Abstract
Introduction
Systemic sclerosis (SSc) and mixed connective tissue disease (MCTD) are chronic immune-mediated disorders complicated by vascular organ damage. The aim of this study was to examine the serum levels of the markers of neoangiogenesis: endostatin and vascular endothelial growth factor (VEGF), in our unselected cohorts of SSc and MCTD.
Methods
Sera of SSc patients (N = 298) and MCTD patients (N = 162) from two longitudinal Norwegian cohorts were included. Blood donors were included as controls (N = 100). Circulating VEGF and endostatin were analyzed by enzyme immunoassay.
Results
Mean endostatin levels were increased in SSc patients 93.7 (37) ng/ml (P < .001) and MCTD patients 83.2 (25) ng/ml (P < .001) compared to controls 65.1 (12) ng/ml. Median VEGF levels were elevated in SSc patients 209.0 (202) pg/ml compared to MCTD patients 181.3 (175) pg/ml (P = .017) and controls 150.0 (145) pg/ml (P < .001). Multivariable analysis of SSc subsets showed that pulmonary arterial hypertension (coefficient 15.7, 95 % CI: 2.2–29.2, P = .023) and scleroderma renal crisis (coefficient 77.6, 95 % CI: 59.3–100.0, P < .001) were associated with elevated endostatin levels. Multivariable analyses of MCTD subsets showed that digital ulcers were associated with elevated endostatin levels (coefficient 10.5, 95 % CI: 3.2–17.8, P = .005). The risk of death increased by 1.6 per SD endostatin increase (95 % CI: 1.2–2.1, P = .001) in the SSc cohort and by 1.6 per SD endostatin increase (95 % CI: 1.0–2.4, P = .041) in the MCTD cohort after adjustments to known risk factors.
Conclusions
Endostatin levels were elevated in patients with SSc and MCTD, particularly SSc patients with pulmonary arterial hypertension and scleroderma renal crisis, and MCTD patients with digital ulcers. Elevated endostatin levels were also associated with increased all-cause mortality during follow-up in both groups of patients. We propose that endostatin might indicate the degree of vascular injury in SSc and MCTD patients.
Electronic supplementary material
The online version of this article (doi:10.1186/s13075-015-0756-5) contains supplementary material, which is available to authorized users.
Introduction
Systemic sclerosis (SSc) is a chronic multiorgan disease characterized by vasculopathy, progressive fibrosis of the skin and internal organs and distinct serum autoantibodies [1, 2]. The primary event in SSc is assumed to be vascular injury [3], which leads to clinical manifestations such as Raynaud’s phenomenon and digital ulcers [4]. Mortality is increased and mainly driven by pulmonary arterial hypertension (PAH) and pulmonary fibrosis [5]. Microangiopathy is thought to be responsible for the life-threatening organ involvement, such as PAH, scleroderma renal crisis (SRC), cardiomyopathy, gastric antrial vascular actasia [3] and possibly also pulmonary fibrosis [6].
Vascular injury has an impact in other connective tissue diseases (CTDs) and is particularly evident in mixed connective tissue disease (MCTD), a chronic immune-mediated disease associated with anti-U1-RNP autoantibodies and clinical features from SSc, systemic lupus erythematosus (SLE) and polymyositis (PM). MCTD appears to be genetically distinct from other CTDs [7], but there is an ongoing debate whether it should be categorized as a distinct disease, an overlap syndrome or an undifferentiated CTD [8]. Even though organ involvement in MCTD is more extensive than initially described, organ involvement is less severe than in SSc [9]. The vasculopathy in MCTD has been found to resemble the vasculopathy found in SSc [10] and it has been suggested that there is an association between pulmonary hypertension (PH) and anti-U1-RNP autoantibodies in SSc [11] and SLE [12]. Identifying SSc and MCTD patients at risk of developing serious vascular organ damage could improve patient outcome. Hence, there is a growing interest in markers that may predict vasculopathy [13].
In healthy tissue vascular injury causes hypoxia, which induces proteins in the vascular endothelial growth factor (VEGF) family. VEGF-A (usually referred to as VEGF) is released by a variety of cells including fibroblasts, macrophages, neutrophils, endothelial cells and T cells, and is involved in numerous steps of neoangiogenesis (13). Endostatin is the most potent inhibitor of VEGF-induced angiogenesis. It is a peptide derived from collagen XVIII, produced by fibroblasts and primarily found in the basement membranes of the skin and lungs [14], both of which are tissues involved in SSc and MCTD. Previous studies have shown increased serum levels of VEGF and endostatin in SSc [15] and MCTD [16], indicating an altered regulation of angiogenesis in these diseases. However, the previous studies of VEGF and endostatin in SSc and MCTD were performed in small-scale cohorts, and the correlation to clinical parameters has been somewhat discrepant [17–19] and some have been contradictive [17, 20]. An additional table shows previous data in more detail (see Additional file 1) [16–24].
The aim of this study was to assess the serum levels of endostatin and VEGF in two large, well-characterized and largely unselected longitudinal CTD cohorts; the Oslo University Hospital (OUH) SSc cohort [25, 26] and the Norwegian nationwide MCTD cohort [27–29]. Serum levels of endostatin and VEGF of SSc and MCTD patients were compared with controls. Our basic hypothesis was that VEGF and endostatin were associated with vasculopathy-related features like digital ulcers, PAH, SRC and possibly also pulmonary fibrosis, in both diseases. Hence, we wanted to explore the associations of these clinical parameters and all-cause mortality with endostatin and VEGF levels.
