Abstract
Objective
To identify the cause of disease in an adult patient presenting with recent onset fevers, chills, urticaria, fatigue, and profound myalgia, who was negative for cryopyrin-associated periodic syndrome (CAPS) NLRP3 mutations by conventional Sanger DNA sequencing.
Methods
We performed whole-exome sequencing and targeted deep sequencing using DNA from the patient’s whole blood to identify a possible NLRP3 somatic mutation. We then screened for this mutation in subcloned NLRP3 amplicons from fibroblasts, buccal cells, granulocytes, negatively-selected monocytes, and T and B lymphocytes and further confirmed the somatic mutation by targeted sequencing of exon 3.
Results
We identified a previously reported CAPS-associated mutation, p.Tyr570Cys, with a mutant allele frequency of 15% based on exome data. Targeted sequencing and subcloning of NLRP3 amplicons confirmed the presence of the somatic mutation in whole blood at a ratio similar to the exome data. The mutant allele frequency was in the range of 13.3%–16.8% in monocytes and 15.2%–18% in granulocytes; Notably, this mutation was either absent or present at a very low frequency in B and T lymphocytes, buccal cells, and in the patient’s cultured fibroblasts.
Conclusion
These data document the possibility of myeloid-restricted somatic mosaicism in the pathogenesis of CAPS, underscoring the emerging role of massively-parallel sequencing in clinical diagnosis.
The cryopyrin-associated periodic syndromes (CAPS) are a group of autoinflammatory disorders including familial cold autoinflammatory syndrome (FCAS), Muckle-Wells syndrome (MWS), and neonatal-onset multisystem inflammatory disease (NOMID; also known as Chronic Infantile Neurological, Cutaneous, and Articular, (CINCA)). These dominantly inherited diseases are caused by heterozygous missense gain-of-function mutations in the NLRP3 (CIAS1) gene encoding NLRP3 (also known as cryopyrin), leading to the activation of the NLRP3 inflammasome and overproduction of IL-1β (1). CAPS patients respond to therapy targeting this cytokine.
Recently, several studies have expanded the genetic basis of NOMID/CINCA/MWS to include NLRP3 mosaicism (2–10). The first case was reported in a NOMID/CINCA patient (2). Subsequent studies suggest that somatic NLRP3 mutations account for up to 70% of NOMID/CINCA patients who test negative for a heterozygous germline mutation (4–6). The estimates of the level of somatic mosaicism vary widely, ranging from as low as 4.2% up to 35.8% (5, 9). Patients with somatic mutations present with symptoms comparable to patients with germline mutations, with slightly older age of disease onset and possibly milder CNS disease (5, 9). The frequency of the mutant allele was reported to be similar in various cell types including myeloid cells, T and B lymphocytes, and epithelial cells (4, 5). A small number of monocytes carrying NLRP3 mutations are sufficient to evoke systemic inflammation (3), and mutant macrophages are predominantly responsible for driving inflammation (7). There is also evidence that in some cases somatic NLRP3 mutations include germ-line cells, with consequent genetic transmission (8). Recently, myeloid lineage-restricted somatic mosaicism of NLRP3 mutations was reported in two patients with the variant-type of Schnitzler syndrome (11).
CAPS almost invariably presents in infancy with fevers and urticarial rash, and other manifestations such as arthropathy, sensorineural hearing loss, and, in more severe cases, central nervous system inflammation. A 52 year-old pediatrician presented to the National Institutes of Health with perimenopausal onset of stress-induced fevers, chills, urticaria, fatigue, and profound myalgia, usually lasting several hours at a time. Occasionally, she developed conjunctivitis and headaches associated with flares. She recalled a similar rash without the other associated symptoms as a child, resolving at puberty (Table 1). She did not have a history of any CNS inflammation such as sensorineural hearing loss, papilledema, or aseptic meningitis nor did she have any bony abnormalities.
Table 1.
