Abstract
Candida species cause a variety of mucosal and invasive infections and are, collectively, the most important human fungal pathogens in the developed world. The majority of these infections result from a few related species within the “CUG clade,” so named because they use a nonstandard translation for that codon. Some members of the CUG clade, such as Candida albicans, present significant clinical problems, whereas others, such as Candida (Meyerozyma) guilliermondii, are uncommon in patients. The differences in incidence rates are imperfectly correlated with virulence in animal models of infection, but comparative analyses that might provide an explanation for why some species are effective pathogens and others are not have been rare or incomplete. To better understand the phenotypic basis for these differences, we characterized eight CUG clade species—C. albicans, C. dubliniensis, C. tropicalis, C. parapsilosis, Clavispora lusitaniae, M. guilliermondii, Debaryomyces hansenii, and Lodderomyces elongisporus—for host-relevant phenotypes, including nutrient utilization, stress tolerance, morphogenesis, interactions with phagocytes, and biofilm formation. Two species deviated from expectations based on animal studies and human incidence. C. dubliniensis was quite robust, grouping in nearly all assays with the most virulent species, C. albicans and C. tropicalis, whereas C. parapsilosis was substantially less fit than might be expected from its clinical importance. These findings confirm the utility of in vitro measures of virulence and provide insight into the evolution of virulence in the CUG clade.
INTRODUCTION
Candida species are the most important fungal pathogens of humans and are collectively responsible for a vast number of infections. These range from superficial mucosal infections such as vulvovaginal candidiasis, and oropharyngeal thrush, to life-threatening infections such as disseminated hematogenous and invasive candidiasis. These latter infections have steadily increased in incidence in the last 30 years and are associated with a stubbornly high mortality rate as a result of the underlying immunodeficiency of the patients and inadequate diagnostics and treatments (1).
Of the approximately 150 species in the genus, 95% of infections are caused by just four species: C. albicans, C. tropicalis, C. parapsilosis, and C. glabrata (2–4). C. albicans is the dominant species, representing about half of disseminated disease and an even greater percentage of mucosal infections. Other clinically relevant species include Clavispora lusitaniae (anamorph: Candida lusitaniae), M. guilliermondii (anamorph: Candida guilliermondii), C. krusei, and C. dubliniensis, while D. hansenii (synonym: Candida famata) and Lodderomyces elongisporus are subjects of rare clinical reports. Because the genus Candida is polyphyletic, a better sense of evolutionary relationships comes in the “CUG clade,” a grouping of species that use an alternative genetic code in which that codon specifies serine rather than leucine (5, 6). The CUG clade encompasses all Candida species commonly isolated from patients other than C. glabrata and C. krusei.
Within the CUG clade there is great diversity in both genotype and phenotype. M. guilliermondii, C. lusitaniae, and D. hansenii are haploid, while the others are diploid. Originally classified as fungi imperfecti, sexual cycles are slowly being identified for most of these species (for a review, see reference 7). Most importantly, clinical incidence of these species is correlated, albeit imperfectly, with virulence potential in animal assays. A series of experiments testing several Candida species in mice grouped C. albicans and C. tropicalis as the most virulent (infections with high inoculums were lethal), followed by C. glabrata and C. lusitaniae (not lethal, but organisms persisted in organs), with C. parapsilosis, C. krusei, and M. guilliermondii as the least virulent, with at least some of the animals clearing even high inoculums from the kidneys (8). This is broadly consistent with a variety of other studies using subsets of these species in mouse models of disseminated or mucosal infections or gastrointestinal colonization (9–13). It is notable that in these models, C. parapsilosis is consistently less virulent than would be predicted from its clinical incidence.
A variety of phenotypes have been correlated with virulence in Candida species, primarily C. albicans, including hyphal growth, adhesion and biofilm formation, resistance to reactive oxygen and nitrogen stresses, use of nonfermentable carbon sources, modulation of macrophage functions, tolerance of a range of extracellular pH, and secreted protease and lipase activity (for a review, see reference 14). Presumably, the ability to cause disease in the mammalian host is the product of a combination of these phenotypes and others, but there are only a few instances in which virulence-related phenotypes were examined systematically across multiple CUG species; for instance, sensitivity to peroxide was assayed for eight species, finding differences that were imperfectly correlated with virulence (15). The phenotypic diversity both in vitro and in animal models comes from a combination of genomic (gene content) and regulatory (expression) variations among these species. The substantial genomic differences between these species have been analyzed primarily in silico (16–18), with interspecies comparisons at a molecular level only beginning to appear.
We assayed eight species of the CUG clade for a variety of host-relevant phenotypes, including interactions with phagocytes, morphology in multiple conditions, nutritional flexibility, and stress resistance. Although there is not a perfect correlation between these in vitro phenotypes and virulence, we found in general that the most pathogenic species have the highest growth rates in a variety of conditions, are most resistant to relevant stresses, and are the most robust when confronted by phagocytes. These findings are an important contribution to the dissection of virulence within this genus and will inform future molecular, genomic, and proteomic studies within this clade.
MATERIALS AND METHODS
Strains and media.
The fungal strains used are listed in Table 1. For each species, the strain chosen was the one used for the respective genome sequencing project, while several additional strains of C. parapsilosis were obtained from G. Butler. Strains were propagated on standard yeast media (19), including YPD (1% yeast extract, 2% peptone, 2% dextrose) and YNB (0.17% yeast nitrogen base, 0.5% ammonium sulfate, 2% dextrose). Media were adjusted as indicated in the text with various stress-inducing agents. SLAD medium (0.17% yeast nitrogen base, 10 mM ammonium sulfate, 2% dextrose, 2% agar) was prepared as described previously (20). Strains were grown at 37°C except for D. hansenii, which grows poorly at that temperature and was propagated at room temperature (25°C) instead.
TABLE 1.
Fungal strains used in this study
| Candida designation | Teleomorph | Strain | Origin of strain | Reference(s) |
|
|---|---|---|---|---|---|
| For origina | For genome sequenceb | ||||
| Candida albicans | None | SC5314 | “Disseminated” | 67 | 16, 68 |
| Candida dubliniensis | None | CD36 | Oral | 69 | 48 |
| Candida tropicalis | None | MYA-3404 | Blood | 70 | 17 |
| Candida lusitaniae | Clavispora lusitaniae | ATCC 42720 | Blood | 71 | 17 |
| Candida guilliermondii | Meyerozyma guilliermondiic | ATCC 6260 | Lungs | 72 | 17 |
| Lodderomyces elongisporusd | None | NRRL YB-4239 | Orange juicee | 73 | 17 |
| Candida famata | Debaryomyces hansenii | CBS767 | Unknownf | 18 | |
| Candida parapsilosis | None | CDC317 | Skin | 47 | 17 |
| CDC173 | Invasive | 47 | |||
| CDC177 | Invasive | 47 | |||
| CLIB214 | Feces | 74 | |||
That is, the reference for the report of the isolation of the strain.
That is, the reference for the report(s) of the complete genome sequence.
In some older literature, the teleomorph is named Pichia guilliermondii.
L. elongisporus has never been classified as a Candida species.
Isolation source per Centraalbureau voor Schimmelcultures for the alias CBS 2065.
Deposited at Centraalbureau voor Schimmelcultures by Carlsberg Laboratory, origin unknown.
Cell culture experiments used the RAW264.7 murine macrophage-like cell line (American Type Culture Collection), which was propagated in RPMI with glutamine and HEPES (Invitrogen) supplemented with 10% fetal bovine serum (FBS; Fisher/HyClone) and grown in a 5% CO2 environment.
Stress sensitivity assays.
To assess growth on various carbon sources, strains were grown overnight in YPD at 30°C and then diluted to an optical density at 600 nm of 0.1 in 8 ml of YPD or in YNB with 2% glucose, ethanol, lactate or Casamino Acids (CAA), or 1% acetate, present as the sole carbon source. Each medium was set to pH 6, except for the CAA media, which was adjusted to pH 4. Cultures were grown with aeration at 37°C for 24 h and monitored by determining the optical density at the indicated times. Aliquots of the CAA cultures were also used for pH measurements to assess alkalinization of the media, as we have reported previously (21, 22). Assays were done at least in triplicate. Doubling times were calculated from the exponential growth phase, between 2 and 6 h after inoculation.
Sensitivities to other stresses were assayed using a BioTek SynergyMX automated plate reader in a 96-well plate format. Strains were grown overnight in YPD at 30°C, washed with water, and resuspended in 200 μl of YPD medium containing the relevant stressor. The plate was incubated at 30°C with periodic agitation, and the optical density was measured every 10 min for up to 16 h. Each experiment had three replicates per condition, and this was repeated at least three times. Doubling times were calculated for rolling 200-min periods every 10 min from 1 to 12 h. The time at which the peak growth rate was achieved differed somewhat by species and condition; the maximal division rate is reported.