Methods
Study cohorts
Sera of SSc patients (N = 298) from the previously described Oslo University Hospital (OUH) SSc cohort were assessed [25, 30, 31]. The OUH SSc cohort is an observational prospective study cohort which includes all consenting SSc patients seen at OUH since 2008. Patients included in the cohort have annual follow-up visits at OUH where clinical parameters, pulmonary function tests (PFTs), lung high-resolution computed tomography (HRCT), echocardiography, and right-sided heart catheterization (RHC) data are systematically recorded and stored in the Norwegian Systemic Connective Tissue Disease and Vasculitides Registry (NOSVAR) [26]. Serum samples are taken at inclusion and stored in the NOSVAR biobank. All SSc patients included in this study fulfilled the 2013 European League Against Rheumatism/American College of Rheumatology (EULAR/ACR) classification criteria for SSc [25, 32].
Sera of MCTD patients (N = 162) from the previously described unselected Norwegian nationwide MCTD cohort [7, 27–29] (n = 135) and NOSVAR (n = 27) were similarly assessed. The Norwegian nationwide MCTD cohort recruited patients from Departments of Rheumatology from 2005 to 2008, while NOSVAR recruited patients from OUH from 2008 to 2012. Inclusion criteria were age 18 and fulfillment of at least one of the three criteria sets of MCTD, the modified Sharp’s criteria [33], the criteria of Alarcón-Segovia or Kasukawa [9], and the exclusion of another CTD [29]. Consenting blood donors from the OUH blood bank were included as controls (N = 100).
Clinical parameters
SSc patients were categorized as diffuse cutaneous SSc (dcSSc) or limited cutaneous SSc (lcSSc) [34]. Disease onset was defined as the onset of the first non-Raynaud’s symptom. Clinical parameters recorded included percentage of predicted full vital capacity (FVC) and percentage of predicted diffusing capacity of the lung for carbon monoxide (DLCO), pulmonary fibrosis (by HRCT: see below), digital ulcers, SRC and PH segregated into two well-defined groups; PAH and PH due to lung disease. In the MCTD cohort, clinical parameters included percentage of predicted FVC and DLCO, pulmonary fibrosis, digital ulcers, PAH and PH due to lung disease. Digital ulcers were scored positive if ulcers were present at least once during the disease course. Precapillary PH was defined according to the updated European Society of Cardiology (ESC) criteria by mean pulmonary artery pressure (mPAP) ≥25 mm HG and pulmonary wedge pressure ≤15 mm HG by RHC at rest [35]. Patients in our SSc cohort have an annual clinical follow-up at OUS, they are referred to RHC when it is clinically indicated based on physical examination, PFT results, 6-minute walk tests and echocardiogram results. Echocardiogram results were available in 294 patients (99 %). RHC was performed in 96 patients (32 %). In the analyses we included patients classified as having pulmonary arterial hypertension (PAH). PAH is characterized by the presence of precapillary PH in the absence of other causes of precapillary PH such as PH due to lung diseases, chronic thromboembolic PH, or other rare diseases [35]. Vital status at the end of the study was obtained from the National Population Register of Norway.
High-resolution computed tomography (HRCT) analysis of the lungs and pulmonary function tests (PFTs)
The presence of fibrosis was evaluated according to the CT criteria of interstitial lung disease recommended by The Nomenclature Committee of the Fleischner Society [36]. HRCT was obtained by inclusion in both cohorts, and reviewed on a Picture Archiving and Communication System (PACS) screen independently and in random order by two experienced readers. HRCT were available in 252 of 298 patients (85 %) in the SSc cohort and in 148 of 162 patients (91 %) in the MCTD cohort. Pulmonary function tests were performed according to established guidelines [37, 38].
Blood samples
Blood samples were centrifuged at room temperature after 30 minutes and serum aliquots were stored at −70 °C until assayed. Circulating VEGF and endostatin were analyzed by enzyme immunoassay (R&D Systems, Stillwater, MN, USA). The detection limit for endostatin was 80 pg/ml and intra- and interassay coefficients <10 %.
Statistical analyses
Serum levels of endostatin were compared by means (M) and standard deviation (SD) in all groups. Statistical differences between MCTD, SSc and controls were analyzed by one-way ANOVA. Post hoc comparisons were performed using Tukey’s test. Serum levels of VEGF did not have a normal distribution and were presented as median (Mdn) and interquartile range (IQR). Comparisons between the three groups were analyzed by Kruskal-Wallis test. Estimations of the effects of various clinical manifestations on serum endostatin levels were performed by linear regression analyses. We included clinical parameters with evident vasculopathy; digital ulcers, PAH and SRC. Since it has been proposed that vasculopathy is involved in pulmonary fibrosis this parameter was included together with the accompanying lung parameters; percentage of predicted FVC and percentage of predicted DLCO. Known risk factors for SRC and PAH was also included in the model. In the linear regression analyses we included patients with established PAH (N = 24) and SRC (N = 11) at the year of serum sampling. Univariable and multivariable logistic regression analyses were performed to explore the predictive value of endostatin. Patients who developed PAH and SRC the same year as serum sampling or later were included in the logistic regression analyses (N = 16 and N = 6, respectively). An additional graph shows when patients were diagnosed with PAH and SRC in relation to serum sampling (Additional file 2). Variables that were significant in univariable analyses were included in multivariable analysis. Using a manual backward elimination procedure, variables at a significant level of P < .25 in the univariable analyses were considered a candidate for the multivariable model in conjunction with age and gender. The association was quantified by the odds ratio (OR) with its 95 % confidence interval (CI). With regard to follow-up time, participants were followed from the date of inclusion in the cohort until death or end of follow-up on 31 October 2014. Kaplan-Meier survival curves were used to determine difference in survival between tertiles of endostatin levels and were estimated by the log-rank test. A multivariable Cox regression model was performed to control for multiple confounders. The proportional hazard assumptions were tested by plotting the logarithm of the integrated hazards (log–log survival plots). The effects were quantified by hazard ratio (HR) with its 95 % CI. Known risk factors were included in the multivariable logistic [39, 40] and Cox regression analyses [30, 41]. The significance level was set at P ≤ .05. Data extraction and analyses were conducted using SPSS version 22 (IBM SPSS, Armonk, NY, USA) and STATA version 22 (StataCorp, College Station, TX, USA).
