Abstract
Phototaxis in flagellated zoospores of the aquatic fungus Blastocladiella emersonii depends on a novel photosensor, Blastocladiella emersonii GC1 (BeGC1), comprising a type I (microbial) rhodopsin fused to a guanylyl cyclase catalytic domain, that produces the conserved second messenger cyclic GMP (cGMP). The rapid and transient increase in cGMP levels during the exposure of zoospores to green light was shown to be necessary for phototaxis and dependent on both rhodopsin function and guanylyl cyclase activity. It is noteworthy that BeGC1 was localized to the zoospore eyespot apparatus, in agreement with its role in the phototactic response. A putative cyclic nucleotide-gated channel (BeCNG1) was also identified in the genome of the fungus and was implicated in flagellar beating via the action of a specific inhibitor (l-cis-diltiazem) that compromised zoospore motility. Here we show that B. emersonii expresses a K+ channel that is activated by cGMP. The use of specific channel inhibitors confirmed the activation of the channel by cGMP and its K+ selectivity. These characteristics are consistent with the function of an ion channel encoded by the BeCNG1 gene. Other blastocladiomycete fungi, such as Allomyces macrogynus and Catenaria anguillulae, possess genes encoding a similar K+ channel and the rhodopsin–guanylyl cyclase fusion protein, while the genes encoding both these proteins are absent in nonflagellated fungi. The presence of these genes as a pair seems to be an exclusive feature of blastocladiomycete fungi. Taken together, these data demonstrate that the B. emersonii cGMP-activated K+ channel is involved in the control of zoospore motility, most probably participating in the cGMP-signaling pathway for the phototactic response of the fungus.
INTRODUCTION
The ability to sense light is essential for the survival of many organisms. The best-characterized photoreceptor cells are vertebrate rods and cones in the retina of the eye. In these cells, light is absorbed by the photoreceptor rhodopsin, activating the G protein transducin and turning on a phosphodiesterase (PDE) that cleaves the second messenger cyclic GMP (cGMP). The decrease in cGMP levels leads to closure of cGMP-gated (CNG) ion channels, blockage of Na+ and Ca2+ influx, and hyperpolarization of the photoreceptor cell plasma membrane, with the resulting electric signal being transmitted to photoreceptor synapses (1). It is noteworthy that in the scallop (Pecten irradians), a distantly related organism, rhodopsin stimulation by light also produces changes in cGMP levels but by a different mechanism, activating an uncharacterized membrane guanylyl cyclase (GC) rather than affecting PDE. Thus, in Pecten, light causes an increase in cGMP levels that leads to the opening of a cGMP-gated channel, which, in this case, is a K+-selective channel virtually impermeable to Ca2+ (2, 3).
Motile microorganisms such as the green alga Chlamydomonas reinhardtii and the blastocladiomycete fungi Allomyces reticulatus and Blastocladiella emersonii also respond to light, in this case by performing phototaxis (4–6). In Chlamydomonas, phototaxis is mediated by two type I (microbial) rhodopsins, both of which have been demonstrated to encode light-gated ion channel activity when heterologously expressed in animal cells and have been shown to act as sensory photoreceptors enabling the influx of cations, triggering the phototactic response (7).
Recently, we have shown that Blastocladiella emersonii zoospores respond to light exposure through the activation of a novel guanylyl cyclase (B. emersonii GC1 [BeGC1]) that encompasses a type I (microbial) rhodopsin domain fused to a GC catalytic domain, which, interestingly, is located next to the eyespot apparatus of the fungus (6). During phototaxis, intracellular cGMP levels in the zoospores show a rapid and transient increase. The changes in cGMP levels and the phototactic response were demonstrated by molecular inhibition experiments to be dependent on both GC activity and rhodopsin function. In addition, the preference of zoospores for green light depended on the presence of retinal A1, the natural chromophore of rhodopsin, contributing to the evidence of BeGC1 involvement in Blastocladiella phototaxis. It should be noted that the rhodopsin–guanylyl cyclase fusion protein BeGC1 is the only rhodopsin encoded in the genome of the fungus (6). Thus, in blastocladiomycete fungi, vertebrates, and the scallop genus Pecten, light produces changes in cGMP levels. Interestingly, in both B. emersonii and the scallop, light leads to increases in cGMP levels, whereas in vertebrates, light produces a decrease in cGMP levels.