Morphological characterization.
For determination of cellular morphology in liquid inducing conditions, strains were grown overnight in YPD at 30°C, washed with water, and diluted into control YPD medium, RPMI (pH 7.4), or 10% FBS in water. After 1 to 4 h at 37°C, the cultures were centrifuged briefly to concentrate the cells before photographing them at ×400 with an Olympus IX-81 inverted microscope.
Cellular and colony morphologies were assessed on solid medium under nitrogen starvation and embedded conditions. Colony morphology was observed on solid SLAD medium in petri dishes. For imaging of cellular morphology, agar pads of the same media were prepared on standard microscope slides. Highly diluted cultures were spotted to these pads, grown for 48 h at 37°C, and imaged using a Zeiss Axiostar microscope fitted with a trinocular camera mount. Cells were embedded in an agar matrix by mixing an average of 100 to 250 cells in YPD top agar (0.5% agar) at 42°C in petri dishes. After solidifying, they were grown 4 days at 37°C and photographed at two resolutions using an Olympus IX-81 inverted microscope.
Fungal-macrophage coculture experiments. (i) Morphology.
RAW264.7 macrophages were seeded on glass coverslips in 12-well plates at a density of 106 cells/well in 1 ml of RPMI plus 10% FBS and allowed to adhere for 2 h at 37°C. Fungal strains were grown overnight in YPD, washed with water, resuspended in phosphate-buffered saline (PBS), and counted using a hemocytometer. Fungal cells were added at ratios of 1:1 to 2.5:1 (with higher ratios used for the weaker pathogens) incubated for 1 h. Cocultures were washed twice with PBS and then treated with 350 ng of calcofluor white/ml for 10 s to stain nonphagocytosed cells. After two washes with PBS, the cells were fixed with paraformaldehyde and permeabilized with 0.1% Triton X-100. A rabbit polyclonal anti-Candida-FITC antibody (LSBio) was then used to stain and visualize both intracellular and extracellular fungi. The proportion of filamentous cells (hyphal or pseudohyphal) was ascertained by scoring morphology in photomicrographs.
(ii) Cytotoxicity.
The ability of the fungal species to kill macrophages was assessed through detection of lactate dehydrogenase (LDH) in the culture supernatant using the CytoTox 96 kit (Promega) as described previously (22). RAW264.7 cells were seeded in 96-well plates at 2.5 × 105 cells/well and allowed to adhere overnight. Fungal overnight cultures were washed and diluted in PBS, added to the macrophages at a 3:1 ratio, and incubated for 5 h before the supernatant was removed and assayed for LDH activity according to the manufacturer's protocol. The ability of each species to induce macrophage damage was expressed as a percentage of the total LDH released from chemically lysed cells.
(iii) NO suppression assay.
RAW264.7 macrophages were seeded at 6 × 105 cells/well in 12-well plates. Fungal species were prepared as described above and added to the wells at fungal cell/macrophage ratios of 1:10 or 1:100. Concurrently, 100 ng of lipopolysaccharide (LPS)/ml and 100 U of gamma interferon (IFN-γ)/ml were added, and the cocultures were incubated at 37°C in 5% CO2 for 24 h. Cell-free culture supernatants were assayed for the presence of nitrite, which spontaneously forms from nitric oxide in aqueous solutions, using Griess reagent, as described previously (23). Assays were performed in triplicate.
Biofilm formation.
To assess biofilm formation, we used a modification of an established assay to measure adherence to polystyrene plates (24). Fungal strains were grown to log phase in YNB plus 2% glucose, washed with PBS, and inoculated to 96-well plates at 106 cells/well. The plate was incubated for 1.5 h with gentle shaking at 37°C. Subsequently, nonadherent cells were removed by aspiration and washing twice with PBS. Biofilms were allowed to develop in YNB plus 2% glucose for 24 h at 37°C. Then the wells were washed twice with PBS and air dried for 45 min before adding 0.4% crystal violet for 45 min. After extensive washing, the wells were destained with 95% ethanol for 45 min. The destain was transferred to a fresh plate, and the absorbance at 595 nm was recorded.
RESULTS
The CUG clade contains highly virulent (and intensively studied) species such as C. albicans and much less virulent and virtually uncharacterized species such as M. guilliermondii and L. elongisporus. The phylogenetic relationships between these species are depicted in Fig. 1 in which the uneven link between phylogeny and virulence is apparent: C. albicans and C. tropicalis are significant pathogens, while C. dubliniensis is not. C. parapsilosis is frequently isolated clinically, L. elongisporus is not. Among the haploid species, C. lusitaniae, although relatively rare clinically, is nonetheless much more common than M. guilliermondii, which in turn is more common than D. hansenii (25). Thus, we sought to add to the existing bioinformatics comparisons based on genome sequence and predicted protein content (17, 26, 27) by understanding the phenotypic differences that may contribute to virulence in these species.
FIG 1.

Phylogenetic relationships between CUG species. Phylogeny was determined using the nucleotide and protein sequences of core set of conserved orthologous genes using the MrBayes 3.1.2 tool (66). (Adapted from Nature [17].)
Utilization of different carbon sources.
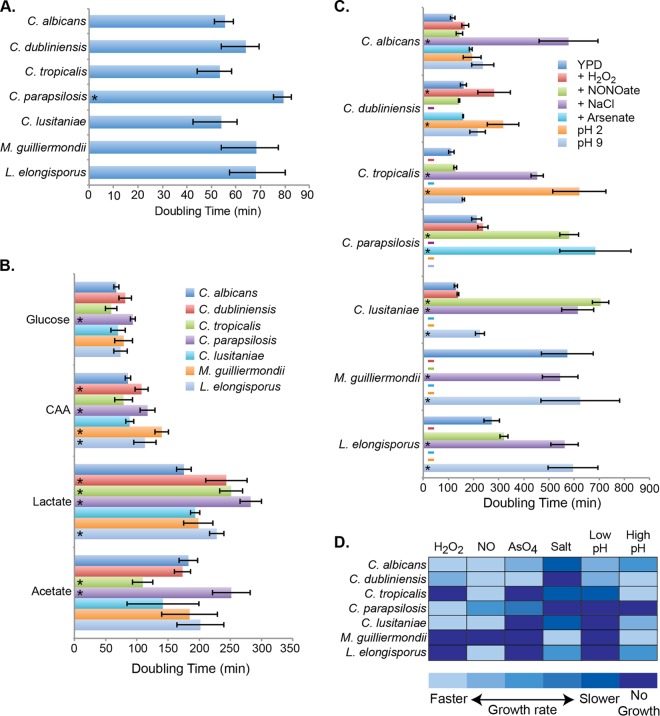
We began the phenotypic assessment of these species by measuring growth rates in standard media. In aerated cultures at 37°C in either rich YPD or minimal YNB medium, both with 2% glucose as the primary (YPD) or sole (YNB) carbon source, each species grew rapidly (Fig. 2A), with the exception of D. hansenii, which grows very poorly at 37°C and was omitted from most of the remaining assays. C. tropicalis had the fastest doubling times in both conditions (53.6 and 59.0 min in YPD and YNB-glucose, respectively), growing slightly more quickly than C. albicans and C. lusitaniae. C. parapsilosis was consistently the slowest growing species, with a cell division time ca. 50% longer than that for C. tropicalis under optimal conditions.
FIG 2.
Growth rates and stress sensitivities for CUG clade species. (A) The exponential doubling time, calculated as described in Materials and Methods, is shown for each species grown at 37°C in aerated (flask) cultures in YPD. An asterisk indicates a significantly different (P < 0.01) doubling time relative to C. albicans. (B) Doubling times for strains grown in aerated cultures in YNB medium containing 2% glucose, Casamino Acids (CAA) or lactate, or 1% acetate. The asterisks represent species with a significantly (P < 0.01) reduced growth rate relative to C. albicans in the same media. (C) The doubling times for each species in a variety of stress conditions are plotted. The strains were grown in 96-well plates in an automated plate reader at 37°C with intermittent shaking in YPD medium containing no additives, 10 mM hydrogen peroxide (H2O2), 8 mM Deta-NONOate (NONOate), 1 M sodium chloride (NaCl), 1 mM arsenate, or media adjusted to pH 2 or 9. Dashes next to the vertical axis indicate no growth (a calculated doubling time greater than 800 min), and asterisks indicate a significant (P < 0.01) reduction in growth rate compared to the same species in the absence of stress. (D) Heat map depiction of the data from panel B in which boxes are shaded according to growth rates in the indicated condition relative to the no-stress control. Darker blue colors indicate lower growth rates (greater growth inhibition by that stressor).