Ethics
The study was approved by the Norwegian Regional Committee for Medical and Health Research Ethics and conducted in accordance with the guidelines of the Helsinki II declaration. All patients have given informed written consent to participate in the study.
Results
Serum endostatin and VEGF levels in the study cohorts
Circulating endostatin and VEGF levels were assessed in the OUH SSc cohort (N = 298) and in the Norwegian MCTD cohort (N = 162) (Table 1). Mean (SD) serum endostatin was higher in SSc 93.7 (37) ng/ml than MCTD 83.2 (25) ng/ml (P = .001) and controls 65.1 (12) ng/ml (P < .001). Mean serum endostatin was also higher in MCTD compared to controls (P < .001) (Fig. 1a). The SSc patients had higher median VEGF 209.0 (IQR 202) pg/ml than both MCTD 181.3 (175) pg/ml (P = .017) and controls 150.4 (145) pg/ml (P < .001). VEGF levels did not differ between MCTD and controls (Fig. 1b).
Table 1.
Demographics and clinical parameters of the MCTD and SSc cohorts
| SSc | MCTD | |
|---|---|---|
| Patients, N | 298 | 162 |
| Females, N (%) | 243 (82) | 128 (79) |
| Diffuse cutaneous SSc, N (%) | 78 (26) | N/A |
| Age at diagnosis, years, M (SD) | 48.3 (15.4) | 35.4 (15.7) |
| Age at blood sampling, M (SD) | 56.0 (13.8) | 44.7 (14.9) |
| Disease duration at sampling, years, Mdn (IQR) | 4.0 (9) | 7.0 (7) |
| Deceased, N (%) | 58 (20) | 14 (9) |
| Observation time, months, M (SD) | 55.0 (28.7) | 98.8 (27.4) |
| Classification criteria: | ||
| ACR/EULAR 2013 SSc, N (%) | 298 (100) | N/A |
| Alarcon, N (%) | N/A | 143 (88) |
| Sharp, N (%) | N/A | 151 (93) |
| Kasukawa, N (%) | N/A | 142 (88) |
| Pulmonary fibrosis at sampling, N (%) | 103/252 (40) | 52/148 (35) |
| % of predicted FVC, N (%) | 297 (100) | 146 (90) |
| % of predicted FVC, M (SD) | 94,7 (20,5) | 92.1 (18.5) |
| % of predicted DLCO, N (%) | 295 (99) | 142 (88) |
| % of predicted DLCO, M (SD) | 68.2 (21.7) | 73.8 (16.4) |
| Digital ulcers, N (%) | 132/278 (44) | 50/155 (32) |
| Precapillary pulmonary hypertension, N (%) | 44 (15) | 3 (.05) |
| Pulmonary arterial hypertension, N (%) | 32 (10.7) | 2 (.04) |
| Scleroderma renal crisis, N (%) | 12 (4) | 0 (0) |
MCTD mixed connective tissue disease, SSc systemic sclerosis, N numbers, N/A not applicable, M mean, SD standard deviation, Mdn median, IQR interquartile range, FVC forced vital capacity, DLCO diffusing capacity of the lungs for carbon monoxide
Fig. 1.

a-b Endostatin and vascular endothelial growth factor (VEGF) serum levels in systemic sclerosis (SSc), mixed connective tissue disease (MCTD) and controls
Association between clinical parameters and serum endostatin and VEGF in the SSc cohort
In univariable analyses dcSSc (Fig. 2a), SRC (Fig. 2b) and PAH (Fig. 2c) were associated with elevated endostatin levels, while percentage of predicted DLCO was negatively associated with endostatin levels (Table 2). In the multiple linear regression analysis, PAH and SRC were significantly associated with elevated endostatin levels (Table 2). The strongest effect was SRC with a mean difference of 77.6 ng/ml in endostatin levels between patients with and without SRC.
Fig. 2.

a, b and c Mean endostatin levels in different systemic sclerosis (SSc) subsets
Table 2.
Association between clinical parameters and circulating endostatin in the SSc cohort
| Clinical manifestations | Univariable | Multivariablea | ||||
|---|---|---|---|---|---|---|
| Regression coefficient | 95 % CI | P value | Regression coefficient | 95 % CI | P value | |
| % of predicted FVC | −.14 | −.34, −.07 | .192 | |||
| % of predicted DLCO | −.33 | −.52, −.15 | .001 | −.18 | −.35, −.002 | .048 |
| dcSSc vs. lcSSc | 10.0 | .6, 19.5 | .037 | |||
| Pulmonary arterial hypertension | 29.3 | 14.4, 44.2 | < .001 | 15.7 | 2.2, 29.2 | .023 |
| Scleroderma renal crisis | 74.6 | 54.1, 95.0 | < .001 | 77.6 | 59.3, 100.0 | < .001 |
SSc systemic sclerosis, CI confidence interval, FVC forced vital capacity, DLCO diffusing capacity of the lungs for carbon monoxide, dsSSc diffuse cutaneous SSc, lcSSc limited cutaneous SSc
aVariables in the final multivariable regression model: percentage of predicted DLCO, pulmonary arterial hypertension, scleroderma renal crisis, age and gender
An inverse association was found between percentage of predicted DLCO and elevated levels of VEGF pg/ml (coefficient - .14, 95 % CI −2.5, −.3, P = .013). No other associations were found between serum VEGF and clinical parameters in the SSc cohort.
