Abstract
Objective
This report evaluates incidence of cardiovascular disease (CVD) morbidity and mortality over 10 years among the >160,000 postmenopausal women in the Women’s Health Initiative (WHI) in relation to self-reported RA, disease modifying anti-rheumatic drugs (DMARD) use, anti-CCP+, RF+, CVD risk factors, joint pain, and inflammation (white blood cell (WBC) count and IL-6.)
Methods
Anti-CCP and RF were measured on a sample (n=9,988) of WHI participants with self-reported RA. RA was classified as self-reported RA plus anti-CCP+ positivity and/or use of DMARDs. Self-reported RA that was both anti-CCP− and DMARD− was classified as “unverified RA.”
Results
Age-adjusted rates of coronary heart disease (CHD), stroke, CVD, fatal CVD and total mortality were higher for women with RA vs. no RA, with multivariable-adjusted HR(95%CI) of 1.46(1.17, 1.83) for CHD, and 2.55(1.86, 3.51) for fatal CVD. Within RA, anti-CCP+ and RF+ were not significantly associated with higher risk of any outcomes, despite slightly higher risk of fatal CVD and death for anti-CCP+ vs. anti-CCP− RA. Joint pain severity and CVD risk factors were strongly associated with CVD risk, even for women with no RA. CVD incidence was increased for RA vs. no RA at almost all risk factor levels, except low levels of joint pain or inflammation. Within RA, inflammation was more strongly associated with fatal CVD and total mortality than CHD or CVD.
Conclusion
Among postmenopausal women, RA was associated with 1.5-2.5 higher CVD risk, strongly associated with CV risk factors, joint pain severity, and inflammation, but similar for anti-CCP+ and RF+.
Clinical Trial Registration
clinicaltrials.gov identifier: NCT00000611
Rheumatoid arthritis (RA) is associated with >1.5-fold increased incidence of coronary heart disease (CHD), stroke, total cardiovascular disease (CVD), fatal CVD and total mortality.(1-3) Despite improved treatment, there is little evidence of reduction in CHD or CVD morbidity or mortality.(4) Risk factors for incident CHD in RA include traditional CVD risk factors, e.g., cigarette smoking, hypertension, diabetes, elevated low density lipoprotein cholesterol (LDL-C),(3, 5-7) and markers of RA severity, including inflammation,(8, 9) joint pain and disability.(9-11) The presence (positivity) of antibodies to cyclic citrullinated proteins (anti-CCP +) is highly sensitive and specific for RA diagnosis among suspected RA patients,(12) but there is increasing evidence of differences in anti-CCP+ vs. anti-CCP− RA. Anti-CCP+RA has been reported to be associated with higher disease activity, and in our study as well as other, with the HLA-DR shared epitope and substantially higher cytokine levels, particularly for anti-CCP+/RF+,(13) and higher mortality.(14) Furthermore, current CVD guidance recommends anti-CCP+ or RF+ as indicators of higher CVD risk in RA.(15) However, the relation of anti-CCP+ and RF+ to a range of CHD and CVD morbidity and mortality outcomes remains unclear.
Among the >160,000 postmenopausal women in the Women’s Health Initiative (WHI), we have conducted the WHI RA Study to evaluate relations of self-reported RA, anti-CCP+, rheumatoid factor (RF), DMARD use, and other risk factors to CVD and mortality outcomes. We have previously reported that RA was associated with a > 2-fold excess mortality compared with no RA,(14) and within the RA group, anti-CCP+ RA had a higher proportion of the HLA-DR shared epitope (SE), higher inflammation as measured by white blood cell (WBC) count and cytokines,(13) and slightly higher total mortality rates than anti-CCP− RA.(14) Furthermore, in multivariable-adjusted models, higher WBC count was associated with mortality for anti-CCP+ and anti-CCP− women, but joint pain severity was associated with mortality primarily among women with anti-CCP+ RA.(14) The objective of the current report is to evaluate the incidence of CHD and CVD outcomes among postmenopausal women in WHI (without baseline CVD) in relation to RA, anti-CCP+, RF+ and risk factors including joint pain severity, inflammation (WBC) count and IL-6) and traditional CHD risk factors. We sought to answer the following questions: 1) Do women classified as RA have increased incidence of CHD, stroke, total and fatal CVD, compared with women without reported RA, or with unverified RA (likely arthritis)? 2) Does the RA-related increased incidence of CVD morbidity and mortality differ by anti-CCP+ or RF+? 3) Is higher CHD and CVD risk modified by levels of traditional CHD risk factors, joint pain, or inflammation?
Patients and Methods
Participants and data collected in WHI
Detailed descriptions of WHI (16) and the WHI RA Study(13, 14) have been published. Briefly, between 1993 and 1997, 40 clinical centers enrolled 161,808 women ages 50-79 into one of the clinical trials (n=68,132) or the observational study (n=93,676).(16) At baseline and follow-up WHI participants were asked if they had arthritis, and if yes, whether it was RA; 16,469 women reported RA at baseline or follow-up. Pharmacological drug histories were also obtained, including DMARDS, both at baseline and follow up, every three years in the clinical trial and at third year of follow up in the observational study. DMARD use was defined as current use of hydroxychloroquine, sulfasalazine, minocycline, methotrexate, leflunomide, azathioprine, cyclosporine, gold, cyclophosphamide use, anti-rheumatic biologic agents, or oral steroids.35, 37 At the baseline WHI examination, women were asked to report joint pain severity (none, mild, moderate, severe) and swelling of joints during the past four weeks, but not the specific joint or number of joints affected. Women reported their current health status, disability, physical functioning, and employment status.(17) Lipids were not routinely measured in WHI participants, so “high cholesterol” is defined as self-reported high cholesterol or use of lipid-lowering medications, as in other WHI publications. WHI participants also reported history of cigarette smoking, hypertension, diabetes, history of CHD at baseline, physical activity, waist circumference, reported general health, age, education, and ethnicity.(18) White blood cell (WBC) count was also measured at baseline on all WHI participants, as described.(19)
Definitions of events
Cardiovascular outcomes and deaths were identified by semiannual or annual follow up with family, friends, medical care providers, the National Death Index, and obituaries. Only about 1-2% of WHI participants have been lost to follow up. Cardiovascular- and cancer-related morbidities in WHI were centrally adjudicated using standardized methods as previously described.(20) Incident CHD was defined as fatal and non-fatal myocardial infarction (MI), angina or coronary revascularization (angioplasty or bypass surgery) or death due to definite or possible CHD. Incident CVD was defined as CHD, stroke, transient ischemic attack, carotid artery surgery, heart failure (HF) or death due to CVD. Fatal CVD included only deaths due to CVD.