The acquisition and utilization of alternative nonfermentable carbon sources such as lactate or amino acids has been proposed to be important in some host niches (21, 22, 28–30); C. albicans mutants lacking the ability to metabolize nonsugar compounds are attenuated in mouse models (22, 31–34). Thus, we assayed growth rates in minimal YNB medium with lactate, acetate, or amino acids (in the form of Casamino Acids) as the sole carbon source. C. albicans, C. tropicalis, and C. lusitaniae utilized amino acids effectively as the carbon source (doubling times of 78.8 to 88.5 min), while the growth of the other species was much slower (Fig. 2B). Growth was uniformly slower in the presence of lactate and acetate (Fig. 2B), with significant variations between species; the doubling times for C. tropicalis, for instance, were far faster in acetate-containing media than other species, but among the slowest in the presence of lactate. Although all species metabolized acetate, there was significant variability between experiments in the lag time before growth began, particularly for C. lusitaniae and M. guilliermondii. C. parapsilosis was the slowest growing species under all of these conditions.
Tolerance to common stresses.
To assess sensitivity to various host-relevant stresses, including reactive oxygen and nitrogen species (hydrogen peroxide and the nitric oxide donor Deta-NONOate), osmotic stress (sorbitol and sodium chloride), pH (pH 2 to 9), and arsenate, we used a 96-well plate format with an automated plate reader (Fig. 2C; see also Fig. S1 in the supplemental material). Growth was generally slower than in the broth cultures but C. tropicalis (111.5 min), C. albicans (117.3 min), and C. lusitaniae (129.4 min) were again the fastest-growing species, followed by C. dubliniensis (159.9 min). The other three species, in contrast, grew less well in the 96-well plates, perhaps due to the more limited aeration in this format. The doubling time for M. guilliermondii was more than eight times longer in the plates compared to broth culture (572.9 versus 68.7 min).
When stressors were added to YPD in the 96-well plate assays, the growth patterns changed markedly. C. albicans and C. dubliniensis were relatively resistant to hydrogen peroxide, NONOate, arsenate (which can induce oxidative stress), and pH extremes (Fig. 2C and D; additional concentrations of stress agents are shown in Fig. S1 in the supplemental material), with doubling times within 2-fold of the control. The addition of 1 M sodium chloride greatly slowed the growth of all species (Fig. 2C and D), although this was not due to osmotic stress, because none of the species were sensitive to 1 M sorbitol (see Fig. S1 in the supplemental material). While C. albicans was fairly resilient under these stress conditions, the other species were sensitive to specific stressors; C. tropicalis, for instance, failed to grow in 10 mM peroxide or 1 mM arsenate and grew slowly at pH 2. In contrast, C. lusitaniae and C. parapsilosis were quite resistant to peroxide but acutely sensitive to reactive nitrogen species. Growth patterns in stress conditions are summarized in the heat map in Fig. 2D.
Modulation of extracellular pH.
We have previously described a phenomenon in which C. albicans neutralizes acidic media through the excretion of ammonia derived from the catabolism of amino acids as a carbon source, and we observed this occurring in other Candida species as well, including C. glabrata (21, 35). Alkalinization occurs optimally in minimal medium with Casamino Acids as the sole source of carbon, a condition in which all species grew fairly well (Fig. 2B); thus, we tested their ability to neutralize the medium (see Fig. S2 in the supplemental material). All seven species rapidly raised the extracellular pH from 4 to about 7.5 in about 12 h and, while there were some differences in the kinetics, this largely correlated with growth rates in this media.
Morphology.
A hallmark of C. albicans is its polymorphic nature and ample evidence indicates that the transition between morphological forms is required for virulence (36, 37). Of the other species, only C. tropicalis and C. dubliniensis form true hyphae, and they do this far less readily than C. albicans (38, 39). We grew each species in standard hyphal-inducing conditions, including in serum or in RPMI (at pH 7.4). Cellular morphology was consistent with previously published results (39), with abundant hyphae seen in C. albicans, some hyphae in C. dubliniensis, and a mix of pseudohyphae and rarer true hyphae in C. tropicalis (see Fig. S3 in the supplemental material). All other species remained in the yeast form.
Nitrogen limitation induces pseudohyphal formation in many yeast species, including C. albicans, C. tropicalis and S. cerevisiae (20, 39–41), so we tested each of the CUG species on solid low ammonia SLAD medium. As previously reported, C. albicans forms pseudohyphae on this medium as observed using a low-magnification stereomicroscope (Fig. 3). The filamentous growth of C. tropicalis and C. lusitaniae was surprisingly robust on this medium. To assess cellular morphology, cells were incubated on thin films of SLAD prepared on microscope slides for 24 to 48 h and then analyzed by higher-resolution differential interference contrast imaging. Although the clarity is compromised by the agar substrate, pseudohyphal cells were seen at least occasionally for all species except M. guilliermondii (Fig. 3). Interestingly, the florid filamentous growth of C. tropicalis cells was largely composed of true hyphae, as evidenced by the parallel cell walls, branched hyphae, and absence of constrictions at septae. It even appeared to form aerial hyphae on SLAD (see Video S1 in the supplemental material). C. tropicalis hyphal forms were far more common under nitrogen limitation than in other reported hyphal-inducing conditions.
FIG 3.
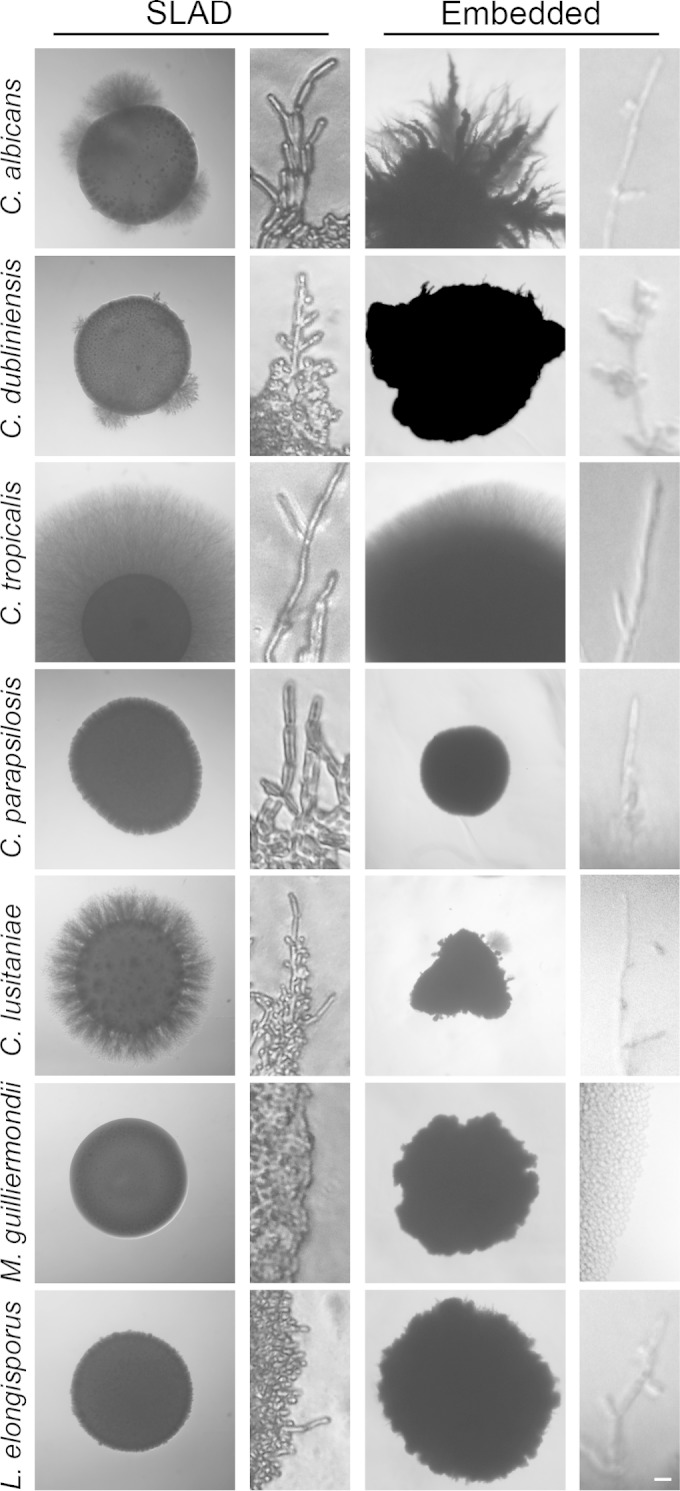
Morphology of CUG species. To assess colony morphology under nitrogen limitation, strains were grown on SLAD medium in standard petri dishes for 4 days at 37°C before imaging with a stereomicroscope at ×20 magnification. To determine cellular morphology, strains were grown at 37°C for 48 h on SLAD agar pads on microscope slides before imaging at ×400. To assess colony morphology under embedded conditions, cells were diluted in YPD-top agar (0.5% agar) at ∼100 cells/plate, followed by incubation at 37°C for 5 days before imaging on the stereomicroscope at ×40. Cellular morphology was determined by imaging the same plates using an inverted microscope. The scale bar (10 μm) in the lower right applies to the insets with the cellular images.
