Abstract
Alcohol-related peripheral neuropathy (ALN) is a potentially debilitating complication of alcoholism that results in sensory, motor, and autonomic dysfunction. Unfortunately, ALN is rarely discussed as a specific disease entity in textbooks because it is widely assumed to primarily reflect consequences of nutritional deficiency. This hypothesis is largely based on observations first made over eight decades ago when it was demonstrated that thiamine deficiency (beriberi) neuropathy was clinically similar to ALN. In recent studies, failure of thiamine treatment to reverse ALN, together with new information demonstrating clinical and electrophysiological distinctions between ALN and nutritional deficiency neuropathies, suggests that alcohol itself may significantly predispose and enhance development of neuropathy in the appropriate clinical setting. We reviewed the evidence on both sides and conclude that ALN should be regarded as a toxic rather than nutritional neuropathy.
Keywords: alcohol, beriberi, neuropathy, nutrition, polyneuropathy, thiamine, toxic
Alcohol-related peripheral neuropathy (ALN) is a potential complication of chronic alcoholism that results in sensory, motor, and autonomic dysfunction, which can lead to significant disability. Patients with ALN sustain repeated injury, infection, and falls that lead to major head trauma and permanent disablement. The disabilities caused by ALN compound the already significant health, social, and economic consequences of chronic alcoholism. The prevalence of ALN is difficult to ascertain because obtaining accurate regular levels of alcohol intake and assessing nutritional status of subjects is problematic. Criteria used to classify and detect neuropathy may also underestimate prevalence. ALN may affect up to half of patients who suffer from alcoholism, but in studies that employ clinical and electrophysiological criteria, 25–66% of chronic alcoholics may be affected.1,2
The etiology of ALN has been debated for nearly a century. ALN was originally considered a toxic neuropathy caused by the effects of ethanol and its metabolites on peripheral nerves. The discovery that nutritional deficiencies caused other neurological diseases such as Wernicke encephalopathy and beriberi, both the result of thiamine deficiency, led to declining enthusiasm about the potential role of alcohol as a neurotoxin. The similar clinical, electrophysiological, and histopathological features of ALN compared with beriberi led to the hypothesis that ALN and beriberi were the same disease and that both were caused by thiamine deficiency.3 Despite these similarities, decades of clinical research have failed to definitively demonstrate that thiamine deficiency is the primary etiologic factor that causes ALN, or that its repletion serves as an effective treatment for ALN.4
Extensive animal and human research of ethanol neurotoxicity in alcoholic brain and liver disease provides a possible mechanism by which ethanol may effect the peripheral nervous system (PNS). The direct toxic effect of ethanol in the central nervous system and liver has been well documented, particularly its effect on insulin and insulin-like growth factor (IGF) resistance and oxidative stress.5–7 Recently, similar results have been obtained in experimental animal models of ALN.8,9 Improved understanding of the role ethanol plays in the pathogenesis of ALN and the mechanism by which it exerts its neurotoxic effects are crucial for developing effective treatments. This information would lead to a more accurate classification of ALN based on its etiology. The primary goal of this article is to review the evidence of proposed mechanisms in the development of ALN, specifically those related to thiamine deficiency and ethanol.
THE THIAMINE STORY
Background
Thiamine deficiency plays a controversial role in the development of ALN. Although there is a relatively clear association between thia-mine deficiency and the onset of some central nervous system manifestations of chronic alcoholism, such as Wernicke encephalopathy, the role it plays in ALN continues to be debated.10 When first described by Lettsom in 1787, the etiology of ALN was ascribed to the direct neurotoxic effect of alcohol.11 For over a century, ethanol was believed to be a neurotoxin that caused what James Jackson, MD, in 1822 referred to as a “peculiar disease resulting from the use of ardent spirits.” He and others observed that this particular disease, which he named arthrodynia a potu, started insidiously, first involving the lower limbs and extending upward to affect the hands and arms. Pain was a predominant symptom that could wax and wane, and at times be excruciating.12 He also described autonomic disturbances of the gastrointestinal tract and cardiovascular system. His treatments for ALN included abstinence from alcohol, the use of opium for pain, warm baths, and relief of constipation to improve some of the debilitating symptoms.12
The hypothesis that alcohol was a direct neurotoxin prevailed until the early twentieth century. Physicians at that time were perplexed by the fact that not all patients exposed to alcohol developed ALN. They believed there had to be another modulating factor. Shattuck suggested ALN was caused by nutritional deficiency of B vitamins, specifically thiamine, based on his observations of the similar clinical presentations of beriberi to ALN.3 His observation was followed by clinical investigations by Strauss, Minot, and Cobb, who concluded that dietary deficiency, specifically of vitamin B1, played an important role in the development and perpetuation of ALN. Their investigations allowed patients to continue their usual intake of alcohol in the setting of a well-balanced diet and supplementation with a non-purified form of B vitamins.13,14 They observed that symptoms improved in all instances and concluded that improvement must be related to thiamine supplementation, although at the time pure vitamin B1 was not available and there were no assays to assess vitamin B levels. Other investigators, including Blankenhorn and Spies, Jolliffe and Colbert, and Wechler, also concluded that vitamin B deficiency and not alcohol was the cause of polyneuropathy in the alcohol addict and thus, dubbed thiamine the anti-neuritic vitamin.15–17
Given the results of the aforementioned studies, the main treatment for ALN included a high-vitamin, high-calorie diet supplemented with yeast and liver extract to ensure a theoretically adequate supply of thiamine.18 Additional evidence supporting the role of thiamine was provided by Victor and Adams.19 Twelve patients with symptoms consistent with ALN were admitted to the hospital, deprived of alcohol, and given diets deficient solely in thiamine. The investigators found that neuritic symptoms persisted in all cases and some worsened, despite alcohol cessation. Thiamine was then added back to the diets. All patients improved, but only 2 patients showed demonstrable improvement in motor and sensory deficits. Given that patients did not improve with alcohol cessation and only improved when thiamine was added to their diet, the investigators concluded ALN must be the result of nutritional deficiency.19 The majority of patients were observed for only 2 weeks and, in nearly all cases, the improvement was purely symptomatic.19 Most of the aforementioned clinical trials were also flawed by relatively short observation periods (usually too short to allow recovery from what was presumed to be an axonal neuropathy), subjective reports of improvement, and unreliable dietary histories. They also failed to account for malnourishment and the improvement that could be seen from enhanced nutritional status. None had reliable methods to objectively assess for thiamine deficiency.