Clinical characteristics of the affected patient and the cryopyrin associated periodic syndromes
| Disease | Age at Disease Onset (years) | Fevers | Flare Duration | Dermatologic | Neurologic | Ocular Features | Musculoskeletal | AA amyloidosis |
|---|---|---|---|---|---|---|---|---|
| FCAS | <1 | Yes | <24 hours | Cold-induced neutrophilic urticaria-like | No | Conjuctivitis | Arthralgias/myalgia | <10% |
| MWS | <20 | Yes | 1–3 days | Neutrophilic urticaria-like | Sensorineural hearing loss | Conjuctivitis | Arthralgias/arthritis | ~30% |
| NOMID/CINCA | <1 | Yes | Continuous | Neutrophilic urticaria-like | Aseptic meningitis, sensorineural hearing loss | Papilledema uveitis | Arthralgias/arthritisepi physeal bone formation | Unknown |
| Patient | Possible symptoms in early childhood, severe symptoms in late 40’s | Stress- induced | Initially <48 hours, increasing to continuous | Stress-induced neutrophilic urticaria-like | No | Conjuctivitis | Arthralgia/Myalgia | No |
Based on the absence of cold-induced symptoms and CNS inflammation, the patient was suspected of having a variant of MWS, although screening in a commercial laboratory was unremarkable for mutations in NLRP3. Nevertheless, her symptoms dramatically improved with daily injections of anakinra, a recombinant IL-1 receptor antagonist. A diagnosis of Schnitzler’s syndrome was considered but the patient did not have an IgM gammopathy nor did she report significant bone pain. Hypothesizing possible NLRP3 somatic mosaicism, we first performed whole-exome sequencing and then targeted deep resequencing of NLRP3 in DNA extracted from whole blood and buccal cells. Upon finding evidence for mosaicism, we interrogated the distribution of the somatic mutation in six different cellular lineages.
MATERIALS AND METHODS
Patient
The patient provided written informed consent as approved by an Institutional Review Board at the National Institutes of Health. The study was performed in accordance to the Declaration of Helsinki. Human peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque density gradient centrifugation. T cells (Pan T Cell Isolation Kit, Miltenyi Biotec), B cells (B Cell Isolation Kit II, Miltenyi Biotec), and monocytes (Monocyte Isolation Kit II, Miltenyi Biotec) were isolated by negative selection using antibody-conjugated supermagnetic beads (MACS) according to the manufacturer’s instructions. Granulocytes were enriched from peripheral blood by sedimentation in 3% dextran in 0.9% saline followed by hypotonic lysis to remove erythrocytes (12). Buccal cells were collected by Easy-Swab (TrimGen), and buccal DNA was extracted by the BuccalQuick kit (TrimGen). Fibroblasts were derived from a skin punch biopsy, which was initially digested with 9 mL of 1 mg/mL collagenase II and 1 mL of 2.5 U/mL dispase for 1 h, then cultured with 2 mL of 20% DMEM/FBS media for 3 weeks prior to collection.
Whole-exome sequencing
Whole-exome sequence derived from the patient’s DNA was obtained using the Illumina TruSeq DNA Sample Preparation Kit on the Illumina HiSeq2000 instrument. Sequencing reads were aligned to the human reference genome hg19 using the Burrows-Wheeler Aligner (BWA). Both SAMtools and the GATK Unified Genotyper (parameters: -stand_call_conf 5.0 -stand_emit_conf 5.0 -dcov 500) were used to identify SNVs and INDELs. Variants were then annotated by ANNOVAR and novel exonic variants were obtained by filtering variants against the dbSNP, 1000 Genomes Project, NHLBI Exome Sequencing Project (ESP6500), and ClinSeq databases.