C. albicans cells embedded in an agar matrix also form abundant hyphae, and this is largely independent of other inducing stimuli, occurring in rich YPD medium even at 25 to 30°C. These conditions also bypass the Cph1p and Efg1p transcription factors classically associated with hyphal growth in vitro and in vivo in certain animal models (42–44). We examined the morphology of each CUG clade species when grown embedded in 0.5% agar in YPD (Fig. 3). Although the three-dimensional nature of these structures presents a challenge to clear photography, matrix embedding stimulated filamentous growth in most of the species, with M. guilliermondii again excepted. C. albicans exhibited the classic “beads-on-a-string” morphology with yeast cells budding from the septa of hyphae that could extend for hundreds of microns. Hyphal growth was also florid in C. tropicalis, but yeast cells were rarely seen budding from hyphae; rather, extensive angular branches produced a dense hyphal network. Pseudohyphal projections were observed at various frequencies in the other species.
Interactions with macrophages.
Avid hyphal growth of C. albicans is also common after phagocytosis by macrophages. We investigated morphology after phagocytosis under standard conditions using cells labeled with fluorescein isothiocyanate (FITC)-concanavalin A (Fig. 4). One hour after initiation of the coculture, germ tube formation was apparent in a majority of C. albicans cells, whereas they were more rarely seen (and were shorter) in C. dubliniensis. Pseudohyphae, but not true hyphae, were sometimes seen in phagocytosed C. tropicalis cells and swollen and/or elongated cells of C. parapsilosis were observed rarely. The remaining species remained exclusively in the yeast form.
FIG 4.
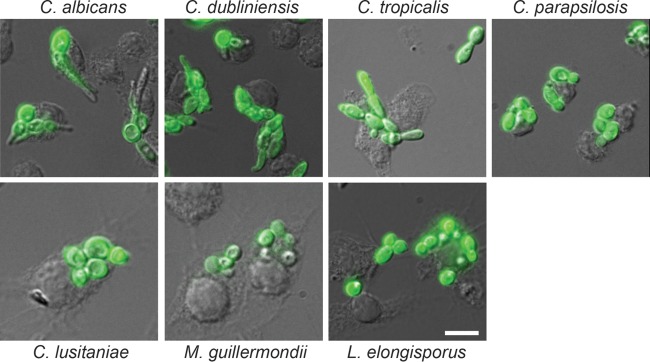
Morphology of phagocytosed cells. RAW264.7 macrophages were allowed to adhere to glass coverslips in 12-well plates and then cocultured with each CUG species for 1 h at 37°C in 5% CO2. After washing, fixing, and permeabilization, the fungal cells were incubated with an α-Candida polyclonal antibody conjugated to FITC before imaging. Scale bar, 10 μm.
We quantitated the cellular morphology in phagocytosed versus nonphagocytosed cells, which were distinguished by staining fixed, nonpermeabilized cells with calcofluor white, which binds to the cell wall of only nonphagocytosed fungal cells. As seen in Fig. 5, the proportion of hyphal or pseudohyphal cells were significantly higher in cells that remained in the media than those that were phagocytosed for C. albicans, C. tropicalis, and C. parapsilosis, indicating that the phagolysosomal environment inhibited some filamentation (the difference was not statistically significant for C. dubliniensis). Filamentous forms were not observed for the other species.
FIG 5.
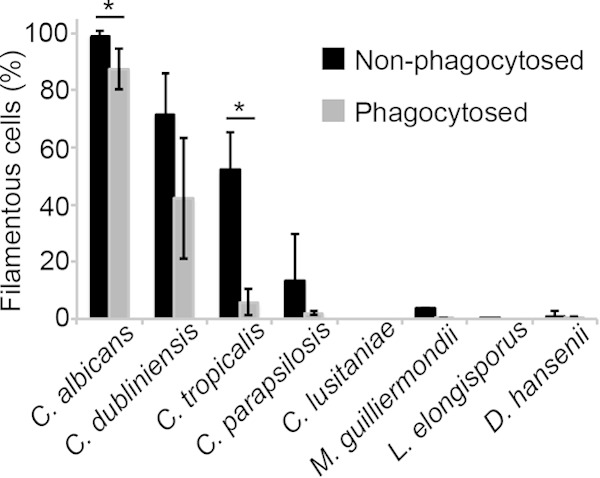
Quantitation of the morphology of phagocytosed cells. Photomicrographs of fungi cocultured with RAW264.7 cells (representative examples are shown in Fig. 4) were scored as filamentous (pseudohyphal or hyphal) or yeast. At least three replicate experiments were performed, from which an aggregate total of at least 100 cells were counted. Asterisks indicate a P value of <0.01 relative to nonphagocytosed cells.
The robust filamentous growth of C. albicans has been proposed to damage macrophages through physical disruption of the membrane, although recent reports ascribe some of the lysis to fungus-induced pyroptosis (45, 46). To assess the ability of the CUG species to lyse macrophages, we utilized a standard assay that measures release of host LDH into the medium. The fungal species were incubated with macrophages at a 3:1 ratio for 5 h before supernatants were assayed for LDH activity (Fig. 6). C. albicans, C. dubliniensis, and C. tropicalis induced nearly as much LDH release as chemically lysed macrophages (the positive control), while macrophage integrity remained high in cocultures with the other species. The species that induce lysis are the only species to filament to a significant degree inside macrophages (Fig. 4 and 5), reinforcing a link between morphogenesis and macrophage damage. However, the substantial variation in the proportion of phagocytosed cells in the hyphal or pseudohyphal form was not reflected in differences in LDH release, suggesting that nonmorphogenetic factors also contribute to lysis.
FIG 6.
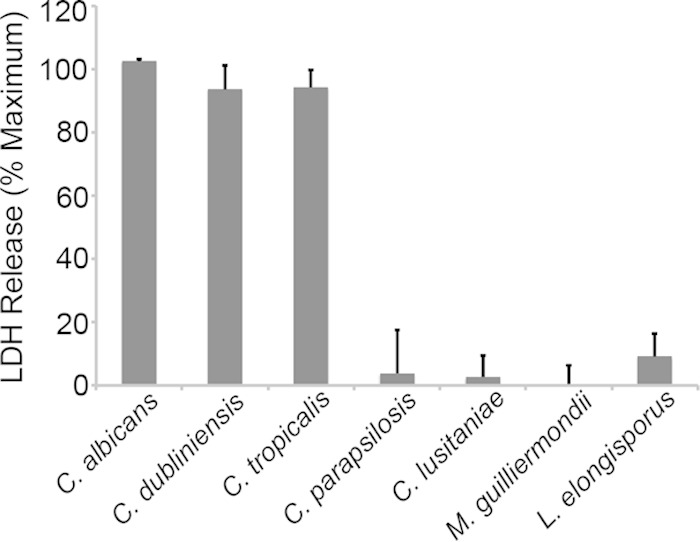
Macrophage damage induced by CUG species. Macrophage membrane integrity was estimated by assaying release of LDH from the cells as described in Materials and Methods and expressed relative to the maximum amount of LDH activity released from chemically lysed cells.
We recently reported that C. albicans cells modulate macrophage function by suppressing the production of reactive nitrogen species via an unknown soluble, secreted factor (23). We tested how broadly this activity was conserved in the CUG clade by assaying NO production in 24-h cocultures. Supernatants were analyzed using the Griess reagent, which detects nitrite, a spontaneous breakdown product of NO in aqueous cultures at neutral pH. As seen in Fig. 7, even at a fungus/macrophage ratio of 1:100, C. albicans and C. dubliniensis effectively suppressed NO production. The other species inhibited NO release modestly at the higher fungus/macrophage ratio, with C. tropicalis and, to a lesser extent, C. lusitaniae being the most effective of this second group.
FIG 7.