Animal Studies
Despite the shortcomings of these early clinical trials, animal studies helped to strengthen the argument of a nutritional versus toxic etiology causing ALN. Windebank et al. evaluated 16 adequately nourished rats, half control and the other half exposed to 16.8 g of alcohol per kilogram of body weight per day. No weight loss occurred in either group over the 9 months of the study. Nerve tissue analyzed at 3 and 9 months failed to show any evidence of neuropathy on 1-μm thin sections or on teased-fiber analysis.20
Primate models provided similar findings. Hallett et al. evaluated the effect of alcohol on 5 male rhesus monkeys as compared with 4 controls over a 5-year period. Four of the monkeys were fed a nutritious diet supplemented with lipotropics, amino acids, and 50% of calories provided by alcohol in place of other carbohydrates. One monkey had a diet deficient in choline and amino acids, also with 50% of calories provided by alcohol. There was no histological or electrophysiological evidence of peripheral neuropathy.21 The same group evaluated 10 female Macaca fascicularis monkeys fed a nutritionally complete diet and 9 age- and gender-matched monkeys fed a diet with 30% of calories provided by alcohol in place of carbohydrate calories over a 3-year span. They, too, did not show any electrophysiological or histological evidence of peripheral neuropathy.21
Biochemical Studies: Thiamine Deficiency vs. Utilization
Although the clinical and animal studies have focused on nutritional deficiency, biochemical studies provide evidence that alcohol may affect thiamine utilization rather than cause thiamine deficiency. Studies have shown that alcohol impairs thiamine absorption through the gastrointestinal tract,22–24 its utilization in tissues,25 its hepatic storage,26,27 and the phosphorylation of thiamine, reducing the availability of the active form, thiamine pyrophosphate.28–30 Paladin and Russo Perez measured plasma thiamine levels and erythrocyte transketolase activity in 30 alcoholics with ALN and 4 with Wernicke–Korsakoff syndrome. Thiamine levels in the ALN group were comparable to those of normal subjects, whereas there was a significantly lower concentration among those in the Wernicke–Korsakoff group. The transketolase activity was lower in both groups as compared with controls.10 The investigators suggested that thiamine utilization rather than lack of thiamine itself was implicated in the development of ALN.
Clinical Trials
Further support for the utilization argument arises from the fact that thiamine has not been proven to be an effective treatment for ALN. Recent clinical trials have shown that administration of vitamin B1 helped some subjective symptoms, such as pain, but there were questionable physical data, with no electrophysiological or histological data, showing that thiamine was beneficial in the treatment of ALN. A randomized, placebo-controlled, double-blind study of 84 patients clinically diagnosed with ALN found no significant reduction of pain after 8 weeks of treatment, but there was a statistically significant improvement in vibration perception threshold of the group treated with the vitamin B preparations.31 A follow-up, 12-week, randomized, double-blind, controlled trial evaluating the utility of vitamin B complex for the treatment of ALN produced similar results.32 A recent Cochrane database review of 13 studies (741 patients), 2 of which were specifically of ALN, found only limited data that vitamin B is effective for treating peripheral neuropathy and concluded that the evidence is insufficient to determine whether vitamin B is beneficial or harmful.4 The Woelk et al. study31 was included in this review, but the Peters et al. study32 was not, as the review only included studies up to 2005. The pooled data indicate that vitamin B may be less efficacious than alpha-lipoic acid, cilotazol, or cytidine triphosphate in short-term improvement of clinical and nerve conduction study outcomes.4
Conclusions about Thiamine in ALN
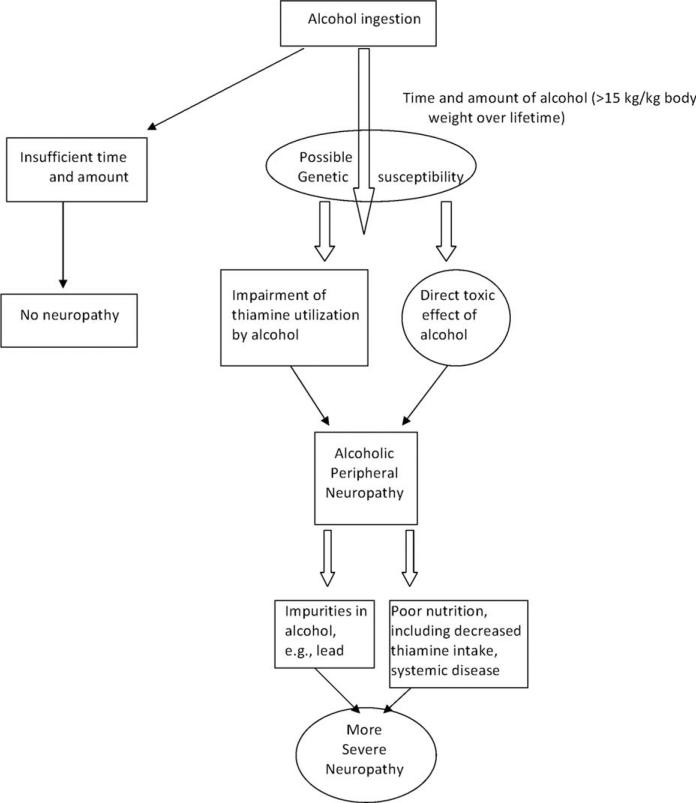
The role thiamine plays in the pathogenesis and treatment of ALN is still unclear. The possibility that thiamine may be a cofactor or modulating factor, but not the main etiologic factor causing ALN, has to be entertained. This possibility opens the door to consideration of other possible causes, including problems with thiamine utilization unique to alcohol abuse or alcohol as a direct neurotoxin in which thiamine deficiency may be a superadded problem (Fig. 1). Platt and Gin hypothesized that patients improved with thiamine administration, because it corrected an underlying metabolic disturbance rather than reversing peripheral nerve damage.33 Some have reconsidered ethanol's possible central role in the genesis of ALN given the indeterminate experimental and biochemical evidence of the role of thiamine deficiency in ALN and the lack of clinical efficacy for treatment of ALN.
FIGURE 1.
Proposed schematic for multifactorial development of alcoholic polyneuropathy.
THE ETHANOL STORY
Background
