Abstract
The overwhelming body of research on T regulatory cells (Tregs) has focused on CD4+CD25+Foxp3+ T cells. However, recent years have witnessed a resurgence in interest in CD4-CD8+, CD4-CD8- (double negative; DN), and CD4+Foxp3- Tr1 Tregs and their role in controlling autoimmune diseases and in promoting the survival of organ allografts and xenografts. CD8+ and DN Tregs can arise spontaneously (natural Tregs) or can be induced in situ. Both CD8+ and DN Tregs have been shown to enhance the survival of organ allografts and xenografts. Additionally, both can suppress alloimmune responses by contact-dependent mechanisms by either inducing apoptosis or mediating direct cytolysis of effector T cells. CD8+, DN, and Tr1 Tregs can also act in a contact-independent manner by elaborating soluble immunosuppressive factors such TGF-β and IL-10. Applying CD8+, DN, and Tr1 Tregs for enhancing the survival of organ allografts and xenografts is still in its infancy but holds significant potential. Furthermore, there is a need for a more comprehensive understanding of how current immunosuppressive therapies applied to organ transplantations affect the wide array of Treg populations.
Introduction
Shortly after the role of T cells in protective immunity was recognized, Gershon and co-workers proposed that a subpopulation of T cells might be capable of suppressing the immune response. Accordingly, the term “suppressor T cell” was coined to describe T cells that were responsible for processes that were previously categorized as tolerance, antigen competition or feedback regulation (1). Initially, suppressor cells were defined largely by their in vitro functional properties. However, the suppressor cell concept quickly fell out of favor due to the absence of distinct surface markers or transcription factors unique to this cell population along with the inability of suppressor cell investigators to identify genes in the putative I-J region in the immune response region of the murine MHC implicated in suppressor cell development (2). As a result, suppressor cell research was largely abandoned for over a decade until it was resurrected by Sakaguchi et al. who provided compelling evidence for the presence of CD4+CD25+ suppressor cells. However, this time around suppressor cells wore the euphemistic moniker “T regulatory cells” (Tregs) (3). Since Sakaguchi's report, hundreds of publications have described and analyzed CD4+T regsand the overwhelming majority of these publications have focused on conventional CD4+CD25+Foxp3+ Tregs. Since numerous review articles have addressed the biology and immunologic properties of CD4+CD25+Foxp3+ Tregs, this review will focus on non-CD4+CD25+Foxp3+Tregs in the context of organ transplantation.
CD8+Tregs
CD8+ Treg phenotypes
The phenotype of Gershon's original suppressor cell was defined by its expression of the CD8 (Lyt-2) surface marker (4). However, in the 40 years since their original description, CD8+ Tregs have been categorized into several distinct phenotypes including: a) Qa-1/HLA-restricted Tregs (5-7); b) CD8+CD122+ Tregs (8); c) CD8+CD28- Tregs (9); d) CD8+Foxp3+ Tregs (10); e) CD8+CD103+ Tregs (11); f) CD8+LAG-3+Foxp3+CTLA-4+ Tregs (12); g) CD8+IL-10+CCR7+CD45RO+ Tregs (13); h) CD8+CD45RClow Tregs (14); i) CD8+CD122+PD-1+ Tregs (15) and j) CD8+CD11chigh Tregs (16) (Table 1). CD8+ Tregs can arise within the thymus as naturally occurring CD8+ Tregs or they can be induced in peripheral tissues by a variety of maneuvers including donor-specific transfusion (17), injection of antigen into the anterior chamber (AC) of the eye (18), anti-idiotype immunization with allospecific T cells or MHC-derived peptides (19, 20), or by in vivo immunization combined with blockade of CD40 co-stimulatory molecules (21). Expression of the IL-2 receptor beta subunit, CD122, is crucial for CD8+ Treg development and function (7). Although CD122+ is also expressed on classical CD8+ memory T cells, expression of PD-1on CD8+CD122+ T cells is limited to Tregs and distinguishes Tregs from memory T cells (22). In addition to the surface markers mentioned above, CD8+ Tregs can also express CD44 and the natural killer (NK) cell inhibitory marker Ly49.
Table 1. Phenotypic markers for non-CD4+CD25+T regs.
| Treg Population | Phenotypic Markers | Reference |
|---|---|---|
| CD8+ Tregs | Qa-1/HLA-restricted | (5-7) |
| CD8+ CD122+ | (8) | |
| CD8+CD28- | (9) | |
| CD8+Foxp3+ | (10) | |
| CD8+CD103+ | (11) | |
| CD8+LAG-3+ Foxp3+CTLA-4+ | (12) | |
| CD8+ IL-10+CCR7+CD45RO+ | (13) | |
| CD8+CD45RClow | (14) | |
| CD8+CD122+PD-1+ | (15) | |
| CD8+CD11chigh | (16) | |
| CD4-CD8- DN Tregs | CD25+CD30+CD28low | (51) |
| CD25+CD28+CD44+CD69+ | (55) | |
| CD25-CD27+CD28low | (56) | |
| CD4+Foxp3- Tr1 Tregs | CD49b+LAG-3+ | (74) |
CD8+ Treg mechanisms of suppression
CD8+ Treg suppression can be mediated by either contact-dependent (23) or contact-independent mechanisms (24) (Table 2). Contact-dependent suppression by CD8+ Tregs involves the direct killing of CD4+ T effector cells by perforin-mediated cytolysis (25, 26) or FasL-induced apoptosis (16). In addition to suppressing effector T cells, CD8+Foxp3+ Tregs are capable of inducing the de novo generation of CD4+Foxp3+ Tregs by a process that is contact-dependent and requires the production of soluble TGF-β (27), and is reminiscent of a phenomenon that has been previously described as “infectious tolerance”. When CD8+ Tregs are in contact with CD4+ T cells suppression is supported by IFN-γ, indoleamine 2,3 dioxygenase (IDO), and fibroleukin-2 (24). However, IDO can also mediate suppression when CD8+ Tregs are not in direct contact with CD4+ effector T cells by inhibiting T cell proliferation (24). CD8+ Tregs can also suppress immune effector responses by elaborating a variety of soluble factors such as TGF-β and IL-10, which inhibit T cell activation and proliferation (28, 29).
Table 2. Mechanisms of suppression for non-CD4+CD25+T regs.
| Treg Population | Contact-Dependent | Contact-Independent | Other |
|---|---|---|---|
| CD8+ Tregs | Perforin | IDO | Promotes generation of CD4+CD25+ Tregs |
| FasL/Fas | IFN-γ | ||
| Fibroleukin-2 (Fgl-2) | |||
| TGF-β | |||
| DN Tregs | Perforin | None reported | Down-regulate costimulatory molecules on APCs |
| FasL/Fas | |||
| Tr1 Tregs | Perforin | IL-10 |
Recent investigations on CD8+ Tregs have demonstrated the importance of the non-classical MHC Ib molecules Qa-1 (mouse) and HLA-E (human) in the induction of CD8+ Tregs (7). Engagement of Qa-1-Qdm peptides expressed on CD4+ Th1 cells with its receptor, NKG2A/CD94 on CD8+ T cells leads to the generation of CD8+ Tregs (7). Qa-1-restriced CD8+ Tregs are believe to arise in the thymus and play a central role in the maintenance of self-tolerance (7). They can also be induced in the periphery by T cell vaccination (30-32) or by introducing alloantigens into the anterior chamber of the eye (33, 34).