The search for other possible components of the signaling pathway involved in B. emersonii phototaxis revealed a gene in the genome of the fungus encoding a putative cyclic nucleotide-gated channel (BeCNG1) that shows similarity to the human rod photoreceptor cGMP-gated channel subunit α1 (33.3% similarity), which transduces the light signals into electric responses (8), and to the K+-selective cGMP-gated ion channel (31.6% similarity to the third repeat module of the channel [9]) that controls the chemosensation of sea urchin sperm (10). In sperm, when chemoattractants bind to a receptor-type guanylyl cyclase, the ensuing rapid rise in the cGMP concentration opens K+-selective CNG channels and thereby hyperpolarizes the cell, leading to changes in the swimming behavior of the sperm (10). Comparison of the putative pore helix region and the cGMP-binding site of BeCNG1 with those of other channels revealed the conservation of important amino acid residues. The K+ selectivity signature GYGD is present in BeCNG1, suggesting that it functions as a K+-selective channel (6). Furthermore, zoospores treated with the CNG channel inhibitor l-cis-diltiazem (11) were observed to stop swimming, in agreement with a possible role for BeCNG1 in the control of flagellar beating. Additionally, investigation of the expression levels of BeCNG1 transcripts during Blastocladiella sporulation revealed the same pattern of variation observed for BeGC1 transcripts, in agreement with a role for BeCNG1 in zoospore phototaxis (6).
The importance of intracellular potassium ions for fungi has been extensively documented in the literature. For instance, potassium flux is involved in turgor recovery after hyperosmotic shock (12, 13) and in the regulation of membrane potential (14) in Neurospora crassa, as well as in ascospore discharge and dissemination in Gibberella zeae (15). For B. emersonii, Van Brunt and Harold (16) reported that zoospores avidly accumulate K+ ions from the medium, attaining intracellular concentrations above 50 mM and a concentration gradient ratio of 3,000; in contrast, sodium ions are expelled. These authors also showed that calcium binds to external sites in the zoospores in an exchangeable form but that zoospores transport little if any calcium across their plasma membranes. Although K+ is necessary for zoospore germination, its role is still not well understood (17). It has been suggested that membrane depolarization could trigger B. emersonii encystment and germination (16). In the case of the oomycete Achlya heterosexualis, it has been shown that the cells contain vacuolar potassium concentrations above 100 mM; most probably, potassium is the most abundant cation in this organism. In addition, potassium ions seem to be connected to the movement of Achlya zoospores, apparently stimulating the circular movement of these cells (18). Appiah and coworkers (19) also showed that changes in K+ concentration alter the speed, frequency of changes of direction, trajectory, and encystment of the zoospores of five species of oomycetes.
In the present work, by the use of specific channel inhibitors, we demonstrate changes in membrane potential induced by potassium and by cGMP in B. emersonii zoospores. Taken together, these data indicate that B. emersonii has a cGMP-activated K+ channel participating in the cGMP-signaling pathway that controls the phototactic response of the zoospore of this fungus.
MATERIALS AND METHODS
Cells and growth conditions.
Cultures of B. emersonii were maintained on PYG agar (0.13% peptone, 0.13% yeast extract, 0.3% glucose, and 1% agar) plates. Large quantities of zoospores were obtained by flooding first-generation cultures grown on PYG agar for 16 to 18 h at 23°C. The zoospore suspension was filtered over nylon mesh (pore size, 30 μm) to remove vegetative-cell debris.
Microfluorimetric measurements of alterations in the membrane potential.