Association between clinical parameters and serum endostatin and VEGF in the MCTD cohort
Pulmonary fibrosis (Fig. 3a) and digital ulcers (Fig 3b) were associated with high endostatin serum levels, while a negative association was found with percentage of predicted FVC and DLCO in the univariable analyses (Table 3). In the multivariable linear regression analyses digital ulcers remained significant, indicating a mean difference of 10.5 ng/ml in endostatin levels in patients with and without digital ulcers. No significant associations were found between clinical parameters and serum VEGF in the MCTD cohort.
Fig. 3.
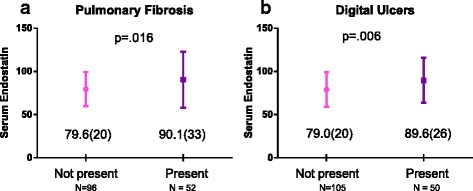
a and b Mean endostatin levels and clinical parameters in mixed connective tissue disease (MCTD)
Table 3.
Association between clinical parameters and circulating endostatin in the MCTD cohort
| Clinical manifestations | Univariable | Multivariablea | ||||
|---|---|---|---|---|---|---|
| Regression coefficient | 95 % CI | P value | Regression coefficient | 95 % CI | P value | |
| Pulmonary fibrosis | 10.5 | 1.9, 19.1 | .017 | |||
| % of predicted FVC | − .33 | −.55, −.12 | .002 | |||
| % of predicted DLCO | −.40 | −.64, −.16 | .001 | |||
| Digital ulcers | 10.7 | 3.2, 18.2 | .006 | 10.5 | 3.2, 17.8 | .005 |
MCTD mixed connective tissue disease, FVC forced vital capacity, DLCO diffusing capacity of the lungs for carbon monoxide
aVariables in the final multivariable regression model: digital ulcers, age and gender
Predictive value of endostatin in SSc
Logistic regression was performed to explore the predictive value of endostatin. When assessing all SSc patients who developed PAH after or within the year of endostatin samples were taken, no significant association was found. However, when including patients that developed PAH within the first 2 years after blood sampling we found for each one SD increase in endostatin levels the odds of developing PAH increased with 70 % (OR = 1.7, 95 % CI: 1.2–2.4, P = .005) (see Additional file 3). The predictive value of endostatin for PAH development was not significant in the multivariable analysis. We then assessed patients who developed SRC after blood sample. All six cases were diagnosed within 2 years of blood sampling. A one SD increase in endostatin level in SSc was associated with a 3.2-fold higher odds (95 % CI: 1.8–5.7, P < .001) of developing SRC. Endostatin was identified as the only predictor of SRC after assessing candidates for multivariable analyses [40] including age, gender, disease duration and dcSSc (see Additional file 3). Analyzing the predictive value of endostatin in the MCTD cohort was not applicable.
All-cause mortality and endostatin levels in the SSc cohort
During 5 years of follow-up 48 patients died and during 10 years of follow-up 58 SSc patients died. SSc patients were divided in tertiles of endostatin levels. The 5-year cumulative survival rate was 94 % (95 % CI: 87–98 %) in the first tertile, 87 % (95 % CI: 77–92 %) in the second tertile and 68 % (95 % CI: 57–76 %). The 10-year cumulative survival rate was 94 % (95 % CI: 90–99 %) in the first tertile, 64 % (95 % CI: 39–86 %) in the second tertile and 28 % (95 % CI: 0–56 %) in the third tertile (log rank P < .001) (Fig. 4). The risk of death increased by 1.6 per SD endostatin in multivariable Cox regression analysis when adjusting for the confounding effects of age, gender, disease duration, dcSSc, pulmonary fibrosis, PH and SRC (95 % CI: 1.2–2.1 %, P = .001).
Fig. 4.

Kaplan-Meier curve for tertiles of endostatin
All-cause mortality and endostatin levels in the MCTD cohort
Similar analyses were performed in the MCTD group. During 10 years of follow-up 14 MCTD patients died and the 10-year cumulative survival rate was 92 % (95 % CI: 85–99 % in the first tertile, 91 % (95 % CI: 77–100 %) in the second tertile and 77 % (95 % CI: 62–92 % in the third tertile. Following multivariable Cox regression a one SD increase in endostatin level increased the risk of death by 1.6 (95 % CI: 1.0–2.4 %, P = .041) when adjusting for the confounding effects of age, gender, disease duration, pulmonary fibrosis and PH.
Discussion
Identifying SSc and MCTD patients at risk of developing serious vascular organ damage could improve patient outcome. The main findings of this study were that increased circulating endostatin, but not VEGF was independently associated with PAH and SRC in SSc patients and with digital ulcers in MCTD patients. Survival analysis showed higher all-cause mortality in both SSc and MCTD patients with increased endostatin levels. Endostatin has been found to be increased in MCTD and SSc compared to controls in previous small-scale studies [16, 18, 19, 23, 24]. The present study extends these previous findings in a much larger sample size and shows a strong association with severe vascular organ damage and mortality during long-term follow-up.