Targeted deep resequencing
We performed targeted deep resequencing of NLRP3 using the DNA from various cell types. NLRP3 exons were amplified using high-fidelity polymerase AccuPrime™ Pfx. Whole blood and buccal cells DNA samples were deep resequenced for all nine NLRP3 exons covered by twelve amplicons. DNA samples from isolated monocytes, granulocytes, T and B lymphocytes, fibroblasts, were deep resequenced only for exon 3 that was covered by four amplicons. Equal volumes of each amplicon were pooled, sheared by sonication, and then prepared using the Illumina TruSeq DNA Sample Preparation Kit or the NuGen Ovation Ultralow Library System. The sequence read data were generated using the MiSeq instrument with 150 bp or 300 bp paired-end reads.
Subcloning and Sequenom genotyping
The amplicon harboring the NLRP3 mutation was prepared from the DNA extracted from various cell types using high-fidelity polymerase AccuPrime™ Pfx and dA addition was performed with LA Taq polymerase. The amplicon was subcloned into pCR2.1-TOPO vector. 192 subclones were randomly selected for each of the six different cell types and further amplified by AccuPrime™ Pfx for individual clone sequencing or the bacterial suspensions were directly subjected to Sequenom genotyping. Genotypes were determined using Typer 4.0 software.
RESULTS
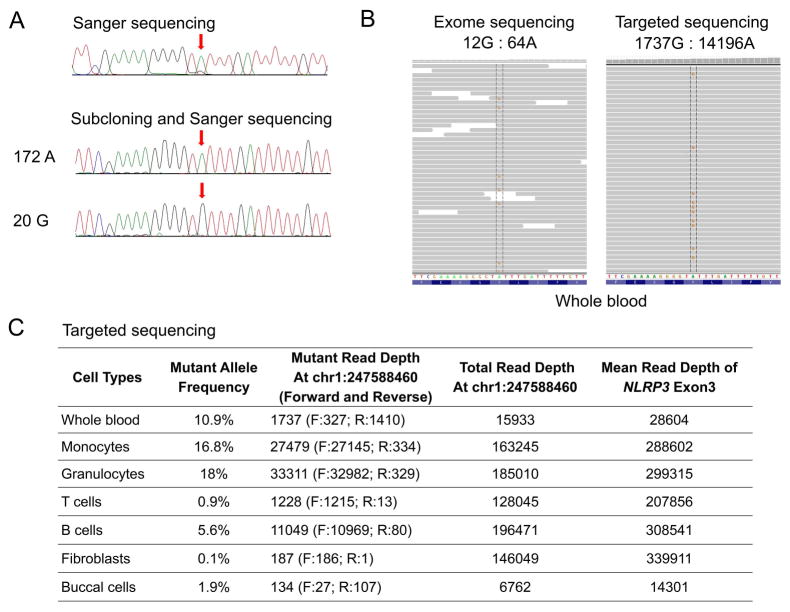
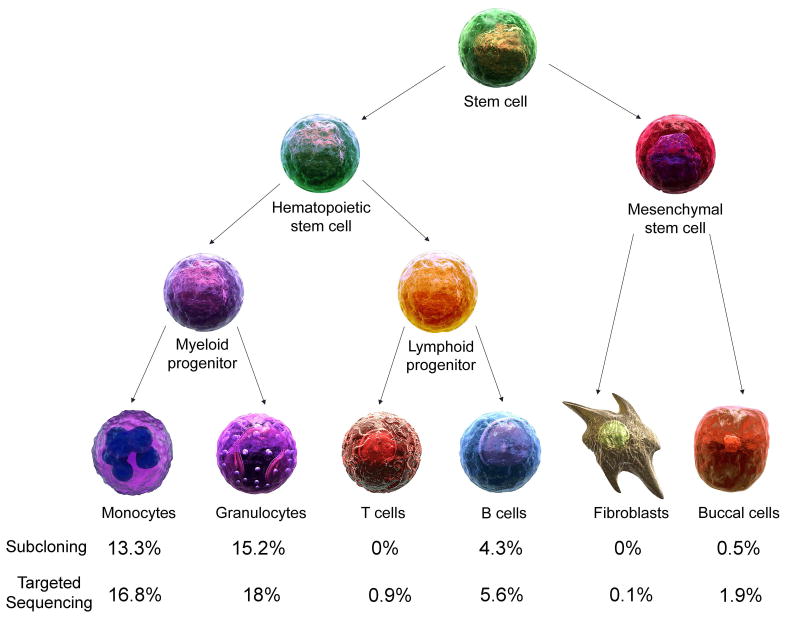
By Sanger sequencing, we confirmed the absence of germline NLRP3 mutations (Figure 1A). We then performed whole-exome sequencing of DNA from the patient’s unfractionated blood. The sequencing generated ~58 million mapped reads with a mean depth of coverage of 72X. The search for a novel disease-causing gene identified 21 novel exonic candidate variants but failed to identify any plausible candidate genes in this patient (Supplementary Table 1). In order to test for possible somatic mutations, we changed our sequence filtering strategy to reduce false negative variant calls, which concomitantly increased the number of false positive calls. In total, 24462 exonic variants were identified. The subsequent filtering for autosomal heterozygous variants that are not present in the public databases resulted in identification of 129 novel variants. Among the variants, we noted a heterozygous change, p.Tyr570Cys (c.1709A>G), in exon 3 of the NLRP3 gene. This mutation has been previously reported in a NOMID/CINCA patient from Australia (13). Exome data showed the somatic mutation with the mutant G allele frequency of 15% (Figure 1B). To