Biomarker testing in WHI RA Study
Anti-CCP2, rheumatoid factor (RF) and anti-nuclear antibodies (ANA) were measured in Phase 1, defined as a sample of 9,988 of the WHI participants who reported RA at baseline or follow-up (limited to white, black and Hispanic women with available blood samples (18), n=15,188).(13, 14) A Phase 2 sample (n=2,993) was selected based on the anti-CCP results from Phase 1, for measurement of cytokines and HLA-DR typing for SE.(13) Detailed descriptions of the sampling and laboratory methodologies have been published.(13, 14) The current report evaluating incident CVD during follow-up is restricted to women who reported RA at baseline (or baseline and follow-up) and without prevalent CVD at baseline.
The following RA-related assays were done on the entire Phase 1 sample of 9,988 women who reported RA in the WHI, as previously described.(13, 14) Anti-CCP2 and RF were assayed in the Rheumatology Clinical Research Laboratory at the University of Colorado using baseline serum samples stored at −70° and not previously thawed. On a subset (n=2,993) of the 9,988 women included in the phase 1 sample, HLA-DR typing was done at the University of Pittsburgh(21) and interleukin-6 (IL-6) was measured on baseline plasma samples stored at -70°, using multiplex cytokine profiling.(13, 22) This paper includes only IL-6, as the other cytokines highly correlated with IL-6 and are being reported in detail in a separate paper. Furthermore, IL-6 is one of the most robust (least variable) of the cytokines, and is the most related to CHD in population studies and relevant to current anti-IL-6 treatments.
Classification of self-reported RA by anti-CCP and DMARD use
A chart-review validation study previously published (19) demonstrated that among WHI participants, the positive predictive value of self-reported RA was 14.8% (similar to other large cohort studies) but was 62.2% when combined with self-reported DMARD use, 80% when combined with anti-CCP positivity, and 100% for both DMARD + and anti-CCP+. The negative predictive value was ~90% for any of the improved definitions.(23, 24) Therefore, as in our previous reports, clinical RA was defined as self- reported RA and anti-CCP+ and/or baseline DMARD+ (not including use of oral steroids).(13, 14) Women who reported RA but were anti-CCP− and did not report DMARD use were unlikely to have clinical RA (94% did not have clinical RA on chart review)(23) and were therefore classified as “Unverified RA.” WHI participants who never reported RA formed the “No RA,” group.
Statistical analyses
Due to the complex sampling design of our study, sampling weights, defined as 1/sampling fraction, were determined for each woman and incorporated in the analyses, as previously described in detail.(13, 14) Analyses were performed with SAS version 9.3 (SAS Institute, Cary, NC). All models were 2-sided at alpha=0.05. Age-adjusted rates and their 95% confidence intervals (CI) were calculated using the direct method with the entire WHI population as the standard population. Cox proportional hazards models were used to assess the association between RA and time to events, which was calculated from baseline to the date of event or to the end of follow up for subjects without events. The proportional hazards assumption was tested using interaction terms with time. For multivariable models assessing RA-related CVD risk, potential effect modification of risk factor associations by RA was assessed by including multiplicative interaction terms for each covariate with RA (yes vs. no), as described in results.
Results
Participant characteristics
As previously reported,(13, 14) CVD risk factors were slightly worse for women with RA than women with no RA in WHI (Supplemental Table 1). Also as previously reported, women with unverified RA (anti-CCP−/DMARD−, who had reported arthritis as well as RA) had higher mean BMI, waist circumference, and a higher prevalence of hypertension, diabetes, and high cholesterol, than women with RA or with no reported RA. Women with RA had higher levels of RF+ and IL-6 than women with unverified RA (RF and IL-6 were not measured on women with no reported RA.) Compared with anti-CCP−/DMARD+ RA, women with anti-CCP+ RA tended to have lower BMI and waist circumference, less diabetes and high cholesterol, but also had more current smoking, strikingly higher levels of IL-6, RF+, higher WBC levels and slightly higher prevalence of fair/poor health than women with anti-CCP− RA. Fair/poor health and severe joint pain were slightly more common for anti-CCP+ DMARD+ vs. anti-CCP+ DMARD− RA.
Comparison of age-adjusted cardiovascular event rates by RA group
Age-adjusted incidence rates/1000 person-years for CVD outcomes and total mortality were calculated for RA (anti-CCP+ or DMARD+), unverified RA (anti-CCP− and DMARD−), and no RA, and 3 RA subsets classified by anti-CCP+ and DMARD use (Table 1.) Women with RA had rates of incident CHD, stroke and total CVD >1.5-fold higher, and rates of fatal CVD and total mortality >2-fold higher (p<0.05 for all) than women with no RA. Women with RA also had higher rates of CVD, fatal CVD and total mortality (p<0.05) compared with women with unverified RA. Age-adjusted CHD and CVD rates were lower, but fatal CVD and total mortality rates were similar or higher, for anti-CCP+RA (DMARD+ or DMARD−) vs. anti-CCP−/DMARD+ RA, although differences were generally not statistically significant(Table 1). Results were similar when DMARD use was redefined as including oral steroids, or as use reported at any time during follow-up rather than at baseline only (not shown).
Table 1.
