Abstract
Background
Achieving tolerance of vascularized composite allografts (VCAs) would improve the risk-to-benefit ratio in patients who undergo this life-enhancing, though not life-saving, transplant. Kidney co-transplantation along with a short course of high-dose immunosuppression enables tolerance of heart allografts across a full MHC mismatch. In this study, we investigated whether tolerance of VCA across full MHC disparities could be achieved in animals already tolerant of heart and kidney allografts.
Methods
Miniature swine that were tolerant of heart and/or kidney allografts long-term underwent transplantation of myocutaneous VCA across the same MHC barrier. Prior to VCA transplant, Group 1 (n=3) underwent Class I-mismatched kidney transplantation; Group 2 (n=3) underwent two sequential Class I-mismatched kidney transplantations; Group 3 (n=2) underwent haploidentical MHC-mismatched heart/kidney transplantation; and Group 4 (n=2) underwent full MHC-mismatched heart/kidney transplantation.
Results
All three animals in Group 1 and two of three animals in Group 2 showed skin rejection ≤85 days; one animal in Group 2 showed prolonged skin survival >200 days. Animals in Groups 3 and 4 showed skin rejection ≤30 days and regained in vitro evidence of donor responsiveness.
Conclusion
This is the first pre-clinical study in which hearts, kidneys, and VCAs have been transplanted into the same recipient. Despite VCA rejection, tolerance of heart and kidney allografts was maintained. These results suggest that regulatory tolerance of skin is possible but not generally achieved by the same level of immunomodulation that is capable of inducing tolerance of heart and kidney allografts. Achieving tolerance of skin may require additional immunomodulatory therapies.
INTRODUCTION
Vascularized composite allograft (VCA) transplantation is an emerging field that provides patients significant functional and psychological benefits over conventional reconstructive techniques (1,2). To date, close to 30 face transplants and 100 hand transplants have been performed world-wide (3-6). However, because VCA transplantation is a life-enhancing rather than life-saving procedure, these benefits are mitigated by the risks of chronic immunosuppression. Recipients of VCAs have suffered from infection, malignancy, metabolic complications and drug toxicity as a result of immunosuppressive therapy, and despite medication compliance, as many as 85% of VCA recipients experience acute rejection episodes within the first year alone (5-7).
One strategy to offset these risks is to apply tolerance induction protocols already successful in clinical kidney transplantation to VCAs (8,9). Establishing immunological tolerance would maximize long-term, rejection-free survival and abrogate the need for chronic immunosuppression. Indeed, our laboratory has recently demonstrated the ability to induce tolerance of VCAs across a haploidentical (single haplotype, Class I and Class II) major histocompatibility (MHC) mismatch in a pre-clinical large animal model using a nonmyeloablative preconditioning regimen to generate durable multilineage mixed chimerism (10,11).
However, in contrast to kidney transplantation, tolerance of VCAs presents a particular set of challenges. First, as deceased donors remain the source of VCAs, tolerance protocols cannot include extensive recipient conditioning prior to transplant. Second, VCAs are composed of tissues that have varying degrees of antigenicity, with skin being the most antigenic (12,13). Third, the morbidity of conditioning protocols, such as the risk of graft-versus-host disease, should be minimized in the context of quality-of-life VCAs (8,9,14-16).
Demonstrating that immunomodulatory mechanisms alone could induce tolerance of VCAs would be a significant step forward in applying a tolerance strategy to clinical VCA transplantation. We previously demonstrated that transplantation of kidney allografts followed by 12 days of high-dose immunosuppression uniformly induces long-term tolerance across Class I alone or full MHC barriers (17,18). We have also demonstrated that kidney co-transplantation allows tolerance of cardiac allografts across a full MHC barrier (19). The mechanism underlying long-term acceptance involves systemic immunomodulation, as evidenced by in vitro studies identifying the necessary presence of a regulatory cell population (20,21) and the finding that long-term tolerant recipients of Class I mismatched renal allografts accepted subsequent donor MHC-matched kidney transplants without further immunosuppression (22).
VCAs placed in kidney recipients who had already achieved tolerance across a Class I alone MHC disparity rejected their skin component in five of the six animals tested (23). In this study, we investigated whether tolerance of VCA could be achieved in recipients already tolerant of kidney and heart allografts, hypothesizing that kidney-induced tolerance of a heart could be extended to a VCA. We compared VCAs that had been transplanted into recipients already tolerant of heart and/or kidney allografts across a (1) Class I alone MHC mismatch; (2) haploidentical MHC mismatch; and (3) full MHC mismatch (two haplotype, Class I and Class II).
MATERIALS AND METHODS
Animals
Transplant donors and recipients were selected from our herd of partially inbred miniature swine (age, 3-12 months; weight, 15-60kg). The immunogenetic characteristics of this herd have been described previously (24). In Group 1, to generate an MHC disparity across Class I but not Class II, SLAdd (class Idd/IIdd) animals received a kidney transplant from an SLAgg (class Icc/IIdd) donor with 12 days of cyclosporine, followed within 70 days by a myocutaneous VCA transplant from an SLAgg (class Icc/IIdd) donor without further immunosuppression (Table I, previously published in (23)). In Group 2, to generate an MHC disparity across Class I but not Class II, SLAdd (class Idd/IIdd) animals received a kidney transplant from an SLAgg (class Icc/IIdd) donor with 12 days of cyclosporine; after 100 days, Group 2 animals underwent nephrectomy of the primary kidney graft and a second donor-matched SLAgg (class Icc/IIdd) kidney transplant without further immunosuppression, followed by a donor-matched SLAgg (class Icc/IIdd) myocutaneous VCA transplant without further immunosuppression more than 100 days after the second kidney transplant (Table I, previously published in (23)). In Group 3, to generate an MHC disparity across a single haplotype with Class I and Class II, SLAac (class Iac/IIac) animals received heart and kidney transplants from an SLAad (class Iad/IIad) donor with 12 days of FK506, followed after 100 days with a donor-matched SLAad (class Iad/IIad) myocutaneous VCA transplant without further immunosuppression (Table I). In Group 4, to generate an MHC disparity across two haplotypes with Class I and Class II, SLAcc (class Icc/IIcc) animals received heart and kidney transplants from an SLAdd (class Idd/IIdd) donor with 12 days of FK506, followed after 100 days with a donor-matched SLAdd (class Idd/IIdd) myocutaneous VCA transplant without further immunosuppression (Table I). All recipients demonstrated significant in vitro anti-donor cytotoxic activity by CML and/or MLR before organ transplantation. All animal care and procedures were approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee and conducted in compliance with the “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources, National Research Council, and published by the National Academy Press.