confirm that p.Tyr570Cys is a true somatic mutation rather than a false positive call, we performed targeted deep resequencing of 9 exons of NLRP3 in DNA obtained from whole blood of the patient. Targeted deep resequencing generated an average depth of coverage as high as 28604X (Figure 1B and 1C). The somatic mutation p.Tyr570Cys was present in the whole blood DNA sample with 1737 reads harboring the mutant G allele and 14196 reads harboring the wild type A allele, indicating a mutant G allele frequency of 10.9% (Figure 1C). This result was consistent with the mutant allele frequency identified by exome sequencing data. Next, we investigated the distribution and frequency of the somatic mutation in various cell types. We subcloned the amplicon potentially harboring this mutation and genotyped 192 colonies from the patient’s monocytes, granulocytes, T cells, B cells, buccal cells, and cultured fibroblasts. The p.Tyr570Cys mutation was identified in monocytes and granulocytes, with the mutant G allele frequency of 13.3% and 15.2%, respectively. The p.Tyr570Cys mutation was not identified in T cells and fibroblasts, and was identified at very low frequency in B cells (4.3%) and buccal cells (0.5%) (Figure 2). Similar frequencies of the somatic mutation were further obtained by targeted sequencing of exon 3 (Figure 1C and Figure 2). As expected, mean depth of coverage for targeted sequencing of exon 3 was significantly higher than when all nine exons of NLRP3 were subjected to targeted sequencing. We suspect that the low level of somatic mutation in non-myeloid cells could be due to contamination in cell preparations. The mutant allele frequency of (subcloning/targeted sequencing) 13.3%/16.8% and 15.2%/18% corresponds to 26.6%/33.6% and 30.4%/36% of monocytes and granulocytes, respectively, mediating the disease.
Figure 1.
(A) Sanger sequencing electropherograms of the patient with somatic NLRP3 mosaicism (c.1709A>G, p.Tyr570Cys) from whole blood DNA (upper panel) and in representative subclones (lower panels). Arrows indicate the position of the somatic mutation. The frequency of the somatic mutant allele in whole blood is 10.4% (172 clones with wild type A allele and 20 clones with mutant G allele, respectively).
(B) Mapped reads from exome sequencing and targeted sequencing of DNA from whole blood support the presence of the mutation c.1709A>G by Integrative Genomics Viewer.
(C) Summary table of the somatic mutation allele frequency and read depth in different cell lineages determined by targeted sequencing.
Figure 2.
