Abstract
Bladder cancer is a common malignancy of the urinary tract, which generally develops in the epithelial lining of the urinary bladder. The specific course of treatment depends on the stage of bladder cancer; however, therapeutic strategies typically involve intravesical drug delivery to reduce toxicity and increase therapeutic effects. Recently, metallic, polymeric, lipid, and protein nanoparticles have been introduced to aid in the treatment of bladder cancer. Nanoparticles are also commonly used as pharmaceutical carriers to improve interactions between drugs and the urothelium. In this review, we classify the characteristics of bladder cancer and discuss the types of nanoparticles used in various treatment modalities. Finally we summarize the potential applications and benefits of various nanoparticles in intravesical therapy.
Keywords: Urothelial cancer, Nanoparticles, Nanotechnology, Photothermal Therapy, Drug delivery
Introduction
Urothelial cancer of urinary bladder is an epithelial cancer in which abnormal cells in the epithelial lining multiply without control [1, 2]. The most common type of bladder cancer is transitional cell carcinoma (TCC), also referred to as urothelial cell carcinoma (UCC). Over the last two decades, various nanoparticle technologies have been used in the detection and treatment of cancers of the breast [3], oral cavity [4], lung [5], cervix [6], and brain [7]. Nanoparticles are also used as pharmaceutical carriers in drug delivery systems comprising organic and inorganic materials [8, 9], and many state-of-the-art techniques incorporate liposomal [10], polymer-drug conjugates [11] and micellar formulations [12]. Furthermore, a considerable number of nanoparticle platforms are currently in the preclinical stages of development [13]. In this review, we classify the various types of bladder cancer according to their clinical characteristics and summarize how various nanoparticles are applied in intravesical bladder cancer therapy. This work is of particular importance at this time, due to recent findings showing that the use of nanoparticles in the treatment of urothelial cancer in the urinary bladder can reduce negative side effects and recurrence rates.
Current Treatment of Urothelial Cancer of the Urinary Bladder
Bladder carcinomas the fourth most common malignancy in America and the fifth most common disease among European males [14]. Furthermore, the prevalence of this malignancy of the urinary tract tends to increase with economic development [15–17]. The most typical symptom of this malignancy is painless hematuria, with microscopic or gross hematuria presenting in more than 85 % of patients [18]. Depending on the severity of hemorrhaging, the color of urine can range from normal, to dark yellow, to bright red or cola [19]. Other symptoms include increased frequency and urgency of urination, dysuria, and abdominal pain [20]. The five-year average survival rate among patients with bladder cancer is approximate 60 %; however, bladder cancer is associated with a high recurrence rate, which results in a longer course of disease with greater per-patient financial cost compared to other cancers [21].
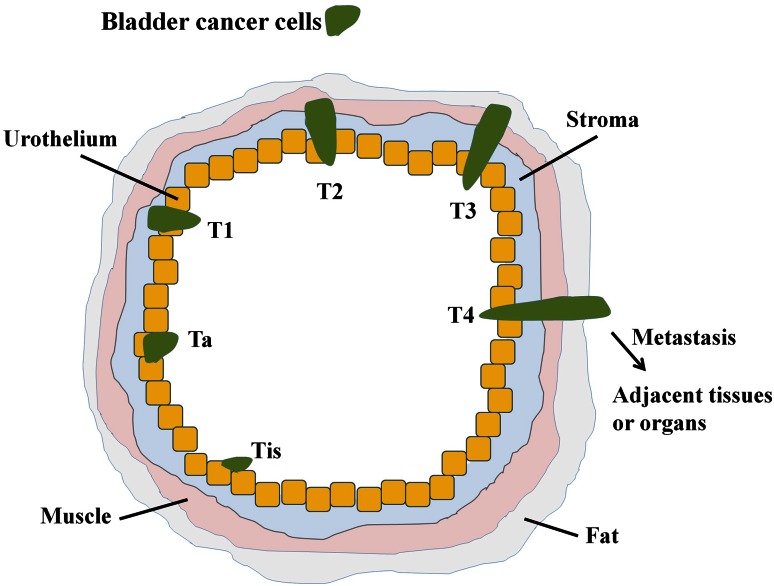
The bladder is a hollow, distensible organ used for the storage of urine [22]. It is composed of mucosa, submucosa, detrusor muscle, and perivesical fat. Bladder cancer typically begins in the mucosa layer, namely, transitional epithelial tissue [23]. TCC comprises over 90 % of bladder cancers; other bladder cancer types include squamous cell carcinoma (SCC, about 7–8 %), adenocarcinoma (1–2 %), and carcinosarcoma (<1 %) [24]. Furthermore, most diagnosed cases (70–85 %) involve a superficial (non-muscle invasive) form of the disease [25–27]. Bladder cancer staging is classified according to the location and spread of tumors (i.e. stage Tis, Ta and T1-4; Fig. 1), and how to directly against superficial bladder cancer is a key issue that must be resolved in order to improve disease prognosis [28–31].
Fig. 1.
A schematic diagram illustrating the classification of bladder cancer: Tis carcinoma in situ (‘flat tumour’); T1 tumour invades subepithelial connective tissue; T2 tumour invades muscle; T3 tumour invades perivesical tissue; T4 tumour invades prostate, uterus, or vagina
Determining the appropriate course of treatment depends on the stage of bladder cancer [32, 33]. In the case of non-invasive bladder tumors, the gold standard of primary therapy is transurethral resection of the bladder tumor (TUR-BT), which allows the bladder to retain functionality; however, TUR-BT commonly results in tumor relapse [21]. This has led to the widespread use of Bacillus Calmette–Guérin (BCG) [34] or chemotherapy agents [35], such as Mitomycin C, Adriamycin, Epirubicin or Thiotepa, as adjuvant therapies through intravesical instillation. Chemical agents [36–38], such as Mitomycin C, can restrain the DNA, RNA, and proteins to suppress the proliferation of cancer cells. However, these agents have a number of negative side effects, such as chemical cystitis and other irritating symptoms. Furthermore, long-term chemical cystitis can lead to bladder contraction and a reduction in functionality. Patients suffering from unfavorable differentiation associated with the recurrences of cancer are generally administered intravesical therapy in the form of immune agent BCG [39, 40]. Adverse reactions to these chemical therapies include cystitis (67 %), fever (25 %), haematuria (23 %), and/or increased frequency of urination (71 %) [41]. In some cases, the use of these treatments has been associated with severe toxicity, leading to septicemia, disseminated intravascular coagulation, and multiple organ failure, which can reduce a patient’s desire to be treated [42–44].
Despite the fact that BCG is currently the most commonly used intravesical therapy in superficial bladder cancer treatment, many studies have reported that this agent is only able to delay early recurrence [45]. Furthermore, the widespread use of BCG and chemotherapy causes bladder cancer patients to suffer from high rates of recurrence and rapid disease progression [46, 47]. Thus, compared with other instillation agents, BCG appears somewhat limited in its therapeutic effectiveness [48]. Conversely, nanoparticle technology, which has been used in recent years, has shown great promise in increasing the efficacy of drugs and preventing adverse reactions. The field of medicine stands to benefit significantly from advances in nanotechnology, specifically from improvements in detection imaging and tumor therapy [49]. Nanoparticle technologies were developed to be controlled drug delivery systems with unique targeting for cancer treatment [50]. Many kinds of delivery systems have been developed for bladder cancer therapy using different materials and types of nanoparticles, such as metal/gold, polymeric, liposome and lipid, and protein nanoparticles.