Changes in membrane potential were determined by microfluorimetry using the FlexStation III microplate reader and the FLIPR membrane potential assay kit (both from Molecular Devices Corp., Sunnyvale, CA) according to the instructions of the manufacturer. This kit is 10 times faster and more stable than those with traditional dyes and provides good correlation with manual patch clamp assays. The FLIPR membrane potential assay kit detects ion channel modulation by increasing or decreasing the fluorescent signal as the cellular membrane potential changes. The signal increases in intensity during membrane depolarization as dye follows the positively charged ions inside the cell. During hyperpolarization, the fluorescent signal decreases in intensity as dye follows the positively charged ions out of the cell. Briefly, B. emersonii zoospores were collected and were seeded into 96-well black microplates with clear bottoms at a concentration of 5 × 106/well in 100 μl of sporulation solution (1 mM Tris-maleate buffer [pH 6.8], 1 mM CaCl2, 1 mM MgCl2) plus a 10-fold dilution of the red dye of the FlexStation membrane potential assay kit (Molecular Devices Corp.) containing 1.8 mM probenecid, in a final volume of 200 μl. The cells were incubated for 60 min at 37°C. The fluorescence of samples was excited at 488 nm, and fluorescence emission was detected by the FLIPR 565 ± 25 nm band-pass emission filter. Samples were measured at 1-s intervals for 120 s, after 30 s of monitoring of the basal fluorescence intensity, as a measure of the membrane potential level of resting cells. A depolarizing agent (60 mM KCl and either 3 mM cGMP or 3 mM cAMP) was added to the cells in the presence or absence of the potassium channel inhibitor tetraethyl ammonium (TEA) chloride at 10 μM or the CNG channel inhibitor l-cis-diltiazem at 10 μM. The responses to agent addition were calculated as the peak fluorescence minus the basal level. Fluorescence intensity was determined using SoftMax2Pro software (Molecular Devices Corp.). Data were expressed as mean values ± standard errors (SE).
Measurement of potassium currents.
A total of 5 × 106 zoospores were loaded with 10 μM potassium-binding benzofuran isophthalate acetoxymethyl ester (PBFI-AM; Molecular Probes) for 60 min at 37°C in sporulation solution (1 mM Tris-maleate buffer [pH 6.8], 1 mM CaCl2, 1 mM MgCl2) containing 1.8 mM probenecid and 0.06% Pluronic F-127 (Sigma-Aldrich), a nonionic surfactant. After loading with PBFI-AM, the cells were washed with sporulation solution and were incubated with 0.1% low-melting-point agarose in sporulation solution. K+ imaging was performed by using the Eclipse Ti-S inverted research microscope (Nikon, Melville, NY) equipped with a 14-bit high-resolution charge-coupled device (CCD) camera (CoolSNAP HQ2; Photometrics, Tucson, AZ), and the images were analyzed with NIS-Elements software (Nikon) using image acquisition rates of two frames per second. Dye fluorescence was excited with a xenon lamp at 340 nm or 380 nm, and the light emitted was detected using a band-pass filter at 515 to 530 nm. Intracellular potassium influx was monitored in cells stimulated with either 60 mM KCl–3 mM cGMP or 60 mM KCl–3 mM cAMP in the presence or absence of the potassium channel inhibitor TEA at 10 μM. About 20 cells were analyzed for each independent experiment. The mean variation between the ratios of the fluorescence intensities obtained by exciting PBFI at these wavelengths (ratio of fluorescence at 340 nm to fluorescence at 380 nm) was then used to determine the concentrations of K+ during the stimulus (F) and the resting state (Fo), normalized to its basal fluorescence.
Phylogenetic analysis of the BeCNG1 amino acid sequence.
To investigate the evolutionary ancestry of the BeGNG1 protein, we calculated a phylogeny. Primarily we used the BeCNG1 amino acid sequence (GenBank accession number KF309500 for the mRNA and AIC07008 for the protein) as a seed sequence for a custom bioinformatic pipeline for generating phylogenies (20). This pipeline initially uses a BLASTp search to recover a set of amino acid sequences (with the gathering threshold set to 1e−10) from a local database of published and publicly available genome databases (see Table S1 in the supplemental material) (21). The sequences retrieved were aligned using MAFFT, version 7.03b, and were masked using trimAl (22). Using this output, a preliminary phylogeny was calculated with FastTree (23). The output tree and alignment were then used as a guide for manual improvement of taxon sampling, sequence alignment, and alignment masking. Taxon sampling was improved by performing additional BLASTp searches of the NCBI nonredundant (nr) database, the Broad Institute Origins of Multicellularity database and Fungal Genome Initiative database, and the JGI Genome Portal. Long branching sequences and closely related sequences from the same genus groups were removed. The alignments were then analyzed using ProtTest 3 (24) to predict the “best-fitting” sequence substitution model for phylogenetic analysis (see the legend to Fig. S1 in the supplemental material). The ProtTest-predicted parameters were then used—where possible—with the RAxML program (with 100 best-known likelihood [BKL] and 1,000 bootstrap [BS] analyses) under the CAT model and with MrBayes, version 3.2 (until the log likelihood reached a plateau for samples from a minimum of 500,000 generations [sampled every 1,000 generations], with burn-in calculated using Tracer, version 1.5) (http://beast.bio.ed.ac.uk/Tracer).