CD8+ Tregsand transplantation
The majority of studies on Tregs have focused on CD4+CD25+ Tregs and it has been assumed by many that CD8+ Tregs were minor players in the maintenance of self-tolerance and in the promotion of allograft survival. However, recent evidence suggests that CD8+CD122+ naturally occurring Tregs play a crucial role in immune homeostasis and contribute to allograft survival (15, 22, 35-37) (Table 3). In fact, there is compelling evidence that CD8+ Tregs are more potent than conventional CD4+CD25+ Tregs in suppressing pancreatic islet allograft rejection (35). In one study, CD8+CD122+ Tregs underwent swifter expansion, produced more IL-10, and suppressed T cell proliferation in vitro more effectively than their CD4+CD25+ Treg counterparts (35). Importantly, adoptively transferred CD8+CD122+ Tregs were able to prolong the survival of pancreatic islet allografts, while similar adoptive transfers of CD4+CD25+ Tregs were unable to prolong allograft survival (35). CD8+CD28- Tregs can promote the generation of tolerogenic dendritic cells (DCs) and are expanded in heart allograft recipients (38). A mouse study of allogenic stem cell transplantation revealed that in addition to the conventional role of CD4+ Foxp3+ Tregs in this model, CD8+Foxp3+ Tregs were able to prevent graft versus host disease (GVHD) mortality (39).
Table 3. Treg Populations Associated with Allograft and Xenograft Tolerance.
| Graft | CD8+ Tregs | DN Tregs | Tr1 Tregs | References |
|---|---|---|---|---|
| Skin allograft | Yes | Yes | ? | (21, 47, 51, 55, 57) |
| Heart allograft | Yes | Yes | ? | (17, 21, 24, 38, 48, 65, 69, 70) |
| Pancreatic islet allograft | Yes | Yes | Yes | (21, 35, 55, 68, 76, 87, 89) |
| Kidney allograft | Yes | ? | Yes | (49, 88) |
| Heart xenograft | Yes | Yes | ? | (21, 58, 71) |
| Corneal xenograft | Yes | ? | ? | (20) |
| Hematopoietic stem cell allograft | Yes | Yes | Yes | (39, 59, 67, 86) |
CD8+ Tregs may also be one of the key elements sustaining immune privilege in the eye. Nominal antigens or alloantigens introduced into the anterior chamber (AC) of the eye elicit the generation of antigen-specific CD8+Tregs through a process called anterior chamber-associated immune deviation (ACAID) (40). Interestingly, two populations of Tregs are induced following AC injection of alloantigens, CD4+ Tregs and CD8+ Tregs (18). The CD8+ Tregs that are generated in the spleen after AC injection of antigen suppress both ocular and systemic immune responses by elaborating TGF-β, IL-10, and IFN-γ (40, 41). Additionally, CD8+ Tregs are also induced in situ by cells lining the AC of the eye. Corneal endothelial cells express membrane-bound TGFβ2, which directly inhibits the activation of effector CD8+ T cells in situ and also induces the conversion of effector CD8+ T cells to Tregs (42) that elaborate TGFβ1. The pigmented epithelial cells of the iris also induce the generation of CD8+ Tregs by B7-1/B7-2 interaction with CTLA-4 on effector T cells (43, 44). It bears noting that injection of donor-specific alloantigenic cells into the anterior chamber of the eye produces ACAID and dramatically enhances corneal allograft survival (45, 46).
While most studies have focused on CD4+CD25+Foxp3+ Tregs in promoting allograft survival, there is a sizeable body of evidence that CD8+ Tregs can also enhance the survival of skin (21, 47), heart (17, 21, 24, 38, 48), pancreatic islet (21, 35), and kidney (49) allografts and heart (21) and corneal (20) xenografts, and hematopoietic allografts (39) (Table 3).
TCRαβ+ CD4-CD8- Double Negative Tregs (DN Treg)
DN Treg phenotypes
In 1989, Strober and colleagues (50) described and cloned a population of spleen cells that did not express either CD4 or CD8, but could suppress T cell proliferation in vitro. The CD4-, CD8- T cell population was termed “double negative” (DN) T cells. DN T cells express the αβ T cell receptor (TCR), yet do not express CD4, CD8, or NK cell markers and represent 1% to 5% of the αβ T cell receptor positive T cells in mice and humans (51, 52). Although no specific marker has been identified for DN Tregs, a lack of Foxp3 expression and patterns of surface markers have been reported (53) (Table 1). The origin of DN T cells is still unclear. Some studies suggest that DN αβ TCR+ T cells can be derived from CD8+ T cells (54) while others have shown that CD4+ T cells can convert to DN Tregs both in vitro and in vivo (55). Cloned DN Tregs from TCR transgenic mice are identified as CD25+CD30+CD28low (51), while DN Tregs arising from CD4+ precursors are CD28+CD25+CD44+CD69+ (55). Isolated human DN Tregs are CD27+CD28lowCD25- (56). Subsequent studies reported that DN Tregs displayed antigen specific suppressive activity both in vitro and in vivo (51). DN Tregs not only suppress CD8+ T cell responses, but also inhibit CD4+ T cells (57), B cells (58), NK cells (59), and dendritic cells (DC) (60).
DN Treg mechanisms of suppression and impact on transplantation
DN Tregs have a unique capacity to acquire the entire MHC-alloantigen complex from APCs' cell membrane via a cell-contact-dependent interaction with the TCR on the DN Tregs via a process termed “trogocytosis” and to express the captured MHC-alloantigen complex on their cell surface (61). The effector CD8+ T cell binds to the captured MHC-alloantigen complex that is now presented on the DN Treg, which culminates in the transmission of a death signal to the CD8+ T cells (61-63). Accordingly, suppression by DN Tregs is antigen-specific (Figure 1).
Figure 1. “Trogocytosis” as a mechanism for suppression mediated by double-negative T cells.
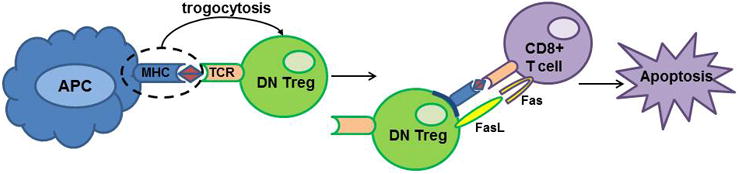
MHC-alloantigen complex on antigen presenting cells (APC) engages the T cell receptor (TCR) on double-negative (DN) Tregs. This is followed by release of MHC/antigen complex by the APC and capture of the MHC/antigen complex by the DN Treg which integrates the complex into its cell membrane. The DN Tregs express this captured MHC/antigen complex on their cell surface. CD8+ T cells bearing the cognate TCR engage the MHC/antigen complex on the DN Tregs. DN Tregs then transmit an apoptotic signal via FasL on the DN Tregs through the Fas receptor on CD8+ T cells. The CD8+ effector T cells undergo apoptosis and are deleted.