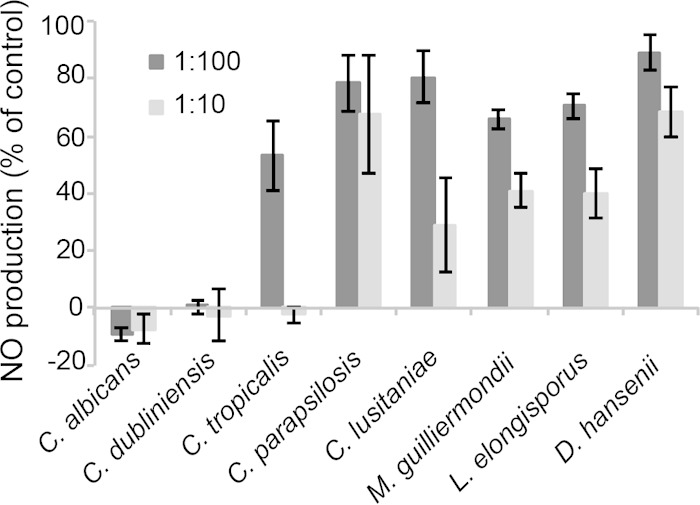
Inhibition of macrophage NO production by CUG species. RAW264.7 macrophages were seeded in 96-well plates and activated by the addition of 100 ng of LPS/ml and 100 U of IFN-γ/ml. At the same time, the fungal species were added at the indicated ratios and incubated for 24 h. NO production was assayed by detecting nitrite in the media using Griess reagent as described in Materials and Methods. Nitrite concentrations are expressed as a percentage of that produced by LPS/IFN-γ-stimulated macrophages incubated in the absence of fungi. Negative values indicate nitrite concentrations lower than the background produced by resting (unstimulated) macrophages.
Biofilm formation.
The formation of biofilms on medical implants, such as venous catheters, is an important risk factor in disseminated candidiasis and poses significant obstacles to effective therapy. C. albicans biofilms are highly polymorphic, and hyphal morphogenesis is required for optimal biofilm formation. To examine biofilms formed by CUG clade species, we used a standard assay that measures adherence to a polystyrene substrate in which biomass is estimated by the binding of the dye crystal violet, using two medium conditions: YNB (Fig. 8A) or RPMI (Fig. 8B) There was a clear distinction between three species that robustly adhered to the polystyrene in both conditions as measured by the retention of crystal violet (C. albicans, C. dubliniensis, and C. tropicalis) and the others, which did not (Fig. 8). Curiously, C. lusitaniae adhered to the plastic surface well when grown in YNB but not when grown in RPMI (Fig. 8).
FIG 8.
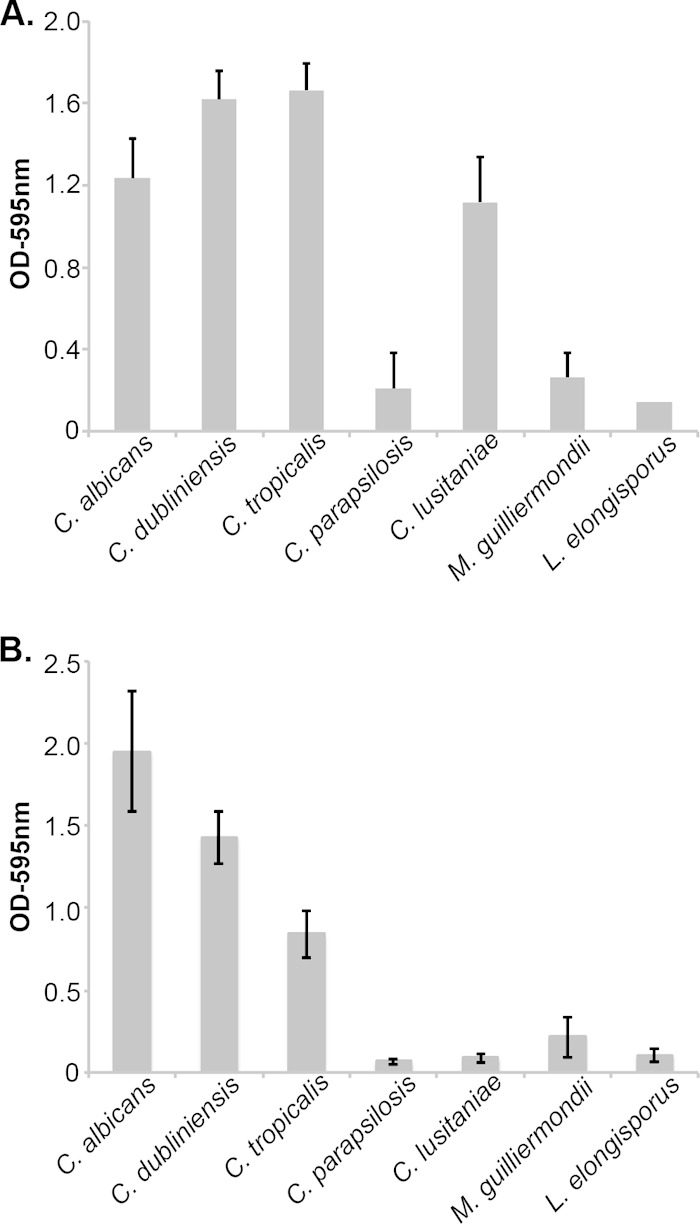
Biofilm adherence in CUG species. The adherence stage of biofilm formation was assayed in a 96-well polystyrene plate format in which biomass is estimated by retention of crystal violet, as described in Materials and Methods.
Strain variation in C. parapsilosis.
Our data clearly group C. parapsilosis with the less virulent species, a significant discrepancy relative to its clinical incidence. The sequenced reference isolate used here, CDC317, was isolated from the skin of a health care worker and, despite the implication of this strain in a hospital outbreak of invasive infection (47), it is possible that it is less robust in these host-related assays than other clinical isolates. To address this question, we examined two strains isolated from invasive infections: one isolated from feces and another CDC317 strain obtained from another laboratory in several of our assays (see Fig. S4 in the supplemental material). Although some strain variation was seen in growth rates and stress sensitivity, all of the C. parapsilosis strains were far less robust than the C. albicans (SC5314) control. One strain, CLIB214, a fecal isolate, was frequently seen in a pseudohyphal form, both in RPMI (see Fig. S4 in the supplemental material) and in YPD (not shown), and formed slightly more robust biofilms, but this had little effect on macrophage interactions or stress tolerance. Notably, in the macrophage lysis assay, our original CDC317 isolate outperformed the other C. parapsilosis strains (see Fig. S4 in the supplemental material). These data suggest that our conclusions based on a single strain are generally warranted.
DISCUSSION
In this study, we have assessed the fitness of eight species of the CUG clade in a variety of in vitro and ex vivo assays often used as proxies for virulence, including assays for resistance to host-associated stresses, hyphal morphogenesis, and interactions with phagocytes. Given the clinical significance of these species and the potential for them to be used to understand the evolution and/or mechanisms of fungal pathogenesis, the lack of comparative studies has been a hindrance. Taken together, our results correlate well with expectations based on animal models of virulence (8, 10, 12, 13), with C. albicans and C. tropicalis as the most robust species, though C. tropicalis was notably sensitive to oxidative stress. Although C. tropicalis is usually cited as the third or fourth most common cause of invasive infections (2, 4, 25), this species performs about as well as C. albicans in animal models, including those of disseminated hematogenous infections, gastrointestinal colonization, and dissemination from the gastrointestinal tract (8, 10, 12, 13). At the other end of the spectrum, M. guilliermondii, L. elongisporus and D. hansenii (when we were able to test it) were generally less stress tolerant and less fit when confronted with macrophages, an observation consistent with their low clinical impact.
The robustness in our assays of three species—C dubliniensis, C. lusitaniae, and C. parapsilosis—deviated from expectations from clinical incidence and animal models. C. dubliniensis, although closely related to C. albicans is a substantially weaker pathogen: only 24 cases of invasive infections from C. dubliniensis were identified among over 4,000 isolates from candidiasis patients (3). The disparity between the general fitness of this species and its low incidence may come from the absence of several particularly important virulence factors found in C. albicans, including the Als3 adhesin/iron acquisition protein, the Sap4-6 proteases, and the invasin Iff4 (48). Thus, a plausible mechanism for the difference in virulence and clinical incidence between these species is a combination of loss of specific genes (in C. dubliniensis) with expansion of gene families that mediate host interactions (in C. albicans).
C. lusitaniae clustered with the more virulent species in some measures and with the less virulent ones in others. In general, it performed well in assays more reflective of in vitro, laboratory conditions, including general growth rates and morphogenesis under nitrogen starvation, but was more sensitive to stresses and macrophage contact. The biofilm assay is illustrative: C. lusitaniae adhered well when grown in the minimal yeast media YNB but not when grown in the tissue culture medium RPMI. Further work will be needed to understand the genetic mechanisms by which this species has adapted to environmental but not host niches.