RESULTS
To investigate BeCNG1 function, changes in membrane potential in zoospores in response to the addition of cGMP were determined. For this purpose, we used a membrane potential assay (see Materials and Methods), which detects a fluorescence signal according to changes in membrane potential. We observed that incubation of zoospores with cGMP, but not cAMP, increased the fluorescence detected, indicating significant membrane depolarization (Fig. 1). The results suggest that external cGMP is taken up into the zoospores, since the cGMP-responsive domain of CNG is predicted to be internal. The resting potential of most cells is determined by the relative potassium levels inside and outside the cell (high potassium levels inside and 4 mM in medium or physiological salt solution). Usually, the addition of high quantities of KCl induces some currents by depolarization. The addition of KCl (positive control) to zoospores also resulted in membrane potential depolarization to the degree shown in Fig. 1. In the presence of the CNG inhibitor l-cis-diltiazem, changes in membrane potential due to the addition of cGMP to zoospores were no longer observed (Fig. 1). The presence of the inhibitor also produced a reduction in the change in membrane potential caused by incubation with KCl, but to a lesser extent (Fig. 1). Furthermore, we observed that addition of 3 mM cGMP or 50 mM KCl to the zoospore suspension causes the cells to stop swimming, whereas addition of 3 mM cAMP does not affect zoospore motility (data not shown). These results indicate that high levels of cGMP and KCl that induce membrane depolarization also inhibit zoospore motility.
FIG 1.
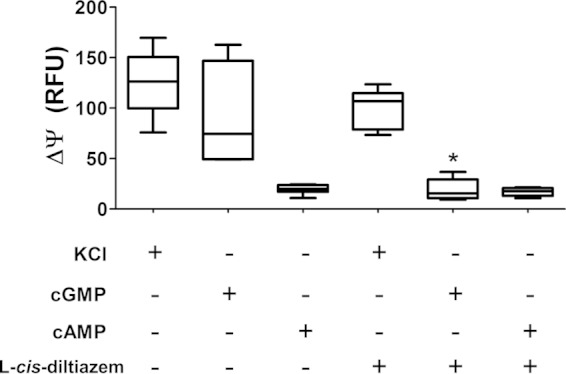
The increase in zoospore membrane potential in response to cGMP depends on a cGMP-activated channel. ΔΨ, change in membrane potential; RFU, relative fluorescence units. KCl (60 mM), cGMP (3 mM), or cAMP (3 mM) was added to the zoospore suspension, and changes in fluorescence emission were recorded in the presence or absence of the CNG channel inhibitor l-cis-diltiazem (at 10 μM). Data are mean values ± SE for three independent experiments performed in triplicate. *, P < 0.05.
To check the hypothesis that BeCNG1 is a potassium-selective channel, we performed the same experiment with the classic potassium channel inhibitor tetraethyl ammonium (TEA) (Fig. 2). Again, as in the experiment for which results are shown in Fig. 1, both KCl (positive control) and cGMP were able to depolarize the membrane potential. However, in the presence of 10 μM TEA, changes in membrane potential were significantly inhibited. Taken together, the results point to the existence of a cGMP-activated potassium channel in B. emersonii zoospores.
FIG 2.
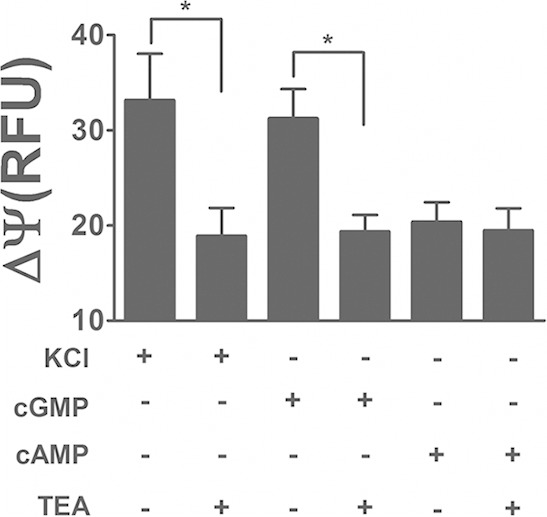
cGMP-dependent depolarization of zoospore membrane potential is due to potassium channel activation. ΔΨ, change in membrane potential; RFU, relative fluorescence units. KCl (60 mM), cGMP (3 mM), or cAMP (3 mM) was added to the zoospore suspension, and changes in fluorescence emission were recorded in the presence or absence of the potassium channel inhibitor TEA. Data are mean values ± SE for three independent experiments performed in triplicate. *, P < 0.05.
