returned to the normal range by two weeks. These data suggest that selective regions of the hippocampus show persistent reductions in CB1 receptor expression and that these reductions are more widespread in female compared to male adolescents.
Keywords: Delta-9-Tetrahydrocannabinol, Sex differences, Adolescence, CB1 receptor expression, Hippocampus
Introduction
Marijuana is the most commonly used and abused illicit drug in the United States. Additionally, since the 1990’s marijuana use has been increasing among teenagers [1]. Marijuana is commonly thought of as having no adverse effects; however marijuana use has been shown to lead to impaired attention and memory in adolescent users [2, 3]. Cognitive deficits are more pronounced when marijuana use is initiated earlier in life [4]. This is in contrast to studies in adults that show few, if any, long term cognitive consequences of marijuana use [3]. Although the body of literature addressing cannabinoid exposure during adolescence is growing, relatively little is known about the biochemical effects of exogenous cannabinoids, such as Δ-9-tetrahydrocannabinol (THC), the primary psychoactive component of marijuana, during adolescence.
Cannabinoid receptors and their endogenous ligands, N-arachidonoylethanolamide (anandamide/AEA) and 2-arachiodonoylglycerol (2-AG), are present in the rat brain as early as gestational days 11–14 [5, 6]. In humans, cannabinoid receptors have been found from week 14 of gestation [7, 8]. The endocannabinoid system influences many processes during development through the modulation of neurotransmitter release and action [9, 10]. Adolescence is a time of dynamic development in the brain, during which neuronal plasticity is enhanced, synapses are refined, and neurotransmitters and their associated receptors mature into adult levels [11]. Endocannabinoid levels, cannabinoid 1 (CB1) receptors, and their activity peak just prior to the onset of puberty [5] possibly rendering this time period particularly susceptible to the effects of exogenous cannabinoids.
Animal studies show a variety of behavioral effects of cannabinoid exposure in juvenile and adolescent animals [for review, 12] but consensus is minimal. Alterations in anxiety and depressive-like measures (which are sex-specific) are commonly reported following adolescent cannabinoid exposure[13–21]. While these effects depend on the specific behavioral task used, deficits in cognitive performance that persist into adulthood following adolescent cannabinoid exposure are the most consistent findings. Lasting recognition memory impairment following adolescent, but not adult, cannabinoid treatment in both male and female rats has been widely reported [22] and we have identified lasting effects on reversal learning following early THC exposure [23].
However, few studies have examined the neurobiological consequences of early cannabinoid exposure which may mediate the observed behavioral effects. Quinn [24] reported that the deficits in novel object recognition in THC treated male adolescent rats were associated with changes in expression of proteins involved in hippocampal cytoarchitecture and mitochondrial function. Additionally, Rubino and colleagues [25, 26] reported that deficits in spatial working memory were accompanied by sex specific changes in protein expression in the prefrontal cortex and hippocampus.
If these observed changes in behavior and biochemical markers of synaptic plasticity are to be attributed to changes in endocannabinoid signaling, the expression of CB1 receptors may also be affected. Lasting changes in CB1 receptor expression and desensitization have been reported into adulthood after adolescent THC exposure in rats. For example, Rubino [19] found region specific CB1 receptor downregulation and desensitization in adult rats following escalating THC dosing during adolescence, with the effect seen more strongly in females than males. Differences were also observed 24 hours after the last drug treatment in the hippocampus and many other areas. By adulthood (P75–80) the downregulation in the hippocampus was no longer observed in either sex [19]. However, downregulation persisted in female animals in the nucleus accumbens, amygdala, and ventral tegmental area while in males downregulation persisted only in the amygdala. This suggests that there are sex differences in CB1 receptor recovery during the drug abstinence period following escalating THC dosing during adolescence.
In order to examine the effect of chronic adolescent THC exposure (P35–41) and abstinence on CB1 receptor expression, this study looked at hippocampal CB1 receptor binding using [3H]CP55,940 autoradiography both 24 hours and 2 weeks post treatment in both male and female adolescent rats.