There is evidence that the death signal delivered by the DN Tregs to the CD8+ T cells in mice is through Fas/FasL (63) while other studies suggest a role for perforin-mediated cytolysis (55) (Table 2). The suppression of CD8+ T cells by DN T cells via recognition of alloantigens and the killing of CD8+ effector T cells is reminiscent of the “veto cell” concept that was proposed 35 years ago (64). Adoptive transfer of DN Tregs following a cardiac allograft in mice prolonged graft survival as well as augmented the Foxp3+ Treg population (65). There is also evidence that murine DN Tregs regulate immune responses at the level of antigen presenting dendritic cells (DC). Gao and co-workers (60) demonstrated that murine DN Tregs expressed high levels of CTLA-4 and down-regulated costimulatory molecules CD80 and CD86 on antigen-presenting mature DCs. Moreover, DN Tregs killed syngeneic antigen-loaded DCs or allogeneic DCs through a Fas-FasL pathway.
DN Tregs can also suppress B cell and NK cell responses. Ma and co-workers found that adoptively transferred DN Tregs prolonged rat heart xenograft survival in mice and induced B cell apoptosis via a perforin-dependent process (58). As might be expected, anti-donor IgM and IgG antibody titers were significantly diminished in recipients of adoptively transferred DN Tregs (58). In a model of murine allogenic bone marrow transfer, DN Tregs supported graft survival through suppressing NK cells by perforin and Fas-FasL dependent pathways (59).
In contrast to murine DN Tregs, human DN Tregs do not kill effector T cells (66). Although cell-cell contact is required for human DN Treg to function, the suppressive activity of human DN Tregs is not Fas/FasL or perforin-mediated. Suppression by DN Treg is reversible and the function of previously suppressed effector T cells can be restored once DN Tregs are removed. Soluble factors such as IL-10 and TGF- β are not involved in suppression mediated by DN Tregs (66). In a study of 40 human patients who received a hematopoietic stem cell allograft, the percent of DN Tregs in the peripheral blood was inversely correlated with risk of graft rejection (67).
DN Tregs have been shown to enhance the survival of skin (51, 55, 57), pancreatic islet (55, 68), and heart allografts (65, 69, 70) and heart xenografts (58, 71) and hematopoietic stem cell allografts (59, 67) (Table 3).
Type 1 Regulatory T Cells (Tr1)
Tr1 phenotypes
Two categories of CD4+ Tregs have been described: conventional CD4+CD25+Foxp3+Tregs (72) and type 1 Tregs (Tr1 cells) (73). Tr1 cells are found in both humans and mice and are characterized by their copious secretion of IL-10 and their lack of Foxp3 expression. Human and mouse Tr1 clones co-express CD49b and lymphocyte activation gene 3 (LAG-3), which distinguishes Tr1 cells from other CD4+ T cells including Th1, Th2, Th17, and Foxp3+ Tregs (74) (Table 1). Tr1 cells are induced in vivo following chronic antigenic stimulation in the presence of IL-10 (75) or in vitro by activating naïve T cells through their TCR in the presence or IL-10 alone or IL-10 in combination with immunosuppressive drugs such as dexamethasone or Rapamycin (73, 76, 77).
A growing body of evidence indicates that IL-27 produced by tolerogenic DCs is a crucial differentiation factor for the development of IL-10-producing Tr1 cells in mice (78-80) and humans (81). However, Jin and co-workers have recently shown that IL-6 can induce the differentiation of murine IL-10-producing Tr1-like Tregs from naïve CD4+ T cells in the absence of IL-27 suggesting that IL-6 produced in inflammatory conditions might serve as a feed-back mechanism for generating Tr1 cells that dampen inflammation and restore homeostasis (82).
Tr1 mechanisms of suppression and impact on transplantation
Tr1 cells mediate immune suppression by secreting IL-10 and by killing antigen presenting cells via a perforin/granzyme-dependent process that requires MHC class I recognition (83) (Table 2). Tr1 also suppress Th17 cells in murine model of colitis by an IL-10-dependent process (84).
The first indication that Tr1 cells might contribute to allograft tolerance came from severe combined immunodeficiency (SCID) patients who developed long-term tolerance to stem cell allografts and expressed cells with Tr1-like properties (85). Subsequent studies showed that Tr1 cells were associated with the induction of mixed chimerism in patients receiving hematopoietic stem cell transplants (86). Investigations in murine allograft models have demonstrated a role for Tr1 cells in tolerance in pancreatic islet transplantation (76, 87). In humans, Tr1 cells can also contribute to transplantation tolerance as Tr1 cells have been detected in patients who spontaneously developed tolerance to kidney or liver allografts (88) and also occur in mice treated with IL-10 in combination with Rapamycin a means of establishing tolerance for pancreatic islet transplants (76). In human subjects, Tr1 cells have been associated with tolerance in kidney (88), pancreatic islet (89), and liver (88) allografts (Table 3).
Effect of Immunosuppressive Agents on Treg Generation and Function
Immunosuppressive drugs such as Cyclosporine A (CsA) and Rapamycin (RPM) have made organ transplantation a feasible and effective therapeutic option for treating end stage organ failure. By contrast, the clinical application of Tregs in promoting the long-term survival of organ transplants is gaining traction but much remains to be improved before it becomes a reality. It is important to address the impact of immunosuppressive drugs in the context of various organ transplantations since they may have varied effects depending on dose, combination treatments, or the type of organ affected. A key issue is whether conventional anti-rejection agents can be combined with Tregs for promoting graft acceptance or if immunosuppressive drugs have untoward effects on Treg function. CsA and RPM have been used successfully to prevent transplant rejection. Although both CsA and RPM target IL-2, they have remarkably different effects on the generation and function of CD4+CD25+Foxp3+ Tregs. CsA inhibits IL-2 transcription and synthesis while RPM acts downstream by blocking T cell responses to IL-2. Using a murine heart allograft model Coenen and co-workers found that CsA treatment resulted in a sharp reduction in peripheral CD4+CD25+Foxp3+ Tregs while RPM treatment did not reduce the generation of these Tregs (90). Moreover, RPM, but not CsA, induces de novo generation of CD4+CD25+Foxp3+ Tregs which potentiate murine skin allograft survival in an alloantigen-specific manner (91). Similar findings were seen in another skin allograft model where RPM treatment supported Tregs and CsA antagonized Treg expansion (92).
Immunosuppression by preventing the egress of T cells from lymphoid organs can be achieved by FTY720 (Fingolimod), a sphingosine-1-phosphate receptor agonist. In a murine corneal allograft model, graft survival was prolonged by topical treatment with CsA or FTY720, with an increase in CD4+ Tregs found in the FTY720 treated mice (93). A model utilizing adoptive transfer for allograft rejection found that either RPM or FTY720 treatment significantly enhanced conversion of CD4+CD25+Foxp3+ Tregs (94).