To confirm that the CNG channel we are monitoring is indeed a potassium channel, the flux of this ion was analyzed in the presence of cGMP by using a fluorescent dye selective for potassium (PBFI-AM; Molecular Probes). The addition of cGMP caused a high influx of potassium (Fig. 3), which was also observed when only KCl was added as a positive control. Nevertheless, the addition of cAMP did not lead to potassium influx, indicating the specificity of the potassium channel for cGMP (Fig. 3). This experiment was also performed in the presence or absence of the potassium channel inhibitor TEA to confirm that the potassium flux observed by the addition of KCl or cGMP was caused by a potassium channel and not by nonspecific flux or diffusion. Again, KCl and cGMP produced increases in potassium influx in all recorded cells (Fig. 4A), but in different ways. KCl induced a rapid increase in potassium flux followed by a slow decrease, whereas cGMP induced a sustained and prolonged increase in potassium influx (Fig. 4B). The addition of cAMP, as expected, did not produce any significant change in potassium flux. In the presence of TEA, the increases in K+ concentrations produced by cGMP and KCl were inhibited, as expected for a potassium channel (Fig. 4B and C). Taken together, our results point to the presence of a potassium-selective channel in B. emersonii zoospores that is specifically activated by cyclic GMP; in the presence of this cyclic nucleotide, the channel opens, creating a potassium influx.
FIG 3.
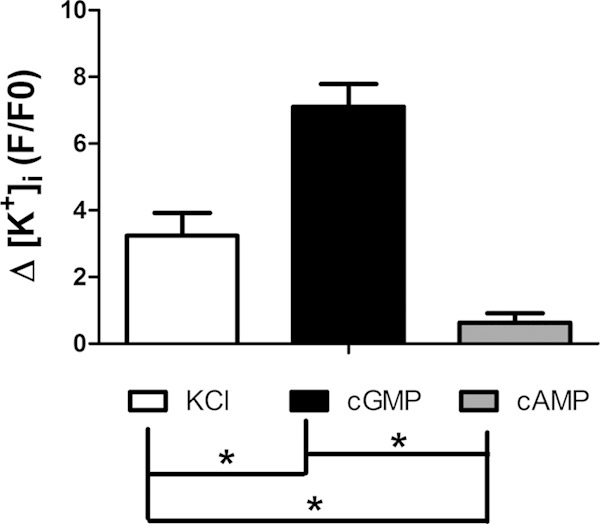
cGMP induces potassium influx in Blastocladiella zoospores. Δ[K+]i, variation in potassium influx; F/F0, peak fluorescence intensity divided by basal fluorescence intensity. KCl (60 mM), cGMP (3 mM), or cAMP (3 mM) was added to the zoospore suspension, and fluorescence emission rates were recorded. Data are mean values ± SE for three independent experiments performed in triplicate. *, P < 0.05.
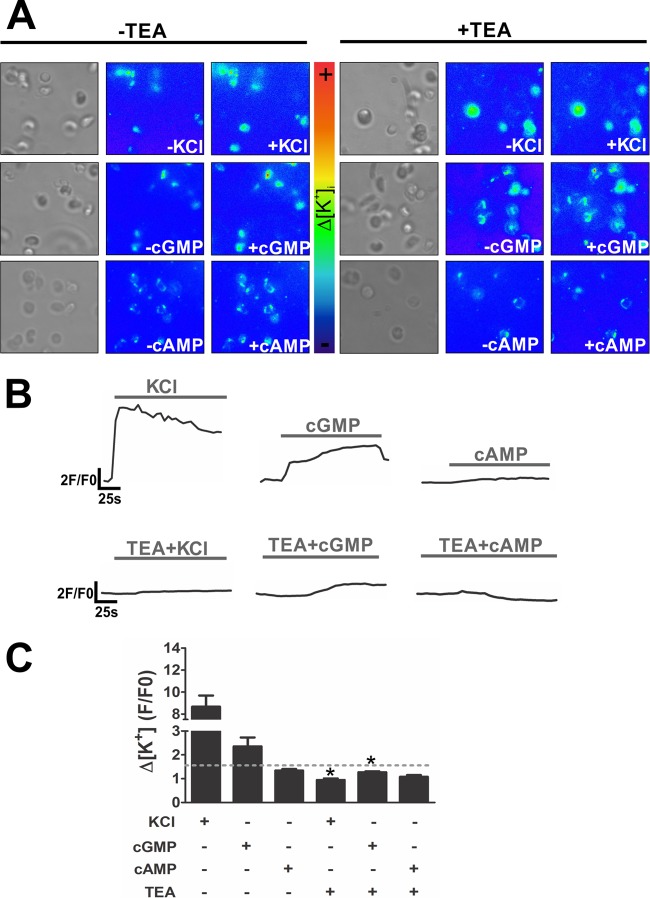
FIG 4.