Table I.
Survival of vascularized composite allografts
| Group | VCA MHC disparity | Animal # | MHC of Allografts | VCA survival (days) | ||||
|---|---|---|---|---|---|---|---|---|
| Donor | Recipient | Epidermis | Dermis/Muscle | |||||
| Heart | Kidney | VCA | ||||||
| 11 | Class I | 19842 | - | GG | GG | DD | 40 | accepted |
| 19941 | - | GG | GG | DD | 28 | accepted | ||
| 20652 | - | GG | GG | DD | 30 | accepted | ||
| 22 | Class I | 18954 | - | GG, GG | GG | DD | >200 | accepted |
| 18958 | - | GG, GG | GG | DD | 85 | accepted | ||
| 18955 | - | GG, GG | GG | DD | 45 | accepted | ||
| 3 | Single haplotype, Class I and II | 21270 | AD | AD | AD | AC | 15 | 29 |
| 21517 | AD | AD | AD | AC | 13 | 31 | ||
| 4 | Two haplotype, Class I and II | 21740 | DD | DD | DD | CC | 14 | 35 |
| 22025 | DD | DD | DD | CC | 30 | 30 | ||
Group 1 animals previously published in Cetrulo et al, Transplantation 2013 (23). Group 1 animals received a Class I mismatched VCA transplant less than 70 days after primary kidney transplant
Group 2 animals previously published in Cetrulo et al, Transplantation 2013 (23). Group 2 animals received a Class I mismatched VCA transplant at least 100 days after kidney re-transplantation, without further immunosuppression (i.e., >200 days after primary transplantation).
Surgical procedures
The surgical procedures used for heart and kidney transplantation have been described in detail previously (25-27). Briefly, the recipients underwent bilateral nephrectomy. The aorta and inferior vena cava were used for end-to-side arterial and venous anastomoses for both the heart and kidney, with the heart placed at least 1 cm caudad to the kidney. The kidney transplantation was completed by performing a vesicoureteral anastomosis. Two indwelling silastic central venous catheters were placed surgically into the external or internal jugular veins. The catheters facilitated immunosuppression administration and frequent blood sampling for in vitro assays and for monitoring of renal function and whole blood tacrolimus or cyclosporine levels.
The surgical procedure used for gracilis myocutaneous VCA transplantation has been described previously (28). The VCA, composed of skin, subcutaneous tissue, muscle and its vascular pedicle (femoral artery and femoral vein) was anastomosed to the recipient's internal jugular vein and internal carotid artery. The animal did not receive immunosuppression post-operatively.
Rejection monitoring
Kidney function was monitored by serial serum creatinine levels. Renal allograft rejection was defined as sustained rise in serum creatinine to >10 mg/dL and/or uremia. Heart function was monitored by daily palpation and electrocardiogram (ECG) using the AliveCor Veterinary Heart Monitor (AliveCor, Inc., San Francisco, CA). Cardiac allograft rejection was defined by either loss of a ventricular impulse on palpation, and/or QRS-wave amplitude of less than 0.3mV, and/or the lack of ventricular contraction on echocardiography (29). The VCA was monitored for viability by checking capillary refill and monitored for rejection by visual inspection and serial biopsies. VCA rejection was defined as the point at which the skin became necrotic and was confirmed by biopsy.
Routine biopsies were performed on all transplant recipients at predetermined time intervals (POD 20-30, 50-60, 90-100) or after a rise in creatinine, a decrease in donor heart palpation/QRS-wave amplitude or a change in VCA appearance. Allograft rejection was confirmed histologically in all cases.
Immunosuppression
Cyclosporine (Novartis Pharmaceutical Corporation, Hanover, NJ) was mixed and administered as an intravenous suspension according to the specifications of the manufacturer. Cyclosporine was given as a daily intravenous infusion over 1 hour (13 to 16 mg/kg/day with target levels 400 to 800 ng/mL) for 12 consecutive days, starting on the day of primary kidney transplantation (day 0) for animals in Groups 1 and 2.
Tacrolimus (Haorui Pharma-Chem Inc., Irvine, CA) was mixed and administered as an intravenous suspension according to the specifications of the manufacturer. Tacrolimus was given as a continuous infusion at a dose of 0.08-0.20 mg/kg (adjusted to maintain a whole blood level of 30-50 ng/ml) for 12 consecutive days, starting on the day of heart and kidney transplantation (day 0) for animals in Groups 3 and 4.
Pathology studies
Core needle biopsies were performed on cardiac allografts. Wedge biopsies were performed on kidney allografts. Kidney biopsies were taken at the same time as the heart samples. 6mm punch biopsies and wedge biopsies were performed on the VCA graft. Tissue was fixed in formalin and embedded in paraffin for routine light microscopy (H&E, PAS). Scoring of rejection was performed without knowledge of the functional status of the graft based on the International Society for Heart and Lung Transplantation System for hearts (30) and the current Banff consensus criteria for kidney (31) and vascularized cutaneous allografts (32). Complete necropsies were done upon completion of the experiments and tissue was similarly processed for pathological examination.
Preparation of peripheral blood leukocytes (PBLs)
Freshly heparinized whole blood was diluted approximately 1:2 with HBSS (Gibco BRL, Grand Island, NY), and the mononuclear cells were obtained by means of gradient centrifugation with Histopaque (Sigma, St. Louis, MO). The mononuclear cells were washed once with HBSS, and contaminating red cells were lysed with ammonium chloride potassium lysing buffer (Bio Whittaker, Inc, Walkersville, MD). Cells were then washed with HBSS and resuspended in tissue culture medium. All cell suspensions were kept at 4°C until used in cellular assays.