Weighted Age-Adjusted Incidence Rates (95%CI)/1000 Person Years (PY) among WHI participants* by RA groups
| Rates/1000 PY | No reported RA | Unverified RA Anti-CCP− and DMARD− |
Total RA Anti-CCP+ and/or DMARD+ |
Anti-CCP− /DMARD+ RA |
RA Subsets Anti-CCP+/ DMARD+ RA |
Anti-CCP+/ DMARD− RA |
|---|---|---|---|---|---|---|
| CHD | 5.78 (5.57, 6.00) | 7.09 (6.00, 8.38)† | 8.56 (5.88, 12.50) ‡ | 11.01 (5.64, 21.56) | 9.17 (4.92, 17.19) | 6.65 (3.45, 13.08) |
| N events/Total | 7909/123,474 | 416/5162 | 80/894 | 25/365 | 29/309 | 26/220 |
| Stroke | 2.57 (2.42, 2.72) | 3.32 (2.60, 4.27)† | 4.53 (2.67, 7.77)‡ | 4.78 (1.74, 13.72) | 3.70 (1.41, 10.63) | 5.18 (2.36, 11.55) |
| N events/Total | 3245/123,481 | 183/5162 | 39/894 | 11/220 | 10/309 | 18/365 |
| Total CVD | 10.03 (9.74, 10.32) | 13.18 (11.66, 14.92)† | 17.31 (13.27, 22.65)‡§ | 23.41 (15.24, 36.16) | 16.02 (10.02, 25.71) | 15.79 (11.40, 21.91)ǁ# |
| N events/Total | 13,415/123,476 | 751/5162 | 156/894 | 49/220 | 49/309 | 58/365 |
| Fatal CVD | 1.71 (1.60, 1.82) | 2.50 (1.78, 3.19)† | 4.06 (2.41, 7.48)‡§ | 3.54 (1.25, 11.49) | 5.53 (2.71, 12.46) | 3.46 (1.42, 8.75) |
| N events/Total | 123/481 | 156/5162 | 39/894 | 9/220 | 16/309 | 13/365 |
| Total Mortality | 7.54 (7.30, 7.79) | 9.91 (8.66, 11.36)† | 17.44 (13.52, 22.58)‡§ | 14.69 (8.65, 25.65) | 20.19 (13.41, 30.64) | 17.39 (11.60, 26.21) |
| N events/Total | 10487/123481 | 611/5162 | 167/894 | 38/220 | 62/309 | 67/365 |
Excluding baseline CVD or RA at follow up only. Between-group comparisons indicated as:
p<0.05 for unverified RA vs. no RA;
p<0.05 for total RA vs. no RA;
p<0.05 for total RA vs. unverified RA;
p=0.06 for anti-CCP+/DMARD+ RA vs. anti-CCP−/DMARD+ RA;
p<0.05 for anti-CCP+ RA(DMARD+ or DMARD−) vs. anti-CCP−/DMARD+ RA;
anti-CCP=anti-cyclic citrullinated peptides; CHD=coronary heart disease; CI=confidence interval; CVD=cardiovascular disease; DMARD=disease-modifying anti-rheumatic drugs; RA=rheumatoid arthritis; WHI=Women’s Health Initiative
Age-adjusted Rates of Incident CVD by Risk Factor Combinations
At each level of CVD risk factors, age-adjusted CVD incidence rates/1000 PYs were ~ 1.5 to 2-fold higher for women with RA compared with no RA, with intermediate rates for unverified RA (Table 2.) Within each group, CVD incidence rates increased >4-fold for those with diabetes and hypertension compared with no risk factors. However, despite these similar relative risks, the difference in absolute risk of CVD (incidence/1000 PYs) was magnified by RA and risk factors. For example, for RA vs. no RA, the excess risk of CVD was 10.75-6.35= 4.4 events/1000 PY for women with no CV risk factors, but 45.72-27.03= ~18.5 events/1000 PYs for women with diabetes and hypertension. Most risk factors showed a similar pattern in relation to CHD incidence, except that among women with RA, CHD rates were not lower for BMI <25 vs. BMI 25-30, and were highest for past smokers, rather than current smokers (Supplemental Table 2.)
Table 2.
Weighted Age-adjusted CVD Incidence Rates(95%CI)/1000 Person-Years (PY) among WHI participants* by Risk Factor Combinations and RA groups
| Total RA ( Anti-CCP+ and/or DMARD+) |
Unverified RA ( Anti-CCP− and DMARD−) |
No Reported RA | |
|---|---|---|---|
| No Smoking, Hypertension, Diabetes, or High Cholesterol | |||
| Rate/1000 PY | 10.75 (5.75, 20.89)†‡ | 8.28 (6.14, 11.20) | 6.35 (5.94, 6.78) |
| N events/Total | 25/217 | 125/1320 | 2480/36299 |
| Smoking Only | |||
| Rate/1000 PY | 16.99 (10.83, 26.78) †‡ | 10.50 (8.09, 13.65) | 8.18 (7.72, 8.66) |
| N events/Total | 56/327 | 163/1445 | 3446/41205 |
| Hypertension Only | |||
| Rate/1000 PY | 16.99 (8.13, 37.74) | 15.36 (11.77, 20.20) | 12.53 (11.72, 13.41) |
| N events/Total | 19/106 | 161/907 | 2540/17297 |
| Hypertension and Smoking Only | |||
| Rate/1000 PY | 27.35 (16.80, 45.21)†‡ | 18.50 (14.39, 23.84) | 16.59 (15.63, 17.61) |
| N events/Total | 45/179 | 186/941 | 3253/18041 |
| Diabetes and Hypertension Only | |||
| Rate/1000 PY | 45.72 (10.98, 216.51) | 37.77 (22.09, 65.29) | 27.03 (23.14, 31.60) |
| N events/Total | 5/16 | 43/124 | 477/1746 |
Excluding baseline CVD or RA at follow up only.
p<0.05 for RA vs. not RA;
p<0.05 for RA vs. No RA;
Anti-CCP=anticyclic citrullinated peptides; CI=confidence interval; CVD=cardiovascular disease; DM=diabetes mellitus; DMARD=disease-modifying antirheumatic drugs; RA=rheumatoid arthritis; WHI=Women’s Health Initiative
Age-adjusted CHD incidence rates/1000 person-years also rose with higher levels of joint pain for women with RA, but also for women with unverified RA, no RA and no RA or arthritis (Table 3.) Higher CHD incidence rates for RA vs. no RA were observed primarily at moderate or severe joint pain severity categories, with little difference at none or mild joint pain. Results were similar for CVD incidence rates (not shown.)
Table 3.
Weighted Age-adjusted CHD Incidence Rates/1000 Person Years (PY) among WHI participants* by Joint Pain Severity and RA Groups
| Joint Pain Severity | Trend p- value |
||||
|---|---|---|---|---|---|
| None | Mild | Moderate | Severe | ||
| Total RA (Anti-CCP+ and/or DMARD+) | |||||
| Rate/1000 PY | 4.33 (0.61, 30.71) | 6.50 (3.10, 13.80) | 10.67 (6.25, 18.29) | 9.27 (4.24, 20.47) | .23 |
| n events/n | 2/38 | 21/295 | 39/356 | 18/198 | |
| Unverified RA (Anti-CCP− and DMARD−) | |||||
| Rate/1000 PY | 5.75 (3.23, 10.49) | 5.55 (4.19, 7.37) | 8.12 (6.15, 10.75) | 10.62 (7.34, 15.42) | <.0001 |
| n events/n | 32/493 | 146/2208 | 150/1653 | 83/754 | |
| No RA Reported | |||||
| Rate/1000 PY | 4.60 (4.26, 4.97) | 5.67 (5.36, 5.99) | 7.27 (6.71, 7.87) | 9.10 (7.87, 10.53) | <.0001 |
| n events/n | 1849/37480 | 3681/57816 | 1800/21982 | 533/5458 | |
| No RA, But Arthritis Reported at Baseline | |||||
| Rate/1000 PY | 5.62 (4.76, 6.65) | 6.13 (5.67, 6.64) | 7.58 (6.92, 8.31) | 9.33 (7.97, 10.93) | <.0001 |
| n events/n | 411/6020 | 1869/25138 | 1384/15600 | 461/4497 | |
| No RA and No arthritis Reported at Baseline | |||||
| Rate/1000 PY | 4.36 (3.99, 4.77) | 5.25 (4.85, 5.69) | 6.65 (5.64, 7.86) | 7.81 (5.15, 11.88) | <.0001 |
| n events/n | 1438/31460 | 1812/32678 | 416/6382 | 72/961 | |
Excluding baseline CVD or RA at follow up only. Anti-CCP=anticyclic citrullinated peptides; CHD=coronary heart disease; cardiovascular disease (CVD); DMARD=disease-modifying antirheumatic drugs; RA=rheumatoid arthritis; WHI=Women’s Health Initiative
Age-adjusted relative risk (HR(95%CIs) for CHD, CVD, fatal CVD, and death in relation to inflammation-related factors (RF+, WBC count and IL-6) were evaluated separately for total RA (anti-CCP+ and/or DMARD+), anti-CCP+ RA, anti-CCP−/DMARD+ RA, and unverified RA (anti-CCP−/DMARD−)(Table 4.) (Women who never reported RA were not sampled for our biomarker cohort, and IL-6 was measured on only a subset (~30%) of the biomarker cohort.(13)) For RF+, HRs for outcomes did not reach statistical significance for any outcomes for RA, RA subsets, or unverified RA, although for anti-CCP+ RA, the HR for CHD was > 2 (Table 4.) In contrast, higher WBC count was associated with higher risk of CHD, CVD, CVD death and death for women with RA and women with unverified RA. For total RA, anti-CCP+ RA, and anti-CCP−/DMARD+RA, HRs per log WBC count were stronger for death than CHD. Similarly, among women with RA or anti-CCP+ RA, log IL-6 was significantly associated with fatal CVD and death, but not CHD or CVD, but was not significantly associated with any outcomes among women with unverified RA.