The potassium influx induced by cGMP is dependent on a potassium channel. For intracellular potassium flux imaging, KCl (60 mM), cGMP (3 mM), or cAMP (3 mM) was added to the zoospore suspension in the presence or absence of the classic potassium channel inhibitor TEA (at 10 μM). (A) Images from one representative experiment. Cells at the rest state and under bright field are shown at the left of each set of panels. (B) For potassium measurements, the time kinetics of changes in fluorescence emission was recorded. Data are from one representative experiment. (C) Bar graph showing variation in the potassium influx (Δ[K+]i), calculated as peak fluorescence intensity divided by basal fluorescence intensity (F/F0). Data are mean values ± SE for three independent experiments performed in triplicate. *, P < 0.05.
DISCUSSION
CNG and K+ channels share significant amino acid sequence identity, and they have been suggested to present a common ancestral 3-dimensional (3D) architecture. However, these two types of channels have quite different selectivity properties: K+ channels are permeable primarily to K+ and Rb+ ions, whereas CNG channels are permeable to all monovalent alkali cations (Li+, Na+, K+, Rb+, and Cs+), to a variety of organic cations, and to some divalent cations, such as Ca2+, Mg2+, Sr2+, and Mn2+. This functional difference is ascribed to the presence of the GYGD motif in the pore of K+ channels, which in CNG channels is replaced by a single glycine (25). As a consequence, the pore in CNG channels is presumably shorter than that in K+ channels and possibly more flexible (25). CNG channels are ubiquitously expressed in sensory and nonsensory cells, and their role in sensory transduction in vertebrate photoreceptors and olfactory sensory neurons has been well established (26). K+-selective CNG (CNGK) channels have been implicated in sea urchin sperm chemosensation and in light signaling by ciliary photoreceptors in the scallop; cGMP is the signaling molecule in both signal transduction pathways, and a rise in cGMP levels opens the K+ channels, causing hyperpolarization of the cells (2, 10).
In this report, we show that the blastocladiomycete fungus B. emersonii expresses a K+ channel that is activated by cGMP. The use of specific channel inhibitors confirms the activation of the channel by cGMP and its K+ selectivity. Interestingly, other blastocladiomycete fungi, which also possess a flagellum during the zoospore phase of their life cycle, such as Allomyces macrogynus and Catenaria anguillulae, possess genes that encode an orthologous putative K+ channel (see Fig. S1 in the supplemental material), as well as the rhodopsin–guanylyl cyclase fusion protein (6). However, both these gene orthologue families are absent in nonflagellated fungi, such as members of the classes Ascomycetes and Basidiomycetes (see Fig. S1) (6). Furthermore, in the flagellated chytridiomycete fungus Batrachochytrium dendrobatidis, a pathogen of amphibians, orthologues of BeGC1 and BeCNG1 are also absent, even though other elements of cGMP signaling are present (27). Current drafts of the genome of yet another chytrid, Spizellomyces punctatus, show the presence of an orthologue of BeCNG1 (see Fig. S1), but no true homologue of BeGC1 was detected (6). Nevertheless, the genome contains several adenylate/guanylate cyclase domains, in contrast to the genomes of fungi that do not form flagella, which lack genes encoding components of cGMP signaling (27). Thus, these observations indicate that the presence of orthologues of both BeGC1 and BeCNG1 genes as a pair may be an exclusive feature of blastocladiomycete fungi. Additionally, these data agree with the hypothesis that cGMP signaling pathways are restricted to motile flagellated fungi (27) and that the loss of flagella within the fungi was accompanied by the loss of cGMP-signaling genes.