We found higher levels of endostatin in SSc than in MCTD, possibly reflecting that the inhibition of angiogenesis is greater in SSc than in MCTD [14]. In line with other studies [17–19, 23], we found serum VEGF levels to be elevated in SSc compared to controls. Elevated VEGF levels in blood and skin of SSc patients have previously been suggested to contribute to the chaotic capillary morphology seen in these patients [42]. In contrast to other studies [16, 17, 22], we did not find associations between VEGF levels and clinical parameters in the SSc or MCTD cohorts. Importantly, our findings support the study by Hummers et al. showing increased levels of endostatin and not VEGF in SSc patients with PH [23].
The mechanisms behind PAH and SRC development in SSc are not fully understood. The pathology in SSc PAH is described as an obliterative vasculopathy with intimal proliferation, medial hyperplasia, and adventitial fibrosis in the small pulmonary arterioles [43], while thrombotic microangiopathic vasculopathy has been observed in SRC [44]. The current endostatin data supports the hypothesis that dysregulated angiogenesis may play a role in both PAH and SRC. Moreover, recent studies have suggested that endostatin may influence the regulation of matrix metalloproteinases [45], which could contribute to vascular remodeling in the SSc target organs [46].
Previous data from a small MCTD cohort with cases selected from referral centers showed that the patient subsets with acrosclerosis and PH had high circulating VEGF, but endostatin were the same levels as controls [16]. In the present larger and population-based MCTD cohort we found an association between digital ulcers and elevated endostatin levels, implying that the level of endostatin might reflect the severity of vasculopathy in MCTD. In the unselected Norwegian MCTD cohort, PAH was less frequent (two cases in total) than other studies have shown [47, 48]. Due to the low number we could not perform analyses involving endostatin and PAH in the MCTD cohort.
For clinical purposes, we found it of interest to explore the potential predictive value of endostatin. These analyses showed that increasing endostatin levels predicted PAH development within 2 years in SSc patients in the univariable analysis, but not in multivariable analysis where age and percentage of predicted DLCO had stronger predictive value. Since both are well-known risk factors for PAH in SSc [39] there is still a possible role for endostatin in predicting PAH, but this needs to be further investigated in cohorts with larger numbers of PAH cases. We found endostatin to be the only predictor of SRC, however due to low number of SRC events (six in total), results must be carefully interpreted.
The present study is, to our knowledge, the first to show an association between endostatin and all-cause mortality in SSc and MCTD. Previous studies have reported elevated endostatin to be associated with increased risk of death in the elderly [49] and a predictor of all-cause mortality in patients with chronic heart failure of ischemic origin and poor renal function [50].
A major strength of this study is the large number of included patients with MCTD and SSc, and the comparison of results between two diseases with partly overlapping clinical features from the two cohorts. There was no loss to follow-up. The cohorts are largely unselected and they have a longitudinal study design that consists of comprehensive clinical data. This gave us the opportunity to assess a number of relevant parameters in the multivariable analyses. In addition to the clinical parameters shown in Tables 2 and 3, we also assessed age, gender, disease duration, digital ulcers and pulmonary fibrosis in both cohorts, and sclerodactyly and puffy hands in the MCTD cohort only.
A limitation of this study was not distinguishing the proangiogenic and antiangiogenic isoforms of VEGF [51]. Unfortunately, we were not able to assess the associations of VEGF and endostatin to the clinical vasculopathy features cardiomyopathy and gastric antrial vascular actasia due to missing data in our SSc cohort. We were not able to compare endostatin to known cardiovascular risk factors in our study and we were not able, due to missing data, to adjust for pro-brain natriuretic peptide serum levels, estimation of glomerular filtration rate levels or anti-RNA polymerase antibodies. For the parameters SRC in SSc and deaths in MCTD the numbers were low, weakening the impact of these findings. Finally, the endostatin and VEGF measurements were performed cross-sectionally at different disease durations.
Conclusions
In this study, performed in largely unselected patient cohorts, we demonstrated that endostatin levels are elevated in SSc and MCTD patients, and associated with SRC and PAH in SSc patients and digital ulcers in MCTD patients. High endostatin levels were also associated with increased all-cause mortality during follow-up in both groups of patients. Taken together our data further underscore the role of dysregulated angiogenesis in SSc and MCTD and suggest that endostatin could reflect the degree of vasculopathy in these disorders. Further studies are warranted to evaluate the potential role of circulating endostatin as a marker for serious vascular organ damage in SSc and MCTD patients.
Acknowledgements
We would like to acknowledge the work of our colleges at the Department of Rheumatology at Oslo University Hospital who participated in collecting clinical data from NOSVAR. The authors have been financially supported by the Institute of Clinical Medicine at the University of Oslo, the Department of Rheumatology at Oslo University Hospital, the Norwegian Women’s Public Health Association, the K. G. Jebsen Thrombosis Research and Expertise Centre at the Arctic University of Norway, the K. G. Jebsen Inflammation Research Centre and the Research Institute of Clinical Medicine at Oslo University Hospital.