Histone deacetylase inhibitors (HDACs) may be a novel route for promoting Treg presence by preventing the conversion of Tregs into effector T cells through enhancing the access to Foxp3 within the chromatin. An HDAC, suberoylanilide hydroxamic acid (SAHA), synergized with low-doses of tacrolimus to prolong cardiac allograft survival in mice by promoting expression of Treg molecules Foxp3 and CTLA-4 as well as increasing apoptosis of T effector cells (95). Furthermore, if the cardiac graft was introduced into Foxp3 deficient recipient mice, SAHA treatment was still able to marginally increase allograft survival suggesting that non-Foxp3 regulatory cells are also impacted by HDACs. Another HDAC, trichostatin A (TSA), in combination with donor-cell transfusion increased CD4+CD25+Foxp3+ Treg numbers and promoted mouse pancreatic islet graft survival (96).
There are still many clinical drugs that could potentially impact Treg numbers and functions. A high-throughput screen, flow cytometry based assay using Foxp3-GFP reporter mice evaluated the in vitro effects of 640 FDA-approved drugs and found that after 3 days in culture 75 drugs significantly increased Treg numbers (97). This study measured Foxp3+ T cell numbers, thus it warrants future investigations to determine if the same drugs, or a different combination of drugs, have similar impacts on non-Foxp3+ Tregs.
Few studies have been conducted with regards to the impact of immunosuppressive agents on the generation or function of non-CD4+CD25+Foxp3+ Tregs. In vitro studies using human peripheral blood mononuclear cells found that RPM caused an increase in the numbers of CD103+CD8+ alloreactive T cells with immunosuppressive properties, while CsA had no significant effect on the percentage of these cells and prednisolone diminished the numbers of these cells (98). CD8+CD28- Treg function was improved in rheumatoid arthritis patients that received TNF-α inhibitor therapy, while patients treated with methotrexate had no effects on the defective CD8+CD28- Treg activity normally found in these patients (99). There is a dearth of published reports on the effects of immunosuppressive agents on Tr1 Tregs and DN T regs activity in the context of allograft and xenograft survival. Thus, there are significant gaps in our knowledge about the effects of immunosuppressive agents and the non-CD4+CD25+Foxp3+ Tregs.
Conclusions
Since Sakaguchi's discovery of CD4+CD25+ Tregs, over 25,000 publications have dealt with the general topic of Tregs. The overwhelming majority of these publications have focused on the role of CD4+CD25+ Tregs. However, in recent years there has been a growing awareness of the importance of CD8+ Tregs, DN Tregs, and Tr1 cells in controlling autoimmune diseases and in enhancing allograft survival. The presence of multiple populations of Tregs is a reflection of the remarkable redundancy and plasticity of the immune system. The importance of CD4+CD25+Tregs for immune homeostasis is well-recognized. Deficiencies in Foxp3 expression invariably lead to lymphoproliferative and multi-organ autoimmune diseases in both humans and mice. It has been suggested that CD4+CD25+ Tregs are generated in response to the initial priming stage of the immune response and act to limit immune-mediated inflammation that inflicts damage to juxtaposed tissues in various organs. By contrast, CD8+ Tregs, and perhaps DN Tregs, are generated from previously activated T cells. In both cases, the Tregs act to suppress immune-mediated inflammation and restore immune homeostasis. In certain conditions, organ allografts and xenografts benefit from the development of these non-conventional Tregs. Harnessing CD4+CD25+, CD4-CD8+, DN Tregs, and Tr1 cells as a comprehensive means of enhancing the survival of allografts and xenografts in patients at high risk of rejecting their grafts is an appealing goal that is still in its early stages of development.
Acknowledgments
Funding: Supported by NIH grants EY007641, EY005631, and EY020799 and Research to Prevent Blindness.
Abbreviations
- ACAID
anterior chamber-associated immune deviation
- APC
antigen presenting cell
- CsA
Cyclosporine
- DC
dendritic cell
- DN
double negative
- DTH
delayed-type hypersensitivity
- HDAC
Histone deacetylase inhibitor
- IDO
indoleamine 2,3 dioxygenase
- MHC
major histocompatibility complex
- NK cell
natural killer cell
- RPM
Rapamycin
- TCR
T cell receptor
- Tregs
T regulatory cells
Footnotes
Disclosure: The authors have no conflict of interest.
AJL and JYN contributed to the writing of the manuscript.
References
- 1.Gershon RK, Cohen P, Hencin R, Liebhaber SA. Suppressor T cells. Journal of Immunology. 1972 Mar;108(3):586–90. Epub 1972/03/01. eng. [PubMed] [Google Scholar]
- 2.Moller G. Do suppressor T cells exist? Scandinavian journal of immunology. 1988 Mar;27(3):247–50. doi: 10.1111/j.1365-3083.1988.tb02344.x. eng. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155(3):1151–64. [PubMed] [Google Scholar]
- 4.Cantor H, Shen FW, Boyse EA. Separation of helper T cells from suppressor T cells expressing different Ly components. II. Activation by antigen: after immunization, antigen-specific suppressor and helper activities are mediated by distinct T-cell subclasses. The Journal of experimental medicine. 1976 Jun 1;143(6):1391–40. doi: 10.1084/jem.143.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu D, Ikizawa K, Lu L, Sanchirico ME, Shinohara ML, Cantor H. Analysis of regulatory CD8 T cells in Qa-1-deficient mice. Nature immunology. 2004 May;5(5):516–23. doi: 10.1038/ni1063. [DOI] [PubMed] [Google Scholar]
- 6.Jiang H, Chess L. Qa-1/HLA-E-restricted regulatory CD8+ T cells and self-nonself discrimination: an essay on peripheral T-cell regulation. Human immunology. 2008 Nov;69(11):721–7. doi: 10.1016/j.humimm.2008.08.279. [DOI] [PubMed] [Google Scholar]