The re-examination of the potential neurotoxic effect of ethanol on peripheral nerves began in the last quarter of the twentieth century. A Danish study evaluated the clinical, electrophysiological, and biopsy findings in 37 patients with ALN as compared to 6 patients with neuropathy associated with post-gastrectomy malnutrition.34 This prospective study included patients who consumed more than 100 g/day of ethanol, mostly beer, for >3 years. Danish beer at the time was fortified with thiamine and vitamin B6. Twenty-three patients in the ALN group showed no evidence of malnutrition, and 14 had a history of weight gain. All of the post-gastrectomy patients had severe weight loss. Clinically, the two groups differed symptomatically in the development of their neuropathies; pain was the predominant symptom in ALN, whereas motor weakness was the primary symptom in the malnutrition group.34 The post-gastrectomy group had marked slowing of motor and sensory nerve conduction velocities, especially in the legs, which was consistent with segmental demyelination observed on nerve biopsy. The alcohol group also showed slowed motor and sensory nerve conduction velocities, but this slowing was commensurate with the marked reduction of amplitudes, suggesting an axonal process. The demyelination observed on nerve biopsy was thought to be secondary to marked axonal degeneration.34
Koike et al. also found significant clinical, electrophysiological, and histological differences between patients with pure ALN, patients with beriberi, and patients with ALN and thiamine deficiency. Like Behse and Buchthal,34 they found that patients with pure ALN developed a slowly progressive, sensory-dominant neuropathy with impaired superficial sensation and pain or burning dysesthesia, whereas those with pure thiamine deficiency developed an acutely progressive motor-dominant neuropathy with impairment of both superficial and deep sensation.35 In the Behse and Buchthal study,34 measurements of vitamin levels, including thiamine, were normal in the patients tested, and vitamin deficiency was not associated with development of ALN. Based on this evidence, both groups of researchers concluded that malnutrition and low vitamin B levels were not prerequisites for the development of ALN.34,35 A study by Poupon et al. confirmed this result.36 They showed that blood thiamine and thiamine phosphate concentrations in excessive drinkers with or without peripheral neuropathy were not significantly different between the two groups or from controls. They concluded that thiamine deficiency was slight or absent in chronic drinkers.36
Animal Studies
Bosch et al. were among the first investigators to provide animal data in support of this clinical evidence. They clearly showed that a neuropathy can be induced in animals that chronically consumed large amounts of alcohol.37 In contrast to the animal studies discussed previously, this group was able to produce chronic high alcohol intake in rats through the use of two procedures: schedule-induced polydipsia and liquid diet technique. The rats ingested approximately 11–12 g of ethanol per kilogram of body weight per day for 16–18 weeks. For both methods, they were able to show morphological evidence of a mild, predominately axonal distal neuropathy in the setting of normal thiamine levels substantiated by normal red blood cell transketolase levels. Not all rats developed the same degree of neuropathy.37 They hypothesized that sensitivity of different animals, either of the same species or of different species, to alcohol could explain the variation of alcohol-induced neuropathic damage. Despite pathological evidence of neuropathy, there was no electrophysiological evidence of neuropathy in these rats.37
In contrast, Juntunen et al. found a statistically significant 12% reduction in conduction velocities of the largest myelinated fibers of the sciatic nerve in rats exposed to half the dose of alcohol but for a much longer period of time, up to 9.5 months.38 They also evaluated the effects of variable dietary thiamine concentrations on the development of ALN in rats exposed for 36 weeks to 10–25% ethanol or water as the sole drinking fluid. They found that the deleterious effect of thiamine deficiency was enhanced with simultaneous consumption of alcohol.39 Their model revealed that the first pathological changes observed were in Schwann cells.40
A more recent study in an established experimental model of alcohol feeding in which adult male rats were pair-fed for 8 weeks with isocaloric diets containing 0% or 37% ethanol by caloric content revealed that chronic alcohol feeding slowed motor nerve conduction velocities in both tibial and peroneal nerves. Amplitudes were not significantly affected.8 Histological evidence showed patchy demyelination of motor and mixed nerves, suggestive of Schwann cell dysfunction.8,9 This finding was supported by an in vitro study of the effects of ethanol in rats, which showed that ethanol affected both Schwann cell proliferation and myelin formation.41 These findings imply different structural targets of ethanol in the development of ALN that can lead to either an axonal- or a demyelinating-predominant picture.