Abbreviations
- CI
confidence interval
- CTD
connective tissue disease
- dcSSc
diffuse cutaneous SSc
- DLCO
diffusing capacity of the lungs for carbon monoxide
- FVC
forced vital capacity
- HRCT
high-resolution computed tomography
- IQR
interquartile range
- lcSSc
limited cutaneous SSc
- M
means
- MCTD
mixed connective tissue disease
- Mdn
median
- NOSVAR
Norwegian Systemic Tissue Disease and Vasculitides Registry
- OR
odds ratio
- OUH
Oslo University Hospital
- PAH
pulmonary arterial hypertension
- PFT
pulmonary function test
- PH
pulmonary hypertension
- RHC
right-sided heart catheterization
- SD
standard deviation
- SLE
systemic lupus erythematosus
- SRC
scleroderma renal crisis
- SSc
systemic sclerosis
- VEGF
vascular endothelial growth factor
Additional files
Previous studies of endostatin and/or vascular endothelial growth factor in SSc and MCTD. Listing of previous studies of endostatin and/or vascular endothelial growth factor levels in SSc and/or MCTD including methods and main results. (PDF 241 kb)
Diagnoses of pulmonary arterial hypertension and scleroderma renal crisis in relation to serum sampling time. A graph showing time of diagnosis of pulmonary arterial hypertension and scleroderma renal crisis in relation to time of serum sampling. (PDF 122 kb)
Results of logistic regression analyses. Known risk factors and endostatin in predicting pulmonary arterial hypertension and scleroderma renal crisis. (PDF 282 kb)
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SR, AMHV and ØM participated in the study design. SR performed the statistical analysis, participated in clinical data collecting and wrote the manuscript. SR, ØM and AMHV drafted and revised the manuscript. RG, AMHV, MBL and TMA participated in collecting the clinical data and revised the manuscript. TG and CB participated in the statistical analysis and revised the manuscript. TU, PA, AM and AA carried out the enzyme immunoassays and revised the manuscript. All writers approved the final manuscript.
Contributor Information
Silje Reiseter, Email: silje.reiseter@medisin.uio.no.
Øyvind Molberg, Email: oyvind.molberg@medisin.uio.no.
Ragnar Gunnarsson, Email: rgunnars@ous-hf.no.
May Brit Lund, Email: mblund@ous-hf.no.
Trond Mogens Aalokken, Email: taalokke@ous-hf.no.
Pål Aukrust, Email: paukrust@ous-hf.no.
Thor Ueland, Email: thor.ueland@medisin.uio.no.
Torhild Garen, Email: tgaren@ous-hf.no.
Cathrine Brunborg, Email: cathrine.brunborg@ous-hf.no.
Annika Michelsen, Email: annika.michelsen@medisin.uio.no.
Aurelija Abraityte, Email: Aurelija.abraityte@rr-research.no.
Anna-Maria Hoffmann-Vold, Email: a.m.hoffmann-vold@medisin.uio.no.
References
- 1.Giordano M, Valentini G, Migliaresi S, Picillo U, Vatti M. Different antibody patterns and different prognoses in patients with scleroderma with various extent of skin sclerosis. J Rheumatol. 1986;13:911–916. [PubMed] [Google Scholar]
- 2.Steen VD, Powell DL, Medsger TA., Jr Clinical correlations and prognosis based on serum autoantibodies in patients with systemic sclerosis. Arthritis Rheum. 1988;31:196–203. doi: 10.1002/art.1780310207. [DOI] [PubMed] [Google Scholar]
- 3.Viswanath V, Phiske MM, Gopalani VV. Systemic sclerosis: current concepts in pathogenesis and therapeutic aspects of dermatological manifestations. Indian J Dermatol. 2013;58:255–268. doi: 10.4103/0019-5154.113930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuwana M, Okazaki Y, Yasuoka H, Kawakami Y, Ikeda Y. Defective vasculogenesis in systemic sclerosis. Lancet. 2004;364:603–610. doi: 10.1016/S0140-6736(04)16853-0. [DOI] [PubMed] [Google Scholar]
- 5.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis. 2007;66:940–944. doi: 10.1136/ard.2006.066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray LA, Rubinowitz A, Herzog EL. Interstitial lung disease: is interstitial lung disease the same as scleroderma lung disease? Curr Opin Rheumatol. 2012;24:656–662. doi: 10.1097/BOR.0b013e3283588de4. [DOI] [PubMed] [Google Scholar]
- 7.Flam ST, Gunnarsson R, Garen T, Norwegian MSG, Lie BA, Molberg O. The HLA profiles of mixed connective tissue disease differ distinctly from the profiles of clinically related connective tissue diseases. Rheumatol. 2015;54:528–535. doi: 10.1093/rheumatology/keu310. [DOI] [PubMed] [Google Scholar]
- 8.Swanton J, Isenberg D. Mixed connective tissue disease: still crazy after all these years. Rheum Dis Clin N Am. 2005;31:421–436. doi: 10.1016/j.rdc.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Ortega-Hernandez OD, Shoenfeld Y. Mixed connective tissue disease: an overview of clinical manifestations, diagnosis and treatment. Best Pract Res Clin Rheumatol. 2012;26:61–72. doi: 10.1016/j.berh.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Grader-Beck T, Wigley FM. Raynaud's phenomenon in mixed connective tissue disease. Rheum Dis Clin N Am. 2005;31:465–481. doi: 10.1016/j.rdc.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Huang J, Li M, Tian Z, Hsieh E, Wang Q, Liu Y, et al. Clinical and laboratory characteristics of systemic sclerosis patients with pulmonary arterial hypertension in China. Clin Experimen Rheumatol. 2014;32:S-115–S-121. [PubMed] [Google Scholar]