Cell-mediated lymphocytotoxicity (CML) assay
Cell-mediated lymphocytotoxicity (CML) assays with porcine cells have been described previously (33). The tissue culture media used for the CML assays consisted of RPMI-1640 (Gibco BRL) supplemented with 6% fetal bovine serum (Sigma Chemical Co, St Louis, MO), 100 U/mL penicillin, 135 mg/mL streptomycin (Gibco BRL), 50 mg/mL gentamicin (Gibco BRL), 10 mmol/L N –2-hydroxyethylpiperazine-N -2-ethanesulfonic acid (HEPES; Fisher Scientific, Pittsburgh, PA), 2 mmol/L L -glutamine (Gibco BRL), 1 mmol/L sodium pyruvate (Bio Whittaker, Inc), nonessential amino acids (Bio Whittaker, Inc), and 5 × 10–5 mol/L β2-mercaptoethanol (Sigma Chemical). The effector phase of the CML assay was performed with Basal Medium Eagle (Gibco BRL) supplemented with 6% controlled processed serum replacement 3 (Sigma Chemical) and 10 mmol/L HEPES. Briefly, lymphocyte cultures containing 4 × 106/mL responder and 4 × 106/mL stimulator PBLs (irradiated with 2500 cGy) were incubated for 6 days at 37° in 5% carbon dioxide and 100% humidity in CML medium. Bulk cultures were harvested, and effectors were tested for cytotoxic activity on chromium 51–labeled (Amersham, Arlington Heights, IL) lymphoblast targets generated from phytohemagglutinin (M-form; Life Technologies, Gaithersburg, MD) stimulation. Effector cells were incubated for 5.5 hours with target cells at effector/target ratios of 100:1, 50:1, 25:1, and 12.5:1. Two target cells were tested in each assay: (1) PBLs SLA matched to the donor and (2) third-party PBLs. Supernatants were then harvested by using the Skatron collection system (Skatron, Sterling, VA), and 51Cr release was determined on a gamma counter (Micromedics, Huntsville, AL). The results were expressed as a percentage of specific lysis and calculated as follows:
Mixed-lymphocyte reaction (MLR) assay
Mixed lymphocyte reaction (MLR) responses to self, donor and third-party were determined in a single assay for each animal. MLR media consisted of RPMI 1640 (Life Technologies) supplemented with 6% fetal pig serum (Sigma; St. Louis, MO, USA), 100 U/mL penicillin (GIBCO-Invitrogen Corporation; Carlsbad, CA, USA), 135 ug/mL streptomycin (GIBCO-Invitrogen Corporation), 50 ug/mL gentamicin (GIBCO-Invitrogen Corporation), 10 mM HEPES (Cellgro Mediatech, Inc.; Manassas, VA, USA), 2 mM l-glutamine (Life Technologies), 1 mM sodium pyruvate (BioWhittaker–Cambrex; East Rutherford, NJ, USA), nonessential amino acids (BioWhittaker–Cambrex) and 5 × 10−5 M 2-beta-mercaptoethanol (Sigma). Cultures containing 4 × 106 responder and 4 × 106 irradiated (2500 cGy) stimulator PBMCs were incubated in 200 uL of media in 96-well flat-bottomed plates (Costar Corning; Lowell, MA, USA) for 5 days at 37°C in 5% CO2 and 100% humidity. After the 5-day incubation, 1 uCi of [3H]-thymidine was added to each well, followed by an additional 5-hr incubation under the same conditions. [3H]-thymidine incorporation was determined in triplicate samples by beta-scintillation counting. Absolute counts were compensated for background and then expressed as stimulation indices (SI), calculated as SI = average counts per minute for a responder– stimulator pair per c.p.m. of the same responder stimulated by an autologous stimulator.
Assessment of alloantibody
The presence of anti-donor immunoglobulin (IgM and IgG) in the serum of experimental swine was examined by indirect flow cytometry using a Becton Dickinson FACScalibur (Sunnyvale, CA) to determine the SLA-binding specificity of the antibody. FITC-labeled goat anti-swine IgM or IgG polyclonal antibodies were used as secondary reagents (Kirkegaard & Perry Laboratories Inc, Gaithersburg, MD). For staining, 1 × 106 cells per tube of donor-type PBLs (SLAdd or SLAad) were resuspended in 100uL HBSS containing 0.1% bovine serum albumin and 0.05% NaN3 and incubated for 30 minutes at 4°C with 10uL decomplemented test sera (neat). After two washes, a saturating concentration of FITC-labeled goat anti-swine IgM or IgG was added and incubated for 30 minutes at 4°C. After a final wash, cells were analyzed by means of flow cytometry with propidium iodide gating to exclude dead cells. Both normal pig serum and pretransplant sera from each experimental animal were used as controls for specific binding.
RESULTS
Early epidermis and muscle loss in recipients of haploidentical MHC-mismatched VCAs
To determine whether tolerance achieved across a single-haplotype Class I and Class II mismatch could confer tolerance to a VCA, two animals (SLAac) who had been long-term tolerant of heart and kidney allografts (heart and kidney allografts from the same SLAad donor) for >100 days underwent VCA transplantation (VCA allograft from a different SLAad donor) (Group 3, Table I). The VCA on Group 3 animal #21270 showed visual and histological signs of epidermis rejection by POD13; VCA dermis and muscle were rejected by POD29. The VCA on Group 3 animal #21517 showed visual and histological signs of epidermis rejection by POD13; VCA dermis and muscle were rejected by POD31 (Figure 1). In contrast, five of six animals tolerant of kidneys across a Class I mismatch who then received a Class I mismatched VCA demonstrated VCA epidermis rejection within 85 days but showed long-term acceptance of VCA dermis and muscle (Groups 1 and 2, Table I) (23).
Figure 1.