Application of Nanoparticles in Urinary Bladder Cancer
Metal/Gold Nanoparticles
Metal nanoparticles are widely used in engineering as well as biomedical sciences [51]. These include magnetic nanoparticles “Fe3O4, Fe-Au alloy” [52, 53] as well as gold [54] and silver [55] nanoparticles, which may be conjugated with antibodies, ligands, or other drugs in order to modify functional groups [56]. Important developments are being made in the field of nanotechnology for applications in magnetic separation, the enrichment of the target analytes, targeted drug delivery, targeted gene delivery, and diagnostic imaging. Important imaging modalities which aid in the visualization of disease states, include MRI, CT, PET, ultrasound, SERS, and optical imaging [57–61].
Because of outstanding biocompatibility, gold was among the first metallic biomaterial to be developed [62]. Furthermore, gold nanoparticles (GNPs) have strong spectral absorption properties when the diameter of the gold particle is smaller than the wavelength of the incoming ray [63]. When nanoparticles absorb energy at a specific wavelength, conduction band electrons from the surface of the particle become polarized and produce instantaneous dipole forces, leading to coherent dipole oscillation in a phenomenon referred to as surface plasma resonance (SPR) [64–66]. Various factors affect the properties of SPR, such as size, shape of the nanoparticles as well as other variables related to chemical structure. The absorption wavelength presents a non-linear red shift associated with the diameter or aspect ratio of the gold nanoparticles.
Laser induced thermotherapy involves the use of a laser to induce heat in tissue, which in-turn leads to coagulative necrosis that destroys tumor cells [67–69]. Plasmonic photothermal therapy (PPTT) applies the optical properties of SPR to assist laser-induced thermotherapy. This technique uses GNPs to enhance the effects of target therapy [69, 70]. Specifically, GNPs absorb specific wavelengths of light suitable for the generation of thermal energy while enhancing spatial selectivity in the application of hyperthermia therapy. One novel adjuvant therapy based on heat effects involves the use of specially designed nanomaterials with high photothermal conversion capability, such as nanospheres, nanoshells, nanorods, nanocages [69]. This treatment provides obvious benefits even after a short treatment time and achieves a hyperthermic state with relatively low laser power, thereby avoiding injury to adjacent healthy tissue.
GNPs provide excellent biocompatibility, modulability, and optical properties [71, 72]. In addition, GNPs are able to modify particular nucleic acids and protein molecules to facilitate the rapid detection of abnormal genes or cancer cells, which makes it possible to diagnose diseases more quickly and easily [73]. The superior photothermal properties of GNPs, compared with other nanoparticles (e.g. core–shell silica nanoparticles, magnetic nanoparticles, cerium oxide “CeO2, TiO2, ZnO”, and quantum dots), has resulted in a gradual shift from these materials to the use of GNPs [69]. GNPs also provide excellent chemical stability and a strong affinity to biomolecules, which facilitates the detection and treatment of cancer. Indeed, the biocompatibility and non-cytotoxic properties of GNPs have the greatest potential for future clinical applications. Thus far, GNPs have been most widely applied in the treatment of breast cancer, oral cavity cancer, lung cancer, cervical and brain cancer [74–76]. Indeed, results from previous studies have revealed that GNPs offer obvious therapeutic benefits to cancer patients. For example, in one study, exposure to a laser was shown to damage cancer cells; however, GNPs require only half the laser power of regular laser treatment [69]. Combining particles with antibodies or proteins that target the overexpressed antigen in the tumor has also been shown to enhance the therapeutic benefits [69].
Previous studies have reported the application of modified gold nanoparticles in bladder cancer. These modified nanoparticles include gum arabic-coated radioactive [77], hyaluronic acid functionalized fluorescent [54], epigallocatechin-3-gallate [75], and antibody-coated silica nanoshells [78, 79]. To destroy tumor cells and preserve normal cells, a targeting system is required. In target therapy, monoclonal antibodies serve as the aiming system of nanoparticles. For different cancer cells, individualized targets are chosen according to the expression of antigens, such as transferring, Her-2, and epidermal growth factor receptor (EGFR) in breast cancer [80–82] and EGFR in oral and epidermal cancers [83, 84]. Globally, bladder cancer is a common cause of carcinomatosis. To target bladder cancer, EGFR, mucin 7, and cytokeratin 20 are commonly used [85–87]. Figure 2, the TEM, shows the bladder cancer cell is targeted by antibody modified GNPs and some endocytosis is found.
Fig. 2.
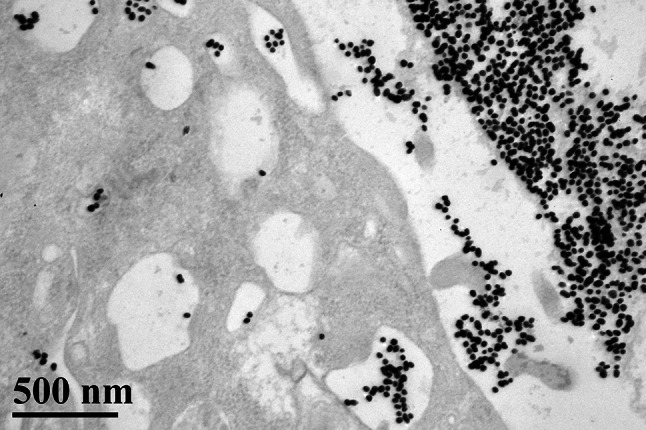
TEM (×60,000) illustrates that the bladder cancer cell is targeted by antibody (anti-EGFR) modified GNPs. Endocytosis is noticed
Polymeric Nanoparticles
Polymeric nanoparticles can be made from a wide range of polymers, including natural or synthetic substances composed of macromolecules such as “poly(lactide-coglycolide)”, “poly(lactic acid)”, “poly(ε-caprolactone)”, “chitosan”, and “poly(alkyl cyanoacrylates)” [88–91]. However, many polymeric nanoparticles are toxic to patients; therefore, improving biocompatibility and reducing cytotoxicity of polymeric nanoparticles is imperative for biomedical applications. The composition of polymeric nanoparticles can be varied for the delivery of specific drugs to the surface of specific cells [91]. The first step in using a polymeric carrier is the design a polymeric structure that is biodegradable to ensure that they retain their properties in vivo only for as long as needed. Specifically, biodegradability ensures that polymeric carriers degrade into small molecules that can be metabolized and excreted from the body. Previous studies on the treatment of bladder cancer with drugs formulated by polymeric nanoparticles have shown considerable promise [92, 93]. Compared with other drug delivery systems, polymeric nano-carriers are easier to synthesize, less expensive, and provide superior biocompatibility and biodegradability. They are also non-immunogenic, non-toxic, and water soluble.
Liposome and Lipid Nanoparticles
Liposomes are artificially-synthesized mono-layer or bi-layer phospholipid vesicles, which have been developed for the transport of molecules, such as drug molecules, nucleotides, protein, and plasmids [94, 95]. Previous studies have indicated that large negatively-charged multilamellar vesicles improve binding affinity and increase the inhibition of four various human bladder tumor cell lines: 253J, J82, T24, and TCCSUP [96, 97].