Taken together, our data indicate that a CNGK channel triggers the phototaxis pathway in B. emersonii zoospores. Analysis of the B. emersonii genome revealed that no other gene encoding an ion channel with these characteristics is present, suggesting that the BeCNG1 channel is a key component of this signaling pathway. The role of a cGMP-activated K+ channel in the scallop phototransduction pathway (2, 3) and in the sea urchin sperm chemotactic response (9, 10) suggests that systems analogous (and possibly paralogous) to BeCNG1 in the B. emersonii phototactic response are present. In our proposed model, the increase in cGMP promoted by the exposure of zoospores to green light leads to activation of the BeCNG1 channel and to K+ influx. The increase in intracellular K+ concentrations in B. emersonii zoospores leads, directly or indirectly, to changes in flagellar beating, producing the phototactic response of the fungus.
It is worth mentioning that the presence in B. emersonii of a K+ channel activated by light due to its association with a photoresponsive guanylyl cyclase can constitute an interesting optogenetic device to be used in eukaryotic cells. The capacity to control a K+ channel by exposure to light could provide the means to manipulate the ability of K+ to terminate excitatory currents within cells. Thus, the rhodopsin–guanylyl cyclase (BeGC1) together with the CNGK channel (BeCNG1) could represent an efficient tool acting in many cellular processes, including neuronal firing and hormone release, as recently discussed by Cosentino and coworkers (28).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and the Provost's Office for Research of the University of São Paulo (grant 2011.1.9333.1.3) (NAPNA-USP), São Paulo, Brazil. G.M.A. and T.G. acknowledge fellowship support by FAPESP. S.L.G. and H.U. were partially supported by CNPq.
We thank Luci D. Navarro and Sandra M. Fernandes for expert technical assistance.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/EC.00087-15.
REFERENCES
- 1.Zhang X, Cote RH. 2005. cGMP signaling in vertebrate retinal photoreceptor cells. Front Biosci 10:1191–1204. doi: 10.2741/1612. [DOI] [PubMed] [Google Scholar]
- 2.Gomez MP, Nasi E. 2000. Light transduction in invertebrate hyperpolarizing photoreceptors: possible involvement of a Go-regulated guanylate cyclase. J Neurosci 20:5254–5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.del Pilar Gomez M, Nasi E. 1995. Activation of light-dependent K+ channels in ciliary invertebrate photoreceptors involves cGMP but not the IP3/Ca2+ cascade. Neuron 15:607–618. doi: 10.1016/0896-6273(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 4.Foster KW, Saranak J, Patel N, Zarilli G, Okabe M, Kline T, Nakanishi K. 1984. A rhodopsin is the functional photoreceptor for phototaxis in the unicellular eukaryote Chlamydomonas. Nature 311:756–759. doi: 10.1038/311756a0. [DOI] [PubMed] [Google Scholar]
- 5.Saranak J, Foster KW. 1997. Rhodopsin guides fungal phototaxis. Nature 387:465–466. doi: 10.1038/387465a0. [DOI] [PubMed] [Google Scholar]
- 6.Avelar GM, Schumacher RI, Zaini PA, Leonard G, Richards TA, Gomes SL. 2014. A rhodopsin-guanylyl cyclase gene fusion functions in visual perception in a fungus. Curr Biol 24:1234–1240. doi: 10.1016/j.cub.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sineshchekov OA, Govorunova EG, Spudich JL. 2009. Photosensory functions of channelrhodopsins in native algal cells. Photochem Photobiol 85:556–563. doi: 10.1111/j.1751-1097.2008.00524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pittler SJ, Lee AK, Altherr MR, Howard TA, Seldin MF, Hurwitz RL, Wasmuth JJ, Baehr W. 1992. Primary structure and chromosomal localization of human and mouse rod photoreceptor cGMP-gated cation channel. J Biol Chem 267:6257–6262. [PubMed] [Google Scholar]
- 9.Bönigk W, Loogen A, Seifert R, Kashikar N, Klemm C, Krause E, Hagen V, Kremmer E, Strunker T, Kaupp UB. 2009. An atypical CNG channel activated by a single cGMP molecule controls sperm chemotaxis. Sci Signal 2:ra68. doi: 10.1126/scisignal.2000516. [DOI] [PubMed] [Google Scholar]