Table 4.
Weighted Age-adjusted Risk (Hazard Ratio (95%CI)) of Events among WHI Participants* by RA-related Variables and RA Groups
| Risk factors | Total RA (Anti-CCP+ and/or DMARD+) |
Anti-CCP+ RA | Anti-CCP− /DMARD+ RA |
Unverified RA (Anti- CCP− and DMARD−) |
|---|---|---|---|---|
| Rheumatoid Factor (Positive vs. Negative) | ||||
| CHD | 1.06 (0.83, 6.39) | 2.31 (0.83, 6.39) | 1.00 (0.40, 2.49) | 1.04 (0.78, 1.39) |
| CVD | 1.11 (0.79, 1.57) | 1.37 (0.76, 2.47) | 1.80 (0.99, 3.27) | 1.11 (0.90, 1.37) |
| Fatal CVD | 1.18 (0.46, 4.18) | 1.39 (0.46, 4.18) | 0.73 (0.14, 4.00) | 1.39 (0.92, 2.10) |
| Death | 1.17 (0.65, 1.70) | 1.05 (0.65, 1.70) | 1.12 (0.54, 2.34) | 1.23 (0.99, 1.53) |
| Log WBC Count (Per SD) | ||||
| CHD | 2.05 (1.03, 4.06) | 2.39 (1.06, 5.40) | 1.93 (0.54, 6.87) | 2.74 (1.99, 3.78) |
| CVD | 1.23 (1.08, 1.41) | 1.16 (0.99, 1.37) | 1.55 (1.19, 2.03) | 1.33 (1.24, 1.43) |
| Fatal CVD | 2.51 (0.96, 6.52) | 1.52 (0.50, 4.57) | 24.24 (2.64, 222.70) | 2.23 (1.29, 3.84) |
| Death | 3.93 (2.48, 6.22) | 3.59 (2.15, 5.99) | 5.48 (1.81, 16.54) | 1.97 (1.49, 2.62) |
| Log IL-6 (Per SD) | ||||
| CHD | 1.12 (0.92, 1.37) | 1.22 (0.96, 1.54) | 1.19 (0.70, 2.01) | 0.92 (0.78, 1.09) |
| CVD | 1.14 (0.99, 1.31) | 1.17 (0.99, 1.38) | 1.50 (1.06, 2.12) | 0.92 (0.81, 1.03) |
| Fatal CVD | 1.39 (1.03, 1.87) | 1.42 (1.01, 1.99) | 1.54 (0.69, 3.47) | 1.21 (0.99, 1.49) |
| Death | 1.31 (1.15, 1.49) | 1.34 (1.16, 1.54) | 1.10 (0.72, 1.69) | 0.95 (0.83, 1.08) |
Excluding baseline CVD or RA at follow up only. IL-6 only measured on a subset. HR(95%CI) significant at p<0.05 are bolded. Anti-CCP, anticyclic citrullinated peptides; DMARD, disease-modifying antirheumatic drugs; IL, interleukin; RA, rheumatoid arthritis; WHI, Women’s Health Initiative
Multivariable Models of Cardiovascular Risk
Associations of RA and risk factors with incident CHD and fatal CVD were evaluated further in multivariable-adjusted Cox proportional hazards regression models (Table 5). Adjusted for age, smoking, hypertension, diabetes, BMI, and high cholesterol, women with RA had a 1.5-fold increased risk of incident CHD, but a 2.5-fold increased risk of CVD death compared with women with no RA (model 1). These increased relative risks were only modestly attenuated with further adjustment for joint pain, or health status, or log WBC count (models 2-4,) each of which was also significantly associated with CHD and with fatal CVD. HRs in models 2 and 3 were similar with further adjustment for log WBC, which also remained significantly associated with CHD and with fatal CVD (not shown.) For incident CHD, only health status had a statistically significant interaction with RA (p=0.015.) Stratified analyses showed that the CHD risk of fair/poor health was stronger for women with RA, as seen in Supplemental Table 2. For fatal CVD, hypertension (p=0.02) and joint pain (p=0.01) had interactions with RA. In stratified analyses, the increased risk of fatal CVD with RA (vs. no RA) was attenuated among women with hypertension (1.58(0.91, 2.75), p=0.11.), similar to CVD results in Table 2. Stratified analyses showed that the risk of fatal CVD for RA vs. no RA was increased at moderate joint pain, HR(95%CI)=2.85(1.77, 4.58) and severe joint pain, 3.56(2.08, 6.08), but was not at no joint pain (HR= 0) or mild joint pain (1.26 (0.56, 2.81.))
Table 5.