- 7.Kim HJ, Cantor H. Regulation of self-tolerance by Qa-1-restricted CD8(+) regulatory T cells. Seminars in immunology. 2011 Dec;23(6):446–52. doi: 10.1016/j.smim.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rifa'i M, Kawamoto Y, Nakashima I, Suzuki H. Essential roles of CD8+CD122+ regulatory T cells in the maintenance of T cell homeostasis. The Journal of experimental medicine. 2004 Nov 1;200(9):1123–34. doi: 10.1084/jem.20040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strioga M, Pasukoniene V, Characiejus D. CD8+ CD28- and CD8+ CD57+ T cells and their role in health and disease. Immunology. 2011 Sep;134(1):17–32. doi: 10.1111/j.1365-2567.2011.03470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahic M, Henjum K, Yaqub S, Bjornbeth BA, Torgersen KM, Tasken K, et al. Generation of highly suppressive adaptive CD8(+)CD25(+)FOXP3(+) regulatory T cells by continuous antigen stimulation. European journal of immunology. 2008 Mar;38(3):640–6. doi: 10.1002/eji.200737529. [DOI] [PubMed] [Google Scholar]
- 11.Uss E, Rowshani AT, Hooibrink B, Lardy NM, van Lier RA, ten Berge IJ. CD103 is a marker for alloantigen-induced regulatory CD8+ T cells. J Immunol. 2006 Sep 1;177(5):2775–83. doi: 10.4049/jimmunol.177.5.2775. [DOI] [PubMed] [Google Scholar]
- 12.Boor PP, Metselaar HJ, Jonge S, Mancham S, van der Laan LJ, Kwekkeboom J. Human plasmacytoid dendritic cells induce CD8(+) LAG-3(+) Foxp3(+) CTLA-4(+) regulatory T cells that suppress allo-reactive memory T cells. European journal of immunology. 2011 Jun;41(6):1663–74. doi: 10.1002/eji.201041229. [DOI] [PubMed] [Google Scholar]
- 13.Wei S, Kryczek I, Zou L, Daniel B, Cheng P, Mottram P, et al. Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer research. 2005 Jun 15;65(12):5020–6. doi: 10.1158/0008-5472.CAN-04-4043. [DOI] [PubMed] [Google Scholar]
- 14.Xystrakis E, Dejean AS, Bernard I, Druet P, Liblau R, Gonzalez-Dunia D, et al. Identification of a novel natural regulatory CD8 T-cell subset and analysis of its mechanism of regulation. Blood. 2004 Nov 15;104(10):3294–301. doi: 10.1182/blood-2004-03-1214. [DOI] [PubMed] [Google Scholar]
- 15.Dai H, Wan N, Zhang S, Moore Y, Wan F, Dai Z. Cutting edge: programmed death-1 defines CD8+CD122+ T cells as regulatory versus memory T cells. J Immunol. 2010 Jul 15;185(2):803–7. doi: 10.4049/jimmunol.1000661. [DOI] [PubMed] [Google Scholar]
- 16.Chen Z, Han Y, Gu Y, Liu Y, Jiang Z, Zhang M, et al. CD11c(high)CD8+ regulatory T cell feedback inhibits CD4 T cell immune response via Fas ligand-Fas pathway. J Immunol. 2013 Jun 15;190(12):6145–54. doi: 10.4049/jimmunol.1300060. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Liu Z, Witkowski P, Vlad G, Manavalan JS, Scotto L, et al. Rat CD8+ FOXP3+ T suppressor cells mediate tolerance to allogeneic heart transplants, inducing PIR-B in APC and rendering the graft invulnerable to rejection. Transplant immunology. 2004 Dec;13(4):239–47. doi: 10.1016/j.trim.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Streilein JW, Niederkorn JY. Characterization of the suppressor cell(s) responsible for anterior chamber-associated immune deviation (ACAID) induced in BALB/c mice by P815 cells. J Immunol. 1985;134(3):1381–7. [PubMed] [Google Scholar]
- 19.Picarda E, Bezie S, Venturi V, Echasserieau K, Merieau E, Delhumeau A, et al. MHC-derived allopeptide activates TCR-biased CD8+ Tregs and suppresses organ rejection. The Journal of clinical investigation. 2014 Jun 2;124(6):2497–512. doi: 10.1172/JCI71533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Jiang S, Shi H, Lin Y, Wang J, Wang X. Prolongation of corneal xenotransplant survival by T-cell vaccination-induced T-regulatory cells. Xenotransplantation. 2008 May-Jun;15(3):164–73. doi: 10.1111/j.1399-3089.2008.00471.x. [DOI] [PubMed] [Google Scholar]
- 21.Guillonneau C, Hill M, Hubert FX, Chiffoleau E, Herve C, Li XL, et al. CD40Ig treatment results in allograft acceptance mediated by CD8CD45RC T cells, IFN-gamma, and indoleamine 2,3-dioxygenase. The Journal of clinical investigation. 2007 Apr;117(4):1096–106. doi: 10.1172/JCI28801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li S, Xie Q, Zeng Y, Zou C, Liu X, Wu S, et al. A naturally occurring CD8(+)CD122(+) T-cell subset as a memory-like Treg family. Cellular & molecular immunology. 2014 Jul;11(4):326–31. doi: 10.1038/cmi.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barbon CM, Davies JK, Voskertchian A, Kelner RH, Brennan LL, Nadler LM, et al. Alloanergization of human T cells results in expansion of alloantigen-specific CD8(+) CD28(-) suppressor cells. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014 Feb;14(2):305–18. doi: 10.1111/ajt.12575. [DOI] [PubMed] [Google Scholar]
- 24.Li XL, Menoret S, Bezie S, Caron L, Chabannes D, Hill M, et al. Mechanism and localization of CD8 regulatory T cells in a heart transplant model of tolerance. J Immunol. 2010 Jul 15;185(2):823–33. doi: 10.4049/jimmunol.1000120. [DOI] [PubMed] [Google Scholar]
- 25.Lu L, Cantor H. Generation and regulation of CD8(+) regulatory T cells. Cellular & molecular immunology. 2008 Dec;5(6):401–6. doi: 10.1038/cmi.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu L, Kim HJ, Werneck MB, Cantor H. Regulation of CD8+ regulatory T cells: Interruption of the NKG2A-Qa-1 interaction allows robust suppressive activity and resolution of autoimmune disease. Proceedings of the National Academy of Sciences of the United States of America. 2008 Dec 9;105(49):19420–5. doi: 10.1073/pnas.0810383105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lerret NM, Houlihan JL, Kheradmand T, Pothoven KL, Zhang ZJ, Luo X. Donor-specific CD8+ Foxp3+ T cells protect skin allografts and facilitate induction of conventional CD4+ Foxp3+ regulatory T cells. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012 Sep;12(9):2335–47. doi: 10.1111/j.1600-6143.2012.04120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Endharti AT, Rifa IM, Shi Z, Fukuoka Y, Nakahara Y, Kawamoto Y, et al. Cutting edge: CD8+CD122+ regulatory T cells produce IL-10 to suppress IFN-gamma production and proliferation of CD8+ T cells. J Immunol. 2005 Dec 1;175(11):7093–7. doi: 10.4049/jimmunol.175.11.7093. [DOI] [PubMed] [Google Scholar]
- 29.Mangalam AK, Luckey D, Giri S, Smart M, Pease LR, Rodriguez M, et al. Two discreet subsets of CD8 T cells modulate PLP(91-110) induced experimental autoimmune encephalomyelitis in HLA-DR3 transgenic mice. Journal of autoimmunity. 2012 Jun;38(4):344–53. doi: 10.1016/j.jaut.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaur A, Ruberti G, Haspel R, Mayer JP, Fathman CG. Requirement for CD8+ cells in T cell receptor peptide-induced clonal unresponsiveness. Science. 1993 Jan 1;259(5091):91–4. doi: 10.1126/science.8418501. [DOI] [PubMed] [Google Scholar]