Electrophysiological and Pathological Differences between ALN and Beriberi Neuropathy
Objective testing with nerve conduction studies has shown that ALN, although not completely distinct from beriberi, does differ in some features. Nerve conduction abnormalities in patients with ALN typically show slowed motor and sensory velocities with moderate to severe reduction in sensory amplitudes.42–45 Koike et al.35 compared the nerve conduction study profiles of patients with pure ALN, pure thiamine deficiency, and those with a combination of ALN and thiamine deficiency. Nerve conduction studies of patients with pure ALN showed moderate reduction of compound muscle action potentials (CMAPs), severe reduction of sensory nerve action potentials (SNAPs), and mild to moderate slowing of motor and sensory nerves affecting mainly the lower extremities, suggesting a length-dependent, sensorimotor axonal neuropathy with secondary demyelination. In contrast, beriberi patients had CMAPs that were significantly reduced, with no evidence of sensory nerve involvement, consistent with a motor axonopathy. Patients with evidence of ALN and thiamine deficiency had a combination of these findings.35
A longitudinal study of nerve conduction velocity in children who had prenatal exposure to greater than 2 oz. of absolute alcohol per day also confirmed the neurotoxicity of alcohol to peripheral nerves.46 Seventeen exposed children were compared with 13 non-exposed children at birth and again at 12–14 months of age. The exposed children had significantly slowed velocities and reduced amplitudes in the ulnar and tibial motor nerves at birth. These abnormalities persisted at 1 year of age. The abnormalities observed in this study and those previously discussed were believed to reflect both myelin (reduced velocity) and axonal damage (decreased amplitude).35,46 The question of whether alcoholic neuropathy is primarily or initially demyelinating versus axonal remains unresolved by electrophysiological data.
Pathological data seems to support that ALN is more consistent with a primary axonal lesion.42,47,48 Electron microscopy has confirmed axonal degeneration of myelinated and unmyelinated fibers with minimal evidence of primary demyelination.34 This evidence was further supported in a prior study conducted by Walsh and McLeod, who found a reduction in the density of myelinated nerve fibers of all diameters in the nerves and teased-fiber preparation, more consistent with axonal degeneration.44 On the other hand, Koike et al. reported that segmental de/remyelination and myelin irregularity was greater with ALN as compared with the large-fiber–predominant axonal loss observed in beriberi.49
Another very interesting finding was that densities of small myelinated and unmyelinated fibers were more severely reduced than the density of large myelinated fibers in the early stages of ALN but not in beriberi.35 It is possible that small-fiber polyneuropathy is a manifestation of early ALN and is more likely to be observed in patients who are younger or have a significantly shorter time of alcohol abuse.50 These studies not only support the direct role of alcohol in the development of ALN but also raise the possibility of a toxic dose effect in the development of ALN.
Dose Effect
Behse and Buchthal proposed that 100 ml of ethyl alcohol, which translates to 3 L of beer or 300 ml of spirits per day for 3 years, is the minimal amount of alcohol consumed by patients who develop polyneuropathy.34 Several clinical studies have shown that the total lifetime amount of alcohol and duration of alcohol consumption are significantly correlated with peripheral neuropathy, but Monforte's study is frequently cited as evidence that ALN is due to the cumulative toxic effect of the total lifetime dose of alcohol.49,51–54 In that study, 107 alcoholic patients and 61 controls provided detailed histories, surveys, and assessments of autonomic function, such as heart rate and blood pressure variations, determinations of sustained hand grip, and nerve conduction studies.54 Reports of nutritional status were provided by the patient and by family members. Similar to Behse and Buchthal,34 they found no relationship between ALN and nutritional status. Only a small portion of their patients exhibited evidence of malnutrition, and almost all had normal transketolase activity. Multivariate analysis revealed that the only independent variable significantly associated with ALN was total lifetime dose of alcohol. No correlation was found with age, smoking habits, active exercise, presence or absence of liver disease, or pancreatitis.54 To put it in practical terms, 31 (41%) of the 75 patients who consumed >15 kg of alcohol per kilogram of body weight over their lifetime met criteria for ALN. This number translates roughly to 300 ml (10 oz.) of 86% proof whiskey per day in a 70-kg man over several years.54