Weighted Multivariable-Adjusted Risk (Hazard Ratio (95%CI)) of CHD or Fatal CVD among WHI Participants* Comparing RA vs. no RA
| Incident CHD | Fatal CVD | |||
|---|---|---|---|---|
| Model 1 | HR (95% CI ) | p-value | HR (95% CI ) | p-value |
| RA vs. no RA | 1.49 (1.20,1.86) | 0.000 | 2.56 (1.86, 3.51) | <.0001 |
| Age, per year | 1.07 (1.07, 1.08) | <.0001 | 1.15 (1.14, 1.16) | <.0001 |
| Smoking (ever vs. never) | 1.25 (1.19, 1.30) | <.0001 | 1.44 (1.32, 1.56) | <.0001 |
| Hypertension (yes vs. no) | 1.69 (1.61, 1.77) | <.0001 | 1.91 (1.75, 2.09) | <.0001 |
| Diabetes (yes vs. no) | 2.42 (2.25, 2.60) | <.0001 | 2.49 (2.19, 2.83) | <.0001 |
| BMI, per 1 unit | 1.02 (1.02, 1.02) | <.0001 | 1.02 (1.01, 1.03) | <.0001 |
| High cholesterol (yes vs. no) | 1.35 (1.28, 1.44) | <.0001 | 0.96 (0.86,1.08) | 0.541 |
| Model 2: Adjusts for Model 1 Covariates + Joint Pain | ||||
| RA vs. no RA | 1.35 (1.08, 1.68) | 0.009 | 2.41 (1.75, 3.32) | <.0001 |
| Joint pain: Mild vs. none | 1.15 (1.09, 1.22) | <.0001 | 0.87 (0.78, 0.96) | <.0001 |
| Moderate vs. none | 1.36 (1.27, 1.45) | 1.00 (0.88, 1.13) | ||
| Severe vs. none | 1.51 (1.37, 1.67) | 1.38 (1.16, 1.64) | ||
| Model 3: Adjusts for Model 1 Covariates + Health Status | ||||
| RA vs. no RA | 1.39 (1.11, 1.73) | 0.004 | 2.24 (1.63,3.08) | <.0001 |
| Fair/poor health vs. other | 1.58 (1.46, 1.70) | <.0001 | 2.22 (1.97,2.51) | <.0001 |
| Model 4: Adjusts for Model 1 Covariates + WBC Count | ||||
| RA vs. no RA | 1.46 (1.17, 1.83) | 0.001 | 2.55 (1.86, 3.51) | <.0001 |
| Log WBC count (per SD) | 1.52 (1.43, 1.62) | <.0001 | 1.59 (1.42, 1.78) | <.0001 |
Excluding baseline CVD or RA at follow up only. Anti-CCP=anticyclic citrullinated peptides; BMI=body mass index; CHD=coronary heart disease; CI=confidence interval; CVD=cardiovascular disease; DMARD =disease-modifying antirheumatic drugs; RA=rheumatoid arthritis; WBC=white blood cell; WHI, Women’s Health Initiative
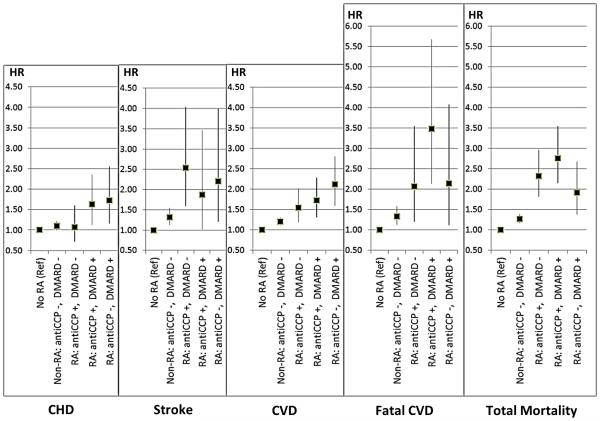
Finally, whether risk factors explained different patterns of CVD morbidity and mortality across RA groups was assessed in Cox models adjusted for age, smoking, hypertension, diabetes, BMI, and (log) WBC, with no RA as the reference group (Figure) Overall, multivariable-adjusted HRs for CHD, stroke, CVD, CVD death and death were higher for all RA categories (anti-CCP+/DMARD−, anti-CCP+/DMARD+, and anti-CCP−/DMARD+) compared with no RA. The only exception was that for anti-CCP+/DMARD− RA, CHD risk was similar to no RA. As with age-adjusted rates, HRs for CHD, stroke and CVD were similar or smaller for anti-CCP+/DMARD+ than anti-CCP−/DMARD+, although HRs for fatal CVD and total mortality risk were somewhat higher for anti-CCP+ RA (both DMARD+ and DMARD−) than anti-CCP−/DMARD+ RA.
Figure.
Multivariable-Adjusted Risk (HR (95%CI) of Cardiovascular Events by RA Group. HR(95%CI)s are calculated from Cox proportional hazards models with no RA as the reference, adjusted for Age, Smoking, Hypertension, Diabetes, BMI, and (log) white blood cell (WBC) count.
Discussion
Few studies have evaluated anti-CCP+ and RF in relation to both cardiovascular morbidity and mortality. In our study of postmenopausal women with self-reported RA, neither anti-CCP+ nor RF+ were significantly associated with higher rates of incident CVD morbidity or mortality, despite being associated with higher levels of inflammation.(13) However, anti-CCP+ RA (DMARD+ or −) had slightly higher rates of CVD death and total mortality and slightly lower rates of CHD and CVD compared with anti-CCP−/DMARD+ RA. Traditional CV risk factors, as well as inflammation (i.e., WBC count), fair/poor health, and joint pain were strongly associated with CHD and fatal CVD, but adjusting for these factors only modestly attenuated the RA-related risk and did not explain differences in CVD morbidity or mortality. Furthermore, as in other studies, anti-CCP+ was associated with a higher prevalence of current and former smoking, but results persisted when adjusted for smoking. A study of 937 patients with RA reported that anti-CCP+ was associated with increased risk of ischemic heart disease (IHD) (OR 2.58, 1.17-5.65), but not heart failure or stroke.(25) That study included men, in whom half of the IHD events occurred. Other studies have reported that anti-CCP+ was significantly associated with total mortality(26, 27) or fatal CVD(27) but not significantly associated with CVD.(26, 28) In a study of outcomes following MI, seropositivity for RF and/or anti-CCP was associated with a marginally increased risk of death, but not heart failure or recurrent ischemia.(29) Although citrullination is found within the atherosclerotic plaque,(30) 2 studies showed no association of higher coronary atherosclerosis with anti-CCP+. (30, 31) Overall, our study results suggest that associations of anti-CCP+ or RF+ with CVD morbidity and mortality may be related to increased inflammation, joint pain and disease activity associated with anti-CCP+ or RF+, rather than anti-CCP+ or RF+ per se. However, given the limitations of the current study, as described below, additional studies will be needed to test this hypothesis.