Oncogene overexpression is one of the major causes of urothelial carcinoma; therefore, the silencing of oncogenes via small interfering RNA (siRNA) coated with liposomes may provide an effective approach to the prevention of bladder cancer [98, 99]. Moreover, the intravesical instillation of liposomes encapsulated with cytotoxic agents has been found to improve the efficacy of intravesical therapies used in the treatment of bladder cancer [100, 101]. Indeed, one highly feasible treatment modality involves intravesical administration of plasmid-containing liposomes, such as IL-2 [102, 103], IL-4 [104], IL-12 [105], interferon-gamma [106], and granulocyte macrophage colony-stimulating factor [107]. Furthermore, in a number of clinical trials, it has been found that intravesical liposomes have similar therapeutic efficacy and can improve the pain score of patients without unanticipated adverse effects [108, 109].
Two lipid nanoparticle systems have previously been applied in cancer therapy: solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs), both of which are composed of lipids instead of phospholipids [110–112]. SLNs are prepared from a single purified lipid and forma crystalline lattice which allows the incorporation of small molecular drugs. NLCs allow a mixture of lipid types to create a lipid matrix as imperfect as possible. Because of their unique size dependent properties, SLNs are at the forefront of the rapidly developing field of nanotechnology, with numerous potential applications in drug delivery [113]. The ability to incorporate drugs into nanocarriers offers a new vehicle for drug delivery that could be used to improve drug targeting. Indeed, over the past few years, nanostructured lipid carriers have been attracting considerable interest as alternative carriers for anticancer pharmaceuticals. However, many anticancer mixtures are limited with regard to solubility and specificity and are toxic to normal tissue [114]. These mixtures are also associated with poor specificity and steadiness, pharmaceutical resistance, rapid degradation, the need for large-scale output procedures, a fast release of the pharmaceutical from its carrier scheme, the residues of the organic solvents utilized in the output method and the toxicity from the polymer with esteem to the carrier scheme are anticipated to be overcome through use of the nanostructured lipid carrier.
Protein Nanoparticles
Colloidal drug carrier systems provide selective drug targeting through the use of modified protein nanoparticles, which reduces the effects of drug toxicity [78, 115]. Protein materials used in vivo solves the enzyme-induced degradable problem and provides considerable advantages over colloidal carriers, such as liposomes and cell ghosts. They are composed of biological components capable of delivering a range of molecules, both large and small. Indeed, protein nanoparticles have already been employed as pharmaceutical carriers in a number of cancer therapies [97, 116]. For example, protein nanoparticles can be used in the delivery of protein therapeutics to the lung. They can also be incorporated into biodegradable polymer microspheres/nanospheres to facilitate the controlled release depot or oral delivery. Many researchers are currently focused on the preparation of nanoparticles using proteins such as albumin, gelatin, gliadin, and legumin. In intravesical therapy, commercial paclitaxel contains Cremophor, which can cause micelle formation and interfere with the transportation of paclitaxel across the urothelium. To improve the delivery of paclitaxel in intravesical therapy against bladder cancer, Lu et al. [117] developed a paclitaxel-loaded gelatin protein nanoparticle. Results from that study as well as other research have demonstrated the potential of protein nanoparticles as drug delivery systems for parenteral, peroral, and ocular administration, and may also be a vaccine adjuvant.
Discussion and Conclusions
The aim of using nanoparticles in cancer treatment is to increase drug specificity and thereby improve treatment outcomes. Indeed, the unique properties of metallic, polymeric, lipid, and protein nanoparticles have been shown to provide considerable benefits in the treatment of superficial urothelial cancer. Intravesical drug delivery is superior to oral therapy in the treatment of bladder cancer, which enables the administration of drugs directly to bladder lesions and reduces the risk of systemic side effects [118]. However, intravesical therapy has limited therapeutic efficacy due to the bladder permeability barrier and periodic bladder discharge. Fortunately, the development of nanoparticles as pharmaceutical carriers has helped to overcome many of these disadvantages. Previous evidence (mostly from animal studies) has supported the application of nanotechnology in intravesical therapy through the retention of drugs in the bladder and the enhancement of drug permeability in bladder cancer [117, 119]. Doubtlessly, nanoparticles will continue to play a dominant role in the coming generations of intravesical therapy against bladder diseases. However, to reduce the risk of distant metastasis, the standard treatment for muscle-invasive bladder cancer remains radical cystectomy, as opposed to regional or systemic drug therapy. As a result, the focus in the application of nanotechnology in bladder cancer treatment remains on non-muscle invasive forms of the disease. Adjuvant therapy in conjunction with nanoparticles lowers the rate of recurrence and reduces the risk of negative side effects associated with traditional intravesical chemotherapy and immune therapy [69].
The excellent photothermal properties of GNPs have led to their application in a variety of treatment techniques which target cancer cells [69, 120, 121]. For example, GNPs can be used to rapidly detect abnormal genes or cancer cells, which improves cancer diagnosis and treatment. In addition, GNPs possess good biocompatibility, good modulability, non-cytotoxicity, and highly specific optical properties. These characteristics suggest that GNPs can benefit a far wider range of clinical applications. Furthermore, differences in electrical charges between GNPs and proteins allow antibody fragments to be conjugated with GNPs [69, 83, 122]. Thus, developing an improved nanoparticle system capable of delivering intact drugs or molecules to the urothelium without severe side effects is a worthy goal for future research.
References
- 1.Wu B, Cao X, Liang X, Zhang X, Zhang W, Sunand G, Wang D. Epigenetic regulation of Elf5 is associated with epithelial-mesenchymal transition in urothelial cancer. PLoS One. 2015;10:e0117510. doi: 10.1371/journal.pone.0117510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katoh T, Kaneko S, Takasawa S, Nagata N, Inatomi H, Ikemura K, Itoh H, Matsumoto T, Kawamotoand T, Bell DA. Human glutathione S-transferase P1 polymorphism and susceptibility to smoking related epithelial cancer; oral, lung, gastric, colorectal and urothelial cancer. Pharmacogenetics and Genomics. 1999;9:165–169. [PubMed] [Google Scholar]
- 3.Zeng X, Morgensternand R, Nystrom AM. Nanoparticle-directed sub-cellular localization of doxorubicin and the sensitization breast cancer cells by circumventing GST-mediated drug resistance. Biomaterials. 2014;35:1227–1239. doi: 10.1016/j.biomaterials.2013.10.042. [DOI] [PubMed] [Google Scholar]