Vittadini et al. agreed there was a cumulative lifetime dose effect, but they also proposed that the type of alcoholic beverage may be a significant factor.55 They studied 296 patients who ingested at least 100 g of alcohol per day. In their patient population 37% of subjects drank wine alone, 42% drank wine as well as other alcoholic beverages, and 21% drank other alcoholic beverages alone or in combination but without wine. The subjective neurological symptoms and electrophysiological findings were more serious in subjects who abused wine alone or in combination with other alcoholic beverages compared with those who solely drank beer or other spirits.55 They and other investigators proposed that the toxic effect of alcohol in wine may have been compounded by the deleterious effects of other impurities, specifically lead.55–57 The mean lead concentration of wine is 50 ng/ml (range 4–254 ng/ml). In chronic alcoholics who drink mostly wine, this may be a toxic contributing factor to the development of polyneuropathy.58
FUTURE DIRECTIONS
There is sufficient evidence to support the proposition that ALN should be classified as a toxic neuropathy rather than a nutritional neuropathy. Given the significant advances made over the past decade or so, it would seem reasonable to conclude that ALN is in fact the result of a multifactorial process primarily mediated by the toxic effect of alcohol modulated by other factors like genetic predisposition, thiamine deficiency, altered thia-mine metabolism, malnutrition, systemic diseases, or impurities such as lead. Alcohol's effect in the central nervous system (CNS) and liver does provide possible clues as to how it may mediate potential effects in the PNS, but the primary targeted structure is still under debate. It has been hypothesized that alcohol interferes with second messenger systems, particularly those activated by acetylcho-line muscarinic receptors.59,60 In vitro and in vivo studies indicated that ethanol inhibits muscarinic receptor–induced phosphoinositide metabolism in an age-specific manner.61,62 In addition, ethanol inhibits axonal transport and cytoskeletal structure maintenance, which could cause or exacerbate either demyelination or the axonal dying-back process.63 As well, acetaldehyde, a major toxic metabolite of ethanol, can also exert direct neurotoxic effects due to increased adduct formation and oxidative stress.64 Other postulated mechanisms of ethanol effect on peripheral nerve include altered lipid peroxidation, activation of atypical protein kinase C, and disruption of the sympathoadrenal and hypothalamic–pituitary axis.65
Ethanol has been linked to insulin/insulin-like growth factor-1 (IGF-1) resistance in the brain in patients with alcoholic dementia and alcoholic liver disease. This mechanism has been investigated in both an adult rat model of chronic ethanol exposure and in human alcoholics. In both, ethanol-mediated neurodegeneration was linked to insulin/IGF-1 resistance, persistent oxidative stress, and impaired acetylcholine homeostasis.64 These pathophysiological processes promote progressive cell loss, degeneration, and impairments in organ/tissue function. The role of insulin/IGF resistance vs. oxidative stress as mediators of ALN is under investigation, but given its role in alcohol-mediated disease in liver and the central nervous system it makes sense that it would also likely be a factor in ALN. By identifying and understanding the primary target and underlying mechanism of alcohol neurotoxicity, more effective—and possibly curative—treatments other than alcohol cessation, improved nutrition, and thiamine treatment may be developed.
CONCLUSIONS
ALN is a potentially significant and debilitating complication of alcoholism. The evidence is accruing that ALN should be reclassified as a toxic, rather than nutritional neuropathy. ALN has clinical and electrophysiological features distinct from but overlapping with neuropathy from pure thia-mine deficiency (beriberi). Thiamine treatment has not been successful in reversing ALN, and the features noted have resulted in re-examination of the 80-year-old theory that ALN is a nutritional rather than a toxic neuropathy. Recent animal and human clinical, electrophysiological and pathological results support a toxic cause, likely affecting small unmyelinated and myelinated fibers early in the course, and progressing to more symptomatic clinical involvement as a large-fiber sensorimotor axonal neuropathy develops. The development of an appropriate therapy will include cessation of alcohol ingestion but will also need to be aimed at the toxic target(s) of alcohol, which is the goal of ongoing research.
Acknowledgments
Research support for this study was received from a special fund from Rhode Island Hospital, Providence, Rhode Island.
Abbreviations
- ALN
alcohol-related peripheral neuropathy
- CMAP
compound muscle action potential
- CNS
central nervous system
- IGF
insulin-like growth factor
- PNS
peripheral nervous system
- SNAP
sensory nerve action potential
REFERENCES
- 1.Ammendola A, Tata MR, Aurilio C, Ciccone G, Gemini D, Ammendola E, et al. Peripheral neuropathy in chronic alcoholism: a retrospective cross sectional study in 76 subjects. Alcohol Alcohol. 2001;36:271–275. doi: 10.1093/alcalc/36.3.271. [DOI] [PubMed] [Google Scholar]
- 2.Laker SR, Sullivan WJ. [May 13, 2009];Alcoholic neuropathy. http://emedicine.medscape.com/article/315159.