Materials and methods
Subjects
Male and female Sprague Dawley rats (Charles River, Wilmington, MA) were housed in a 12-hour reverse light/dark cycle (lights out at 11am) with ad libitum access to water and food. Pups arrived in natural litters at postnatal day (P)14–15, were weaned on P21, and then housed in same-sex pairs. Rats were from 11 unique litters which arrived over the period of a year. On average, 2 litters (3–6 rats) were represented in each group. All procedures were carried out in accordance with NIH-approved standards under IACUC approval.
Drug treatment
Rats were given a once-daily intraperitoneal injection of 15mg/kg Δ9-tetrahydrocannabinol (THC; RTI, Research Triangle Park, North Carolina) in pluronic acid (Sigma-Alrich, Inc, St. Louis, MO)/saline [prepared as described in 27], or vehicle from P35–41, a period approximating mid-adolescence in humans [11].
Tissue preparation
24 hours (P42) or 2 weeks following the last administration of THC (P56) rats were decapitated immediately following testing on the elevated plus maze [45] and brains were quickly removed and frozen in methylbutane (Fisher Scientific) kept at −20°C on dry ice, the methylbutane evaporated and the brains stored at −80°C. 20μM-thick coronal sections containing the dorsal hippocampus (according to [28]) were made on a Hacker cryostat microtome (3 sections/subject). Tissue was mounted on positively charged slides and stored at −80°C.
Cannabinoid receptor autoradiography
Frozen sections were thawed and incubated for 2h with 3nM [3H]CP55,940 in binding buffer (50mM Tris-HCl, pH 7.4, with 5% BSA), as described previously [29, 30]. Sections were washed 4× 30min in ice-cold buffer (50mM Tris-HCl with 1% BSA), followed by 5 min at 25°C in buffer containing 50mM Tris-HCl with 0.5% formaldehyde solution. Slides were then dipped quickly in ice-cold deionized water and dried. Nonspecific binding was defined as binding in the presence of 10μM CP55,940. Slides and standards (3H-labeled microscales, Amersham Corp., Arlington Heights, IL) were exposed to Kodak Biomax MR film for 18 days. Following development, binding densities were quantified in regions shown in figure 1 using curves generated from the labeled standards. Data were analyzed using NIH ImageJ software.
Figure 1.
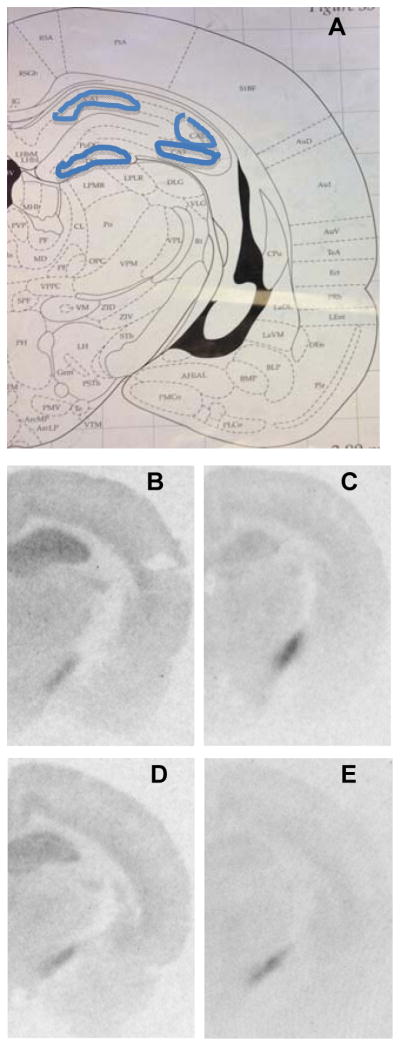
Representative autoradiograms for day 42 showing [3H] CP55,940 binding. (A) Outline of sections analyzed for autoradiography (Plate 35, −3.8 mm from Bregma, from Paxinos and Watson, Fourth Edition, 1998). (B) Male Control. (C) Male 15 THC. (D) Female Control. (E) Female 15 THC.