In contrast, C. parapsilosis performed poorly in nearly every assay, a finding consistent with published reports indicating that it is much less virulent than C. albicans in animal models (8, 9, 49), despite its clinical incidence. Although intraspecies strain variation can be significant (see, for instance, references 50, 51, 52, and 53), several additional C. parapsilosis species fared no better than did CDC317, the sequenced reference isolate used in most of our studies. Another recent study that looked at a larger set of C. parapsilosis clinical isolates also concluded that, while there were phenotypic differences in vitro, the effects on host interactions were modest (54). The discrepancy between the lab and the clinic might be explained if C. parapsilosis were more common as a commensal or more easily transmitted from person to person, such that patients were exposed to it more frequently. Indeed, there is some evidence for this: C. parapsilosis is more commonly isolated from skin than C. albicans, with one study identifying it as the most commonly isolated yeast species on hands (55–57; see also reference 58). A few studies have linked cases in neonatal intensive care units to transmission from health care workers (59–61), including the type strain CDC317 (47). The prevalence of C. parapsilosis fungemia in neonates suggests that this population is particularly susceptible to this species (3, 61, 62) and that the risk factors for these patients may not be modeled effectively in vitro or in mouse models.
The adaptations to the host environment that contribute to pathogenesis come from the many changes in gene content between species, which has been addressed in bioinformatic detail (17), but not at a mechanistic level for the most part. They also derive from changes in patterns of regulation, which are only beginning to be understood. A recent analysis of transcription factors in C. parapsilosis using a small library of mutant strains demonstrated significant rewiring of regulatory networks in biofilm formation and the hypoxic response relative to C. albicans (63). C. dubliniensis differentiates into hyphae less readily than C. albicans, partly because it lacks a close homolog of the hyphal regulatory transcription factor Sfl2 (64). These regulatory changes, and likely many others, contribute to phenotypic differences relevant to virulence, including hyphal and biofilm formation and responses to oxidative and hypoxic stresses. These might be more subtle than our assays can detect; indeed, C. dubliniensis does poorly when incubated competitively with C. albicans in several broth and biofilm assays (63–65).
As for any complex trait, the ability to cause pathology in a mammalian host is a multifactorial phenomenon, and the analysis of closely related species with vastly different inherent virulence is a potentially powerful tool for understanding which genetic and regulatory adaptations are particularly important. Our study directly compares a large number of species across this spectrum in numerous host-related assays for the first time, providing greater insight into the evolution of virulence in this species complex. Further study will be needed to understand the mechanistic basis for the phenotypic differences we observe and to determine how to exploit these to improve outcomes for patients.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to D. Soll, A. Brown, J. Heitman, and G. Butler for the gift of strains. We thank other members of the Lorenz laboratory, especially S. Vylkova, H. Danhof, P. Miramón, and E. Vesely for advice and helpful discussions.
This study was supported by Public Health Service grant R21AI105651 to M.C.L.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/EC.00062-15.
REFERENCES
- 1.Pfaller MA, Diekema DJ. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diekema D, Arbefeville S, Boyken L, Kroeger J, Pfaller M. 2012. The changing epidemiology of healthcare-associated candidemia over three decades. Diagn Microbiol Infect Dis 73:45–48. doi: 10.1016/j.diagmicrobio.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Pfaller M, Neofytos D, Diekema D, Azie N, Meier-Kriesche HU, Quan SP, Horn D. 2012. Epidemiology and outcomes of candidemia in 3,648 patients: data from the Prospective Antifungal Therapy (PATH Alliance(R)) registry, 2004-2008. Diagn Microbiol Infect Dis 74:323–331. doi: 10.1016/j.diagmicrobio.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Wisplinghoff H, Ebbers J, Geurtz L, Stefanik D, Major Y, Edmond MB, Wenzel RP, Seifert H. 2014. Nosocomial bloodstream infections due to Candida spp. in the USA: species distribution, clinical features and antifungal susceptibilities. Int J Antimicrob Agents 43:78–81. doi: 10.1016/j.ijantimicag.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Massey SE, Moura G, Beltrao P, Almeida R, Garey JR, Tuite MF, Santos MA. 2003. Comparative evolutionary genomics unveils the molecular mechanism of reassignment of the CTG codon in Candida spp. Genome Res 13:544–557. doi: 10.1101/gr.811003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santos MA, Tuite MF. 1995. The CUG codon is decoded in vivo as serine and not leucine in Candida albicans. Nucleic Acids Res 23:1481–1486. doi: 10.1093/nar/23.9.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett RJ. 2009. A Candida-based view of fungal sex and pathogenesis. Genome Biol 10:230. doi: 10.1186/gb-2009-10-7-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arendrup M, Horn T, Frimodt-Moller N. 2002. In vivo pathogenicity of eight medically relevant Candida species in an animal model. Infection 30:286–291. doi: 10.1007/s15010-002-2131-0. [DOI] [PubMed] [Google Scholar]
- 9.Bistoni F, Vecchiarelli A, Cenci E, Sbaraglia G, Perito S, Cassone A. 1984. A comparison of experimental pathogenicity of Candida species in cyclophosphamide-immunodepressed mice. Sabouraudia 22:409–418. doi: 10.1080/00362178485380661. [DOI] [PubMed] [Google Scholar]
- 10.de Repentigny L, Phaneuf M, Mathieu LG. 1992. Gastrointestinal colonization and systemic dissemination by Candida albicans and Candida tropicalis in intact and immunocompromised mice. Infect Immun 60:4907–4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howlett JA. 1976. The infection of rat tongue mucosa in vitro with five species of Candida. J Med Microbiol 9:309–316. doi: 10.1099/00222615-9-3-309. [DOI] [PubMed] [Google Scholar]
- 12.Mellado E, Cuenca-Estrella M, Regadera J, Gonzalez M, Diaz-Guerra TM, Rodriguez-Tudela JL. 2000. Sustained gastrointestinal colonization and systemic dissemination by Candida albicans, Candida tropicalis, and Candida parapsilosis in adult mice. Diagn Microbiol Infect Dis 38:21–28. doi: 10.1016/S0732-8893(00)00165-6. [DOI] [PubMed] [Google Scholar]
- 13.Wingard JR, Dick JD, Merz WG, Sandford GR, Saral R, Burns WH. 1982. Differences in virulence of clinical isolates of Candida tropicalis and Candida albicans in mice. Infect Immun 37:833–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayer FL, Wilson D, Hube B. 2013. Candida albicans pathogenicity mechanisms. Virulence 4:119–128. doi: 10.4161/viru.22913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abegg MA, Alabarse PV, Casanova A, Hoscheid J, Salomon TB, Hackenhaar FS, Medeiros TM, Benfato MS. 2010. Response to oxidative stress in eight pathogenic yeast species of the genus Candida. Mycopathologia 170:11–20. doi: 10.1007/s11046-010-9294-5. [DOI] [PubMed] [Google Scholar]