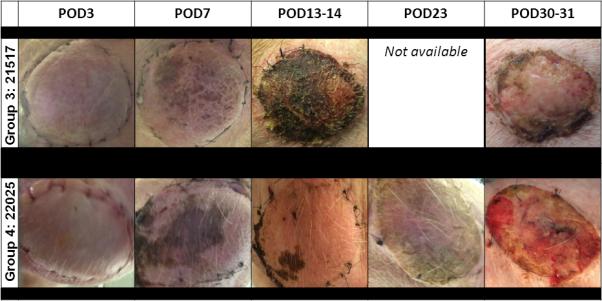
Gross appearance of VCA. Representative clinical images of VCAs from Group 3 animal #21517 (top row) and Group 4 animal #22026 (bottom row) by postoperative day. Animals in both groups showed patchy areas of necrosis starting on postoperative day 7 that progressed to epidermal sloughing.
Early epidermis and muscle loss in recipients of full MHC-mismatched VCAs
To determine whether tolerance achieved across a two-haplotype Class I and Class II mismatch could confer tolerance to a VCA, two animals (SLAcc) who had been long-term tolerant of heart and kidney allografts (heart and kidney allografts from the same SLAdd donor) for >100 days underwent VCA transplantation (VCA allograft from a different SLAdd donor) (Group 4, Table I). The VCA on Group 4 animal #21740 showed visual and histological signs of epidermis rejection by POD14; VCA dermis and muscle were rejected by POD35. The VCA on Group 4 animal #22025 showed visual and histological signs of epidermis, dermis, and muscle rejection by POD30 (Figure 1).
Donor-MHC-specific responsiveness is regained after VCA transplant
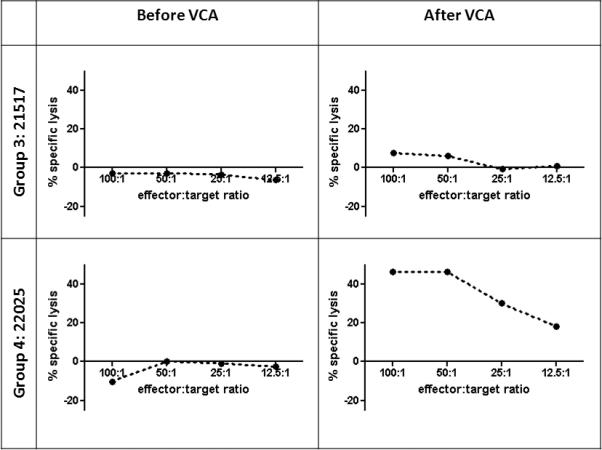
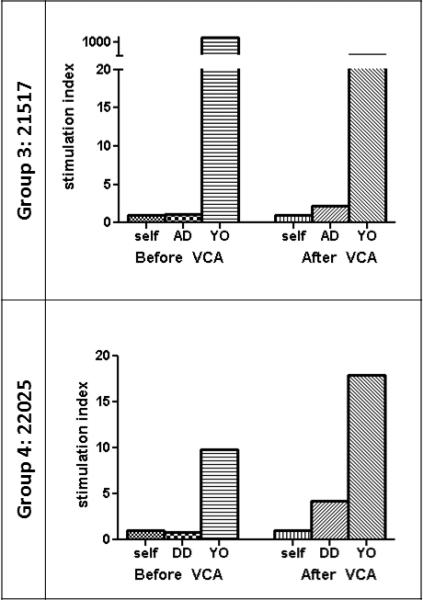
To assess immune competence in recipients before and after VCA transplant, MLR and CML assays were performed. Group 3 animal #21517, which had been donor-specific unresponsive by CML and MLR prior to VCA transplant, regained donor-responsiveness after VCA transplant (Figures 3 and 4). Group 3 animal #21270 already showed positive donor-specific response by CML and MLR at the time of VCA transplant (data not shown). Both Group 4 animals showed donor-specific unresponsiveness by CML and MLR prior to VCA transplant. After VCA transplant, Group 4 animal #22025 regained donor-responsiveness by CML and MLR (Figures 3 and 4); Group 4 animal #21740 also regained donor-responsiveness by MLR, but remained unresponsive to donor by CML (data not shown). Animals in Groups 1 and 2 displayed donor-specific hyporesponsiveness or unresponsiveness by CML before and after VCA epidermis rejection (23).
Figure 3.
MLR assays from VCA recipients. Stimulation indices to self, donor-type (SLAad or SLAdd), and third-party (YO) peripheral blood mononuclear cells before VCA transplant and 30-31 days after VCA transplant. MLR, mixed-lymphocyte reaction. YO, York.
Figure 4.
CML assays from VCA recipients. Percent specific lysis is plotted as a function of effector:target ratio. Response against donor-type (SLAad or SLAdd) targets before VCA transplant and 30-31 days after VCA transplant. CML, cell-mediated lympholysis.
Lack of circulating alloantibody following VCA rejection
To determine whether rejection of VCA led to alloantibody formation, flow cytometry analysis of anti-donor antibodies was performed. Animals in Group 3 and Group 4 did not develop any detectable circulating levels of anti-donor IgM or IgG antibody before or after VCA transplant (Figure 5). Animals in Groups 1 and 2 also did not develop alloantibody after VCA transplant (23).
Figure 5.
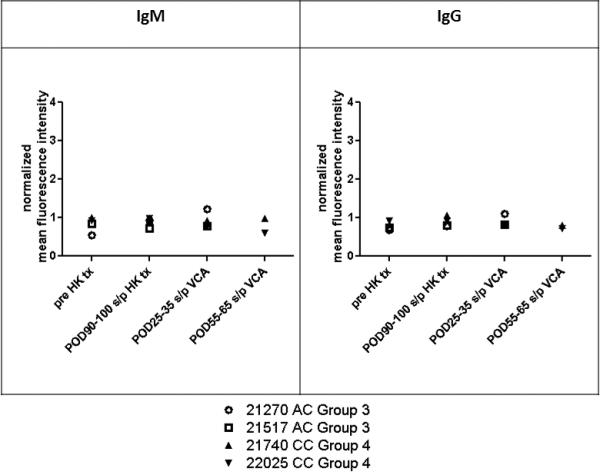
Alloantibody response. Levels of circulating IgM and IgG alloantibody were measured by flow cytometry in recipients in Groups 3 and 4. Data were normalized to the mean fluorescence intensity of negative control values to plot normalized mean fluorescence intensity as a function of postoperative day (POD). HK tx, heart kidney transplant.