Second, RA was associated with a larger relative risk of fatal CVD and total mortality, than CVD or CHD, and among women with RA, inflammation markers, i.e., WBC count and IL-6, had stronger associations with fatal CVD and total mortality than with CVD or CHD. Our previous report from this study showed that the > 2-fold excess mortality for RA vs. no RA was associated with higher baseline WBC count among subgroups in the WHI RA study, independent of CVD risk factors.(14) In WHI, WBC count was associated with nonfatal MI, stroke, total mortality, but particularly with fatal CVD, over 6.1 mean years follow-up, independent of CVD risk factors.(19) Similar results have been reported in studies of HIV studies, i.e., stronger associations of IL-6 with mortality(32) than with CVD.(33) There are several possible explanations. Sudden cardiac death may be increased as the initial manifestation of CVD among RA patients.(34) Poor renal function may contribute to fatal CVD in RA patients.(35) Finally, RA patients may have a higher prevalence of unrecognized MI (UMI)(36) or other silent myocardial damage or lung disease(37-39) related to systemic inflammation and disease activity(40)that increase mortality independent of CHD, and which may also differ by anti-CCP+.(37, 41)
A third important finding of this study is the association of self-reported joint pain severity with higher CHD incidence among women with RA, but also among women with unverified RA, with other arthritis, and among women with neither RA nor arthritis. In multivariable-adjusted models, the CHD risk for moderate and for severe joint pain categories was similar or larger than that of RA vs. no RA. In contrast, for fatal CVD, only severe joint pain was significantly associated risk in multivariable models including women with RA and no RA. Furthermore, the 2-fold increased risk for CHD and CVD for RA vs. no RA was seen at most CVD risk factor levels, even no CVD risk factors, but was not observed among women with low levels of joint pain or inflammation (WBC count or IL-6). Furthermore, joint pain and WBC remained significantly associated with CHD and fatal CVD when both were included in the multivariable models (not shown.) These results suggest that joint pain and inflammation are key risk markers and possible therapeutic targets for the reduction of CHD and CVD, among women without RA as well as women with RA. Our results agree with several recent RA studies, showing strong associations of cumulative exposure to disease flares, joint pain, and DAS28 with increased CVD risk.(42, 43) Furthermore, with anti-IL-6 treatment, greater reductions in DAS28, and swollen and tender joint counts were independently related to lower risk of MACE during follow-up.(43) Higher levels of joint symptomatology may adversely affect adherence to risk factor modification, i.e., increasing physical activity, weight loss, and smoking cessation, or pharmacological treatment of hypertension and dyslipidemia.(44) Further studies are needed to elucidate the mechanisms between joint pain and CVD risk among women with unverified RA and no RA as well as RA. Given that anti-inflammatory medications such as methotrexate reduce symptomatology,(45) our results suggest that clinical trials of anti-inflammatory therapies to reduce CVD among non-RA populations should evaluate joint pain as a possible mediator.
Strengths and Limitations
There are several strengths of the current study. It was conducted within the very large, longitudinal WHI study of postmenopausal women from the community, not clinic, with standardized data collection, follow-up and central adjudication of CVD events. The large sample size allowed stratification by anti-CCP+ and DMARD use, and the relatively unique “unverified RA” comparison group of women who reported RA (and arthritis) and had higher levels of joint pain than women who never reported RA. Furthermore, study baseline and follow-up occurred prior to widespread use of powerful new biological RA treatments, which reduces potential confounding from those medications. However, our results, particularly comparisons of anti-CCP+ vs. anti-CCP− RA, must be cautiously interpreted in light of study limitations, described below, and should be considered as hypothesis-generating rather than definitive. Foremost, in this very large study of women from the community, RA was not diagnosed by clinical exam, and we have no information on duration of disease. Furthermore, our definition of anti-CCP− RA requires reported DMARD use, which does not allow us to evaluate anti-CCP−/DMARD− RA. However, anti-CCP+ is fairly sensitive and highly specific for RA, and our key comparisons of anti-CCP+ vs. anti-CCP− were based on DMARD+ groups. Furthermore, show that anti-CCP positivity is associated with a more severe disease course, as also seen in our study, since anti-CCP+/DMARD+ had higher levels of joint pain, worse general health and similar or higher risk of CHD, fatal CVD and total mortality compared with anti-CCP+/DMARD− RA. However, compared with anti-CCP−/DMARD+ RA, CHD and CVD morbidity were lower, yet total and CVD mortality were similar or higher for both anti-CCP+/DMARD+ and anti-CCP+/DMARD− groups. A small proportion of the anti-CCP−/DMARD− women we classified as “unverified RA” may have had RA, and likely had osteoarthritis or other issues, but this potential misclassification also does not explain our key results. There is also high potential for confounding by medication use in this study. In sensitivity analyses oral steroids were included in the definition and did not materially change our results. Our results may have been affected by survival bias, i.e., anti-CCP+ women may have had non-fatal or fatal CHD or events prior to entering the study that removed them from our analytic cohort. We cannot evaluate survival bias prior to entering the WHI, but within the WHI, the proportion of women excluded from the current analysis due to pre-existing CVD was similar across anti-CCP+/DMARD+, anti-CCP+/DMARD−, anti-CCP−/DMARD+RA and anti-CCP−/DMARD− groups. Furthermore, results were similar when women with baseline CVD were included in the analyses (not shown.) Another limitation is that since lipids were not routinely measured in WHI participants, the standard definition of high cholesterol is defined by self-reported high cholesterol or use of lipid-lowering medications. Therefore, high cholesterol was not a focus of our study, and our results add little clarity to the important question of the relation of lipids and lipoproteins and changes in them to CVD outcomes in RA.
In conclusion, among postmenopausal women in the WHI, RA was associated with higher risk of CHD, stroke, CVD (HR ~1.5) but even higher risk of fatal CVD (~2.5) or total mortality, particularly for anti-CCP+ RA. CVD morbidity and mortality was not significantly related to anti-CCP+ or RF+, but CHD and CVD risk were related to CVD risk factors, and joint pain, and among women with RA, inflammation was associated with fatal CVD and mortality. Interestingly, joint pain severity was associated with higher CHD risk among women with no RA and no arthritis as well as RA and unverified RA. These results suggest that the treatment of traditional CHD risk factors among RA patients likely remains an important priority for reducing CHD risk.(8, 15, 46-48) In addition, the relationship between joint symptoms and CHD/CVD supports continued focus on whether reduction of joint symptomatology, even in patients without RA, may partially mediate improved CVD-related outcomes, especially in relation to anti-inflammatory therapies.
Supplementary Material
Acknowledgments
Funding Sources: The Women’s Health Initiative program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221. This WHI Ancillary Study work was funded by BAA NHLBI-WH-09-01 Contract No. HHSN268200960006C.
Footnotes
Disclosures: Dr. Mackey has received honoraria from the National Lipid Association related to educational, not promotional, endeavors. Other authors: None.