- 3.Shattuck GC. The relation of beri-beri to polyneuritis from other causes. Am J Trop Med. 1928;8:539–543. [Google Scholar]
- 4.Ang CD, Alviar MJM, Dans AL, Bautista-Velez GGP, Villaruz-Sulit MVC, Tan JJ, et al. Vitamin B for treating peripheral neuropathy [review]. Cochrane Database Syst Rev. 2008;3:CD004573. doi: 10.1002/14651858.CD004573.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de la Monte SM, Xu XJ, Wands JR. Ethanol inhibits insulin expression and actions in the developing brain. Cell Mol Life Sci. 2005;62:1131–1145. doi: 10.1007/s00018-005-4571-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de la Monte SM, Yeon JE, Tong M, Longato L, Chaudhry R, Pang MY, et al. Insulin resistance in experimental alcohol-induced liver disease. J Gastroenterol Hepatol. 2008;23:e477–486. doi: 10.1111/j.1440-1746.2008.05339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de la Monte SM, Tong M, Cohen AC, Sheedy D, Harper C, Wands JR. Insulin and insulin-like growth factor resistance in alcoholic neurodegeneration. Alcohol Clin Exp Res. 2008;32:1630–1644. doi: 10.1111/j.1530-0277.2008.00731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mellion ML, Nguyen VA, Tong M, Mark P, Wands JR, Gilchrist JM, et al. Alcohol-related peripheral neuropathy is not caused by nutritional deficiency. Neurology. 2010;74(suppl 2):A520. [Google Scholar]
- 9.Nguyen VA, Mellion ML, Tong M, Gilchrist JM, de la Monte SM. Poster session presented at the annual meeting of the American Academy of Neuropathologists. Philadelphia, Pennsylvania: Jun, 2010. Alcohol-related peripheral neuropathy—characterization in an experimental animal model. [Google Scholar]
- 10.Paladin F, Russo Perez G. The haematic thiamine level in the course of alcoholic neuropathy. Eur Neurol. 1987;26:129–133. doi: 10.1159/000116324. [DOI] [PubMed] [Google Scholar]
- 11.Lettsom JC. Some remarks on the effects of lignum quassiae amarae. Memoirs Med Soc Lond. 1787;1:128. [Google Scholar]
- 12.Jackson J. On a peculiar disease resulting from the use of ardent spirits. N Engl J Med Surg. 1822;11:351–353. [Google Scholar]
- 13.Minot GR, Strauss MB, Cobb S. Alcoholic polyneuritis: dietary deficiency as a factor in its production. N Engl J Med. 1933;208:1244–1249. [Google Scholar]
- 14.Strauss MB. The etiology of alcoholic polyneuritis. Am J Med Sci. 1935;189:378–382. [Google Scholar]
- 15.Blankenhorn MA, Spies TD. Prevention, treatment and possible nature of the peripheral neurtis associated with pellagra and chronic alcoholism. Trans A Am Physicians. 1935;50:164–166. [Google Scholar]
- 16.Jolliffe N, Colbert CN. The etiology of polyneuritis in the alcohol addict. JAMA. 1936;107:642–647. [Google Scholar]
- 17.Wechler IS. Etiology of polyneuritis. Arch Neurol Psychiatry. 1933;29:813–827. [Google Scholar]
- 18.Meiklejohn AP. Is thiamine the antineuritic vitamin? N Engl J Med. 1940;223:265–273. [Google Scholar]
- 19.Victor M, Adams RD. On the etiology of alcoholic neurologic diaseases with special reference to the role of nutrition. Am J Clin Nutr. 1961;9:379–397. doi: 10.1093/ajcn/9.4.379. [DOI] [PubMed] [Google Scholar]
- 20.Windebank AJ. Polyneuropathy due to nutritional deficiency and alcoholism. In: Thomas PK, Griffin JW, Dyck PJ, editors. Peripheral neuropathy. 3rd ed. Saunders; Philadelphia: 1993. pp. 1310–1321. [Google Scholar]
- 21.Hallett M, Fox JG, Rogers AE, Nicolosi R, Schoene W, Goolsby HA, et al. Controlled studies on the effects of alcohol ingestion on peripheral nerves of macaque monkeys. J Neurol Sci. 1987;80:65–71. doi: 10.1016/0022-510x(87)90221-8. [DOI] [PubMed] [Google Scholar]
- 22.Hoyumpa AM. Mechanism of thiamine deficiency in chronic alcoholism. Am J Clin Nutr. 1980;3:2759–2761. doi: 10.1093/ajcn/33.12.2750. [DOI] [PubMed] [Google Scholar]
- 23.Thomson AD, Baker H, Levy CM. Patterns of 35S-thiamine hydro-chloride absorption in the malnourished alcoholic patient. J Lab Clin Med. 1970;76:34–45. [PubMed] [Google Scholar]
- 24.Tomasulo PA, Katner RM, Iber FL. Impairment of thiamine absorption in alcoholism. Am J Clin Nutr. 1968;21:1340–1344. doi: 10.1093/ajcn/21.11.1341. [DOI] [PubMed] [Google Scholar]
- 25.Hoyumpa AM, Breen KJ, Schenker S, Wilson FA. Thiamine transport across rat intestine. II. Effect of ethanol. J Lab Clin Med. 1975;86:803–816. [PubMed] [Google Scholar]
- 26.Frank O, Luisasa-Opper MF, Sorrell A, Thompson D, Baker H. Vita-min deficits in severe alcohol fatty liver of man calculated from multiple reference points. Exp Mol Pathol. 1971;15:191–197. doi: 10.1016/0014-4800(71)90098-0. [DOI] [PubMed] [Google Scholar]