- 4.Damascelli B, Patelli G, Ticha V, Di Tolla G, Frigerio LF, Garbagnati F, Lanocita R, Marchiano A, Spreafico C, Mattavelli F, Brunoand A, Zunino F. Feasibility and efficacy of percutaneous transcatheter intraarterial chemotherapy with paclitaxel in albumin nanoparticles for advanced squamous-cell carcinoma of the oral cavity, oropharynx, and hypopharynx. Journal of Vascular and Interventional Radiology. 2007;18:1395–1403. doi: 10.1016/j.jvir.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Wehrungand D, Oyewumi MO. Antitumor effect of novel gallium compounds and efficacy of nanoparticle-mediated gallium delivery in lung cancer. Journal of Biomedical Nanotechnology. 2012;8:161–171. doi: 10.1166/jbn.2012.1361. [DOI] [PubMed] [Google Scholar]
- 6.Das S, Jagan L, Isiah R, Rajesh B, Backianathanand S, Subhashini J. Nanotechnology in oncology: Characterization and in vitro release kinetics of cisplatin-loaded albumin nanoparticles: Implications in anticancer drug delivery. Indian Journal of Pharmacology. 2011;43:409–413. doi: 10.4103/0253-7613.83111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yin PT, Shahand BP, Lee KB. Combined magnetic nanoparticle-based microRNA and hyperthermia therapy to enhance apoptosis in brain cancer cells. Small (Weinheim an der Bergstrasse, Germany) 2014;10:4106–4112. doi: 10.1002/smll.201400963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wuand D, Navrotsky A. Probing the energetics of organic-nanoparticle interactions of ethanol on calcite. Proceedings of the National Academy of Sciences. 2015;112(17):5314–5318. doi: 10.1073/pnas.1505874112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu Y, Dorinand RM, Wiesner U. Asymmetric organic-inorganic hybrid membrane formation via block copolymer-nanoparticle co-assembly. Nano Letters. 2013;13:5323–5328. doi: 10.1021/nl402829p. [DOI] [PubMed] [Google Scholar]
- 10.Tamaru M, Akita H, Kajimoto K, Sato Y, Hatakeyamaand H, Harashima H. An apolipoprotein E modified liposomal nanoparticle: ligand dependent efficiency as a siRNA delivery carrier for mouse-derived brain endothelial cells. International Journal of Pharmaceutics. 2014;465:77–82. doi: 10.1016/j.ijpharm.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 11.Appel EA, Tibbitt MW, Webber MJ, Mattix BA, Veisehand O, Langer R. Self-assembled hydrogels utilizing polymer-nanoparticle interactions. Nature Communications. 2015;6:6295. doi: 10.1038/ncomms7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao H, Stefanick JF, Jia X, Jing X, Kiziltepe T, Zhangand Y, Bilgicer B. Micellar nanoparticle formation via electrostatic interactions for delivering multinuclear platinum(II) drugs. Chemical Communications (Camb) 2013;49:4809–4811. doi: 10.1039/c3cc39119a. [DOI] [PubMed] [Google Scholar]
- 13.Alexis F, Pridgen EM, Langerand R, Farokhzad OC. Nanoparticle technologies for cancer therapy. Handbook of Experimental Pharmacology. 2010;197:55–86. doi: 10.1007/978-3-642-00477-3_2. [DOI] [PubMed] [Google Scholar]
- 14.Aziz A, Shariat SF, Roghmann F, Brookman-May S, Stief CG, Rink M, Chun FK, Fisch M, Novotny V, Froehner M, Wirth MP, Schnabel MJ, Fritsche HM, Burger M, Pycha A, Brisuda A, Babjuk M, Vallo S, Haferkamp A, Roigas J, Noldus J, Stredele R, Volkmer B, Bastian PJ, Xylinasand E, May M. Prediction of cancer-specific survival after radical cystectomy in pT4a urothelial carcinoma of the bladder—development of a tool for clinical decision-making. BJU International. 2014 doi: 10.1111/bju.12984. [DOI] [PubMed] [Google Scholar]
- 15.Sengupta N, Siddiquiand E, Mumtaz FH. Cancers of the bladder. The Journal of the Royal Society for the Promotion of Health. 2004;124:228–229. doi: 10.1177/146642400412400520. [DOI] [PubMed] [Google Scholar]
- 16.Siegel R, Naishadhamand D, Jemal A. Cancer statistics, 2012. CA: A Cancer Journal for Clinicians. 2012;62:10–29. doi: 10.1017/S0009840X11002678. [DOI] [PubMed] [Google Scholar]
- 17.Hongand YM, Loughlin KR. Economic impact of tumor markers in bladder cancer surveillance. Urology. 2008;71:131–135. doi: 10.1016/j.urology.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Wakuiand M, Shiigai T. Urinary tract cancer screening through analysis of urinary red blood cell volume distribution. International Journal of Urology. 2000;7:248–253. doi: 10.1046/j.1442-2042.2000.00184.x. [DOI] [PubMed] [Google Scholar]
- 19.Salawuand OT, Odaibo AB. Urogenital schistosomiasis and urological assessment of hematuria in preschool-aged children in rural communities of Nigeria. Journal of Pediatric Urology. 2014;10:88–93. doi: 10.1016/j.jpurol.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Gardner JM, Khurana H, Leach FS, Ayala AG, Zhaiand J, Ro JY. Adenocarcinoma in ectopic prostatic tissue at dome of bladder: a case report of a patient with urothelial carcinoma of the bladder and adenocarcinoma of the prostate. Archives of Pathology & Laboratory Medicine. 2010;134:1271–1275. doi: 10.5858/2009-0338-CR.1. [DOI] [PubMed] [Google Scholar]
- 21.Kondylis FI, Demirci S, Ladaga L, Kolmand P, Schellhammer PF. Outcomes after intravesical bacillus Calmette-Guerin are not affected by substaging of high grade T1 transitional cell carcinoma. The Journal of Urology. 2000;163:1120–1123. doi: 10.1016/S0022-5347(05)67706-3. [DOI] [PubMed] [Google Scholar]
- 22.Korossis S, Bolland F, Ingham E, Fisher J, Kearney J, Southgate J. Review: tissue engineering of the urinary bladder: considering structure-function relationships and the role of mechanotransduction. Tissue Engineering. 2006;12:635–644. doi: 10.1089/ten.2006.12.635. [DOI] [PubMed] [Google Scholar]
- 23.Amling CL. Diagnosis and management of superficial bladder cancer. Current Problems in Cancer. 2001;25:219–278. doi: 10.1067/mcn.2001.117539. [DOI] [PubMed] [Google Scholar]
- 24.Leung HY, Griffithsand TR, Neal DE. Bladder cancer. Postgraduate Medical Journal. 1996;72:719–724. doi: 10.1136/pgmj.72.854.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puntoni M, Zanardi S, Branchi D, Bruno S, Curotto A, Varaldo M, Bruzziand P, Decensi A. Prognostic effect of DNA aneuploidy from bladder washings in superficial bladder cancer. Cancer Epidemiology Biomarkers & Prevention. 2007;16:979–983. doi: 10.1158/1055-9965.EPI-06-0538. [DOI] [PubMed] [Google Scholar]
- 26.Kirkali Z, Chan T, Manoharan M, Algaba F, Busch C, Cheng L, Kiemeney L, Kriegmair M, Montironi R, Murphy WM, Sesterhenn IA, Tachibana M, Weider J. Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology. 2005;66:4–34. doi: 10.1016/j.urology.2005.07.062. [DOI] [PubMed] [Google Scholar]
- 27.Quintero A, Alvarez-Kindelan J, Luque RJ, Gonzalez-Campora R, Requena MJ, Montironiand R, Lopez-Beltran A. Ki-67 MIB1 labelling index and the prognosis of primary TaT1 urothelial cell carcinoma of the bladder. Journal of Clinical Pathology. 2006;59:83–88. doi: 10.1136/jcp.2004.022939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tavora F, Epstein JI. Bladder cancer, pathological classification and staging. BJU International. 2008;102:1216–1220. doi: 10.1111/j.1464-410X.2008.07962.x. [DOI] [PubMed] [Google Scholar]
- 29.Lamm D. Bladder cancer: improving care with better classification and risk stratification. Journal of Urology. 2007;178:1146–1147. doi: 10.1016/j.juro.2007.07.065. [DOI] [PubMed] [Google Scholar]