- 10.Strünker T, Weyand I, Bönigk W, Van Q, Loogen A, Brown JE, Kashikar N, Hagen V, Krause E, Kaupp UB. 2006. A K+-selective cGMP-gated ion channel controls chemosensation of sperm. Nat Cell Biol 8:1149–1154. doi: 10.1038/ncb1473. [DOI] [PubMed] [Google Scholar]
- 11.Plachetzki DC, Fong CR, Oakley TH. 2010. The evolution of phototransduction from an ancestral cyclic nucleotide gated pathway. Proc Biol Sci 277:1963–1969. doi: 10.1098/rspb.2009.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lew RR, Levina NN, Shabala L, Anderca MI, Shabala SN. 2006. Role of a mitogen-activated protein kinase cascade in ion flux-mediated turgor regulation in fungi. Eukaryot Cell 5:480–487. doi: 10.1128/EC.5.3.480-487.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lew RR, Nasserifar S. 2009. Transient responses during hyperosmotic shock in the filamentous fungus Neurospora crassa. Microbiology 155:903–911. doi: 10.1099/mic.0.023507-0. [DOI] [PubMed] [Google Scholar]
- 14.Lew RR. 2007. Ionic currents and ion fluxes in Neurospora crassa hyphae. J Exp Bot 58:3475–3481. doi: 10.1093/jxb/erm204. [DOI] [PubMed] [Google Scholar]
- 15.Trail F, Gaffoor I, Vogel S. 2005. Ejection mechanics and trajectory of the ascospores of Gibberella zeae (anamorph Fusarium graminearum). Fungal Genet Biol 42:528–533. doi: 10.1016/j.fgb.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Van Brunt J, Harold FM. 1980. Ionic control of germination of Blastocladiella emersonii zoospores. J Bacteriol 141:735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soll DR, Sonneborn DR. 1972. Zoospore germination in Blastocladiella emersonii. IV. Ion control over cell differentiation. J Cell Sci 10:315–333. [DOI] [PubMed] [Google Scholar]
- 18.Thomas DD, Butler DL. 1989. Cationic interactions regulate the initiation and termination of zoospore activity in the water mould Achlya heterosexualis. J Gen Microbiol 135:1917–1922. [Google Scholar]
- 19.Appiah AA, van West P, Osborne MC, Gow NA. 2005. Potassium homeostasis influences the locomotion and encystment of zoospores of plant pathogenic oomycetes. Fungal Genet Biol 42:213–223. doi: 10.1016/j.fgb.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Richards TA, Soanes DM, Foster PG, Leonard G, Thornton CR, Talbot NJ. 2009. Phylogenomic analysis demonstrates a pattern of rare and ancient horizontal gene transfer between plants and fungi. Plant Cell 21:1897–1911. doi: 10.1105/tpc.109.065805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Capella-Gutiérrez S, Silla-Martinez JM, Gabaldon T. 2009. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25:1972–1973. doi: 10.1093/bioinformatics/btp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Price MN, Dehal PS, Arkin AP. 2010. FastTree 2—approximately maximum-likelihood trees for large alignments. PLoS One 5:e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Darriba D, Taboada GL, Doallo R, Posada D. 2011. ProtTest 3: fast selection of best-fit models of protein evolution. Bioinformatics 27:1164–1165. doi: 10.1093/bioinformatics/btr088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazzolini M, Marchesi A, Giorgetti A, Torre V. 2010. Gating in CNGA1 channels. Pflugers Arch 459:547–555. doi: 10.1007/s00424-009-0751-2. [DOI] [PubMed] [Google Scholar]
- 26.Matulef K, Zagotta WN. 2003. Cyclic nucleotide-gated ion channels. Annu Rev Cell Dev Biol 19:23–44. doi: 10.1146/annurev.cellbio.19.110701.154854. [DOI] [PubMed] [Google Scholar]
- 27.Johnson JL, Leroux MR. 2010. cAMP and cGMP signaling: sensory systems with prokaryotic roots adopted by eukaryotic cilia. Trends Cell Biol 20:435–444. doi: 10.1016/j.tcb.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Cosentino C, Alberio L, Gazzarrini S, Aquila M, Romano E, Cermenati S, Zuccolini P, Petersen J, Beltrame M, Van Etten JL, Christie JM, Thiel G, Moroni A. 2015. Optogenetics. Engineering of a light-gated potassium channel. Science 348:707–710. doi: 10.1126/science.aaa2787. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.