- 27.Leevy CM, Baker H, Ten Hove W, Frank O, Cherrick GR. B-complex vitamins in liver disease of the alcoholic. Am J Clin Nutr. 1965;16:339–346. doi: 10.1093/ajcn/16.4.339. [DOI] [PubMed] [Google Scholar]
- 28.Abe T. Itokawa. Effect of ethanol administration on thiamine metabolism and transketolase activity in rats. Int J Vitamin Nutr. 1977;47:307–314. [PubMed] [Google Scholar]
- 29.Fennelly K, Frank O, Baker H, Leevy CM. Red blood cell transketolase activity in malnourished alcoholics with cirrhosis. Am J Clin Nutr. 1967;20:946–949. doi: 10.1093/ajcn/20.9.946. [DOI] [PubMed] [Google Scholar]
- 30.Rindi G, Cominciolo V, Reggiani C, Patrini C. Nervous tissue thia-mine metabolism in vivo. III. Influence of ethanol intake on the dynamics of thiamine and its phosphoesthers in different brain regions and sciatic nerve of the rat. Brain Res. 1987;413:21–25. doi: 10.1016/0006-8993(87)90150-8. [DOI] [PubMed] [Google Scholar]
- 31.Woelk H, Lehrl S, Bitsch R, Federlin K. Benfotiamine in treatment of alcoholic polyneuropathy: an 8-week randomised controlled study. Alcohol Alcohol. 1998;33:631–638. doi: 10.1093/alcalc/33.6.631. [DOI] [PubMed] [Google Scholar]
- 32.Peters TJ, Kotowicz J, Nyka W, Kozubski W, Kuznetsov V, Vanderbist F, et al. Treatment of alcoholic polyneuropathy with vitamin B complex: a randomised controlled trial. Alcohol Alcohol. 2006;41:636–642. doi: 10.1093/alcalc/agl058. [DOI] [PubMed] [Google Scholar]
- 33.Platt B, Gin SY. Some observations on a preliminary study of beriberi in Shanghai. Trans IX Cong Far East A Trop Med. 1934;2:407–413. [Google Scholar]
- 34.Behse F, Buchthal F. Alcoholic neuropathy: clinical, electrophysio-logical and biopsy findings. Ann Neurol. 1977;2:95–110. [Google Scholar]
- 35.Koike H, Iijima M, Sugiura M, Mori K, Hattori N, Ito H, et al. Alcoholic neuropathy is clinicopathologically distinct from thiamine deficiency. Ann Neurol. 2003;54:19–29. doi: 10.1002/ana.10550. [DOI] [PubMed] [Google Scholar]
- 36.Poupon RE, Gervaise G, Riant P, Houin G, Tilement JP. Blood thia-mine and thiamine phosphate concentrations in excessive drinkers with or without peripheral neuropathy. Alcohol Alcohol. 1990;25:605–611. doi: 10.1093/oxfordjournals.alcalc.a045056. [DOI] [PubMed] [Google Scholar]
- 37.Bosch EP, Pelham RW, Rasool CG, Chatterjee A, Lash RW, Brown L, et al. Animal models of alcoholic neuropathy: morphologic, electrophysiologic and biochemical findings. Muscle Nerve. 1979;2:133–144. doi: 10.1002/mus.880020208. [DOI] [PubMed] [Google Scholar]
- 38.Juntunen J, Teravainen H, Eriksson K, Panula P, Larsen A. Experimental alcoholic neuropathy in the rat: histological and electrophysiological study on myoneural junctions and peripheral nerves. Acta Neuropathol (Berl) 1978;41:131–137. doi: 10.1007/BF00689764. [DOI] [PubMed] [Google Scholar]
- 39.Juntunen J, Teravainen H, Eriksson K, Larsen A, Hillbom M. Peripheral neuropathy and myopathy: an experimental study of rats on alcohol and variable dietary thiamine. Virchows Arch A Path Anat Histol. 1979;383:241–252. doi: 10.1007/BF00430243. [DOI] [PubMed] [Google Scholar]
- 40.Juntunen J, Matikainen E, Nickels J, Ylikahri R, Sarviharju M. Alcoholic neuropathy and hepatopathy in mice, an experimental study. Acta Pathol Microbiol Immunol Scand. 1983;91:137–144. doi: 10.1111/j.1699-0463.1983.tb02738.x. [DOI] [PubMed] [Google Scholar]
- 41.Mithen FA, Reiker MM, Birchem R. Effects of ethanol on rat Schwann cell proliferation and myelination in culture. In Vitro Cell Dev Biol. 1990;26:129–139. doi: 10.1007/BF02624103. [DOI] [PubMed] [Google Scholar]
- 42.Blackstock E, Rushworth G, Garth D. Electophysiological studies in alcoholism. J Neurol Neurosurg Psychiatry. 1972;35:326–334. doi: 10.1136/jnnp.35.3.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Casey EB, Le Quesne PM. Electrophysiological evidence for a distal lesion in alcoholic neuropathy. J Neurol Neurosurg Psychiatry. 1972;35:624–630. doi: 10.1136/jnnp.35.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walsh JC, McLeod JG. Alcoholic neuropathy: an electrophysiological and histological study. J Neurol Sci. 1970;10:457–469. doi: 10.1016/0022-510x(70)90025-0. [DOI] [PubMed] [Google Scholar]
- 45.Mawdsley C, Mayer RF. Nerve conduction in alcoholic polyneuropathy. Brain. 1965;88:335–356. doi: 10.1093/brain/88.2.335. [DOI] [PubMed] [Google Scholar]
- 46.de los Angeles Avaria M, Mills JL, Kleinsteuber K, Aros S, Conley MR, Cox C, et al. Peripheral nerve conduction abnormalities in children exposed to alcohol in utero. J Pediatr. 2004;144:338–343. doi: 10.1016/j.jpeds.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 47.Dyck PJ, Gutrecht JA, Bastron JA, Karnes WE, Dale AJ. Histologic and teased-fiber measurements of sural nerve disorders in lower motor and primary sensory neurons. Proc Mayo Clin. 1968;43:81–123. [PubMed] [Google Scholar]