- 16.Braun BR, van het Hoog M, d'Enfert C, Martchenko M, Dungan J, Kuo A, Inglis DO, Uhl MA, Hogues H, Berriman M, Lorenz MC, Levitin A, Oberholzer U, Bachewich C, Harcus D, Marcil A, Dignard D, Iouk T, Zito R, Frangeul L, Tekaia F, Rutherford K, Wang E, Gow NA, Hoyer LL, Kohler G, Morschhauser J, Newport G, Znaidi S, Raymond M, Turcotte B, Sherlock G, Costanzo M, Ihmels J, Berman J, Sanglard D, Agabian N, Mitchell AP, Johnson AD, Whiteway M, Nantel A. 2005. A human-curated annotation of the Candida albicans genome. PLoS Genet 1:38–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butler G, Rasmussen MD, Lin MF, Santos MA, Sakthikumar S, Munro CA, Rheinbay E, Grabherr M, Forche A, Reedy JL, Agrafioti I, Arnaud MB, Bates S, Brown AJ, Brunke S, Costanzo MC, Fitzpatrick DA, de Groot PW, Harris D, Hoyer LL, Hube B, Klis FM, Kodira C, Lennard N, Logue ME, Martin R, Neiman AM, Nikolaou E, Quail MA, Quinn J, Santos MC, Schmitzberger FF, Sherlock G, Shah P, Silverstein KA, Skrzypek MS, Soll D, Staggs R, Stansfield I, Stumpf MP, Sudbery PE, Srikantha T, Zeng Q, Berman J, Berriman M, Heitman J, Gow NA, Lorenz MC, Birren BW, Kellis M, Cuomo CA. 2009. Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature 459:657–662. doi: 10.1038/nature08064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dujon B, Sherman D, Fischer G, Durrens P, Casaregola S, Lafontaine I, De Montigny J, Marck C, Neuveglise C, Talla E, Goffard N, Frangeul L, Aigle M, Anthouard V, Babour A, Barbe V, Barnay S, Blanchin S, Beckerich JM, Beyne E, Bleykasten C, Boisrame A, Boyer J, Cattolico L, Confanioleri F, De Daruvar A, Despons L, Fabre E, Fairhead C, Ferry-Dumazet H, Groppi A, Hantraye F, Hennequin C, Jauniaux N, Joyet P, Kachouri R, Kerrest A, Koszul R, Lemaire M, Lesur I, Ma L, Muller H, Nicaud JM, Nikolski M, Oztas S, Ozier-Kalogeropoulos O, Pellenz S, Potier S, Richard GF, Straub ML, Suleau A, Swennen D, Tekaia F, Wesolowski-Louvel M, Westhof E, Wirth B, Zeniou-Meyer M, Zivanovic I, Bolotin-Fukuhara M, Thierry A, Bouchier C, Caudron B, Scarpelli C, Gaillardin C, Weissenbach J, Wincker P, Souciet JL. 2004. Genome evolution in yeasts. Nature 430:35–44. doi: 10.1038/nature02579. [DOI] [PubMed] [Google Scholar]
- 19.Sherman F. 1991. Getting started with yeast. Methods Enzymol 194:3–21. [DOI] [PubMed] [Google Scholar]
- 20.Gimeno CJ, Ljungdahl PO, Styles CA, Fink GR. 1992. Unipolar cell divisions in the yeast Saccharomyces cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell 68:1077–1090. doi: 10.1016/0092-8674(92)90079-R. [DOI] [PubMed] [Google Scholar]
- 21.Vylkova S, Carman AJ, Danhof HA, Collette JR, Zhou H, Lorenz MC. 2011. The fungal pathogen Candida albicans autoinduces hyphal morphogenesis by raising extracellular pH. mBio 2:e00055-11. doi: 10.1128/mBio.00055-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vylkova S, Lorenz MC. 2014. Modulation of phagosomal pH by Candida albicans promotes hyphal morphogenesis and requires Stp2p, a regulator of amino acid transport. PLoS Pathog 10:e1003995. doi: 10.1371/journal.ppat.1003995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collette JR, Zhou H, Lorenz MC. 2014. Candida albicans suppresses nitric oxide generation from macrophages via a secreted molecule. PLoS One 9:e96203. doi: 10.1371/journal.pone.0096203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin Y, Yip HK, Samaranayake YH, Yau JY, Samaranayake LP. 2003. Biofilm-forming ability of Candida albicans is unlikely to contribute to high levels of oral yeast carriage in cases of human immunodeficiency virus infection. J Clin Microbiol 41:2961–2967. doi: 10.1128/JCM.41.7.2961-2967.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfaller MA, Andes DR, Diekema DJ, Horn DL, Reboli AC, Rotstein C, Franks B, Azie NE. 2014. Epidemiology and outcomes of invasive candidiasis due to non-albicans species of Candida in 2,496 patients: data from the Prospective Antifungal Therapy (PATH) registry 2004-2008. PLoS One 9:e101510. doi: 10.1371/journal.pone.0101510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maguire SL, OhEigeartaigh SS, Byrne KP, Schroder MS, O'Gaora P, Wolfe KH, Butler G. 2013. Comparative genome analysis and gene finding in Candida species using CGOB. Mol Biol Evol 30:1281–1291. doi: 10.1093/molbev/mst042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maguire SL, Wang C, Holland LM, Brunel F, Neuveglise C, Nicaud JM, Zavrel M, White TC, Wolfe KH, Butler G. 2014. Zinc finger transcription factors displaced SREBP proteins as the major sterol regulators during Saccharomycotina evolution. PLoS Genet 10:e1004076. doi: 10.1371/journal.pgen.1004076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ene IV, Adya AK, Wehmeier S, Brand AC, MacCallum DM, Gow NA, Brown AJ. 2012. Host carbon sources modulate cell wall architecture, drug resistance and virulence in a fungal pathogen. Cell Microbiol 14:1319–1335. doi: 10.1111/j.1462-5822.2012.01813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ene IV, Cheng SC, Netea MG, Brown AJ. 2013. Growth of Candida albicans cells on the physiologically relevant carbon source lactate affects their recognition and phagocytosis by immune cells. Infect Immun 81:238–248. doi: 10.1128/IAI.01092-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lorenz MC, Bender JA, Fink GR. 2004. Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot Cell 3:1076–1087. doi: 10.1128/EC.3.5.1076-1087.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barelle CJ, Priest CL, Maccallum DM, Gow NA, Odds FC, Brown AJ. 2006. Niche-specific regulation of central metabolic pathways in a fungal pathogen. Cell Microbiol 8:961–971. doi: 10.1111/j.1462-5822.2005.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lorenz MC, Fink GR. 2001. The glyoxylate cycle is required for fungal virulence. Nature 412:83–86. doi: 10.1038/35083594. [DOI] [PubMed] [Google Scholar]
- 33.Piekarska K, Mol E, van den Berg M, Hardy G, van den Burg J, van Roermund C, Maccallum D, Odds F, Distel B. 2006. Peroxisomal fatty acid β-oxidation is not essential for virulence of Candida albicans. Eukaryot Cell 5:1847–1856. doi: 10.1128/EC.00093-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramirez MA, Lorenz MC. 2007. Mutations in alternative carbon utilization pathways in Candida albicans attenuate virulence and confer pleiotropic phenotypes. Eukaryot Cell 6:280–290. doi: 10.1128/EC.00372-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kasper L, Seider K, Gerwien F, Allert S, Brunke S, Schwarzmuller T, Ames L, Zubiria-Barrera C, Mansour MK, Becken U, Barz D, Vyas JM, Reiling N, Haas A, Haynes K, Kuchler K, Hube B. 2014. Identification of Candida glabrata genes involved in pH modulation and modification of the phagosomal environment in macrophages. PLoS One 9:e96015. doi: 10.1371/journal.pone.0096015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lo HJ, Kohler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR. 1997. Nonfilamentous Candida albicans mutants are avirulent. Cell 90:939–949. doi: 10.1016/S0092-8674(00)80358-X. [DOI] [PubMed] [Google Scholar]
- 37.Saville SP, Lazzell AL, Monteagudo C, Lopez-Ribot JL. 2003. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot Cell 2:1053–1060. doi: 10.1128/EC.2.5.1053-1060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwon-Chung KJ, Bennett JE. 1992. Medical mycology. Lea & Febiger, Philadelphia, PA. [Google Scholar]
- 39.Lackey E, Vipulanandan G, Childers DS, Kadosh D. 2013. Comparative evolution of morphological regulatory functions in Candida species. Eukaryot Cell 12:1356–1368. doi: 10.1128/EC.00164-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen YL, Yu SJ, Huang HY, Chang YL, Lehman VN, Silao FG, Bigol UG, Bungay AA, Averette A, Heitman J. 2014. Calcineurin controls hyphal growth, virulence, and drug tolerance of Candida tropicalis. Eukaryot Cell 13:844–854. doi: 10.1128/EC.00302-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Csank C, Schroppel K, Leberer E, Harcus D, Mohamed O, Meloche S, Thomas DY, Whiteway M. 1998. Roles of the Candida albicans mitogen-activated protein kinase homolog, Cek1p, in hyphal development and systemic candidiasis. Infect Immun 66:2713–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown DH Jr, Giusani AD, Chen X, Kumamoto CA. 1999. Filamentous growth of Candida albicans in response to physical environmental cues and its regulation by the unique CZF1 gene. Mol Microbiol 34:651–662. doi: 10.1046/j.1365-2958.1999.01619.x. [DOI] [PubMed] [Google Scholar]