The schematic diagram shows cell lineage development from hematopoietic and mesenchymal stem cells. The somatic NLRP3 mutation, c.1709A>G, is restricted to monocytes and granulocytes and comparable data from two sequencing techniques suggest that the somatic event likely occurred in myeloid progenitor cells.
DISCUSSION
This manuscript reports on lineage-specific NLRP3 mosaicism in patients with late-onset urticaria, fevers, and arthralgias but lacking the monoclonal gammopathy and bone pain associated with Schnitzler’s syndrome. The p.Tyr570Cys mutation has been previously reported to occur in patients with NOMID, but this patient lacked the CNS-related inflammation and bony changes that usually associated with NOMID patients. In previous reports of early-onset CAPS patients, the cellular/tissue distribution of mosaicism was either not examined or ubiquitous (2, 4, 5, 8–10). Our patient is unique in that the mutation was mainly present in myeloid cells, the major drivers of disease in CAPS, confirming a new paradigm in the etiology of autoinflammation and possibly other diseases. Based on recent reports, patient suspected to have CAPS, Schnitzler’s syndrome and Blau syndrome who are negative for germ-line mutations should be investigated for somatic mutations (11, 14). Whereas high frequency somatic mutations might be detected by whole exome sequencing, low frequency mutations can be only detected by targeted deep resequencing of respective exons/genes.
The tissue distribution of mosaicism is largely determined by normal embryological processes, and thus when mutational events occur early in embryogenesis, the clonal expansion of mutations is broadly dispersed into different types of cells or tissues. However, when mutational events happen during a later stage of embryogenesis or after birth, the expansion of the mutation may be more limited. The mutational event in the patient reported here may have occurred later, possibly during the development of myeloid progenitor cells, considering the high frequency of mosaicism in monocytes and granulocytes and relative absence of the mutation in lymphocytes (Figure 2). Another possibility is that the mutation arose in hematopoietic stem cells, with a fitness advantage in myeloid, rather than lymphoid progenitor cells. The patient has four adult children with no medical histories consistent with CAPS, suggesting the absence of mosaicism in the germ line.
Given the uncertain significance of her childhood episodes of urticaria, it is difficult to estimate the time when her somatic mutation may have arisen. There are no pre-symptomatic tissue or blood samples available nor are there subsequent samples, aside from our recent collections, that could demonstrate potential expansion of the somatic mutation. Nevertheless, the current report underscores the utility of massively parallel sequencing in establishing a new molecular basis of CAPS in cases presenting after infancy, but who fortunately are also responsive to IL-1 inhibitory treatment.
Supplementary Material
Acknowledgments
Supported by the Intramural Research Programs of the National Human Genome Research Institute and the National Heart, Lung, and Blood Institute.
We would like to thank the affected patient for her support in this research study.
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Aksentijevich and Dr. Kastner had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Aksentijevich, Kastner
Acquisition of data. Zhou, Aksentijevich, Wood, Walts, Hoffmann, Remmers, Ombrello
Analysis and interpretation of data. Zhou, Aksentijevich
References
- 1.Ozen S, Bilginer Y. A clinical guide to autoinflammatory diseases: familial Mediterranean fever and next-of-kin. Nat Rev Rheumatol. 2014;10(3):135–47. doi: 10.1038/nrrheum.2013.174. [DOI] [PubMed] [Google Scholar]