Statistical analysis
Three sections from each animal were processed and readings taken from both right and left sides yielding 6 values per animal. These were averaged to create one value for each rat for each hippocampal region. Group averages were then calculated for each hippocampal region.
A mixed linear model was constructed, with fixed effects being region, sex, treatment, and day of abstinence. 2- and 3-way interactions of these factors were modeled, but not the 4-way due to lack of adequate sample size. Litter was introduced as a random factor. The dependent variable was log-10 transformed to reduce skew. Intra-subject covariance was modeled as compound symmetry. Model residuals were inspected for skew and outliers. SAS (SAS Institute, Cary, NC) Release 9.2 software was used. Follow up analyses (ANOVAs) were conducted using Systat v.12 (SigmaPlot, San Jose, CA).
Results
One outlying observation was excluded from analysis. None of the 3-way interactions was significant (p> 0.2 in each case); the model was re-run without these effects to increase efficiency in the small sample. The litter effect was significant (p<0.001) suggesting that litter contributed significantly to the observed variance, however the mixed linear model controls for this effect. There were significant main effects for region, day, and treatment; and a 2-way interaction between region and treatment. Simple effects analysis of this interaction showed a significant treatment effect in cornu ammon region 1 (CA1) (p=0.003), cornu ammon region 2 (CA2) (p=0.005), cornu ammon region 3 (CA3) (p=0.005) and dentate gyrus (DG) (p=0.011) when collapsed across day and sex.
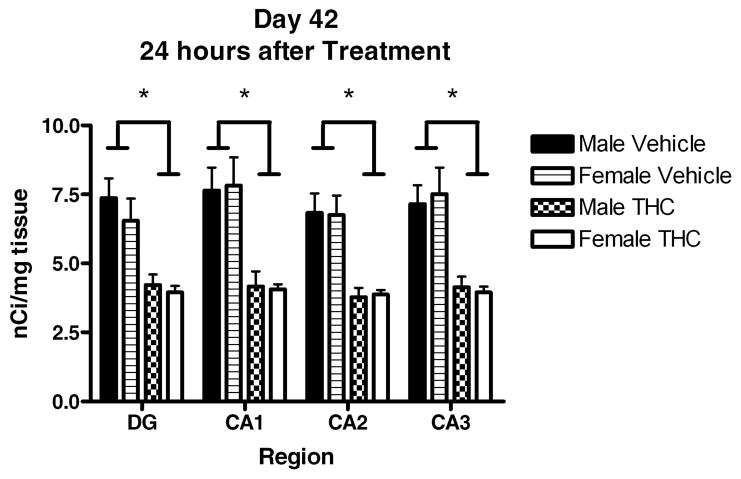
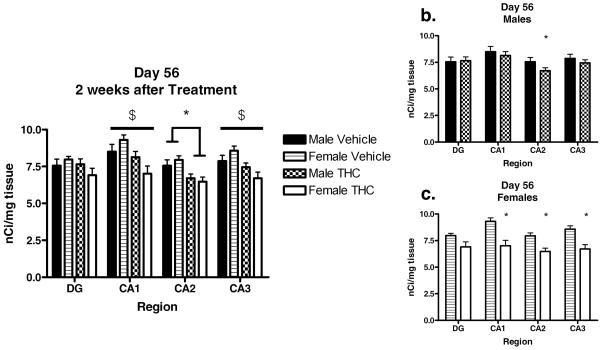
In order to further characterize interaction of region and treatment, CB1 receptor densities for each region at each time point were analyzed separately using 2-way ANOVAs with fixed effects for sex and treatment and their interaction. On P42, there were significant treatment effects in regions CA1 [F(1,14)=17.485, p=0.001], CA2 [F(1,14)=17.906, p=0.001], CA3 [F(1,14)=19.745, p=0.001], and DG [F(1,14)=15.146, p=0.002] where THC treatment resulted in a downregulation of CB1 receptors. No significant sex differences or interactions were observed on P42 (Figure 1 and 2). On P56, significant downregulation persisted in CA2 [F(1,20)=13.262, p=0.002] in both sexes, while a significant sex by treatment interaction was observed in CA1 [F(1,20)=4.900, p=0.039] and CA3 [F(1,20)=3.174, p=0.05]. Tukey post hoc analysis revealed that THC treated females still showed significant (CA1, p=0.007; CA3, p=0.006) CB1 receptor downregulation compared to vehicle treated females whereas males no longer showed this effect (Figures 3b and 3c). In the dentate gyrus, the downregulation had normalized in both males and females (Figure 3a).