- 12.Artim-Esen B, Cene E, Sahinkaya Y, Ertan S, Pehlivan O, Kamali S, et al. Cluster analysis of autoantibodies in 852 patients with systemic lupus erythematosus from a single center. J Rheumatol. 2014;41:1304–1310. doi: 10.3899/jrheum.130984. [DOI] [PubMed] [Google Scholar]
- 13.Becker MO, Kill A, Kutsche M, Guenther J, Rose A, Tabeling C, et al. Vascular receptor autoantibodies in pulmonary arterial hypertension associated with systemic sclerosis. Am J Respir Crit Care Med. 2014;190:808–817. doi: 10.1164/rccm.201403-0442OC. [DOI] [PubMed] [Google Scholar]
- 14.Seppinen L, Pihlajaniemi T. The multiple functions of collagen XVIII in development and disease. Matrix Biol. 2011;30:83–92. doi: 10.1016/j.matbio.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Liakouli V, Cipriani P, Marrelli A, Alvaro S, Ruscitti P, Giacomelli R. Angiogenic cytokines and growth factors in systemic sclerosis. Autoimmun Rev. 2011;10:590–594. doi: 10.1016/j.autrev.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 16.Distler JH, Strapatsas T, Huscher D, Dees C, Akhmetshina A, Kiener HP, et al. Dysbalance of angiogenic and angiostatic mediators in patients with mixed connective tissue disease. Ann Rheum Dis. 2011;70:1197–1202. doi: 10.1136/ard.2010.140657. [DOI] [PubMed] [Google Scholar]
- 17.Choi JJ, Min DJ, Cho ML, Min SY, Kim SJ, Lee SS, et al. Elevated vascular endothelial growth factor in systemic sclerosis. J Rheumatol. 2003;30:1529–1533. [PubMed] [Google Scholar]
- 18.Farouk HM, Hamza SH, El Bakry SA, Youssef SS, Aly IM, Moustafa AA, et al. Dysregulation of angiogenic homeostasis in systemic sclerosis. Int J Rheum Dis. 2013;16:448–454. doi: 10.1111/1756-185X.12130. [DOI] [PubMed] [Google Scholar]
- 19.Hebbar M, Peyrat JP, Hornez L, Hatron PY, Hachulla E, Devulder B. Increased concentrations of the circulating angiogenesis inhibitor endostatin in patients with systemic sclerosis. Arthritis Rheum. 2000;43:889–893. doi: 10.1002/1529-0131(200004)43:4<889::AID-ANR21>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 20.Dziankowska-Bartkowiak B, Waszczykowska E, Zalewska A, Sysa-Jedrzejowska A. Correlation of endostatin and tissue inhibitor of metalloproteinases 2 (TIMP2) serum levels with cardiovascular involvement in systemic sclerosis patients. Mediat Inflamm. 2005;2005:144–149. doi: 10.1155/MI.2005.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Santis M, Bosello SL, Capoluongo E, Inzitari R, Peluso G, Lulli P, et al. A vascular endothelial growth factor deficiency characterises scleroderma lung disease. Ann Rheum Dis. 2012;71:1461–1465. doi: 10.1136/annrheumdis-2011-200657. [DOI] [PubMed] [Google Scholar]
- 22.Distler O, Del Rosso A, Giacomelli R, Cipriani P, Conforti ML, Guiducci S, et al. Angiogenic and angiostatic factors in systemic sclerosis: increased levels of vascular endothelial growth factor are a feature of the earliest disease stages and are associated with the absence of fingertip ulcers. Arthritis Res. 2002;4:R11. doi: 10.1186/ar596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hummers LK, Hall A, Wigley FM, Simons M. Abnormalities in the regulators of angiogenesis in patients with scleroderma. J Rheumatol. 2009;36:576–582. doi: 10.3899/jrheum.080516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dziankowska-Bartkowiak B, Waszczykowska E, Dziankowska-Zaboroszczyk E, de Graft-Johnson JE, Zalewska A, Luczynska M, et al. Decreased ratio of circulatory vascular endothelial growth factor to endostatin in patients with systemic sclerosis--association with pulmonary involvement. Clin Exp Rheumatol. 2006;24:508–513. [PubMed] [Google Scholar]
- 25.Hoffmann-Vold AM, Gunnarsson R, Garen T, Midtvedt O, Molberg O. Performance of the 2013 American College of Rheumatology/European League Against Rheumatism classification criteria for systemic sclerosis (SSc) in large, well-defined cohorts of SSc and mixed connective tissue disease. J Rheumatol. 2015;42:60–63. doi: 10.3899/jrheum.140047. [DOI] [PubMed] [Google Scholar]
- 26.Hoffmann-Vold AM, Aaløkken TM, Lund MB, Garen T, Midtvedt O, Brunborg C, et al. Predictive value of serial HRCT analyses and concurrent lung function tests in systemic sclerosis. Arthritis Rheumatol. 2015;67:2205–2212. doi: 10.1002/art.39166. [DOI] [PubMed] [Google Scholar]
- 27.Gunnarsson R, Molberg O, Gilboe IM, Gran JT, Group PS. The prevalence and incidence of mixed connective tissue disease: a national multicentre survey of Norwegian patients. Ann Rheum Dis. 2011;70:1047–1051. doi: 10.1136/ard.2010.143792. [DOI] [PubMed] [Google Scholar]
- 28.Gunnarsson R, Andreassen AK, Molberg O, Lexberg AS, Time K, Dhainaut AS, et al. Prevalence of pulmonary hypertension in an unselected, mixed connective tissue disease cohort: results of a nationwide, Norwegian cross-sectional multicentre study and review of current literature. Rheumatol. 2013;52:1208–1213. doi: 10.1093/rheumatology/kes430. [DOI] [PubMed] [Google Scholar]