Heart and kidney allograft tolerance maintained despite VCA rejection
Animals in Groups 3 and 4 were monitored by serial biopsies to determine whether VCA rejection affected tolerance of heart or kidney allografts. Heart and kidney allografts were biopsied up to 40 days after VCA transplant in Group 3 and up to 65 days after VCA transplant in Group 4. Animals in both groups showed no clinical or histological signs of heart rejection (Table II). Animals in Group 3 showed no signs of kidney rejection, though animal #21270 developed pyelonephritis with a corresponding increase in creatinine (Table II). Group 4 animal #22025 showed no signs of kidney rejection; however, Group 4 animal #21740 had chronic rejection changes present prior to VCA transplantation (Table II). Nodular lymphocytic infiltrates associated with the vasculature of the kidney defined as “Treg-rich organized lymphoid structures” were found in all animals on kidney biopsies before VCA transplantation and after VCA rejection (Figure 6).
Table II.
Histology and function of heart and kidney allografts before and after VCA transplantation
| Group | VCA MHC disparity | Animal # | Heart Histology1/Function2 | Kidney Histology1/Function2 | ||
|---|---|---|---|---|---|---|
| Before VCA | After VCA | Before VCA | After VCA | |||
| 3 | Single haplotype, Class I and II | 21270 | 0/sinus | 0/sinus | 0/1.7 | 1/23.23 |
| 21517 | 0/sinus | 0/sinus | 1/1.7 | 1/1.8 | ||
| 4 | Two haplotype, Class I and II | 21740 | 0/sinus | 1/sinus | chronic/4.24 | chronic/8.44 |
| 22025 | 0/sinus | 0/sinus | 0/1.3 | 0/0.7 | ||
Grading of heart acute rejection from 0 (no rejection) to 3 (severe rejection) based on ISHLT scoring system (28); grading of kidney acute rejection from 0 (no rejection) to 3 (severe rejection) based on Banff classification (29).
Function of heart allografts assessed by ECG and palpation; function of kidney allografts assessed by creatinine measurements (mg/dL)
Animal #21270 developed pyelonephritis.
Animal #21740 had chronic rejection of the kidney allograft before and after VCA transplantation.
Figure 6.
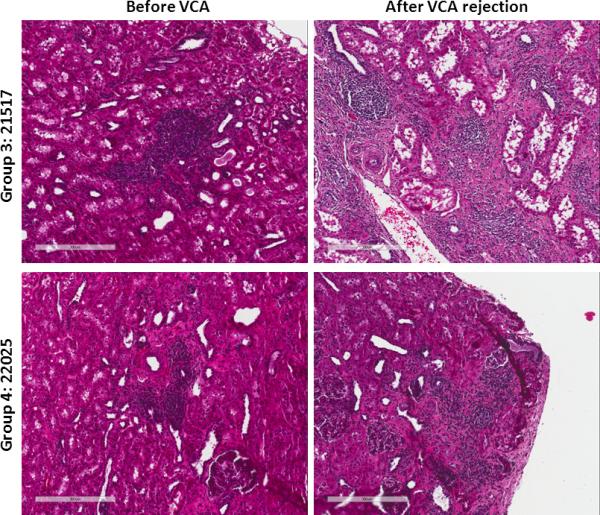
Histology from representative kidney samples taken prior to VCA transplantation and after VCA rejection. Group 3 animal #21517 (top row) and Group 4 animal #22025 (bottom row) demonstrate the presence of organized lymphoid structures before and after VCA rejection.
DISCUSSION
Because VCA transplantation is a life-enhancing rather than life-saving procedure, any risk to the patient must be minimized. Inducing tolerance of VCAs would alleviate the burden of chronic immunosuppression (5-7). The use of deceased donors and a small donor pool preclude MHC antigen matching, implying that tolerance of VCAs needs to be applicable across full Class I and Class II MHC disparities. However, current clinical and pre-clinical protocols for achieving tolerance across full MHC mismatches contain risks that are unacceptable for the VCA recipient. For example, tolerance of MHC-mismatched kidneys was achieved in humans through conditioning regimens that generate mixed chimerism, which carry up to a 34% risk of GvHD (8,9,34-36). In a pre-clinical large animal model, a mixed chimerism-based protocol resulted in tolerance of haploidentical VCAs but incurred the morbidity of GvHD in 12% of animals (11). Therefore, achieving tolerance of VCA using immunomodulatory pathways alone would be an important building block from which alternative strategies that do not require stable chimerism or intense conditioning can be developed.
Here we investigated whether immunomodulatory mechanisms alone are sufficient to induce tolerance of VCAs. Immunomodulation occurring during the maintenance phase of kidney-induced cardiac allograft tolerance across a full MHC mismatch is known to allow indefinite survival of heart allografts without the presence of kidney grafts and prolong donor skin graft survival (19). In this study, we found that animals who received hearts/kidneys and a delayed VCA across a haploidentical or full MHC barrier showed rejection of the skin component, rejection of the muscle and return of donor responsiveness by CML and MLR (Groups 3 and 4, Table I, Figures 3 and 4). In comparison, animals in Groups 1 and 2, who received kidneys and a delayed VCA across a Class I MHC barrier, showed rejection of the skin component, long-term acceptance of the muscle and maintenance of donor-specific unresponsiveness in vitro (23).
These findings demonstrate that (1) immunomodulatory mechanisms that maintain tolerance of heart and kidney allografts are usually, but not always, insufficient to induce tolerance of VCAs and (2) inducing tolerance across a full MHC barrier is more difficult than inducing tolerance across a lesser MHC disparity (e.g. prolonged skin survival in Group 1 and 2 versus Group 3 and 4 recipients). Indeed, in 1 of 3 animals who achieved tolerance across a Class I alone MHC mismatch (#18954), VCA epidermis survival was prolonged for over 200 days (Group 2, (23)). This finding indicates that tolerance of VCA via immunomodulatory mechanisms alone, although rare in these studies, is possible and can be potentially due, in the case of animal #18954, to augmented T regulatory cell activity (23), the reason for which may be worthy of further investigation.