Figure 2.
Average CB1R Density in Hippocampal Sub-Regions 24 hours after final THC (15mg/kg/day) exposure. * indicates significantly (ANOVA, p<0.05) lower than vehicle group. (n=3–6/group). Values expressed as mean ± SEM.
Figure 3.
Average CB1R Density in Hippocampal Sub-Regions 2 weeks after final THC (15mg/kg/day) exposure. (a.) Data for all sex and treatment groups * indicates significantly lower than vehicle group (ANOVA, p<0.05). $ indicates a significant sex by treatment interaction (n=3–6/group). Data for males (b.) and females (c.). * indicates significantly less than same sex vehicle treated group (Tukey, p<0.05). Values expressed as mean ± SEM.
Discussion
In light of increasing evidence of long lasting behavioral effects of adolescent cannabinoid exposure in both humans [for review, 3] and animals [for review, 12], it is of interest to study the underlying biochemical changes that may be present after cannabinoid exposure. To this end, we examined the effects of chronic adolescent THC exposure on CB1 receptor density in male and female adolescent rats. 24 hours after being exposed to 15 mg/kg THC from P35–41, both male and female rats showed significant downregulation of CB1 receptors in the dentate gyrus, and areas CA1, CA2, and CA3 of the hippocampus (Figures 1 and 2). This is consistent with the findings of others [31, 32] that the hippocampus shows a greater magnitude of downregulation and desensitization and slower recovery following abstinence compared to many brain regions [e.g. 33, 34]. Interestingly, two weeks after THC administration, the downregulation of CB1 persisted in both sexes only in area CA2 of the hippocampus. Sex differences in CB1 receptor expression in CA1 and CA3 emerged at this time, with THC treated females still showing lower CB1 receptor expression in areas CA1 and CA3 compared to vehicle treated females, while THC treated males no longer differed from their vehicle treated counterparts (Figure 3b and 3c). Binding within the dentate gyrus returned to control levels in both sexes. The regional selectivity of these changes relates well to the existing literature. The dentate gyrus is known to undergo extensive neurogenesis and during adolescence, in particular, undergoes robust neurogenesis compared to other hippocampal regions [35]. The rapid turnover of cells within the dentate gyrus may contribute to the normalization of binding in this structure. Decades of work on the hippocampus support the “trisynaptic circuit” of the dorsal hippocampus: entorhinal cortex-dentate gyrus-CA3-CA1 as being central to mediating spatial cognition [e.g. 36, 37]. Our data support a persistent alteration in this circuit only in females. That adolescent cannabinoids alter spatial cognition long after drug administration has ended is widely reported [e.g. 4, 23] and the present findings provide a substrate for this effect. Interestingly, binding in the CA2 region of the hippocampus remained decreased in both sexes 2 weeks after treatment ended. The CA2 region of the dorsal hippocampus has been reported to be essential for social memory [38] suggesting that adolescent cannabinoid treatment may have more profound effects on this component of memory compared to spatial memory.
While sex differences in CB1 receptor binding density in the hippocampus of adolescent animals have previously been reported with females showing lower CB1 binding in most regions studied [19, 39], the present study found no differences between control male and female subjects with respect to CB1 receptor density in any area examined. This could be due to differences in methodology or perhaps because animals in the current study were shipped at P14. Dow-Edwards et al. [40] reported an increase in hippocampal CB1 receptor expression in females shipped at P21 relative to non-shipped females, a difference not observed in males. This could be a contributing factor in the lack of observed sex differences in the present control animals.