- 30.Nishiyama N, Kitamura H, Maeda T, Takahashi S, Masumori N, Hasegawa T, Tsukamoto T. Clinicopathological analysis of patients with non-muscle-invasive bladder cancer: prognostic value and clinical reliability of the 2004 WHO classification system. Japanese Journal of Clinical Oncology. 2013;43:1124–1131. doi: 10.1093/jjco/hyt120. [DOI] [PubMed] [Google Scholar]
- 31.Sacristan R, Gonzalez C, Fernandez-Gomez JM, Fresno F, Escafand S, Sanchez-Carbayo M. Molecular classification of non-muscle-invasive bladder cancer (pTa low-grade, pT1 low-grade, and pT1 high-grade subgroups) using methylation of tumor-suppressor genes. The Journal of Molecular Diagnostics. 2014;16:564–572. doi: 10.1016/j.jmoldx.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 32.Kamat AM, Witjes JA, Brausi M, Soloway M, Lamm D, Persad R, Buckley R, Bohle A, Colombel M, Palou J. Defining and treating the spectrum of intermediate risk nonmuscle invasive bladder cancer. The Journal of Urology. 2014;192:305–315. doi: 10.1016/j.juro.2014.02.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anastasiadisand A, De Reijke TM. Best practice in the treatment of nonmuscle invasive bladder cancer. Therapeutic Advances in Urology. 2012;4:13–32. doi: 10.1177/1756287211431976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel SG, Cohen A, Weinerand AB, Steinberg GD. Intravesical therapy for bladder cancer. Expert Opinion on Pharmacotherapy. 2015;16:889–901. doi: 10.1517/14656566.2015.1024656. [DOI] [PubMed] [Google Scholar]
- 35.Sirohi B, Singh A, Jagannathand P, Shrikhande SV. Chemotherapy and targeted therapy for gall bladder cancer. Indian Journal of Surgical Oncology. 2014;5:134–141. doi: 10.1007/s13193-014-0317-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xin Y, Lyness G, Chen D, Song S, Wientjes MG, Au JL. Low dose suramin as a chemosensitizer of bladder cancer to mitomycin C. The Journal of Urology. 2005;174:322–327. doi: 10.1097/01.ju.0000161594.86931.ea. [DOI] [PubMed] [Google Scholar]
- 37.Staalesen V, Leirvaag B, Lillehaugand JR, Lonning PE. Genetic and epigenetic changes in p21 and p21B do not correlate with resistance to doxorubicin or mitomycin and 5-fluorouracil in locally advanced breast cancer. Clinical Cancer Research. 2004;10:3438–3443. doi: 10.1158/1078-0432.CCR-03-0796. [DOI] [PubMed] [Google Scholar]
- 38.He LF, Guan KP, Yan Z, Ye HY, Xu KX, Renand L, Hou SK. Enhanced sensitivity to mitomycin C by abating heat shock protein 70 expression in human bladder cancer cell line of BIU-87. Chinese Medical Journal-Beijing-English Edition. 2005;118:1965–1972. [PubMed] [Google Scholar]
- 39.Ehdaie B, Sylvesterand R, Herr HW. Maintenance bacillus Calmette-Guerin treatment of non-muscle-invasive bladder cancer: a critical evaluation of the evidence. European Urology. 2013;64:579–585. doi: 10.1016/j.eururo.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 40.Sylvester RJ. Bacillus Calmette-Guerin treatment of non-muscle invasive bladder cancer. International Journal of Urology. 2011;18:113–120. doi: 10.1111/j.1442-2042.2010.02678.x. [DOI] [PubMed] [Google Scholar]
- 41.Shelley MD, Kynaston H, Court J, Wilt TJ, Coles B, Burgonand K, Mason MD. A systematic review of intravesical bacillus Calmette-Guerin plus transurethral resection vs transurethral resection alone in Ta and T1 bladder cancer. BJU International. 2001;88:209–216. doi: 10.1046/j.1464-410x.2001.02306.x. [DOI] [PubMed] [Google Scholar]
- 42.Bohle A, Jochamand D, Bock PR. Intravesical bacillus Calmette-Guerin versus mitomycin C for superficial bladder cancer: a formal meta-analysis of comparative studies on recurrence and toxicity. Journal of Urology. 2003;169:90–95. doi: 10.1016/S0022-5347(05)64043-8. [DOI] [PubMed] [Google Scholar]
- 43.Schreinemachers LM, Van Der Meijden AP, Wagenaar J, Steerenberg PA, Feitz WF, Groothuis DG, Tiesjema RH, De Jong WH, Debruyneand FM, Ruitenberg EJ. Intravesical and intradermal Bacillus Calmette-Guerin application. A phase I study to the toxicity of a Dutch Bacillus Calmette-Guerin preparation in patients with superficial bladder cancer. European Urology. 1988;14:15–21. [PubMed] [Google Scholar]
- 44.Filardi MJ, Codish SD, Civerchia L, Howardand RK, Mckneally MF. Toxicity of intrapleural Bacillus Calmette-Guerin treatment in animals. Cancer Research. 1979;39:3673–3676. [PubMed] [Google Scholar]
- 45.Gardmark T, Jahnson S, Wahlquist R, Wijkstromand H, Malmstrom PU. Analysis of progression and survival after 10 years of a randomized prospective study comparing mitomycin-C and bacillus Calmette-Guerin in patients with high-risk bladder cancer. BJU International. 2007;99:817–820. doi: 10.1111/j.1464-410X.2006.06706.x. [DOI] [PubMed] [Google Scholar]
- 46.Lodde M, Mian C, Mayr R, Comploj E, Trenti E, Melotti R, Campodonico F, Maffezzini M, Fritscheand HM, Pycha A. Recurrence and progression in patients with non-muscle invasive bladder cancer: prognostic models including multicolor fluorescence in situ hybridization molecular grading. International Journal of Urology. 2014;21:968–972. doi: 10.1111/iju.12509. [DOI] [PubMed] [Google Scholar]
- 47.Takashi M, Wakai K, Hattori T, Onoand Y, Ohshima S. Evaluation of multiple recurrence events in superficial bladder cancer patients treated with intravesical bacillus Calmette-Guerin therapy using the Andersen-Gill’s model. International Urology and Nephrology. 2002;34:329–334. doi: 10.1023/A:1024431519652. [DOI] [PubMed] [Google Scholar]
- 48.Sylvester RJ, Brausi MA, Kirkels WJ, Hoeltl W, Calais Da Silva F, Powell PH, Prescott S, Kirkali Z, Van De Beek C, Gorlia T, De Reijkeand TM, E. G.-U. T. C. Group Long-term efficacy results of EORTC genito-urinary group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus Calmette-Guerin, and bacillus Calmette-Guerin plus isoniazid in patients with intermediate- and high-risk stage Ta T1 urothelial carcinoma of the bladder. European Urology. 2010;57:766–773. doi: 10.1016/j.eururo.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aliabadi HM, Shahin M, Brocksand DR, Lavasanifar A. Disposition of drugs in block copolymer micelle delivery systems: from discovery to recovery. Clinical Pharmacokinetics. 2008;47:619–634. doi: 10.2165/00003088-200847100-00001. [DOI] [PubMed] [Google Scholar]
- 50.Dinauer N, Balthasar S, Weber C, Kreuter J, Langerand K, Von Briesen H. Selective targeting of antibody-conjugated nanoparticles to leukemic cells and primary T-lymphocytes. Biomaterials. 2005;26:5898–5906. doi: 10.1016/j.biomaterials.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 51.Nossier AI, Eissa S, Ismail MF, Hamdyand MA, Azzazy HM. Direct detection of hyaluronidase in urine using cationic gold nanoparticles: a potential diagnostic test for bladder cancer. Biosensors & Bioelectronics. 2014;54:7–14. doi: 10.1016/j.bios.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 52.Zhang D, Sun P, Li P, Xue A, Zhang X, Zhangand H, Jin X. A magnetic chitosan hydrogel for sustained and prolonged delivery of Bacillus Calmette-Guerin in the treatment of bladder cancer. Biomaterials. 2013;34:10258–10266. doi: 10.1016/j.biomaterials.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 53.Chung, R.-J., Wangand, H.-Y., Wu, K.-T. (2014). Preparation and characterization of Fe-Au alloy nanoparticles for hyperthermia application. Journal of Medical and Biological Engineering.