The ability of systemic versus local factors to induce tolerance depends on the tolerogenicity of the organ implanted. For example, with a tolerogenic organ such as the kidney, regulatory mechanisms that maintain long-term tolerance of kidneys (18,20,37-39) are sufficient to induce tolerance. Long-term tolerant recipients of Class I mismatched renal allografts accepted second transplants from donors MHC matched to the donors of the first renal grafts without additional immunosuppression (22). Here we attempted to exploit the robust tolerance achieved across a full MHC mismatch for heart and kidney allografts to achieve tolerance of VCA. Tolerance across a full MHC mismatch, despite being more difficult to induce, appears harder to abrogate once gained. This hypothesis is based on recent work showing that recipients of Class I alone MHC mismatched heart and kidney allografts who undergo kidney graftectomy and subsequent skin grafting demonstrate heart rejection with severe cardiac allograft vasculopathy whereas recipients of full MHC mismatched heart and kidney allografts who are subject to the same immunologic challenge remain tolerant of their heart allografts indefinitely (Michel et al, manuscript in preparation). However, in the present study, despite robust tolerance across highly disparate MHC barriers, VCAs placed in a delayed fashion showed en-bloc rejection. One possibility is that the state of tolerance was not robust enough to induce tolerance of VCA. Another more likely possibility is that the VCA itself, due to tissue-specific or minor antigens, is not as tolerogenic as a kidney and requires additional conditioning for acceptance. Indeed, a 28-day course of high-dose FK506, which is sufficient to induce kidney allograft tolerance (18), could not induce tolerance of VCA (2 animals, Torabi et al, unpublished data).
Interestingly, despite en-bloc rejection of the VCA and return of donor-specific responsiveness in vitro (Figures 3 and 4), animals in Groups 3 and 4 remained tolerant of their heart and kidney allografts. This finding suggests the presence of intra-graft suppressive phenomena that maintain tolerance of hearts and kidneys despite sensitization to donor MHC (22,38,40,41). Indeed, nodular lymphocytic infiltrates associated with the vasculature of the kidney, termed “Treg-rich organized lymphoid structures” (42) were found in kidney samples taken before and after VCA rejection (Figure 6). A key difference between the tolerant heart/kidney grafts and rejected VCA grafts is timing of transplantation. Future work will investigate whether kidney transplantation at the time of VCA transplant (day 0 protocol) would generate sufficient systemic and local immunomodulatory mechanisms to enable tolerance of VCA.
Long-term acceptance of the skin component of VCAs without incurring risk of comorbidity remains an elusive goal. Several groups have attempted to offset risk by minimizing maintenance immunosuppression or by the addition of costimulatory blockade (43-47). Current mixed chimerism protocols, on the other hand, contain the risk of GvHD (8,11,35). Demonstrating that immunomodulatory mechanisms alone could induce tolerance of VCAs would be a significant step forward. To our knowledge, this is the first pre-clinical study in which hearts, kidneys, and VCAs have been transplanted into the same recipients. We demonstrate that a robust immunomodulatory milieu alone, which is able to induce tolerance of kidneys, is insufficient to achieve tolerance of VCA in most cases. In this regard, tolerance of VCA, especially skin, is more difficult to achieve than tolerance of kidneys or hearts co-transplanted with kidneys, and will require additional strategies that address both the need for tolerance across highly disparate MHC and for minimizing the risks of a life-enhancing procedure.
Figure 2.
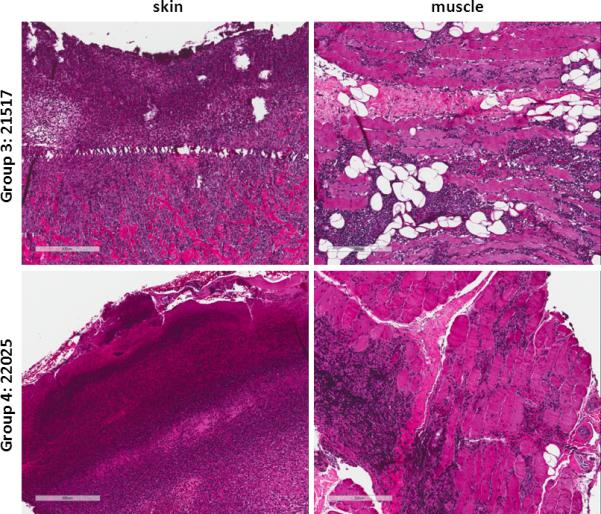
Histology from representative VCA biopsies taken on postoperative day 30-31. VCA biopsy from Group 3 animal #21517 (top row) shows grade 4 rejection of the epidermis and acute rejection of the muscle with endarteritis. VCA biopsy from Group 4 animal #22025 (bottom row) shows grade 4 rejection of the epidermis and acute rejection of the muscle with endarteritis.
ACKNOWLEDGEMENTS
We are indebted to Mr. J. Scott Arn for management of the swine herd. We thank Nicole Brousaides for preparing biopsy samples for histological analysis.
Funding:
This work was supported in part by grants from the National Heart, Lung, and Blood Institute (P01HL18646) and the National Institute of Allergy and Infectious Disease (R01AI84657) of the National Institutes of Health. Dr. Madariaga is an Edward D. Churchill Surgical Research Fellow, Massachusetts General Hospital and recipient of a fellowship from the International Society for Heart and Lung Transplantation and a National Research Service Award from the National Heart, Lung, and Blood Institute of the National Institutes of Health (F32HL117540). Dr. Michel is a recipient of the 2013 ASTS-Novartis Scientist Scholarship Grant. Dr. Leonard is a recipient of the 2012 AST-Genentech Basic Scientist Scholarship and the 2013 ASTS-Novartis Scientist Scholarship Grant. We acknowledge C06RR020135-01 for construction of the facility utilized for production and maintenance of miniature swine and Novartis for generous supply of cyclosporine used in preparation of chimeric donors and in treatment of lung recipients.
Abbreviations
- ACR
acute cellular rejection
- CML
cell-mediated lympholysis
- HKtx
heart kidney transplant
- HSC
hematopoietic stem cell
- MHC
major histocompatibility complex
- MLR
mixed-lymphocyte reaction
- PAA
pig allelic antigen
- PBL
peripheral blood leukocytes
- POD
postoperative day
- PSL
percent specific lysis
- SLA
swine lymphocyte antigen
- VCA
vascularized composite allograft.