Our findings of a downregulation of CB1 receptor density in all hippocampal regions 24 hours post THC treatment are similar to studies in adult animals, which show significant downregulation of CB1 receptors 24 hours after chronic treatment [31–33, 41, 42]. Additionally, long term changes in CB1 receptor expression following adolescent cannabinoid treatment have been reported [19, 43, 44]. The downregulation of CB1 receptors observed by Rubino et al [19] 24 hours post-THC treatment was no longer seen in adult animals of either sex suggesting that during drug abstinence, CB1 receptor expression in the hippocampus undergoes a recovery to control levels within 30 days. However, the manner by which this recovery occurs is still unclear. The findings of the current study indicate that this recovery process differs based on the sex of the animal, however, a full understanding of the mechanisms for these differences has yet to be revealed.
Studies in adult humans [34] and mice [33], both show region-specific downregulation and recovery rates of CB1 receptor expression following chronic cannabinoid treatment. Previous work in our laboratory has shown significant sex differences in both locomotor activity and anxiety (as measured by the elevated plus maze) during the drug abstinence period immediately following adolescent (P35–41) THC exposure [45]. Similarly treated animals also show deficits in reversal learning in the hippocampal-dependent active place avoidance task [23]. Receptor expression is only one aspect of changes to the endocannabinoid system. Further study must be done to assess changes in the functionality of these receptors. This is especially pertinent to the sex effects seen with adolescent cannabinoid exposure as females are generally more susceptible to receptor desensitization than males [19, 39].
Conclusions
The findings of the current study add to the growing body of evidence suggesting that males and females respond to cannabinoid treatment and abstinence differently. Additionally, the sex and region specific effects during the abstinence period suggest different patterns of endocannabinoid system dysregulation following adolescent THC exposure. Further characterizing these sex specific effects, especially during the abstinence period, will give us a better understanding of the neurobiology of male and female responses to cannabinoid exposure and may lead to sex-specific avenues for treatment of drug abuse and relapse.
Highlights.
Adolescent male and female rats were given Δ-9-tetrahydrocannabinol at 15mg/kg.
Cannabinoid receptor 1 expression was examined 24 hours and 2 weeks post-treatment.
Males and females both showed CB1R downregulation 24 hours post-treatment.
CB1R downregulation persisted in CA1 and CA3 in females 2 weeks post-treatment.
Normalization of CB1R expression occurred in dentate gyrus and not CA2 in both sexes
Acknowledgments
The authors would like to thank Dr. Jeremy Weedon for his statistical analysis and Dr. Steven Fox for helpful comments. This work was supported by NIH grants RO1 DA019348, P50 DA04584-0001, and P50 DA04584-0002
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.United States. Substance Abuse and Mental Health Services Administration. Center for Behavioral Health Statistics and Quality. Treatment episode data set (TEDS) 1999–2009 : state admissions to substance abuse treatment services, Center for Behavioral Health Statistics and Quality. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2011. p. xiv.p. 163. [Google Scholar]
- 2.Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schilling L, Gigerenzer G, Hoehe MR. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology. 1999;142:295–301. doi: 10.1007/s002130050892. [DOI] [PubMed] [Google Scholar]