- 54.Cheng D, Han W, Yang K, Song Y, Jiangand M, Song E. One-step facile synthesis of hyaluronic acid functionalized fluorescent gold nanoprobes sensitive to hyaluronidase in urine specimen from bladder cancer patients. Talanta. 2014;130:408–414. doi: 10.1016/j.talanta.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 55.Boucher W, Stern JM, Kotsinyan V, Kempuraj D, Papaliodis D, Cohenand MS, Theoharides TC. Intravesical nanocrystalline silver decreases experimental bladder inflammation. Journal of Urology. 2008;179:1598–1602. doi: 10.1016/j.juro.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 56.Wang AZ, Gu F, Zhang L, Chan JM, Radovic-Moreno A, Shaikhand MR, Farokhzad OC. Biofunctionalized targeted nanoparticles for therapeutic applications. Expert Opinion on Biological Therapy. 2008;8:1063–1070. doi: 10.1517/14712598.8.8.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muir BW, Acharya DP, Kennedy DF, Mulet X, Evans RA, Pereira SM, Wark KL, Boyd BJ, Nguyen TH, Hinton TM, Waddington LJ, Kirby N, Wright DK, Wang HX, Eganand GF, Moffat BA. Metal-free and MRI visible theranostic lyotropic liquid crystal nitroxide-based nanoparticles. Biomaterials. 2012;33:2723–2733. doi: 10.1016/j.biomaterials.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 58.Mizukoshi Y, Tsuru Y, Tominaga A, Seino S, Masahashi N, Tanabeand S, Yamamoto TA. Sonochemical immobilization of noble metal nanoparticles on the surface of maghemite: mechanism and morphological control of the products. Ultrasonics Sonochemistry. 2008;15:875–880. doi: 10.1016/j.ultsonch.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 59.Imran M, Lee KG, Imtiaz Q, Kim BK, Han M, Choand BG, Kim DH. Metal-oxide-doped silica nanoparticles for the catalytic glycolysis of polyethylene terephthalate. Journal of Nanoscience and Nanotechnology. 2011;11:824–828. doi: 10.1166/jnn.2011.3201. [DOI] [PubMed] [Google Scholar]
- 60.Liuand S, Han MY. Silica-coated metal nanoparticles. Chemistry, An Asian Journal. 2010;5:36–45. doi: 10.1002/asia.200900228. [DOI] [PubMed] [Google Scholar]
- 61.Ando J, Yano TA, Fujitaand K, Kawata S. Metal nanoparticles for nano-imaging and nano-analysis. Physical Chemistry Chemical Physics: PCCP. 2013;15:13713–13722. doi: 10.1039/c3cp51806j. [DOI] [PubMed] [Google Scholar]
- 62.Santos-Martinez MJ, Rahme K, Corbalan JJ, Faulkner C, Holmes JD, Tajber L, Medina C, Radomski MW. Pegylation increases platelet biocompatibility of gold nanoparticles. Journal of Biomedical Nanotechnology. 2014;10:1004–1015. doi: 10.1166/jbn.2014.1813. [DOI] [PubMed] [Google Scholar]
- 63.Aiboushev A, Gostev F, Shelaev I, Kostrov A, Kanaev A, Museur L, Traore M, Sarkisovand O, Nadtochenko V. Spectral properties of the surface plasmon resonance and electron injection from gold nanoparticles to TiO2 mesoporous film: femtosecond study. Photochemical & Photobiological Sciences. 2013;12:631–637. doi: 10.1039/C2PP25227A. [DOI] [PubMed] [Google Scholar]
- 64.Leeand KS, El-Sayed MA. Gold and silver nanoparticles in sensing and imaging: sensitivity of plasmon response to size, shape, and metal composition. The Journal of Physical Chemistry B. 2006;110:19220–19225. doi: 10.1021/jp062536y. [DOI] [PubMed] [Google Scholar]
- 65.Springer T, Homola J. Biofunctionalized gold nanoparticles for SPR-biosensor-based detection of CEA in blood plasma. Analytical and Bioanalytical Chemistry. 2012;404:2869–2875. doi: 10.1007/s00216-012-6308-9. [DOI] [PubMed] [Google Scholar]
- 66.Gao H, Shen W, Lu C, Liangand H, Yuan Q. Surface plasmon resonance additivity of gold nanoparticles for colorimetric identification of cysteine and homocysteine in biological fluids. Talanta. 2013;115:1–5. doi: 10.1016/j.talanta.2013.03.073. [DOI] [PubMed] [Google Scholar]
- 67.Huang X, Jain PK, El-Sayedand IH, El-Sayed MA. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers in Medical Science. 2008;23:217–228. doi: 10.1007/s10103-007-0470-x. [DOI] [PubMed] [Google Scholar]
- 68.Huang X, Jain PK, El-Sayedand IH, El-Sayed MA. Gold nanoparticles: interesting optical properties and recent applications in cancer diagnostics and therapy. Nanomedicine (London). 2007;2:681–693. doi: 10.2217/17435889.2.5.681. [DOI] [PubMed] [Google Scholar]
- 69.Chen CH, Wuand YJ, Chen JJ. Gold nanotheranostics: photothermal therapy and imaging of mucin 7 conjugated antibody nanoparticles for urothelial cancer. BioMed Research International. 2015;2015:813632. doi: 10.1155/2015/813632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nergiz SZ, Gandra N, Tadepalliand S, Singamaneni S. Multifunctional hybrid nanopatches of graphene oxide and gold nanostars for ultraefficient photothermal cancer therapy. ACS Applied Materials & Interfaces. 2014;6:16395–16402. doi: 10.1021/am504795d. [DOI] [PubMed] [Google Scholar]
- 71.Pooja D, Panyaram S, Kulhari H, Rachamallaand SS, Sistla R. Xanthan gum stabilized gold nanoparticles: characterization, biocompatibility, stability and cytotoxicity. Carbohydrate Polymers. 2014;110:1–9. doi: 10.1016/j.carbpol.2014.03.041. [DOI] [PubMed] [Google Scholar]
- 72.Osvath Z, Deak A, Kertesz K, Molnar G, Vertesy G, Zambo D, Hwang C, Biro LP. The structure and properties of graphene on gold nanoparticles. Nanoscale. 2015;7:5503–5509. doi: 10.1039/C5NR00268K. [DOI] [PubMed] [Google Scholar]
- 73.Larguinhoand M, Baptista PV. Gold and silver nanoparticles for clinical diagnostics—from genomics to proteomics. Journal of Proteomics. 2012;75:2811–2823. doi: 10.1016/j.jprot.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 74.Eissa S, Shawky SM, Matboli M, Mohamedand S, Azzazy HM. Direct detection of unamplified hepatoma upregulated protein RNA in urine using gold nanoparticles for bladder cancer diagnosis. Clinical Biochemistry. 2014;47:104–110. doi: 10.1016/j.clinbiochem.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 75.Hsieh DS, Wang H, Tan SW, Huang YH, Tsai CY, Yehand MK, Wu CJ. The treatment of bladder cancer in a mouse model by epigallocatechin-3-gallate-gold nanoparticles. Biomaterials. 2011;32:7633–7640. doi: 10.1016/j.biomaterials.2011.06.073. [DOI] [PubMed] [Google Scholar]
- 76.Peng G, Tisch U, Adams O, Hakim M, Shehada N, Broza YY, Billan S, Abdah-Bortnyak R, Kutenand A, Haick H. Diagnosing lung cancer in exhaled breath using gold nanoparticles. Nature Nanotechnology. 2009;4:669–673. doi: 10.1038/nnano.2009.235. [DOI] [PubMed] [Google Scholar]