Footnotes
Disclosure:
The authors declare no conflict of interest.
Reference List
- 1.Diaz-Siso JR, Bueno EM, Sisk GC, Marty FM, Pomahac B, Tullius SG. Vascularized composite tissue allotransplantation--state of the art. Clin Transplant. 2013;27:330–337. doi: 10.1111/ctr.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pribaz JJ, Caterson EJ. Evolution and limitations of conventional autologous reconstruction of the head and neck. J Craniofac Surg. 2013;24:99–107. doi: 10.1097/SCS.0b013e31827104ab. [DOI] [PubMed] [Google Scholar]
- 3.Shanmugarajah K, Hettiaratchy S, Clarke A, Butler PE. Clinical outcomes of facial transplantation: a review. Int J Surg. 2011;9:600–607. doi: 10.1016/j.ijsu.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Khalifian S, Brazio PS, Mohan R, et al. Facial transplantation: the first 9 years. Lancet. 2014 doi: 10.1016/S0140-6736(13)62632-X. [DOI] [PubMed] [Google Scholar]
- 5.Petruzzo P, Dubernard JM. The International Registry on Hand and Composite Tissue allotransplantation. Clin Transpl. 2011:247–253. [PubMed] [Google Scholar]
- 6.Petruzzo P, Lanzetta M, Dubernard JM, et al. The International Registry on Hand and Composite Tissue Transplantation. Transplantation. 2010;90:1590–1594. doi: 10.1097/TP.0b013e3181ff1472. [DOI] [PubMed] [Google Scholar]
- 7.Petit F, Minns AB, Dubernard JM, Hettiaratchy S, Lee WP. Composite tissue allotransplantation and reconstructive surgery: first clinical applications. Ann Surg. 2003;237:19–25. doi: 10.1097/00000658-200301000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawai T, Sachs DH, Sykes M, Cosimi AB. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2013;368:1850–1852. doi: 10.1056/NEJMc1213779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horner BM, Randolph MA, Duran-Struuck R, et al. Induction of tolerance to an allogeneic skin flap transplant in a preclinical large animal model. Transplant Proc. 2009;41:539–541. doi: 10.1016/j.transproceed.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leonard DA, Kurtz JM, Mallard C, et al. Vascularized composite allograft tolerance across MHC barriers in a large animal model. Am J Transplant. 2014;14:343–355. doi: 10.1111/ajt.12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee WP, Yaremchuk MJ, Pan YC, Randolph MA, Tan CM, Weiland AJ. Relative antigenicity of components of a vascularized limb allograft. Plast Reconstr Surg. 1991;87:401–411. doi: 10.1097/00006534-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Murray JE. Organ transplantation (skin, kidney, heart) and the plastic surgeon. Plast Reconstr Surg. 1971;47:425–431. doi: 10.1097/00006534-197105000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Markmann JF, Kawai T. The quest for transplantation tolerance: have we finally sipped from the cup? Sci Transl Med. 2012;4:124fs5. doi: 10.1126/scitranslmed.3003678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leventhal J, Abecassis M, Miller J, et al. Chimerism and tolerance without GVHD or engraftment syndrome in HLA-mismatched combined kidney and hematopoietic stem cell transplantation. Sci Transl Med. 2012;4:124ra28. doi: 10.1126/scitranslmed.3003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scandling JD, Busque S, Dejbakhsh-Jones S, et al. Tolerance and withdrawal of immunosuppressive drugs in patients given kidney and hematopoietic cell transplants. Am J Transplant. 2012;12:1133–1145. doi: 10.1111/j.1600-6143.2012.03992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosengard BR, Ojikutu CA, Guzzetta PC, et al. Induction of specific tolerance to class I disparate renal allografts in miniature swine with cyclosporine. Transplantation. 1992;54:490–497. doi: 10.1097/00007890-199209000-00020. [DOI] [PubMed] [Google Scholar]
- 18.Utsugi R, Barth RN, Lee RS, et al. Induction of transplantation tolerance with a short course of tacrolimus (FK506): I. Rapid and stable tolerance to two-haplotype fully mhc-mismatched kidney allografts in miniature swine. Transplantation. 2001;71:1368–1379. doi: 10.1097/00007890-200105270-00003. [DOI] [PubMed] [Google Scholar]
- 19.Madariaga ML, Michel SG, Tasaki M, et al. Induction of cardiac allograft tolerance across a full MHC barrier in miniature sqine by donor kidney co-transplantation. American Journal of Transplantation. 2013;13:2558–2566. doi: 10.1111/ajt.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ierino FL, Yamada K, Hatch T, Rembert J, Sachs DH. Peripheral tolerance to class I mismatched renal allografts in miniature swine: donor antigen-activated peripheral blood lymphocytes from tolerant swine inhibit antidonor CTL reactivity. J Immunol. 1999;162:550–559. [PubMed] [Google Scholar]
- 21.Wu A, Yamada K, Ierino FL, Vagefi PA, Sachs DH. Regulatory mechanism of peripheral tolerance: in vitro evidence for dominant suppression of host responses during the maintenance phase of tolerance to renal allografts in miniature swine. Transpl Immunol. 2003;11:367–374. doi: 10.1016/S0966-3274(03)00006-6. [DOI] [PubMed] [Google Scholar]
- 22.Gianello PR, Yamada K, Fishbein JM, et al. Long-term acceptance of primarily vascularized renal allografts in miniature swine. Systemic tolerance versus graft adaptation. Transplantation. 1996;61:503–506. doi: 10.1097/00007890-199602150-00032. [DOI] [PubMed] [Google Scholar]