References
- 1.Meune C, Touze E, Trinquart L, Allanore Y. High risk of clinical cardiovascular events in rheumatoid arthritis: Levels of associations of myocardial infarction and stroke through a systematic review and meta-analysis. Arch Cardiovasc Dis. 2010;103(4):253–61. doi: 10.1016/j.acvd.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Avina-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 2008;59(12):1690–7. doi: 10.1002/art.24092. [DOI] [PubMed] [Google Scholar]
- 3.Solomon DH, Karlson EW, Rimm EB, Cannuscio CC, Mandl LA, Manson JE, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107(9):1303–7. doi: 10.1161/01.cir.0000054612.26458.b2. [DOI] [PubMed] [Google Scholar]
- 4.Bergstrom U, Jacobsson LT, Turesson C. Cardiovascular morbidity and mortality remain similar in two cohorts of patients with long-standing rheumatoid arthritis seen in 1978 and 1995 in Malmo, Sweden. Rheumatology (Oxford) 2009;48(12):1600–5. doi: 10.1093/rheumatology/kep301. [DOI] [PubMed] [Google Scholar]
- 5.Boyer JF, Gourraud PA, Cantagrel A, Davignon JL, Constantin A. Traditional cardiovascular risk factors in rheumatoid arthritis: a meta-analysis. Joint Bone Spine. 2011;78(2):179–83. doi: 10.1016/j.jbspin.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 6.Solomon DH, Curhan GC, Rimm EB, Cannuscio CC, Karlson EW. Cardiovascular risk factors in women with and without rheumatoid arthritis. Arthritis Rheum. 2004;50(11):3444–9. doi: 10.1002/art.20636. [DOI] [PubMed] [Google Scholar]
- 7.Kremers HM, Crowson CS, Therneau TM, Roger VL, Gabriel SE. High ten-year risk of cardiovascular disease in newly diagnosed rheumatoid arthritis patients: a population-based cohort study. Arthritis Rheum. 2008;58(8):2268–74. doi: 10.1002/art.23650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solomon DH, Kremer J, Curtis JR, Hochberg MC, Reed G, Tsao P, et al. Explaining the cardiovascular risk associated with rheumatoid arthritis: traditional risk factors versus markers of rheumatoid arthritis severity. Ann Rheum Dis. 2010;69(11):1920–5. doi: 10.1136/ard.2009.122226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.del Rincon I, Freeman GL, Haas RW, O'Leary DH, Escalante A. Relative contribution of cardiovascular risk factors and rheumatoid arthritis clinical manifestations to atherosclerosis. Arthritis Rheum. 2005;52(11):3413–23. doi: 10.1002/art.21397. [DOI] [PubMed] [Google Scholar]
- 10.Farragher TM, Lunt M, Bunn DK, Silman AJ, Symmons DP. Early functional disability predicts both all-cause and cardiovascular mortality in people with inflammatory polyarthritis: results from the Norfolk Arthritis Register. Ann Rheum Dis. 2007;66(4):486–92. doi: 10.1136/ard.2006.056390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Metsios GS, Stavropoulos-Kalinoglou A, Panoulas VF, Wilson M, Nevill AM, Koutedakis Y, et al. Association of physical inactivity with increased cardiovascular risk in patients with rheumatoid arthritis. Eur J Cardiovasc Prev Rehabil. 2009;16(2):188–94. doi: 10.1097/HJR.0b013e3283271ceb. [DOI] [PubMed] [Google Scholar]
- 12.Whiting PF, Smidt N, Sterne JA, Harbord R, Burton A, Burke M, et al. Systematic review: accuracy of anti-citrullinated Peptide antibodies for diagnosing rheumatoid arthritis. Ann Intern Med. 2010;152(7):456–64. doi: 10.7326/0003-4819-152-7-201004060-00010. W155-66. [DOI] [PubMed] [Google Scholar]
- 13.Kuller LH, Mackey RH, Walitt BT, Deane KD, Holers VM, Robinson WH, et al. Rheumatoid Arthritis in the Women's Health Initiative: Methods and Baseline Evaluation. Am J Epidemiol. 2014 doi: 10.1093/aje/kwu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuller LH, Mackey RH, Walitt BT, Deane KD, Holers VM, Robinson WH, et al. Determinants of Mortality Among Postmenopausal Women in the Women's Health Initiative Who Report Rheumatoid Arthritis. Arthritis Rheumatol. 2014;66(3):497–507. doi: 10.1002/art.38268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peters MJ, Symmons DP, McCarey D, Dijkmans BA, Nicola P, Kvien TK, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69(2):325–31. doi: 10.1136/ard.2009.113696. [DOI] [PubMed] [Google Scholar]
- 16.Design of the Women's Health Initiative clinical trial and observational study The Women's Health Initiative Study Group. Control Clin Trials. 1998;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 17.Lynch CP, McTigue KM, Bost JE, Tinker LF, Vitolins M, Adams-Campbell L, et al. Excess weight and physical health-related quality of life in postmenopausal women of diverse racial/ethnic backgrounds. J Womens Health (Larchmt) 2010;19(8):1449–58. doi: 10.1089/jwh.2009.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McTigue KM, Chang YF, Eaton C, Garcia L, Johnson KC, Lewis CE, et al. Severe obesity, heart disease, and death among white, African American, and Hispanic postmenopausal women. Obesity (Silver Spring) 2014;22(3):801–10. doi: 10.1002/oby.20224. [DOI] [PubMed] [Google Scholar]
- 19.Margolis KL, Manson JE, Greenland P, Rodabough RJ, Bray PF, Safford M, et al. Leukocyte count as a predictor of cardiovascular events and mortality in postmenopausal women: the Women's Health Initiative Observational Study. Arch Intern Med. 2005;165(5):500–8. doi: 10.1001/archinte.165.5.500. [DOI] [PubMed] [Google Scholar]
- 20.Curb JD, McTiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, et al. Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Ann Epidemiol. 2003;13(9 Suppl):S122–8. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 21.Ng J, Hurley CK, Baxter-Lowe LA, Chopek M, Coppo PA, Hegland J, et al. Large-scale oligonucleotide typing for HLA-DRB1/3/4 and HLA-DQB1 is highly accurate, specific, and reliable. Tissue Antigens. 1993;42(5):473–9. doi: 10.1111/j.1399-0039.1993.tb02191.x. [DOI] [PubMed] [Google Scholar]
- 22.Chandra PE, Sokolove J, Hipp BG, Lindstrom TM, Elder JT, Reveille JD, et al. Novel multiplex technology for diagnostic characterization of rheumatoid arthritis. Arthritis Res Ther. 2011;13(3):R102. doi: 10.1186/ar3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walitt B, Mackey R, Kuller L, Deane KD, Robinson W, Holers VM, et al. Predictive Value of Autoantibody Testing for Validating Self-reported Diagnoses of Rheumatoid Arthritis in the Women's Health Initiative. Am J Epidemiol. 2013 doi: 10.1093/aje/kws310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walitt BT, Constantinescu F, Katz JD, Weinstein A, Wang H, Hernandez RK, et al. Validation of self-report of rheumatoid arthritis and systemic lupus erythematosus: The Women's Health Initiative. J Rheumatol. 2008;35(5):811–8. [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez-Longo FJ, Oliver-Minarro D, de la Torre I, Gonzalez-Diaz de Rabago E, Sanchez-Ramon S, Rodriguez-Mahou M, et al. Association between anti-cyclic citrullinated peptide antibodies and ischemic heart disease in patients with rheumatoid arthritis. Arthritis Rheum. 2009;61(4):419–24. doi: 10.1002/art.24390. [DOI] [PubMed] [Google Scholar]