- 77.Axiak-Bechtel SM, Upendran A, Lattimer JC, Kelsey J, Cutler CS, Selting KA, Bryan JN, Henry CJ, Boote E, Tate DJ, Bryan ME, Kattiand KV, Kannan R. Gum arabic-coated radioactive gold nanoparticles cause no short-term local or systemic toxicity in the clinically relevant canine model of prostate cancer. International Journal of Nanomedicine. 2014;9:5001–5011. doi: 10.2147/IJN.S67333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Szlachcic A, Pala K, Zakrzewska M, Jakimowicz P, Wiedlocha A, Otlewski J. FGF1-gold nanoparticle conjugates targeting FGFR efficiently decrease cell viability upon NIR irradiation. International Journal of Nanomedicine. 2012;7:5915–5927. doi: 10.2147/IJN.S36575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cheng FY, Chenand CT, Yeh CS. Comparative efficiencies of photothermal destruction of malignant cells using antibody-coated silica@Au nanoshells, hollow Au/Ag nanospheres and Au nanorods. Nanotechnology. 2009;20:425104. doi: 10.1088/0957-4484/20/42/425104. [DOI] [PubMed] [Google Scholar]
- 80.Press MF, Finn RS, Cameron D, Di Leo A, Geyer CE, Villalobos IE, Santiago A, Guzman R, Gasparyan A, Ma Y, Danenberg K, Martin AM, Williams L, Oliva C, Stein S, Gagnon R, Arbushitesand M, Koehler MT. HER-2 gene amplification, HER-2 and epidermal growth factor receptor mRNA and protein expression, and lapatinib efficacy in women with metastatic breast cancer. Clinical Cancer Research. 2008;14:7861–7870. doi: 10.1158/1078-0432.CCR-08-1056. [DOI] [PubMed] [Google Scholar]
- 81.Eghtedari M, Liopo AV, Copland JA, Oraevskyand AA, Motamedi M. Engineering of hetero-functional gold nanorods for the in vivo molecular targeting of breast cancer cells. Nano Letters. 2009;9:287–291. doi: 10.1021/nl802915q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li JL, Wang L, Liu XY, Zhang ZP, Guo HC, Liuand WM, Tang SH. In vitro cancer cell imaging and therapy using transferrin-conjugated gold nanoparticles. Cancer Letters. 2009;274:319–326. doi: 10.1016/j.canlet.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 83.El-Sayed IH, Huangand X, El-Sayed MA. Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Letters. 2006;239:129–135. doi: 10.1016/j.canlet.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 84.O’neal DP, Hirsch LR, Halas NJ, Payne JD, West JL. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Letters. 2004;209:171–176. doi: 10.1016/j.canlet.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 85.Bhatia A, Dey P, Kumar Y, Gautam U, Kakkar N, Srinivasan R, Nijhawan R. Expression of cytokeratin 20 in urine cytology smears: a potential marker for the detection of urothelial carcinoma. Cytopathology : official Journal of the British Society for Clinical Cytology. 2007;18:84–86. doi: 10.1111/j.1365-2303.2006.00432.x. [DOI] [PubMed] [Google Scholar]
- 86.Kinjo M, Okegawa T, Horie S, Nutaharaand K, Higashihara E. Detection of circulating MUC7-positive cells by reverse transcription-polymerase chain reaction in bladder cancer patients. International Journal of Urology : Official Journal of the Japanese Urological Association. 2004;11:38–43. doi: 10.1111/j.1442-2042.2004.00739.x. [DOI] [PubMed] [Google Scholar]
- 87.Villares GJ, Zigler M, Blehm K, Bogdan C, Mcconkey D, Colinand D, Bar-Eli M. Targeting EGFR in bladder cancer. World Journal of Urology. 2007;25:573–579. doi: 10.1007/s00345-007-0202-7. [DOI] [PubMed] [Google Scholar]
- 88.Kimand J, Van Der Bruggen B. The use of nanoparticles in polymeric and ceramic membrane structures: review of manufacturing procedures and performance improvement for water treatment. Environmental Pollution. 2010;158:2335–2349. doi: 10.1016/j.envpol.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 89.Connolly D, Currivanand S, Paull B. Polymeric monolithic materials modified with nanoparticles for separation and detection of biomolecules: a review. Proteomics. 2012;12:2904–2917. doi: 10.1002/pmic.201200142. [DOI] [PubMed] [Google Scholar]
- 90.Beck RC, Ourique AF, Guterres SS, Pohlmann AR. Spray-dried polymeric nanoparticles for pharmaceutics: a review of patents. Recent Patents on Drug Delivery & Formulation. 2012;6:195–208. doi: 10.2174/187221112802652651. [DOI] [PubMed] [Google Scholar]
- 91.Bian S, Lu W, Xu C, Fanand Y, Zhang X. In vitro cartilage tissue engineering using porous collagen/PLLA nanoparticle hybrid scaffold. Journal of Medical and Biological Engineering. 2014;34:36–43. doi: 10.5405/jmbe.1439. [DOI] [Google Scholar]
- 92.Huang C, Neoh KG, Xu L, Kangand ET, Chiong E. Polymeric nanoparticles with encapsulated superparamagnetic iron oxide and conjugated cisplatin for potential bladder cancer therapy. Biomacromolecules. 2012;13:2513–2520. doi: 10.1021/bm300739w. [DOI] [PubMed] [Google Scholar]
- 93.Yan X, Al-Hayek S, Huang H, Zhu Z, Zhuand W, Guo H. Photodynamic effect of 5-aminolevulinic acid-loaded nanoparticles on bladder cancer cells: a preliminary investigation. Scandinavian Journal of Urology. 2013;47:145–151. doi: 10.3109/00365599.2012.713000. [DOI] [PubMed] [Google Scholar]
- 94.Riaz M. Review article : stability and uses of liposomes. Pakistan Journal of Pharmaceutical Sciences. 1995;8:69–79. [PubMed] [Google Scholar]
- 95.Baileyand AL, Sullivan SM. Efficient encapsulation of DNA plasmids in small neutral liposomes induced by ethanol and calcium. Biochimica et Biophysica Acta. 2000;1468:239–252. doi: 10.1016/S0005-2736(00)00264-9. [DOI] [PubMed] [Google Scholar]
- 96.Johnson JW, Nayar R, Killion JJ, Von Eschenbachand AC, Fidler IJ. Binding of liposomes to human bladder tumor epithelial cell lines: implications for an intravesical drug delivery system for the treatment of bladder cancer. Selective Cancer Therapeutics. 1989;5:147–155. doi: 10.1089/sct.1989.5.147. [DOI] [PubMed] [Google Scholar]
- 97.Guhasarkarand S, Banerjee R. Intravesical drug delivery: Challenges, current status, opportunities and novel strategies. Journal of Controlled Release. 2010;148:147–159. doi: 10.1016/j.jconrel.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 98.Wu XR. Urothelial tumorigenesis: a tale of divergent pathways. Nature Reviews Cancer. 2005;5:713–725. doi: 10.1038/nrc1697. [DOI] [PubMed] [Google Scholar]
- 99.Barr FA, Silljeand HH, Nigg EA. Polo-like kinases and the orchestration of cell division. Nature Reviews Molecular Cell Biology. 2004;5:429–440. doi: 10.1038/nrm1401. [DOI] [PubMed] [Google Scholar]