- 31.Koh DR, Fung-Leung WP, Ho A, Gray D, Acha-Orbea H, Mak TW. Less mortality but more relapses in experimental allergic encephalomyelitis in CD8-/- mice. Science. 1992 May 22;256(5060):1210–3. doi: 10.1126/science.256.5060.1210. [DOI] [PubMed] [Google Scholar]
- 32.Varthaman A, Khallou-Laschet J, Clement M, Fornasa G, Kim HJ, Gaston AT, et al. Control of T cell reactivation by regulatory Qa-1-restricted CD8+ T cells. J Immunol. 2010 Jun 15;184(12):6585–91. doi: 10.4049/jimmunol.0903109. [DOI] [PubMed] [Google Scholar]
- 33.Cone RE, Chattopadhyay S, Sharafieh R, Lemire Y, O'Rourke J. The suppression of hypersensitivity by ocular-induced CD8(+) T cells requires compatibility in the Qa-1 haplotype. Immunology and cell biology. 2009 Mar-Apr;87(3):241–8. doi: 10.1038/icb.2008.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D'Orazio TJ, Mayhew E, Niederkorn JY. Ocular immune privilege promoted by the presentation of peptide on tolerogenic B cells in the spleen. II. Evidence for presentation by Qa-1. J Immunol. 2001;166(1):26–32. doi: 10.4049/jimmunol.166.1.26. [DOI] [PubMed] [Google Scholar]
- 35.Dai Z, Zhang S, Xie Q, Wu S, Su J, Li S, et al. Natural CD8+CD122+ T cells are more potent in suppression of allograft rejection than CD4+CD25+ regulatory T cells. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014 Jan;14(1):39–48. doi: 10.1111/ajt.12515. [DOI] [PubMed] [Google Scholar]
- 36.Guillonneau C, Picarda E, Anegon I. CD8+ regulatory T cells in solid organ transplantation. Current opinion in organ transplantation. 2010 Dec;15(6):751–6. doi: 10.1097/MOT.0b013e32834016d1. [DOI] [PubMed] [Google Scholar]
- 37.Wan N, Dai H, Wang T, Moore Y, Zheng XX, Dai Z. Bystander central memory but not effector memory CD8+ T cells suppress allograft rejection. J Immunol. 2008 Jan 1;180(1):113–21. doi: 10.4049/jimmunol.180.1.113. [DOI] [PubMed] [Google Scholar]
- 38.Colovai AI, Mirza M, Vlad G, Wang S, Ho E, Cortesini R, et al. Regulatory CD8+CD28- T cells in heart transplant recipients. Human immunology. 2003 Jan;64(1):31–7. doi: 10.1016/s0198-8859(02)00742-5. [DOI] [PubMed] [Google Scholar]
- 39.Beres AJ, Haribhai D, Chadwick AC, Gonyo PJ, Williams CB, Drobyski WR. CD8+ Foxp3+ regulatory T cells are induced during graft-versus-host disease and mitigate disease severity. J Immunol. 2012 Jul 1;189(1):464–74. doi: 10.4049/jimmunol.1200886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niederkorn JY. See no evil, hear no evil, do no evil: the lessons of immune privilege. Nature immunology. 2006 Apr;7(4):354–9. doi: 10.1038/ni1328. eng. [DOI] [PubMed] [Google Scholar]
- 41.Cone RE, Li X, Sharafieh R, O'Rourke J, Vella AT. The suppression of delayed-type hypersensitivity by CD8+ regulatory T cells requires interferon-gamma. Immunology. 2007 Jan;120(1):112–9. doi: 10.1111/j.1365-2567.2006.02486.x. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamada Y, Sugita S, Horie S, Yamagami S, Mochizuki M. Mechanisms of immune suppression for CD8+ T cells by human corneal endothelial cells via membrane-bound TGFbeta. Investigative ophthalmology & visual science. 2010 May;51(5):2548–57. doi: 10.1167/iovs.09-4233. [DOI] [PubMed] [Google Scholar]
- 43.Sugita S, Keino H, Futagami Y, Takase H, Mochizuki M, Stein-Streilein J, et al. B7+ iris pigment epithelial cells convert T cells into CTLA-4+, B7-expressing CD8+ regulatory T cells. Investigative ophthalmology & visual science. 2006 Dec;47(12):5376–84. doi: 10.1167/iovs.05-1354. [DOI] [PubMed] [Google Scholar]
- 44.Sugita S, Ng TF, Lucas PJ, Gress RE, Streilein JW. B7+ iris pigment epithelium induce CD8+ T regulatory cells; both suppress CTLA-4+ T cells. J Immunol. 2006 Jan 1;176(1):118–27. doi: 10.4049/jimmunol.176.1.118. [DOI] [PubMed] [Google Scholar]
- 45.Niederkorn JY. Anterior chamber-associated immune deviation and its impact on corneal allograft survival. Current opinion in organ transplantation. 2006;11:360–5. [Google Scholar]
- 46.Niederkorn JY, Mellon J. Anterior chamber-associated immune deviation promotes corneal allograft survival. Investigative ophthalmology & visual science. 1996 Dec;37(13):2700–7. eng. [PubMed] [Google Scholar]
- 47.Sireci G, Barera A, Macaluso P, Di Sano C, Bonanno CT, Pio La Manna M, et al. A continuous infusion of a minor histocompatibility antigen-immunodominant peptide induces a delay of male skin graft rejection. Immunobiology. 2009;214(8):703–11. doi: 10.1016/j.imbio.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 48.Kapp JA, Honjo K, Kapp LM, Xu X, Cozier A, Bucy RP. TCR transgenic CD8+ T cells activated in the presence of TGFbeta express FoxP3 and mediate linked suppression of primary immune responses and cardiac allograft rejection. International immunology. 2006 Nov;18(11):1549–62. doi: 10.1093/intimm/dxl088. [DOI] [PubMed] [Google Scholar]
- 49.Zhou J, Appleton SE, Stadnyk A, Lee TD, Nashan BA. CD8+ gammadelta T regulatory cells mediate kidney allograft prolongation after oral exposure to alloantigen. Transplant international: official journal of the European Society for Organ Transplantation. 2008 Jul;21(7):679–87. doi: 10.1111/j.1432-2277.2008.00669.x. [DOI] [PubMed] [Google Scholar]
- 50.Strober S, Dejbachsh-Jones S, Van Vlasselaer P, Duwe G, Salimi S, Allison JP. Cloned natural suppressor cell lines express the CD3+CD4-CD8- surface phenotype and the alpha, beta heterodimer of the T cell antigen receptor. J Immunol. 1989 Aug 15;143(4):1118–22. [PubMed] [Google Scholar]
- 51.Zhang ZX, Yang L, Young KJ, DuTemple B, Zhang L. Identification of a previously unknown antigen-specific regulatory T cell and its mechanism of suppression. Nature medicine. 2000 Jul;6(7):782–9. doi: 10.1038/77513. [DOI] [PubMed] [Google Scholar]
- 52.Zhang ZX, Young K, Zhang L. CD3+CD4-CD8- alphabeta-TCR+ T cell as immune regulatory cell. Journal of molecular medicine. 2001 Aug;79(8):419–27. doi: 10.1007/s001090100238. [DOI] [PubMed] [Google Scholar]