- 3.Hall W, Degenhardt L. Adverse health effects of non-medical cannabis use. Lancet. 2009;374:1383–1391. doi: 10.1016/S0140-6736(09)61037-0. [DOI] [PubMed] [Google Scholar]
- 4.Pope HG, Jr, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug and alcohol dependence. 2003;69:303–310. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez de Fonseca F, Ramos JA, Bonnin A, Fernandez-Ruiz JJ. Presence of cannabinoid binding sites in the brain from early postnatal ages. Neuroreport. 1993;4:135–138. doi: 10.1097/00001756-199302000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Berrendero F, Sepe N, Ramos JA, Di Marzo V, Fernandez-Ruiz JJ. Analysis of cannabinoid receptor binding and mRNA expression and endogenous cannabinoid contents in the developing rat brain during late gestation and early postnatal period. Synapse. 1999;33:181–191. doi: 10.1002/(SICI)1098-2396(19990901)33:3<181::AID-SYN3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 7.Glass M, Dragunow M, Faull RL. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Dow-Edwards D, Keller E, Hurd YL. Preferential limbic expression of the cannabinoid receptor mRNA in the human fetal brain. Neuroscience. 2003;118:681–694. doi: 10.1016/s0306-4522(03)00020-4. [DOI] [PubMed] [Google Scholar]
- 9.Viveros MP, Llorente R, Moreno E, Marco EM. Behavioural and neuroendocrine effects of cannabinoids in critical developmental periods. Behavioural pharmacology. 2005;16:353–362. doi: 10.1097/00008877-200509000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Gaffuri AL, Ladarre D, Lenkei Z. Type-1 cannabinoid receptor signaling in neuronal development. Pharmacology. 2012;90:19–39. doi: 10.1159/000339075. [DOI] [PubMed] [Google Scholar]
- 11.Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and biobehavioral reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 12.Rubino T, Zamberletti E, Parolaro D. Adolescent exposure to cannabis as a risk factor for psychiatric disorders. Journal of psychopharmacology. 2012;26:177–188. doi: 10.1177/0269881111405362. [DOI] [PubMed] [Google Scholar]
- 13.O’Shea M, Singh ME, McGregor IS, Mallet PE. Chronic cannabinoid exposure produces lasting memory impairment and increased anxiety in adolescent but not adult rats. Journal of psychopharmacology. 2004;18:502–508. doi: 10.1177/026988110401800407. [DOI] [PubMed] [Google Scholar]
- 14.Schneider M, Drews E, Koch M. Behavioral effects in adult rats of chronic prepubertal treatment with the cannabinoid receptor agonist WIN 55,212–2. Behavioural pharmacology. 2005;16:447–454. doi: 10.1097/00008877-200509000-00018. [DOI] [PubMed] [Google Scholar]
- 15.O’Shea M, McGregor IS, Mallet PE. Repeated cannabinoid exposure during perinatal, adolescent or early adult ages produces similar longlasting deficits in object recognition and reduced social interaction in rats. Journal of psychopharmacology. 2006;20:611–621. doi: 10.1177/0269881106065188. [DOI] [PubMed] [Google Scholar]
- 16.Bambico FR, Gobbi G. The cannabinoid CB1 receptor and the endocannabinoid anandamide: possible antidepressant targets. Expert opinion on therapeutic targets. 2008;12:1347–1366. doi: 10.1517/14728222.12.11.1347. [DOI] [PubMed] [Google Scholar]
- 17.Biscaia M, Marin S, Fernandez B, Marco EM, Rubio M, Guaza C, Ambrosio E, Viveros MP. Chronic treatment with CP 55,940 during the peri-adolescent period differentially affects the behavioural responses of male and female rats in adulthood. Psychopharmacology. 2003;170:301–308. doi: 10.1007/s00213-003-1550-7. [DOI] [PubMed] [Google Scholar]
- 18.Wegener N, Koch M. Behavioural disturbances and altered Fos protein expression in adult rats after chronic pubertal cannabinoid treatment. Brain research. 2009;1253:81–91. doi: 10.1016/j.brainres.2008.11.081. [DOI] [PubMed] [Google Scholar]