- 100.Killion JJ, Fan D, Bucana CD, Frangos DN, Priceand JE, Fidler IJ. Augmentation of antiproliferative activity of interferon alfa against human bladder tumor cell lines by encapsulation of interferon alfa within liposomes. Journal of the National Cancer Institute. 1989;81:1387–1392. doi: 10.1093/jnci/81.18.1387. [DOI] [PubMed] [Google Scholar]
- 101.Frangos DN, Killion JJ, Fan D, Fishbeck R, Von Eschenbachand AC, Fidler IJ. The development of liposomes containing interferon alpha for the intravesical therapy of human superficial bladder cancer. Journal of Urology. 1990;143:1252–1256. doi: 10.1016/s0022-5347(17)40248-5. [DOI] [PubMed] [Google Scholar]
- 102.Connor J, Bannerji R, Saito S, Heston W, Fairand W, Gilboa E. Regression of bladder tumors in mice treated with interleukin 2 gene-modified tumor cells. Journal of Experimental Medicine. 1993;177:1127–1134. doi: 10.1084/jem.177.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Porgador A, Gansbacher B, Bannerji R, Tzehoval E, Gilboa E, Feldmanand M, Eisenbach L. Anti-metastatic vaccination of tumor-bearing mice with IL-2-gene-inserted tumor cells. International Journal of Cancer. 1993;53:471–477. doi: 10.1002/ijc.2910530320. [DOI] [PubMed] [Google Scholar]
- 104.Golumbek PT, Lazenby AJ, Levitsky HI, Jaffee LM, Karasuyama H, Bakerand M, Pardoll DM. Treatment of established renal cancer by tumor cells engineered to secrete interleukin-4. Science. 1991;254:713–716. doi: 10.1126/science.1948050. [DOI] [PubMed] [Google Scholar]
- 105.Brunda MJ, Luistro L, Warrier RR, Wright RB, Hubbard BR, Murphy M, Wolfand SF, Gately MK. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. Journal of Experimental Medicine. 1993;178:1223–1230. doi: 10.1084/jem.178.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Porgador A, Bannerji R, Watanabe Y, Feldman M, Gilboaand E, Eisenbach L. Antimetastatic vaccination of tumor-bearing mice with two types of IFN-gamma gene-inserted tumor cells. The Journal of Immunology. 1993;150:1458–1470. [PubMed] [Google Scholar]
- 107.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardolland D, Mulligan RC. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proceedings of the National Academy of Sciences. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chuang YC, Leeand WC, Chiang PH. Intravesical liposome versus oral pentosan polysulfate for interstitial cystitis/painful bladder syndrome. Journal of Urology. 2009;182:1393–1400. doi: 10.1016/j.juro.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 109.Lee WC, Chuangand YC, Chiang PH. Safety and dose flexibility clinical evaluation of intravesical liposome in patients with interstitial cystitis or painful bladder syndrome. Kaohsiung Journal of Medical Sciences. 2011;27:437–440. doi: 10.1016/j.kjms.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 110.Dasand S, Chaudhury A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech. 2011;12:62–76. doi: 10.1208/s12249-010-9563-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Das S, Ngand WK, Tan RB. Sucrose ester stabilized solid lipid nanoparticles and nanostructured lipid carriers. II. Evaluation of the imidazole antifungal drug-loaded nanoparticle dispersions and their gel formulations. Nanotechnology. 2014;25:105102. doi: 10.1088/0957-4484/25/10/105102. [DOI] [PubMed] [Google Scholar]
- 112.Souto EB, Wissing SA, Barbosaand CM, Muller RH. Comparative study between the viscoelastic behaviors of different lipid nanoparticle formulations. Journal of Cosmetic Science. 2004;55:463–471. [PubMed] [Google Scholar]
- 113.Kang MR, Yang G, Place RF, Charisse K, Epstein-Barash H, Manoharanand M, Li LC. Intravesical delivery of small activating RNA formulated into lipid nanoparticles inhibits orthotopic bladder tumor growth. Cancer Research. 2012;72:5069–5079. doi: 10.1158/0008-5472.CAN-12-1871. [DOI] [PubMed] [Google Scholar]
- 114.Nakamura T, Fukiage M, Higuchi M, Nakaya A, Yano I, Miyazaki J, Nishiyama H, Akaza H, Ito T, Hosokawa H, Nakayamaand T, Harashima H. Nanoparticulation of BCG-CWS for application to bladder cancer therapy. Journal of Controlled Release. 2014;176:44–53. doi: 10.1016/j.jconrel.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 115.Lohcharoenkal W, Wang L, Chen YC, Rojanasakul Y. Protein nanoparticles as drug delivery carriers for cancer therapy. BioMed Research International. 2014;2014:180549. doi: 10.1155/2014/180549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Weber C, Coester C, Kreuter J, Langer K. Desolvation process and surface characterisation of protein nanoparticles. International Journal of Pharmaceutics. 2000;194:91–102. doi: 10.1016/S0378-5173(99)00370-1. [DOI] [PubMed] [Google Scholar]
- 117.Lu Z, Yeh TK, Tsai M, Auand JL, Wientjes MG. Paclitaxel-loaded gelatin nanoparticles for intravesical bladder cancer therapy. Clinical Cancer Research. 2004;10:7677–7684. doi: 10.1158/1078-0432.CCR-04-1443. [DOI] [PubMed] [Google Scholar]
- 118.Zhou D, Zhangand G, Gan Z. c(RGDfK) decorated micellar drug delivery system for intravesical instilled chemotherapy of superficial bladder cancer. Journal of Controlled Release. 2013;169:204–210. doi: 10.1016/j.jconrel.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 119.Chang LC, Wu SC, Tsai JW, Yuand TJ, Tsai TR. Optimization of epirubicin nanoparticles using experimental design for enhanced intravesical drug delivery. International Journal of Pharmaceutics. 2009;376:195–203. doi: 10.1016/j.ijpharm.2009.04.045. [DOI] [PubMed] [Google Scholar]
- 120.Lu W, Arumugam SR, Senapati D, Singh AK, Arbneshi T, Khan SA, Yuand H, Ray PC. Multifunctional oval-shaped gold-nanoparticle-based selective detection of breast cancer cells using simple colorimetric and highly sensitive two-photon scattering assay. ACS Nano. 2010;4:1739–1749. doi: 10.1021/nn901742q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Von Maltzahn G, Park JH, Agrawal A, Bandaru NK, Das SK, Sailorand MJ, Bhatia SN. Computationally guided photothermal tumor therapy using long-circulating gold nanorod antennas. Cancer Research. 2009;69:3892–3900. doi: 10.1158/0008-5472.CAN-08-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ackerson CJ, Sykesand MT, Kornberg RD. Defined DNA/nanoparticle conjugates. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:13383–13385. doi: 10.1073/pnas.0506290102. [DOI] [PMC free article] [PubMed] [Google Scholar]