- 53.Juvet SC, Zhang L. Double negative regulatory T cells in transplantation and autoimmunity: recent progress and future directions. Journal of molecular cell biology. 2012 Feb;4(1):48–58. doi: 10.1093/jmcb/mjr043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balomenos D, Rumold R, Theofilopoulos AN. The proliferative in vivo activities of lpr double-negative T cells and the primary role of p59fyn in their activation and expansion. J Immunol. 1997 Sep 1;159(5):2265–73. [PubMed] [Google Scholar]
- 55.Zhang D, Yang W, Degauque N, Tian Y, Mikita A, Zheng XX. New differentiation pathway for double-negative regulatory T cells that regulates the magnitude of immune responses. Blood. 2007 May 1;109(9):4071–9. doi: 10.1182/blood-2006-10-050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fischer K, Voelkl S, Heymann J, Przybylski GK, Mondal K, Laumer M, et al. Isolation and characterization of human antigen-specific TCR alpha beta+ CD4(-)CD8- double-negative regulatory T cells. Blood. 2005 Apr 1;105(7):2828–35. doi: 10.1182/blood-2004-07-2583. [DOI] [PubMed] [Google Scholar]
- 57.Ford MS, Young KJ, Zhang Z, Ohashi PS, Zhang L. The immune regulatory function of lymphoproliferative double negative T cells in vitro and in vivo. The Journal of experimental medicine. 2002 Jul 15;196(2):261–7. doi: 10.1084/jem.20020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma Y, He KM, Garcia B, Min W, Jevnikar A, Zhang ZX. Adoptive transfer of double negative T regulatory cells induces B-cell death in vivo and alters rejection pattern of rat-to-mouse heart transplantation. Xenotransplantation. 2008 Feb;15(1):56–63. doi: 10.1111/j.1399-3089.2008.00444.x. [DOI] [PubMed] [Google Scholar]
- 59.He KM, Ma Y, Wang S, Min WP, Zhong R, Jevnikar A, et al. Donor double-negative Treg promote allogeneic mixed chimerism and tolerance. European journal of immunology. 2007 Dec;37(12):3455–66. doi: 10.1002/eji.200737408. [DOI] [PubMed] [Google Scholar]
- 60.Gao JF, McIntyre MS, Juvet SC, Diao J, Li X, Vanama RB, et al. Regulation of antigen-expressing dendritic cells by double negative regulatory T cells. European journal of immunology. 2011 Sep;41(9):2699–708. doi: 10.1002/eji.201141428. [DOI] [PubMed] [Google Scholar]
- 61.Ford McIntyre MS, Young KJ, Gao J, Joe B, Zhang L. Cutting edge: in vivo trogocytosis as a mechanism of double negative regulatory T cell-mediated antigen-specific suppression. J Immunol. 2008 Aug 15;181(4):2271–5. doi: 10.4049/jimmunol.181.4.2271. [DOI] [PubMed] [Google Scholar]
- 62.Thomson CW, Lee BP, Zhang L. Double-negative regulatory T cells: non-conventional regulators. Immunologic research. 2006;35(1-2):163–78. doi: 10.1385/IR:35:1:163. [DOI] [PubMed] [Google Scholar]
- 63.Young KJ, Zhang L. The nature and mechanisms of DN regulatory T-cell mediated suppression. Human immunology. 2002 Oct;63(10):926–34. doi: 10.1016/s0198-8859(02)00446-9. [DOI] [PubMed] [Google Scholar]
- 64.Miller RG. An immunological suppressor cell inactivating cytotoxic T-lymphocyte precursor cells recognizing it. Nature. 1980 Oct 9;287(5782):544–6. doi: 10.1038/287544a0. [DOI] [PubMed] [Google Scholar]
- 65.Zhang ZX, Lian D, Huang X, Wang S, Sun H, Liu W, et al. Adoptive transfer of DNT cells induces long-term cardiac allograft survival and augments recipient CD4(+)Foxp3(+) Treg cell accumulation. Transplant immunology. 2011 Jan 15;24(2):119–26. doi: 10.1016/j.trim.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 66.Voelkl S, Gary R, Mackensen A. Characterization of the immunoregulatory function of human TCR-alphabeta+ CD4- CD8- double-negative T cells. European journal of immunology. 2011 Mar;41(3):739–48. doi: 10.1002/eji.201040982. [DOI] [PubMed] [Google Scholar]
- 67.McIver Z, Serio B, Dunbar A, O'Keefe CL, Powers J, Wlodarski M, et al. Double-negative regulatory T cells induce allotolerance when expanded after allogeneic haematopoietic stem cell transplantation. British journal of haematology. 2008 Apr;141(2):170–8. doi: 10.1111/j.1365-2141.2008.07021.x. [DOI] [PubMed] [Google Scholar]
- 68.Zhang D, Zhang W, Ng TW, Wang Y, Liu Q, Gorantla V, et al. Adoptive cell therapy using antigen-specific CD4(-)CD8(-)T regulatory cells to prevent autoimmune diabetes and promote islet allograft survival in NOD mice. Diabetologia. 2011 Aug;54(8):2082–92. doi: 10.1007/s00125-011-2179-4. [DOI] [PubMed] [Google Scholar]
- 69.Chen W, Ford MS, Young KJ, Zhang L. Infusion of in vitro-generated DN T regulatory cells induces permanent cardiac allograft survival in mice. Transplantation proceedings. 2003 Nov;35(7):2479–80. doi: 10.1016/j.transproceed.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 70.Hill M, Thebault P, Segovia M, Louvet C, Beriou G, Tilly G, et al. Cell therapy with autologous tolerogenic dendritic cells induces allograft tolerance through interferon-gamma and epstein-barr virus-induced gene 3. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011 Oct;11(10):2036–45. doi: 10.1111/j.1600-6143.2011.03651.x. [DOI] [PubMed] [Google Scholar]
- 71.Chen W, Ford MS, Young KJ, Cybulsky MI, Zhang L. Role of double-negative regulatory T cells in long-term cardiac xenograft survival. J Immunol. 2003 Feb 15;170(4):1846–53. doi: 10.4049/jimmunol.170.4.1846. [DOI] [PubMed] [Google Scholar]
- 72.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003 Feb 14;299(5609):1057–61. doi: 10.1126/science.1079490. Epub 2003/01/11. eng. [DOI] [PubMed] [Google Scholar]
- 73.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389(6652):737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 74.Gagliani N, Magnani CF, Huber S, Gianolini ME, Pala M, Licona-Limon P, et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nature medicine. 2013 Jun;19(6):739–46. doi: 10.1038/nm.3179. [DOI] [PubMed] [Google Scholar]
- 75.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunological reviews. 2006 Aug;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 76.Battaglia M, Stabilini A, Draghici E, Gregori S, Mocchetti C, Bonifacio E, et al. Rapamycin and interleukin-10 treatment induces T regulatory type 1 cells that mediate antigen-specific transplantation tolerance. Diabetes. 2006 Jan;55(1):40–9. [PubMed] [Google Scholar]
- 77.Volchenkov R, Karlsen M, Jonsson R, Appel S. Type 1 regulatory T cells and regulatory B cells induced by tolerogenic dendritic cells. Scandinavian journal of immunology. 2013 Apr;77(4):246–54. doi: 10.1111/sji.12039. [DOI] [PubMed] [Google Scholar]