- 43.Rosenbach A, Dignard D, Pierce JV, Whiteway M, Kumamoto CA. 2010. Adaptations of Candida albicans for growth in the mammalian intestinal tract. Eukaryot Cell 9:1075–1086. doi: 10.1128/EC.00034-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.White SJ, Rosenbach A, Lephart P, Nguyen D, Benjamin A, Tzipori S, Whiteway M, Mecsas J, Kumamoto CA. 2007. Self-regulation of Candida albicans population size during GI colonization. PLoS Pathog 3:e184. doi: 10.1371/journal.ppat.0030184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Meara TR, Veri AO, Ketela T, Jiang B, Roemer T, Cowen LE. 2015. Global analysis of fungal morphology exposes mechanisms of host cell escape. Nat Commun 6:6741. doi: 10.1038/ncomms7741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wellington M, Koselny K, Sutterwala FS, Krysan DJ. 2014. Candida albicans triggers NLRP3-mediated pyroptosis in macrophages. Eukaryot Cell 13:329–340. doi: 10.1128/EC.00336-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clark TA, Slavinski SA, Morgan J, Lott T, Arthington-Skaggs BA, Brandt ME, Webb RM, Currier M, Flowers RH, Fridkin SK, Hajjeh RA. 2004. Epidemiologic and molecular characterization of an outbreak of Candida parapsilosis bloodstream infections in a community hospital. J Clin Microbiol 42:4468–4472. doi: 10.1128/JCM.42.10.4468-4472.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jackson AP, Gamble JA, Yeomans T, Moran GP, Saunders D, Harris D, Aslett M, Barrell JF, Butler G, Citiulo F, Coleman DC, de Groot PW, Goodwin TJ, Quail MA, McQuillan J, Munro CA, Pain A, Poulter RT, Rajandream MA, Renauld H, Spiering MJ, Tivey A, Gow NA, Barrell B, Sullivan DJ, Berriman M. 2009. Comparative genomics of the fungal pathogens Candida dubliniensis and Candida albicans. Genome Res 19:2231–2244. doi: 10.1101/gr.097501.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barrett-Bee K, Hayes Y, Wilson RG, Ryley JF. 1985. A comparison of phospholipase activity, cellular adherence and pathogenicity of yeasts. J Gen Microbiol 131:1217–1221. [DOI] [PubMed] [Google Scholar]
- 50.Abi-Chacra EA, Souza LO, Cruz LP, Braga-Silva LA, Goncalves DS, Sodre CL, Ribeiro MD, Seabra SH, Figueiredo-Carvalho MH, Barbedo LS, Zancope-Oliveira RM, Ziccardi M, Santos AL. 2013. Phenotypical properties associated with virulence from clinical isolates belonging to the Candida parapsilosis complex. FEMS Yeast Res 13:831–848. doi: 10.1111/1567-1364.12092. [DOI] [PubMed] [Google Scholar]
- 51.Li X, Yan Z, Xu J. 2003. Quantitative variation of biofilms among strains in natural populations of Candida albicans. Microbiology 149:353–362. doi: 10.1099/mic.0.25932-0. [DOI] [PubMed] [Google Scholar]
- 52.MacCallum DM, Castillo L, Nather K, Munro CA, Brown AJ, Gow NA, Odds FC. 2009. Property differences among the four major Candida albicans strain clades. Eukaryot Cell 8:373–387. doi: 10.1128/EC.00387-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pannanusorn S, Ramirez-Zavala B, Lunsdorf H, Agerberth B, Morschhauser J, Romling U. 2014. Characterization of biofilm formation and the role of BCR1 in clinical isolates of Candida parapsilosis. Eukaryot Cell 13:438–451. doi: 10.1128/EC.00181-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nemeth T, Toth A, Szenzenstein J, Horvath P, Nosanchuk JD, Grozer Z, Toth R, Papp C, Hamari Z, Vagvolgyi C, Gacser A. 2013. Characterization of virulence properties in the Candida parapsilosis sensu lato species. PLoS One 8:e68704. doi: 10.1371/journal.pone.0068704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bonassoli LA, Bertoli M, Svidzinski TI. 2005. High frequency of Candida parapsilosis on the hands of healthy hosts. J Hosp Infect 59:159–162. doi: 10.1016/j.jhin.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 56.Findley K, Oh J, Yang J, Conlan S, Deming C, Meyer JA, Schoenfeld D, Nomicos E, Park M, Kong HH, Segre JA, Program NIHISCCS. 2013. Topographic diversity of fungal and bacterial communities in human skin. Nature 498:367–370. doi: 10.1038/nature12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saiman L, Ludington E, Dawson JD, Patterson JE, Rangel-Frausto S, Wiblin RT, Blumberg HM, Pfaller M, Rinaldi M, Edwards JE, Wenzel RP, Jarvis W, National Epidemiology of Mycoses Study Group. 2001. Risk factors for Candida species colonization of neonatal intensive care unit patients. Pediatr Infect Dis J 20:1119–1124. doi: 10.1097/00006454-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 58.van Asbeck EC, Clemons KV, Stevens DA. 2009. Candida parapsilosis: a review of its epidemiology, pathogenesis, clinical aspects, typing and antimicrobial susceptibility. Crit Rev Microbiol 35:283–309. doi: 10.3109/10408410903213393. [DOI] [PubMed] [Google Scholar]
- 59.Hernandez-Castro R, Arroyo-Escalante S, Carrillo-Casas EM, Moncada-Barron D, Alvarez-Verona E, Hernandez-Delgado L, Torres-Narvaez P, Lavalle-Villalobos A. 2010. Outbreak of Candida parapsilosis in a neonatal intensive care unit: a health care workers source. Eur J Pediatr 169:783–787. doi: 10.1007/s00431-009-1109-7. [DOI] [PubMed] [Google Scholar]
- 60.Lupetti A, Tavanti A, Davini P, Ghelardi E, Corsini V, Merusi I, Boldrini A, Campa M, Senesi S. 2002. Horizontal transmission of Candida parapsilosis candidemia in a neonatal intensive care unit. J Clin Microbiol 40:2363–2369. doi: 10.1128/JCM.40.7.2363-2369.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Asbeck EC, Huang YC, Markham AN, Clemons KV, Stevens DA. 2007. Candida parapsilosis fungemia in neonates: genotyping results suggest health care workers hands as source, and review of published studies. Mycopathologia 164:287–293. doi: 10.1007/s11046-007-9054-3. [DOI] [PubMed] [Google Scholar]
- 62.Pammi M, Holland L, Butler G, Gacser A, Bliss JM. 2013. Candida parapsilosis is a significant neonatal pathogen: a systematic review and meta-analysis. Pediatr Infect Dis J 32:e206–216. doi: 10.1097/INF.0b013e3182863a1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Holland LM, Schroder MS, Turner SA, Taff H, Andes D, Grozer Z, Gacser A, Ames L, Haynes K, Higgins DG, Butler G. 2014. Comparative phenotypic analysis of the major fungal pathogens Candida parapsilosis and Candida albicans. PLoS Pathog 10:e1004365. doi: 10.1371/journal.ppat.1004365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spiering MJ, Moran GP, Chauvel M, Maccallum DM, Higgins J, Hokamp K, Yeomans T, d'Enfert C, Coleman DC, Sullivan DJ. 2010. Comparative transcript profiling of Candida albicans and Candida dubliniensis identifies SFL2, a C. albicans gene required for virulence in a reconstituted epithelial infection model. Eukaryot Cell 9:251–265. doi: 10.1128/EC.00291-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kirkpatrick WR, Lopez-Ribot JL, McAtee RK, Patterson TF. 2000. Growth competition between Candida dubliniensis and Candida albicans under broth and biofilm growing conditions. J Clin Microbiol 38:902–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fitzpatrick DA, Logue ME, Stajich JE, Butler G. 2006. A fungal phylogeny based on 42 complete genomes derived from supertree and combined gene analysis. BMC Evol Biol 6:99. doi: 10.1186/1471-2148-6-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gillum AM, Tsay EY, Kirsch DR. 1984. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet 198:179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- 68.Jones T, Federspiel NA, Chibana H, Dungan J, Kalman S, Magee BB, Newport G, Thorstenson YR, Agabian N, Magee PT, Davis RW, Scherer S. 2004. The diploid genome sequence of Candida albicans. Proc Natl Acad Sci U S A 101:7329–7334. doi: 10.1073/pnas.0401648101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sullivan DJ, Westerneng TJ, Haynes KA, Bennett DE, Coleman DC. 1995. Candida dubliniensis sp. nov.: phenotypic and molecular characterization of a novel species associated with oral candidosis in HIV-infected individuals. Microbiology 141(Pt 7):1507–1521. [DOI] [PubMed] [Google Scholar]
- 70.Joly S, Pujol C, Schroppel K, Soll DR. 1996. Development of two species-specific fingerprinting probes for broad computer-assisted epidemiological studies of Candida tropicalis. J Clin Microbiol 34:3063–3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Holzschu DL, Presley HL, Miranda M, Phaff HJ. 1979. Identification of Candida lusitaniae as an opportunistic yeast in humans. J Clin Microbiol 10:202–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Castellani AJ. 1912. Observations on the fungi found in tropical bronchomycosis. Lancet 179:13–15. doi: 10.1016/S0140-6736(00)51698-5. [DOI] [Google Scholar]
- 73.Recca J, Mrak EM. 1952. Yeasts occurring in citrus products. Food Technol 6:450–454. [Google Scholar]
- 74.Ashford BK. 1928. Certain conditions of the gastro-intestinal tract in Porto Rico and their relation to tropical sprue. Am J Trop Med Hyg 8:507–538. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.