- 19.Rubino T, Vigano D, Realini N, Guidali C, Braida D, Capurro V, Castiglioni C, Cherubino F, Romualdi P, Candeletti S, Sala M, Parolaro D. Chronic delta 9-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: behavioral and biochemical correlates. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:2760–2771. doi: 10.1038/sj.npp.1301664. [DOI] [PubMed] [Google Scholar]
- 20.Higuera-Matas A, Botreau F, Miguens M, Del Olmo N, Borcel E, Perez-Alvarez L, Garcia-Lecumberri C, Ambrosio E. Chronic periadolescent cannabinoid treatment enhances adult hippocampal PSA-NCAM expression in male Wistar rats but only has marginal effects on anxiety, learning and memory. Pharmacology, biochemistry, and behavior. 2009;93:482–490. doi: 10.1016/j.pbb.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 21.Bambico FR, Nguyen NT, Katz N, Gobbi G. Chronic exposure to cannabinoids during adolescence but not during adulthood impairs emotional behaviour and monoaminergic neurotransmission. Neurobiology of disease. 2010;37:641–655. doi: 10.1016/j.nbd.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 22.Schneider M, Koch M. Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2003;28:1760–1769. doi: 10.1038/sj.npp.1300225. [DOI] [PubMed] [Google Scholar]
- 23.Harte LC, Dow-Edwards D. Sexually dimorphic alterations in locomotion and reversal learning after adolescent tetrahydrocannabinol exposure in the rat. Neurotoxicology and teratology. 2010;32:515–524. doi: 10.1016/j.ntt.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quinn HR, Matsumoto I, Callaghan PD, Long LE, Arnold JC, Gunasekaran N, Thompson MR, Dawson B, Mallet PE, Kashem MA, Matsuda-Matsumoto H, Iwazaki T, McGregor IS. Adolescent rats find repeated Delta(9)-THC less aversive than adult rats but display greater residual cognitive deficits and changes in hippocampal protein expression following exposure. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:1113–1126. doi: 10.1038/sj.npp.1301475. [DOI] [PubMed] [Google Scholar]
- 25.Rubino T, Realini N, Braida D, Alberio T, Capurro V, Vigano D, Guidali C, Sala M, Fasano M, Parolaro D. The depressive phenotype induced in adult female rats by adolescent exposure to THC is associated with cognitive impairment and altered neuroplasticity in the prefrontal cortex. Neurotoxicity research. 2009;15:291–302. doi: 10.1007/s12640-009-9031-3. [DOI] [PubMed] [Google Scholar]
- 26.Rubino T, Realini N, Braida D, Guidi S, Capurro V, Vigano D, Guidali C, Pinter M, Sala M, Bartesaghi R, Parolaro D. Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus. 2009;19:763–772. doi: 10.1002/hipo.20554. [DOI] [PubMed] [Google Scholar]
- 27.Campbell KA, Foster TC, Hampson RE, Deadwyler SA. delta 9-Tetrahydrocannabinol differentially affects sensory-evoked potentials in the rat dentate gyrus. The Journal of pharmacology and experimental therapeutics. 1986;239:936–940. [PubMed] [Google Scholar]
- 28.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press/Elsevier; Amsterdam ; Boston: 2007. [Google Scholar]
- 29.Oviedo A, Glowa J, Herkenham M. Chronic cannabinoid administration alters cannabinoid receptor binding in rat brain: a quantitative autoradiographic study. Brain research. 1993;616:293–302. doi: 10.1016/0006-8993(93)90220-h. [DOI] [PubMed] [Google Scholar]
- 30.Werling LL, Reed SC, Wade D, Izenwasser S. Chronic nicotine alters cannabinoid-mediated locomotor activity and receptor density in periadolescent but not adult male rats. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2009;27:263–269. doi: 10.1016/j.ijdevneu.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Vogt LJ, Sim-Selley LJ. Chronic delta9-tetrahydrocannabinol treatment produces a time-dependent loss of cannabinoid receptors and cannabinoid receptor-activated G proteins in rat brain. Journal of neurochemistry. 1999;73:2447–2459. doi: 10.1046/j.1471-4159.1999.0732447.x. [DOI] [PubMed] [Google Scholar]