- 78.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nature immunology. 2007 Dec;8(12):1380–9. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 79.Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nature immunology. 2007 Dec;8(12):1372–9. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 80.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nature immunology. 2007 Dec;8(12):1363–71. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 81.Murugaiyan G, Mittal A, Lopez-Diego R, Maier LM, Anderson DE, Weiner HL. IL-27 is a key regulator of IL-10 and IL-17 production by human CD4+ T cells. J Immunol. 2009 Aug 15;183(4):2435–43. doi: 10.4049/jimmunol.0900568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jin JO, Han X, Yu Q. Interleukin-6 induces the generation of IL-10-producing Tr1 cells and suppresses autoimmune tissue inflammation. Journal of autoimmunity. 2013 Feb;40:28–44. doi: 10.1016/j.jaut.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Magnani CF, Alberigo G, Bacchetta R, Serafini G, Andreani M, Roncarolo MG, et al. Killing of myeloid APCs via HLA class I, CD2 and CD226 defines a novel mechanism of suppression by human Tr1 cells. European journal of immunology. 2011 Jun;41(6):1652–62. doi: 10.1002/eji.201041120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huber S, Gagliani N, Esplugues E, O'Connor W, Jr, Huber FJ, Chaudhry A, et al. Th17 cells express interleukin-10 receptor and are controlled by Foxp3(-) and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity. 2011 Apr 22;34(4):554–65. doi: 10.1016/j.immuni.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bacchetta R, Bigler M, Touraine JL, Parkman R, Tovo PA, Abrams J, et al. High levels of interleukin 10 production in vivo are associated with tolerance in SCID patients transplanted with HLA mismatched hematopoietic stem cells. The Journal of experimental medicine. 1994 Feb 1;179(2):493–502. doi: 10.1084/jem.179.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Serafini G, Andreani M, Testi M, Battarra M, Bontadini A, Biral E, et al. Type 1 regulatory T cells are associated with persistent split erythroid/lymphoid chimerism after allogeneic hematopoietic stem cell transplantation for thalassemia. Haematologica. 2009 Oct;94(10):1415–26. doi: 10.3324/haematol.2008.003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gagliani N, Jofra T, Stabilini A, Valle A, Atkinson M, Roncarolo MG, et al. Antigen-specific dependence of Tr1-cell therapy in preclinical models of islet transplant. Diabetes. 2010 Feb;59(2):433–9. doi: 10.2337/db09-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.VanBuskirk AM, Burlingham WJ, Jankowska-Gan E, Chin T, Kusaka S, Geissler F, et al. Human allograft acceptance is associated with immune regulation. The Journal of clinical investigation. 2000;106(1):145–55. doi: 10.1172/JCI9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huurman VA, Velthuis JH, Hilbrands R, Tree TI, Gillard P, van der Meer-Prins PM, et al. Allograft-specific cytokine profiles associate with clinical outcome after islet cell transplantation. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009 Feb;9(2):382–8. doi: 10.1111/j.1600-6143.2008.02479.x. [DOI] [PubMed] [Google Scholar]
- 90.Coenen JJ, Koenen HJ, van Rijssen E, Kasran A, Boon L, Hilbrands LB, et al. Rapamycin, not cyclosporine, permits thymic generation and peripheral preservation of CD4+ CD25+ FoxP3+ T cells. Bone marrow transplantation. 2007 May;39(9):537–45. doi: 10.1038/sj.bmt.1705628. [DOI] [PubMed] [Google Scholar]
- 91.Gao W, Lu Y, El Essawy B, Oukka M, Kuchroo VK, Strom TB. Contrasting effects of cyclosporine and rapamycin in de novo generation of alloantigen-specific regulatory T cells. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2007 Jul;7(7):1722–32. doi: 10.1111/j.1600-6143.2007.01842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lim DG, Koo SK, Park YH, Kim Y, Kim HM, Park CS, et al. Impact of immunosuppressants on the therapeutic efficacy of in vitro-expanded CD4+CD25+Foxp3+ regulatory T cells in allotransplantation. Transplantation. 2010 Apr 27;89(8):928–36. doi: 10.1097/TP.0b013e3181d3c9d4. [DOI] [PubMed] [Google Scholar]
- 93.Liu Y, Jiang J, Xiao H, Wang X, Li Y, Gong Y, et al. Topical application of FTY720 and cyclosporin A prolong corneal graft survival in mice. Molecular vision. 2012;18:624–33. [PMC free article] [PubMed] [Google Scholar]
- 94.Wu T, Zhang L, Xu K, Sun C, Lei T, Peng J, et al. Immunosuppressive drugs on inducing Ag-specific CD4(+)CD25(+)Foxp3(+) Treg cells during immune response in vivo. Transplant immunology. 2012 Aug;27(1):30–8. doi: 10.1016/j.trim.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 95.Zhang X, Han S, Kang Y, Guo M, Hong S, Liu F, et al. SAHA, an HDAC inhibitor, synergizes with tacrolimus to prevent murine cardiac allograft rejection. Cellular & molecular immunology. 2012 Sep;9(5):390–8. doi: 10.1038/cmi.2012.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sugimoto K, Itoh T, Takita M, Shimoda M, Chujo D, SoRelle JA, et al. Improving allogeneic islet transplantation by suppressing Th17 and enhancing Treg with histone deacetylase inhibitors. Transplant international: official journal of the European Society for Organ Transplantation. 2014 Apr;27(4):408–15. doi: 10.1111/tri.12265. [DOI] [PubMed] [Google Scholar]
- 97.Mao R, Xiao W, Liu H, Chen B, Yi B, Kraj P, et al. Systematic evaluation of 640 FDA drugs for their effect on CD4(+)Foxp3(+) regulatory T cells using a novel cell-based high throughput screening assay. Biochemical pharmacology. 2013 May 15;85(10):1513–24. doi: 10.1016/j.bcp.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 98.Uss E, Yong SL, Hooibrink B, van Lier RA, ten Berge IJ. Rapamycin enhances the number of alloantigen-induced human CD103+CD8+ regulatory T cells in vitro. Transplantation. 2007 Apr 27;83(8):1098–106. doi: 10.1097/01.tp.0000259555.29762.f0. [DOI] [PubMed] [Google Scholar]
- 99.Ceeraz S, Hall C, Choy EH, Spencer J, Corrigall VM. Defective CD8+CD28+ regulatory T cell suppressor function in rheumatoid arthritis is restored by tumour necrosis factor inhibitor therapy. Clinical and experimental immunology. 2013 Oct;174(1):18–26. doi: 10.1111/cei.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]