- 29.Gunnarsson R, Aalokken TM, Molberg O, Lund MB, Mynarek GK, Lexberg AS, et al. Prevalence and severity of interstitial lung disease in mixed connective tissue disease: a nationwide, cross-sectional study. Ann Rheum Dis. 2012;71:1966–1972. doi: 10.1136/annrheumdis-2011-201253. [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann-Vold AM, Molberg O, Midtvedt O, Garen T, Gran JT. Survival and causes of death in an unselected and complete cohort of Norwegian patients with systemic sclerosis. J Rheumatol. 2013;40:1127–1133. doi: 10.3899/jrheum.121390. [DOI] [PubMed] [Google Scholar]
- 31.Hoffmann-Vold AM, Midtvedt O, Molberg O, Garen T, Gran JT. Prevalence of systemic sclerosis in south-east Norway. Rheumatol. 2012;51:1600–1605. doi: 10.1093/rheumatology/kes076. [DOI] [PubMed] [Google Scholar]
- 32.van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2013;72:1747–1755. doi: 10.1136/annrheumdis-2013-204424. [DOI] [PubMed] [Google Scholar]
- 33.Sharp GC, Irvin WS, Tan EM, Gould RG, Holman HR. Mixed connective tissue disease--an apparently distinct rheumatic disease syndrome associated with a specific antibody to an extractable nuclear antigen (ENA) Am J Med. 1972;52:148–159. doi: 10.1016/0002-9343(72)90064-2. [DOI] [PubMed] [Google Scholar]
- 34.Hachulla E, Launay D. Diagnosis and classification of systemic sclerosis. Clin Rev Allergy Immunol. 2011;40:78–83. doi: 10.1007/s12016-010-8198-y. [DOI] [PubMed] [Google Scholar]
- 35.Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur Heart J. 2009;30:2493–2537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 36.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Muller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246:697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 37.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 38.Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26:720–735. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 39.Yaqub A, Chung L. Epidemiology and risk factors for pulmonary hypertension in systemic sclerosis. Curr Rheumatol Rep. 2013;15:302. doi: 10.1007/s11926-012-0302-2. [DOI] [PubMed] [Google Scholar]
- 40.Bose N, Chiesa-Vottero A, Chatterjee S. Scleroderma renal crisis. Semin Arthritis Rheum. 2015;44:687–694. doi: 10.1016/j.semarthrit.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Tyndall AJ, Bannert B, Vonk M, Airo P, Cozzi F, Carreira PE, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis. 2010;69:1809–1815. doi: 10.1136/ard.2009.114264. [DOI] [PubMed] [Google Scholar]
- 42.Distler O, Distler JH, Scheid A, Acker T, Hirth A, Rethage J, et al. Uncontrolled expression of vascular endothelial growth factor and its receptors leads to insufficient skin angiogenesis in patients with systemic sclerosis. Circ Res. 2004;95:109–116. doi: 10.1161/01.RES.0000134644.89917.96. [DOI] [PubMed] [Google Scholar]
- 43.Chatterjee S. Pulmonary hypertension in systemic sclerosis. Semin Arthritis Rheum. 2011;41:19–37. doi: 10.1016/j.semarthrit.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 44.Saketkoo LA, Distler O. Is there evidence for vasculitis in systemic sclerosis? Curr Rheumatol Rep. 2012;14:516–525. doi: 10.1007/s11926-012-0296-9. [DOI] [PubMed] [Google Scholar]
- 45.Dodd T, Wiggins L, Hutcheson R, Smith E, Musiyenko A, Hysell B, et al. Impaired coronary collateral growth in the metabolic syndrome is in part mediated by matrix metalloproteinase 12-dependent production of endostatin and angiostatin. Arterioscler Thromb Vasc Biol. 2013;33:1339–1349. doi: 10.1161/ATVBAHA.113.301533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peng WJ, Yan JW, Wan YN, Wang BX, Tao JH, Yang GJ, et al. Matrix metalloproteinases: a review of their structure and role in systemic sclerosis. J Clin Immunol. 2012;32:1409–1414. doi: 10.1007/s10875-012-9735-7. [DOI] [PubMed] [Google Scholar]
- 47.Hajas A, Szodoray P, Nakken B, Gaal J, Zold E, Laczik R, et al. Clinical course, prognosis, and causes of death in mixed connective tissue disease. J Rheumatol. 2013;40:1134–1142. doi: 10.3899/jrheum.121272. [DOI] [PubMed] [Google Scholar]
- 48.Szodoray P, Hajas A, Kardos L, Dezso B, Soos G, Zold E, et al. Distinct phenotypes in mixed connective tissue disease: subgroups and survival. Lupus. 2012;21:1412–1422. doi: 10.1177/0961203312456751. [DOI] [PubMed] [Google Scholar]
- 49.Arnlov J, Ruge T, Ingelsson E, Larsson A, Sundstrom J, Lind L. Serum endostatin and risk of mortality in the elderly: findings from 2 community-based cohorts. Arterioscler Thromb Vasc Biol. 2013;33:2689–2695. doi: 10.1161/ATVBAHA.113.301704. [DOI] [PubMed] [Google Scholar]
- 50.Ueland T, Aukrust P, Nymo SH, Kjekshus J, McMurray JJ, Wikstrand J, et al. Predictive value of endostatin in chronic heart failure patients with poor kidney function. Cardiology. 2014;130:17–22. doi: 10.1159/000368220. [DOI] [PubMed] [Google Scholar]
- 51.Manetti M, Guiducci S, Ibba-Manneschi L, Matucci-Cerinic M. Impaired angiogenesis in systemic sclerosis: the emerging role of the antiangiogenic VEGF(165)b splice variant. Trends Cardiovasc Med. 2011;21:204–210. doi: 10.1016/j.tcm.2012.05.011. [DOI] [PubMed] [Google Scholar]