- 48.Tredici G, Minazzi M. Alcoholic neuropathy. An electron microscopic study. J Neurol Sci. 1975;25:333–346. doi: 10.1016/0022-510x(75)90155-0. [DOI] [PubMed] [Google Scholar]
- 49.Koike H, Mori K, Misu K, Hattori N, Ito H, Hirayama M, Sobue G. Painful alcoholic neuropathy with predominant small-fiber loss and normal thiamine status. Neurology. 2001;56:1727–1732. doi: 10.1212/wnl.56.12.1727. [DOI] [PubMed] [Google Scholar]
- 50.Zambelis T, Karandreas N, Tzavellas E, Kokotis P, Liappas J. Large and small fiber neuropathy in chronic alcohol dependent subjects. J Periph Nerv Sys. 2005;10:375–381. doi: 10.1111/j.1085-9489.2005.00050.x. [DOI] [PubMed] [Google Scholar]
- 51.Hilz MJ, Zimmermann P, Claus D, Neundorfer B. Thermal threshold determination in alcoholic polyneuropathy: an improvement of diagnosis. Acta Neurol Scand. 1995;91:389–393. doi: 10.1111/j.1600-0404.1995.tb07026.x. [DOI] [PubMed] [Google Scholar]
- 52.Angelink MW, Malessa R, Weisser U, Lemmer W, Zeit T, Majewski T, et al. Alcoholism, peripheral neuropathy (PNP), and cardiovascular autonomic neuropathy (CAN). J Neurol Sci. 1998;161:135–142. doi: 10.1016/s0022-510x(98)00266-4. [DOI] [PubMed] [Google Scholar]
- 53.Meldgaard B, Anderson K, Ahlgren P, Danielsen UT, Sorensen H. Peripheral neuropathy, cerebral atrophy, and intellectual impairment in chronic alcoholics. Acta Neurol Scand. 1984;70:336–344. doi: 10.1111/j.1600-0404.1984.tb00834.x. [DOI] [PubMed] [Google Scholar]
- 54.Monforte R, Estruch R, Valls-Sole J, Nicolas J, Villalta J, Urnamo-Marquez A. Autonomic and peripheral neuropathies in patients with chronic alcoholism: a dose-related toxic effect of alcohol. Arch Neurol. 1995;52:45–51. doi: 10.1001/archneur.1995.00540250049012. [DOI] [PubMed] [Google Scholar]
- 55.Vittadini G, Buonocore M, Colli G, Terzi M, Fonte R, Biscaldi G. Alcoholic polyneuropathy: a clinical and epidemiological study. Alcohol Alcohol. 2001;36:393–400. doi: 10.1093/alcalc/36.5.393. [DOI] [PubMed] [Google Scholar]
- 56.Minoia C, Sabbioni E, Ronchi A, Gatti A, Pietra R, Nicoletti A, et al. Trace element reference values in tissues from inhabitants of the European community. IV. Influence of dietary factors. Sci Total Environ. 1994;141:181–195. [Google Scholar]
- 57.Cezard C, Demarquilly C, Boniface M, Heguenor JM. Influence of the degree of exposure of lead on relations between alcohol consumption and the biological indices of lead exposure: epidemiological study in a lead acid battery factory. Br J Indust Med. 1992;49:645–647. doi: 10.1136/oem.49.9.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ostapczuk P, Eschnauer HR, Scollary GR. Determination of cadmium, lead and copper in wine by potentiometric stripping analysis. Fresenius J Anal Chem. 1997;358:723–727. [Google Scholar]
- 59.Balduini W, Reno F, Costa LG, Cattabeni F. Developmental neurotoxicity of ethanol: further evidence for an involvement of muscarinic receptor-stimulated phosphoinositide metabolism. Eur J Pharmacol Mol Pharmacol. 1994;266:283–289. doi: 10.1016/0922-4106(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 60.Costa LG, Guizzetti M, Lu H, Bordi F, Vitalone A, Tita B, et al. Intra-cellular signal transduction pathways as targets for neurotoxicants. Toxicology. 2001;160:19–26. doi: 10.1016/s0300-483x(00)00435-2. [DOI] [PubMed] [Google Scholar]
- 61.Balduini W, Costa LG. Effect of ethanol on muscarinic receptor stimulated phosphoinositide metabolism during brain development. J Pharmacol Exp Ther. 1989;250:541–547. [PubMed] [Google Scholar]
- 62.Balduini W, Camdura SM, Manzo L, Cattabeni F, Costa LG. Time-, concentration-, and age-dependent inhibition of muscarinic receptor-stimulated phosphoinositide metabolism by ethanol in the developing rat brain. Neurochem Res. 1991;16:1235–1240. doi: 10.1007/BF00966701. [DOI] [PubMed] [Google Scholar]
- 63.Malatova Z, Cizkova D. Effect of ethanol on axonal transport of cholinergic enzymes in the rat sciatic nerve. Alcohol. 2002;26:115–120. doi: 10.1016/s0741-8329(01)00207-5. [DOI] [PubMed] [Google Scholar]
- 64.Cohen AC, Tong M, Wands JR, de la Monte SM. Insulin and insulin-like growth factor resistance with neurodegeneration in an adult chronic ethanol exposure model. Alcohol Clin Exp Res. 2007;31:1558–1573. doi: 10.1111/j.1530-0277.2007.00450.x. [DOI] [PubMed] [Google Scholar]
- 65.Dina OA, Sachia GK, Alessandri-Haber N, Green PG, Messing RO, Levine JD. Alcohol-induced stress in painful alcoholic neuropathy. Eur J Neurosci. 2008;27:83–92. doi: 10.1111/j.1460-9568.2007.05987.x. [DOI] [PubMed] [Google Scholar]