- 2.Saito M, Fujisawa A, Nishikomori R, Kambe N, Nakata-Hizume M, Yoshimoto M, et al. Somatic mosaicism of CIAS1 in a patient with chronic infantile neurologic, cutaneous, articular syndrome. Arthritis Rheum. 2005;52(11):3579–85. doi: 10.1002/art.21404. [DOI] [PubMed] [Google Scholar]
- 3.Saito M, Nishikomori R, Kambe N, Fujisawa A, Tanizaki H, Takeichi K, et al. Disease-associated CIAS1 mutations induce monocyte death, revealing low-level mosaicism in mutation-negative cryopyrin-associated periodic syndrome patients. Blood. 2008;111(4):2132–41. doi: 10.1182/blood-2007-06-094201. [DOI] [PubMed] [Google Scholar]
- 4.Arostegui JI, Lopez Saldana MD, Pascal M, Clemente D, Aymerich M, Balaguer F, et al. A somatic NLRP3 mutation as a cause of a sporadic case of chronic infantile neurologic, cutaneous, articular syndrome/neonatal-onset multisystem inflammatory disease: Novel evidence of the role of low-level mosaicism as the pathophysiologic mechanism underlying mendelian inherited diseases. Arthritis Rheum. 2010;62(4):1158–66. doi: 10.1002/art.27342. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka N, Izawa K, Saito MK, Sakuma M, Oshima K, Ohara O, et al. High incidence of NLRP3 somatic mosaicism in patients with chronic infantile neurologic, cutaneous, articular syndrome: results of an International Multicenter Collaborative Study. Arthritis Rheum. 2011;63(11):3625–32. doi: 10.1002/art.30512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Izawa K, Hijikata A, Tanaka N, Kawai T, Saito MK, Goldbach-Mansky R, et al. Detection of base substitution-type somatic mosaicism of the NLRP3 gene with >99.9% statistical confidence by massively parallel sequencing. DNA Res. 2012;19(2):143–52. doi: 10.1093/dnares/dsr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka T, Takahashi K, Yamane M, Tomida S, Nakamura S, Oshima K, et al. Induced pluripotent stem cells from CINCA syndrome patients as a model for dissecting somatic mosaicism and drug discovery. Blood. 2012;120(6):1299–308. doi: 10.1182/blood-2012-03-417881. [DOI] [PubMed] [Google Scholar]
- 8.Jimenez-Trevino S, Gonzalez-Roca E, Ruiz-Ortiz E, Yague J, Ramos E, Arostegui JI. First report of vertical transmission of a somatic NLRP3 mutation in cryopyrin-associated periodic syndromes. Ann Rheum Dis. 2013;72(6):1109–10. doi: 10.1136/annrheumdis-2012-202913. [DOI] [PubMed] [Google Scholar]
- 9.Nakagawa K, Gonzalez-Roca E, Souto A, Kawai T, Umebayashi H, Campistol JM, et al. Somatic NLRP3 mosaicism in Muckle-Wells syndrome. A genetic mechanism shared by different phenotypes of cryopyrin-associated periodic syndromes. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2013-204361. [DOI] [PubMed] [Google Scholar]
- 10.Omoyinmi E, Melo Gomes S, Standing A, Rowczenio DM, Eleftheriou D, Klein N, et al. Brief Report: whole-exome sequencing revealing somatic NLRP3 mosaicism in a patient with chronic infantile neurologic, cutaneous, articular syndrome. Arthritis Rheumatol. 2014;66(1):197–202. doi: 10.1002/art.38217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Koning HD, van Gijn ME, Stoffels M, Jongekrijg J, Zeeuwen PL, Elferink MG, et al. Myeloid lineage-restricted somatic mosaicism of NLRP3 mutations in patients with variant Schnitzler syndrome. J Allergy Clin Immunol. 2015;135(2):561–4. e4. doi: 10.1016/j.jaci.2014.07.050. [DOI] [PubMed] [Google Scholar]
- 12.Coligan John E, AMK, Margulies David H, Shevach Ethan M, Strober Warren. Current Protocols in Immunology. New York, NY: John Wiley & Sons; 1999. [Google Scholar]
- 13.Aksentijevich I, Nowak M, Mallah M, Chae JJ, Watford WT, Hofmann SR, et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis Rheum. 2002;46(12):3340–8. doi: 10.1002/art.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Inocencio J, Mensa-Vilaro A, Tejada-Palacios P, Enriquez-Merayo E, Gonzalez-Roca E, Magri G, et al. Somatic NOD2 mosaicism in Blau syndrome. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2014.12.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.