- 32.Sim-Selley LJ. Regulation of cannabinoid CB1 receptors in the central nervous system by chronic cannabinoids. Critical reviews in neurobiology. 2003;15:91–119. doi: 10.1615/critrevneurobiol.v15.i2.10. [DOI] [PubMed] [Google Scholar]
- 33.Sim-Selley LJ, Schechter NS, Rorrer WK, Dalton GD, Hernandez J, Martin BR, Selley DE. Prolonged recovery rate of CB1 receptor adaptation after cessation of long-term cannabinoid administration. Molecular pharmacology. 2006;70:986–996. doi: 10.1124/mol.105.019612. [DOI] [PubMed] [Google Scholar]
- 34.Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, Morse C, Pike VW, Volkow ND, Huestis MA, Innis RB. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Molecular psychiatry. 2012;17:642–649. doi: 10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He J, Crews FT. Neurogenesis decreases during brain maturation from adolescence to adulthood. Pharmacology, biochemistry, and behavior. 2007;86:327–333. doi: 10.1016/j.pbb.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Bird CM, Burgess N. The hippocampus and memory: insights from spatial processing. Nature reviews Neuroscience. 2008;9:182–194. doi: 10.1038/nrn2335. [DOI] [PubMed] [Google Scholar]
- 37.Nakashiba T, Cushman JD, Pelkey KA, Renaudineau S, Buhl DL, McHugh TJ, Rodriguez Barrera V, Chittajallu R, Iwamoto KS, McBain CJ, Fanselow MS, Tonegawa S. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell. 2012;149:188–201. doi: 10.1016/j.cell.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hitti FL, Siegelbaum SA. The hippocampal CA2 region is essential for social memory. Nature. 2014;508:88–92. doi: 10.1038/nature13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burston JJ, Wiley JL, Craig AA, Selley DE, Sim-Selley LJ. Regional enhancement of cannabinoid CB1 receptor desensitization in female adolescent rats following repeated Delta-tetrahydrocannabinol exposure. British journal of pharmacology. 2010;161:103–112. doi: 10.1111/j.1476-5381.2010.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dow-Edwards D, Frank A, Wade D, Izenwasser S. Prenatal/earlypostnatal history affects cannabinoid receptors in adolescent rats. Neurobehavioral and Teratological Society Annual Meeting; 2013. [Google Scholar]
- 41.Rodriguez de Fonseca F, Gorriti MA, Fernandez-Ruiz JJ, Palomo T, Ramos JA. Downregulation of rat brain cannabinoid binding sites after chronic delta 9-tetrahydrocannabinol treatment. Pharmacology, biochemistry, and behavior. 1994;47:33–40. doi: 10.1016/0091-3057(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 42.Lazenka MF, Selley DE, Sim-Selley LJ. DeltaFosB induction correlates inversely with CB(1) receptor desensitization in a brain region-dependent manner following repeated Delta(9)-THC administration. Neuropharmacology. 2014;77:224–233. doi: 10.1016/j.neuropharm.2013.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mateos B, Borcel E, Loriga R, Luesu W, Bini V, Llorente R, Castelli MP, Viveros MP. Adolescent exposure to nicotine and/or the cannabinoid agonist CP 55,940 induces gender-dependent long-lasting memory impairments and changes in brain nicotinic and CB(1) cannabinoid receptors. Journal of psychopharmacology. 2011;25:1676–1690. doi: 10.1177/0269881110370503. [DOI] [PubMed] [Google Scholar]
- 44.Lopez-Gallardo M, Lopez-Rodriguez AB, Llorente-Berzal A, Rotllant D, Mackie K, Armario A, Nadal R, Viveros MP. Maternal deprivation and adolescent cannabinoid exposure impact hippocampal astrocytes, CB1 receptors and brain-derived neurotrophic factor in a sexually dimorphic fashion. Neuroscience. 2012;204:90–103. doi: 10.1016/j.neuroscience.2011.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harte-Hargrove LC, Dow-Edwards DL. Withdrawal from THC during adolescence: sex differences in locomotor activity and anxiety. Behavioural brain research. 2012;231:48–59. doi: 10.1016/j.bbr.2012.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]