- 23.Cetrulo CL, Jr., Torabi R, Scalea JR, et al. Vascularized composite allograft transplant survival in miniature Swine: is MHC tolerance sufficient for acceptance of epidermis? Transplantation. 2013;96:966–974. doi: 10.1097/TP.0b013e3182a579d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sachs DH, Leight G, Cone J, Schwartz S, Stuart L, Rosenberg S. Transplantation in miniature swine. I. Fixation of the major histocompatibility complex. Transplantation. 1976;22:559–567. doi: 10.1097/00007890-197612000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Madsen JC, Yamada K, Allan JS, et al. Transplantation tolerance prevents cardiac allograft vasculopathy in major histocompatibility complex class I-disparate miniature swine. Transplantation. 1998;65:304–313. doi: 10.1097/00007890-199802150-00002. [DOI] [PubMed] [Google Scholar]
- 26.Madsen JC, Sachs DH, Fallon JT, Weissman NJ. Cardiac allograft vasculopathy in partially inbred miniature swine. I. Time course, pathology, and dependence on immune mechanisms. J Thorac Cardiovasc Surg. 1996;111:1230–1239. doi: 10.1016/s0022-5223(96)70226-x. [DOI] [PubMed] [Google Scholar]
- 27.Kirkman RL, Colvin MW, Flye GS, et al. Transplantation in miniature swine. VI. Factors influencing survival of renal allografts. Transplantation. 1979;28:18–23. [PubMed] [Google Scholar]
- 28.Leto Barone AA, Leonard DA, Torabi R, et al. The gracilis myocutaneous free flap in swine: an advantageous preclinical model for vascularized composite allograft transplantation research. Microsurgery. 2013;33:51–55. doi: 10.1002/micr.21997. [DOI] [PubMed] [Google Scholar]
- 29.Avitall B, Payne DD, Connolly RJ, et al. Heterotopic heart transplantation: electrophysiologic changes during acute rejection. J Heart Transplant. 1988;7:176–182. [PubMed] [Google Scholar]
- 30.Mehra MR, Crespo-Leiro MG, Dipchand A, et al. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy-2010. J Heart Lung Transplant. 2010;29:717–727. doi: 10.1016/j.healun.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 31.Haas M, Sis B, Racusen LC, et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14:272–283. doi: 10.1111/ajt.12590. [DOI] [PubMed] [Google Scholar]
- 32.Cendales LC, Kanitakis J, Schneeberger S, et al. The Banff 2007 working classification of skin-containing composite tissue allograft pathology. Am J Transplant. 2008;8:1396–1400. doi: 10.1111/j.1600-6143.2008.02243.x. [DOI] [PubMed] [Google Scholar]
- 33.Kirkman RL, Colvin RB, Flye MW, Williams GM, Sachs DH. Transplantation in miniature swine. VII. Evidence for cellular immune mechanisms in hyperacute rejection of renal allografts. Transplantation. 1979;28:24–30. [PubMed] [Google Scholar]
- 34.Kawai T, Sachs DH, Hoshino T, et al. Graft-vs-host tolerance in mixed allogeneic chimerism. Transplant Proc. 1997;29:1222–1223. doi: 10.1016/s0041-1345(96)00566-0. [DOI] [PubMed] [Google Scholar]
- 35.Leventhal J, Abecassis M, Miller J, et al. Tolerance induction in HLA disparate living donor kidney transplantation by donor stem cell infusion: durable chimerism predicts outcome. Transplantation. 2013;95:169–176. doi: 10.1097/TP.0b013e3182782fc1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luznik L, O'Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okumi M, Scalea JR, Gillon BC, et al. The Induction of Tolerance of Renal Allografts by Adoptive Transfer in Miniature Swine. Am J Transplant. 2013 doi: 10.1111/ajt.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scalea JR, Okumi M, Villani V, et al. Abrogation of Renal Allograft Tolerance in MGH Miniature Swine: The Role of Intra-Graft and Peripheral Factors in Long-Term Tolerance. Am J Transplant. 2014 doi: 10.1111/ajt.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu A, Yamada K, Ierino FL, Vagefi PA, Sachs DH. Regulatory mechanism of peripheral tolerance: in vitro evidence for dominant suppression of host responses during the maintenance phase of tolerance to renal allografts in miniature swine. Transpl Immunol. 2003;11:367–374. doi: 10.1016/S0966-3274(03)00006-6. [DOI] [PubMed] [Google Scholar]
- 40.Rosengard BR, Ojikutu CA, Guzzetta PC, et al. Renal transplantation in miniature swine: preliminary evidence that graft infiltrating leukocytes suppress donor-specific cell-mediated lymphocytotoxicity in co-culture. Transplant Proc. 1991;23:189–191. [PubMed] [Google Scholar]
- 41.Rosengard BR, Fishbein JM, Gianello P, et al. Retransplantation in miniature swine. Lack of a requirement for graft adaptation for maintenance of specific renal allograft tolerance. Transplantation. 1994;57:794–799. [PubMed] [Google Scholar]
- 42.Miyajima M, Chase CM, Alessandrini A, et al. Early acceptance of renal allografts in mice is dependent on foxp3(+) cells. Am J Pathol. 2011;178:1635–1645. doi: 10.1016/j.ajpath.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gorantla VS, Brandacher G, Schneeberger S, et al. Favoring the risk-benefit balance for upper extremity transplantation--the Pittsburgh Protocol. Hand Clin. 2011;27:511–51x. doi: 10.1016/j.hcl.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 44.Breidenbach WC, Gonzales NR, Kaufman CL, Klapheke M, Tobin GR, Gorantla VS. Outcomes of the first 2 American hand transplants at 8 and 6 years posttransplant. J Hand Surg Am. 2008;33:1039–1047. doi: 10.1016/j.jhsa.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 45.Schneeberger S, Gorantla VS, Brandacher G, et al. Upper-extremity transplantation using a cell-based protocol to minimize immunosuppression. Ann Surg. 2013;257:345–351. doi: 10.1097/SLA.0b013e31826d90bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siemionow M, Ozturk C. An update on facial transplantation cases performed between 2005 and 2010. Plast Reconstr Surg. 2011;128:707e–720e. doi: 10.1097/PRS.0b013e318230c77b. [DOI] [PubMed] [Google Scholar]
- 47.Wachtman GS, Wimmers EG, Gorantla VS, et al. Biologics and donor bone marrow cells for targeted immunomodulation in vascularized composite allotransplantation: a translational trial in swine. Transplant Proc. 2011;43:3541–3544. doi: 10.1016/j.transproceed.2011.10.010. [DOI] [PubMed] [Google Scholar]