- 26.Liang KP, Kremers HM, Crowson CS, Snyder MR, Therneau TM, Roger VL, et al. Autoantibodies and the risk of cardiovascular events. J Rheumatol. 2009;36(11):2462–9. doi: 10.3899/jrheum.090188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Humphreys JH, van Nies J, Chipping J, Marshall T, Mil A, Symmons D, et al. Rheumatoid factor and anti-citrullinated protein antibody positivity, but not level, are associated with increased mortality in patients with rheumatoid arthritis: results from two large independent cohorts. Arthritis Res Ther. 2014;16(6):483. doi: 10.1186/s13075-014-0483-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Innala L, Moller B, Ljung L, Magnusson S, Smedby T, Sodergren A, et al. Cardiovascular events in early RA are a result of inflammatory burden and traditional risk factors: a five year prospective study. Arthritis Res Ther. 2011;13(4):R131. doi: 10.1186/ar3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCoy SS, Crowson CS, Maradit-Kremers H, Therneau TM, Roger VL, Matteson EL, et al. Longterm outcomes and treatment after myocardial infarction in patients with rheumatoid arthritis. J Rheumatol. 2013;40(5):605–10. doi: 10.3899/jrheum.120941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sokolove J, Brennan MJ, Sharpe O, Lahey LJ, Kao AH, Krishnan E, et al. Brief report: citrullination within the atherosclerotic plaque: a potential target for the anti-citrullinated protein antibody response in rheumatoid arthritis. Arthritis Rheum. 2013;65(7):1719–24. doi: 10.1002/art.37961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karpouzas GA, Malpeso J, Choi TY, Li D, Munoz S, Budoff MJ. Prevalence, extent and composition of coronary plaque in patients with rheumatoid arthritis without symptoms or prior diagnosis of coronary artery disease. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2013-203617. [DOI] [PubMed] [Google Scholar]
- 32.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duprez DA, Neuhaus J, Kuller LH, Tracy R, Belloso W, De Wit S, et al. Inflammation, Coagulation and Cardiovascular Disease in HIV-Infected Individuals. PLoS One. 2012;7(9):e44454. doi: 10.1371/journal.pone.0044454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maradit-Kremers H, Crowson CS, Nicola PJ, Ballman KV, Roger VL, Jacobsen SJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 2005;52(2):402–11. doi: 10.1002/art.20853. [DOI] [PubMed] [Google Scholar]
- 35.Lertnawapan R, Bian A, Rho YH, Kawai VK, Raggi P, Oeser A, et al. Cystatin C, renal function, and atherosclerosis in rheumatoid arthritis. J Rheumatol. 2011;38(11):2297–300. doi: 10.3899/jrheum.110168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schelbert EB, Cao JJ, Sigurdsson S, Aspelund T, Kellman P, Aletras AH, et al. Prevalence and prognosis of unrecognized myocardial infarction determined by cardiac magnetic resonance in older adults. JAMA. 2012;308(9):890–6. doi: 10.1001/2012.jama.11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giles JT, Malayeri AA, Fernandes V, Post W, Blumenthal RS, Bluemke D, et al. Left ventricular structure and function in patients with rheumatoid arthritis, as assessed by cardiac magnetic resonance imaging. Arthritis Rheum. 2010;62(4):940–51. doi: 10.1002/art.27349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crowson CS, Nicola PJ, Kremers HM, O'Fallon WM, Therneau TM, Jacobsen SJ, et al. How much of the increased incidence of heart failure in rheumatoid arthritis is attributable to traditional cardiovascular risk factors and ischemic heart disease? Arthritis Rheum. 2005;52(10):3039–44. doi: 10.1002/art.21349. [DOI] [PubMed] [Google Scholar]
- 39.Myasoedova E, Davis JM, 3rd, Crowson CS, Roger VL, Karon BL, Borgeson DD, et al. Brief report: rheumatoid arthritis is associated with left ventricular concentric remodeling: results of a population-based cross-sectional study. Arthritis Rheum. 2013;65(7):1713–8. doi: 10.1002/art.37949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kobayashi Y, Giles JT, Hirano M, Yokoe I, Nakajima Y, Bathon JM, et al. Assessment of myocardial abnormalities in rheumatoid arthritis using a comprehensive cardiac magnetic resonance approach: a pilot study. Arthritis Res Ther. 2010;12(5):R171. doi: 10.1186/ar3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yin Y, Liang D, Zhao L, Li Y, Liu W, Ren Y, et al. Anti-cyclic citrullinated Peptide antibody is associated with interstitial lung disease in patients with rheumatoid arthritis. PLoS One. 2014;9(4):e92449. doi: 10.1371/journal.pone.0092449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Myasoedova E, Chandran A, Ilhan B, Major BT, Michet CJ, Matteson EL, et al. The role of rheumatoid arthritis (RA) flare and cumulative burden of RA severity in the risk of cardiovascular disease. Ann Rheum Dis. 2015 doi: 10.1136/annrheumdis-2014-206411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rao VU, Pavlov A, Klearman M, Musselman D, Giles JT, Bathon JM, et al. An evaluation of risk factors for major adverse cardiovascular events during tocilizumab therapy. Arthritis Rheumatol. 2015;67(2):372–80. doi: 10.1002/art.38920. [DOI] [PubMed] [Google Scholar]
- 44.Akkara Veetil BM, Myasoedova E, Matteson EL, Gabriel SE, Crowson CS. Use of lipid-lowering agents in rheumatoid arthritis: a population-based cohort study. J Rheumatol. 2013;40(7):1082–8. doi: 10.3899/jrheum.121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Micha R, Imamura F, Wyler von Ballmoos M, Solomon DH, Hernan MA, Ridker PM, et al. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol. 2011;108(9):1362–70. doi: 10.1016/j.amjcard.2011.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chung CP, Oeser A, Avalos I, Gebretsadik T, Shintani A, Raggi P, et al. Utility of the Framingham risk score to predict the presence of coronary atherosclerosis in patients with rheumatoid arthritis. Arthritis Res Ther. 2006;8(6):R186. doi: 10.1186/ar2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peters MJ, van Halm VP, Voskuyl AE, Smulders YM, Boers M, Lems WF, et al. Does rheumatoid arthritis equal diabetes mellitus as an independent risk factor for cardiovascular disease? A prospective study. Arthritis Rheum. 2009;61(11):1571–9. doi: 10.1002/art.24836. [DOI] [PubMed] [Google Scholar]
- 48.Gomez-Vaquero C, Robustillo M, Narvaez J, Rodriguez-Moreno J, Gonzalez-Juanatey C, Llorca J, et al. Assessment of cardiovascular risk in rheumatoid arthritis: impact of the new EULAR recommendations on the score cardiovascular risk index. Clin Rheumatol. 2012;31(1):35–9. doi: 10.1007/s10067-011-1774-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.